Abstract
Exposure to arsenic (As) in drinking water is a widespread public health problem leading to increased risk for multiple outcomes such as cancer, cardiovascular disease, and possibly renal disease; potential mechanisms include inflammation and oxidative stress. We tested the hypothesis that As exposure is associated with increased inflammation and decreased estimated glomerular filtration rate (eGFR) and examined whether the effects of As were modified by plasma glutathione (GSH), glutathione disulfide (GSSG), or the reduction potential of the GSSG/2GSH pair (EhGSH). In a cross-sectional study of N = 374 Bangladeshi adults having a wide range of As exposure, we measured markers of inflammation (plasma C-reactive protein (CRP), α-1 acid glycoprotein (AGP)), renal function (eGFR), GSH, and GSSG. In covariate-adjusted models, a 10% increase in water As, urinary As adjusted for specific gravity (uAs), or blood As (bAs) was associated with a 0.74% (p = 0.01), 0.90% (p = 0.16), and 1.39% (p = 0.07) increase in CRP, respectively; there was no association with AGP. A 10% increase in uAs or bAs was associated with an average reduction in eGFR of 0.16 (p = 0.12) and 0.21 ml/min/1.73 m2 (p = 0.08), respectively. In stratified analyses, the effect of As exposure on CRP was observed only in participants having EhGSH > median (uAs pWald = 0.03; bAs pWald = 0.05). This was primarily driven by stronger effects of As exposure on CRP in participants with lower plasma GSH. The effects of As exposure on eGFR were not modified significantly by EhGSH, GSH, or GSSG. These data suggest that participants having lower plasma GSH and a more oxidized plasma EhGSH are at increased risk for As-induced inflammation. Future studies should evaluate whether antioxidant treatment lowers plasma EhGSH and reduces risk for As-induced diseases.
Keywords: Arsenic, Bangladesh, Inflammation, C-reactive protein, α-1 acid glycoprotein, Glutathione, Redox, Kidney, Glomerular filtration rate, Cystatin C, Oxidative stress, Free radicals
Globally, the World Health Organization estimates that more than 200 million people are chronically exposed to arsenic (As)-contaminated drinking water above the safety standard of 10 μg/L [1]. Arsenic exposure is associated with increased risk for skin, lung, and bladder cancer, as well as noncancer outcomes including cardiovascular disease (CVD)1, respiratory illness, and neurologic deficits [2]. Chronic kidney disease (CKD) is also emerging as a potential As-induced disease outcome [3]. We have observed in a cross-sectional study in Bangladesh that total urinary As (uAs), but not water As (wAs), was marginally associated with decreased estimated glomerular filtration rate (eGFR) [4].
Arsenic exposure has been shown to cause inflammation; this may be a mechanism for As-induced diseases, as inflammation is involved in the pathogenesis of many chronic diseases, including CVD, metabolic syndrome [5], CKD [6], and cancer [7]. Chronic exposure of mice to 100 ppb sodium arsenite in drinking water resulted in an increase in the acute-phase protein C-reactive protein (CRP) in the liver and kidney [8]. Increases in proinflammatory cytokines have also been observed in serum of arsenic-exposed animals [9,10]. Additionally, As exposure in humans has been associated with increased serum proinflammatory cytokines interleukin-6 (IL-6) and IL-8 [11] and CRP [12].
Numerous in vitro and in vivo studies have demonstrated that As toxicity is mediated by induction of oxidative stress [13]. Reactive oxygen species (ROS) that have increased in response to As include the superoxide anion radical and hydrogen peroxide [14]. Oxidative stress and inflammation are connected in a complex feedback cycle in which ROS can activate transcription factors that upregulate the expression of proinflammatory cytokines and both pro-oxidant and antioxidant enzymes [15]. Additionally, phagocytic leukocytes recruited to sites of inflammation express enzymes that contribute to oxidative damage, which can further amplify the inflammation [16]. Oxidative stress and inflammation are linked as causal agents in a variety of chronic diseases including cancer, CVD, and diabetes [17,18].
There is evidence to suggest that oxidative stress may mediate both As-induced inflammation and As-induced renal dysfunction. In certain mammalian cell models, arsenite exposure induces oxidative stress (i.e., increased ROS) and activates the proinflammatory transcription factor nuclear factor-κB (NF-κB), which can be prevented by administration of the antioxidant N-acetylcysteine (NAC) [19–21]. Arsenic exposure in rodent studies has resulted in increased ROS, lipid peroxidation, and protein carbonylation; decreased reduced glutathione (GSH) and increased glutathione disulfide (GSSG); and reduced antioxidant enzyme activity in kidney tissue [22–26]. The above-mentioned studies have also demonstrated that treatment of As-exposed rodents with antioxidants can either mitigate or prevent the oxidative and nephrotoxic effects observed with As administration alone. Collectively, this evidence suggests that antioxidants may protect against the inflammatory and nephrotoxic effects of As.
The Folate and Oxidative Stress (FOX) study [27] is a data-rich cross-sectional study of 378 Bangladeshi adults that was initially designed to assess the dose–response relationship between As exposure and oxidative stress. In this study, we previously found As exposure to be negatively associated with blood GSH concentrations, but not with blood GSSG [27], the plasma GSH/GSSG ratio [28], urinary 8-oxoG, or plasma protein carbonyls [29]. Plasma GSH and GSSG may correlate with tissue GSH and GSSG concentrations, and the reduction potential of the GSSG/2GSH couple (EhGSH) may reflect the balance of pro-oxidants and antioxidants within tissues [30]. In the current analyses, we examine markers of inflammation and renal function in these study participants to test the hypotheses that As exposure is associated with inflammation and that the effects of As exposure on inflammation and renal function are greater among people with a more oxidized plasma EhGSH. We also wished to examine whether inflammation mediates the effect of As on renal function, as inflammation is involved in the deterioration of renal function [6].
