Abstract
Lung transplant survival is limited by obliterative bronchiolitis (OB), but the mechanisms of OB development are unknown. Previous studies in a mouse model of orthotopic lung transplantation suggested a requirement for IL-17. We have used this orthotopic mouse model to investigate the source of IL-17A and the requirement for T cells producing IL-17A. The major sources of IL-17A were CD4+ T cells and γδ T cells. Depletion of CD4+ T cells led to a significantly decreased frequency and number of IL-17A+ lymphocytes and was sufficient to prevent acute rejection and OB. However, mice with STAT3-deficient T cells, which are unable to differentiate into Th17 cells, rejected lung allografts and developed OB similar to control mice. The frequency of IL-17A+ cells was not decreased in mice with STAT3-deficient T cells due mainly to the presence of IL-17A+ γδ T cells. Deficiency of γδ T cells also did not affect the development of airway fibrosis. Our data suggest that CD4+ T cells are required for OB development and expansion of IL-17A responses in the lung, while Th17 and γδ T cells are not absolutely required and may compensate for each other.
Keywords: CD4, T-cells, lung transplantation, Th17 cells, STAT3, γδ T-cells
Introduction
Lung transplantation is a valuable therapeutic option for patients with end-stage lung diseases. The major obstacle limiting lung transplant survival and function is chronic lung allograft dysfunction (CLAD), with one of the major manifestations being obliterative bronchiolitis (OB) with fibrous obliteration of the airways (1). Progressive airway injury mediated by ongoing alloimmune and nonalloimmune responses to the lung allograft is thought to be the precursor of subsequent graft fibrosis (2). OB/BOS remains one of the major limitations to long term success of lung transplant with approximately 50% of lung transplant recipients affected by 5 years (3).
Recently, IL-17 and T helper 17 (Th17) cells have been linked to OB/BOS development after lung transplantation (4). Expression of IL-17 in the BAL positively correlated with OB in lung transplant recipients and Th17 cells were associated with OB/BOS in humans (5, 6). Using a murine MHC minor mismatch orthotopic lung transplantation model, our group previously found that systemic IL-17A protein and mRNA levels were much higher in mice with OB compared to those without OB (7). Furthermore, mice treated with an IL-17RA:Fc fusion protein had decreased cellular rejection scores and did not develop OB. However, the cellular sources of IL-17A in OB have not been investigated in an intact orthotopic lung transplant model. A major source of IL-17 is CD4+ T cells (Th17), but other cells are known to produce IL-17A including innate lymphocytes (8-11).
In the present study, we have investigated the source and timing of IL-17A producing cells and their requirement to promote graft fibrosis and OB. We reasoned that identifying particular subsets may allow the design of more targeted and effective therapies that have less unintended consequences. Our results suggest CD4+ T cells are required for promoting the IL-17A response to transplant and the development of OB in this model. In contrast, deficiency of either major IL-17A-producing subset, Th17 or γδ T cells alone is not sufficient to prevent airway fibrosis. Each subset may be compensating for the lack of the other to produce IL-17 and induce airway fibrosis.
Materials and Methods
Animals
C57BL/6N (H-2b) and C57BL/10 (H-2b) mice were purchased from Harlan Laboratories (Indianapolis, IN). C57Bl/6N.Stat3fl/fl. CD4-Cre (STAT3CD4−/−) mice were previously described (12, 13). C57Bl/6J.TCRδ−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed under specific pathogen-free conditions in the animal care facility at Indiana University or University of Illinois at Chicago. Male animals weighing 24-30g were used as both donors and recipients. All experimental mouse protocols were reviewed and approved by the Indiana University School of Medicine and the University of Illinois at Chicago Institutional Animal Care and Use Committee
Orthotopic lung transplant
A mouse model of orthotopic minor histocompatibility antigen mismatch left lung transplant was used and has been previously described in detail (7).
CD4 T cell depletion in vivo
CD4+ T cells were depleted in vivo by injection of rat anti-mouse CD4 (GK1.5) monoclonal antibody (Bio-X-cell), 250 μg on days -2, 0 and twice per week after transplantation. Untreated allografts or isotype treated wild-type recipients were used as controls.
Histology
Lungs were inflated via the trachea with 10% neutral buffered formalin solution (Sigma-Aldrich, Missouri, USA), followed by embedding in paraffin. Tissue sections were prepared and stained with H&E or Masson's trichrome stain. Standard clinical criteria for scoring vascular lung rejection were used according to ISHLT guidelines and scoring was done blinded (14). For the severity of fibrosis an arbitrary scale was used (15). Presence of OB lesions was also determined.
Preparation of tissue
Lung single cell suspensions were collected by digestion with collagenase I (Life Technologies). Lymph nodes (LN) or spleen were dissociated mechanically. Single cell suspensions were treated with red blood cell lysis buffer (BioLegend).
Flow cytometry
Cells were stained with antibodies for CD4, CD8, TCRβ, TCR γδ, CD3, IL-17A, IFN- γ and NK1.1 (Biolegend) or CD1d tetramer (NIH Tetramer facility, Emory University). For staining of intracellular cytokines, cells were stained first with surface markers, fixed with formaldehyde and permeabilized prior to stimulation with PMA and ionomycin. Cells were acquired on a LSRII (BD Biosciences) and data analysis was performed with Flowjo software (Tree Star, Ashland, OR).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Differences were considered significant at P<0.05. All data are expressed as mean ± (SEM).
Additional material and methods can be found in the supplements.
Results
IL-17A producing CD4+ T cells and IFNγ producing CD4+ and CD8+ T cells infiltrate lung allografts
A minor mismatch model of orthotopic left lung transplantation using C57Bl/10 (B10) mice as donors and C57Bl/6 (B6) mice as recipients was used as previously described (7). To further characterize the immune response to transplant in this model, we determined the frequency of CD4+ and CD8+ T cells infiltrating the lung at different time-points after transplant. On day 7 post-transplant, very few lymphocytes had infiltrated the lungs and the production of cytokines was minimal (data not shown). Compared to B6→B6 syngeneic lung transplants, CD4+ and CD8+ T cells were increased at day 21 in B10→B6 lung allografts (Figure S1). By day 28 post-transplant, the frequency of CD4+ and CD8+ T cells in the lung allografts was comparable to the syngeneic lung transplants.
