This large prospective study found that approximately 15% of cancer patients receiving chemotherapy with a normal adrenal response showed suppressed adrenal responses after antiemetic dexamethasone therapy. This result was particularly significant for patients cotreated with megestrol acetate.
Keywords: Adrenal, Antiemetic, Cancer, Dexamethasone, Suppression
Abstract
Background.
In a previous pilot study, adrenal suppression was found to be common after antiemetic dexamethasone therapy in cancer patients. The objective of this large prospective multicenter study was to confirm the incidence and factors associated with secondary adrenal suppression related to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy.
Methods.
Chemotherapy-naïve patients who were scheduled to receive at least three cycles of highly or moderately emetogenic chemotherapy with dexamethasone as an antiemetic were enrolled. Patients with a suppressed adrenal response before chemotherapy or those administered corticosteroids within 6 months of enrollment in the study were excluded.
Results.
Between October 2010 and August 2014, 481 patients receiving chemotherapy underwent the rapid adrenocorticotropic hormone (ACTH) stimulation test to assess eligibility; 350 of these patients were included in the final analysis. Fifty-six patients (16.0%) showed a suppressed adrenal response in the rapid ACTH stimulation test at 3 or 6 months after the start of the first chemotherapy. The incidence of adrenal suppression was affected by age, performance status, stage, and use of megestrol acetate in univariate analysis. Multivariate analysis revealed that secondary adrenal suppression associated with antiemetic dexamethasone therapy was significantly associated with megestrol acetate treatment (odds ratio: 3.06; 95% confidence interval: 1.60 to 5.86; p < .001).
Conclusion.
This large prospective study indicates that approximately 15% of cancer patients receiving chemotherapy with a normal adrenal response show suppressed adrenal responses after antiemetic dexamethasone therapy. This result was particularly significant for patients cotreated with megestrol acetate.
Implications for Practice:
This large prospective multicenter study indicates that approximately 15% of cancer patients receiving chemotherapy with a normal adrenal response show secondary adrenal suppression after antiemetic dexamethasone therapy. Adrenal suppression was particularly significant for patients cotreated with megestrol acetate. Clinicians need increased awareness of the potential for adrenal insufficiency secondary to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy. These findings should help encourage prospective studies designed to determine the adequate doses and durations of antiemetic dexamethasone therapy required to reduce dexamethasone-related adverse effects while controlling chemotherapy-induced nausea and vomiting.
Introduction
Corticosteroids are effective in the prevention of chemotherapy-induced nausea and vomiting (CINV) in both the acute and delayed phases [1, 2]. The antiemetic mechanisms of action of corticosteroids are incompletely understood but are thought to include inhibition of prostaglandin synthesis, anti-inflammatory efficacy, and serotonin receptor antagonism [3‒5]. Although no randomized study has compared the antiemetic potency of available corticosteroids, the most frequently used steroid as an antiemetic is dexamethasone. Dexamethasone is usually used in combination with serotonin (5-hydroxytryptamine 3 [5-HT3]) or neurokinin-1 receptor antagonists for highly or moderately emetogenic chemotherapy or as monotherapy for mildly emetogenic chemotherapy [6‒9].
The repeated and chronic use of high doses of dexamethasone is associated with many adverse systemic effects including insomnia, epigastric discomfort, agitation, weight gain, and hyperglycemia, although these adverse effects can be managed and are thought to be outweighed by the benefits of dexamethasone [10]. One of the most serious adverse events of dexamethasone is secondary adrenal insufficiency, which manifests as various symptoms such as fatigue, loss of appetite, nausea, vomiting, and diarrhea [11, 12]. However, the incidence of secondary adrenal suppression associated with the repeated use of dexamethasone for the prevention of CINV has not been reported. We conducted a pilot study to assess the incidence of adrenal suppression in 103 patients receiving chemotherapy with dexamethasone as an antiemetic; it was found that adrenal suppression is common (43.7%) after antiemetic dexamethasone therapy [13]. We designed a larger prospective multicenter study to confirm the incidence of and factors associated with secondary adrenal suppression related to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy.
Materials and Methods
Study Design and Patients
The present study was conducted to confirm the incidence and factors associated with secondary adrenal suppression related to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy. This study was a prospective multicenter study involving nine institutions of the Korean South West Oncology Group (KSWOG). All patients provided written informed consent to participate in the study, which was reviewed and approved by the institutional review board of each participating hospital.
Chemotherapy-naïve patients with histologically confirmed cancer who were scheduled to receive highly or moderately emetogenic chemotherapy with antiemetic dexamethasone for at least 3 days per cycle were enrolled. Patients were required to have a life expectancy of ≥3 months and adequate hematologic, hepatic, and renal function. Exclusion criteria included a suppressed baseline adrenal response before chemotherapy, corticosteroids administered within 6 months of enrollment in the study, history of autoimmune disease, serious concurrent infection or nonmalignant illness, and history of adrenalectomy or adrenal metastasis.