1. Materials and methods
1.1. Study participants
The FOX study was a cross-sectional study designed to examine the dose–response relationship between As exposure and oxidative stress, as described previously [27]. Briefly, N = 378 adults living in Araihazar, Bangladesh, were recruited in 2008 for this study. Individuals had to be between the ages of 30 and 65; not pregnant; not taking nutritional supplements; free of known diabetes, cardiovascular or renal disease, chronic obstructive pulmonary disease, or cancer; and drinking from their current well for at least 3 months. Participants were selected based on the As concentration of their wells, to include a wide range of As exposures (Group A, 0–10 μg/L (N = 76); Group B, 10–100 μg/L (N = 104); Group C, 100–200 μg/L (N = 86); Group D, 200–300 μg/L (N = 67); Group E, >300 μg/L (N = 45)). Participants missing information on wAs, BMI, specific gravity, plasma EhGSH, or α-1 acid glycoprotein (AGP) were excluded from the current analysis, leaving a final sample size of N = 374.
Oral informed consent was obtained by our Bangladeshi field staff physicians, who read an approved assent form to the study participants. This study was approved by the Bangladesh Medical Research Council and the institutional review board of Columbia University Medical Center.
1.2. Collection of biospecimens
Spot urine samples were collected into 50-ml acid-washed polypropylene tubes and frozen at −20 °C. After initial processing of blood samples in the field clinic, blood and plasma aliquots were immediately frozen at −80 °C. Samples were transported to Dhaka on dry ice and again stored at −80 °C (blood and plasma) or −20 °C (urine). Samples were then packed on dry ice and flown to Columbia University.
1.3. Well-water As
Water samples were analyzed by high-resolution inductively coupled plasma mass spectrometry [31]. Water samples were collected in 20-ml polyethylene scintillation vials and acidified to 1% with high-purity Optima HCl (Fisher Scientific, Pittsburg, PA, USA) at least 48 h before analysis. A standard with an As concentration of 51 μg/L was run multiple times in each batch. The intra- and interassay coefficients of variation (CVs) for this standard were 6.01 and 3.76%, respectively.
1.4. Total uAs and specific gravity
The arsenic metabolites (arsenite (AIII), arsenate (AsV), monomethylarsonous acid plus monomethylarsonic acid (MMAIII+V), and dimethylarsinous acid plus dimethylarsinic acid (DMAIII+V)) were measured in urine by coupling HPLC to dynamic reaction cell inductively coupled plasma mass spectrometry, as described previously [32]. Total uAs was calculated as the sum of AsIII, AsV, MMAIII+V, and DMAIII+V. The intra-assay CVs for urinary AsIII, AsV, MMA, and DMA were 3.6, 4.5, 1.5, and 0.6%, respectively; the interassay CVs were 9.7, 10.6, 3.5, and 2.8%, respectively. We measured specific gravity by refractometer. Urinary As was adjusted for specific gravity (SG) using the following formula: uAs × (overall mean SG − 1)/(measured SG − 1). We refer to this adjusted variable as uAs-SG.
1.5. Total blood As (bAs)
As described previously [33], bAs concentrations were measured using a PerkinElmer Elan DRC II ICP-MS equipped with an AS 93+ autosampler. The intra- and interassay CVs were 2.1 and 4.9%, respectively.
1.6. Plasma glutathione and glutathione disulfide
Whole blood and plasma GSH and GSSG were measured by HPLC with fluorescence detection [34], as previously described [27]. Intra-assay CVs ranged from 5 to 10% and interassay CVs ranged from 11 to 18%.
1.7. Calculation of the redox potential
The EhGSH was calculated using the Nernst equation, Eh = Eo + RT/nF ln[disulfide]/[thiol]2. In this equation, Eo is the standard potential for the redox couple (−264 mV), R is the gas constant, T is body temperature in Kelvin, n is 2 for the number of electrons transferred, and F is Faraday’s constant [30]. The Eh represents the two-electron half-cell reduction potential of the GSSG/2GSH couple, and as such a more positive Eh value reflects a more oxidized redox state.
1.8. Plasma nutrients
Plasma folate and B12 were measured by radio-protein binding assay (SimulTRAC-S, MP Biomedicals) as previously described [27]. The intra- and interassay CVs for folate were 9 and 14%, respectively, and those for B12 were 5 and 9%, respectively. Plasma total homocysteine (tHcys) was measured by HPLC with fluorescence detection [35]. The intra- and interassay CVs for tHcys were 2 and 9%, respectively.
1.9. C-reactive protein and α-1 acid glycoprotein
CRP and AGP were assayed by ELISA with Quantikine ELISA kits from R&D Systems (Minneapolis, MN, USA). The intra- and inter-assay CVs were 4 and 3% for CRP, respectively, and 5 and 6% for AGP, respectively.
1.10. Plasma cystatin C and eGFR
Cystatin C was measured by ELISA according to the manufacturer’s protocol (R&D Systems Human Cystatin C Duoset, Catalog No. DY1196). We used a six-point standard curve with a high standard of 3000 pg/ml. Samples were diluted 1:2000 in phosphate-buffered saline with 10% fetal bovine serum (Sigma–Aldrich F6178). Recovery of the IFCC-certified reference material for serum cystatin C (ERM-DA 471/IFCC) was 104%. The intra- and interassay CVs were 7 and 10%, respectively. We calculated eGFR using the 2012 CKD-EPI Cystatin C equation [36].
1.11. Statistical analysis
We calculated descriptive statistics (means ± standard deviations for continuous variables and frequencies for categorical variables) for the total study population and stratified by the median of plasma EhGSH. To reduce variation in plasma GSH and GSSG, we adjusted these variables for plasma GSH laboratory batch as a categorical variable using the residual method; these batch-adjusted variables were used in the calculation of plasma EhGSH. Differences in characteristics between the EhGSH strata were tested using the Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables. We used Spearman correlation coefficients to explore bivariate associations between CRP, AGP, eGFR, GSH, GSSG, and EhGSH, adjusting for age, sex, and BMI, which are strongly associated with eGFR and the inflammatory biomarkers. We also used Spearman correlations to examine associations between the measures of As exposure (wAs, uAs-SG, and bAs) and GSH, GSSG, and EhGSH, adjusting for covariates that were associated with at least one measure of As exposure (p < 0.1) and at least one glutathione variable (p < 0.1) in bivariate correlations.
