SUMMARY
The control of promoter-proximal pausing and the release of RNA polymerase II (Pol II) is a widely used mechanism for regulating gene expression in metazoans, especially for genes that respond to environmental and developmental cues. Here, we identify Pol II-associated Factor 1 (PAF1) to possess an evolutionarily conserved function in metazoans in the regulation of promoter-proximal pausing. Reduction in PAF1 levels leads to an increased release of paused Pol II into gene bodies at thousands of genes. PAF1 depletion results in increased nascent and mature transcripts and increased levels of phosphorylation of Pol II’s C-terminal domain on serine 2 (Ser2P). These changes can be explained by the recruitment of the Ser2P kinase Super Elongation Complex (SEC) effecting increased release of paused Pol II into productive elongation, thus establishing a novel function for PAF1 as a regulator of promoter-proximal pausing by Pol II.
Graphical abstract

INTRODUCTION
It has long been known that a major regulatory step of transcription by RNA Pol II activity in metazoans is the control of the release of promoter-proximal pausing (Kwak and Lis, 2013; Luo et al., 2012b; Peterlin and Price, 2006; Smith and Shilatifard, 2013). Stress-inducible genes such as Hsp70 in Drosophila, the proto-oncogene MYC, and the HIV-1 provirus, were found to be regulated at the transition from transcription initiation by Pol II to productive elongation (Bentley and Groudine, 1986; Gilmour and Lis, 1986; Kao et al., 1987; Toohey and Jones, 1989). This promoter-proximal pausing by Pol II is characterized by an engaged polymerase transcribing 20–60 nt downstream of the transcription start site (TSS) (Gilmour and Lis, 1986; Rougvie and Lis, 1988). Recently, genome-wide studies using Pol II chromatin immunoprecipitation (ChIP-chip and ChIP-seq), nuclear run-on (GRO-seq), and the sequencing of short transcripts demonstrated that most genes in Drosophila and mammalian cells are characterized by a rate-limiting step of the transition of Pol II from the initiated state to productive elongation (Levine, 2011).
In vitro transcription systems were used to identify factors that were able to establish or release paused Pol II, including DRB sensitivity inducible factor (DSIF), negative elongation factor (NELF) and positive transcription elongation factor b (P-TEFb) (Marshall and Price, 1995; Wada et al., 1998; Yamaguchi et al., 1999). DSIF, which consists of Spt4 and Spt5, promotes pausing in conjunction with NELF and also serves as a factor required for increasing the catalytic rate of transcription elongation by Pol II. P-TEFb is a kinase that phosphorylates the C-terminal domain (CTD) of RPB1, the largest subunit of Pol II, the C-terminal domain of SPT5, and the NELF-E subunit to promote the transition to transcription elongation (Fujinaga et al., 2004; Marshall et al., 1996; Yamada et al., 2006).
The RPB1 CTD contains a heptad repeat (consensus Tyr1–Ser2–Pro3–Thr4–Ser5–Pro6–Ser7) that is subject to extensive post-translational modifications, most notably phosphorylation at Ser2 and Ser5 residues (Smith and Shilatifard, 2013). In contrast to Ser5-phosphorylated Pol II (Ser5P), that peaks around the TSS and is associated with paused Pol II, Ser2-phophorylated Pol II (Ser2P) accumulates throughout gene bodies and is associated with elongating Pol II. Phosphorylation of Ser2, which is implemented by P-TEFb, is considered to be essential for the release of paused Pol II and for productive transcription elongation (Eick and Geyer, 2013; Ni et al., 2008).
Most P-TEFb in cells is sequestered in an inactive complex with the 7SK snRNA and the proteins HEXIM1 or HEXIM2, LARP7 and MEPCE (Zhou et al., 2012). Upon cellular signals, P-TEFb is released from the 7SK snRNP complex, allowing it to interact with bromodomain-containing protein 4 (BRD4) or to incorporate into the super elongation complex (SEC) with other transcription elongation factors such as ELL2 and AFF4 (Lin et al., 2010; Sobhian et al., 2010; He et al., 2010; Lou et al., 2012b). Among the three P-TEFb containing complexes, the most active forms were observed within the SEC family (Luo et al., 2012a). SEC, but not BRD4, is required for the release of paused Pol II at developmental genes in response to retinoic acid (RA) induction (Lin et al., 2011), as well as for the Tat-mediated activation of HIV-1 proviral transcription (Li et al., 2013b). It was also demonstrated that the association of SEC with a given gene and not paused Pol II is a major indication of rapid transcriptional induction (Lin et al., 2011).
Although the in vitro function of P-TEFb within its complex SEC has been extensively validated by in vivo studies, the function of NELF has been more enigmatic. Studies in vitro led to a model where the role of NELF was to restrain Pol II in the paused state while in vivo studies revealed that loss of NELF led to more downregulation of gene expression rather than upregulation (Gilchrist et al., 2008). Subsequent studies demonstrating that knockdown of NELF can lead to a global reduction of Pol II occupancy at promoter proximal regions (Core et al., 2012), which can then lead to nucleosomes moving into the region to prevent re-initiation of Pol II (Core et al., 2012). Thus, how promoter-proximal paused Pol II is restrained 20–60 nt downstream of the promoters, is currently unknown.
To identify factors required for the regulated release of promoter-proximal paused Pol II, we set up an RNAi screen of Pol II elongation factors followed by Pol II ChIP-seq. Here, we report that PAF1 as an evolutionarily conserved factor that contributes significantly to the process of pause-release by Pol II. PAF1 was originally identified biochemically as a factor co-purifying with Pol II from yeast extracts (Shi et al., 1996; Wade and Jaehning, 1996) and the role of PAF1 and its complex, in serving as a platform for co-transcriptional histone modifying enzymes and RNA processing factors, is highly conserved from yeast to humans (Krogan et al., 2003; Shilatifard 2006). Our studies in mammalian cells reveal that the loss of PAF1 leads to widespread release of the promoter-proximal paused Pol II into gene bodies. The loss of PAF1 is associated with an increased Ser2P form of Pol II and increased nascent transcription. Most significantly, the super elongation complex (SEC), which is required for the rapid transcriptional induction and release of promoter-proximal paused Pol II into gene bodies (Lin et al., 2010; Lin et al., 2011), is rapidly recruited to Pol II upon PAF1 loss. This study reveals an evolutionarily conserved role for PAF1 as a regulator of promoter-proximal pausing by RNA Pol II in metazoans.
