Abstract
Glioblastoma multiforme (GBM) is notoriously resistant to therapy, and the development of a durable cure will require the identification of broadly relevant regulators of GBM cell tumorigenicity and survival. Here, we identify Sprouty2 (SPRY2), a known regulator of receptor tyrosine kinases (RTKs), as one such regulator. SPRY2 knockdown reduced proliferation and anchorage-independent growth in GBM cells and slowed xenograft tumor growth in mice. SPRY2 knockdown also promoted cell death in response to co-inhibition of the epidermal growth factor receptor (EGFR) and the c-MET receptor in GBM cells, an effect that involved regulation of the ability of the p38 mitogen activated protein kinase (MAPK) to drive cell death in response to inhibitors. Analysis of clinical tumor specimens further demonstrated that SPRY2 protein is definitively expressed in GBM tissue, that SPRY2 expression is elevated in GBM tumors expressing EGFR variant III (EGFRvIII), and that elevated SPRY2 mRNA expression portends reduced GBM patient survival. Overall, these results identify SPRY2 and the pathways it regulates as novel candidate biomarkers and therapeutic targets in GBM.
Implications: SPRY2, counter to its roles in other cancer settings, promotes glioma cell and tumor growth and cellular resistance to targeted inhibitors of oncogenic RTKs; thus, making SPRY2 and the cell signaling processes it regulates potential novel therapeutic targets in glioma.
Keywords: epidermal growth factor receptor, c-MET receptor, p38 mitogen activated protein kinase, gefitinib
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common adult malignancy of the brain (1). Standard treatment includes surgical resection, radiotherapy, and chemotherapy, resulting in an average survival of just 12–15 months (2). Targeted approaches based on the molecular pathogenesis of GBM have shown some promise in clinical trials but have thus far failed to demonstrate significant benefit over traditional treatment approaches (3, 4). Significant challenges limiting the effectiveness of available treatments are the diffuse spreading of GBM tumors and heterogeneity in the expression of oncogenic proteins among GBM tumor cells (5, 6). Identification of broadly relevant regulators of tumorigenicity and therapeutic resistance is therefore necessary to improve patient outcomes. Here, we identify Sprouty2 (SPRY2) as one such protein.
SPRY2 is a regulator of receptor tyrosine kinase signaling whose most well-characterized role is regulation of extracellular signal-regulated kinase (ERK) activity (7) and whose expression is promoted by ERK activity (8, 9). Studies in different systems have demonstrated varied, and sometimes conflicting, roles for SPRY2. SPRY2 inhibits ERK activity downstream of several receptor tyrosine kinases through regulation of RAS, by preventing growth factor receptor-bound protein 2 (GRB2)-son of sevenless (SOS) binding (10) or by preventing RAS activation downstream of GRB2-SOS (11), or through regulation of RAF (7), depending on the cellular context. In contrast, SPRY2 has also been reported to potentiate epidermal growth factor (EGF)-induced ERK activation by interfering with CBL-mediated EGF receptor (EGFR) downregulation (12). Downstream of the fibroblast growth factor receptor, SPRY2 specifically inhibits ERK activation without effect on p38 or JUN N-terminal kinase (JNK) activation (7), two other members of the mitogen-activated protein (MAP) kinase family. However, results showing that interferon-stimulated p38 phosphorylation is enhanced in SPRY1/SPRY2/SPRY4 knockout murine embryonic fibroblasts (13) could indicate an ability for SPRY2 (or other Sprouty family members) to regulate other MAP kinases more broadly.
SPRY2 expression has been reported to be reduced in cancers of the breast, prostate, lung, and liver compared to normal tissues, and SPRY2 expression can suppress the proliferative and tumorigenic capacity of hepatocellular carcinoma and non-small cell lung cancer cell lines (14–16). These and other findings have led some investigators to propose SPRY2 as a tumor suppressor. However, as with its signaling regulatory functions, SPRY2 expression patterns and SPRY2-mediated control of key phenotypes also appear to be context dependent. In colon cancer cells, for example, SPRY2 expression appears to be elevated compared to normal tissue and to promote metastasis (17). In terms of regulating response to therapy, SPRY2 expression promotes response to EGFR inhibition in colon cancer cells (18) but drives resistance to EGFR inhibition in non-small cell lung cancer cell lines (19). In GBM, little has been done to investigate the functional role of SPRY2. There has been one report of SPRY2 downregulation via microRNA-21 expression in high grade gliomas (20). As will be shown here, however, SPRY2 protein is expressed at functionally meaningful levels in glioblastoma primary tumor samples and cell lines.
Here, we investigated the function and expression of SPRY2 in GBM cell lines, tumor xenografts, and human tumor samples. In a panel of GBM cell lines, SPRY2 knockdown reduced proliferation, antagonized colony formation in soft agar, and potentiated response to co-inhibition of EGFR and c-MET (the receptor for hepatocyte growth factor). In some cell lines, SPRY2 control of cell death response to inhibitors involved regulation of the ability of p38, which was phosphorylated in response to EGFR and c-MET co-inhibition, to promote cell death. In a mouse tumor xenograft model, SPRY2 knockdown significantly impaired tumor growth, confirming the relevance of SPRY2 in vivo. Analysis of gene expression from primary human tumor samples and rat tumor allografts revealed that SPRY2 mRNA is upregulated in EGFR variant III (EGFRvIII)-positive tumors compared to EGFRvIII-negative tumors. This is of interest because EGFRvIII is present in 41–67% of GBMs with EGFR amplification (21–23), promotes an invasive and proliferative GBM cell phenotype (24, 25), and is associated with especially poor prognosis for patients with EGFR amplification (26). Immunohistochemical analyses confirmed SPRY2 protein expression in human GBMs with or without EGFRvIII expression, with a tendency for greater SPRY2 expression in EGFRvIII-positive tumors. Analysis of SPRY2 protein from patient-derived xenograft and glioma stem cell samples demonstrated SPRY2 expression levels at least as high as those found in GBM cell lines where SPRY2 knockdown produced substantial phenotypic effects. Through an analysis of The Cancer Genome Atlas (TCGA) GBM data set, we further found that elevated SPRY2 expression portends reduced patient survival, regardless of EGFRvIII status. Overall, our study identifies SPRY2 and the pathways it regulates as potentially useful prognostic biomarkers and candidate therapeutic targets in GBM.
MATERIALS AND METHODS
Cell culture
U87MG and U373MG cells expressing EGFRvIII or kinase-dead EGFRvIII (27, 28) and parental U87MG cells were provided by Dr. Frank Furnari (UCSD, La Jolla, CA, USA). U251 and SF188 cells were gifts from Dr. Gary Kuo and Craig Thompson (University of Pennsylvania, Philadelphia, PA, USA), respectively. LN18 and U118MG cells were obtained from the American Type Culture Collection. All cells were maintained in DMEM supplemented with 10% fetal bovine serum, 1 mM L-Glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin (Life Technologies).