For our primary analysis, we used linear regression models to examine the effect of the As exposure variables, wAs, uAs-SG, and bAs, on the outcomes CRP, AGP, and eGFR, controlling for potential confounders. We a priori decided to adjust for age, sex, and BMI in these models. Other covariates considered for inclusion were plasma folate, plasma B12, smoking, betel nut chewing, television ownership, education, systolic blood pressure, and diastolic blood pressure. Covariates were considered based on their Spearman correlations with the As exposure variables and the outcome variables, adjusted for age and sex. The control variables in the final models were those that were associated with CRP, AGP, or eGFR and with any one As exposure variable in bivariate correlations (p < 0.1), and resulted in an appreciable (>10%) change in the regression coefficient for the association between a predictor and an outcome. Variables with skewed distributions (wAs, bAs, uAs-SG, age, BMI, CRP, and AGP) were natural log transformed for linear regression analyses. To aid interpretation, for the models in which both the predictor of As exposure and the outcome (CRP or AGP) were log-transformed, we estimated the percentage change in the geometric mean of the outcome for a 10% increase in the As exposure variable. For the outcome of eGFR in its original scale, with log-transformed As variables as predictors, we estimated the mean change in eGFR for a 10% increase in the As exposure.
Effect modification by plasma EhGSH was examined by repeating the regression analyses in strata of plasma EhGSH (above and below the median). We used the Wald test to detect differences in the covariate-adjusted associations of As exposure with the outcome variables between plasma EhGSH strata. To explore whether the effect modification was dependent on the cut-point for EhGSH, we conducted a sensitivity analysis in which we repeated the linear regression analysis above and below every unique EhGSH cut-point, starting at the 20th percentile of EhGSH and ending at the 80th percentile, for a total of 224 cut-points. In the same manner we also checked effect modification by GSH and GSSG plasma concentrations.
All p values were two-sided. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA) and R version 3.0.2.
2. Results
Characteristics of the study sample are reported in Table 1 for the entire study sample and separately by plasma GSH redox potential (EhGSH) strata, which were derived by dividing plasma EhGSH at the median. On average, participants having a more oxidized plasma EhGSH (> −99.14) were older (p = 0.05), were more frequent betel nut chewers (p = 0.02), and had higher plasma B12 (p = 0.06), lower eGFR (p = 0.04), and higher systolic (p = 0.06) and diastolic blood pressure (p = 0.02) than participants having a less oxidized plasma EhGSH (≤ −99.14). Of these factors, only age and plasma B12 were significantly independent predictors of continuous plasma EhGSH, together explaining 5.5% of the variation in EhGSH.
Table 1.
General characteristics of the study population.
| Total sample (N = 374; mean±SD or %) | Plasma EhGSH ≤ −99.14 (N = 187)
|
Plasma EhGSH > −99.14 (N = 187)
|
pa | |||
|---|---|---|---|---|---|---|
| Mean±SD or % | Range | Mean±SD or % | Range | |||
| Age | 43±8 | 42±8 | 30, 60 | 44±9 | 31, 63 | 0.05 |
| Male (%) | 48.4 | 44.4 | 52.4 | 0.12 | ||
| BMI (kg/m2) | 20.4±3.5 | 20.2±3.3 | 14.2, 31.9 | 20.7±3.7 | 13.8, 35.3 | 0.21 |
| Education (years) | 3.4±3.6 | 3.2±3.3 | 0, 16 | 3.6±3.9 | 0, 14 | 0.79 |
| Smokers (ever) (%) | 36.1 | 35.3 | 36.9 | 0.75 | ||
| Betel nut use (ever) (%) | 42.2 | 36.4 | 48.1 | 0.02 | ||
| Own TV (%) | 58.3 | 55.1 | 61.5 | 0.21 | ||
| Water arsenic (μg/L) | 138±124 | 146±132 | 0.4, 700 | 130±115 | 0.4, 457 | 0.32 |
| Urinary As (μg/L) | 205±236 | 201±219 | 3.7, 1340 | 210±253 | 2.3, 1800 | 0.79 |
| Urinary As (SG adjusted) | 232±205 | 250±233 | 13, 1088 | 215±171 | 13, 742 | 0.45 |
| Blood arsenic (μg/L) | 13±10 | 14±10 | 1.6, 57 | 13±9.1 | 1.2, 44 | 0.56 |
| Plasma folateb (nmol/L) | 13±7.2 | 13±7.9 | 2.4, 61 | 13±6.5 | 3.4, 48 | 0.42 |
| Plasma B12c (pmol/L) | 203±113 | 190±89 | 47, 490 | 216±132 | 44, 1183 | 0.06 |
| Plasma tHcysd (μmol/L) | 11±13 | 12±16 | 3, 170 | 11±10 | 3, 120 | 0.39 |
| Plasma cystatin C (ng/ml) | 906±258 | 871±224 | 454, 1800 | 941±284 | 447, 2000 | 0.04 |
| eGFR (ml/min/1.73 m2) | 94±25 | 97±23 | 35, 144 | 91±26 | 32, 142 | 0.04 |
| Proteinuriae (%) | 1.6 | 2.2 | 1.1 | 0.43 | ||
| Systolic blood pressuree (mm Hg) | 108±14 | 107±11 | 80, 140 | 110±15 | 83, 165 | 0.06 |
| Diastolic blood pressuree (mm Hg) | 72±9 | 71±8 | 48, 98 | 73±9 | 50, 100 | 0.02 |
| Plasma C-reactive protein (mg/L) | 2.3±4.4 | 2.6±5.4 | 0.036, 46 | 2.0±3.3 | 0.017, 32 | 0.50 |
| Plasma α-1 acid glycoprotein (μg/ml) | 558±192 | 541±180 | 233, 1570 | 575±203 | 205, 1340 | 0.09 |
| Plasma GSH (μmol/L) | 2.6±0.7 | 3.1±0.6 | 2.0, 5.5 | 2.1±0.5 | 1.0, 3.4 | <0.0001 |
| Plasma GSSG (μmol/L) | 2.1±0.6 | 2.0±0.6 | 0.8, 4.7 | 2.2±0.6 | 1.0, 4.0 | 0.001 |
| Plasma EhGSH (mV) | −98.7±7.3 | −104.2±3.9 | −116.1, −99.2 | −93.1±5.1 | −99.1, −73.7 | <0.0001 |
p for difference between EhGSH strata from Wilcoxon rank-sum test for continuous variables and χ2 test for categorical variables.