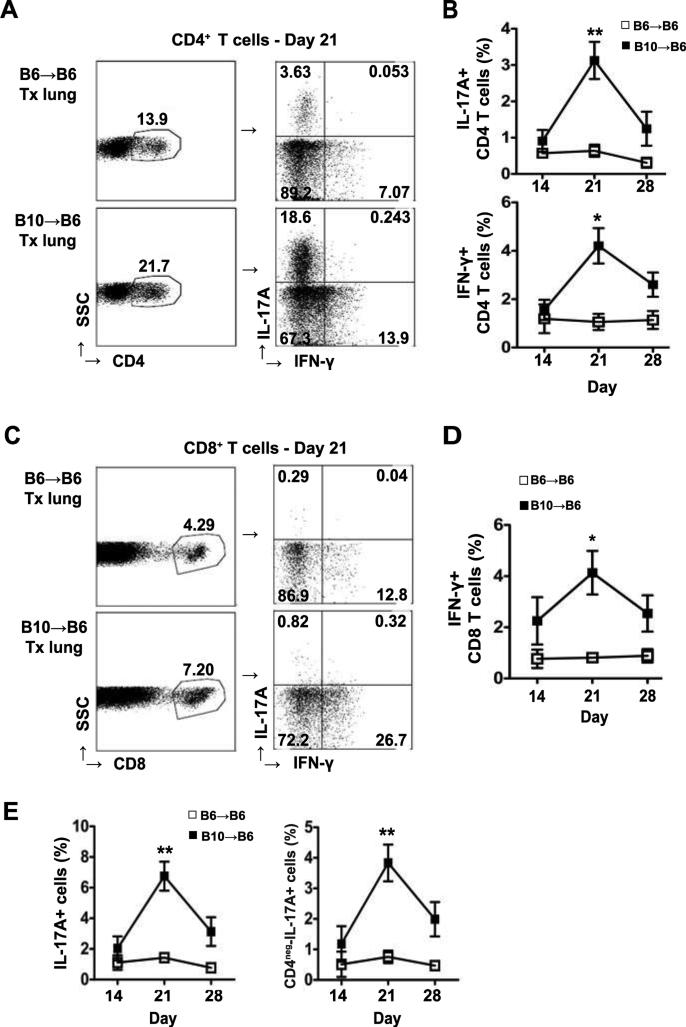
CD4+ T cells were found to be either IFN-γ+ or IL-17A+ and the frequency of both subsets of cytokine-producing cells was increased compared to syngeneic grafts peaking at Day 21 (Figure 1A and B). The frequency of IFN-γ+CD8+ T cells was also significantly increased in lung allografts and peaked at day 21 (Figure 1C and D). Very few CD8+ T cells produced IL-17A in either allografts or syngeneic grafts (Figure 1C). However, the percentage of IL-17A+ cells was not completely accounted for by CD4+ T cells (Figure 1E). Both IL-17A producing CD4+ and CD4-negative lymphocytes contributed to an increased frequency of IL-17A+ lymphocytes in lung allografts.
Figure 1. IL-17A and IFN-γ producing T cells are increased in allografts.
Transplanted lungs from B6→B6 and B10→B6 transplants were harvested at different time-points and analyzed by flow cytometry. (A) Representative flow cytometry plots with frequency of IL-17A+ and IFN-γ+ populations (right plot) gated on CD4+ T cells (left plot). (B) Frequency of IL-17A+ and IFN-γ+ CD4+ T cells in lymphocytes gated by size. (C) Representative dot plots with frequency of IL-17A+ and IFN-γ+ populations (right) gated on CD8+ T cells (left). (D) The overall frequency of IFN-γ+ CD8+ T cells in lymphocyte gate. (E) Percentage of IL-17A+ cells (left) and non-CD4 IL-17A+ cells (right) in lymphocyte gate. Number in dot plots represents percentage. For day 14 (n = 2 for B6→B6, n = 4 for B10→B6); day 21 (n = 5 for B6→B6, n = 12 for B10→B6) and day 28 (n = 3 for B6→B6, n = 5 for B10→B6). For IFN-γ+CD4+ and IFN-γ+CD8+ T cells, n=4 for B6→B6 at day 21. Data were analyzed by unpaired t test compared to B6→B6 at same time-point, *P<0.05, **P<0.01.
γδ T cells and CD4+ T cells are the majority of IL-17A+ cells in allografts
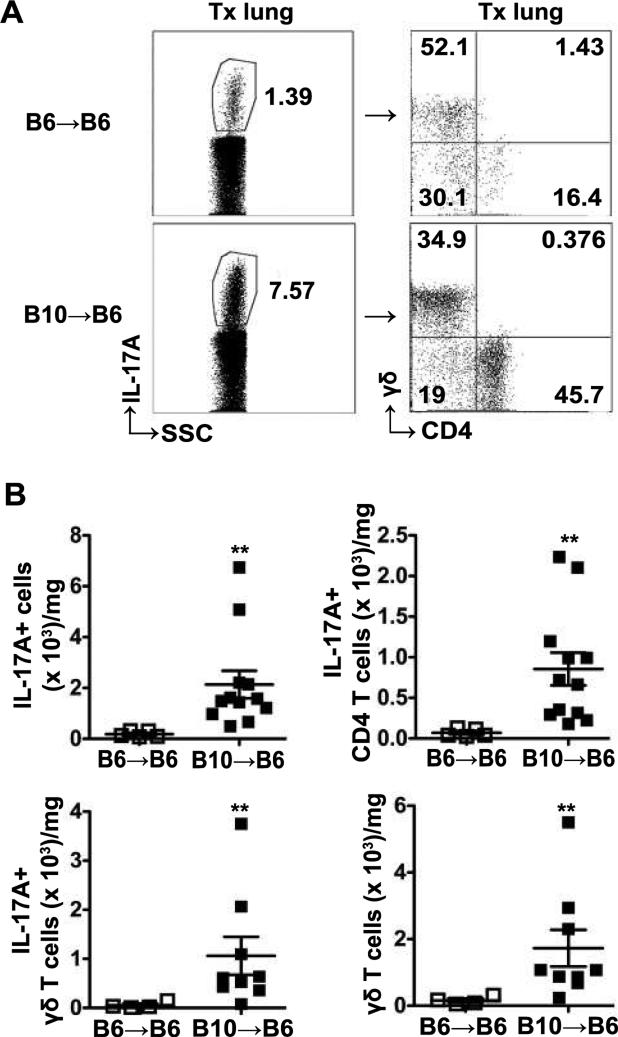
We found that γδ T cells were the other major population of IL-17A producing lymphocytes in B10→B6 lung allografts (Figure 2A). In B6→B6 syngeneic grafts, they were the majority of IL-17A+ lymphocytes, as well as in the native right lungs (Figure 2A and data not shown). In addition to CD4+ T cells and γδ T cells, we found a small proportion of non-T cells producing IL-17A, which were not NK cells, and are likely innate lymphoid cells (ILC) (Figure S2). Among IL-17A+TCRβ+ T cells, CD4+ T cells were the majority of cells followed by CD4− CD8− T cells (Figure S2). CD8+, NK1.1+ or invariant NK T cells were not a major proportion of the IL-17A+ cells (Figure S2).
