Identification and characterization of predisposition syndromes for gastric carcinomas require a multidisciplinary effort. This article reviews the molecular genetics, clinical and pathologic features, surveillance guidelines, and preventive measures of common and less common hereditary syndromes to facilitate a better understanding of the biology of these conditions and allow early identification and intervention.
Keywords: Stomach, Cancer, Hereditary, Familial, Syndromic
Abstract
Although the majority of gastric carcinomas are sporadic, approximately 10% show familial aggregation, and a hereditary cause is determined in 1%–3% cases. Of these, hereditary diffuse gastric cancer is the most recognized predisposition syndrome. Although rare, the less commonly known syndromes also confer a markedly increased risk for development of gastric cancer. Identification and characterization of these syndromes require a multidisciplinary effort involving oncologists, surgeons, genetic counselors, biologists, and pathologists. This article reviews the molecular genetics, clinical and pathologic features, surveillance guidelines, and preventive measures of common and less common hereditary gastric cancer predisposition syndromes.
Implications for Practice:
Although the majority of gastric adenocarcinomas are sporadic with many of those related to chronic Helicobacter pylori infection, approximately 10% of the cases show familial aggregation, and a specific hereditary cause is determined in 1%–3% cases. This review describes the molecular genetics, clinical and pathologic features, surveillance guidelines, and preventive measures of common and less common hereditary gastric cancer predisposition syndromes. Ultimately, a better understanding of the biology of these conditions should allow early identification and intervention as part of a multidisciplinary approach involving oncologists, surgeons, genetic counselors, and pathologists.
Abstract
摘要
尽管大多数胃癌为散发性,但约有10%表现出家族聚集性,约1%∼3%的患者确诊为遗传性病因。在这些患者中,遗传性弥漫型胃癌是最为人熟知的易感综合征。而一些较不为人知的综合征尽管少见,同样可明显增加发生胃癌的风险。识别和鉴定这些综合征需要包括肿瘤科医生、外科医生、遗传学专家、生物学家和病理学家的多学科协作。本文对常见和较少见的遗传性胃癌易感综合征的分子遗传学、临床和病理特征、监测指南和预防措施进行了综述。The Oncologist 2015;20:1365–1377
对临床实践的提示:尽管多数胃腺癌为散发性,并且其中许多与慢性幽门螺杆菌感染有关,但大约有10%的患者表现为家族聚集性,1%∼3%的病例可确认特异性遗传性病因。本综述描述了常见和较少见遗传性胃癌易感综合征的分子遗传学、临床和病理特征、监测指南和预防措施。最后,更好地理解这些病症的生物学有助于多学科手段的早期识别和干预,这些学科涉及了肿瘤科医生、外科医生、遗传学专家和病理学家。
Introduction
Family history is a well-recognized risk factor for gastric cancer, with the most famous example of hereditary transmission of gastric carcinoma being the family of Napoleon Bonaparte. Napoleon had five first degree relatives affected by gastric carcinoma, affecting three consecutive generations [1].
The term “familial gastric cancer” has been used to describe families with 2 first- or second-degree relatives with gastric cancer before the age of 50 years or 3 first- or second-degree relatives with gastric cancer independent of age [2]. Clustering of gastric cancer can be seen in such families in approximately 10% of cases. However, a gene defect can be determined in only 1%–3% of cases [3, 4]. A better understanding of the biology of these predisposition syndromes would allow early identification and intervention and improve life expectancy.
Familial gastric cancer syndromes can be classified into two categories: (a) hereditary gastric cancer with polyps and (b) hereditary gastric cancer without polyps. Polyp-associated syndromes may endoscopically present as “polyposis,” with polyps carpeting the wall of the stomach. Histologically, like sporadic cancer, hereditary neoplasms can be broadly classified as intestinal, diffuse, or mixed. In this review, we discuss the features of hereditary gastric cancers, with a focus on molecular genetics, pathologic features, surveillance guidelines, and preventive measures.
Hereditary Gastric Cancer Associated with Polyps
Familial Adenomatous Polyposis/Attenuated Familial Adenomatous Polyposis
Overview and Inclusion Criteria
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder. The presence of gastric polyps is a known manifestation of FAP, with a reported incidence varying from 51% [5] to 88% [6]. The incidence of gastric polyps in attenuated FAP has been reported to be even higher (93%) in a series of 16 patients [7]. The pediatric population is affected, with gastric polyps reported in 81% of syndromic children, 31% of them harboring dysplasia [8]. The risk of gastric carcinoma in FAP varies geographically. A high risk has been reported in Japan (4.5%–13.6%) [9] but has not been confirmed in the West, where the risk of gastric carcinoma is low (0.6%–4.2%) [10, 11]. Overall, it has been estimated that Korean and Japanese FAP patients are 7–10 times more likely to develop gastric carcinoma than nonsyndromic patients [12, 13]. The reason for this difference is currently not known.
It is worth noting that although neither gastric polyps nor carcinoma is a defining feature of FAP or attenuated FAP in the West, gastric cancer is considered an extracolonic manifestation of FAP in the East. Another criterion establishing FAP is the presence of more than 100 adenomatous colorectal polyps. If the number is less than 100, a diagnosis of attenuated FAP (AFAP) may be suggested if (a) there are at least 2 family members with 10–99 adenomas at age greater than 30 years or (b) an individual with 10–99 adenomas at age greater than 30 years and 1 first-degree relative with colorectal cancer (CRC) and few adenomas [14]. Other features of FAP or AFAP include extraintestinal abnormalities (e.g., congenital hypertrophy of retinal pigment epithelium, osteomas, epidermoid cysts, desmoid tumor, adrenal adenoma, or thyroid carcinoma, hepatoblastoma, and brain tumors) [13].
It is worth noting that although neither gastric polyps nor carcinoma is a defining feature of FAP or attenuated FAP in the West, gastric cancer is considered an extracolonic manifestation of FAP in the East. Another criterion establishing FAP is the presence of more than 100 adenomatous colorectal polyps.
Molecular Genetics
The FAP and AFAP syndromes are autosomal dominant disorders with a high penetrance [15] and are caused by heterozygous mutation in the adenomatous polyposis coli (APC) tumor suppressor gene on Chr 5q21. Several mechanisms of germline inactivation of APC have been described, most of which (>90%) lead to truncation of APC protein [16]. These mutations have been detected in approximately 67% of FAP patients and include cases of both point mutations/substitutions (43% cases) and insertion deletions (indels) (57%). Although no mutational hotspots have been identified for point mutations, 97% of the indels were found in exon 15 [16].
The clinical phenotype and severity are determined not only by the loss of function of the APC gene and type of second hit (somatic mutation), but also by the position of the germline mutations [17]. For example, mutations toward the 5′ and the 3′ ends (codons 1,982–1,983) have been associated with profuse gastric fundic gland polyposis [18]. Somatic mutations involving codon 1,554–1,556 also have been reported in 51% of FAP-associated familial gastric polyposis (FGP) and 50% of gastric adenomas (GAs). Finally, because of a higher risk of upper gastrointestinal polyposis, one group has suggested more aggressive upper gastrointestinal screening in patients with mutations in codons 1,099–1,694 [5]. However, no difference has been observed between gastric or duodenal location of the polyps and mutation sites [19]. With regard to the subtype, mutations in exons 10–15H (codon 564–1,465) have been seen with a significantly higher frequency in GAs yet are undetected in FGPs [20]. Finally, a few studies have reported mutational variation in dysplastic and malignant FAP-associated gastric polyps, with rare KRAS mutations in codon 12 seen in FGPs harboring low grade [21] and mutations in exon 4 of the FAP gene reported in gastric polyposis and early-onset gastric carcinoma in AFAP patients [22].
Clinical Features and Pathology
The age of onset of gastric manifestations is variable. Although gastric adenocarcinomas typically develop long after colectomy (often greater than 20 years) [23], FGPs have been detected as early as 8 years of age [24], and gastric carcinoma as early as 11 years of age [5]. The types of benign gastric lesions detected include FGPs (reported in 26% [25] to 85% [6] of FAP cases), GAs (reported in 2% [25] to 41% [5]), and, rarely, hyperplastic polyps [26] and pyloric adenomas [27]. Although FGPs are limited to the body/fundus, GAs are commonly present at the junction of the body/antrum [26]. In fact, when GAs are detected in the body/fundus, they are commonly associated with adenomatous change in FGPs. Another feature associated with syndromic FGPs is their multiplicity, endoscopically presenting as gastric polyposis (arbitrarily defined as >20 polyps) [26]. Instead, adenomas tend to be less likely multiple [28] and are generally flat or sessile [29], making the endoscopic identification more challenging.
Syndromic FGPs have a higher incidence of harboring incipient dysplasia (25% [30] to 44% [31]) than sporadic FGPs (∼1%) [30], and low grade dysplasia is observed more commonly than high grade. The risk of developing dysplasia is directly proportional to the size of the polyp and the presence of antral gastritis [6]. The risk of carcinoma is low. Reported gastric adenocarcinomas are of the World Health Organization tubular type (Lauren intestinal type) [23, 32]. Multicentric or metachronous lesions have been described [23]. Gastric cancers can also arise in the absence of precursor lesions [33].
Surveillance and Clinical Management
There are no standard surveillance guidelines for upper endoscopy in FAP patients. However, current data suggest that it should be started at 21–30 years of age [8] and performed at intervals of 3–5 years [34].
Nonsteroidal anti-inflammatory drugs and acid-suppressive therapy have been associated with regression and reduction in the number of gastric polyps [35] and incidence of dysplasia [6]. However, the impact of this on the development of malignancy and overall survival is not known. In severe polyposis causing symptoms, surgical intervention may be considered to control the disease [36].
Mutyh-Associated Polyposis
Overview and Inclusion Criteria
Unlike other polyposis syndromes, Mutyh-associated polyposis (MAP) [37] is an autosomal recessive polyposis syndrome. The diagnosis can be established only after exclusion of FAP syndrome by demonstrating an absence of APC mutation. It has been estimated that the prevalence in MUTYH is 1 in 40,000 and 1 in 20,000 (clinical and subclinical carriers) [38]. Gastric involvement (i.e., polyps and carcinoma) is uncommon, but the incidence of duodenal involvement (especially duodenal carcinoma) is comparable to FAP [39]. Affected individuals are also predisposed to developing colorectal, breast, ovary, skin and sebaceous, and bladder carcinomas [39].