Antiemetic Treatments
According to the National Comprehensive Cancer Network guidelines [9], all patients treated with highly emetogenic chemotherapy received 125 mg of an oral aprepitant plus a 5-HT3 receptor antagonist and 10‒12 mg dexamethasone on day 1, 80 mg of an oral aprepitant and 7‒8 mg of daily oral dexamethasone on days 2 and 3, and 7‒8 mg of dexamethasone on day 4. Patients treated with moderately emetogenic chemotherapy received a 5-HT3 receptor antagonist and 10‒12 mg of dexamethasone on day 1, followed by 7‒8 mg of daily dexamethasone on days 2 to 3. These antiemetics were administered every 2‒4 weeks according to the chemotherapy schedule.
Rapid Adrenocorticotropic Hormone Stimulation Test
Adrenal function was assessed using the rapid adrenocorticotropic hormone (ACTH) stimulation test before chemotherapy and 3 and 6 months after the start of chemotherapy. The low-dose (1 μg) ACTH stimulation test is more sensitive than the standard-dose (250 μg) ACTH stimulation test for establishing a diagnosis of secondary adrenal suppression [14]; however, the standard-dose ACTH stimulation test was used because it has been used as the standard test in the initial assessment of adrenal function, and the low-dose ACTH stimulation test is physician intensive. Patients showing suppressed adrenal response in the rapid ACTH stimulation test before chemotherapy were excluded. To minimize the direct effect of chemotherapeutic agents and the transient inhibition of adrenal function by dexamethasone, ACTH stimulation tests were performed between 8 and 11 a.m. just before chemotherapy. Baseline serum cortisol and ACTH levels were obtained, and then 250 μg of tetracosactide (Synacthen; Novartis, Basel, Switzerland, https://www.novartis.com), a synthetic ACTH, was administered by bolus intravenous injection. Blood was then drawn at 30 and 60 minutes after injection to determine serum cortisol level. Secondary adrenal suppression was defined by standard criteria as a failure of stimulated serum cortisol to rise above 18 μg/dL (500 nmol/L) after ACTH administration [15, 16].
Assessment of Symptoms Associated With Adrenal Insufficiency and Quality of Life After Prednisolone Replacement
If response to the rapid ACTH stimulation test was suppressed at 3 or 6 months, the symptoms associated with adrenal insufficiency were investigated using a self-report questionnaire focused on the six most common symptoms—weakness, anorexia, nausea, vomiting, abdominal pain, and diarrhea [16]—each scored using a 15-cm visual analog scale (VAS). Zero centimeters was labeled “no symptom,” and 15 cm was labeled “worst symptom you can imagine.” The patient placed a mark on the line at the point that represented his or her own perception of the current state. The score is determined by measuring the distance in centimeters from the left end of the line to the point marked by the patient. Health-related quality of life was also measured with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30). Patients with suppressed adrenal response received 7.5 mg of prednisolone replacement daily for 1‒2 weeks; after prednisolone treatment, the symptoms associated with adrenal insufficiency and EORTC QLQ-C30 were followed using the same self-report questionnaire.
Statistical Analysis
The sample size was calculated under the following assumptions. The incidence of adrenal suppression occurring in cancer patients receiving systemic chemotherapy with antiemetic dexamethasone therapy was expected to be approximately 35% (p = .35) based on a pilot study [13]. The number of patients required can be assessed using the following formula with 95% confidence interval and 5% sampling error: n ≥ (2 × Z1−α/2 √p(1 − p) / (2 × sampling error) = (2 × 1.96 √0.35(1 − 0.35)) / (2 × 0.05) [17, 18]. We estimated that 350 patients needed to be enrolled to assess the incidence of adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy.
The Student t test was used to compare means when there were two categories, and one-way analysis of variance was used when there were more than two categories. Proportions were compared using two-way tables and chi-square tests. The paired t test was used to assess the changes in symptoms associated with adrenal insufficiency and the EORTC QLQ-C30 scale before and after prednisolone replacement. Univariate and multivariate regression analyses were performed to investigate the relationship between the incidence of adrenal suppression and clinical variables. Potential explanatory variables were age (<60 or ≥60 years), sex (female or male), Eastern Cooperative Oncology Group (ECOG) performance status (0, 1, or 2‒3), primary site of tumor (lung, breast, stomach, colorectal, or others), stage (I, II, II, or IV), intent of first chemotherapy (adjuvant, palliative, or neoadjuvant and concurrent chemoradiation), emetic risk of first chemotherapy (high or moderate), chemotherapeutic agents (platinum or nonplatinum based), and the use of megestrol acetate (yes or no). A p-value <.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows software, version 17.0 (IBM Corp., Armonk, NY, http://www.ibm.com).