Figure 2. Increased IL-17A+ cells in allografts are CD4+ T cells and γδ T cells.
Transplanted lungs from B6→B6 and B10→B6 transplants were harvested at day 21 post-transplant and analyzed by flow cytometry. (A) Representative dot plots indicating frequency of IL-17A+ cells (left) of total lymphocytes and frequency of CD4+ T cells and γδ T cells (right) in IL-17A+ lymphocytes in transplanted left lung. (B) The absolute numbers of IL-17A+ lymphocytes, IL-17A+CD4+ T cells, IL-17A+γδ T cells and total γδ T cells in transplanted left lung. Absolute number of cells is normalized to the weight of the lung. For IL-17A+ cells and IL-17A+CD4+ T cells, n=5 for B6→B6, n=12 for B10→B6; for IL-17A+γδ T cells and total γδ T cells, n=4 for B6→B6, n=9 for B10→B6; data analyzed by Mann-Whitney U test, ** P<0.01.
The absolute number of total IL-17A+ lymphocytes was significantly increased only in B10→B6 allografts and not B6→B6 syngeneic grafts (Figure 2B). In addition, the absolute numbers of IL-17A+ CD4+ T cells and IL-17A+γδ T cells were significantly higher in the allografts (Figure 2B). The total number of γδ T cells was also significantly increased in allografts compared with syngeneic grafts (Figure 2B). These data suggested that both CD4+ and γδ T cells were the major sources of IL-17A after lung transplant.
Depletion of CD4+ T cells prevents chronic rejection and OB and affects expansion of IL-17A+ cells
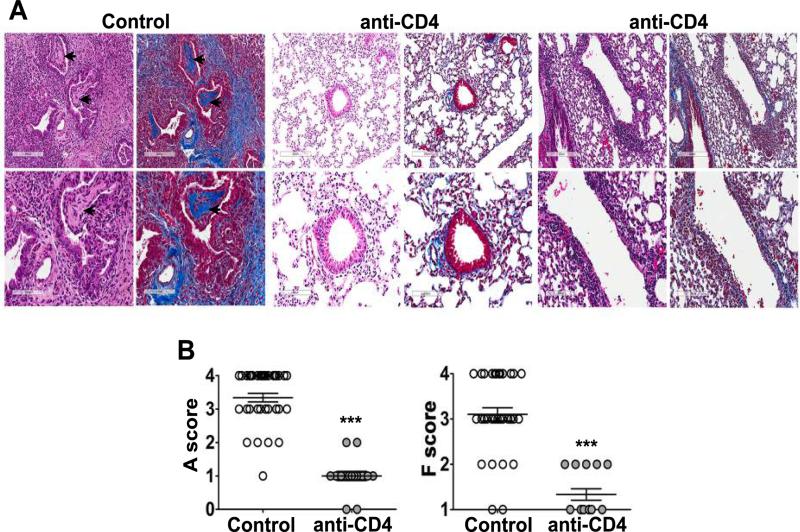
To determine if CD4+ T cells were necessary for cellular rejection and airway fibrosis after orthotopic lung transplant, recipient mice were treated with a CD4-depleting antibody. On day 21 after transplant, allografts from CD4-depleted mice displayed a normal gross appearance, while untreated control allografts were grossly darker in color and shrunken in size (data not shown). Remarkably, none of the CD4-depleted allografts developed OB, while 44% of control allografts developed OB-like lesions, a similar incidence to what was reported previously (7) (Table 1). Based on established clinical rejection criteria, we observed either no acute rejection (grade A0) or minimal (grade A1) to mild acute rejection (grade A2) with a mean grade of A1 in the CD4-depleted allografts on day 21 (Figure 3). Histological analysis of untreated control allografts demonstrated mild (grade A1) to severe (A4) acute rejection with a mean score of A3 (Figure 3). In addition, CD4-depleted allografts developed very little fibrosis, while untreated control allografts had minimal (F1) to severe fibrosis (F4) with a mean score of moderate fibrosis (F3) (Figure 3). These data provide evidence that CD4+ T cells play a crucial role in chronic rejection and OB development in the lungs of a minor mismatch model.
Table 1.
Depletion of CD4+ T cells inhibits OB development.
| Group | OB/total mice | OB (%) |
|---|---|---|
| B10→B6 | 15/34* | 44 |
| B10→B6 CD4 depleted | 0/10 | 0 |
Data represent the number and percentage of mice that developed OB in each group.
p < 0.01 using two-tailed Fisher's exact test.
Figure 3. CD4+ T cells are required for rejection and the development of OB.
B6 recipients of B10 left lungs were treated with CD4-depleting mAb (GK1.5) two days prior to transplant and twice a week post-transplant, lungs were harvested and analyzed on Day 21. Untreated B10→B6 allografts were used as control. (A) H&E and Masson's trichrome staining of lung sections with no evidence of rejection in anti-CD4 treated allografts (middle panels) compared to cellular rejection and OB (arrow) found in the control allografts (left panels), an anti-CD4 treated allograft with mild cellular rejection and fibrosis has also been shown (right panels). magnification, 10X and 20X. (B) Acute rejection (A) scores and fibrosis (F) scores in transplanted left lung as described in methods, n=39 (control) and n=15 (anti-CD4), ***P<0.001 with Mann-Whitney U test. H&E, hematoxylin and eosin.