Knockdown of SPRY2, MKP-1, and MKP-5
Oligonucleotides encoding hairpins targeting nucleotides 2061-2079 (main sequence used; shRNA #1) or 1195-1213 (shRNA #2) of human SPRY2, nucleotides 2041-2059 of human MKP-1, or nucleotides 935-953 of human MKP-5 were purchased from IDT and inserted into pSicoR.puro (Dr. Tyler Jacks, MIT, Cambridge, MA, USA; (29)). A control shRNA was created using a hairpin that does not target a known human mRNA. Lentivirus was produced by calcium-phosphate-mediated transfection of 293FT cells (Life Technologies) with the pSicoR.puro plasmid as well as the pCMV-VSVg, pMDL-gp-RRE, and pRSV-Rev plasmids (Dr. Marilyn Farquhar, UCSD, La Jolla, CA, USA). Virus-containing supernatant was filtered and target cells were selected in 1–2 µg/mL puromycin (Sigma).
Retroviral protein expression
MEK2DD cDNA in the pBabe.puro vector was provided by Dr. Sylvain Meloche (Université de Montréal, Montreal, Quebec, Canada). Y55F SPRY2 cDNA (Dr. Dafna Bar-Sagi, NYU, New York, NY, USA) and MKK3 cDNA (Dr. Margaret Chou, University of Pennsylvania, Philadelphia, PA, USA) were sub-cloned into pBabe.hygro. For all constructs, retrovirus was produced by calcium phosphate-mediated transfection of amphotropic Phoenix cells (Dr. Gary Nolan, Stanford University, Stanford, CA, USA). Virus-containing supernatant was filtered, and target cells were selected in 150 µg/mL hygromycin B (Sigma) or 2 µg/mL puromycin (Sigma).
Mouse tumor xenografts
8 female NIH-III mice (Charles River) were subcutaneously injected in each flank with 2 million control (left side) or SPRY2-depleted (right side) U87MG-L cells. Tumors were measured with calipers starting 7 days after cell injection (when measurable tumors had formed) and every 2–3 days thereafter. Tumor volumes were calculated as π/6 × A × B2, where A and B are the larger and smaller tumor diameters, respectively. After final caliper measurements were made, animals were sacrificed and dissected tumors from the 6 animals bearing measurable control and SPRY2 knockdown tumors were weighed. Note that in 2 of the 8 animals injected with cells, SPRY2 knockdown cells failed to form apparent tumors; no tumors were dissected from these animals. All experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and performed in accordance with NIH guidelines.
Western blotting
Whole cell lysates were prepared and western blotting was performed as described previously (19). Details, including information on antibodies used, are provided in Supplemental Methods.
Flow cytometry
Floating and adherent cells were pooled and stained with ToPro3 (Life Technologies). Cells were analyzed using a Becton Dickinson FACS-Calibur cytometer.
Proliferation measurements
To measure cellular proliferation, cells were seeded at 50,000 cells per well in 6-well plates and counted with a hemocytometer 5 days later.
Inhibitors
Gefitinib, U0126, SB203580, SP600125 (all from LC Laboratories), and PHA665752 (Santa Cruz Biotechnology) were reconstituted in DMSO and added to cells in complete media.
Quantitative real-time PCR
RNA was extracted from cells using the RNeasy kit (Qiagen) with on-column DNase I digestion. Equal amounts of RNA were reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). PCR was performed using SYBR Green PCR Master Mix (Life Technologies) on an Applied Biosystems 7300 Real-Time PCR System. Relative amounts of mRNA were determined using the comparative CT method.
Anchorage-independent growth assay
5,000 cells were seeded per 35 mm dish in 0.3% low melting temperature agarose (Lonza) on top of a bottom layer of 0.6% agarose. Media was replaced every three days with or without inhibitors. At three weeks, plates were stained with 0.1% crystal violet (Sigma) and colonies were counted.
Immunohistochemistry
GBM sections were obtained from samples banked through the University of Pennsylvania Brain Tumor Tissue Bank, with the EGFRvIII status of individual tumors determined by PCR. Immunohistochemistry of formalin fixed and paraffin embedded tissues was performed on a Leica Bond instrument using the Bond Polymer Refine Detection System using the SPRY2 antibody. Heat-induced epitope retrieval was required for SPRY2 and done for 20 min with ER1 solution (Leica Microsystems).
Analysis of TCGA GBM data set
Methods for preprocessing of TCGA exon-array data and analysis to determine EGFRvIII status are described in Supplemental Methods. A two-sided student’s t-test was applied to determine whether SPRY2 expression varied among GBM subtypes and normal brain samples using TCGA exon-array data. A student’s t-test was also used to determine whether SPRY2 was differentially expressed between EGFRvIII-positive and EGFRvIII-negative samples based on TCGA RNA-seq samples. The upper quartile normalized RSEM (30) count estimates were base-10 log transformed before the t-test. The R package “survival” was used to analyze the TCGA survival data (31). The log-rank test was applied to test for differences between the survival curves.
Statistics
Data were analyzed by a student’s t-test, and differences with p-values < 0.05 were considered statistically significant.
Accession numbers
Microarray data presented in this study were deposited in the National Center for Biotechnology Information’s GEO database (GSE51062, GSE51147).