Results
Patient Characteristics
From October 2010 to August 2014, 481 patients underwent the baseline rapid ACTH stimulation test for eligibility before first chemotherapy, and 131 of these patients were excluded because they either showed a suppressed adrenal response to the baseline rapid ACTH stimulation test (41 patients), did not receive at least 3 cycles of highly or moderately emetogenic chemotherapy (40 patients), were lost to follow-up (23 patients), died before the rapid ACTH stimulation test at 3 months after first chemotherapy (18 patients), failed to comply with at least one entry criterion (6 patients), or withdrew their consent (3 patients). Consequently, 350 and 196 patients underwent the rapid ACTH stimulation test at 3 and 6 months, respectively, after the start of the first chemotherapy with dexamethasone as an antiemetic (Fig. 1).
Figure 1.
Flow diagram of the patients.
Abbreviation: ACTH, adrenocorticotropic hormone.
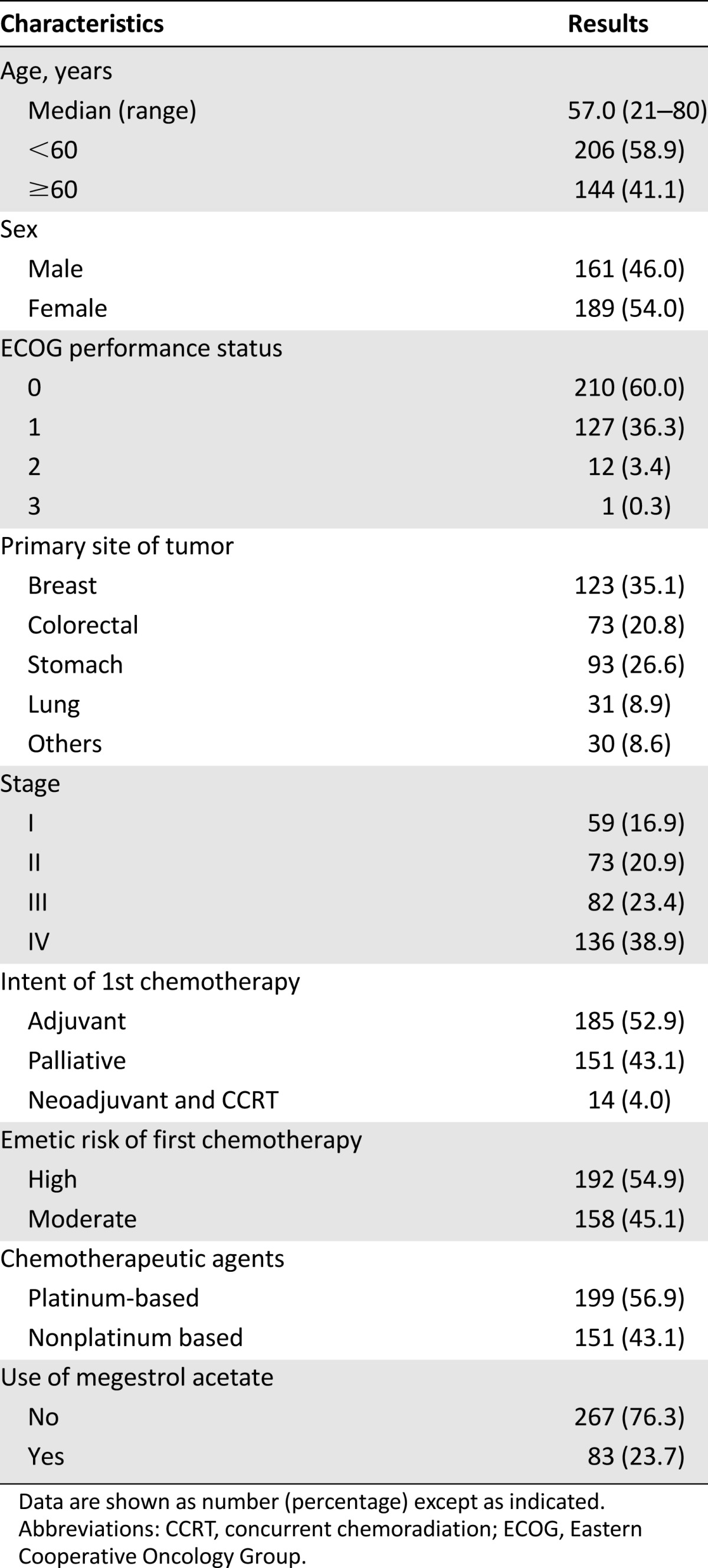
The baseline characteristics of these 350 patients are described in Table 1. The median age was 57 years (range: 21‒80 years), and 337 patients (96.6%) had good performance status (ECOG 0–1). A total of 136 patients (38.9%) had stage IV disease, and 151 patients (43.1%) received palliative chemotherapy. In addition, 199 patients (56.9%) received a platinum-based chemotherapeutic agent, and 83 patients (23.7%) received megestrol acetate to relieve symptoms of anorexia before the ACTH stimulation test at 3 or 6 months.
Table 1.
