Abstract
We have recently proposed a model for subtyping schizophrenia based on antipsychotic (AP) treatment response. Evidence suggests that APs, both old and new, are comparable in terms of efficacy; however, one AP, clozapine, is uniquely effective in one subgroup of patients (that is, those with treatment-resistant schizophrenia [TRS]). This permits us to subdivide schizophrenia into 3 specific groups: AP responsive, clozapine responsive, and clozapine resistant. Here, we integrate this model with current criteria related to TRS and ultraresistant schizophrenia, the latter referred to in our model as clozapine resistant. We suggest several modifications to existing criteria, in line with current evidence and practice patterns, particularly emphasizing the need to focus on positive symptoms. While APs can favourably impact numerous dimensions related to schizophrenia, it is their effect on positive symptoms that distinguishes them from other psychotropics. Further, it is positive symptoms that are central to AP and clozapine resistance, and it is these people that place the greatest demands on acute and long-term inpatient resources. In moving AP development forward, we advocate specifically focusing on positive symptoms and capitalizing on the evidence we have of 3 subtypes of psychosis (that is, positive symptoms) based on treatment response, implicating 3 distinguishable forms of underlying pathophysiology. Conversely, pooling these groups risks obfuscating potentially identifiable differences. Such a position does not challenge the importance of dopamine D2 receptor blockade, but rather highlights the need to better isolate those other subgroups that require something more or entirely different.
Keywords: antipsychotic, clozapine, treatment-resistant, ultraresistant, psychosis
Abstract
Nous avons récemment proposé un modèle de sous-typage de la schizophrénie basé sur la réponse au traitement antipsychotique (AP). Les données probantes suggèrent que les AP, anciens comme nouveaux, sont d’efficacité comparable; cependant, un AP, la clozapine, a une efficacité unique dans un sous-groupe de patients (c’est-à-dire, ceux qui souffrent de schizophrénie résistante au traitement [SRT]). Ceci nous permet de subdiviser la schizophrénie en 3 groupes spécifiques : réceptive aux AP, réceptive à la clozapine, et résistante à la clozapine. Ici, nous intégrons ce modèle à des critères actuels liés à la SRT et à la schizophrénie ultrarésistante, cette dernière étant identifiée dans notre modèle comme étant résistante à la clozapine. Nous suggérons plusieurs modifications aux critères existants, conformément aux données probantes et aux modèles de pratique actuels, particulièrement en insistant sur le besoin de mettre l’accent sur les symptômes positifs. Bien que les AP puissent avoir un effet favorable sur de nombreuses dimensions liées à la schizophrénie, leur effet sur les symptômes positifs est ce qui les distingue des autres psychotropes. En outre, ce sont les symptômes positifs qui jouent un rôle central dans la résistance aux AP et à la clozapine, et ce sont les personnes qui présentent ces symptômes qui exigent le plus de ressources de soins actifs et d’hospitalisations à long terme. Pour faire avancer le développement des AP, nous réclamons spécifiquement de mettre l’accent sur les symptômes positifs et de capitaliser sur la preuve que nous détenons des 3 sous-types de psychose (c’est-à-dire, les symptômes positifs) d’après la réponse au traitement, ce qui implique 3 formes reconnaissables de pathophysiologie sous-jacente. À l’inverse, mettre en commun ces groupes risque d’obscurcir des différences potentiellement identifiables. Cette position ne conteste pas l’importance du blocage des récepteurs D2 de la dopamine, mais met plutôt en évidence le besoin de mieux isoler ces autres sous-groupes qui nécessitent quelque chose de plus ou d’entièrement différent.
Treatment-resistant schizophrenia is a familiar concept to today’s clinicians, and closely linked to clozapine. This is not coincidental, as seminal work involving clozapine in the late 1980s established the current framework for defining TRS. Drawing on similar criteria, a substantive body of evidence has evolved since clozapine’s reintroduction, and while modifications have been suggested, persistent symptoms and a prolonged period of poor functioning, despite several AP trials, remain fundamental. Subsequent advances demand that we revisit the concept of TRS to ensure it aligns with current thinking.
Treatment-Resistant Schizophrenia: A Historical Perspective
It is believed that the first report of TRS occurred in the context of insulin resistance.1 However, it was following the introduction of CPZ and APs in the 1950s that the term gained momentum, and within a decade, therapy refractory was discussed.2 Established criteria defining TRS were first used in 1966 in a paper2 addressing therapy-resistant schizophrenia, which included the following: active psychotic symptoms, despite 2 years of AP exposure, including 6 months of phenothiazine treatment at doses greater than CPZ 600 mg or trifluoperazine 80 mg daily.