RESULTS
PAF1 depletion leads to a redistribution of Pol II from promoters to gene bodies
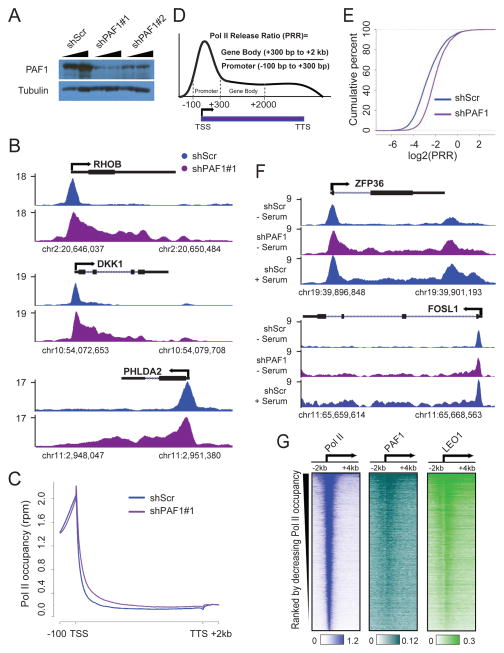
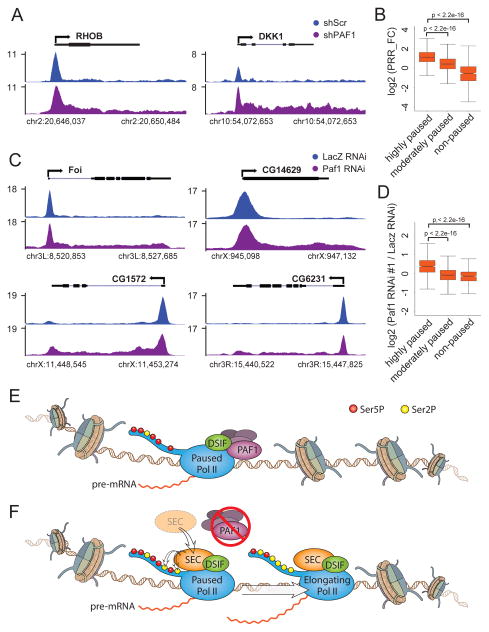
PAF1 was identified as a factor copurifying with RNA Pol II (Shi et al., 1996; Wade et al., 1996) and in vitro approaches have shown that PAF1 can facilitate transcription elongation cooperatively with TFIIS, DSIF and Tat-SF1 (Chen et al., 2009; Kim et al., 2010). Furthermore, in yeast we demonstrated that PAF1 functions by regulating histone modifications associated with elongating forms Pol II (Krogan et al., 2003). To identify factors required for the regulation of release of promoter-proximal paused Pol II, we set up an RNAi screen of Pol II elongation factors followed by Pol II ChIP-seq. Our screen identified a role for PAF1 in this process. Pol II ChIP-seq following PAF1 depletion using two independent short hairpin RNAs (shRNA) targeting PAF1 (shPAF1#1 and shPAF1#2) in HCT116 cells is shown (Figure 1A). For many genes, exemplified by RHOB, DKK1 and PHLDA2, reduction in PAF1 levels leads to an increase of Pol II within gene bodies (Figure 1B). To examine whether this type of change in the Pol II pattern is a general occurrence, genome-wide analysis was applied to genes occupied by Pol II in the control condition, as defined by having a peak called by MACS (Model-based Analysis of ChIP-Seq) (Zhang et al., 2008) overlapping the region from the TSS to 500 bp downstream of the TSS, and this region having reads per million (rpm) > 1. Genes also had to be greater than 2 kb in length and more than 1 kb from any other gene in order to be considered for analysis. As shown in a plot of average gene occupancy of the resulting 9,333 genes, the increase of Pol II within gene bodies after PAF1 knockdown is observed for both shRNAs targeting PAF1 (Figure 1C and S1A), suggesting that PAF1 has a genome-wide role in controlling the procession of Pol II into gene bodies.
Figure 1. Depletion of PAF1 results in a redistribution of Pol II from promoters to gene bodies.
(A) Cells transduced with shScr or two independent shRNAs targeting PAF1 (shPAF1#1 and shPAF1#2) were subjected to western blotting with antibodies against PAF1 and β-Tubulin.
(B) Representative genome browser track examples of total Pol II ChIP-seq in HCT116 cells transduced with shScr or shPAF1#1 for the indicated genes. The x-axis indicates the chromosome position, and the y-axis represents normalized read density in reads per million (rpm).
(C) Metagene analysis showing total Pol II occupancy measured by ChIP-seq in cells transduced with shScr or shPAF1#1. The 9,333 genes included for analysis meet the criteria of having Pol II (rpm > 1) at promoters in the control condition, are longer than 2 kb, and do not have nearby genes within 1 kb to avoid the inclusion of reads for Pol II from nearby genes.
(D) Schematic representation of the modified traveling ratio or body/promoter ratio used to calculate the Pol II Release Ratio (herein called the PRR). The promoter is defined as the region covering 100 bp upstream to 300 bp downstream of the TSS; The gene body is defined as the region from 300 bp to 2 kb downstream of the TSS.
(E) The empirical cumulative distribution function (ECDF) plot of the PRR distribution in cells transduced with shScr or shPAF1.
(F) Track examples of total Pol II ChIP-seq in cells transduced with shScr or shPAF1 in starved (− serum) or serum-induced (+ serum) conditions for the indicated genes.
(G) Heatmaps of Pol II, PAF1 and LEO1 occupancy around the TSS region. Rows are sorted by decreasing Pol II occupancy in the −2 kb to +4 kb region. Color-scaled intensities are in units of rpm.
PAF1 has a broad role in the maintenance of promoter-proximal Pol II pausing
Genome-wide studies in Drosophila and mammals have shown that Pol II accumulates at the 5′ end of most genes, and collectively is referred to as promoter-proximal pausing. The prevalence of Pol II pausing in metazoans rather than yeast implies that Pol II release after recruitment at promoters is an essential step of transcription regulation in higher organisms (Kwak and Lis, 2013; Smith and Shilatifard, 2013). The observed changes in the Pol II pattern after PAF1 depletion suggest defects in Pol II pausing control. To verify this, we calculated the ratio of Pol II occupancy between the gene body and its promoter (Figure 1D), a modified traveling ratio or body/promoter calculation (Core et al., 2008; Reppas et al., 2006; Thornton et al., 2014) that for convenience we refer to as the “Pol II Release Ratio” (PRR). Specifically, the promoter is defined as 100 bp upstream and 300 bp downstream of the TSS, and the gene body is defined as the region from 300 bp to 2 kb downstream of the TSS. These regions were chosen to measure the release of Pol II from promoters while avoiding complications from potential roles of PAF1 in Pol II processivity in gene bodies and in transcription termination. Correlations of PRR fold change (PRR_FC) between biological replicates (Figure S1B), and between independent shRNAs targeting PAF1 (Figure S1C), reveal a consistent role of PAF1 in controlling Pol II pausing.