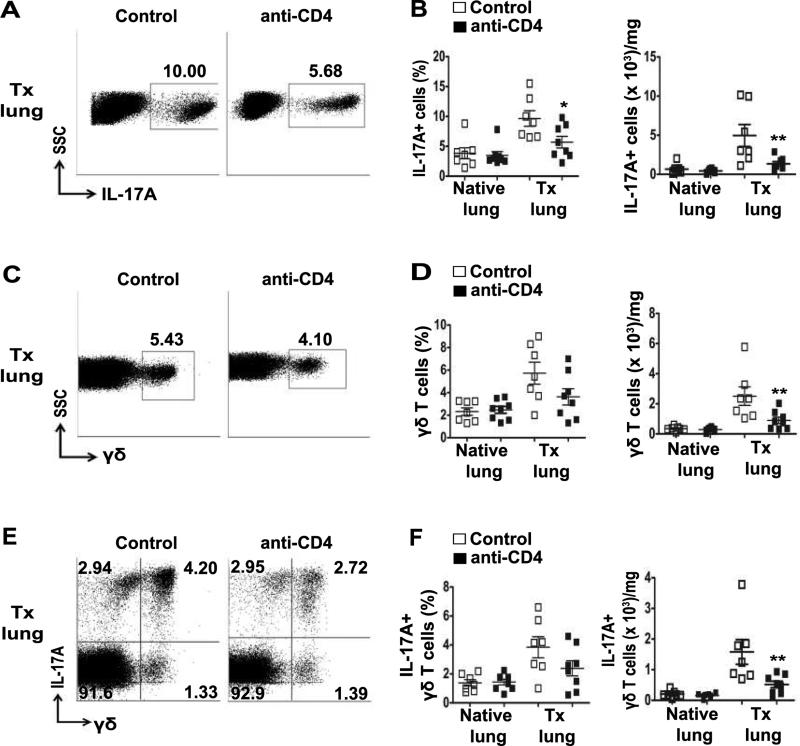
In allografts depleted of CD4+ T cells, we found a decrease in all IL-17A+ cells when compared to control allografts. The decrease was almost to the levels found in the native right lungs of the recipients (Figure 4A and B). The majority of the IL-17A+ cells in the absence of CD4+ T cells were still T cells with a similar proportion of TCRβ+ T cells and γδ T cells as in the presence of CD4+ T cells (Figure S2). The residual IL-17A+TCRβ+ were mostly CD8+ T cells (Figure S2). Interestingly, CD4-depletion had an effect on both the total number of γδ T cells and the number of IL-17A+γδ T cells in the allografts (Figure 4D and F). The frequency of γδ T cells and IL-17A+γδ T cell was not significantly affected (Figure 4C and D). Thus, allo-reactive CD4+ T cells are necessary for the recruitment or expansion of γδ T cells and other IL-17A-producing subsets during allograft rejection.
Figure 4. CD4+ T cell depletion decreases IL-17A and γδ T cell response to lung transplant.
B6 recipients of B10 left lungs were treated with CD4-depleting mAb (GK1.5) two days prior to transplant and twice a week post-transplant, lungs were harvested and analyzed on Day 21. Isotype antibody treated B10→B6 allografts were used as control. (A) Representative dot plots showing frequency of IL-17A+ cells of total lymphocytes in transplanted left lungs. (B) Frequency and absolute number of IL-17A+ lymphocytes of total lymphocytes in transplanted left lungs. (C) Dot plots representing frequency of γδ T cells on gated lymphocytes. (D) Frequency and absolute number of γδ T cells of total lymphocytes in the transplanted left lungs. (E) Representative dot plots indicating proportion of IL-17A+ γδ T cells after CD4+ T cell depletion on lymphocyte size gate. (F) Frequency and absolute number of IL-17A+ γδ T cells in the transplanted lungs. Unpaired t test for frequency and Mann-Whitney U test for absolute number, *P<0.05, **P<0.01, n=7 (control), n=8 (anti-CD4). The absolute number of cells has been normalized to the weight of the lung.
STAT3 signaling in CD4+ T cells and Th17 are not required for OB development
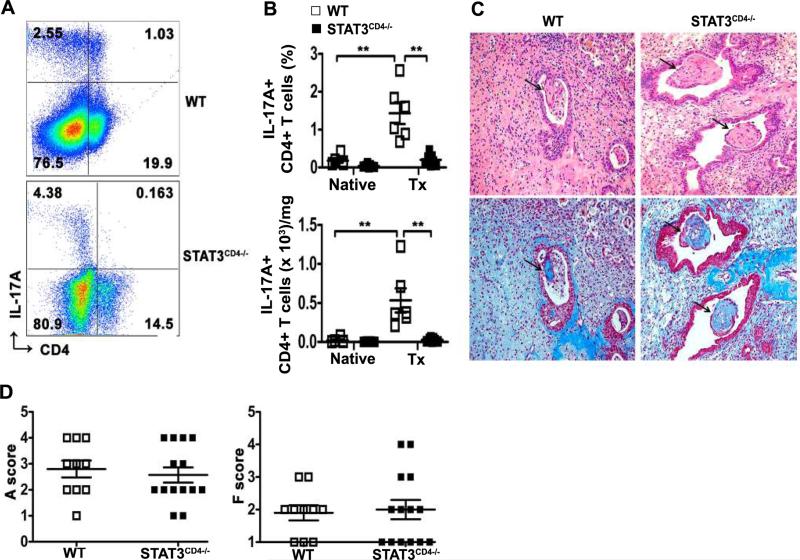
While CD4+ T cells were required for the cellular rejection and airway fibrosis in our model, we hypothesized that Th17 cells were the key CD4+ T cells for driving the rejection response. STAT3 is the major transcription factor required for the initiation of Th17 differentiation (12, 16, 17). Previous studies found that mice with STAT3-deficient T cells did not develop Th17 immune responses in vivo (17-19). To test the hypothesis that STAT3 and Th17 were required for OB development, we used STAT3CD4−/− mice as recipients in our model. Prior to transplant, IL-17A+CD4+ T cells were almost undetectable in the lungs of un-manipulated mice consistent with prior studies (data not shown). B10 lungs were transplanted into STAT3CD4−/− or littermate wild-type controls. Left lung allografts from STAT3CD4−/− mice had virtually no IL-17A+ CD4+ T cells on day 21 after transplant (Figures 5A and B). Similarly, mediastinal lymph node T cells from STAT3CD4−/− mice demonstrated almost no detectable IL-17A+ CD4+ T cells (Figure S3). Despite the lack of Th17 cells in the lungs and draining lymph nodes, STAT3CD4−/− mice developed OB lesions with 4 of 14 (28.5%) STAT3CD4−/− mice having evidence of OB (Figure 5C). The incidence of OB in the littermate controls was 1 out of 10 (10%). Acute rejection scores in these mice were also comparable to the left lung allografts from littermate controls (Figure 5D). There was no difference in the overall fibrosis scores between the STAT3CD4−/− mice and controls (Figure 5D). Our data suggest that STAT3 expression by CD4+ T cells and differentiation to Th17 are not required for OB.