RESULTS
SPRY2 knockdown slows cellular proliferation, reduces anchorage-independent growth, and enhances cellular sensitivity to EGFR and c-MET co-inhibition
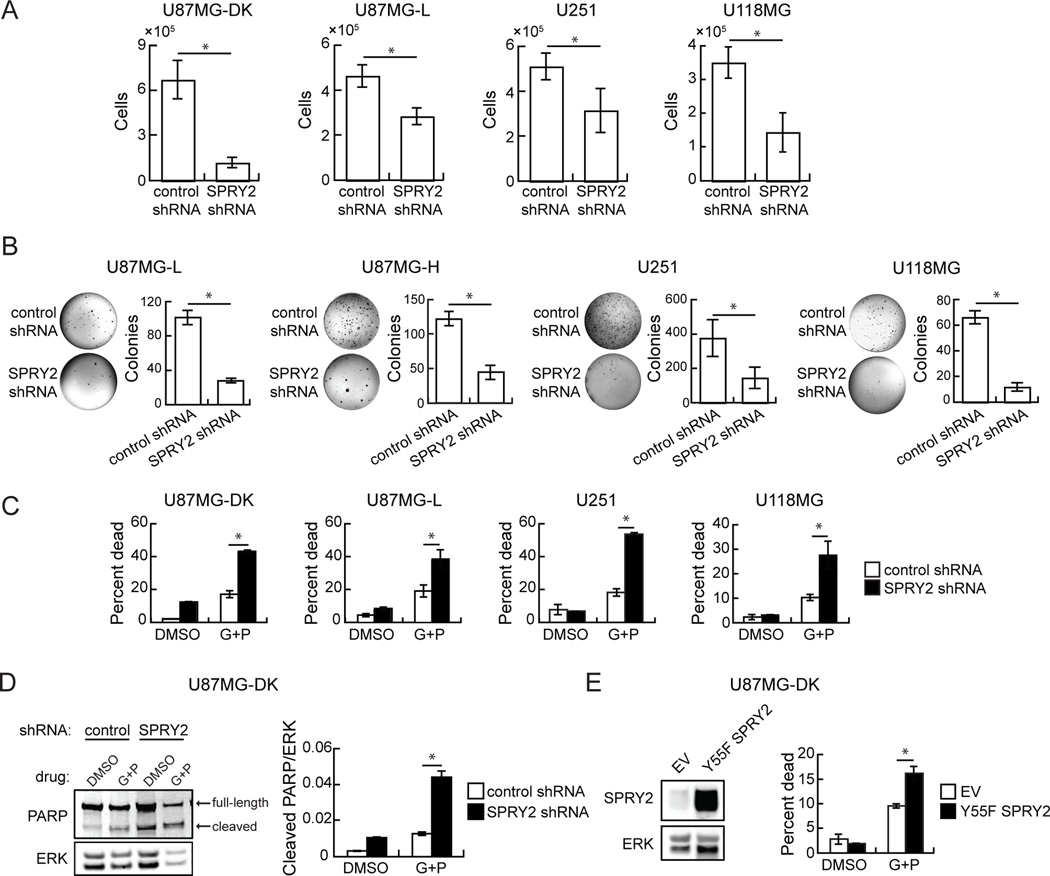
Using a set of common GBM cell lines, including U251, U118MG, and U87MG cells as well as U87MG cells engineered with “low” (U87MG-L, ~1×106 EGFR/cell) or “high” (U87MG-H, ~2×106 EGFR/cell) ectopic EGFRvIII expression or high expression of a control dead kinase EGFRvIII (U87MG-DK; K721M) (27), we sought to investigate the effects of SPRY2 expression on several cellular phenotypes. SPRY2 was depleted by stable shRNA expression in the cell lines, with knockdown validated by western blot (Supplemental Figure 1). SPRY2 knockdown reduced proliferation rates in U87MG-DK, U87MG-L, U251, and U118MG cells grown on tissue culture plastic (Figure 1A). Colony formation in soft agar was also reduced by >50% in U87MG-L, U87MG-H, U251, and U118MG cells with SPRY2 knockdown compared to controls (Figure 1B). A second independent shRNA expressed in a subset of the cell lines produced similar effects on proliferation and anchorage-independent growth (Supplemental Figure 2A,B). Reductions in colony formation were also observed in a panel of EGFRvIII-expressing cell lines transiently transfected with SPRY2 siRNA (Supplemental Figure 2C). Note that colony formation data is not available for U87MG-DK cells because that cell line does not form colonies in soft agar.
Figure 1. SPRY2 knockdown reduces cellular proliferation and anchorage-independent growth and enhances cellular sensitivity to EGFR and c-MET co-inhibition.
(A) Cellular proliferation and (B) colony formation in soft agar were measured with expression of control or SPRY2-targeting shRNA (shRNA #1) in a panel of GBM cell lines including U87MG cells expressing dead kinase (DK), low (L), or high (H) EGFRvIII. (C) The indicated cell lines expressing control or SPRY2-targeting shRNA (shRNA #1) were treated with DMSO or a combination of gefitinib and PHA665752 (G+P; 10 µM gefitinib + 5 µM PHA665752 for U87MG-DK, U87MG-L, U118MG; 10 µM gefitinib + 2 µM PHA665752 for U251) for 48 hrs prior to flow cytometry analysis for ToPro3 permeability. (D) U87MG cells expressing DK EGFRvIII and control or SPRY2-targeting shRNA were treated with DMSO or G+P (20 µM gefitinib + 2 µM PHA665752) for 72 hrs. Whole cell lysates were probed by western blot using antibodies against the indicated proteins. (E) Whole cell lysates of U87MG cells expressing DK EGFRvIII and transduced with an empty vector (EV) or Y55F SPRY2 were probed by western blot using antibodies against the indicated proteins. Cells were also treated with DMSO or G+P (10 µM gefitinib + 5 µM PHA665752) for 48 hrs prior to flow cytometry analysis for ToPro3 permeability. Throughout the panels, data are represented as the average of three independent experiments ± s.e.m., and asterisks indicate p < 0.05.
Despite frequent EGFR overexpression and mutation in GBM, EGFR kinase inhibitors are not clinically effective (22, 32–34). GBM cell lines are also generally resistant to EGFR kinase inhibitors, but co-inhibition of c-MET can augment response (22, 27, 35). We tested whether SPRY2 knockdown affects cellular response to EGFR and c-MET co-inhibition using the EGFR inhibitor gefitinib and the c-MET inhibitor PHA665752. In multiple cell lines, cell death in response to gefitinib and PHA665752 increased significantly with SPRY2 knockdown (Figure 1C). In one of the cell lines from Figure 1C, we probed for markers of apoptosis and found that SPRY2 knockdown also promoted cleavage of poly ADP ribose polymerase (PARP) in response to EGFR and c-MET co-inhibition (Figure 1D). The same general effect on response to EGFR and c-MET inhibitors was also observed in parental U87MG cells with stable SPRY2 knockdown and U87MG-DK cells with transient SPRY2 knockdown (Supplemental Figure 3A, B). Because the expression of functional phosphatase and tensin homolog (PTEN) has been shown to regulate GBM cell response to EGFR inhibition (22, 36), we also investigated whether PTEN status influenced the effects of SPRY2 knockdown. Ectopic expression of wild-type PTEN in U87MG cells, which do not express PTEN, did not reverse the increased cellular sensitivity to gefitinib and PHA665752 observed with SPRY2 knockdown (Supplemental Figure 3C). In two cell lines that express wild-type PTEN, LN18 and SF188, SPRY2 knockdown also increased death response to the inhibitors (Supplemental Figure 3D). Consistent with the effects of SPRY2 knockdown, expression of dominant negative Y55F SPRY2 in U87MG-DK cells also enhanced cellular sensitivity to EGFR and c-MET co-inhibition (Figure 1E). This effect also repeated in multiple cell lines expressing the second non-overlapping SPRY2-targeting shRNA (Supplemental Figure 3E). Enhanced response to EGFR and c-MET inhibitors due to SPRY2 knockdown was also observed in a glioma stem cell (GSC) cell line maintained in non-adherent sphere culture conditions (Supplemental Figure 4).