Advances in understanding related to dopamine’s role in psychosis, specifically dopamine D2 receptor blockade in AP activity, translated to a focus on high-potency conventional APs. Notably, these drugs were not more effective than their low-potency counterparts, although their improved side effect profile from a cardiovascular perspective propagated the practice of high-dose therapy.3 The guidelines for evaluation of clozapine in TRS captured this, with CPZ equivalents dosing almost twice that of the 1966 criteria.2,4
Clinical Implications
APs treat positive symptoms; their impact (including atypical agents) on other major symptom domains is modest at best.
Clozapine initiation should be based on failure of prior AP trials in achieving a response in positive symptoms; criteria specific to illness and functional recovery should be removed.
Up to 20% of people with schizophrenia could be resistant to current APs, including clozapine, and there should be increased emphasis on distinguishing this subpopulation, in an effort to develop alternative APs and (or) treatment strategies.
Limitations
AP response may best be viewed along a spectrum, rather than as a discrete outcome.
More work needs to be done to understand the development, trajectory, and biology of AP resistance.
Treatment-Resistant Schizophrenia: Operational Criteria
By the late 1970s, evidence was being published regarding the comparison of different treatment approaches, including nonpharmacological, providing further evidence that a smaller subgroup would remain treatment resistant.5 In the late 1980s, a text was published on this topic,6 including a chapter by Philip May, underscoring the need for a systematic approach that would consider social adaptation as well as symptom reduction.7 Indeed, May and Dencker,8 one of the text’s editors, had been instrumental through the 1980s in bringing together an international group to address treatment refractoriness, a process that resulted in criteria highlighting 4 domains: positive symptoms, negative symptoms, functional deficits, and behavioural excess. They highlighted that these domains could be impacted differentially, and advocated that this framework be superimposed on criteria addressing course of illness and treatment response. Specifically, people must be ill for 2 years to define chronicity; additionally, 3 AP trials were required, each at doses of 1000 mg/day CPZ equivalents or more for 6 weeks or longer at steady state levels. An argument was made for either TDM or a trial of depot APs, as well as continuous psychosocial interventions.
Seven levels of response refractoriness were identified and defined: clinical remission, partial remission, slight resistance, moderate resistance, severe resistance, refractory, and severely refractory.8 Scales to assess functioning were discussed, but no clear-cut thresholds were defined; rather, authors favoured clinical judgment regarding functional capacity.
Criteria for clozapine in TRS captured the essence of this definition, with slight modifications.4 They aligned with moderate resistance but permitted 3 trials during 5 years, fitting with their prolongation of the illness’ negative impact on functioning. Criteria also specified trials from 2 chemical classes but omitted requirements related to TDM or depot APs. Subsequent treatment studies have embraced these criteria.
Two other sets of criteria have since been published, both using existing criteria for clozapine as a foundation (Table 1).9,10
Table 1.
Comparison of published criteria for treatment resistance in schizophrenia with proposed criteria for clozapine eligibility
| Published criteria | Itil et al2 | Brenner et al8 | Kane et al4 | Conley and Kelly10 | Suzuki et al9 | Proposed criteria for clozapine eligibility |
|---|---|---|---|---|---|---|
| Number of AP trials | Not mentioned | 3 (different chemical classes) | 3 (2 different chemical classes) | 2 | 2 | 2 |
| Adequate dose, CPZeq | 600 mg or Trifluoperazine 80 mg | ≥1000 mg | ≥1000 mg | 400 to 600 mg | ≥600 mg | Upper half of the recommended dosing rangea |
| Adequate duration, weeks | 24 | 6 | 6 | 4 to 6 | 6 | 6, at adequate dose |
| No significant improvement | Not mentioned | Not mentioned | <20% decrease on BPRS, and either CGI-S ≥ 4 or BPRS ≥ 45 | No clinical improvement | CGI-I ≥ 3, or <20 point increase in GAF and FACT-Sz, or <20% decrease of BPRS and PANSS | CGI-SCH positive change >2 (2 = much improved)b |
| Current illness severity | Active psychotic symptoms | Persistent positive and negative symptoms; disability in social, self-care, and occupational domains | BPRS ≥ 45, and CGI-S ≥ 4, and ≥4 on at least 2 out of 4 positive items | BPRS > 45, and CGI-S > 4, and >4 on at least 2 out of 4 positive items | CGI-S ≥ 4, and FACT-Sz ≤ 49 or GAF ≤ 50 | CGI-SCH positive ≥ 4 (4 = moderately ill)c |
| Duration of illness with poor functioning, years | 2 | 2 | 5 | 5 | Not mentioned |
AP = antipsychotic; BPRS = Brief Psychiatric Rating Scale; CGI-S = Clinical Global Impression–Severity; CGI-SCH = Clinical Global Impression–Schizophrenia; CPZeq = chlorpromazine equivalents; GAF = Global Assessment of Functioning; FACT-Sz = Functional Assessment for Comprehensive Treatment of Schizophrenia; PANSS = Positive and Negative Syndrome Scale
A CGI-SCH positive change score of 2 = Notably better with significant reduction of symptoms, but some symptoms remain; increase in the level of functioning.30
A CGI-SCH positive symptom score of 4 = Some prominent symptoms with some interference in the level of daily functioning.30
Redefining Treatment Resistance in Schizophrenia
Since clozapine was reintroduced, a new class of atypical APs thought to capture its unique pharmacology was spawned.11 With several decades of clinical experience and research now available, it is timely to reflect on existing evidence.