Figure 5. STAT3 expression in CD4+ T cells is not required for OB development.
Left lungs from B10 mice were transplanted into STAT3fl/flCD4-Cre negative (WT) or STAT3CD4−/− littermates and analyzed at day 21 post-transplant. (A) Representative dot plots for intracellular staining for IL-17A+ lung lymphocytes in transplanted allografts, gated on lymphocyte size, percentage of cells noted in quadrants. (B) Frequency (top) and absolute number (bottom) of IL-17A+CD4+ T cells of total lymphocytes in the native and transplanted left lungs, absolute number normalized to the weight of the lung, n=6 for WT, n=7 for STAT3CD4−/−, analyzed by Mann-Whitney U test, **P<0.005. (C) H&E (top) and Masson's trichrome (bottom) staining demonstrated the presence of OB-like lesions (arrows) in transplanted left lungs from WT and STAT3CD4−/− mice, magnification, 20X. (D) Acute rejection (A) scores and fibrosis (F) scores in left lung allografts as described in Methods, n=10 (WT), n=14 (STAT3CD4−/−).
Since IL-17A+ and IFNγ+ CD4+ T cells share a reciprocal relationship, we determined if STAT3CD4−/− allografts had augmented IFNγ+ CD4+ T cells, which may be compensating for the loss of Th17 (16, 20). As expected, we found a significantly higher proportion of IFNγ+CD4+ T cells in the STAT3CD4−/− allografts as compared to WT allografts (Figure S4). However, the absolute number of IFNγ+ CD4+ T cells remained similar between STAT3CD4−/− and WT allografts (Figure S4). Expression of IFNγ, TGF-β, IL-6 and IL-10 were also not altered in STAT3CD4−/− allografts (Figure S4).
IL-17A production by γδ T cells in the absence of Th17
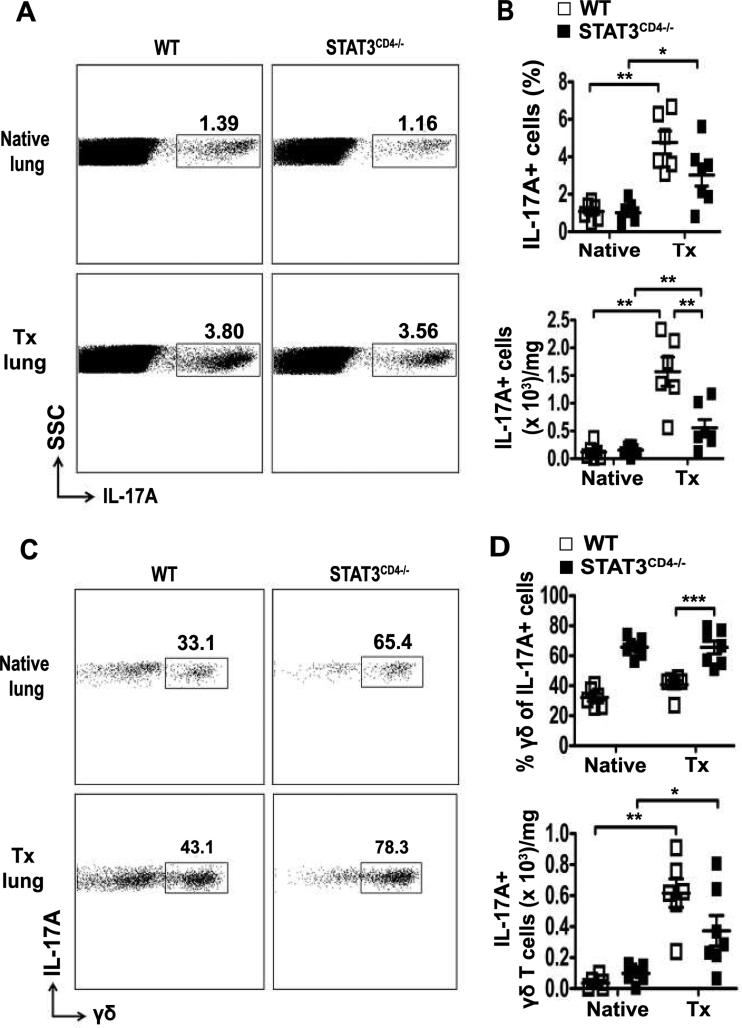
Since airway fibrosis and OB developed in the left lung allografts in STAT3CD4−/− mice, the loss of IL-17A production from CD4+ T cells was possibly compensated for by γδ T cells or other cells. However, previous work had suggested that γδ T cells may be unable to develop into IL-17A producing cells in the absence of Th17 cells (21). Interestingly, the overall percentage of IL-17A+ cells was no different between STAT3CD4−/− recipients compared to WT recipients (Figure 6A and B). In STAT3CD4−/− recipients the predominant population of IL-17A+ cells was γδ T cells and the absolute number of IL-17A+ γδ T cells remained comparable between wild-type and STAT3 CD4−/− recipients (Figure 6C and D). The mediastinal lymph nodes also had no decrease in the frequency of IL-17A+ cells in STAT3CD4−/− mice and γδ T cells were the major cell type (Figure S3). While there was no difference in frequency, a significant decrease in the number of IL-17A+ cells was found in the lung allografts of STAT3CD4−/− mice (Figure 6B). Thus, in the absence of STAT3 expression by CD4+ T cells, γδ T cells are the majority source of IL-17A in lung allografts and IL-17A+ γδ T cells are unaffected by the lack of Th17 differentiation.