SPRY2 controls p38’s ability to regulate anchorage-independent growth and response to inhibitors
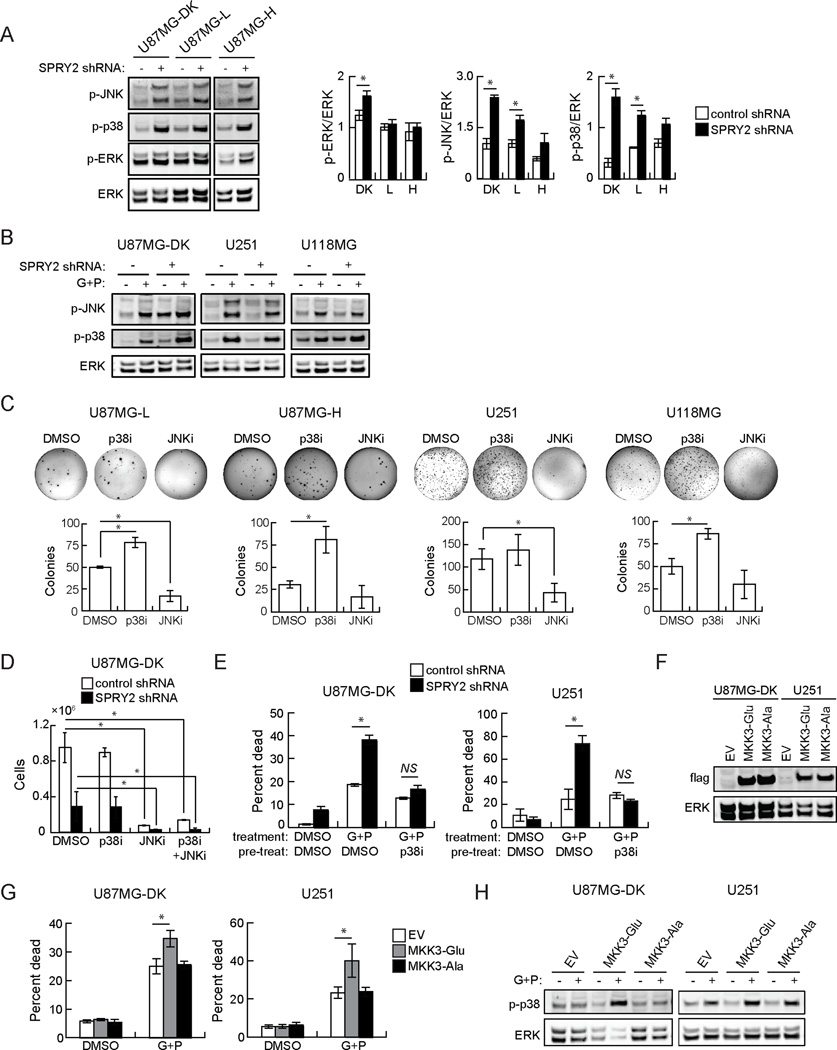
In multiple cellular settings, SPRY2 regulates signaling through the ERK pathway (7, 10, 11). In the panel of U87MG cells, SPRY2 knockdown produced a small increase in basal ERK phosphorylation in U87MG-DK cells, but did not affect ERK phosphorylation in cells expressing active EGFRvIII (Figure 2A), where SPRY2 knockdown also produced phenotypic effects. Although SPRY2 has been reported to specifically inhibit ERK among the MAP kinase pathways (7), a recent study found enhanced p38 phosphorylation in SPRY1/SPRY2/SPRY4 knockout murine embryonic fibroblasts (13). We thus hypothesized that JNK and/or p38 MAP kinases could be affected by SPRY2 knockdown. Indeed, basal JNK and p38 phosphorylation were increased in U87MG cells with SPRY2 knockdown compared to controls (Figure 2A). We did not generally observe similar basal increases in p38 or JNK phosphorylation in other GBM cell lines with SPRY2 knockdown (with the exception of p38 in U118MG), but we did observe clearly increased p38 and JNK phosphorylation in U87MG-DK, U251, and U118MG cells in response to EGFR and c-MET co-inhibition (Figure 2B, Supplemental Figure 5A). We also observed increased p38 and JNK phosphorylation in U87MG cells expressing Y55F SPRY2 (Supplemental Figure 5B).
Figure 2. p38 and JNK phosphorylation are basally increased in some cells with SPRY2 knockdown and are uniformly increased in cells co-treated with EGFR and c-MET inhibitors, and SPRY2 regulates the ability of p38 to promote cell death and antagonize proliferation.
(A) Whole cell lysates from U87MG cells expressing dead kinase (DK), low (L), or high (H) EGFRvIII and control shRNA (−) or SPRY2-targeting shRNA (+; shRNA #1) were probed by western blot using antibodies against the indicated proteins, and blots were analyzed by densitometry. Note that all lanes shown were on the same blot, but that extraneous lanes were removed from the image. (B) The indicated cell lines expressing control shRNA (−) or SPRY2-targeting shRNA (+; shRNA #1) were treated with DMSO or a combination of gefitinib and PHA665752 (G+P; 10 µM gefitinib + 5 µM PHA665752 for U87MG and U118MG; 10 µM gefitinib + 2 µM PHA665752 for U251) for 48 hrs. Whole cell lysates were probed by western blot using antibodies against the indicated proteins. (C) Colony formation in soft agar was measured in the indicated cell lines treated with DMSO, 20 µM SB203580 (p38i), or 20 µM SP600125 (JNKi). (D) Cellular proliferation was measured in U87MG cells expressing DK EGFRvIII and control shRNA or SPRY2-targeting shRNA (shRNA #1) treated with DMSO, 20 µM p38i, 20 µM JNKi, or both 20 µM p38i and 20 µM JNKi (p38i+JNKi). (E) U87MG DK EGFRvIII and U251 cells expressing control shRNA or SPRY2-targeting shRNA (shRNA #1) were treated with DMSO or 20 µM p38i for 24 hrs and then treated with DMSO or G+P (10 µM gefitinib + 5 µM PHA665752 for U87MG; 10 µM gefitinib + 3 µM PHA665752 for U251) for 48 hrs prior to flow cytometry analysis for ToPro3 permeability. (F) Whole cell lysates from U87MG DK EGFRvIII and U251 cells expressing control empty vector (EV), constitutively active MKK3 (MKK3-Glu), or inactive MKK3 (MKK3-Ala) were probed by western blot with a Flag antibody to detect Flag-MKK3 expression. (G) MKK3-expressing cells were treated with DMSO or G+P (10 µM gefitinib + 5 µM PHA665752 for U87MG; 10 µM gefitinib + 3 µM PHA665752 for U251) for 48 hrs prior to flow cytometry analysis for ToPro3 permeability. (H) Whole cell lysates from the indicated EV control or MKK3-expressing cells were probed by western blot using antibodies against the indicated proteins. Throughout the panels, data are represented as the average of at least three independent experiments ± s.e.m. Asterisks indicate p < 0.05, and “NS” indicates lack of statistical significance.