Although not entirely consistent, current consensus holds that clozapine is clinically superior in TRS12; however, its effectiveness remains limited, ranging from 30% to 70%.13 Moreover, early expectations that clozapine and subsequent APs substantially impact the multiple symptom domains characterizing schizophrenia (for example, negative symptoms or cognitive deficits) have been challenged.14
These findings necessitate changes in how treatment resistance is framed, and we recently proposed a new paradigm for classifying schizophrenia based on AP treatment response.15 We also argued that the scope of APs was becoming blurred inasmuch as claims regarding their efficacy broadened markedly with the advent of the newer atypical agents, only to be tempered over subsequent years.16 We highlighted the uniqueness of clozapine, which appears true in only one form of the illness, TRS. Finally, we raised concerns that efforts to better understand the biological underpinnings of schizophrenia risk being compromised, and the development of new treatments delayed, if these clearly identifiable clinical differences are not acknowledged in research strategies.
The model we propose revolves around positive symptoms, and the premise behind isolating them is straightforward. Available evidence indicates APs are not magic bullets; it seems naive to envisage that a single drug is capable of effectively treating the multiple symptom domains of schizophrenia.17 Just as the field of psychopharmacology has shifted to the development of selective compounds for these other domains, the search for better drugs for positive symptoms should do likewise. We have evidence of at least 3 subtypes of positive symptoms, and discuss how this might be integrated with existing criteria specifically related to TRS and URS in the goal of accelerating AP development.
Classification Based on Treatment Response
Three types of schizophrenia were proposed based on treatment response: AP responsive, clozapine responsive, and clozapine resistant.15 The latter 2 subtypes form the clozapine eligible subgroup, that is, not AP responsive. It is possible that the clozapine-resistant population represents more than one subgroup; we simply have insufficient information on this group.18
Schizophrenia is described as heterogeneous19 and such an approach represents a valuable portal for advancing this line of thinking. A clearly detailed underlying framework is required to ensure clinicians and researchers speak the same language for subtyping by treatment response; such an approach represents a clinical, not biological, marker and its use relies on specificity. To this end, we now focus on recommendations requiring conceptual shifts in current approaches to evaluating treatment response and resistance.
Framework for Classifying Treatment Response
While our model hinges on positive symptoms, we strongly advocate an approach that ensures different symptom domains are individually evaluated from the earliest stages of intervention. Evidence suggests that positive symptoms represent the end stage of illness, with cognitive and negative symptoms identifiable in schizophrenia’s prodrome.20,21 We also emphasize the need to differentially evaluate measures of functional outcome and subjective well-being from the illness’ outset.22–24
Regarding positive symptoms, there are criteria defining both TRS and URS.4,9,10,25 Regarding our model, criteria for clozapine eligibility align with people who are not AP responsive, a frequently encountered subgroup in clinical practice characterized by 2 failed AP trials, that is, clozapine eligible. More recently, criteria for URS have been proposed, and these parallel what we describe as clozapine resistant.
Building on existing literature related to TRS and URS, tables 1 and 2 summarize published criteria and our proposal. Notably, we avoid the terms TRS and URS. TRS is somewhat of a misnomer, as these people are not necessarily treatment resistant. All are clozapine eligible and some will be clozapine responsive; URS is vague, as this subsample actually represents people who are clozapine resistant. Our approach incorporates 3 changes representing conceptual shifts in thinking supported by more recent evidence, most importantly: focus on positive symptoms, removal of functioning, and removal of duration of illness in establishing treatment eligibility at any stage.
Table 2.