Figure 6. IL-17A is primarily produced by γδ T cells in absence of TH17 in STAT3CD4−/− mice.
As in Figure 4, transplanted left lungs and native lungs were analyzed at day 21 post-transplant. (A) Representative dot plots showing percentage of IL-17A+ lymphocytes on gated lymphocytes from WT and STAT3CD4−/− mice. Number in dot plots indicates percentage. (B)Percentage and absolute number of IL-17A+ lymphocytes in right and transplanted left lungs gated on lymphocytes, analyzed using Mann-Whitney U test, *P<0.05, **P <0.01. (C) Dot plots showing analysis percentage of γδ T cells in the IL-17A+ population in the lung. Number in the dot plots indicates percentage. (D) Frequency of γδ T cells in the IL-17A+ population and absolute number of IL-17A+γδ T cells; one-way ANOVA with post hoc Newman-Keuls multiple comparison test for frequency and Mann-Whitney U test for absolute number, *P<0.05, **P<0.01, ***P<0.001 n=6 for WT, n=7 for STAT3CD4−/−. Data on absolute number of cells are presented after normalization to the weight of the lung.
γδ T cells are not necessary for OB development
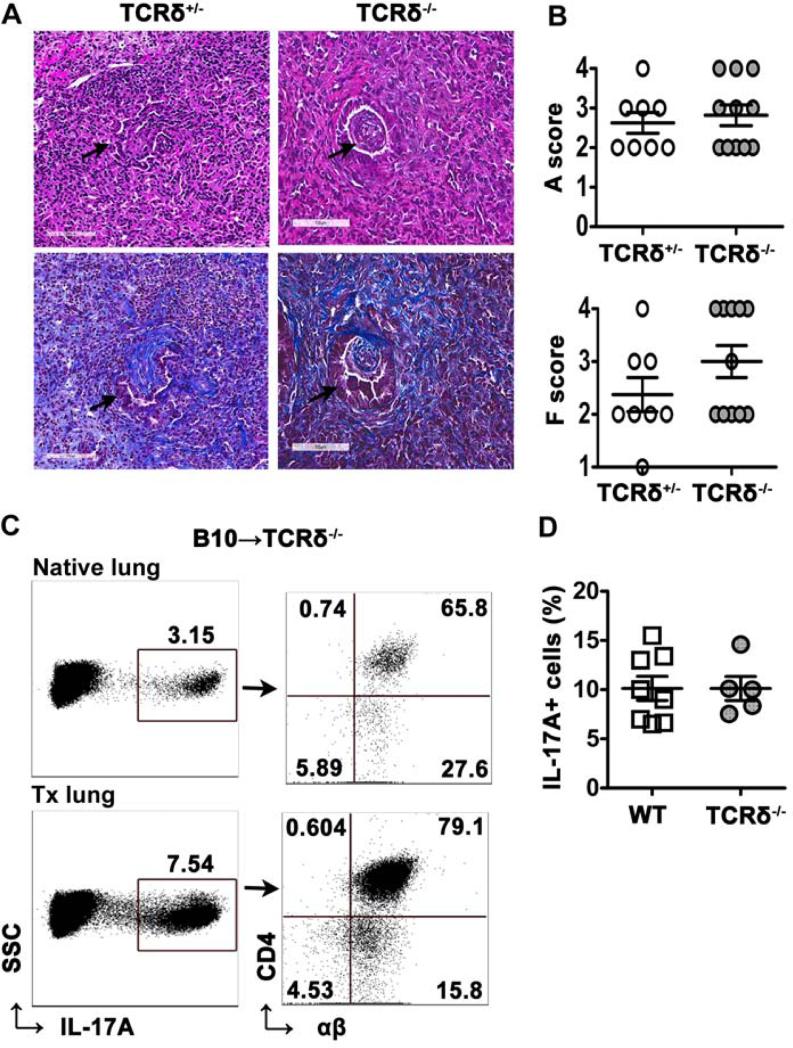
Our data suggested that γδ T cells may participate in OB development through production of IL-17A. Earlier studies found that γδ T cell deficiency was associated with prolonged survival of cardiac allografts (22, 23). To determine whether γδ T cells were required for OB development, TCRδ−/− mice were used as recipients. However, there was no apparent effect on cellular rejection or fibrosis in the absence of γδ T cells (Figure 7A and B). TCRδ−/− recipients developed OB-like lesions similar to TCRδ+/− controls with 3 out of 11 TCRδ−/− mice (27.2%) developing OB compared to 2 of 8 mice (25%) developing OB in the controls. In the absence of γδ T cells, the majority of IL-17A+ cells were CD4+ T cells in the lung allografts and the native lungs (Figure 7C). At the time of sacrifice, we did not find evidence of residual donor γδ T cells in the lungs (data not shown). As with the STAT3CD4−/− mice, TCRδ−/− allografts had a frequency of IL-17A+ cells similar to WT recipients (Figure 7D). These data suggest IL-17A production is compensated for by CD4+ T cells in the absence of γδ T cells and γδ T cells are not absolutely required for cellular rejection and the development of OB in this model.
Figure 7. γδ T cell-deficiency does not prevent OB.
Left lungs from B10 mice were transplanted into TCRδ−/− or TCRδ+/− mice and analyzed at day 21 post-transplant. (A) H&E (top) and Masson's trichrome (bottom) stained lung allografts from TCRδ+/− and TCRδ−/− mice demonstrated OB-like lesions (arrows) in the airway, magnification, 20X. (B) Analysis of acute rejection (A) scores and fibrosis (F) scores in left lung allografts as described in methods, n=8 (TCRδ+/−), n=11 (TCRδ−/−). (C) Representative dot plots showing percentage of IL-17A+ lymphocytes on gated lymphocytes (left) and percentage of CD4+TCRβ+ T cells of IL-17A+ lymphocytes (right) in native lungs and transplanted left lungs from TCRδ−/− mice (n=5). (D) Frequency of IL-17A+ lymphocytes in transplanted left lungs from wild-type mice (n=7) as in Figure 4 and TCRδ−/− mice.