To determine whether JNK and/or p38 activity control the cellular phenotypes affected by SPRY2 knockdown, we treated cells with the p38 inhibitor SB203580 and/or the JNK inhibitor SP600125. For cells grown in soft agar, p38 inhibition promoted colony formation, while JNK inhibition decreased colony formation (Figure 2C). When p38 and JNK inhibitors were combined, colony formation was reduced compared to DMSO-treated controls but was increased compared to JNK inhibition alone (Supplemental Figure 5C). For cells grown on tissue culture plastic, p38 inhibition was not able to rescue the decreased cellular proliferation observed in U87MG-DK cells with SPRY2 knockdown, but JNK inhibition decreased cellular proliferation relative to DMSO-treated controls for cells with or without SPRY2 knockdown (Figure 2D). The ability of p38 inhibition to promote proliferation in soft agar, but not on tissue culture plastic, may suggest a greater level of p38 activity or a greater impact of p38 activity on proliferation in the soft agar context compared to plastic. Given the ability for p38 activity to antagonize proliferation in at least some culture settings, we hypothesized that p38 activity might also promote cell death in response to EGFR and c-MET co-inhibition. Consistent with this hypothesis, pretreatment with the p38 inhibitor eliminated the increase in cell death in response to gefitinib and PHA665752 in U87MG-DK and U251 cells with SPRY2 knockdown (Figure 2E). Conversely, expression of constitutively active mitogen-activated protein kinase kinase 3 (MKK3; (37)), the kinase for p38, enhanced cell death response to EGFR and c-MET co-inhibition in U87MG-DK and U251 cells (Figure 2F, G). Expression of an inactive MKK3 mutant did not affect drug response in either cell line. As expected, expression of constitutively active MKK3 increased p38 phosphorylation in cells where EGFR and c-MET were co-inhibited (Figure 2H, Supplemental Figure 5D). Note that U87MG-DK cells were used for these studies because they exhibited a larger increase in cell death due to SPRY2 knockdown than U87MG cells with active EGFRvIII expression.
In U87MG cells, SPRY2 regulates p38 and JNK phosphorylation through regulation of dual-specificity phosphatase expression
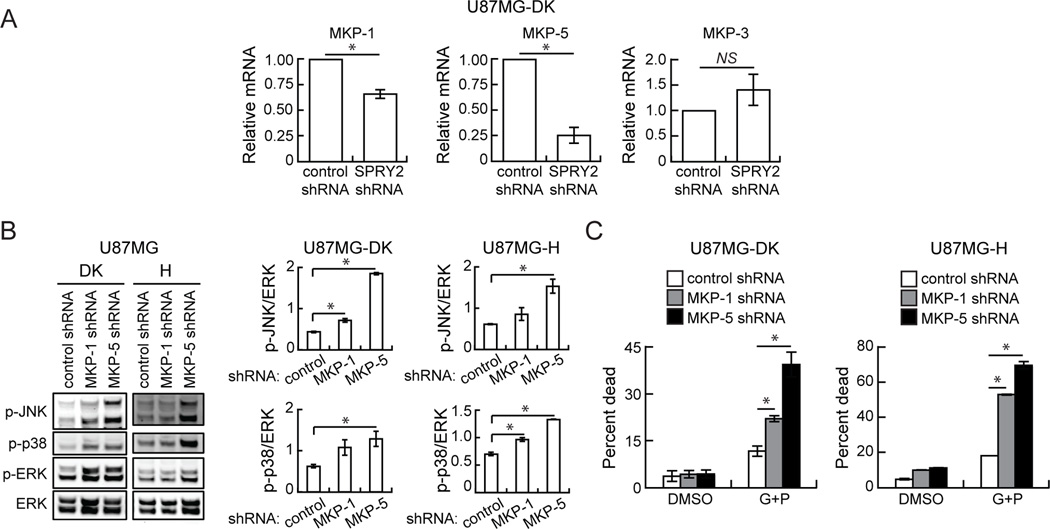
Although we observed basal increases in p38 and JNK phosphorylation in some cell lines with SPRY2 knockdown, there is no known mechanism for SPRY2-mediated inhibition of p38 or JNK. We postulated that SPRY2 could potentially antagonize JNK and p38 phosphorylation by promoting expression of regulatory phosphatases, similar to what has been found for the protein tyrosine phosphatase PTP1B in HeLa cells (38). Indeed, mRNA levels of the dual-specificity MAP kinase phosphatases MKP-1 and MKP-5, which act upon JNK and p38 (39, 40), were reduced in U87MG-DK cells with SPRY2 knockdown compared to controls (Figure 3A). There was no change in MKP-3 mRNA levels, which primarily acts upon ERK (39, 40). Knockdown of MKP-1 or MKP-5 in U87MG-DK or U87MG-H cells enhanced phosphorylation of p38, JNK, and ERK (Figure 3B and Supplemental Figure 6) and significantly increased cell death response to EGFR and c-MET co-inhibition (Figure 3C). The effects were greatest with MKP-5 knockdown, perhaps due to a more efficient knockdown of that target (Supplemental Figure 6).
Figure 3. In U87MG cells, SPRY2 knockdown reduces MKP-1 and MKP-5 mRNA expression, and MKP-1 or MKP-5 knockdown enhances cellular response to EGFR and c-MET co-inhibition.
(A) MKP-1, MKP-5, and MKP-3 mRNA levels were measured in U87MG dead kinase (DK) EGFRvIII cells expressing control shRNA or SPRY2-targeting shRNA (shRNA #1). (B) Whole cell lysates from U87MG DK or high (H) EGFRvIII cells expressing a control, MKP-1-targeting, or MKP-5-targeting shRNA were probed by western blot using antibodies against the indicated proteins, and blots were analyzed by densitometry. (C) The indicated cell lines were treated with DMSO or 10 µM gefitinib + 5 µM PHA665752 (G+P) for 48 hrs prior to flow cytometry analysis for ToPro3 permeability. Throughout the panels, data are represented as the average of three independent experiments ± s.e.m. Asterisks indicate p < 0.05, and “NS” indicates lack of statistical significance.
SPRY2 knockdown inhibits tumor xenograft growth
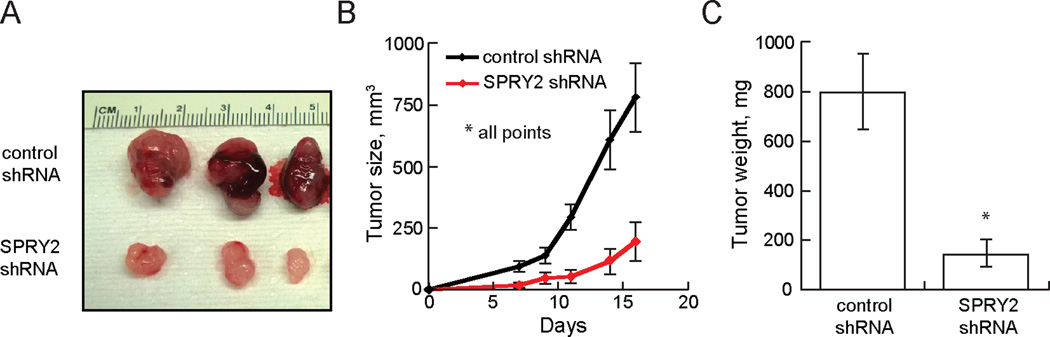
To determine the functional role of SPRY2 in an in vivo model, female NIH-III mice were injected subcutaneously with U87MG-L cells expressing control or SPRY2-targeting shRNA. Tumors arising from SPRY2 knockdown cells were significantly smaller than those arising from control cells throughout the course of the experiment (Figure 4). Strikingly, SPRY2 knockdown cells failed to give rise to discernable tumors in 2 of 8 mice. At 16 days post-injection, the average volume and weight of tumors from SPRY2 knockdown cells was approximately one-quarter of that for control tumors.