A diagnosis is established only after confirmation of MUTYH mutation [40] in a suspected individual on the basis of the following criteria: (a) family history of CRC with an autosomal recessive mode of inheritance, (b) >100 colon polyps in the absence of germline APC mutation, (c) 10–100 colon polyps (including adenomas and hyperplastic type), (d) 1–10 colon adenomas in an individual younger than 10 years of age, or (e) CRC with a specific somatic KRAS mutation (c.34G→T) in codon 12.
Molecular Genetics
MAP is caused by biallelic mutations in MUTYH (mutY homolog [Escherichia coli]) gene, located at Chr locus 1p34.3-p32.1 [40], which plays an important role in DNA base-excision repair. MUTYH is a DNA glycosylase that excises the misincorporated bases as a result of DNA damage caused by ionizing radiation or chemical oxidants [41]. Biallelic mutations result in the formation of truncated protein [42]. Interestingly, ethnic clustering of mutational hotspots is reported. For example, a higher frequency of biallelic loss at p.Y179C and p.G396D is seen in Caucasians but has not been noted in other populations. This can likely be explained by founder mutation resulting in selective overexpression of a mutational hotspot [43].
Clinical and Pathologic Features (Including Associated Other Neoplastic Lesions)
Gastric polyps are noted in 11% of cases, diagnosed at a median age of 49 years (range, 14–67 years). These include both adenomas and fundic gland polyps [39]. The risk of gastric cancer is low, seen in 2% of cases diagnosed at a median age of 38 years (range, 17–48 years). However, the incidence of duodenal cancer is significantly increased (occurring in approximately 17% of cases) [39].
Surveillance and Clinical Management
Surveillance guidelines for families with MUTYH germline mutations recommend that upper endoscopy be performed starting between the ages of 30 and 35 years and then subsequently at intervals of 3–5 years. The onset of colonoscopy surveillance has been recommended to be initiated at an earlier age (25–30 years) and repeated more frequently (every 1–2 years). Others have recommended initiation of upper gastrointestinal screening at 25 years of age, to be repeated at 30 years and then subsequently every 2 years if the results are normal [44]. Screening and testing minors is not recommended because of low risk.
Peutz-Jeghers Syndrome
Overview and Inclusion Criteria
Peutz-Jeghers syndrome (PJS) is an autosomal dominant disorder characterized by multiple gastrointestinal hamartomatous polyps (most commonly jejunal) and melanocytic macules [45]. Estimated incidence is approximately 1 in 200,000 [46] live births. It is estimated that PJS patients have a relative lifetime risk of 89% for developing cancer and are predisposed to developing neoplasms of the gut, pancreas, breast, uterus, cervix, testis, ovary, and lung [47].
A diagnosis of classic PJS can be established if two of the following features are present: (a) small bowel polyposis; (b) hyperpigmentation of lips, buccal mucosa, and digits (which usually fades by puberty); and (c) positive family history, along with histologically confirmed hamartomatous polyps [48]. The detection of the STK11 mutation is not a prerequisite, because mutations are identified in only 70% of cases [49].
Molecular Genetics
Germline mutations of tumor suppressor gene STK11 (serine threonine kinase 1, also known as liver kinase B1, LKB1) located on Chr 19p13.3 are seen in 70% of individuals with PJS [50, 51]. Additional genetic alterations (LOH of 17p and 18q) are present in subsequent adenocarcinomas [49], indicating that STK11 may be an “initiator” mutation regulating the development of hamartomas and that secondary somatic “driver” mutations underlie the progression to adenocarcinoma [52]. However, it has been proposed that the proliferating stroma, instead of the epithelial component, is responsible for induction of malignancy by a phenomenon called “landscaper effect.” This has been proven in other hamartomatous polyps in which a clonal genetic abnormality is present only in the stroma and not in the epithelium [53]. However, in PJS polyps, allelic imbalance at the LKM1 locus has been detected in the epithelial component, supporting a hamartoma-adenoma-carcinoma model [54].
It has been suggested that the site and type of STK11 gene mutations are predictors of development of gastric polyps and malignancies; individuals with truncating mutations or no mutations develop an earlier onset of gastric polyps in comparison with those with missense mutations [55]. Notably, mutations in the ATP binding and catalysis area of the gene result in a nonmalignant clinical phenotype, whereas mutations in the substrate recognition area are associated with malignancies [56].
Surveillance guidelines for families with MUTYH germline mutations recommend that upper endoscopy be performed starting between the ages of 30 and 35 years and then subsequently at intervals of 3–5 years. The onset of colonoscopy surveillance has been recommended to be initiated at an earlier age (25–30 years) and repeated more frequently (every 1–2 years)
Clinical Features and Pathology
Polyps are detected along the entire gastrointestinal tract and at extraintestinal sites and are seen more commonly in the small bowel, colon, and stomach (with a prevalence of ∼70%–90%, 50% and 25% of the cases, respectively). The median age of onset of gastric polyps is 16 years [55]. Gastric polyps can involve the antrum and pylorus [57]. They can reach a large size and present as a single polyp mimicking carcinoma [58]. Associated symptomatology may include bleeding, abdominal pain, intussusception, or even obstruction, depending on the site and size. PJS polyps are characterized by an arborizing pattern of the muscularis mucosa, underscoring a villous-like profile well developed in small intestinal polyps but less so in the stomach [59].
Although reported as early as 12 years of age [60], gastric carcinoma usually develops after a long latency (greater than 25 years in one study) [61]. The carcinoma may be associated with giant fold gastritis, making the detection difficult despite annual screening [62]. Because of the rarity of cases, detailed histologic studies are lacking. However, in the few reported cases, the histologic pattern has been intestinal type gastric adenocarcinoma [60].
Surveillance and Clinical Management
Because gastric polyps develop at a young age, endoscopic surveillance should be initiated early, with baseline endoscopy at the age of 8 years; henceforth, the screening interval may be tailored based on the findings of the first endoscopy. If polyposis is detected, screening should be performed at 2–3-year intervals; if no polyps are seen, it is suggested that screening be reinitiated at 18 years of age. More rigorous screening (every 1–2 years) should be performed after the age of 50 years [48]. Screening colonoscopy has been recommended starting at 20–25 years of age and performed at intervals of 2–5 years.
Prevention and Treatment
Because mammalian target of rapamycin (mTOR) is the final downstream effector of LKB1 inactivation, rapamycin (mTOR inhibitor) could be tested as a potential therapeutic agent [63]. Other drugs that have been suggested to decrease polyp burden include COX2 inhibition and metformin [64, 65].
Juvenile Polyposis Syndrome/Hereditary Hemorrhagic Telangiectasia
Overview and Inclusion Criteria
Juvenile polyposis syndrome (JPS) is an autosomal dominant disorder associated with the development of multiple polyps throughout the entire gastrointestinal tract. The incidence of JPS is 1 in 16,000 to 1 in 100,000 [48].
The inclusion criteria are (a) more than five juvenile polyps in the colon or rectum, (b) juvenile polyps throughout the gastrointestinal tract, or (c) more than one juvenile polyp with a family history of juvenile polyps [66]. However, individuals with mutations in SMAD4 or BMPR1A may exhibit a mixed polyposis phenotype similar to individuals with hereditary mixed polyposis syndrome (HMPS). Hence, JPS and HMPS are regarded, at least in part, as allelic entities [67].
Molecular Genetics
JPS is caused by mutations in several genes, most commonly SMAD4 (MADH4 or DPC4) on Chr 18q21.1 (20% of the cases) and in BMPRS1 on Chr 10q22.23 (20%–25% of the cases) [68–70]. Severe gastric polyposis has been associated with mutations in SMAD4, but not with BMPR1A mutations [70].
Germline mutations in PTEN (which controls function of the PI3K/AKT signaling pathway) and possibly ENG genes have been described [70, 71]. Of note, ENG encodes for endoglin, which is a transforming growth factor-β protein, which when mutated is responsible for hereditary hemorrhagic telangiectasia (HHT). Besides the common signaling pathway, there is also morphologic overlap in the phenotypic expression of these two conditions, and consequently, it is suggested that all HHT patients be screened for gastric polyposis [70].
Clinical and Pathologic Features (Including Associated Other Neoplastic Lesions)
Gastric polyps are commonly diagnosed in adults (median age of 41 years), whereas colorectal polyps are detected earlier in life (median age of 16 years) [70]. Polyposis also may develop, resulting in obstructive symptoms and hypergastrinemia [72]. The polyps may be associated with gastromegaly, severe anemia, hematemesis, and protein-losing enteropathy [73]. In such cases, the clinical presentation overlaps with Ménétrier disease. Interestingly, mutations in SMAD4 have also been detected in some cases of Ménétrier disease, and because the pathogenesis of both diseases involves dysregulation of transforming growth factor-β signaling pathway, some authors have suggested that Ménétrier disease could represent a variant of JPS in which another etiology (e.g., cytomegalovirus or Helicobacter pylori) would result in the expression of the clinical phenotype [74].
JPS polyps are pedunculated and present a smooth surface, ranging in size from 5 to 50 mm. Although JPS polyps are classically hamartomatous, they may exhibit morphologic heterogeneity, hence the term “mixed polyposis” referring to the hyperplastic, fundic gland, or inflammatory pseudopolyp phenotypes [75]. Gastric adenocarcinoma has been reported in up to 21% of gastric polyps [76]. Similar to polyps, SMAD4 mutations have been reported, but not BMPR1A mutations. Phenotypically, the gastric carcinomas were both intestinal and diffuse type [75].
Surveillance and Clinical Management
Upper and lower endoscopy have been recommended to be initiated in midteens or when symptoms begin, whichever is earlier, and repeated every 3 years if no polyps are found [48]. Annual screening is recommended if one to a few polyps are detected, which may be followed by screening every 3 years after no polyps are found. Gastrectomy is recommended for symptomatic patients with many polyps or gastric polyposis [76].
Familial Gastric Polyposis
Overview and Inclusion Criteria
Familial gastric polyposis is a rare autosomal dominant syndrome reported essentially only in Portuguese families, characterized by the development of a gastric hyperplastic polyposis, a high incidence of gastric carcinoma, and cutaneous psoriasis [77]. It is unclear whether the association with cutaneous psoriasis represents two distinct disorders or pleiotropic manifestations of one syndrome. Given the rarity of this syndrome, no tested inclusion criteria have been established.