N=373.
N=367.
Total homocysteine.
N=368.
We calculated Spearman correlations between plasma CRP, plasma AGP, eGFR, plasma GSH, GSSG, and EhGSH, adjusting for age, sex, and BMI. CRP and AGP were positively correlated with each other (r = 0.42, p < 0.0001), and AGP was negatively correlated with eGFR (r = −0.16, p = 0.002). CRP was negatively associated with eGFR as well; however, this correlation was not significant (r = −0.07, p = 0.21). Plasma GSH, GSSG, and EhGSH were not correlated with CRP, AGP, or eGFR (|r| ≤ 0.05, p ≥ 0.37). Additionally, plasma GSH, GSSG, and EhGSH were not significantly correlated with any of the As exposure measures (|r| ≤ 0.08, p ≥ 0.13), adjusting for sex, BMI, smoking, and betel nut use.
Water As, uAs-SG, and bAs were all positive predictors of plasma CRP, adjusting for age, sex, and BMI (Table 2). A 10% increase in wAs, uAs-SG, or bAs was associated with a 0.74% (p = 0.01), 0.90% (p = 0.16), and 1.39% (p = 0.07) increase in CRP, respectively. The As exposure measures were not significant predictors of plasma AGP. We observed that uAs-SG and bAs were associated with decreased eGFR, adjusting for age, sex, BMI, and smoking, although these associations did not reach statistical significance at p < 0.05; wAs was not associated with eGFR. A 10% increase in uAs-SG or bAs was associated with an average reduction in eGFR of 0.16 ml/min/1.73 m2 (p = 0.12) and 0.21 ml/min/1.73 m2 (p = 0.08), respectively. Adjustment for CRP attenuated the effect of uAs-SG on eGFR by 4.0% and the effect of bAs on eGFR by 4.2%. Adjustment for AGP did not attenuate the effect of uAs-SG or bAs on eGFR.
Table 2.
Covariate adjusted effect-size estimates for associations between measures of As exposure and CRP, AGP, and eGFR, in the total sample and stratified by plasma EhGSH.
| Predictor | CRPa
|
AGPa
|
eGFRb
|
ΔR2 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percentage change (95% CI) | p | ΔR2 (%)c | Percentage change (95% CI) | p | ΔR2 (%) | Mean change (95% CI) | p | ||
| Total sample (N = 374) | |||||||||
| Water As | 0.74 (0.16, 1.32) | 0.01 | 1.44 | −0.01 (−0.16, 0.14) | 0.86 | 0.01 | −0.03 (−0.12, 0.07) | 0.56 | 0.06 |
| Urinary As-SGd | 0.90 (−0.37, 2.19) | 0.16 | 0.45 | −0.04 (−0.37, 0.29) | 0.82 | 0.01 | −0.16 (−0.37, 0.04) | 0.12 | 0.42 |
| Blood As | 1.39 (−0.10, 2.90) | 0.07 | 0.77 | −0.12 (−0.50, 0.27) | 0.55 | 0.09 | −0.21 (−0.45, 0.03) | 0.08 | 0.54 |
| EhGSH ≤ −99.14 mV (N = 187) | |||||||||
| Water As | 0.17 (−0.71, 1.04) | 0.71 | 0.06 | −0.06 (−0.27, 0.14) | 0.55 | 0.18 | −0.05 (−0.19, 0.09) | 0.47 | 0.22 |
| Urinary As-SG | −0.52 (−2.33, 1.31) | 0.57 | 0.14 | −0.26 (−0.69, 0.17) | 0.24 | 0.69 | −0.10 (−0.38, 0.19) | 0.50 | 0.19 |
| Blood As | −0.25 (−2.42, 1.96) | 0.82 | 0.02 | −0.35 (−0.87, 0.16) | 0.18 | 0.91 | −0.16 (−0.50, 0.18) | 0.35 | 0.36 |
| EhGSH > −99.14 mV (N = 187) | |||||||||
| Water As | 1.13 (0.37, 1.90) | 0.004 | 3.93 | 0.03 (−0.20, 0.25) | 0.82 | 0.03 | −0.02 (−0.15, 0.11) | 0.80 | 0.02 |
| Urinary As-SG | 2.31 (0.55, 4.10) | 0.01 | 3.10 | 0.19 (−0.32, 0.71) | 0.46 | 0.28 | −0.26 (−0.56, 0.04) | 0.09 | 0.92 |
| Blood As | 2.76 (0.75, 4.80) | 0.007 | 3.38 | 0.09 (−0.49, 0.68) | 0.75 | 0.05 | −0.31 (−0.65, 0.03) | 0.07 | 1.01 |
Models with the outcomes CRP and AGP are adjusted for log(age), sex, and log(BMI); predictor parameters are expressed as the expected % change in the geometric mean of CRP or AGP for a 10% increase in exposure.