Comparison of published and proposed criteria for clozapine resistance in schizophrenia
| Published criteria | Mouaffak et al25 | Proposed criteria for clozapine resistance |
|---|---|---|
| Adequate dose | Plasma levels > 350 ng/mL | Plasma levelsa ≥ 350 ng/mL for once a day dosing; ≥ 250 ng/mL for equal divided dosing, or oral dose ≥ 400 mg a dayb |
| Adequate duration, weeks | 8 | 8, at adequate dosec |
| No significant improvement | <20% decrease on BPRS | CGI-SCH positive change > 2 (2 = much improved) |
| Current illness severity | BPRS ≥ 45, CGI-S ≥ 4, and ≥ 4 on at least 2 out of 4 positive items on the BPRS | CGI-SCH positive > 4 (4 = moderately ill) |
| Duration of illness with no good functioning, years | 5 |
BPRS = Brief Psychiatric Rating Scale; CGI-S = Clinical Global Impression–Severity; CGI-SCH = Clinical Global Impression–Schizophrenia
Plasma levels should be taken after 5 days of unchanged clozapine dosing and 12 hours from last clozapine dose.
A daily clozapine dose of 400 mg has been shown to achieve a threshold of 350 ng/mL in various trials, and lies within the dose range advocated for by a field of experts for acute and maintenance treatment.64–66
A study identified all clozapine responders within 8 weeks of a change in dose, indicating no increased benefits with continuing people on a particular dose longer to establish benefits.67
Focus on Positive Symptoms
While APs exert a generalized effect that impacts symptom domains beyond psychosis,26 the magnitude of effect is not comparable across domains. The success of APs in impacting either negative or cognitive symptoms is modest, leading to efforts to develop compounds selectively addressing these other features.14
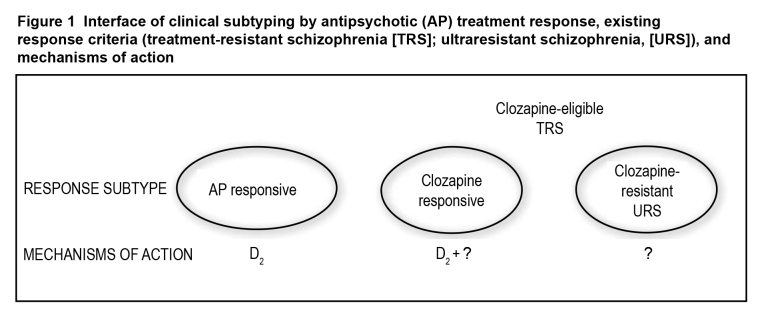
There is a greater chance for gains in AP development if a focus on positive symptoms remains central. APs are used to control positive symptoms, the crux of TRS and highlighted in all published TRS criteria (Table 1). It has been argued that dopamine D2 antagonism is central to AP effects,27 but we have at least 2 other forms of psychosis, clozapine responsive and clozapine resistant; in both cases, D2 antagonism alone is insufficient for establishing response (Figure 1).28
Figure 1. Interface of clinical subtyping by antipsychotic (AP) treatment response, existing response criteria (treatment-resistant schizophrenia [TRS]; ultraresistant schizophrenia, [URS]), and mechanisms of action.
In focusing on positive symptoms, we believe there is value in employing the CGI-SCH scale.29,30 The CGI itself has been shown to be as sensitive as the BPRS in detecting efficacy differences between APs.31 As recommended by different sources,32,33 the CGI-SCH also permits independent evaluation of multiple key symptom domains associated with schizophrenia (positive, negative, depressive, cognitive, and overall severity), simultaneously allowing for extraction of a value specific to positive symptoms. It is comparable with the PANSS in terms of sensitivity to change, and easier to administer, crucial if the objective includes clinical use.29 The CGI-SCH permits scoring of severity and change, the latter also accommodating relative values, compared with absolute thresholds. It is meant to include delusions, hallucinations, and bizarre behaviour, comparable with the positive symptom dimensions of psychoticism (reality distortion) and disorganization captured in more recent remission criteria.32
Removal of Functioning in Evaluating Treatment Response
All available criteria for TRS and URS incorporate functional impairment (tables 1 and 2). It seems intuitive that clinical recovery or remission32 would translate to functional recovery, although some evidence refutes this; for example, patients with first-episode schizophrenia meeting criteria for remission continue to manifest marked deficits in functional outcome.34 Similarly, patients meeting criteria for TRS frequently demonstrate a favourable response to clozapine, but this is not necessarily matched by improvements in functional measures.22,35 Longer-term, follow-up studies have also demonstrated that clinical and functional recovery do not parallel each other across time.23
More recently, it has been argued that other symptom domains may play a more critical role in functional outcome.36,37 Because APs fall short in effective treatment of these domains,14 we recommend that treatment response be confined to AP efficacy (positive symptoms) only.