Patient characteristics
Results of the Rapid ACTH Stimulation Test
Compared with the baseline cortisol level before the first chemotherapy, a significant decrease in cortisol level was observed at 3 and 6 months after the start of the first chemotherapy using antiemetic dexamethasone. Cortisol levels (mean ± SD) were 15.5 ± 7.1 μg/dL at baseline, 12.2 ± 7.8 μg/dL at 3 months (p < .001), and 12.3 ± 7.0 μg/dL at 6 months (p < .001). Stimulated serum cortisol levels after 30 minutes were 28.2 ± 7.9 μg/dL at baseline, 25.4 ± 10.2 μg/dL at 3 months (p < .001), and 26.5 ± 9.7 μg/dL at 6 months (p = .044). Stimulated serum cortisol levels after 60 minutes were 33.7 ± 9.8 μg/dL at baseline, 30.4 ± 12.1 μg/dL at 3 months (p < .001), and 31.8 ± 11.6 μg/dL at 6 months (p = .058).
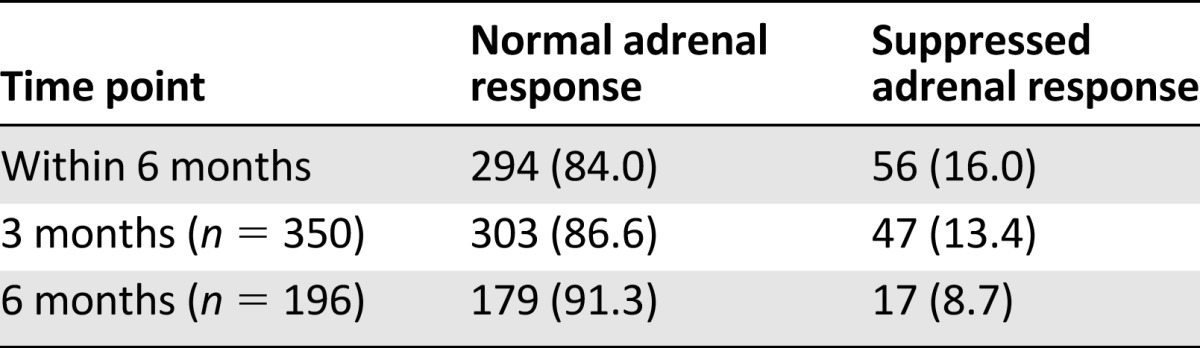
The incidence of adrenal suppression is presented in Table 2. If secondary adrenal suppression was defined as a failure of stimulated serum cortisol to rise above 18 μg/dL after ACTH administration, 56 of the 350 patients (16.0%) showed a suppressed adrenal response to ACTH stimulation within 6 months of starting first chemotherapy using dexamethasone as an antiemetic. At 3 and 6 months after the start of the first chemotherapy, 47 of the 350 patients (13.4%) and 17 of the 196 patients (8.7%) showed a suppressed adrenal response to ACTH stimulation.
Table 2.
The incidence of adrenal suppression as determined by the rapid adrenocorticotropic hormone stimulation test
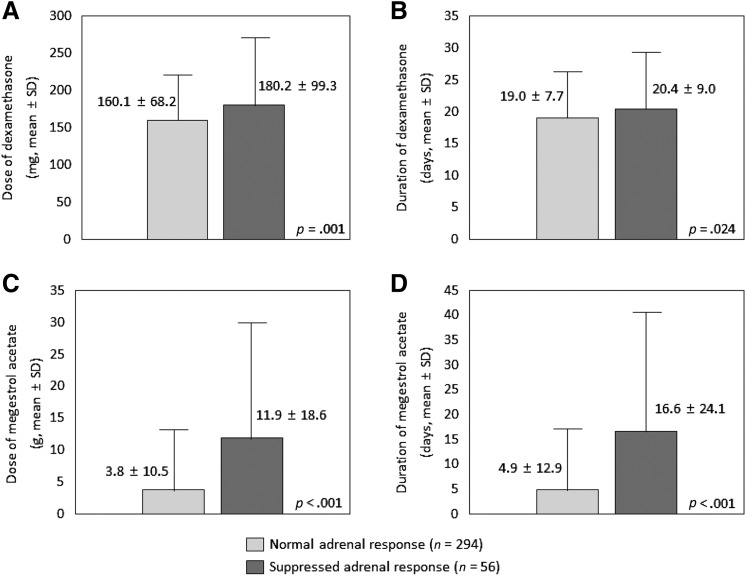
Cumulative Dose and Duration of Dexamethasone and Megestrol Acetate Therapy
The patients with a suppressed adrenal response received a larger cumulative dexamethasone dose (mean ± SD: 160.1 ± 68.2 vs. 180.2 ± 99.3 mg; p = .001) (Fig. 2A) and received dexamethasone for a longer period (mean ± SD: 19.0 ± 7.7 vs. 20.4 ± 9.0 days; p = .024) (Fig. 2B) than patients with a normal adrenal response. Likewise, the patients with a suppressed adrenal response received a larger cumulative megestrol acetate dose (mean ± SD: 3.8 ± 10.5 vs. 11.9 ± 18.6 g; p < .001) (Fig. 2C) and received megestrol acetate for a longer period (mean ± SD: 4.9 ± 12.9 vs. 16.6 ± 24.1 days; p < .001) than patients with a normal adrenal response (Fig. 2D).