Models with the outcome eGFR are adjusted for log(age), sex, log(BMI), and ever smoking; predictor parameters are expressed as the expected mean change in eGFR (ml/min/1.73 m2) for a 10% increase in exposure.
The change in R2 after adding As exposure variable to model.
Urinary As adjusted for specific gravity.
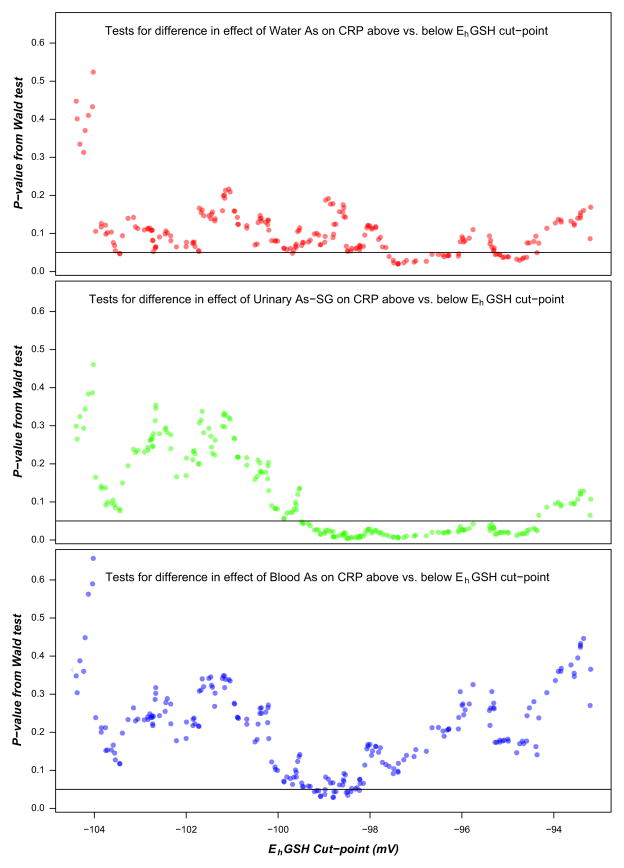
To examine modification of the effect of As exposure on CRP by plasma EhGSH, we repeated the regression analyses within strata of plasma EhGSH above or below the median (EhGSH = −99.14), representing participants having relatively more or less oxidized plasma redox status, respectively (Table 2). The effect of As exposure on CRP was only significant in the higher EhGSH strata, in which a 10% increase in wAs, uAs-SG, or bAs was associated with a 1.13% (p = 0.004), 2.31% (p = 0.01), and 2.76% (p = 0.007) increase in CRP, respectively. The regression coefficient estimates for the effect of As exposure on CRP differed between the EhGSH strata by the Wald test, particularly for uAs-SG (p = 0.03) and bAs (p = 0.05) and less so for wAs (p = 0.10). In a sensitivity analysis, we examined the effect of As exposure on CRP in participants above and below EhGSH cut-points ranging from the 20th to the 80th percentile of plasma EhGSH. We found that As exposure was associated with increased CRP in groups of participants having relatively more oxidized plasma redox status (EhGSH > cut-point), with regression coefficients and 95% CIs above zero for a wide range of EhGSH cut-points (Fig. 1). In contrast, As exposure was not associated with CRP in groups of participants having relatively less oxidized plasma redox status (EhGSH < cut-point); the regression coefficients had estimates around zero and the 95% CIs included zero for all EhGSH cut-points examined. Additionally, we found that there was a range of EhGSH cut-points with small p values from the Wald test, indicating significant differences in the effect of As exposure on CRP between groups of participants having relatively more and less oxidized plasma redox status defined by these cut-points (Fig. 2). The range of cut-points resulting in significant group differences varied by the measure of As exposure, with uAs-SG having more cut-points with small p values.
Fig. 1.
Plots of the estimated regression coefficients (B) and 95% CIs for the effect of As exposure (top, water As; middle, urinary As-SG; bottom, blood As) on log(CRP) in more and less oxidized groups, defined by different plasma EhGSH cut-points. All models were adjusted for log(age), log(BMI), and sex.
Fig. 2.
Plots of p values from Wald tests for the difference in the effect of log(water As) (top), log(urinary As-SG) (middle), or log(blood As) (bottom) on log(CRP) between more and less oxidized groups, defined by different plasma EhGSH cut-points. The effect of As exposure was the regression coefficient of the As variables adjusted for log (age), log(BMI), and sex.
We also found that plasma GSH concentrations modified the effect of As exposure on CRP; As exposure was associated with increased CRP (95% CIs above zero) only in participants with relatively lower plasma GSH, and there were significant differences in the effect of As exposure on CRP between plasma GSH strata by the Wald test for a wide range of plasma GSH cut-points (data not shown). Plasma GSSG concentration did not significantly modify the effect of As exposure on CRP, although significant positive associations between As exposure and CRP were observed only in participants with relatively higher plasma GSSG (data not shown).
We did not find any effect of As exposure on AGP within EhGSH strata, defined by the median EhGSH (Table 2) or by any other EhGSH cut-point, nor did we find any effect of As exposure on AGP in strata defined by plasma GSH or GSSG concentrations (data not shown). For the outcome of eGFR, the negative effects of uAs-SG and bAs (but not wAs) tended to be larger in magnitude in participants with a more oxidized plasma EhGSH (> median), compared to participants with a less oxidized EhGSH (< median), with marginally significant regression coefficients for uAs-SG and bAs in the high EhGSH strata (Table 2). However, the regression coefficients for the effect of As exposure on eGFR did not differ significantly between EhGSH strata by the Wald test. Consistent results were seen in the EhGSH cut-point sensitivity analysis for eGFR (data not shown). Likewise, we did not observe significant modification of the effect of As exposure on eGFR when stratifying by plasma GSH or GSSG concentrations (data not shown).