Figure 4. SPRY2 depletion suppresses tumor xenograft growth.
NIH-III mice were subcutaneously injected with U87MG cells expressing low EGFRvIII and either control shRNA or SPRY2-targeting shRNA (shRNA #1). (A) 16 days after injection of cells, animals were sacrificed, and representative images of resected tumors were taken. (B) Tumor volumes were measured every 2–3 days (n = 8 mice; p < 0.05 at all times). (C) Weights of resected tumors were measured (n = 6 mice with both control and SPRY2 knockdown tumors; p < 0.05). All data are represented as the mean ± s.e.m.
SPRY2 protein is expressed in human GBM, and elevated SPRY2 mRNA is present in EGFRvIII-positive tumors
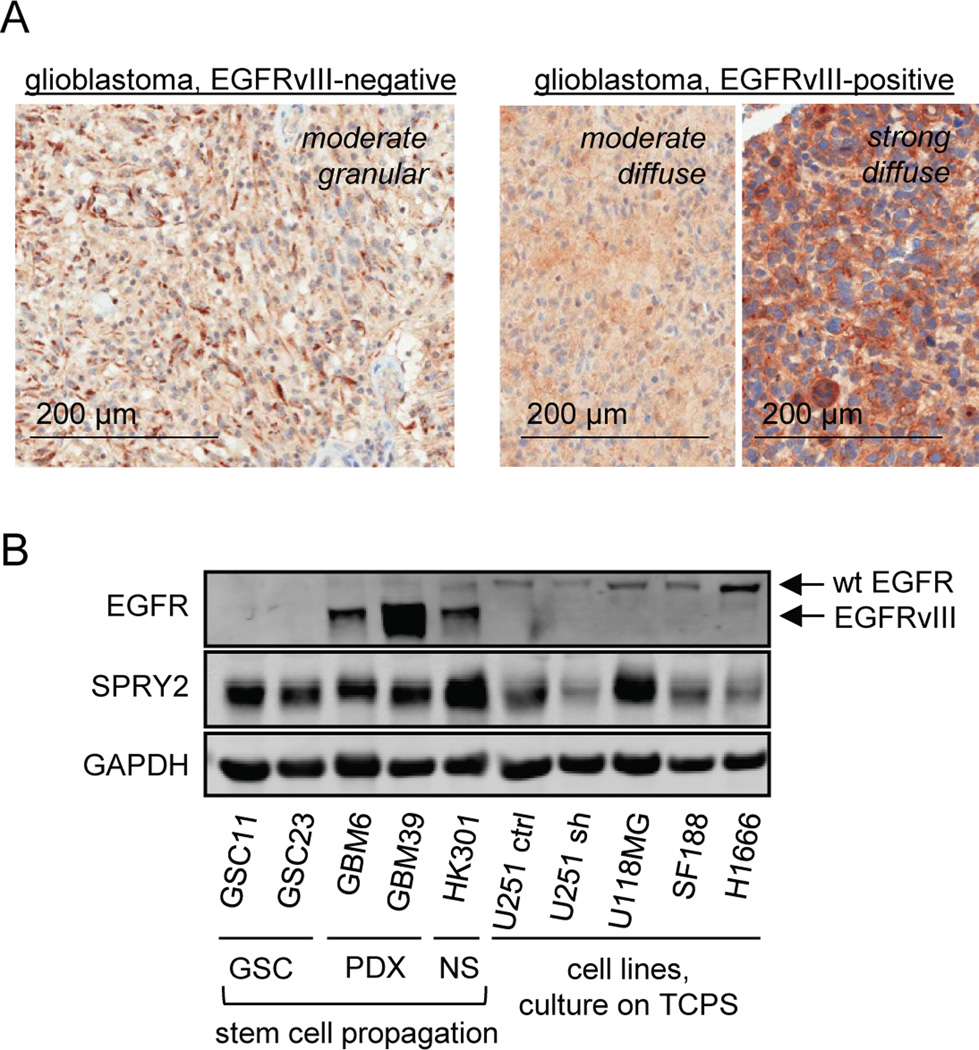
To confirm the presence of SPRY2 protein in human tumors, we analyzed tissue from banked GBMs expressing or lacking EGFRvIII by immunohistochemical staining. To validate our antibody for immunohistochemistry, SPRY2 protein expression was first probed in sections of kidney and cerebellum (Supplemental Figure 7). In GBM samples, all EGFRvIII-positive and -negative tumors analyzed showed the presence of SPRY2 protein. In some cases, SPRY2 staining was strong throughout the tumor section, while in others SPRY2 staining was localized in patches. In areas of SPRY2 expression, staining patterns included moderate focal, moderate granular, and strong diffuse staining (Figure 5A). A semi-quantitative H-score analysis revealed a tendency for more intense SPRY2 staining in sections of EGFRvIII-positive GBM (Supplemental Table 1), in qualitative agreement with the results of transcript analysis. To further assess SPRY2 protein expression in samples that should correspond well with what would be observed in the tumor environment, we probed for SPRY2 by western blot in samples of patient-derived xenografts, GSC cell lines, and a neurosphere culture (samples kindly provided by Dr. Frank Furnari, UCSD) (Figure 5B). SPRY2 expression in those samples was comparable to or greater than that observed in representative GBM cell lines where we observed phenotypic effects of SPRY2 knockdown or to that observed in a lung carcinoma cell line (H1666) where we previously documented effects of SPRY2 knockdown (19).
Figure 5. SPRY2 protein is expressed in human GBM.
(A) SPRY2 protein expression was probed in sections of EGFRvIII-positive (n = 10) or EGFRvIII-negative (n = 10) glioblastomas. In both tumor types, all sections analyzed showed definitive SPRY2 staining, with a tendency for stronger SPRY2 straining in EGFRvIII-positive tumors revealed by an H-score analysis (Supplemental Table 1). (B) SPRY2 protein expression in patient-derived xenografts (PDX) exhibiting EGFRvIII expression was compared against that observed in two glioma stem cell (GSC) lines, a neurosphere (NS) line with EGFRvIII expression, and in a panel of GBM cell lines and one lung cancer cell line (H1666) cultured on tissue culture polystyrene (TCPS). For U251 cells, lysates are shown for cells transduced with control (ctrl) or SPRY2-targeting shRNA (sh).