We calculated the degree of overlap of genes showing increased PRR values (PRR_FC > 1) following PAF1 depletion by two independent shRNAs (Figure S1D). shPAF1#1, which is more efficient for depleting PAF1 levels (Figure 1A), leads to a larger number of genes exhibiting an increased PRR value (5,122 vs. 3,420). However, 84.7% (2,897 of 3,420) of genes with increased PRR values after PAF1 depletion using the less efficient shPAF1#2 are also affected by shPAF1#1, indicating that the phenomenon of increased PRR is due to PAF1 depletion. Therefore, subsequent analysis was restricted to the stronger shPAF1#1 (abbreviated as shPAF1 hereafter). An empirical cumulative distribution function (ECDF) plot reveals that ~ 90% of genes have a PRR value less than 0.5 (log2 (PRR) < −1), indicating the prevalence of promoter-proximal pausing regulation (Figure 1E). The knockdown of PAF1 leads to a significant increase (Kolmogorov-Smirnov test of distributions, p-value < 2.2e-16, D value = 0.2664) of PRR values on average (Figure 1E and S1E).
In order to ask whether the increased Pol II in gene bodies was due to a general amplification in transcription as opposed to release of promoter-proximal paused Pol II, we performed Pol II ChIP-seq in serum-starved HCT116 cells that had been transduced with shScr or shPAF1. During serum starvation, genes such as ZFP36 and FOSL1 are highly paused (Donner et al., 2010; Lin et al., 2011). However, in the PAF1 knockdown, increased Pol II can be seen throughout the gene body of each of these genes, sometimes to the level of release obtained with 30 minutes of serum stimulation in the shScr HCT116 cells (Figure 1F).
In order to determine whether PAF1 has a direct effect on the regulation of Pol II at the promoter-proximal region, ChIP-seq was performed. Heatmaps ranked by decreasing Pol II occupancy show a positive correlation with PAF1 and Pol II (Figure 1G). To corroborate these findings, we also performed ChIP-seq with LEO1, as this was the only other antibody that we tested that gave any significant ChIP-seq signal. Like PAF1, LEO1 is found throughout gene bodies with particular enrichment around the TSS (Figure 1G). Interestingly, LEO1 was not broadly lost from chromatin upon PAF1 knockdown, but the distribution was altered, including reduced occupancy in the promoter-proximal region (Figure S1F).
Highly paused genes are more affected by the loss of PAF1
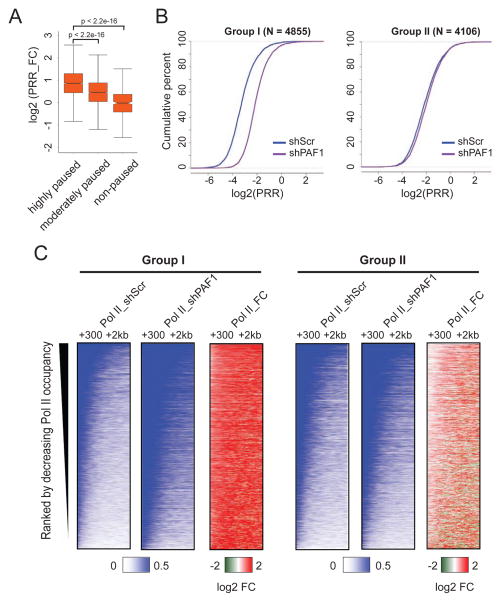
There are many degrees of Pol II pausing, with some genes such as Drosophila Hsp70 exhibiting a rare release of Pol II into gene bodies, while at other genes, such as beta actin, Pol II pauses briefly before being released into gene bodies (Boehm et al., 2003; Chen et al., 2015). In order to determine if PAF1’s effect on the redistribution of Pol II is related to pausing we separated the 9,333 genes into three groups based on the previously described pausing index, the ratio of Pol II density at the promoter over Pol II in gene bodies (Core et al., 2008; Muse et al., 2007; Reppas et al., 2006). We calculated the PRR_FC following PAF1 knockdown for each class of genes. We find that the highly-paused genes have the largest increase in PRR_FC values (median value: 1.82) and the non-paused genes have the smallest change (median value: 0.98) (Figures 2A and S2A), indicating that highly paused genes have a strong dependence on PAF1 in maintaining Pol II pausing.
Figure 2. PAF1 depletion has a greater effect on genes that are highly paused.
(A) Box plot analysis of PRR fold change (PRR_FC) after PAF1 depletion for genes with different degrees of pausing as measured by pausing index (the ratio of Pol II occupancy at promoters to occupancy over gene bodies). The 9,333 genes were divided into three groups: highly paused (pausing index >4; n=4184), moderately paused (1 < pausing index < 4; n=4743), and non-paused (pausing index < 1; n=406). PRR values were then plotted in pausing index groups and p-values were then calculated with a two-sided t test.
(B) ECDF plots of PRR for more affected (group I) and less affected (group II) genes in cells transduced with shScr or shPAF1. Group I constitutes 4,855 genes with a more than 1.5 increase of PRR_FC (log2 (PRR_FC) > 0.585). Group II constitutes 4,106 genes with a PRR_FC less than 1.5 in either direction (0.585 > log2 (PRR_FC) > −0.585).
(C) Heatmaps of Pol II occupancy within the gene body regions, 300 bp to 2 kb downstream of the TSS, in cells transduced with shScr or shPAF1. Rows are sorted by decreasing Pol II occupancy in the shScr condition. Color-scaled intensities are in units of rpm. For the heatmaps of fold changes (Pol II_FC), the color bars depict log2 values.
To further investigate the differential response to PAF1, genes were classified into more affected and less affected genes based on the fold change of PRR after PAF1 knockdown. We found that 4,855 genes show a more than a 1.5 increase of PRR_FC (log2 (PRR_FC) > 0.585) which we refer to as group I, while 4,106 genes have a less than 1.5 fold change of PRR_FC (0.585 > log2 (PRR_FC) > −0.585) and are referred to as group II. Only 372 genes exhibit a more than 1.5 fold decrease in PRR_FC and these genes are not considered for further analysis. ECDF plots illustrate that group I genes are more affected than group II genes by loss of PAF1 (Figures 2B and S2B). Heatmaps and metagene plots reveal that group I genes have both a higher occupancy of Pol II at promoters in the control condition and a greater increase of Pol II within gene bodies after PAF1 knockdown (Figures 2C and S2C). Consistent with the higher level of Pol II at group I genes, higher occupancy for PAF1 at group I genes is observed (Figure S2D).