3. Discussion
In this study of Bangladeshi adults chronically exposed to a wide range of As concentrations through drinking water, As exposure was associated with increased plasma CRP, particularly in participants with lower plasma GSH or a relatively more oxidized plasma EhGSH, but had no effect on plasma AGP. Our finding that As exposure was associated with increased CRP is in agreement with another study in Bangladesh [12] and also supports the recent evidence that As exposure is associated with risk for CVD [37], as CRP is a strong predictor of CVD. The finding that As exposure is associated with increased CRP in a relatively healthy population has many future health implications, as CRP is probably not only a biomarker of inflammation, but an active agent in disease pathogenesis, as has been observed for atherosclerosis [38] and insulin-related diseases [39].
CRP and AGP are both acute-phase proteins, and it is not clear why we did not observe an effect of As exposure on AGP. A potential explanation lies in the different transcription factors required for CRP and AGP activation during the acute-phase response. NF-κB family proteins, STAT3, and C/EBPβ bind to the CRP promoter to activate CRP transcription in response to IL-6 and IL-1β [40,41], whereas AGP expression in response to IL-6 involves binding of C/EBPβ and the glucocorticoid receptor to the AGP promoter [42]. Because arsenite can bind to and inhibit the glucocorticoid receptor [43], this could potentially be a reason we observe no net effect of As exposure on plasma AGP. Alternatively, the explanation may relate to differences in magnitude and duration of CRP and AGP expression during the acute-phase response. Plasma CRP increases 1000- to 10,000-fold quickly and relatively briefly after inflammatory insult, whereas AGP increases 2- to 5-fold and remains elevated longer than CRP [44]. It is possible that in a situation of chronic exposure to As, CRP is more apt to reflect the effect of variation in As exposure than AGP. Also, AGP has a much smaller range than CRP, which may hinder detection of an association.
Arsenic has been suggested to cause oxidative stress through several different pathways, including (a) increasing ROS within tissues via various proposed mechanisms [14], (b) binding to thiol groups and inhibiting regulatory proteins [45], or (c) binding directly to GSH. In pathway (a), ROS-scavenging antioxidants (e.g., vitamin C, vitamin E) would protect against the effect of As; in pathways (b) and (c), thiol-containing antioxidants (e.g., GSH, NAC, α-lipoic acid) would be necessary to increase thiol status and protect against the effect of As. Arsenic exposure has not been directly associated with oxidative stress in the FOX study [27–29], and we confirm here that As exposure is not associated with plasma GSH biomarkers. This observation is consistent with a recent study in mini pigs showing that plasma GSH biomarkers were unresponsive to oxidative stress induced by lipopolysaccharide exposure [46]. However, plasma GSH biomarkers may still reflect inherent interindividual variability in the pro-oxidant to antioxidant balance of tissues [30]. This interindividual variability may result from inheritance [47], age [48], diet [49], and other factors unrelated to As exposure. We hypothesized that participants having a more oxidized plasma EhGSH would be at greater risk for As-induced inflammation. Indeed, we observed here that the As-induced increase in plasma CRP was apparent only in participants having relatively lower plasma GSH or a relatively more oxidized EhGSH. The observations that plasma GSH and EhGSH modify the effect of As exposure on CRP may suggest that higher GSH and/or greater antioxidant capacity are important in protecting against As-induced inflammation.
To our knowledge, the specific mechanism for induction of CRP expression by As has been investigated in only one study by Druwe et al. [8]. In that study, low-level arsenite exposure to HepG2 cells increased CRP expression and secretion, whereas ROS remained unchanged [8], suggesting that As can induce CRP independent of ROS. As-induced activation of NF-κB, a transcription factor involved in CRP expression [41], was prevented by pretreatment with NAC in cultured aortic endothelial cells, suggesting that oxidation of thiols was responsible for the activation of NF-κB [19]. Other transcription factors involved in CRP expression, including C/EBPβ and STAT3 [40], may also be activated by As [50,51]. Because the redox environment can modulate activation of NF-κB [52], STAT3 [53,54], and possibly C/EBPβ [55], it is possible that in a low GSH/more oxidized redox environment, As-induced redox changes mediate the effect of As on CRP, in accordance with our results. Additional research is necessary to determine the exact mechanism by which As may induce CRP expression and whether the effect of As on CRP can be inhibited by ROS-scavenging and/or thiol-containing antioxidants.
Alternatively, our findings may relate to the involvement of GSH in biliary excretion of As. Arsenite and monomethylarsonous acid form conjugates with GSH (AsIII(GS)3 and MMAIII(GS)2), which are excreted in the bile of rats via the multidrug-resistant protein efflux transporters [56]. Additionally, arsenite and selenite enhance each other’s elimination by forming a complex with GSH ([(GS)2AsSe]−) which is excreted in bile [56]. Whereas current understanding of the involvement of GSH in As excretion in humans is limited, it is possible that participants with lower GSH may have impaired biliary As elimination, resulting in greater susceptibility to As-induced inflammation. However, the primary route of As excretion is through urine, so the magnitude of such an effect is unknown.
In the present study, As exposure, as measured by As in urine and blood, was associated with reduced eGFR, although these associations did not reach statistical significance at p < 0.05. The finding that uAs, but not wAs, was negatively associated with eGFR is consistent with our previous study [4] and with other studies that have observed negative associations between uAs and eGFR [57,58]. The lack of a significant effect of As exposure on eGFR in the current study may be related to the study design, as individuals with known CKD, CVD, or diabetes were excluded from participation; these exclusion criteria would reduce the variation in eGFR in the study population. Although adjustment for CRP resulted in a very small attenuation (<5%) of the negative effect of uAs-SG and bAs on eGFR, we cannot rule out the possibility that As-induced inflammation may mediate the effects of As on renal function, because (a) the effect of As exposure on eGFR in this study was very small and (b) plasma CRP is a systemic marker of inflammation largely produced in the liver and, as such, may not specifically reflect inflammation within other tissues. Although we tested here whether inflammation may be on the causal pathway from As exposure to renal dysfunction, it is important to note that inflammation may be positively associated with cystatin C, our biomarker of GFR, independent of actual GFR [59,60]. As such, we cannot distinguish in this study whether inflammation and cystatin C-based eGFR are related through an inflammation-induced decrease in GFR or through a mechanism independent of actual GFR.