Given that EGFRvIII-positive tumors tend to be especially aggressive and that SPRY2 appeared to promote proliferation and resistance to therapy, we went on to analyze EGFRvIII-dependent gene signatures in primary human GBM tumors and orthotopic rat tumor allografts to probe for any evidence that SPRY2 mRNA might be upregulated in EGFRvIII-positive tumors. We first examined gene expression in 52 primary GBM tumors, which were stratified based on the presence or absence of EGFRvIII expression using EGFRvIII-specific RT-PCR (41). Extracted RNA samples from these tumors were subjected to microarray analysis using HG-U133 2.0 Plus Affymetrix gene chips. This analysis revealed 355 gene probes whose expression was significantly increased (p < 0.05) by at least 1.5-fold in EGFRvIII-positive tumors compared to EGFRvIII-negative tumors. For the analysis in rats, 12 animals received intracranial injections of 9L rat gliosarcoma cells expressing EGFRvIII or an empty vector control. Three weeks after implantation, tumors were analyzed for gene expression profiling using the RatRef12 Illumina chip array. This analysis revealed 1498 gene probes that were increased (p < 0.05) by at least 1.5-fold in EGFRvIII-expressing tumors compared to empty vector control tumors. These gene probes were aligned against the gene probes upregulated in EGFRvIII-positive human tumors. SPRY2 was one of 9 upregulated genes that were shared by human EGFRvIII-expressing GBMs and rat 9L EGFRvIII-expressing tumors (Supplemental Table 2), suggesting the possibility that SPRY2 expression may be elevated in EGFRvIII-positive GBM.
Based on the rat and human tumor transcript analysis, we proceeded to analyze SPRY2 expression patterns in a panel of GBM cell lines. Consistent with the documented ability of ERK to regulate SPRY2 expression (8, 9), we observed a positive correlation between SPRY2 expression and ERK phosphorylation that was supported by results of pharmacological or genetic perturbations (Supplemental Figure 8A–D). Among cell lines engineered to express EGFRvIII, we also observed good correlation between SPRY2 expression and ERK phosphorylation, but we did not observe a consistent trend between EGFRvIII and SPRY2 expression (Supplemental Figure 8E). The apparent lack of SPRY2/EGFRvIII expression correlation may arise because of the limited number of isogenic cell backgrounds tested or because the method used to drive EGFRvIII expression in cell lines differs drastically from the way that EGFRvIII expression is regulated in GBM (42). Whatever the explanation for the discrepancy, the results in this section provide overall support for the notion that SPRY2 is expressed at functionally relevant levels in tumors with or without EGFRvIII expression. In the future, a more detailed analysis of potential SPRY2 protein expression differences between EGFR-negative and EGFRvIII-positive settings should be undertaken using tumor tissues.
Analysis of TCGA data reveals that elevated SPRY2 expression correlates with EGFRvIII expression, the classical GBM subtype, and reduced patient survival
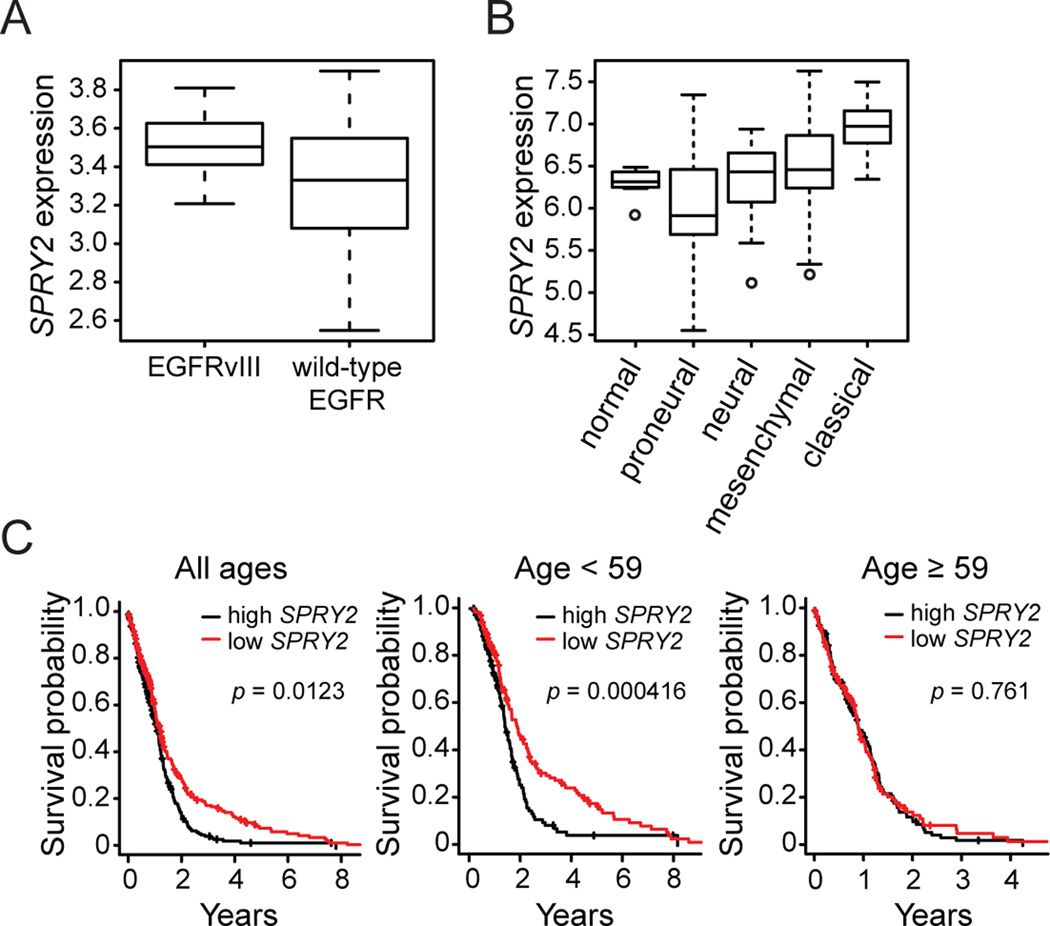
We probed the TCGA GBM data set to ask if SPRY2 expression correlates with EGFRvIII expression in a larger set of patient samples and to determine whether SPRY2 expression correlates with previously defined GBM tumor subtypes and patient survival. TCGA samples were classified by EGFRvIII expression status based on RNA-seq data, which was available for 161 samples. 41/161 samples (25%) were EGFRvIII-positive. SPRY2 expression was increased in EGFRvIII-positive samples compared to EGFRvIII-negative samples (p = 1.00×10−8), with a fold difference of 1.49 (Figure 6A), consistent with the results in Supplemental Table 2. Based on the definitions of four clinically relevant GBM subtypes determined by gene signatures (43) and using TCGA GBM exon-array data (173 “core” representative samples), we further found that SPRY2 expression was lower in the proneural subtype than the other three subtypes (p = 8.06×10−9 and fold difference of 0.60) (Figure 6B). SPRY2 expression was highest in the classical subtype (p < 2.20×10−16 and fold difference of 1.58 for comparison to other subtypes), which exhibits a high rate of EGFR amplification and mutation.
Figure 6. TCGA GBM data reveals that elevated SPRY2 expression correlates with EGFRvIII expression, the classical GBM subtype, and reduced patient survival.