Removal of Duration of Illness in Evaluating Treatment Response
Existing criteria for TRS and URS incorporate duration of illness (2 to 5 years) in establishing candidacy for clozapine (tables 1 and 2). Earlier, TRS and chronicity were seen as inextricably linked4,8; however, safety concerns regarding clozapine in the early stages of reintroduction framed it as a treatment of last resort.4 More recently, focus has shifted to earlier diagnosis and treatment, bolstered by evidence that early, effective treatment improves outcome.34 Suggesting that TRS requires a minimum 2-year duration is contrary to this evidence and current arguments that clozapine use is, in fact, inappropriately delayed.38,39 By current guidelines, clozapine can be implemented as early as 2 to 3 months after treatment onset.40,41
Strengths and Limitations
We have previously spoken of the model’s advantages.15 Arguably, this is the most ecologically valid model currently available for advancing AP development, in that it uses existing clinical evidence as its foundation. It reminds us why we still prescribe APs, highlighting the central role of positive symptoms. Finally, it acknowledges clinical reality; the profound impact of persistent psychosis despite existing treatments, including clozapine, on people and resources demands a continued focus on better APs, even as we turn our attention to other symptom domains. The criteria we propose have fidelity in this regard and best optimize our chances to advance AP development.
That we distinguish our groups clinically, rather than biologically (at least at this point), represents the greatest limitation to subtyping. Nowhere is this more evident than in decision making regarding thresholds for response. One means of dealing with this has been the establishment of response thresholds, although as a strategy this has been challenged.42 Here we propose criteria on 2 dimensions: true response (severity) and relative response (change). However, this is necessary to also capture clinical reality, especially with clozapine, which is confined to people who are more ill. There are people who demonstrate complete, or almost complete, resolution of symptoms, even after multiple AP trials, but there are also those who remain quite symptomatic despite substantially greater benefits following clozapine. Without better options, these people remain on clozapine and are, relatively speaking, responders. More recent literature on response trajectories speaks to the notion of differential treatment response and comes closest to what is observed clinically.43–46
At first glance, our proposal to subtype schizophrenia clinically may appear to contradict the recent removal of clinical subtypes in DSM-533; however, these 2 approaches of clinical subtyping have notable differences. The previous clinical subtypes (paranoid, disorganized, catatonic, undifferentiated, and residual) relied on symptom presentation at a specific point, and symptoms have been known to vary across various phases of the illness; hence it suffered from a lack of diagnostic stability, reliability, and validity. Conversely, the proposed subtyping by treatment response, with a specific focus on positive symptoms, is based on decades of clinical and research experience with APs, and is in line with DSM-5′s dimensional approach for symptoms. Further, a growing body of biological evidence supports such an approach. For example, recent positron emission tomography imaging studies47,48 have reported normal dopamine but elevated glutamate levels in the brains of patients with TRS, while genetic studies,49,50 too, have identified promising markers for TRS. Taken together, these research findings strongly suggest TRS to be a biologically distinct subtype.
The CGI-SCH has not gained the widespread use of scales such as the BPRS and PANSS, and undoubtedly the use of these other scales will continue. While we advocate for further studies comparing each scale, at least one report has proposed comparative values between the CGI, BPRS, and PANSS based on several large databases arising from clinical trials.31,51,52 In terms of CGI–Severity, it was proposed that CGI of 4 or more is roughly equivalent to a total BPRS of 40 or more, and PANSS of 80 or more, while for CGI–Change a CGI of more than 2 represents improvement of less than 50% on the BPRS and PANSS, respectively.
Finally, the focus on a single symptom domain flies in the face of a progressive shift to view schizophrenia in the context of multiple symptom domains,30,32,33 just as it seems at odds with the increased use of APs in other diagnoses and even off label.53 However, the notion of focusing drug development on specific symptoms is also gaining momentum,14 and measurement tools, such as the DSM-533 and CGI-SCH,30 provide a means of achieving both. Again, we do not disavow the benefits of APs in symptoms beyond positive features; what we underscore is the magnitude of response (or lack thereof) and the logic of focusing on positive symptoms if our goal is one of better APs. Evidence has not supported their stand-alone efficacy in other key symptom domains, nor in clinical practice where extensive use of additional psychotropics in schizophrenia (for example, anxiolytics, antidepressants, or mood stabilizers) further highlights the limitations of APs beyond positive symptoms.54
Conclusions
What we have detailed builds on existing criteria for TRS and URS, with minor modifications to accommodate advances in the field. Current practice patterns actually reflect several of these; clozapine is advocated and used in people much earlier in the course of illness, with decision making regarding initiation of clozapine and evaluation of response guided by psychopathology.55,56 Our choice to focus decision making purely on positive symptoms is shaped by the belief that AP development would best be served using this as the yardstick as it homes in on AP activity per se. We highlight pharmacotherapy here, but note that nonpharmacological interventions can be evaluated by the same standard.