Clinical and Pathology Features (Including Associated Other Neoplastic Lesions)
Gastric manifestations are seen in young patients, with polyposis involving the entire gastric wall. The polyps acquire a striking villous configuration and display exuberant globoid features. The epithelium is made up of either prominent foveolar hyperplasia or hyperplastic polyps with or without cytologic atypia [78]. No adenomas or fundic gland polyps are seen [77]. Poorly cohesive gastric adenocarcinoma has been observed arising from the dysplastic foveolar epithelium.
Molecular Genetics
The syndrome is inherited as an autosomal dominant trait with incomplete penetrance with reported healthy carriers [79].
Clinical Management, Surveillance, and Prevention
No data are available on surveillance and prevention at this time.
Gastric Adenocarcinoma and Proximal Polyposis Syndrome
Overview and Inclusion Criteria
Gastric adenocarcinoma and proximal polyposis syndrome (GAPPS) is a recently described syndrome with increased risk of gastric carcinoma, characterized by multiple FGPs, with areas of multifocal dysplasia and subsequent development of carcinoma. The diagnosis can be established only after exclusion of other polyposis syndromes [80, 81].
The following diagnostic criteria have been recommended: (a) >100 gastric polyps in the index case or over 30 polyps in a first-degree relative of a known case, (b) polyps restricted to the body and fundus of the stomach, (c) absence of colorectal or duodenal polyposis, (d) morphologically confirmed FGPs with areas of dysplasia or carcinoma, and (d) autosomal dominant inheritance.
Molecular Genetics
The etiology of this autosomal dominant disorder with incomplete penetrance is yet undetermined; however, coding mutations in APC, MUTYH, CDH1, SMAD4, BMPR1A, STK11, and PTEN have been excluded.
Clinical and Pathologic Features (Including Associated Other Neoplastic Lesions)
In the two series reported to date, the gastric manifestations were evident as early as 10 years of age, and gastric carcinoma was seen at 33 years of age. It appears to be more common in females.
Extensive polyposis is seen in the body and fundus with sparing of the lesser curvature. The polyps are small (<10 mm in size) and resemble sporadic FGPs. In fact, it is recommended that endoscopic biopsies be repeated after the patient is off proton pump inhibitor therapy to exclude sporadic FGPs. The polyps are associated with areas of dysplasia and mixed morphology, with combined adenomatous and hyperplastic polyp-like areas noted. Gastric carcinomas have been detected in 12.7% of patients and have all been gland-forming.
Although some patients were diagnosed with a few colorectal adenomas, none had colonic polyposis or colorectal carcinoma. Finally, as in sporadic FGPs, an inverse relationship has seen been between H. pylori infection and gastric manifestations of GAPPS.
Surveillance and Clinical Management
Management ought to be decided on a case-by-case basis, taking into consideration the risk of gastric cancer in the individual family. The presence of gastric polyposis presents difficulties with endoscopic surveillance, and patients may opt for total gastrectomy. Supporting this approach is the report of young patients (33 and 48 years of age), relatives of the proband, who despite endoscopic surveillance and biopsies developed and subsequently died of metastatic gastric carcinoma [81].
Familial Gastric Cancer Syndromes Without Polyps
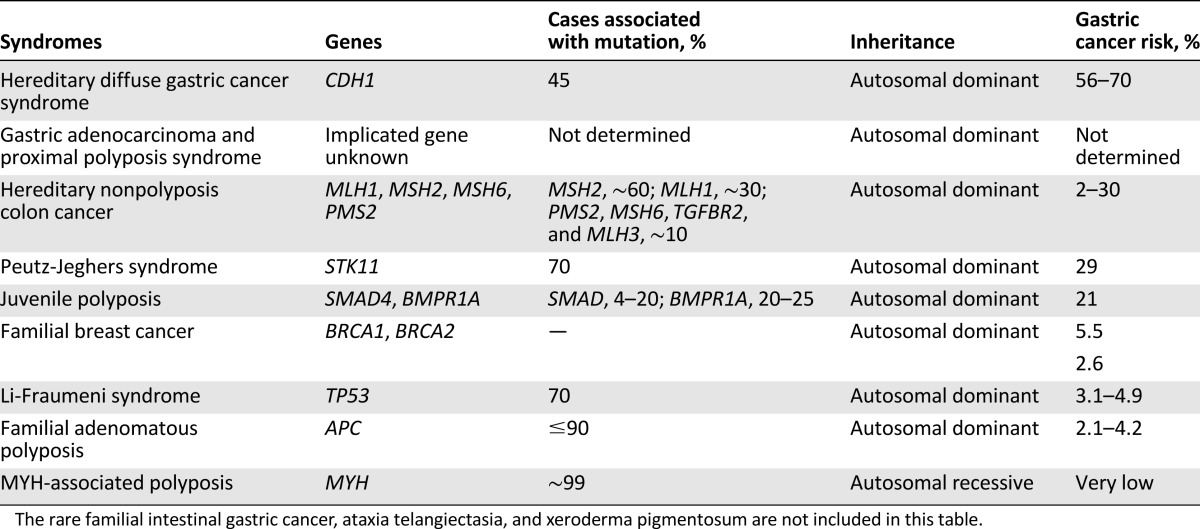
As a result of increased application of genomic analysis, a better understanding of molecular anomalies associated with gastric cancer has emerged. Among these, hereditary diffuse gastric cancer syndrome has been the focus of significant research since the 1998 identification of the E-cadherin protein by Guilford et al. [82]. In addition, identification of genes for other nonpolyposis syndromes predisposing to gastric carcinoma is discussed below (Table 1).
Table 1.
Gastric cancer predisposing polyposis and nonpolyposis syndromes with characteristic molecular and cytogenetic features
Hereditary Diffuse Gastric Cancer
Overview and Inclusion Criteria
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant cancer predisposition syndrome characterized by an increased risk of diffuse gastric cancer and breast carcinoma [83]. The prevalence in the general population is less than 0.1 per 100,000 and less than 1% among individuals with gastric cancer [84]. The lifetime risk of developing gastric carcinoma in male carriers is 70% (95% confidence interval [CI], 59%–80%) and 56% for female carriers (95% CI, 44%–69%). Similarly, the risk for lobular breast carcinoma, initially reported at 60% in female carriers, has been noted subsequently to vary from 39% [85] to 52% [86].
The 1999 guidelines proposed that a diagnosis of HDGC could be established in families with (a) ≥2 documented cases of diffuse gastric cancer in first and second degree relatives with at least one diagnosed <50 years of age or (b) ≥3 documented cases of diffuse gastric cancer in first and second degree relatives regardless of the age of onset [87]. In 2010, the guidelines were updated to expand the spectrum of clinical and pathologic findings triggering genetic testing for CDH1 mutations; that is, (a) pathologic confirmation of diffuse type gastric carcinoma now required only in 1 family member, (b) individuals with diffuse type gastric cancer diagnosed <40 years of age (even without a family history), (c) addition of lobular breast carcinoma to the prior guidelines, and (d) detection of in situ signet ring cells and/or pagetoid spread of signet ring cells adjacent to diffuse type gastric cancer. Finally, testing for large genomic rearrangements of CDH1 was recommended in addition to direct sequencing [88]. The latest guidelines propose merging the first two criteria into a new criteria: ≥2 documented cases of gastric carcinoma (at least one confirmed diffuse gastric cancer) in first and second degree relatives, irrespective of age [89]. Some have proposed that the criteria laid out by the first workshop be called “Clinical HDGC” and that the expanded criteria be referred to as “Probable HDGC” [2]. A recent study suggested that a personal or family history of 2 histologically proven lobular breast cancers before age 50, after exclusion of a germline mutation in BRCA1 or BRCA2, should be added to the criteria for CDH1 gene testing [90]. Testing is also recommended in individuals with family history of cleft lip/palate and diffuse gastric carcinoma [89].
Molecular Genetics
HDGC syndrome is caused by heterozygous mutation in the calcium-dependent adhesion protein (CDH1, Uromodulin) gene located on Chr 16q22.1 [78], inherited as an autosomal dominant disorder with a incomplete clinical penetrance [88]. CDH1 is a tumor suppressor gene encoded by 16 exons [91], but no mutational hotspots are identified, unlike sporadic discohesive gastric carcinoma [82]. CDH1 gene testing should be performed for the entire open reading frame, including intron-exon boundary and copy number analysis [89]. Recognized mutations include frameshift mutations, insertions, and deletions that are most frequently reported (∼30% of HDGC families); other mutation types include splice-site mutations (∼25%), nonsense mutations (∼20%), and missense mutations (∼20%) [92]. Large deletions account for 4% of cases, and 1% are a result of in-frame deletions and germline-promoter methylation [84]. These mutations lead to an altered or absent expression of E-cadherin protein, which plays an important role in cell polarity and intercellular adhesion. Notably, CDH1 mutations we previously reported to be detected in approximately 45% of HDGC individuals; however, a recent study reports a decrease in the frequency of CDH1 mutations to 19% after application of the new criteria [89, 92].
Several mechanisms have been proposed for inactivation of the second allele (the second hit), including promotor hypermethylation (which may explain the absence of loss of heterozygosity of the CDH1 allele) [93], intragenic deletions of the wild allele, and, less commonly, somatic mutations in CDH1 [94]. Germline mutations in CTNNA1 gene have been identified in a family of individuals meeting the criteria for HDGC; however, there is insufficient information regarding the penetrance at this point [89].
Clinical and Pathologic Features
Gastric carcinoma has been seen as early as 14 years of age and as late as 85 years [4]. The topographic distribution of HDGCs varies. Several series have shown clustering of signet ring cell carcinomas in the cardia and proximal stomach, especially in oxyntic type mucosa [95]; however, families from New Zealand were reported to have early onset carcinomas clustered in the distal stomach and antral-body transitional zone [96]. Currently, there are no apparent genetic alterations explaining the differences. Nevertheless, it has been suggested that the confirmation of uneven topographic distribution may increase the diagnostic yield during surveillance [95].