Pol II release due to PAF1 RNAi generates increased nascent and mature transcripts
The increased levels of Pol II found in gene bodies after PAF1 RNAi could conceivably be explained by Pol II getting stuck in gene bodies due to arrest or backtracking of Pol II resulting from loss of an elongation activity of PAF1 or associated factors. To address this question, we performed global run-on sequencing (GRO-seq) in control and PAF1 shRNA treated cells (Figure S3A). GRO-seq measures the level and location of engaged polymerases, but not arrested or backtracked polymerases, on a gene at the time of nuclear isolation (Core et al., 2008). The genes shown in Figure 1B, with increased Pol II in gene bodies after PAF1 knockdown, also exhibit increased gene body GRO-seq signal (Figure 3A). This increase in GRO-seq signal in gene bodies can also be observed by metagene analysis (Figure 3B). The PRR calculations based on GRO-seq signal demonstrate a significant increase (D value = 0.2528) of PRR following PAF1 knockdown (Figure 3C), further demonstrating a global defect in pausing control. Heatmap and metagene analyses of GRO-seq signal were broken out by group I and group II genes, with group I exhibiting a more significant increase of nascent RNA level with the gene bodies (Figure 3D and S3B)
Figure 3. Pause-release following PAF1 depletion represented by GRO-seq.
(A) Genome browser tracks of GRO-seq in cells transduced with shScr or shPAF1 for the same set of genes shown in Figure 1B. Positive and negative values on the y-axis represent normalized reads (rpm) mapping to the positive and negative strands, respectively.
(B) Metagene analysis showing Pol II occupancy as measured by GRO-seq in cells transduced with shScr or shPAF1.
(C) Box plots of log2 PRR values of GRO-seq signal in cells transduced with shScr or shPAF1.
(D) Heatmaps of GRO-seq signal around TSS regions in cells transduced with shScr or shPAF1 for group I and group II genes. Rows are sorted by decreasing Pol II occupancy in the −2 kb to +4 kb region in the shScr condition. Color-scaled intensities are in units of rpm.
As a complementary approach to GRO-seq, we also performed nascent RNA-seq in control and PAF1-depleted cells. Nascent RNA-seq takes advantage of the high affinity of engaged Pol II with DNA and associated nascent transcripts to wash away mature RNA before performing RNA extraction (Khodor et al., 2011). High correlations between replicates indicate the reproducibility of nascent RNA-seq (Figures 4A and S4A). Genes shown in Figures 1B and 3A, with increased Pol II in gene bodies after PAF1 knockdown, also exhibit increased nascent transcripts (Figure 4A). Since increased levels of nascent transcripts could reflect more transient pausing of Pol II in gene bodies, we also performed total RNA-seq after PAF1 knockdown (Figures 4B and S4B). By comparing group I genes with group II genes for changes in GRO-seq, nascent-seq or total RNA-seq, we observed that group I genes generally had higher levels of nascent transcription after PAF1 RNAi while group II genes were downregulated (Figures 4C–E). Hierarchical clustering demonstrates that GRO-seq, nascent-seq and total RNA-seq show similar changes in expression after PAF1 knockdown (Figure 4F). Importantly, by all three measures, we observed that group I genes were more likely to show increased expression while group II genes were more likely to be down-regulated (Figure S4C). Therefore, since the total RNA-seq analysis, which is primarily a measure of mature stable transcripts, concurs with the Gro-seq and nascent RNA-seq results, we can conclude that the observed increase of Pol II in gene bodies for group I genes is due to more release of Pol II into productive elongation.
Figure 4. PAF1 depletion leads to deregulation of gene expression.
(A–B) Genome browser tracks of nascent RNA-seq (A) and total RNA-seq (B) in cells transduced with shScr or shPAF1 for the same set of genes shown in Figure 1B. Positive and negative values on the y-axis represent normalized reads (rpm) mapping to the positive and negative strands, respectively.
(C–E) Box plots showing the log2 fold change of RNA levels from GRO-seq (C), nascent RNA-seq (D) and total RNA-seq (E) following the knockdown of PAF1 in group I and group II.
(F) Hierarchical clustering demonstrating similarities in the log2 fold changes of RNA levels as determined by GRO-seq, nascent RNA-seq and total RNA-seq following PAF1 depletion.
Pausing regulation by PAF1 and H2B monoubiquitination
In an attempt to determine the mechanism by which PAF1 loss led to the release of paused Pol II, we considered PAF1’s role in promoting the monoubiquitination of histone H2B at lysine 120 (H2Bub) by directly interacting with the RAD6-BRE1A/B, E2-E3, ubiquitin ligases that implement H2Bub (Wood et al., 2003) (Krogan et al., 2003). To evaluate the relationship between PAF1-dependent H2Bub and the effect of PAF1 on pausing, we performed H2Bub ChIP-seq in control and PAF1 depleted cells. Heatmaps reveal that the H2Bub levels are much higher in group I than group II (Figure S5A, compare columns 1 and 4), suggesting that group I genes are more highly transcribed than group II genes, as H2Bub levels correlate with transcription levels (Lee et al., 2012; Minsky et al., 2008) (Krogan et al., 2003). However, the decrease of H2Bub appears equally evident for both group I and group II (Figure S5A, compare columns 3 and 6), indicating that changes in H2Bub may not simply explain the observed changes in the Pol II distribution seen after PAF1 RNAi.
To directly test the link between H2Bub and promoter-proximal Pol II pausing, ChIP-seq of total Pol II was performed in cells transduced with either control or BRE1A shRNAs. We originally discovered Bre1 in yeast as the E3 ligase required for H2B monoubiquitination and H3K4 trimethylation (Wood et al., 2003b). In mammalian cells, Bre1 functions within both Bre1A and Bre1B genes (Wood et al., 2003b; Shilatifard 2012). Western analysis shows efficient knockdown of BRE1A for two independent shRNAs. Although PAF1 depletion does not affect the bulk level of BRE1A, knockdown of either BRE1A or PAF1 results in a bulk loss of H2Bub (Figure 5A). However, ECDF and metagene analyses demonstrate that BRE1A knockdown does not result in a major redistribution of Pol II as seen with PAF1 depletion when comparing all genes (Figures S5B–C) or when comparing group I and group II genes (Figure 5B).
Figure 5. Changes in CTD phosphorylation, but not H2B monoubiquitination, are associated with the release of Pol II into gene bodies in PAF1-depleted cells.
(A) Cells transduced with shScr, shPAF1, or two independent shRNAs targeting BRE1A (shBRE1A#1 and shBRE1A#2) were subjected to Western blotting with antibodies against H2Bub, BRE1A, PAF1 and β-Tubulin.