Subtyping schizophrenia by treatment response affords us a distinct advantage, and there is already evidence of this. Neuroimaging data confirm that suboptimal response to conventional APs is not simply a function of suboptimal dopamine blockade.57 Regarding clozapine response, the role of dopamine D2 antagonism has been challenged based on its low affinity58; however, it has also been identified that clozapine’s level of D2 occupancy may exceed proposed thresholds associated with clinical response, albeit short-lived.59 This does not absolutely prove a role for dopamine in clozapine’s unique efficacy, although there is additional preclinical work that indicates modifying the D2 profile of clozapine can attenuate its atypical profile.60
Unfortunately, there has not yet been a systematic effort to isolate those demonstrating poor response to clozapine for comparison purposes with AP and clozapine responders. This clearly represents an important next step. Clozapine augmentation strategies have been ineffective, indicating that this third type of psychosis requires a novel approach. Given how little we know about this population, it is even presumptuous at present to assume it represents a single population in terms of pathophysiology. As a starting point, though, we have clear evidence of 3 subtypes of psychosis, which may represent the best opportunity currently available for advancing our understanding and treatment of positive symptoms.
Acknowledgments
Dr Lee has served as a consultant for Roche, and is currently supported by the Singapore Ministry of Health’s National Medical Research Council under its Transition Award (grant no NMRC/TA/002/2012). Dr Takeuchi has received fellowship grants from the Canadian Institutes of Health Research (CIHR), the Centre for Addiction and Mental Health Foundation, the Japanese Society of Clinical Neuropsychopharmacology, and Astellas Foundation for Research on Metabolic Disorders; speaker’s honoraria from Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceuticals, Meiji Seika Pharma, and Otsuka Pharmaceutical; and manuscript fees from Dainippon Sumitomo Pharma within the past 5 years. Dr Foussias has served on advisory boards for Hoffman-La Roche, received speaker’s fees from Novartis, Hoffman-La Roche, and Lundbeck, and received research support from the CIHR, the Ontario Mental Health Foundation, the American Psychiatric Association, Brain and Behaviour Research Foundation, the Canada Foundation for Innovation, and the Schizophrenia Society of Ontario. Dr Agid has received research support from Pfizer Inc and Janssen-Ortho; consultant fees from Janssen-Ortho, Eli Lilly and Company (US), Eli Lilly Canada, Sepracor, Sunovion, and Lundbeck; and speaker’s fees from Janssen-Ortho, Eli Lilly and Company (US), Eli Lilly Canada, Novartis, Sepracor, and Sunovion. Dr Remington has received research support, consulting fees, or speaker’s fees from the Canadian Diabetes Association, CIHR, Hoffman-La Roche, Laboratorios Farmacéuticos Rovi, Medicure, Neurocrine Biosciences, Novartis Canada, Research Hospital Fund– Canada Foundation for Innovation, and the Schizophrenia Society of Ontario. The other authors have no competing interests to disclose.
Abbreviations
- AP
antipsychotic
- BPRS
Brief Psychiatric Rating Scale
- CGI
Clinical Global Impression
- CGI-SCH
CGI–Schizophrenia
- CPZ
chlorpromazine
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- PANSS
Positive and Negative Syndrome Scale
- TDM
therapeutic drug monitoring
- TRS
treatment-resistant schizophrenia
- URS
ultraresistant schizophrenia
References
- 1.Vanelle J. Refractory schizophrenia: historical and currently prevailing criteria and definitions. Eur Psychiatry. 1997;12(Suppl 5):S321–S326. doi: 10.1016/S0924-9338(97)83575-4. [DOI] [PubMed] [Google Scholar]
- 2.Itil TM, Keskiner A, Fink M. Therapeutic studies in “therapy resistant” schizophrenic patients. Compr Psychiatry. 1966;7(6):488–493. doi: 10.1016/s0010-440x(66)80028-7. [DOI] [PubMed] [Google Scholar]
- 3.McCreadie RG, MacDonald IM. High dosage haloperidol in chronic schizophrenia. Br J Psychiatry. 1977;131:310–316. doi: 10.1192/bjp.131.3.310. [DOI] [PubMed] [Google Scholar]
- 4.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 5.Tuma AH, May PR. And if that doesn’t work, what next . . . ? A study of treatment failures in schizophrenia. J Nerv Ment Dis. 1979;167(9):566–571. doi: 10.1097/00005053-197909000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dencker SJ, Kulhanek F, editors. Treatment resistance in schizophrenia. Braunschweig (DE): Vieweg Verlag; 1988. [Google Scholar]
- 7.May PRA, Dencker SJ, Hubbard JW. A systematic approach to treatment resistance in schizophrenic disorders. In: Dencker SJ, Kulhanek F, editors. Treatment resistance in schizophrenia. Braunschweig (DE): Vieweg Verlag; 1988. pp. 22–23. [Google Scholar]