DISCUSSION
In the current study, we investigated the requirement for IL-17 producing T cells to mediate chronic rejection and the development of OB in a mouse lung transplant model previously found to be ameliorated by IL-17 neutralization (7). We found that CD4+ T cells were required for rejection and fibrosis, as depletion of CD4+ T cells significantly decreased the severity of cellular infiltration, the lung IL-17A response, and prevented the development of OB in the mouse. While CD4+ IL-17A+ T cells were significantly increased in lung allografts, Th17 cells were not required for rejection and the development of OB. In the absence of Th17, γδ T cells were the major IL-17A producing population in lung allografts. However, γδ T cell-deficient mice developed airway fibrosis and an IL-17A cellular response in the lungs similar to wild-type recipients. In the absence of γδ T cells, the IL-17A response appears to be compensated for by CD4+ T cells. Our data suggest CD4+ T cells are required to promote the IL-17A+ immune response, cellular rejection and airway fibrosis in mouse lung allografts. Targeting either of the major IL-17A producing cells, Th17 or γδ T cells alone, is not sufficient to affect rejection and fibrosis after lung transplant.
The absence of direct allo-recognition in the minor mismatch model, which is important for CD8+ T cell activation, is likely the reason for the requirement for CD4+ T cells (24). In contrast, CD8+ T cells alone were sufficient to induce rejection in a major mismatch model of orthotopic lung transplant (25). Interestingly, we found that in the absence of CD4+ T cells, there was a reduction in the IL-17A response, mainly IL-17A+γδ T cells, in the allografts. The overall γδ T cell response was also diminished after CD4+ T cell depletion. Our data suggest that alloreactive CD4+ T cells are promoting expansion of the innate γδ T cell response and other IL-17A-producing cells during allograft rejection. In an acute lung injury model, CD4+TCRβ+ T cells were also found to promote γδ T cell recruitment and production of IL-17A (26). Our data are consistent with this work and support a role for allo-reactive CD4+ T cells in expanding or recruiting innate IL-17A producing cells through cytokine and chemokine production either directly or indirectly from CD4+ T cells.
Since CD4+ T cells were a major source of IL-17A and Th17 cells had previously been linked to human OB, we expected that CD4+ T cell differentiation to Th17 would be required for the development of OB (5, 6). Contrary to our expectations, conditional deletion of STAT3 in CD4+ T cells did not affect cellular rejection or the development of OB. It is unlikely that there is significant residual IL-17A production from CD4+ T cells, as CD4+ T cells from the lungs or lymphoid tissue of un-manipulated or post-transplant STAT3CD4−/− mice did not produce IL-17A when stimulated ex vivo. Further, these mice have been used by others to evaluate Th17 responses in vivo without evidence of IL-17A production by CD4+ T cells (17, 19). We also did not find evidence of a donor source. In the absence of Th17, we did not find a significant increase in the number of IFN-γ+CD4+ T cells in STAT3CD4−/− mice recipients. We also found no significant difference in expression of IFN-γ, IL-6, TGFβ or IL-10. These data suggest there is not a major expansion of Th1 or these other cytokines compensating for the loss of STAT3 in the T cells. In addition to affecting IL-17A production, STAT3 expression by CD4+ T cells is necessary for production of the cytokines IL-17F, IL-21 and IL-22 (12). The Th17 lineage in general does not appear to be required for mediating OB in this model.
γδ T cells were the predominant source of IL-17A in the absence of Th17 cells. The defects in the STAT3CD4−/− mice are restricted to αβ T cells and would not affect STAT3 expression in γδ T cells (27). However, we were surprised to find an intact IL-17A γδ T cell response in the absence of Th17 cells, as a recent study suggested that Th17 cells were required for the maintenance of IL-17-producing γδ T cells in vivo in a TGF-β dependent manner (21). Instead, we found that γδ T cells from the lungs or draining lymph nodes had no defect in IL-17A production either in un-manipulated mice (unpublished data) or after transplant in the absence of Th17. It is possible STAT3CD4−/− T cells produce enough TGF-β to support maintenance of IL-17A+γδ T cells during homeostasis.
In the TCRδ−/− recipients, we found that fibrosis and OB was not decreased compared to controls. We did not use antibody depletion of γδ T cells, as the antibodies have been found to be non-depleting in vivo and may be activating γδ T cells (28, 29). However, we did not find residual γδ T cells to suggest expansion of donor γδ T cells was confounding our results. Instead, CD4+ T cells appeared to compensate for the lack of IL-17A+γδ T cells, as the frequency of IL-17A+ cells in the lungs was similar to wild-type mice. Thus γδ T cells are not absolutely required as a source of IL-17A to promote OB in this model and the cells producing IL-17A appear to be able to respond to deficiency of other subsets.
Interestingly, we found differences in the prevalence of OB-like pathology in wild-type littermates of STAT3CD4−/− mice, bred in house on a C57Bl/6N background, compared to mice purchased from Harlan Labs. The previous study with this model found a similar incidence of OB, also using C57Bl/6N mice from Harlan Labs (7). However, a recent study did not find evidence of OB using C57Bl/10 lung transplanted to C57Bl/6J mice from Jackson labs (30). We also found the TCRδ−/− mice from Jackson and TCRδ+/− mice, mixed C57Bl/6N and C57Bl/6J background, had a decreased incidence of OB at 25%, although we still found similar pathology to the previous study (7). The different results may be due to differences in microbiota or differences in genetic background between the strains. Procedural and technical variations also cannot be ruled out. An important caveat in our model is that the airway fibrosis pattern differs from the constrictive OB that can be found in humans after lung transplant. The pathology in our model includes fibrous plugs, with loss of overlying epithelium and only partial airway obliteration (7, 31). Nevertheless, the model allows an understanding of the processes by which the alloreactive immune response and IL-17A can promote fibrosis after lung transplant without the need for immunosuppression as in a full mismatch model (32).