Figure 2.
Cumulative dose and duration of antiemetic dexamethasone and megestrol acetate according to the adrenal response. (A): Dose of dexamethasone (in milligrams). (B): Duration of dexamethasone (in days). (C): Dose of megestrol acetate (in grams). (D): Duration of megestrol acetate (in days).
Univariate and Multivariate Analysis of Adrenal Suppression
The incidence of adrenal suppression based on clinical variables is shown in Table 3. The incidence of adrenal suppression was significantly affected by age, ECOG performance status, stage, and the use of megestrol acetate in univariate analysis. The incidence of adrenal suppression in patients aged <60 and ≥60 years was 11.7% and 22.2%, respectively (p = .012). The incidence of adrenal suppression for patients with ECOG performance 0, 1, and 2‒3 was 11.9%, 21.3%, and 30.8%, respectively (p = .007). Patients with advanced tumor stage (19.5% for stage III and 20.6% for stage IV) also showed adrenal suppression more often than patients with less advanced stage (10.2% for stage I and 8.2% for stage II) (p = .014). Twenty-nine of the 267 patients (10.9%) who did not receive megestrol acetate had a suppressed adrenal response, whereas 27 of the 83 patients (32.5%) who received megestrol acetate had a suppressed adrenal response (p < .001).
Table 3.
Univariate and multivariate analysis of adrenal suppression
The use of megestrol acetate was a significant factor only for the incidence of secondary adrenal suppression associated with antiemetic dexamethasone therapy (p = .001); other clinical characteristics were not found to be significantly associated with adrenal suppression in multivariate analysis (Table 3).
Changes in Symptoms Associated With Adrenal Insufficiency and QOL After Corticosteroid Treatment
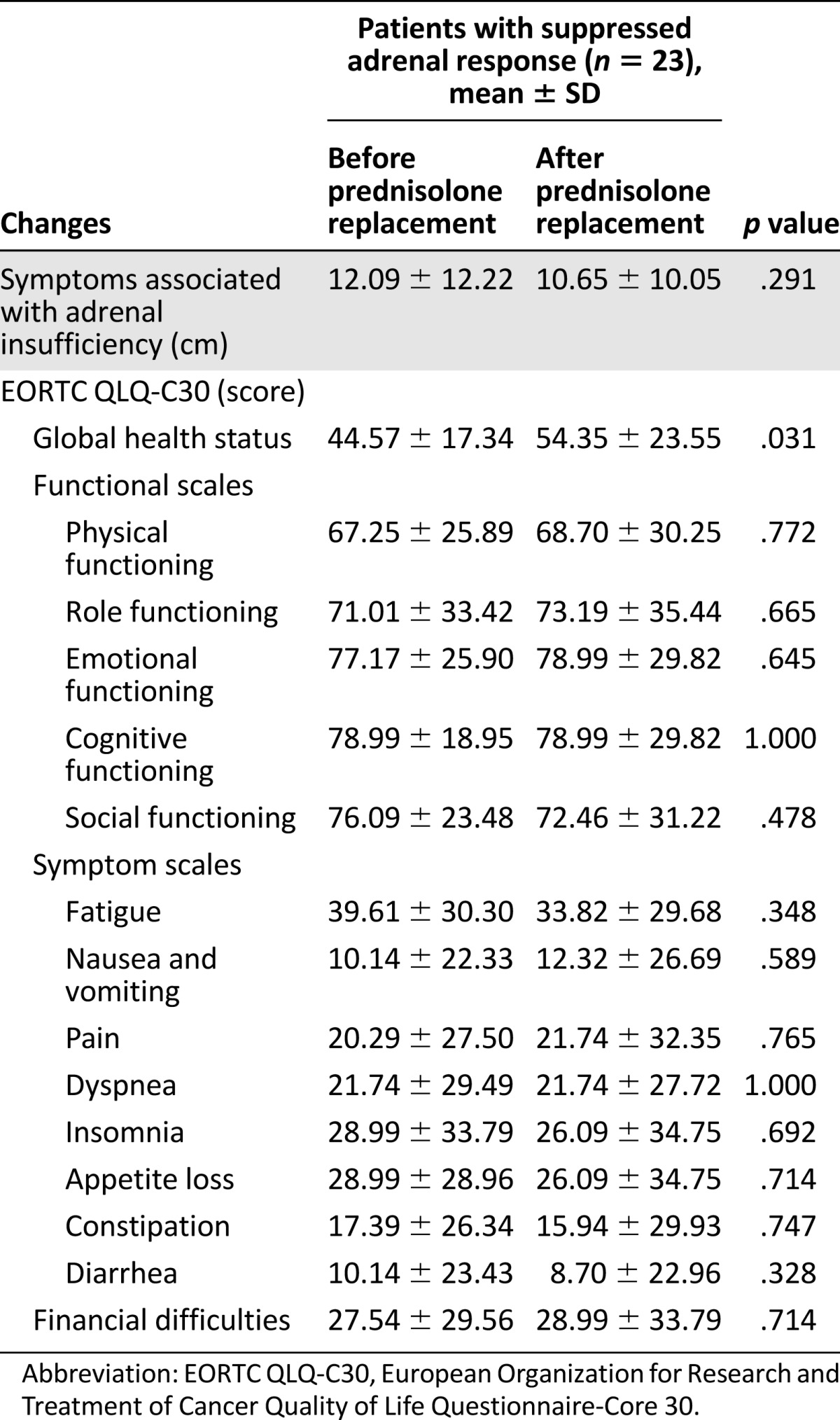
Twenty-three (41.1%) of the 56 patients with suppressed adrenal response received prednisolone replacement for 1‒2 weeks and were assessed for symptoms associated with adrenal insufficiency and EORTC QLQ-C30 before and after corticosteroid treatment (Table 4). The symptoms of adrenal insufficiency as assessed by VAS (mean ± SD) were 12.09 ± 12.22 and 10.65 ± 10.05 cm before and after 1‒2 weeks of prednisolone replacement, respectively; however, this difference was not statistically significant (p = .291). Furthermore, the functional and symptom scales of EORTC QLQ C-30 also showed no statistically significant differences after prednisolone replacement. There was an improvement in the EORTC QLQ C-30 global health status scale after prednisolone replacement (mean ± SD: 44.57 ± 17.34 to 54.35 ± 23.55; p = .031).
Table 4.
Changes in symptoms associated with adrenal insufficiency and quality of life after corticosteroid treatment
Discussion