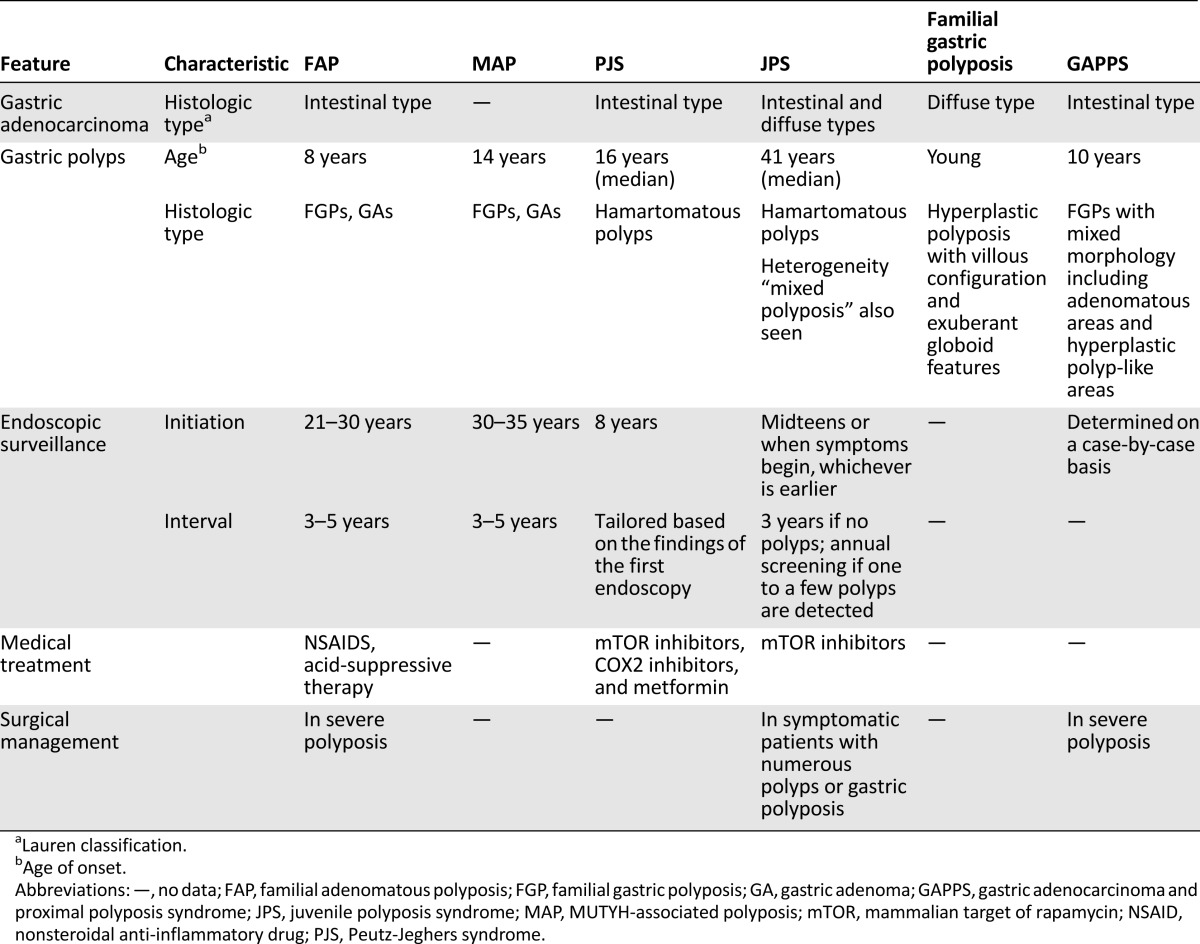
Surveillance biopsies and prophylactic gastrectomies of CDH1 patients have allowed recognition of early stages of diffuse type gastric carcinoma. Microscopic foci of invasive signet ring cells develop just underneath the surface mucosal epithelium, with preservation of the overall architecture of the tissue. Alternatively, individual tumor cells may display a pagetoid spread underneath the preserved epithelium of pits and foveolae but still within the basement membrane. Immunohistochemically, the neoplastic cells show reduced expression or absence of E-cadherin protein (Table 2).
Table 2.
Clinicopathologic features and management of polyposis-associated gastric cancer predisposing syndromes
Surveillance and Clinical Management
Carriers are advised to consider prophylactic gastrectomy after obtaining a baseline endoscopy to exclude the presence of macroscopic lesions. Prophylactic gastrectomies exhibit close to 100% histologic penetrance, unlike “clinical penetrance,” which is seen in ∼80% of HDGC cases [88].
It is advised that endoscopic surveillance performed annually be offered to young individuals (<20 years), to mutation-positive individuals who decide to decline surgery, and to those with mutations of uncertain significance. Multiple biopsies are recommended, including sampling of any endoscopically visible lesion and at least 5 random biopsies from the 6 anatomical zones (cardia, fundus, body, antrum, transitional zone, and prepyloric area, a total of 30 biopsies) [88, 89]. It has been estimated that to achieve a 90% detection rate of at least one neoplastic focus on biopsy, theoretically, approximately 1,768 biopsies (range 50–5,832) will be required [97].
Prevention and Treatment
Prophylactic gastrectomy (now reported as “risk-reduction gastrectomy,” given the high prevalence of microscopic carcinoma) [89] is the treatment of choice for carriers of pathogenic (truncating) CDH1 mutations. Endoscopic surveillance is an option offered to carriers of pathogenic mutations who opt not to have gastrectomy, individuals carrying mutations of undetermined significance, and individuals with a strong family history of gastric carcinoma but who test negative for CDH1 mutations [98]. The decision to perform genetic testing must be based on the earliest age of cancer onset in the family, but it is recommended that screening be initiated in the late teens or early twenties [97]. In women, annual mammography and breast magnetic resonance imaging are recommended after the age of 35 years. There are insufficient data for consideration of prophylactic mastectomy [84].
The prognosis of individuals undergoing prophylactic gastrectomy is excellent [84]. The surgical procedure includes total gastrectomy with end-to-side Roux-en-Y esophagojejunostomy.
Hereditary Nonpolyposis Colorectal Cancer
Overview and Inclusion Criteria
Hereditary nonpolyposis colorectal cancer (HNPCC) is the most common form of inherited CRC syndrome, accounting for 2%–4% of all CRC. It is caused by mutations in DNA repair genes resulting in errors in repetitive DNA sequences throughout the genome (microsatellite instability [MSI]) [99]. The syndrome has been subdivided into (a) Lynch syndrome I, predisposing primarily to colonic carcinoma, and (b) Lynch syndrome II, predisposing to other neoplasms in addition to CRC, including those arising in the endometrium, stomach, pancreaticobiliary tract, prostate, and genitourinary tract. Muir-Torre syndrome (MTS) is a variant characterized by HNPCC-related tumors and sebaceous neoplasms.
The lifetime risk for developing gastric cancer varies geographically. It is in fact the most frequent extracolonic carcinoma in countries with a high prevalence of sporadic gastric carcinoma, with a lifetime risk of 30% in Korea [100] and 44.4% in China [101], whereas it is reported at 2.1% in the Netherlands [102]. Only three cases have been reported in association with MTS [103, 104].
Two sets of criteria are used to establish a diagnosis of HNPCC: (a) Amsterdam criteria and (b) Bethesda criteria. Gastric carcinoma is not a defining criterion for HNPCC in either classification. The 1999 revision of the Amsterdam criteria is characterized by the inclusion of extracolonic carcinomas such as endometrial, small bowel, ureter, or renal pelvis in at least 3 relatives with CRC meeting these criteria: (a) 1 relative should be a first degree relative of the other 2, (b) CRC or HNPCC-related carcinoma affects 2 successive generations, and (c) at least 1 carcinoma should be before the age of 50 years. Histopathologic verification and exclusion of a diagnosis of FAP are cardinal [99].
The Bethesda criteria provide a more complete list of all clinical presentations of HNPCC. The 2002 revision includes additional features of MSI tumors. The criteria include (a) CRC diagnosed in an individual younger than 50 years of age; (b) presence of synchronous or metachronous colorectal or other HNPCC-related tumors, irrespective of age; (c) CRC with MSI-H histology diagnosed at <60 years of age; (d) individuals with CRC with at least 1 first-degree relative with CRC or HNPCC-related tumor, diagnosed at <50 years of age; (e) individuals with CRC with at least 2 first- or second-degree relatives with CRC or HNPCC-related tumor, irrespective of age. If an individual meets the above criteria, they are referred for molecular and immunohistochemical testing for MSI, because some individuals may meet the clinical criteria but are microsatellite stable on testing, an exclusionary characteristic [99].
Molecular Genetics
The most common defect is seen in MSH2, which accounts for ∼60% of HNPCC cases (also known as HNPCC1), and MLH1 accounts for ∼30% of the cases (also known as HNPCC2). Mutations in PMS2, MSH6, TGFBR2, and MLH3 account for the remaining 10% of the cases. Epigenetic silencing of MSH2 caused by deletions in upstream EPCAM gene results in another variant [105]. It is unclear whether there is a significant variation in the incidence of gastric carcinoma between MSH2 and MLH1 mutated HNPCC cases; conflicting reports have described higher incidences clustering in one subtype versus the other (T33, T34) [106, 107]. Some phenotypic variations are observed, especially between HNPCC1 (MSH2 mutated) and other HNPCC types [105, 108].
Clinical and Pathologic Features
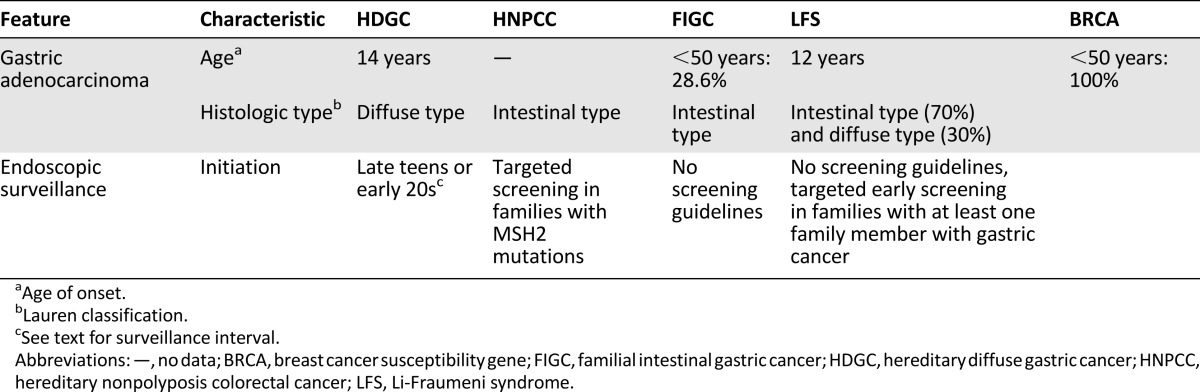
It is noteworthy that the original report of HNPCC by Warthin in 1913 presented a family with clustering gastric carcinoma [109]. The cumulative incidence of gastric carcinoma in HNPCC is 13% by 70 years of age [107]. Compared with sporadic tumors, 52% of gastric carcinomas (GCAs) in HNPCC are diagnosed in individuals younger than 50 years (90% of sporadic GCAs are diagnosed after the age of 55 years) [106]. The carcinomas are reported to have an intestinal phenotype [110] (Table 3).