(B) ECDF plots of the PRR distributions in cells transduced with shScr or shBRE1A#1 for group I and group II.
(C) Western blotting with antibodies against total Pol II (N20), Ser2P (3E10) and β-Tubulin for cells transduced with shScr or shPAF1.
(D) Box plots showing the ratio of Ser2P and total Pol II at promoters for group I and group II genes.
(E) Heatmaps of Ser2P levels around TSS regions in cells transduced with shScr or shPAF1 for all genes. Rows are sorted by decreasing Pol II occupancy in the –2 kb to +4 kb region in the shScr condition. Color-scaled intensities are in units of rpm. For the heatmaps of fold changes (Ser2P_FC), the color bars depict log2 values.
(F) Metagene analysis showing average Ser2P occupancy as measured by ChIP-seq in cells transduced with shScr or shPAF1 for group I and group II genes.
PAF1 as a negative-regulatory factor for CTD phosphorylation
To further substantiate that the increased Pol II in gene bodies in PAF1 knockdown cells is due to the release of paused Pol II into the elongating form, we measured the levels of Pol II CTD phosphorylation at serine 2 (Ser2P), the elongating form of Pol II, by Western blotting and ChIP-seq in cells transduced with shScr or shPAF1. Western analysis indicates that PAF1 has no effect on the bulk levels of Ser2P or on total Pol II levels (Figure 5C). However, measuring the ratio of Ser2P to total Pol II shows that the relative levels of Ser2P to total Pol II are increased at promoters of group I genes but not group II genes (Figure 5D). Heatmaps and metagene analysis of Ser2P occupancy indicates that this is a widespread phenomenon, with group I genes exhibiting a greater increase of Ser2P at promoters and in gene bodies (Figure 5E–F).
PAF1 regulates CTD phosphorylation through the recruitment of SEC
To determine if the increased release of paused Pol II into gene bodies is due to increased recruitment of SEC containing the most active version of P-TEFb that phosphorylates serine 2 of the RPB1 CTD to release Pol II from the paused state (Lin et al., 2010; Luo et al., 2012a; Luo et al., 2012b), we performed ChIP-seq of the SEC subunits AFF4, ELL2 and CDK9 in cells transduced with shScr or shPAF1. Western blotting shows that PAF1 depletion does not affect the global protein levels for AFF4, ELL2 or CDK9 (Figure 6A). However, at those genes with increased PRR, such as RHOB, DKK1 and PHLDA2, the levels of SEC subunits are increased (Figure 6B). Metagene and heatmap analyses demonstrate that increased SEC occupancy following knockdown of PAF1 is a widespread occurrence (Figures 6C–E and S6A–C). Furthermore, analyses of group I and group II genes demonstrate that SEC occupancy is increased more at group I genes and this increase occurs at promoter-proximal regions, and to a lesser extent in gene bodies (Figure 6F–H). Consistent with an increase in the release of Pol II in PAF1 knockdowns occurring through P-TEFb activity, treatment with the P-TEFb inhibitor flavopiridol (FP) resulted in restoration of the paused state in PAF1 knockdown cells (Figure S6D).
Figure 6. PAF1 regulates CTD phosphorylation of elongating Pol II through recruitment of SEC.
(A) Western blotting with antibodies against SEC subunits (CDK9, AFF4 and ELL2) and β-Tubulin for cells transduced with shScr or shPAF1.
(B) Track examples of AFF4, ELL2 and CDK9 occupancy in cells transduced with shScr or shPAF1 for the same set of genes shown in Figure 1B.
(C–E) Heatmaps of AFF4 (C), ELL2 (D) and CDK9 (E) levels around TSS regions in cells transduced with shScr or shPAF1 for all genes. Rows are sorted by decreasing Pol II occupancy in the −2 kb to +4 kb region in the shScr condition. Color-scaled intensities are in units of rpm.
(F–H) Metagene analyses showing the occupancies of AFF4 (F), ELL2 (G) and CDK9 (H) as measured by ChIP-seq in cells transduced with shScr or shPAF1.
Functional conservation of pausing maintenance by PAF1 in Drosophila and mammals
To rule out the possibility that PAF1- dependent regulation of pausing is a cell type- specific occurrence, ChIP-seq of total Pol II in MCF7 cells was performed. We found a similar increase in pause release as seen in HCT116 cells (Figure 7A). As represented by PRR_FC, the highly paused genes have the largest degree in pause release, while the non-paused genes show the least change (Figure 7B). Therefore, PAF1’s role in the regulation of pausing is likely to be widespread.
Figure 7. The role of PAF1 in regulating Pol II pausing in metazoans.
(A) Track examples of total Pol II ChIP-seq in MCF7 cells transduced with shScr or shPAF1 for the indicated genes.
(B) Box plot analysis of PRR fold change (PRR_FC) after PAF1 depletion for genes with different degrees of pausing as measured by pausing index (the ratio of Pol II occupancy at promoters to occupancy over gene bodies) in MCF7 cells.
(C) Track examples of total Pol II ChIP-seq in Drosophila S2 cells with non-targeting (LacZ) or Paf1 RNAi for the indicated genes.
(D) Box plot analysis of PRR fold change (PRR_FC) after PAF1 depletion by dsRNA#1 targeting Paf1 for genes with different degrees of pausing as measured by pausing index (the ratio of the Pol II occupancy at promoters to the occupancy over gene bodies). Genes that have Pol II (rpm > 1) at promoters in the control condition, are longer than 1 kb, and do not have nearby genes within 200 bp were selected and then divided into three groups: highly paused (pausing index >10; n=1003), moderately paused (2 < pausing index < 10; n=3089), and non-paused (pausing index < 2; n=625). PRR values were then plotted in pausing index groups and p-values were then calculated with a two-sided t test.
(E) In metazoans, Pol II is frequently found to be pausing near the promoter and is phosphorylated at serine 5 of the CTD (Ser5P, red circles). The release into gene bodies is restrained by PAF1, a component of the PAF complex, which is recruited to direct interactions with Pol II and DSIF.
(F) Upon depletion of PAF1, the CTD kinase SEC is recruited and subsequently phosphorylates Pol II on serine 2 (Ser2P, yellow circles), which facilitates release of paused Pol II into productive elongation.
Pol II pausing is also pervasive in Drosophila, especially for genes involved in the response to environmental and developmental cues. To investigate whether pausing maintenance by PAF1 is conserved in Drosophila, ChIP-seq of Rpb1, the largest subunit of Pol II, was performed in control and Paf1 RNAi (#1 and #2) S2 cells. We found that the increased release of paused Pol II was also observed for a subset of genes in Drosophila, as exemplified by Foi, CG14629, CG1572 and CG6231 (Figure 7C). The PRR values were calculated for genes grouped by different degrees of pausing as measured by the pausing index. As observed in mammalian cells, highly paused genes displayed a significant increase in pause release after Paf1 RNAi (Figures 7D), indicating a conserved role of Paf1 in regulating the release of Pol II from promoter-proximal pausing. Together, these findings suggest that the increased recruitment of SEC by PAF1 depletion is responsible for the enhanced phosphorylation of Pol II and for its release into transcription elongation (Figure 7E–F).