Although the negative effect of As exposure on eGFR was larger in magnitude in participants with a more oxidized plasma EhGSH, the effect of As exposure on eGFR did not significantly differ between low and high plasma EhGSH strata. Antioxidants are protective against As-induced renal injury and dysfunction in several animal studies [23–26]. In a human study, low levels of the antioxidant lycopene combined with high uAs were associated with increased odds of CKD, above the odds associated with either factor alone [58]. The mechanism for As-induced renal dysfunction may involve oxidative stress, however, we may not have had sufficient power to detect this in the current study. It is also possible that other biomarkers of renal injury, such as markers of proximal tubule damage, may be more appropriate for assessing As-induced nephrotoxicity.
4. Conclusions
In summary, we observed that As exposure was associated with increased CRP. Upon stratifying our analyses by plasma EhGSH or GSH, we observed that the As-induced increase in CRP was apparent only in participants with a relatively more oxidized EhGSH or a lower GSH concentration, suggesting that increased GSH or a higher antioxidant to pro-oxidant balance may protect against As-induced inflammation. Our conclusions are limited by the cross-sectional nature of this study, as we cannot rule out reverse causality or account for potential biases due to unmeasured confounding. Additionally, the EhGSH strata differed on variables such as age and plasma B12 and probably differ on other unmeasured variables. Therefore, our EhGSH strata may represent more than antioxidant capacity alone (although the majority of variation in EhGSH in this study was not explained by measured variables). Randomized controlled trials in As-exposed humans are necessary to confirm the potential benefit of antioxidants against As-induced inflammation. Because inflammation is involved in the pathogenesis of many diseases, antioxidant treatment may represent a viable option for preventing As-induced diseases.
Highlights.
Arsenic exposure may cause oxidative stress, inflammation, and renal dysfunction.
We analyzed plasma GSH, GSSG, CRP, AGP, and cystatin C in 374 As-exposed adults.
Arsenic exposure was associated with significantly increased CRP.
Plasma GSH and EhGSH significantly modified the effect of arsenic on CRP.
Higher GSH/lower EhGSH may protect against As-induced inflammation.
Acknowledgments
This work was supported by funding from NIH Grants R01CA133595, R01ES017875, P42ES010349, P30ES009089, and T32ES007322.
Abbreviations
- AGP
α-1 acid glycoprotein
- bAs
blood arsenic
- BMI
body mass index
- CKD
chronic kidney disease
- CRP
C-reactive protein
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- EhGSH
reduction potential of the plasma GSSG/2GSH redox pair
- FOX
Folate and Oxidative Stress study
- GFR
glomerular filtration rate
- GSH
glutathione
- GSSG
glutathione disulfide
- NF-κB
nuclear factor κB
- ROS
reactive oxygen species
- uAs
urinary arsenic
- uAs-SG
urinary arsenic adjusted for specific gravity
- wAs
water arsenic
References
- 1.World Health Organization. Guidelines for Drinking-Water Quality, Vol. 1, Recommendations. incorporating first and second addenda. 3. Geneva: WHO Press; 2008. [PubMed] [Google Scholar]
- 2.National Research Council. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. Washington, DC: National Academies Press; 2013. [Google Scholar]
- 3.Zheng L, Kuo CC, Fadrowski J, Agnew J, Weaver V, Navas-Acien A. Arsenic and chronic kidney disease: a systematic review. Curr Environ Health Rep. 2014;1:192–207. doi: 10.1007/s40572-014-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters BA, Hall MN, Liu X, Neugut YD, Pilsner JR, Levy D, Ilievski V, Slavkovich V, Islam T, Factor-Litvak P, Graziano JH, Gamble MV. Creatinine, arsenic metabolism, and renal function in an arsenic-exposed population in Bangladesh. PLoS One. 2014;9:e113760. doi: 10.1371/journal.pone.0113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immunity. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 6.Leemans JC, Kors L, Anders HJ, Florquin S. Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol. 2014;10:398–414. doi: 10.1038/nrneph.2014.91. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druwe IL, Sollome JJ, Sanchez-Soria P, Hardwick RN, Camenisch TD, Vaillancourt RR. Arsenite activates NFkappaB through induction of C-reactive protein. Toxicol Appl Pharmacol. 2012;261:263–270. doi: 10.1016/j.taap.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Kumar D, Lal K, Raisuddin S, Sahu AP. Adverse health effects due to arsenic exposure: modification by dietary supplementation of jaggery in mice. Toxicol Appl Pharmacol. 2010;242:247–255. doi: 10.1016/j.taap.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 11.Das N, Paul S, Chatterjee D, Banerjee N, Majumder NS, Sarma N, Sau TJ, Basu S, Banerjee S, Majumder P, Bandyopadhyay AK, States JC, Giri AK. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health. 2012;12:639. doi: 10.1186/1471-2458-12-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim MR, Rahman M, Islam K, Mamun AA, Hossain S, Hossain E, Aziz A, Yeasmin F, Agarwal S, Hossain MI, Saud ZA, Nikkon F, Hossain M, Mandal A, Jenkins RO, Haris PI, Miyataka H, Himeno S, Hossain K. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci. 2013;135:17–25. doi: 10.1093/toxsci/kft130. [DOI] [PubMed] [Google Scholar]
- 13.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 15.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 17.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflammation. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-κB activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 20.Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol. 2005;19:67–77. doi: 10.1002/jbt.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Cai JF, Chiu JF. Arsenic induces oxidative stress and activates stress gene expressions in cultured lung epithelial cells. J Cell Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- 22.Roy A, Manna P, Sil PC. Prophylactic role of taurine on arsenic mediated oxidative renal dysfunction via MAPKs/NF-kappaB and mitochondria dependent pathways. Free Radic Res. 2009;43:995–1007. doi: 10.1080/10715760903164998. [DOI] [PubMed] [Google Scholar]