Table 3.
Clinicopathologic features and management of non-polyposis-associated gastric cancer predisposing syndromes
Clinical Management: Surveillance, Prevention, and Treatment
There are no consensus guidelines regarding upper gastrointestinal screening in individuals with HNPCC. Some authors recommend screening in individuals for whom a family history of gastric cancer is present or in countries with a higher incidence [111, 112]. Also, based on the clustering of gastric cancer seen in families with MSH2 mutations, it has been proposed that screening should be implemented in this subset of families [106]. Eradication of H. pylori in HNPCC patients may reduce the risk of gastric carcinoma [111]. Routine testing by immunohistochemistry is recommended for screening all colorectal carcinomas in major academic centers and can detect up to 95% of MMR related CRCs [113]. There are no current screening guidelines at this time for identification and screening of MSI-H gastric carcinoma by immunohistochemical testing.
Familial Intestinal Gastric Cancer
Overview and Inclusion Criteria
Familial intestinal gastric cancer (FIGC) is a poorly characterized genetic predisposition for gastric cancer of intestinal phenotype and lacking CDH1 mutation [88].
The recommended criteria vary geographically and are based on the local incidence of gastric cancer [114]. Guidelines analogous to the Amsterdam criteria for colorectal carcinoma have been used in countries with high incidence. In countries with low incidence, FIGC has been defined as intestinal gastric cancer in 2 or more first- or second-degree relatives, with at least one diagnosis by age 50 or 3 cases or more in first- or second-degree relatives, independent of age.
Molecular Genetics
The mode of inheritance is autosomal dominant, but the genetic factors involved are unclear. These tumors do not exhibit mutations in TP53, DNA mismatch repair genes, or CDH1 [115]. However, epigenetic methylation of CDH1 is reported in approximately 17% of cases, and loss of heterozygosity is reported in 9.4% of cases [114].
Clinical and Pathologic Features (Including Associated Other Neoplastic Lesions)
According to a Japanese study, after applying the Amsterdam criteria to a large cohort of 3,632 families with gastric carcinoma, only 31 (0.9%) met the criteria for FIGC. Gastric carcinoma was seen in 28.6% of individuals before the age of 50.
Clinical Management, Surveillance, Prevention, and Treatment
Based on the experience of the optical endoscopic interval surveillance of gastric cancer, particularly among patients with a family history, Corso et al. [116] suggest a yearly endoscopic examination starting at the age of 60.
Li-Fraumeni Syndrome
Overview and Inclusion Criteria
Li-Fraumeni syndrome (LFS) is an autosomal dominant inherited cancer syndrome characterized by an increased risk of developing sarcomas (index tumors), breast carcinoma, leukemias, and other neoplasms in children and young adults [117]. In contrast to other inherited cancer syndromes, the carriers do not exhibit site-specific tumors but present with multiple primary tumors of divergent phenotype. Gastric carcinoma is detected in 1.8% [118] to 4.9% [119] of LFS carriers. Overall, it is reported that 22.6% of LFS families have at least one member with gastric carcinoma [119].
Classic LFS is defined as a proband diagnosed with the following criteria: (a) sarcoma before 45 years of age, (b) a first-degree relative with cancer before this same age, and (c) another first- or second-degree relative in the lineage with any cancer before this age or sarcoma at any age [120]. Neoplasms that are more commonly seen, besides sarcomas, include breast carcinomas, brain tumors, and adrenal cortical carcinomas. Gastric carcinoma is a less common malignancy [121].
Molecular Genetics
LFS is caused by heterozygous mutations in the TP53 gene on Chr 17p13.1, with germline mutations in TP53 present in ∼70% of patients [120, 122]. Mutations in gastric carcinoma cases generally have been reported to be predominantly in exons 5–8 (of the 11-exon gene), compromising the DNA-binding domain [123–126]. However, another group has reported mutations in exons 4–10 with no genotype-phenotype correlation [119]. A very high penetrance is seen, with cancer risk approaching 100% in females and ∼73% in males [120].
Clinical and Pathologic Features
Gastric carcinoma has been reported in a child as young as 12 years of age [127]. However, the mean age at diagnosis of gastric carcinoma is 36 years (range, 24–74 years), with most patients under 50 years of age (e.g., 19% <30 years, 24% <40 years, and 57% <50 years), which is significantly younger compared with the mean age of sporadic gastric cancer in the SEER data set (71 years) [119]. Most of the tumors have been located in the proximal stomach (∼50%) compared with the antrum (∼30%) and fundus (∼10%), and approximately 70% display an intestinal phenotype [119].
Surveillance and Clinical Management
Phenotypic diversity among carriers complicates the formulation of effective screening strategies. The National Comprehensive Cancer Network has proposed surveillance guidelines that include screening for breast and colorectal neoplasms [128]. However, it has been suggested that periodic screening gastroscopy of LFS carriers with at least one family member affected by gastric cancer should be considered. Given the early onset of gastric carcinoma, the screening should be initiated at an early age [119].
BRCA1 and BRCA2 Hereditary Breast and Ovarian Cancer
Overview and Inclusion Criteria
The clinical criteria for genetic testing include: 3 or more breast and/or ovarian cancer cases, at least one before the age of 50 years; 2 breast cancer cases before the age of 40 years; male breast cancer and ovarian cancer or early onset female breast cancer; Ashkenazi Jew with breast cancer before the age of 60 years; young onset bilateral breast cancer; and breast and ovarian cancer in the same patient. Certain histologic features may trigger genetic testing, such as breast medullary carcinoma and triple negative phenotype of breast carcinoma in women younger than 50 [129].
In addition, melanoma as well as gastric and pancreatic carcinomas have been associated with BRCA1 and BRCA2 syndromes [130–132]. Gastric cancer has been reported to be one of the most frequent cancers in the families of probands with BRCA mutations in one study, and its incidence before the age of 70 years is twice as common in these patients compared with the general population [133, 134]. The association of gastric carcinoma is reported to be stronger with BRCA2 than BRCA1, with an increased relative risk of gastric cancer in BRCA2 mutation carriers (2.59; 95% CI = 1.46–4.61) [131]. The frequency of gastric carcinoma is 5 times higher than the general population particularly in Ashkenazi Jews with BRCA2 mutations (5.7%) [132]. It appears from some studies [135] that the presence of a family history of gastric cancer doubled the probability/risk of BRCA1/2 carrier (23.8% vs. 11.8%), which would suggest that testing for BRCA mutations ought to be performed in all patients with a suggestive history.
Gastric cancer has been reported to be one of the most frequent cancers in the families of probands with BRCA mutations in one study, and its incidence before the age of 70 years is twice as common in these patients compared with the general population.
Molecular Genetics
BRCA1 and BRCA2 are autosomal recessive syndromes caused by mutations in BRCA1 located on Chr 17q.21.31 or BRCA2 on 13q.13.1. BRCA2 syndrome has a lower level of penetrance than BRCA1 syndrome [136]. Certain mutations have been seen to result in clustering of gastric carcinoma in BRCA families. These include BRCA1 mutation at c.3,936 C→T, which results in a stop codon at 1,273, resulting in more deleterious effects [137]. Mutation in BRCA2 at 6174delT also has been reported in a higher frequency of gastric carcinoma [132]. Notably, the most common mechanism of BRCA1 inactivation in sporadic gastric carcinoma is microsatellite instability or loss of heterozygosity rather than point mutations as seen in hereditary cases [131, 137, 138].
Clinical and Pathologic Features
All BRCA-related gastric carcinomas were diagnosed under the age of 55 years (range, 27–54 years) in a Polish study [139]. No reports are available studying the histopathologic features of BRCA syndrome-associated gastric carcinoma; however, sporadic carcinoma with BRCA mutations have been reported to be associated with diffuse phenotype, higher tumor grades, and advanced clinical stage [140].
Prevention, Treatment, Clinical Management, and Surveillance
Screening guidelines are not available for surveillance of gastric carcinoma in BRCA carriers [129].
Other Genomic Instability Syndromes With Reported Predisposition to Gastric Cancer: Ataxia Telangiectasia and Xeroderma Pigmentosum
Overview and Inclusion Criteria of Ataxia Telangiectasia
Ataxia telangiectasia is an autosomal recessive disorder characterized by cerebellar ataxia, multiple telangiectasia, immune defects, and multiple primary carcinomas [141]. The clinical diagnosis is straightforward, with the presence of oculocutaneous telangiectasia qualifying for the diagnosis. The presence of neoplasia is not required for the diagnosis [141].
Molecular Genetics
The syndrome is caused by extensive DNA damage as a result of the inherent susceptibility of DNA to radiation secondary to mutations in the DNA repair gene, ataxia telangiectasia-mutated on Chr 11q22.3. Mutations are frequently seen in 10 exons and 2 cDNA fragments (38.5%).
Clinical and Pathologic Features
There have been 10 reports of gastric carcinoma, all detected in the first or second decade of life (range, 14–26 years) and at an advanced stage [142]. It has been postulated that gastritis developing in the setting of the immunodeficiency reported in these patients predisposes to carcinoma [143]. A varied histopathologic spectrum of adenocarcinomas has been reported, including mucinous adenocarcinomas, adenocarcinoma not otherwise specified, and signet ring cell carcinoma.
Surveillance
A systematic screening program involving upper gastrointestinal endoscopy could be considered in any patient over the age of 10 years with nonspecific gastrointestinal manifestations [143].
Overview and Inclusion Criteria of Xeroderma Pigmentosum
Xeroderma pigmentosum carriers are predisposed to developing a high incidence of cutaneous carcinomas in exposed areas and ocular neoplasms. Carriers are at greater than 10,000-fold increased risk of neoplasms [144]. The diagnosis can be established based on family history and a constellation of clinical findings including (a) extreme sun sensitivity, (b) ocular, and (c) neurologic manifestations [145].
Molecular Genetics
The XPS phenotype may be caused by mutation in one of the eight alleles of the XPS gene, which codes for a protein that plays a significant role in global genome nucleotide excision repair as a result of ultraviolet radiation or chemical carcinogens [105, 146, 147]. Certain single nucleotide polymorphisms in the XP gene have been seen with a higher frequency in sporadic gastric carcinomas and have been proposed to be useful markers for identifying high-risk individuals [148].