DISCUSSION
In vitro studies had previously identified factors promoting pausing, including NELF, DSIF and GDOWN1 (Smith and Shilatifard, 2013). DSIF and NELF cooperate to establish pausing in vitro (Yamaguchi et al., 1999), but since DSIF has a second role as an essential factor for processive transcription (Swanson et al., 1991; Wada et al., 1998), most attention has focused on NELF as the key factor for the maintenance of Pol II in the paused state. However, knockdown of NELF in cells led to more genes with decreased than increased expression when assayed with gene expression microarrays (Gilchrist et al., 2008). More recently, GRO-seq was used to demonstrate that while knockdown of NELF leads to significant reductions in occupancy of promoter-proximal Pol II at a large number of genes, the nascent Pol II in gene bodies was also reduced (Core et al., 2012). Thus, loss of NELF does not lead to a release of paused Pol II into gene bodies, even though NELF has a clear role in the establishment of Pol II at promoters of paused genes (Core et al., 2012; Li et al., 2013a).
The PAF1 complex (PAF1C) has a well-established role as a factor promoting transcription elongation in vitro (Wood et al., 2003; Krogan et al., 2003)(Chen et al., 2009; Kim et al., 2010; Pavri et al., 2006). The in vivo evidence for PAF1C facilitating transcription elongation largely consists of the PAF1C-dependent histone modifications such as H2Bub, H3K4me3 and H3K79me3 that occur subsequent to transcriptional activation, as well as defects in RNA processing that could be downstream consequences of the PAF1C-dependent histone modifications (Krogan et al., 2003)(Jaehning, 2010; Shilatifard, 2006; Sims et al., 2004). Our studies suggest that the effects on Pol II release into gene bodies after PAF1 knockdown cannot simply be explained by changes in histone H2B monoubiquitination levels (Figure 5 and S5). In addition to a role for Paf1 complex in the regulation of H2B monoubqiuitination levels, our previous studies demonstrated that Paf1 complex in yeast is also required for proper H3K36 methylation by yeast Set2 (Krogan et al., 2003). There are several Set2 related factors in human cells including Set2, ASH1, NSD1, NSD2 and NSD3. A possible role for these factors individually or collectively in the regulation of Pol II pausing through PAF1 should also be considered.
Most in vivo studies of PAF1C function have been performed in yeast, which lack promoter proximal pausing as seen in metazoans. Yeast also lack NELF and SEC, factors involved in setting up pausing and facilitating pause release, respectively. Nonetheless, biochemical and genetic studies in yeast have provided us with the idea of PAF1C being a platform for interactions with numerous factors connecting its function to multiple transcriptional processes, from transcription elongation to transcription termination (Jaehning, 2010; Krogan et al., 2002; Shilatifard, 2006) (Krogan et al., 2003). Therefore, it is not surprising that PAF1C in metazoans would have additional roles in regulating promoter-proximal pausing.
The increase in SEC recruitment observed after PAF1 depletion suggests that the presence of PAF1 restricts access to SEC in some manner (Fig 6). This could occur through interactions between PAF1 and Pol II and/or associated factors that occlude binding surfaces for SEC. Under this scenario, upon removal of PAF1, SEC can find its phosphorylation substrates on the Pol II and SPT5 CTDs leading to more Pol II release into gene bodies. Under normal activating conditions, signaling pathways could communicate through PAF1 to allow access to SEC, after which both PAF1C and SEC can participate in transcription elongation and associated processes.
In the past few years, several factors with known roles in transcription termination, namely XRN2, TTF2 and the Integrator complex, have been linked to the control of promoter-proximal pausing (Gardini et al., 2014; Stadelmayer et al., 2014). Interestingly, Gdown1 can inhibit the termination activity of TTF2 in vitro, and loss of Gdown1 leads to decreased promoter-proximal Pol II and increased Pol II in gene bodies (Cheng et al., 2012). Since PAF1C has known roles in transcription termination (Crisucci and Arndt, 2011), it is possible that PAF1 interacts with termination machineries at promoter-proximal regions to help regulate the paused Pol II state. However, whether there is widespread termination of Pol II in promoter-proximal regions is still under debate (Jonkers and Lis, 2015). An interesting area for future investigation will be to determine to what extent some of these factors work together at the same genes and respond to the same cellular signals. Nonetheless, these studies emphasize the need for in vivo functional approaches to screen for factors, which can either complement or extend the in vitro approaches that have dominated studies of promoter-proximal pausing in metazoans.
EXPERIMENTAL PROCEDURES
Antibodies
Human Pol II (N-20) and CDK9 (C20) antibodies for ChIP-seq were purchased from Santa Cruz, anti-PAF1 (ab137519) was from Abcam, anti-LEO1 (A300-175A) was from Bethyl Laboratories, anti-H2Bub (mAb #5546) and anti-BRE1A (mAb #9425) were from Cell Signaling, anti-Ser2P (3E10) was from Millipore. Anti-CDK9 (Figure 6A), anti-AFF4, anti-ELL2 and anti-Drosophila Rpb1 were made in house and were previously described (Lin et al., 2011; Lin et al., 2010). Anti-β-tubulin E7 monoclonal antibody was purchased from the Developmental Hybridoma Studies Bank.
Cell lines and RNA interference (RNAi)
HCT116 and MCF7 cells were grown in DMEM supplemented with 10% FBS. The cells were infected with lentivirus containing short-hairpin RNAs in the presence of 8 μg/ml Polybrene (Sigma) for 24 h in DMEM supplemented with 10% FBS. The infected cells were selected with 2 μg/ml puromycin for an extra 48 h before harvest. The shRNAs were purchase from Open Biosystems. The clone IDs for shPAF1 are TRCN0000010939 (#1) and TRCN0000005454 (#2). The clone IDs for shBRE1A are TRCN0000033875 (#1) and TRCN0000033877 (#2).
For serum starvation experiments, HCT116 cells were transduced with shScr or shPAF1, then 24 h later cells were serum starved for 48 h. For serum stimulation, starved cells transduced with shScr were fed back DMEM supplemented with 10% FBS for 30 min.
For flavopiridol inhibition of pause-release, HCT116 cells transduced with shPAF1 for 3 days were treated with 1 μM flavopiridol (Sigma) for 30 min.