- 23.Bera AK, Rana T, Das S, Bhattacharya D, Pan D, Bandyopadhyay S, Das SK. Mitigation of arsenic-mediated renal oxidative stress in rat by Pleurotus florida lectin. Hum Exp Toxicol. 2011;30:940–951. doi: 10.1177/0960327110384521. [DOI] [PubMed] [Google Scholar]
- 24.Ince S, Kucukkurt I, Turkmen R, Demirel HH, Sever E. Dietary Yucca schidigera supplementation reduces arsenic-induced oxidative stress in Swiss albino mice. Toxicol Ind Health. 2013;29:904–914. doi: 10.1177/0748233712446730. [DOI] [PubMed] [Google Scholar]
- 25.Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep. 2012;39:11201–11216. doi: 10.1007/s11033-012-2029-6. [DOI] [PubMed] [Google Scholar]
- 26.Sinha M, Manna P, Sil PC. Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology. 2008;15:147–156. doi: 10.1016/j.pathophys.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, Ilievski V, Levy D, Siddique AB, Parvez F, Mey JL, van Geen A, Graziano J, Gamble MV. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect. 2013;121:1068–1074. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedzwiecki MM, Hall MN, Liu X, Slavkovich V, Ilievski V, Levy D, Alam S, Siddique AB, Parvez F, Graziano JH, Gamble MV. Interaction of plasma glutathione redox and folate deficiency on arsenic methylation capacity in Bangladeshi adults. Free Radic Biol Med. 2014;73:67–74. doi: 10.1016/j.freeradbiomed.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper KN, Liu X, Hall MN, Ilievski V, Oka J, Calancie L, Slavkovich V, Levy D, Siddique A, Alam S, Mey JL, van Geen A, Graziano JH, Gamble MV. A dose–response study of arsenic exposure and markers of oxidative damage in Bangladesh. J Occup Environ Med. 2014;56:652–658. doi: 10.1097/JOM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 32.Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, Graziano J. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225:225–233. doi: 10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma: evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–292. [PubMed] [Google Scholar]
- 36.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS Investigators C-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, Ahmed A, Argos M, Islam T, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, Graziano J, Ahsan H. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121:832–838. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paffen E, DeMaat MP. C-reactive protein in atherosclerosis: a causal factor? Cardiovasc Res. 2006;71:30–39. doi: 10.1016/j.cardiores.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Pravenec M, Kajiya T, Zidek V, Landa V, Mlejnek P, Simakova M, Silhavy J, Malinska H, Oliyarnyk O, Kazdova L, Fan J, Wang J, Kurtz TW. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension. 2011;57:731–737. doi: 10.1161/HYPERTENSIONAHA.110.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young DP, Kushner I, Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J Immunol (Baltimore, Md 1950) 2008;181:2420–2427. doi: 10.4049/jimmunol.181.4.2420. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-kappaB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3. Immunology. 2003;108:539–547. doi: 10.1046/j.1365-2567.2003.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam T, Mifflin MR, Hsieh RC, Ge CC, Papaconstantinou XJ. Transactivation of the alpha 1-acid glycoprotein gene acute phase responsive element by multiple isoforms of C/EBP and glucocorticoid receptor. J Biol Chem. 1993;268:15681–15688. [PubMed] [Google Scholar]
- 43.Kaltreider RC, Davis AM, Lariviere JP, Hamilton JW. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ Health Perspect. 2001;109:245–251. doi: 10.1289/ehp.01109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. doi: 10.1258/acb.2007.007167. [DOI] [PubMed] [Google Scholar]
- 45.Kitchin KT, Wallace K. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J Inorg Biochem. 2008;102:532–539. doi: 10.1016/j.jinorgbio.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Kadiiska MB, Peddada S, Herbert RA, Basu S, Hensley K, Jones DP, Hatch GE, Mason RP. Biomarkers of oxidative stress study VI. Endogenous plasma antioxidants fail as useful biomarkers of endotoxin-induced oxidative stress. Free Radic Biol Med. 2015;81:100–106. doi: 10.1016/j.freeradbiomed.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van’t Erve TJ, Wagner BA, Ryckman KK, Raife TJ, Buettner GR. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic Biol Med. 2013;65:742–749. doi: 10.1016/j.freeradbiomed.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 49.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88:1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L, Stringfield TM, Shi X, Chen Y. Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem J. 2005;392:93–102. doi: 10.1042/BJ20050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong CH, Lee CH, Chen GS, Chang KL, Yu HS. STAT3-dependent VEGF production from keratinocytes abrogates dendritic cell activation and migration by arsenic: a plausible regional mechanism of immunosuppression in arsenical cancers. Chem-Biol Interact. 2015;227:96–103. doi: 10.1016/j.cbi.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signaling. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Cheung SH, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70:8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 55.Kim JW, Tang QQ, Li X, Lane MD. Effect of phosphorylation and S–S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc Natl Acad Sci USA. 2007;104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leslie EM. Arsenic–glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs) J Inorg Biochem. 2012;108:141–149. doi: 10.1016/j.jinorgbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 57.Chen JW, Chen HY, Li WF, Liou SH, Chen CJ, Wu JH, Wang SL. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere. 2011;84:17–24. doi: 10.1016/j.chemosphere.2011.02.091. [DOI] [PubMed] [Google Scholar]
- 58.Hsueh YM, Chung CJ, Shiue HS, Chen JB, Chiang SS, Yang MH, Tai CW, Su CT. Urinary arsenic species and CKD in a Taiwanese population: a case–control study. Am J Kidney Dis. 2009;54:859–870. doi: 10.1053/j.ajkd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grubb AO. Cystatin C—properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]