Clinical and Pathologic Features
Of interest, there is only a single morphologic evaluation of gastric carcinoma in a 3-year-old patient with poorly cohesive adenocarcinoma and widespread metastasis [149].
Surveillance and Clinical Management
XPS patients receive prevention for skin, ocular, and neurologic manifestations, but there are no guidelines for surveillance of gastric carcinoma [145].
Conclusion
Despite better understanding and control over known risk factors, gastric adenocarcinoma remains one of the most common cancers worldwide. Recently, awareness of familial gastric cancer syndromic predisposition has been emphasized, because though these syndromes are uncommon, they bear major management implications for the patients and their families. This review, while providing concise information regarding the molecular and histopathologic characteristics of these syndromes, also aimed to offer updated management guidelines, including follow-up and surveillance.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Gregory Y. Lauwers
Provision of study material or patients: Jeffrey W. Clark, Dan G. Duda, Theodore S. Hong, Eunice L. Kwak, John T. Mullen
Collection and/or assembly of data: Namrata Setia, Jeffrey W. Clark, Dan G. Duda, Theodore S. Hong, Eunice L. Kwak, John T. Mullen
Data analysis and interpretation: Namrata Setia, Jeffrey W. Clark, Dan G. Duda, Theodore S. Hong, Eunice L. Kwak, John T. Mullen, Gregory Y. Lauwers
Manuscript writing: Namrata Setia, Jeffrey W. Clark, Dan G. Duda, Theodore S. Hong, Eunice L. Kwak, John T. Mullen
Final approval of manuscript: Gregory Y. Lauwers
Disclosures
Dan G. Duda: Hexal (C/A), Merrimack, HealthCare Pharma (RF); Theodore S. Hong: Eisai (C/A), Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bevan S, Houlston RS. Genetic predisposition to gastric cancer. QJM. 1999;92:5–10. doi: 10.1093/qjmed/92.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Kluijt I, Sijmons RH, Hoogerbrugge N, et al. Familial gastric cancer: Guidelines for diagnosis, treatment and periodic surveillance. Fam Cancer. 2012;11:363–369. doi: 10.1007/s10689-012-9521-y. [DOI] [PubMed] [Google Scholar]
- 3.Carneiro F, Lauwers GY.Epithelial tumors of the stomach. In: Shepherd NA, Warren BF, Williams GT. et al., eds. Morson and Dawson’s Gastrointestinal Pathology. 5th ed Hoboken, NJ: Wiley-Blackwell, 2013:182. [Google Scholar]
- 4.Carneiro F, Charlton A, Huntsman DG.Hereditary diffuse gastric cancer. In: Bosman FT, Carneiro F, Hruban RH. et al., eds. WHO Classification of Tumours of the Digestive System. 4th ed Lyon, France: World Health Organization International Agency for Research on Cancer, 2010:59. [Google Scholar]
- 5.Attard TM, Cuffari C, Tajouri T, et al. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familial adenomatous polyposis. Am J Gastroenterol. 2004;99:681–686. doi: 10.1111/j.1572-0241.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180–185. doi: 10.1016/j.cgh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Lynch HT, Smyrk T, McGinn T, et al. Attenuated familial adenomatous polyposis (AFAP): A phenotypically and genotypically distinctive variant of FAP. Cancer. 1995;76:2427–2433. doi: 10.1002/1097-0142(19951215)76:12<2427::aid-cncr2820761205>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Attard TM, Yardley JH, Cuffari C. Gastric polyps in pediatrics: An 18-year hospital-based analysis. Am J Gastroenterol. 2002;97:298–301. doi: 10.1111/j.1572-0241.2002.05461.x. [DOI] [PubMed] [Google Scholar]
- 9.Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs: Its rational treatment. Ann Surg. 1993;217:101–108. doi: 10.1097/00000658-199302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–1982. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 11.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 12.Utsunomiya J, Miki Y, Kuroki T, et al. [Recent trends in studies on carcinogenesis in familial adenomatous polyposis] Gan To Kagaku Ryoho. 1988;15:185–191. [PubMed] [Google Scholar]
- 13.Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen M, Hes FJ, Nagengast FM, et al. Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet. 2007;71:427–433. doi: 10.1111/j.1399-0004.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 15.MIM number 175100: Familial Adenomatous Polyposis 1; FAP1. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/175100. Accessed July 8, 2013.
- 16.Miyoshi Y, Ando H, Nagase H, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA. 1992;89:4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamlum H, Ilyas M, Rowan A, et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: A new facet to Knudson’s “two-hit” hypothesis. Nat Med. 1999;5:1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 18.Gardner RJ, Kool D, Edkins E, et al. The clinical correlates of a 3′ truncating mutation (codons 1982–1983) in the adenomatous polyposis coli gene. Gastroenterology. 1997;113:326–331. doi: 10.1016/s0016-5085(97)70111-2. [DOI] [PubMed] [Google Scholar]
- 19.Groves C, Lamlum H, Crabtree M, et al. Mutation cluster region, association between germline and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol. 2002;160:2055–2061. doi: 10.1016/S0002-9440(10)61155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enomoto M, Konishi M, Iwama T, et al. The relationship between frequencies of extracolonic manifestations and the position of APC germline mutation in patients with familial adenomatous polyposis. Jpn J Clin Oncol. 2000;30:82–88. doi: 10.1093/jjco/hyd017. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SC, Nobukawa B, Giardiello FM, et al. Fundic gland polyps in familial adenomatous polyposis: Neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol. 2000;157:747–754. doi: 10.1016/S0002-9440(10)64588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao H, Shinmura K, Yamada H, et al. Identification of 5 novel germline APC mutations and characterization of clinical phenotypes in Japanese patients with classical and attenuated familial adenomatous polyposis. BMC Res Notes. 2010;3:305. doi: 10.1186/1756-0500-3-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata C, Ogawa H, Miura K, et al. Clinical characteristics of gastric cancer in patients with familial adenomatous polyposis. Tohoku J Exp Med. 2013;229:143–146. doi: 10.1620/tjem.229.143. [DOI] [PubMed] [Google Scholar]
- 24.Attard TM, Giardiello FM, Argani P, et al. Fundic gland polyposis with high-grade dysplasia in a child with attenuated familial adenomatous polyposis and familial gastric cancer. J Pediatr Gastroenterol Nutr. 2001;32:215–218. doi: 10.1097/00005176-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Sarre RG, Frost AG, Jagelman DG, et al. Gastric and duodenal polyps in familial adenomatous polyposis: A prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306–314. doi: 10.1136/gut.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seow-Choen F, Ho JM, Wong J, et al. Gross and histological abnormalities of the foregut in familial adenomatous polyposis: A study from a South East Asian Registry. Int J Colorectal Dis. 1992;7:177–183. doi: 10.1007/BF00341216. [DOI] [PubMed] [Google Scholar]
- 27.Wood LD, Salaria SN, Cruise MW, et al. Upper GI tract lesions in familial adenomatous polyposis (FAP): Enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38:389–393. doi: 10.1097/PAS.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham SC, Montgomery EA, Singh VK, et al. Gastric adenomas: Intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol. 2002;26:1276–1285. doi: 10.1097/00000478-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ngamruengphong S, Boardman LA, Heigh RI, et al. Gastric adenomas in familial adenomatous polyposis are common, but subtle, and have a benign course. Hered Cancer Clin Pract. 2014;12:4. doi: 10.1186/1897-4287-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu TT, Kornacki S, Rashid A, et al. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998;22:293–298. doi: 10.1097/00000478-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Bertoni G, Sassatelli R, Nigrisoli E, et al. Dysplastic changes in gastric fundic gland polyps of patients with familial adenomatous polyposis. Ital J Gastroenterol Hepatol. 1999;31:192–197. [PubMed] [Google Scholar]
- 32.Shimoyama S, Aoki F, Kawahara M, et al. Early gastric cancer development in a familial adenomatous polyposis patient. Dig Dis Sci. 2004;49:260–265. doi: 10.1023/b:ddas.0000017448.58196.dc. [DOI] [PubMed] [Google Scholar]
- 33.Ravoire A, Faivre L, Degrolard-Courcet E, et al. Gastric adenocarcinoma in familial adenomatous polyposis can occur without previous lesions. J Gastrointest Cancer. 2014 doi: 10.1007/s12029-013-9504-8. [DOI] [PubMed] [Google Scholar]
- 34.Sawada T, Muto T. Familial adenomatous polyposis: Should patients undergo surveillance of the upper gastrointestinal tract? Endoscopy. 1995;27:6–11. doi: 10.1055/s-2007-1005625. [DOI] [PubMed] [Google Scholar]
- 35.Hirata K, Okazaki K, Nakayama Y, et al. Regression of gastric polyps in Gardner’s syndrome with use of indomethacin suppositories: A case report. Hepatogastroenterology. 1997;44:918–920. [PubMed] [Google Scholar]
- 36.Srinivasa D, Wray CJ. Total gastrectomy with isoperistaltic jejunal interposition flap for symptomatic management of gastric polyposis from familial adenomatous polyposis. J Gastrointest Oncol. 2014;5:E18–E21. doi: 10.3978/j.issn.2078-6891.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MIM number 608456: Familial adenomatous polyposis 2; FAP2. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/608456. Accessed October 11, 2011.
- 38.Aretz S, Genuardi M, Hes FJ. Clinical utility gene card for: MUTYH-associated polyposis (MAP), autosomal recessive colorectal adenomatous polyposis, multiple colorectal adenomas, multiple adenomatous polyps (MAP): Update 2012. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2012.163. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976–1985. doi: 10.1053/j.gastro.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 40.MIM number 604933: MutY, E. coli, homolog of; MUTYH. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/604933. Accessed October 11, 2013.
- 41.Isidro G, Laranjeira F, Pires A, et al. Germline MUTYH (MYH) mutations in Portuguese individuals with multiple colorectal adenomas. Hum Mutat. 2004;24:353–354. doi: 10.1002/humu.9282. [DOI] [PubMed] [Google Scholar]
- 42.Lipton L, Tomlinson I. The multiple colorectal adenoma phenotype and MYH, a base excision repair gene. Clin Gastroenterol Hepatol. 2004;2:633–638. doi: 10.1016/s1542-3565(04)00286-1. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen M, Morreau H, Vasen HF, et al. MUTYH-associated polyposis (MAP) Crit Rev Oncol Hematol. 2011;79:1–16. doi: 10.1016/j.critrevonc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Buecher B, Bonaïti C, Buisine MP, et al. French experts report on MUTYH-associated polyposis (MAP) Fam Cancer. 2012;11:321–328. doi: 10.1007/s10689-012-9511-0. [DOI] [PubMed] [Google Scholar]
- 45.MIM number 175200: Peutz-Jeghers Syndrome; PJS. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/175200. Accessed October 12, 2013.