- 8.Brenner HD, Dencker SJ, Goldstein MJ, et al. Defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16(4):551–561. doi: 10.1093/schbul/16.4.551. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Remington G, Mulsant BH, et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197(1–2):1–6. doi: 10.1016/j.psychres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 11.Grunder G, Hippius H, Carlsson A. The ‘atypicality’ of antipsychotics: a concept re-examined and re-defined. Nat Rev Drug Discov. 2009;8(3):197–202. doi: 10.1038/nrd2806. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SW, Barnes TR, Davies L, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane JM. Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry Suppl. 1992;(17):41–45. [PubMed] [Google Scholar]
- 14.Citrome L. Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. J Clin Psychiatry. 2014;75(Suppl 1):21–26. doi: 10.4088/JCP.13049su1c.04. [DOI] [PubMed] [Google Scholar]
- 15.Farooq S, Agid O, Foussias G, et al. Using treatment response to subtype schizophrenia: proposal for a new paradigm in classification. Schizophr Bull. 2013;39(6):1169–1172. doi: 10.1093/schbul/sbt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remington G. Understanding antipsychotic “atypicality”: a clinical and pharmacological moving target. J Psychiatry Neurosci. 2003;28(4):275–284. [PMC free article] [PubMed] [Google Scholar]
- 17.Biedermann F, Fleischhacker WW. Antipsychotics in the early stage of development. Curr Opin Psychiatry. 2009;22(3):326–330. doi: 10.1097/YCO.0b013e328329cd73. [DOI] [PubMed] [Google Scholar]
- 18.Dold M, Leucht S. Pharmacotherapy of treatment-resistant schizophrenia: a clinical perspective. Evid Based Ment Health. 2014;17(2):33–37. doi: 10.1136/eb-2014-101813. [DOI] [PubMed] [Google Scholar]
- 19.Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, “just the facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophr Res. 2011;127(1–3):3–13. doi: 10.1016/j.schres.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 21.Piskulic D, Addington J, Cadenhead KS, et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196(2–3):220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler A, Humberstone V, Robinson G. Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009;23(8):957–965. doi: 10.1177/0269881108093588. [DOI] [PubMed] [Google Scholar]
- 23.Robinson DG, Woerner MG, McMeniman M, et al. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(3):473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 24.Fervaha G, Agid O, Takeuchi H, et al. Effect of antipsychotic medication on overall life satisfaction among individuals with chronic schizophrenia: findings from the NIMH CATIE study. Eur Neuropsychopharmacol. 2014;24(7):1078–1085. doi: 10.1016/j.euroneuro.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Mouaffak F, Tranulis C, Gourevitch R, et al. Augmentation strategies of clozapine with antipsychotics in the treatment of ultraresistant schizophrenia. Clin Neuropharmacol. 2006;29(1):28–33. doi: 10.1097/00002826-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Marques TR, Levine SZ, Reichenberg A, et al. How antipsychotics impact the different dimensions of schizophrenia: a test of competing hypotheses. Eur Neuropsychopharmacol. 2014;24(8):1279–1288. doi: 10.1016/j.euroneuro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50(11):873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- 28.Wenthur CJ, Lindsley CW. Classics in chemical neuroscience: clozapine. ACS Chem Neurosci. 2013;4(7):1018–1025. doi: 10.1021/cn400121z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haro JM, Edgell ET, Jones PB, et al. The European Schizophrenia Outpatient Health Outcomes (SOHO) study: rationale, methods and recruitment. Acta Psychiatr Scand. 2003;107(3):222–232. doi: 10.1034/j.1600-0447.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 30.Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression–Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;(416):16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 31.Leucht S, Kane JM, Etschel E, et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31(10):2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Carpenter WT, Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): APA; 2013. [Google Scholar]
- 34.Alvarez-Jimenez M, Gleeson JF, Henry LP, et al. Road to full recovery: longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychol Med. 2012;42(3):595–606. doi: 10.1017/S0033291711001504. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Takeuchi H, Fervaha G, et al. Relationship between clinical improvement and functional gains with clozapine in schizophrenia. Eur Neuropsychopharmacol. 2014;24(10):1622–1629. doi: 10.1016/j.euroneuro.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 38.Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481–485. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 39.Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186–192. doi: 10.1176/appi.ps.201300180. [DOI] [PubMed] [Google Scholar]
- 40.Agid O, Foussias G, Singh S, et al. Where to position clozapine: re-examining the evidence. Can J Psychiatry. 2010;55(10):677–684. doi: 10.1177/070674371005501007. [DOI] [PubMed] [Google Scholar]