This large prospective multicenter study showed that the incidence of secondary adrenal suppression was approximately 15% after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy. Furthermore, secondary adrenal suppression after antiemetic dexamethasone therapy was significantly affected by megestrol acetate treatment.
No previous study has reported adrenal insufficiency after antiemetic dexamethasone therapy, despite the fact that dexamethasone is the most frequently used antiemetic for the prevention of CINV in cancer patients receiving chemotherapy. In a previous pilot study, we assessed the incidence of adrenal suppression related to antiemetic dexamethasone therapy in cancer patients [13]. Adrenal suppression after antiemetic dexamethasone therapy was demonstrated biochemically in 45 of 103 patients (43.7%) and was more common in patients receiving megestrol acetate concomitantly. The incidence of adrenal suppression in the present prospective study (16.0%) was much lower than that in the pilot study (43.7%), and that could be explained by several factors. First, the previous pilot study did not include any baseline ACTH stimulation test before chemotherapy. This present multicenter study excluded not only patients with clinical factors associated with suppressed adrenal response (history of adrenalectomy, adrenal metastasis, or the use of corticosteroid within 6 months of enrollment) but also patients who showed a suppressed adrenal response in the ACTH stimulation test before chemotherapy. In the baseline ACTH stimulation test, approximately 10% (41 patients) of all assessed patients were found to have a suppressed adrenal response before chemotherapy. These patients, who were excluded, were older (median: 67.0 vs. 57 years), had a poorer ECOG performance status (ECOG performance status 1 and 2, 70.7% vs. 40.0%), and many had a more advanced tumor stage (stage IV, 56.1% vs. 38.9%) than the patients who were included in the study. Second, possible differences in baseline characteristics between the two studies may have affected the adrenal response. The pilot study included older patients (median: 63.5 vs. 57.0 years), more patients whose ECOG performance status was 2 or 3 (25.3% vs. 3.4%), and more patients receiving palliative chemotherapy (73.8% vs. 43.1%) than the present study. Third, the pilot study showed a significantly higher mean cumulative dose of dexamethasone (251.8 vs. 158.7 mg), mean cumulative duration of dexamethasone (25.5 vs. 19.5 days), percentage of patients receiving megestrol acetate (56.3% vs. 23.7%), mean cumulative dose of megestrol acetate (22.7 vs. 5.1 g), and mean cumulative duration of megestrol acetate (28.3 vs. 6.8 days) than the present study. Consequently, based on the results in this study, the incidence of secondary adrenal suppression after antiemetic dexamethasone therapy is associated with age, performance status, advanced tumor stage, cumulative dose and duration of dexamethasone, and cumulative dose and duration of megestrol acetate treatment.