- 46.Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. 2006;4:408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Turpin A, Cattan S, Leclerc J, et al. Hereditary predisposition to cancers of the digestive tract, breast, gynecological and gonadal: Focus on the Peutz-Jeghers. Bull Cancer. 2014;101:813–822. doi: 10.1684/bdc.2014.1942. [DOI] [PubMed] [Google Scholar]
- 48.Chun N, Ford JM. Genetic testing by cancer site: Stomach. Cancer J. 2012;18:355–363. doi: 10.1097/PPO.0b013e31826246dc. [DOI] [PubMed] [Google Scholar]
- 49.Gruber SB, Entius MM, Petersen GM, et al. Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res. 1998;58:5267–5270. [PubMed] [Google Scholar]
- 50.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 51.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 52.Miyaki M, Iijima T, Hosono K, et al. Somatic mutations of LKB1 and beta-catenin genes in gastrointestinal polyps from patients with Peutz-Jeghers syndrome. Cancer Res. 2000;60:6311–6313. [PubMed] [Google Scholar]
- 53.Jacoby RF, Schlack S, Cole CE, et al. A juvenile polyposis tumor suppressor locus at 10q22 is deleted from nonepithelial cells in the lamina propria. Gastroenterology. 1997;112:1398–1403. doi: 10.1016/s0016-5085(97)70156-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZJ, Taylor F, Churchman M, et al. Genetic pathways of colorectal carcinogenesis rarely involve the PTEN and LKB1 genes outside the inherited hamartoma syndromes. Am J Pathol. 1998;153:363–366. doi: 10.1016/S0002-9440(10)65579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amos CI, Keitheri-Cheteri MB, Sabripour M, et al. Genotype-phenotype correlations in Peutz-Jeghers syndrome. J Med Genet. 2004;41:327–333. doi: 10.1136/jmg.2003.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumacher V, Vogel T, Leube B, et al. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. J Med Genet. 2005;42:428–435. doi: 10.1136/jmg.2004.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Payson BA, Moumgis B. Metastasizing carcinoma of the stomach in Peutz-Jeghers syndrome. Ann Surg. 1967;165:145–151. doi: 10.1097/00000658-196701000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westerman AM, van Velthuysen ML, Bac DJ, et al. Malignancy in Peutz-Jeghers syndrome? The pitfall of pseudo-invasion. J Clin Gastroenterol. 1997;25:387–390. doi: 10.1097/00004836-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 59.Lam-Himlin D, Park JY, Cornish TC, et al. Morphologic characterization of syndromic gastric polyps. Am J Surg Pathol. 2010;34:1656–1662. doi: 10.1097/PAS.0b013e3181f2b1f1. [DOI] [PubMed] [Google Scholar]
- 60.Schneider C, Simon T, Hero B, et al. [18F]Fluorodeoxyglucose positron emission tomography/computed tomography-positive gastric adenocarcinoma in a 12-year-old girl with Peutz-Jeghers syndrome. J Clin Oncol. 2012;30:e140–e143. doi: 10.1200/JCO.2011.39.7422. [DOI] [PubMed] [Google Scholar]
- 61.Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 62.Al-Taie OH, Adam P, Kraus MR, et al. Giant fold gastritis with consecutive gastric carcinoma in a patient with Peutz-Jeghers syndrome. Z Gastroenterol. 2005;43:653–656. doi: 10.1055/s-2005-858144. [DOI] [PubMed] [Google Scholar]
- 63.Kuwada SK, Burt R. A rationale for mTOR inhibitors as chemoprevention agents in Peutz-Jeghers syndrome. Fam Cancer. 2011;10:469–472. doi: 10.1007/s10689-011-9471-9. [DOI] [PubMed] [Google Scholar]
- 64.Udd L, Katajisto P, Rossi DJ, et al. Suppression of Peutz-Jeghers polyposis by inhibition of cyclooxygenase-2. Gastroenterology. 2004;127:1030–1037. doi: 10.1053/j.gastro.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 65.Huang X, Wullschleger S, Shpiro N, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 66.Jass JR, Williams CB, Bussey HJ, et al. Juvenile polyposis: A precancerous condition. Histopathology. 1988;13:619–630. doi: 10.1111/j.1365-2559.1988.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 67.Cao X, Eu KW, Kumarasinghe MP, et al. Mapping of hereditary mixed polyposis syndrome (HMPS) to chromosome 10q23 by genomewide high-density single nucleotide polymorphism (SNP) scan and identification of BMPR1A loss of function. J Med Genet. 2006;43:e13. doi: 10.1136/jmg.2005.034827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sayed MG, Ahmed AF, Ringold JR, et al. Germline SMAD4 or BMPR1A mutations and phenotype of juvenile polyposis. Ann Surg Oncol. 2002;9:901–906. doi: 10.1007/BF02557528. [DOI] [PubMed] [Google Scholar]
- 69.Howe JR, Sayed MG, Ahmed AF, et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet. 2004;41:484–491. doi: 10.1136/jmg.2004.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MIM number 174900: Juvenile polyposis syndrome; JPS. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/174900. Accessed August 26, 2013.
- 71.van Hattem WA, Brosens LA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A and PTEN in juvenile polyposis. Gut. 2008;57:623–627. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 72.Papay KD, Falck VG, Poulsen SS, et al. Juvenile polyposis of the stomach: A novel cause of hypergastrinemia. Nat Rev Gastroenterol Hepatol. 2010;7:583–588. doi: 10.1038/nrgastro.2010.138. [DOI] [PubMed] [Google Scholar]
- 73.Wong-Chong N, Kidanewold WH, Kirsch R, et al. Giant stomach secondary to juvenile polyposis syndrome. J Gastrointest Surg. 2012;16:669–672. doi: 10.1007/s11605-011-1714-4. [DOI] [PubMed] [Google Scholar]
- 74.Piepoli A, Mazzoccoli G, Panza A, et al. A unifying working hypothesis for juvenile polyposis syndrome and Ménétrier’s disease: Specific localization or concomitant occurrence of a separate entity? Dig Liver Dis. 2012;44:952–956. doi: 10.1016/j.dld.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome. J Med Genet. 2007;44:702–709. doi: 10.1136/jmg.2007.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen Haidle J, Howe JR.Juvenile polyposis syndrome. In: Pagon RA, Adam MP, Ardinger HH. et al., eds. GeneReviews(R) Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 77.Carneiro F, David L, Seruca R, et al. Hyperplastic polyposis and diffuse carcinoma of the stomach. A study of a family. Cancer. 1993;72:323–329. doi: 10.1002/1097-0142(19930715)72:2<323::aid-cncr2820720204>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 78.MIM number 192090: Cadherin 1; CDH1. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/192090. Accessed July 24, 2013.
- 79.dos Santos JG, de Magalhães J. Familial gastric polyposis. A new entity. J Genet Hum. 1980;28:293–297. [PubMed] [Google Scholar]
- 80.Carneiro F. Hereditary gastric cancer. Pathologe. 2012;33(suppl 2):231–234. doi: 10.1007/s00292-012-1677-6. [DOI] [PubMed] [Google Scholar]
- 81.Worthley DL, Phillips KD, Wayte N, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut. 2012;61:774–779. doi: 10.1136/gutjnl-2011-300348. [DOI] [PubMed] [Google Scholar]
- 82.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 83.MIM number 137215: Gastric cancer, hereditary diffuse; HDGC. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/137215. Accessed July 24, 2013.
- 84.Oliveira C, Seruca R, Hoogerbrugge N, et al. Clinical utility gene card for: Hereditary diffuse gastric cancer (HDGC) Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2012.247. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guilford P, Humar B, Blair V. Hereditary diffuse gastric cancer: Translation of CDH1 germline mutations into clinical practice. Gastric Cancer. 2010;13:1–10. doi: 10.1007/s10120-009-0531-x. [DOI] [PubMed] [Google Scholar]
- 86.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–2372. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 87.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: Overview and guidelines for management. J Med Genet. 1999;36:873–880. [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–374. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benusiglio PR, Malka D, Rouleau E, et al. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: A multicentre study. J Med Genet. 2013;50:486–489. doi: 10.1136/jmedgenet-2012-101472. [DOI] [PubMed] [Google Scholar]
- 91.Berx G, Staes K, van Hengel J, et al. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1) Genomics. 1995;26:281–289. doi: 10.1016/0888-7543(95)80212-5. [DOI] [PubMed] [Google Scholar]
- 92.Oliveira C, Senz J, Kaurah P, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet. 2009;18:1545–1555. doi: 10.1093/hmg/ddp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grady WM, Willis J, Guilford PJ, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 94.Pinheiro H, Bordeira-Carriço R, Seixas S, et al. Allele-specific CDH1 downregulation and hereditary diffuse gastric cancer. Hum Mol Genet. 2010;19:943–952. doi: 10.1093/hmg/ddp537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujita H, Lennerz JK, Chung DC, et al. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: Biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol. 2012;36:1709–1717. doi: 10.1097/PAS.0b013e31826ca204. [DOI] [PubMed] [Google Scholar]
- 96.Charlton A, Blair V, Shaw D, et al. Hereditary diffuse gastric cancer: Predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–820. doi: 10.1136/gut.2002.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lauwers GY, Mullen JT, Chelcun Schreiber KE, et al. Familial gastric cancers: A review with focus on hereditary diffuse gastric cancer syndrome. Pathol Case Rev. 2014;19:66–73. [Google Scholar]
- 98.Pinheiro H, Oliveira C, Seruca R, et al. Hereditary diffuse gastric cancer - pathophysiology and clinical management. Best Pract Res Clin Gastroenterol. 2014;28:1055–1068. doi: 10.1016/j.bpg.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Vasen HF. Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)] Fam Cancer. 2005;4:219–225. doi: 10.1007/s10689-004-3906-5. [DOI] [PubMed] [Google Scholar]
- 100.Park YJ, Shin KH, Park JG. Risk of gastric cancer in hereditary nonpolyposis colorectal cancer in Korea. Clin Cancer Res. 2000;6:2994–2998. [PubMed] [Google Scholar]
- 101.Cai SJ, Xu Y, Cai GX, et al. Clinical characteristics and diagnosis of patients with hereditary nonpolyposis colorectal cancer. World J Gastroenterol. 2003;9:284–287. doi: 10.3748/wjg.v9.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: A study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001;19:4074–4080. doi: 10.1200/JCO.2001.19.20.4074. [DOI] [PubMed] [Google Scholar]
- 103.Akhtar S, Oza KK, Khan SA, et al. Muir-Torre syndrome: Case report of a patient with concurrent jejunal and ureteral cancer and a review of the literature. J Am Acad Dermatol. 1999;41:681–686. doi: 10.1016/s0190-9622(99)70001-0. [DOI] [PubMed] [Google Scholar]
- 104.Shellenberger MJ, Deivert DE, Komar MJ. Genes are more than skin deep: A case of Muir-Torre syndrome. Gastrointest Endosc. 2008;68:608–610. doi: 10.1016/j.gie.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 105.MIM number 120435: Lynch syndrome I. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/120435. Accessed August 6, 2013.