S2 cells were maintained at 2-10e6 cells/mL in SFX medium (containing 1% penicillin/streptomycin) and incubated at 28° C prior to RNAi treatment. Cells were plated at 5e5 cells/mL in 20 mL SFX per T75 flask and treated with 100 μg dsRNA for 5.5 days. dsRNA was generated using T7 RiboMAX Large Scale RNA Production Kit (Promega). Oligo sequences are available in the Extended Experimental Procedures.
ChIP-seq
5 × 107 cells were used for each ChIP assay as described (Chen et al., 2015; Lee et al., 2006). ChIP-sequencing libraries were prepared with Illumina’s Tru-seq DNA sample prep kit.
Nascent RNA-seq
The nascent RNA isolation procedure was previously described (Chen et al., 2015; Khodor et al., 2011). Libraries were made with the TruSeq RNA sample Prep Kit (Illumina).
Gro-seq
The global nuclear run-on procedure and the preparation of Gro-seq libraries were previously described (Core et al., 2008; Gardini et al., 2014).
ChIP-Seq analysis
ChIP-seq reads were aligned to the human genome (UCSC hg19) for HCT116 and MCF7 cells, and the Drosophila genome (UCSC dm3) for S2 cells, using Bowtie version 1.0.0 (Langmead et al., 2009). Only uniquely mapping reads with up to two mismatches within the entire length of the read were considered for further analysis. The resulting reads were extended to 150 bases toward the interior of the sequenced fragment and normalized to total reads aligned (reads per million, rpm). Peak detection for Pol II was done with MACS (model-based analysis of ChIP-Seq) (Zhang et al., 2008) version 1.4.2 using default parameters. Gene annotations and transcript start site information for human and Drosophila genes were from Ensembl release 72.
Total Pol II ChIP-seq analysis
Genes considered for analysis had peaks of Pol II with a p-value < 1e-5 in the scrambled short hairpin control condition (shScr) or dsLacZ for human or Drosophila, respectively. For human data, the peaks had to overlap the transcription start site (TSS) (from the TSS to 500 bp downstream of the TSS), and the reads per million (rpm) had to be > 1 for the same window, and genes had to be > 2 kb in length and be > 1kb from neighboring genes to avoid potential artifacts of analyzing reads from nearby gene promoters or transcription termination sites (TTS). For genes with multiple TSS’s only the highest occupied was used. For Drosophila data, genes had to be > 1 kb in length and be > 200 bp from neighboring genes.
Promoter release ratios (PRR) were calculated for the resulting 9,333 genes by taking the average coverage in rpm in the gene body (300bp downstream of the TSS to 2kb downstream of the TSS) divided by the average coverage of the promoter region (100 upstream of the TSS to 300 downstream of the TSS). Genes were then placed into two groups based on the fold change of PRR values (PRR_FC). Group I (more affected) genes had a PRR_FC greater than 1.5 fold (n=4855); group II (less affected) genes had a PRR_FC less than 1.5 fold in either direction (n=4106); 372 genes had a more than 1.5 fold decrease of PRR_FC (p-values were then calculated with a two-sided Kolmogorov-Smirnov test). The percent overlap was calculated for shRNA replicates by taking the common peaks for shScr in both batches that overlapped the TSS (TSS to 500 bp downstream, 7354 peaks) and finding intersecting genes with a PRR _FC > 1 (log2(PRR_FC) > 0).
Additional details on ChIP-seq, nascent and total RNA-seq, and Gro-seq can be found in the Extended Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
PAF1 loss results in release of Pol II into gene bodies at thousands of genes.
Genes exhibiting high degrees of pausing are more affected by loss of PAF1.
Redistribution of Pol II is associated with increased transcription.
PAF1 depletion leads to increased recruitment of the Super Elongation Complex.
Acknowledgments
We thank the Molecular Biology core facility at the Stowers Institute for library preparation and next generation sequencing, and the Stowers Institute Tissue Culture core facility help with cell culture. We thank Laura Shilatifard for editorial assistance. These studies were supported in part by a grant from the National Institutes of Health R01GM069905 to A.S.
Footnotes
ACCESSION NUMBERS
ChIP-seq, RNA-seq and Gro-seq data are available at GEO under accession number GSEXXXXX.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentley DL, Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986;321:702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Boehm AK, Saunders A, Werner J, Lis JT. Transcription factor and polymerase recruitment, modification, and movement on dhsp70 in vivo in the minutes following heat shock. Molecular and cellular biology. 2003;23:7628–7637. doi: 10.1128/MCB.23.21.7628-7637.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Gao X, Shilatifard A. Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide. Genes Dev. 2015;29:39–47. doi: 10.1101/gad.246173.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yamaguchi Y, Tsugeno Y, Yamamoto J, Yamada T, Nakamura M, Hisatake K, Handa H. DSIF, the Paf1 complex, and Tat-SF1 have nonredundant, cooperative roles in RNA polymerase II elongation. Genes Dev. 2009;23:2765–2777. doi: 10.1101/gad.1834709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Hu X, Gnatt A, et al. Functional association of Gdown1 with RNA polymerase II poised on human genes. Molecular cell. 2012;45:38–50. doi: 10.1016/j.molcel.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Gilchrist DA, Fargo DC, Kwak H, Adelman K, Lis JT. Defining the status of RNA polymerase at promoters. Cell reports. 2012;2:1025–1035. doi: 10.1016/j.celrep.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisucci EM, Arndt KM. The Roles of the Paf1 Complex and Associated Histone Modifications in Regulating Gene Expression. Genet Res Int. 2011;2011 doi: 10.4061/2011/707641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol. 2010;17:194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D, Geyer M. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev. 2013;113:8456–8490. doi: 10.1021/cr400071f. [DOI] [PubMed] [Google Scholar]
- Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Molecular and cellular biology. 2004;24:787–795. doi: 10.1128/MCB.24.2.787-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini A, Baillat D, Cesaroni M, Hu D, Marinis JM, Wagner EJ, Lazar MA, Shilatifard A, Shiekhattar R. Integrator regulates transcriptional initiation and pause release following activation. Molecular cell. 2014;56:128–139. doi: 10.1016/j.molcel.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist DA, Nechaev S, Lee C, Ghosh SK, Collins JB, Li L, Gilmour DS, Adelman K. NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev. 2008;22:1921–1933. doi: 10.1101/gad.1643208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour DS, Lis JT. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Molecular and cellular biology. 1986;6:3984–3989. doi: 10.1128/mcb.6.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol Cell. 2010;38:428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaehning JA. The Paf1 complex: platform or player in RNA polymerase II transcription? Biochimica et biophysica acta. 2010;1799:379–388. doi: 10.1016/j.bbagrm.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16:167–177. doi: 10.1038/nrm3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Khodor YL, Rodriguez J, Abruzzi KC, Tang CH, Marr MT, 2nd, Rosbash M. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Genes Dev. 2011;25:2502–2512. doi: 10.1101/gad.178962.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guermah M, Roeder RG. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell. 2010;140:491–503. doi: 10.1016/j.cell.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, Canadien V, Richards DP, Beattie BK, Emili A, Boone C, Shilatifard A, et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2003;23:4207–4218. doi: 10.1128/MCB.23.12.4207-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, Emili A, Shilatifard A, Buratowski S, Greenblatt JF. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Molecular and cellular biology. 2002;22:6979–6992. doi: 10.1128/MCB.22.20.6979-6992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, Lis JT. Control of transcriptional elongation. Annu Rev Genet. 2013;47:483–508. doi: 10.1146/annurev-genet-110711-155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Garrett AS, Yen K, Takahashi YH, Hu D, Jackson J, Seidel C, Pugh BF, Shilatifard A. Codependency of H2B monoubiquitination and nucleosome reassembly on Chd1. Genes Dev. 2012;26:914–919. doi: 10.1101/gad.186841.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145:502–511. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Y, Rhee HS, Ghosh SK, Bai L, Pugh BF, Gilmour DS. Kinetic competition between elongation rate and binding of NELF controls promoter-proximal pausing. Molecular cell. 2013a;50:711–722. doi: 10.1016/j.molcel.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Guo J, Wu Y, Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic acids research. 2013b;41:277–287. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Garrett AS, De Kumar B, Smith ER, Gogol M, Seidel C, Krumlauf R, Shilatifard A. Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC) Genes Dev. 2011;25:1486–1498. doi: 10.1101/gad.2059211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Molecular cell. 2010;37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Lin C, Guest E, Garrett AS, Mohaghegh N, Swanson S, Marshall S, Florens L, Washburn MP, Shilatifard A. The super elongation complex family of RNA polymerase II elongation factors: gene target specificity and transcriptional output. Molecular and cellular biology. 2012a;32:2608–2617. doi: 10.1128/MCB.00182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Lin C, Shilatifard A. The super elongation complex (SEC) family in transcriptional control. Nat Rev Mol Cell Biol. 2012b;13:543–547. doi: 10.1038/nrm3417. [DOI] [PubMed] [Google Scholar]
- Marshall NF, Peng J, Xie Z, Price DH. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- Marshall NF, Price DH. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J Biol Chem. 1995;270:12335–12338. doi: 10.1074/jbc.270.21.12335. [DOI] [PubMed] [Google Scholar]
- Minsky N, Shema E, Field Y, Schuster M, Segal E, Oren M. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, Adelman K. RNA polymerase is poised for activation across the genome. Nat Genet. 2007;39:1507–1511. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Saunders A, Fuda NJ, Yao J, Suarez JR, Webb WW, Lis JT. P-TEFb is critical for the maturation of RNA polymerase II into productive elongation in vivo. Molecular and cellular biology. 2008;28:1161–1170. doi: 10.1128/MCB.01859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Molecular cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Reppas NB, Wade JT, Church GM, Struhl K. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Molecular cell. 2006;24:747–757. doi: 10.1016/j.molcel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Rougvie AE, Lis JT. The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988;54:795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- Shi X, Finkelstein A, Wolf AJ, Wade PA, Burton ZF, Jaehning JA. Paf1p, an RNA polymerase II-associated factor in Saccharomyces cerevisiae, may have both positive and negative roles in transcription. Molecular and cellular biology. 1996;16:669–676. doi: 10.1128/mcb.16.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhian B, Laguette N, Yatim A, Nakamura M, Levy Y, Kiernan R, Benkirane M. HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol Cell. 2010;38:439–451. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annual review of biochemistry. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Sims RJ, 3rd, Belotserkovskaya R, Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 2004;18:2437–2468. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- Smith E, Shilatifard A. Transcriptional elongation checkpoint control in development and disease. Genes Dev. 2013;27:1079–1088. doi: 10.1101/gad.215137.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmayer B, Micas G, Gamot A, Martin P, Malirat N, Koval S, Raffel R, Sobhian B, Severac D, Rialle S, et al. Integrator complex regulates NELF-mediated RNA polymerase II pause/release and processivity at coding genes. Nat Commun. 2014;5:5531. doi: 10.1038/ncomms6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Malone EA, Winston F. SPT5, an essential gene important for normal transcription in Saccharomyces cerevisiae, encodes an acidic nuclear protein with a carboxy-terminal repeat. Molecular and cellular biology. 1991;11:3009–3019. doi: 10.1128/mcb.11.6.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JL, Westfield GH, Takahashi YH, Cook M, Gao X, Woodfin AR, Lee JS, Morgan MA, Jackson J, Smith ER, et al. Context dependency of Set1/COMPASS-mediated histone H3 Lys4 trimethylation. Genes Dev. 2014;28:115–120. doi: 10.1101/gad.232215.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey MG, Jones KA. In vitro formation of short RNA polymerase II transcripts that terminate within the HIV-1 and HIV-2 promoter-proximal downstream regions. Genes Dev. 1989;3:265–282. doi: 10.1101/gad.3.3.265. [DOI] [PubMed] [Google Scholar]
- Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog GA, Winston F, et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA, Jaehning JA. Transcriptional corepression in vitro: a Mot1p-associated form of TATA-binding protein is required for repression by Leu3p. Molecular and cellular biology. 1996;16:1641–1648. doi: 10.1128/mcb.16.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA, Werel W, Fentzke RC, Thompson NE, Leykam JF, Burgess RR, Jaehning JA, Burton ZF. A novel collection of accessory factors associated with yeast RNA polymerase II. Protein expression and purification. 1996;8:85–90. doi: 10.1006/prep.1996.0077. [DOI] [PubMed] [Google Scholar]
- Wood A, Schneider J, Dover J, Johnston M, Shilatifard A. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J Biol Chem. 2003a;278:34739–34742. doi: 10.1074/jbc.C300269200. [DOI] [PubMed] [Google Scholar]
- Wood A, Krogan N, Dover J, Schneider J, Heidt J, Boateng MA, Dean K, Golshani A, Zhang Y, Greenblatt JF, Johnston M, Shilatifard A. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol Cell. 2003b;11:267–274. doi: 10.1016/s1097-2765(02)00802-x. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yamaguchi Y, Inukai N, Okamoto S, Mura T, Handa H. P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Molecular cell. 2006;21:227–237. doi: 10.1016/j.molcel.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li T, Price DH. RNA polymerase II elongation control. Annual review of biochemistry. 2012;81:119–143. doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.