Megestrol acetate, a synthetic progestational agent has been used in the treatment of metastatic breast cancer and endometrial cancer [19‒21] and has also been used for stimulating appetite in patients with wasting illness, including patients with cancer-associated anorexia and cachexia [22, 23]. Megestrol acetate has glucocorticoid activity, and high doses or a prolonged treatment period with megestrol acetate reduce plasma ACTH and cortisol secretion, leading to adrenal insufficiency secondary to prolonged suppression of the hypothalamic-pituitary-adrenal axis [24‒27]. In both the pilot and this study, megestrol acetate treatment was significantly associated with adrenal suppression on antiemetic dexamethasone therapy. The incidence of adrenal suppression in cancer patients cotreated with antiemetic dexamethasone and megestrol acetate was remarkable: 30 of 58 patients (51.7%) in the pilot study and 27 of 83 patients (32.5%) in this study showed a suppressed adrenal response in the ACTH stimulation test. It is very impressive that the frequency of adrenal suppression is high in such cases because dexamethasone and megestrol acetate are commonly used in many cancer patients as an antiemetic and as an aperient against anorexia, respectively.
To prevent or reduce CINV, cancer patients on chemotherapy are repeatedly administered dexamethasone over long periods of time, and that substantially increases the risk of adrenal insufficiency; however, some of the symptoms of adrenal insufficiency, such as fatigue, weakness, anorexia, nausea, vomiting, and diarrhea, are also common in patients with advanced malignancies for other reasons (e.g., tumor itself, chemotherapy-related adverse events, certain medicines, metabolic imbalance, and psychologic problems). Consequently, the diagnosis of adrenal insufficiency is frequently delayed unless there is a high degree of clinical suspicion or adrenal function tests are performed, and the condition is probably under-recognized clinically. Furthermore, many cancer patients receiving chemotherapy often suffer from chemotherapy-related adverse events including severe myelosuppression and infection. The potential impact of even mild adrenal insufficiency in these patients can be hazardous because cortisol has vital circulatory effects in response to stress [28].
The present study has several limitations. A biochemically suppressed ACTH stimulation test does not always mean overt clinical adrenal insufficiency. The improvement in symptoms associated with adrenal insufficiency was observed in the pilot study when patients with a suppressed adrenal response were treated with prednisolone, but no improvement was observed in the most common symptoms associated with adrenal insufficiency or in the symptom scales of the EORTC QLQ C-30 questionnaire used in this study, although there was a significant improvement in the EORTC QLQ C-30 global health status scale. In the present study, only 23 of 56 patients (41.1%) with suppressed adrenal function received prednisolone replacement and were assessed for symptoms associated with adrenal insufficiency and QOL before and after prednisolone replacement, and these patients were not likely to have had overt clinical adrenal insufficiency. Furthermore, glucocorticoid replacement was given for a minimum of ≥2 weeks in previous longitudinal studies investigating health-related QOL in patients with adrenal insufficiency [29]; therefore, the relatively shorter period of 1 or 2 weeks of prednisolone treatment used in the present study may have been why we observed no improvement in the symptoms of adrenal insufficiency or in the symptom scales of EORTC QLQ C-30. Moreover, multiagent chemotherapy could have influenced adrenal function; however, we performed an ACTH stimulation test just before the next cycle after a cycle of chemotherapy to minimize the direct effect of chemotherapeutic agents. Finally, we could not compare adrenal suppression for all chemotherapeutic agents but compared the incidence of adrenal suppression based on the use of platinum-based chemotherapy, which is already known to affect adrenal function [30], and found no significant difference.
Conclusion
This large prospective multicenter study indicates that approximately 15% of cancer patients receiving chemotherapy with a normal adrenal response show secondary adrenal suppression after antiemetic dexamethasone therapy for the prevention of CINV. Adrenal suppression was affected by age, performance status, and stage and was particularly significant for patients cotreated with megestrol acetate. Clinicians need increased awareness of the potential for adrenal insufficiency secondary to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
We thank the patients who took part in this study, the coordinators, and the investigators from each institute.
Author Contributions
Conception/Design: Hye Sook Han, Ki Hyeong Lee
Provision of study material or patients: Hye Sook Han, Ji Chan Park, Suk Young Park, Kyu Taek Lee, Sang Byung Bae, Han Jo Kim, Samyoung Kim, Hwan Jung Yun, Woo Kyun Bae, Hyun-Jeong Shim, Jun-Eul Hwang, Sang-Hee Cho, Moo-Rim Park, Hyeok Shim, Ki Hyeong Lee
Collection and/or assembly of data: Hye Sook Han, Ji Chan Park, Han Jo Kim, Hwan Jung Yun, Woo Kyun Bae, Hyeok Shim, Ki Hyeong Lee
Data analysis and interpretation: Hye Sook Han, Kyu Taek Lee, Hwan Jung Yun, Jihyun Kwon, Moon Ki Choi, Seung Taik Kim, Ki Hyeong Lee
Manuscript writing: Hye Sook Han, Jihyun Kwon, Moon Ki Choi, Seung Taik Kim, Ki Hyeong Lee
Final approval of manuscript: Hye Sook Han, Ki Hyeong Lee
Disclosures
The authors indicated no financial relationships.