- 106.Geary J, Sasieni P, Houlston R, et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC) Fam Cancer. 2008;7:163–172. doi: 10.1007/s10689-007-9164-6. [DOI] [PubMed] [Google Scholar]
- 107.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 108.Scott RJ, McPhillips M, Meldrum CJ, et al. Hereditary nonpolyposis colorectal cancer in 95 families: Differences and similarities between mutation-positive and mutation-negative kindreds. Am J Hum Genet. 2001;68:118–127. doi: 10.1086/316942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Classics in Oncology. Heredity with Reference to Carcinoma as Shown by the Study of the Cases Examined in the Pathological Laboratory of the University of Michigan, 1895–1913. By Aldred Scott Warthin. 1913. CA Cancer J Clin 1985;35:348–359. [DOI] [PubMed]
- 110.Cristofaro G, Lynch HT, Caruso ML, et al. New phenotypic aspects in a family with Lynch syndrome II. Cancer. 1987;60:51–58. doi: 10.1002/1097-0142(19870701)60:1<51::aid-cncr2820600110>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 111.Koornstra JJ, Mourits MJ, Sijmons RH, et al. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10:400–408. doi: 10.1016/S1470-2045(09)70041-5. [DOI] [PubMed] [Google Scholar]
- 112.Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44:353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 114.Corso G, Carvalho J, Marrelli D, et al. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868–875. doi: 10.1200/JCO.2012.44.4612. [DOI] [PubMed] [Google Scholar]
- 115.Shinmura K, Kohno T, Takahashi M, et al. Familial gastric cancer: Clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127–1131. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- 116.Corso G, Marrelli D, Roviello F. Clinical management of familial gastric cancer. In: Corso G, Roviello F, editors. Spotlight on Familial and Hereditary Gastric Cancer. New York, NY: Springer; 2013. pp. 183–190. [Google Scholar]
- 117.Li FP, Fraumeni JF., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969;71:747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- 118.The International Agency for Research on Cancer International Agency for Research on Cancer. Lyon, France: International Agency for Research on Cancer; 2007. TP53 mutation data database, R12 release. [Google Scholar]
- 119.Masciari S, Dewanwala A, Stoffel EM, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–657. doi: 10.1097/GIM.0b013e31821628b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MIM number 151623: Li-Fraumeni syndrome 1; LFS1. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/151623. Accessed April 2, 2013.
- 121.Sereno M, Aguayo C, Guillén Ponce C, et al. Gastric tumours in hereditary cancer syndromes: Clinical features, molecular biology and strategies for prevention. Clin Transl Oncol. 2011;13:599–610. doi: 10.1007/s12094-011-0705-y. [DOI] [PubMed] [Google Scholar]
- 122.Oliveira C, Ferreira P, Nabais S, et al. E-Cadherin (CDH1) and p53 rather than SMAD4 and Caspase-10 germline mutations contribute to genetic predisposition in Portuguese gastric cancer patients. Eur J Cancer. 2004;40:1897–1903. doi: 10.1016/j.ejca.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 123.Chompret A. The Li-Fraumeni syndrome. Biochimie. 2002;84:75–82. doi: 10.1016/s0300-9084(01)01361-x. [DOI] [PubMed] [Google Scholar]
- 124.Horio Y, Suzuki H, Ueda R, et al. Predominantly tumor-limited expression of a mutant allele in a Japanese family carrying a germline p53 mutation. Oncogene. 1994;9:1231–1235. [PubMed] [Google Scholar]
- 125.Pötzsch C, Voigtländer T, Lübbert M. p53 Germline mutation in a patient with Li-Fraumeni syndrome and three metachronous malignancies. J Cancer Res Clin Oncol. 2002;128:456–460. doi: 10.1007/s00432-002-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sugano K, Taniguchi T, Saeki M, et al. Germline p53 mutation in a case of Li-Fraumeni syndrome presenting gastric cancer. Jpn J Clin Oncol. 1999;29:513–516. doi: 10.1093/jjco/29.10.513. [DOI] [PubMed] [Google Scholar]
- 127.da Silva EM, Achatz MI, Martel-Planche G, et al. TP53 mutation p.R337H in gastric cancer tissues of a 12-year-old male child: Evidence for chimerism involving a common mutant founder haplotype: Case report. BMC Cancer. 2011;11:449. doi: 10.1186/1471-2407-11-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fort Washington, PA: National Comprehensive Cancer Network; 2010. NCCN. Practice Guidelines in Oncology-V1.2010: Genetic/Familial High-Risk Assessment: Breast and Ovarian: Li-Fraumeni Syndrome. [Google Scholar]
- 129.Balmaña J, Díez O, Rubio IT, et al. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(suppl 6):vi31–vi34. doi: 10.1093/annonc/mdr373. [DOI] [PubMed] [Google Scholar]
- 130.Gutiérrez Espeleta GA, Llacuachaqui M, García-Jiménez L, et al. BRCA1 and BRCA2 mutations among familial breast cancer patients from Costa Rica. Clin Genet. 2012;82:484–488. doi: 10.1111/j.1399-0004.2011.01774.x. [DOI] [PubMed] [Google Scholar]
- 131.Breast Cancer Linkage Consortium Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 132.Figer A, Irmin L, Geva R, et al. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001;84:478–481. doi: 10.1054/bjoc.2000.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lorenzo Bermejo J, Hemminki K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann Oncol. 2004;15:1834–1841. doi: 10.1093/annonc/mdh474. [DOI] [PubMed] [Google Scholar]
- 134.Gallardo M, Silva A, Rubio L, et al. Incidence of BRCA1 and BRCA2 mutations in 54 Chilean families with breast/ovarian cancer, genotype-phenotype correlations. Breast Cancer Res Treat. 2006;95:81–87. doi: 10.1007/s10549-005-9047-1. [DOI] [PubMed] [Google Scholar]
- 135.Li WF, Hu Z, Rao NY, et al. The prevalence of BRCA1 and BRCA2 germline mutations in high-risk breast cancer patients of Chinese Han nationality: Two recurrent mutations were identified. Breast Cancer Res Treat. 2008;110:99–109. doi: 10.1007/s10549-007-9708-3. [DOI] [PubMed] [Google Scholar]
- 136.Boyd J. BRCA2 as a low-penetrance cancer gene. J Natl Cancer Inst. 1996;88:1408–1409. doi: 10.1093/jnci/88.19.1408-b. [DOI] [PubMed] [Google Scholar]
- 137.Chen XR, Zhang WZ, Lin XQ, et al. Genetic instability of BRCA1 gene at locus D17S855 is related to clinicopathological behaviors of gastric cancer from Chinese population. World J Gastroenterol. 2006;12:4246–4249. doi: 10.3748/wjg.v12.i26.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Semba S, Yokozaki H, Yasui W, et al. Frequent microsatellite instability and loss of heterozygosity in the region including BRCA1 (17q21) in young patients with gastric cancer. Int J Oncol. 1998;12:1245–1251. doi: 10.3892/ijo.12.6.1245. [DOI] [PubMed] [Google Scholar]
- 139.Jakubowska A, Nej K, Huzarski T, et al. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br J Cancer. 2002;87:888–891. doi: 10.1038/sj.bjc.6600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang ZZ, Liu YJ, Yin XL, et al. Loss of BRCA1 expression leads to worse survival in patients with gastric carcinoma. World J Gastroenterol. 2013;19:1968–1974. doi: 10.3748/wjg.v19.i12.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.MIM number 208900: Ataxia-telangiectasia; AT. Online Mendelian Inheritance in Man. Available at http://omim.org/entry/208900. Accessed October 7, 2013.
- 142.Patiroglu T, Eke Gungor H, Arslan D, et al. Gastric signet ring carcinoma in a patient with ataxia-telangiectasia: A case report and review of the literature. J Pediatr Hematol Oncol. 2013;35:e341–e343. doi: 10.1097/MPH.0b013e318279b3f7. [DOI] [PubMed] [Google Scholar]
- 143.Otabor IA, Abdessalam SF, Erdman SH, et al. Gastric outlet obstruction due to adenocarcinoma in a patient with Ataxia-Telangiectasia syndrome: A case report and review of the literature. World J Surg Oncol. 2009;7:29. doi: 10.1186/1477-7819-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kraemer KH, DiGiovanna JJ.Xeroderma pigmentosum. In: Pagon RA, Adam MP, Ardinger HH. et al., eds. GeneReviews(R) Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 146.Giglia G, Dumaz N, Drougard C, et al. p53 mutations in skin and internal tumors of xeroderma pigmentosum patients belonging to the complementation group C. Cancer Res. 1998;58:4402–4409. [PubMed] [Google Scholar]
- 147.Kraemer KH. Xeroderma pigmentosum. In: Demis DJ, Dobson RL, McGuire J, eds. Clinical Dermatology, ed. Vol. 4ed. Hagerstown:Harper and Row, 1980:1–33.
- 148.Dong Z, Guo W, Zhou R, et al. Polymorphisms of the DNA repair gene XPA and XPC and its correlation with gastric cardiac adenocarcinoma in a high incidence population in North China. J Clin Gastroenterol. 2008;42:910–915. doi: 10.1097/MCG.0b013e3180f6262c. [DOI] [PubMed] [Google Scholar]
- 149.Puig L, Martí R, Matías-Guiu X, et al. Gastric adenocarcinoma in a patient with xeroderma pigmentosum. Br J Dermatol. 1985;113:632–633. doi: 10.1111/j.1365-2133.1985.tb02394.x. [DOI] [PubMed] [Google Scholar]