- 41.Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146–151. doi: 10.1176/appi.ajp.2012.12060778. [DOI] [PubMed] [Google Scholar]
- 42.Leucht S, Davis JM, Engel RR, et al. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scand Suppl. 2009;(438):7–14. doi: 10.1111/j.1600-0447.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 43.Levine SZ, Rabinowitz J, Case M, et al. Treatment response trajectories and their antecedents in recent-onset psychosis: a 2-year prospective study. J Clin Psychopharmacol. 2010;30(4):446–449. doi: 10.1097/JCP.0b013e3181e68e80. [DOI] [PubMed] [Google Scholar]
- 44.Levine SZ, Lurie I, Kohn R, et al. Trajectories of the course of schizophrenia: from progressive deterioration to amelioration over three decades. Schizophr Res. 2011;126(1–3):184–191. doi: 10.1016/j.schres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Schennach R, Meyer S, Seemuller F, et al. Response trajectories in “real-world” naturalistically treated schizophrenia patients. Schizophr Res. 2012;139(1–3):218–224. doi: 10.1016/j.schres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Johnston JA, Kinon BJ, et al. The longitudinal interplay between negative and positive symptom trajectories in patients under antipsychotic treatment: a post hoc analysis of data from a randomized, 1-year pragmatic trial. BMC Psychiatry. 2013;13:320. doi: 10.1186/1471-244X-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demjaha A, Egerton A, Murray RM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–e13. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Demjaha A, Murray RM, McGuire PK, et al. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169(11):1203–1210. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 49.Liou YJ, Wang HH, Lee MT, et al. Genome-wide association study of treatment refractory schizophrenia in Han Chinese. PLoS One. 2012;7(3):e33598. doi: 10.1371/journal.pone.0033598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Meltzer HY. A genetic locus in 7p12.2 associated with treatment resistant schizophrenia. Schizophr Res. 2014;159(2–3):333–339. doi: 10.1016/j.schres.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 51.Leucht S, Kane JM, Kissling W, et al. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005;187:366–371. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- 52.Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 2009;28(5):w770–w781. doi: 10.1377/hlthaff.28.5.w770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26(2):208–213. doi: 10.1097/YCO.0b013e32835d9efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agid O, Remington G, Kapur S, et al. Early use of clozapine for poorly responding first-episode psychosis. J Clin Psychopharmacol. 2007;27(4):369–373. doi: 10.1097/jcp.0b013e3180d0a6d4. [DOI] [PubMed] [Google Scholar]
- 56.Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- 57.Wolkin A, Barouche F, Wolf AP, et al. Dopamine blockade and clinical response: evidence for two biological subgroups of schizophrenia. Am J Psychiatry. 1989;146(7):905–908. doi: 10.1176/ajp.146.7.905. [DOI] [PubMed] [Google Scholar]
- 58.Coward DM, Imperato A, Urwyler S, et al. Biochemical and behavioural properties of clozapine. Psychopharmacology (Berl) 1989;99:S6–S12. doi: 10.1007/BF00442552. [DOI] [PubMed] [Google Scholar]
- 59.Suhara T, Okauchi T, Sudo Y, et al. Clozapine can induce high dopamine D(2) receptor occupancy in vivo. Psychopharmacology (Berl) 2002;160(1):107–112. doi: 10.1007/s00213-001-0967-0. [DOI] [PubMed] [Google Scholar]
- 60.Kapur S, McClelland RA, VanderSpek SC, et al. Increasing D2 affinity results in the loss of clozapine’s atypical antipsychotic action. Neuroreport. 2002;13(6):831–835. doi: 10.1097/00001756-200205070-00019. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Remington G. Adequate dosing for second-generation antipsychotics in establishing treatment resistance in schizophrenia. Am J Psychiatry. 2014;171(1):118–119. doi: 10.1176/appi.ajp.2013.13070965. [DOI] [PubMed] [Google Scholar]
- 62.Lee J, Takeuchi H, Remington G. Comparing dopamine D(2) receptor occupancies for use in clinical practice: attractive proposition but fraught with pitfalls. J Clin Psychopharmacol. 2014;34(4):530–532. doi: 10.1097/JCP.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 63.Patel MX, Arista IA, Taylor M, et al. How to compare doses of different antipsychotics: a systematic review of methods. Schizophr Res. 2013;149(1–3):141–148. doi: 10.1016/j.schres.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 64.Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(Suppl 12):5–19. [PubMed] [Google Scholar]
- 65.Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 66.VanderZwaag C, McGee M, McEvoy JP, et al. Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153(12):1579–1584. doi: 10.1176/ajp.153.12.1579. [DOI] [PubMed] [Google Scholar]
- 67.Conley RR, Carpenter WT, Jr, Tamminga CA. Time to clozapine response in a standardized trial. Am J Psychiatry. 1997;154(9):1243–1247. doi: 10.1176/ajp.154.9.1243. [DOI] [PubMed] [Google Scholar]