Previously it was found that blockade of IL-17 using IL-17R:Fc protein abrogated OB (7). We have expanded on these data and find that graft infiltrating IL-17A+ cells are largely CD4+ Th17 cells and γδ T cells. A minor subset was TCRβ+CD4−CD8− T cells, which may be Type II NKT cells that do not recognize the CD1d tetramer used, or they may have lost expression of CD4 or CD8 (10). While these other cells can produce IL-17A, CD4+ and γδ T cells were the predominant populations found in wild-type mice and were increased in the lung after transplant. The absence of either Th17 or γδ T cells did not protect from airway fibrosis or OB in this model. These data are consistent with findings in a tracheal transplant mouse model that suggested redundancy for IL-17A production from CD4+ T cells and γδ T cells (33). In addition, we have found a role for CD4+ T cells in the recruitment or expansion of γδ T cells and other IL-17A+ cells in the lung. However, we have also found that Th17 or γδ T cells are compensatory sources of IL-17A when the other T cell subset is deficient. These data suggest that IL-17A-producing cells respond to similar signals and may co-regulate each other. Others have proposed that IL-17 regulates the homeostasis of IL-17+γδ T cells during development and our work suggests the regulation extends to an inflammatory response (34). In conclusion, our data support a model where CD4+ T cells initiate the alloimmune response to minor antigens and promote airway fibrosis. Absence of CD4+ T cells diminishes the IL-17A+ cell response during allograft rejection and the expansion of γδ T cells. Targeting CD4+ T cells early after transplant may prevent the development of deleterious IL-17 responses. A better understanding of the signals that regulate IL-17 producing cells may lead to novel therapies to improve outcomes after lung transplantation.
Supplementary Material
Acknowledgements
The research was supported by NIH grants to M.H.K. (R03AI101628), D.S.W (R01HL060797-08 and 5P01AI084853-03) and R.A.S (R01HL109310). We would like to thank Drs. Marisa Alegre and Shreevrat Goenka, Amanda Fisher, Elizabeth Mickler and Kathleen Heidler, as well as members of the Wilkes and Kaplan labs for helpful discussions and advice and the staff of the Flow Cytometry and Histology core facilities at Indiana University School of Medicine and University of Illinois at Chicago for their technical assistance.
Abbreviations
- ANOVA
analysis of variance
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- CD
cluster of differentiation
- CLAD
chronic lung allograft dysfunction
- H&E
hematoxylin and eosin
- IFN-γ
interferon gamma
- IL
interleukin
- ILC
innate lymphoid cells
- miHA
minor histocompatibility antigen
- OB
obliterative bronchiolitis
- PMA
phorbol myristate acetate
- STAT
signal transducer and activator of transcription
- Th
T helper cell
- WT
wild type
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Weigt SS, Derhovanessian A, Wallace WD, Lynch JP, 3rd, Belperio JA. Bronchiolitis obliterans syndrome: the achilles’ heel of lung transplantation. Semin Respir Crit Care Med. 2013;34:336–51. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714–8. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–86. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant rejection. Semin Immunopathol. 2011;33:129–34. doi: 10.1007/s00281-011-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–20. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 6.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007 doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–22. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price AE, Reinhardt RL, Liang HE, Locksley RM. Marking and quantifying IL-17A-producing cells in vivo. PLoS One. 2012;7:e39750. doi: 10.1371/journal.pone.0039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nature medicine. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 13.Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96:2846–51. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG. Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol. 1999;20:924–34. doi: 10.1165/ajrcmb.20.5.3526. [DOI] [PubMed] [Google Scholar]
- 16.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 17.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radojcic V, Pletneva MA, Yen HR, Ivcevic S, Panoskaltsis-Mortari A, Gilliam AC, et al. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J Immunol. 2010;184:764–74. doi: 10.4049/jimmunol.0903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nature immunology. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Do JS, Visperas A, O'Brien RL, Min B. CD4 T cells play important roles in maintaining IL-17-producing gammadelta T-cell subsets in naive animals. Immunol Cell Biol. 2012;90:396–403. doi: 10.1038/icb.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura N, Nakae S, Itoh S, Merk DR, Wang X, Gong Y, et al. Potential role of gammadelta T cell-derived IL-17 in acute cardiac allograft rejection. The Annals of thoracic surgery. 2012;94:542–8. doi: 10.1016/j.athoracsur.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–40. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 24.Simpson E, Roopenian D. Minor histocompatibility antigens. Curr Opin Immunol. 1997;9:655–61. doi: 10.1016/s0952-7915(97)80045-3. [DOI] [PubMed] [Google Scholar]
- 25.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180:4754–62. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Colpitts SL, Menoret A, Budelsky AL, Lefrancois L, Vella AT. Rapid alphabeta T-cell responses orchestrate innate immunity in response to Staphylococcal enterotoxin A. Mucosal immunology. 2013;6:1006–15. doi: 10.1038/mi.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman DR, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–41. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 28.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol. 2009;39:372–9. doi: 10.1002/eji.200838741. [DOI] [PubMed] [Google Scholar]
- 29.Blink SE, Caldis MW, Goings GE, Harp CT, Malissen B, Prinz I, et al. gammadelta T cell subsets play opposing roles in regulating experimental autoimmune encephalomyelitis. Cellular immunology. 2014;290:39–51. doi: 10.1016/j.cellimm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama S, Sato M, Loisel-Meyer S, Matsuda Y, Oishi H, Guan Z, et al. Lentivirus IL-10 gene therapy down-regulates IL-17 and attenuates mouse orthotopic lung allograft rejection. Am J Transplant. 2013;13:1586–93. doi: 10.1111/ajt.12230. [DOI] [PubMed] [Google Scholar]
- 31.Kreisel D, Gelman AE, Palmer SM. In pursuit of new experimental models of obliterative bronchiolitis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:882–3. doi: 10.1111/j.1600-6143.2011.03483.x. [DOI] [PubMed] [Google Scholar]
- 32.De Vleeschauwer S, Jungraithmayr W, Wauters S, Willems S, Rinaldi M, Vaneylen A, et al. Chronic rejection pathology after orthotopic lung transplantation in mice: the development of a murine BOS model and its drawbacks. PLoS One. 2012;7:e29802. doi: 10.1371/journal.pone.0029802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemaître PH, Vokaer B, Charbonnier LM, Iwakura Y, Estenne M, Goldman M, et al. IL-17A mediates early post transplant lesions after heterotopic trachea allotransplantation in mice. Plos One. 2013;8:e70236. doi: 10.1371/journal.pone.0070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas JD, Ravens S, Duber S, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.