References
- 1.Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: A meta-analysis of randomized evidence. J Clin Oncol. 2000;18:3409–3422. doi: 10.1200/JCO.2000.18.19.3409. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 3.Ho CM, Ho ST, Wang JJ, et al. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg. 2004;99:734–739. doi: 10.1213/01.ANE.0000130003.68288.C7. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Sugimoto M, Koyama H, et al. Inhibitory effect of glucocorticoids on human-cloned 5-hydroxytryptamine3A receptor expressed in xenopus oocytes. Anesthesiology. 2004;101:660–665. doi: 10.1097/00000542-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Tanihata S, Oda S, Nakai S, et al. Antiemetic effect of dexamethasone on cisplatin-induced early and delayed emesis in the pigeon. Eur J Pharmacol. 2004;484:311–321. doi: 10.1016/j.ejphar.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: Dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–240. doi: 10.1093/annonc/mdl347. [DOI] [PubMed] [Google Scholar]
- 7.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 8.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN Clinical Practice Guidelines in Oncology. Antiemesis, version 2, 2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed March 1, 2015.
- 10.Vardy J, Chiew KS, Galica J, et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94:1011–1015. doi: 10.1038/sj.bjc.6603048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335:1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 12.Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 13.Han HS, Shim YK, Kim JE, et al. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support Care Cancer. 2012;20:1565–1572. doi: 10.1007/s00520-011-1248-z. [DOI] [PubMed] [Google Scholar]
- 14.Tordjman K, Jaffe A, Trostanetsky Y, et al. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: Sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf) 2000;52:633–640. doi: 10.1046/j.1365-2265.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 15.Seravalli L. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;361:824–825; author reply 825. [PubMed] [Google Scholar]
- 16.Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Companies, Inc.; 2008. [Google Scholar]
- 17.Piantadosi S. Clinical Trials: A Methodologic Perspective. 2nd ed. New York, NY: John Wiley and Sons, Inc., 2005. [Google Scholar]
- 18.Pocock SJ. Clinical Trials: A Practical Approach. New York, NY: John Wiley and Sons, Inc.; 1983. [Google Scholar]
- 19.Muss HB, Wells HB, Paschold EH, et al. Megestrol acetate versus tamoxifen in advanced breast cancer: 5-Year analysis--a phase III trial of the Piedmont Oncology Association. J Clin Oncol. 1988;6:1098–1106. doi: 10.1200/JCO.1988.6.7.1098. [DOI] [PubMed] [Google Scholar]
- 20.Schacter LP, Rozencweig M, Canetta R, et al. Overview of hormonal therapy in advanced breast cancer. Semin Oncol. 1990;17(suppl 9):38–46. [PubMed] [Google Scholar]
- 21.Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 1996;14:357–361. doi: 10.1200/JCO.1996.14.2.357. [DOI] [PubMed] [Google Scholar]
- 22.Leśniak W, Bała M, Jaeschke R, et al. Effects of megestrol acetate in patients with cancer anorexia-cachexia syndrome--a systematic review and meta-analysis. Pol Arch Med Wewn. 2008;118:636–644. [PubMed] [Google Scholar]
- 23.Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;3:CD004310. doi: 10.1002/14651858.CD004310.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann M, Koller E, Murgo A, et al. Glucocorticoidlike activity of megestrol. A summary of Food and Drug Administration experience and a review of the literature. Arch Intern Med. 1997;157:1651–1656. doi: 10.1001/archinte.157.15.1651. [DOI] [PubMed] [Google Scholar]
- 25.Naing KK, Dewar JA, Leese GP. Megestrol acetate therapy and secondary adrenal suppression. Cancer. 1999;86:1044–1049. [PubMed] [Google Scholar]
- 26.Ron IG, Soyfer V, Goldray D, et al. A low-dose adrenocorticotropin test reveals impaired adrenal function in cancer patients receiving megestrol acetate therapy. Eur J Cancer. 2002;38:1490–1494. doi: 10.1016/s0959-8049(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 27.Raedler TJ, Jahn H, Goedeken B, et al. Acute effects of megestrol on the hypothalamic-pituitary-adrenal axis. Cancer Chemother Pharmacol. 2003;52:482–486. doi: 10.1007/s00280-003-0697-6. [DOI] [PubMed] [Google Scholar]
- 28.Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337:1285–1292. doi: 10.1056/NEJM199710303371807. [DOI] [PubMed] [Google Scholar]
- 29.Johannsson G, Falorni A, Skrtic S, et al. Adrenal insufficiency: Review of clinical outcomes with current glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2015;82:2–11. doi: 10.1111/cen.12603. [DOI] [PubMed] [Google Scholar]
- 30.Morrow GR, Hickok JT, Andrews PL, et al. Reduction in serum cortisol after platinum based chemotherapy for cancer: A role for the HPA axis in treatment-related nausea? Psychophysiology. 2002;39:491–495. doi: 10.1017.S0048577202991195. [DOI] [PubMed] [Google Scholar]