(A) SPRY2 expression (log base 10 transformed RPKM) is higher in EGFRvIII-positive RNA-seq samples (n = 41) than EGFRvIII-negative RNA-seq samples (n = 120) (p = 1.00×10−8; fold difference of 1.49). (B) SPRY2 expression (log base 2 transformed data) is shown for normal brain samples (n = 10) and the four GBM subtypes: proneural (n = 54, p = 8.06×10−9 and fold difference of 0.60 compared to other three subtypes), neural (n = 27), mesenchymal (n = 55), and classical (n = 37, p < 2.20×10−16 and fold difference of 1.58 compared to other three subtypes). The p-value for tumor versus normal brain samples is 0.154 with a fold difference of 1.13. (C) Survival probability is shown as a function of time after diagnosis classified by SPRY2 expression with the median SPRY2 expression used as a cutoff for all patients and patients parsed by age using the median age (59 yrs) as a cutoff.
We also determined the relationship between SPRY2 expression and patient survival (Figure 6C), using median SPRY2 expression as a cutoff between low and high expression. Across all patients, low SPRY2 expression was associated with reduced mortality compared to high SPRY2 expression (p = 0.0123), with median survival for low or high SPRY2 expression of 469 or 393 days, respectively. Because age is an established important prognostic factor in GBM (44, 45), we further looked at the effect of SPRY2 expression in patients stratified by age. Parsing patients into age groups using the median age (59 yrs) as a cutoff, we found that the effect of SPRY2 expression differences was more pronounced in the younger patient cohort (p = 0.000416), with median survival of 631 or 451 days for low or high SPRY2 expression, respectively, and that there was no effect of SPRY2 expression on survival in the older cohort. The difference in survival was even further increased using an age cutoff of 40 yrs (p = 0.00316), with median survival of 1024 or 538 days for low or high SPRY2 expression, respectively (Supplemental Figure 9). The larger difference in survival for patients 40 years of age and younger may be observed because younger patients survive longer overall, allowing for a greater time to observe the effects of differential SPRY2 expression.
Our findings that SPRY2 expression is highest in the classical subtype and that elevated SPRY2 expression portends reduced patient survival need not be viewed as in conflict with the finding by Verhaak et al. (43) that patients in the classical subtype responded best to “more intensive treatment,” defined by the authors as “concurrent chemo- and radiotherapy or more than three subsequent cycles of chemotherapy,” versus patients undergoing non-concurrent therapies or short regimens. Our analysis does not parse patients by therapeutic approach. Of course, the analysis of Verhaak et al. is fully consistent with our findings in that overall median survival was highest for the proneural subtype, where we found the lowest SPRY2 expression.
DISCUSSION
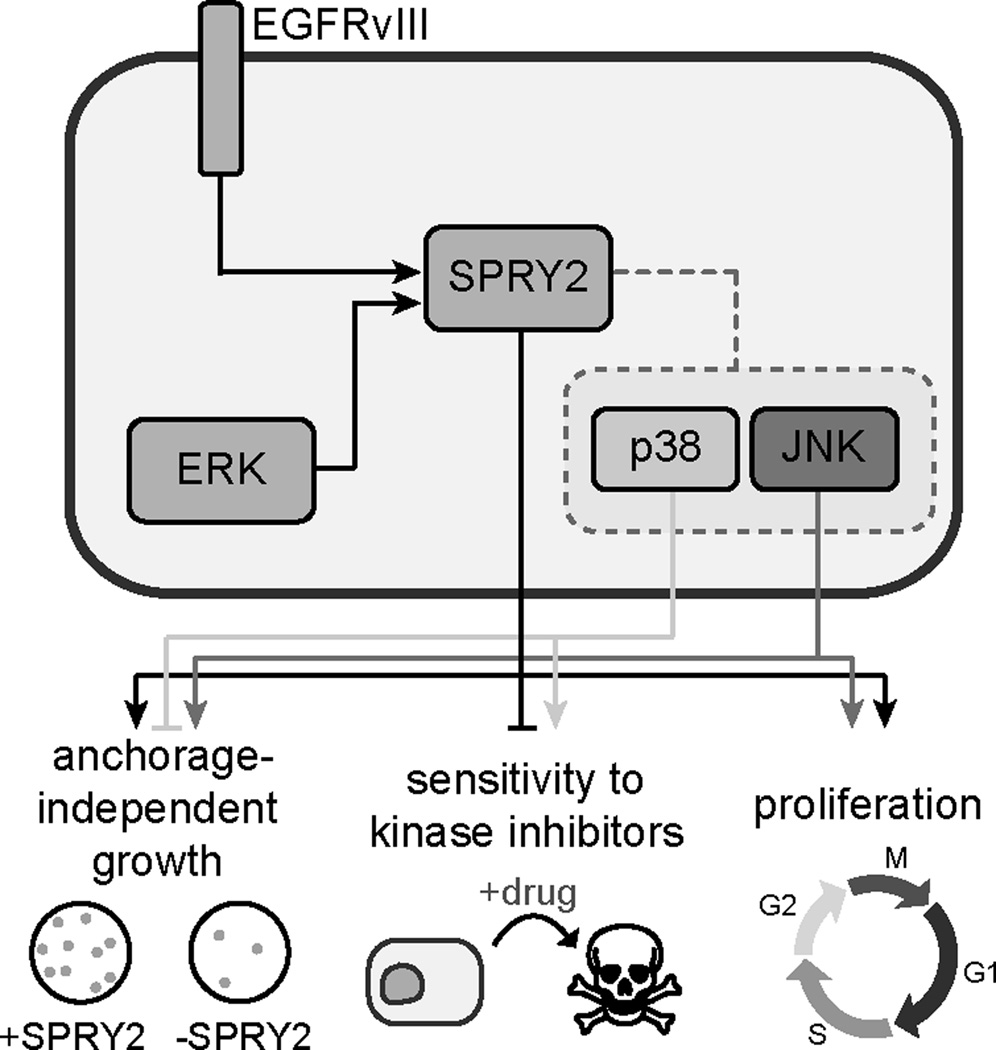
The basic findings of our study are summarized schematically in Figure 7. In GBM cell lines with or without EGFRvIII expression, we found that SPRY2 surprisingly acted as a driver of GBM cell proliferation, anchorage-independent growth, and resistance to inhibition of receptor tyrosine kinases that promote GBM survival. SPRY2 also promoted the growth of subcutaneous tumor xenografts, demonstrating the relevance of SPRY2 in vivo. In at least some cell lines, SPRY2 exerted control over phenotypes by regulating the ability of p38 and JNK to influence cell outcomes. In one cell line context, U87MG cells, this control over p38 and JNK resulted at least in part through regulation of MKP1 and MKP5 expression, which altered baseline p38 and JNK phosphorylation. However, the ability of SPRY2 to control the effects of p38 activity on cell phenotypes in cell lines where baseline p38 or JNK phosphorylation levels were not altered by SPRY2 knockdown suggests that the ability of SPRY2 to regulate GBM cell phenotypes is likely to be more complex than we have uncovered thus far. Through analysis of human GBM samples, rat tumor allografts, and lysates from patient-derived xenografts and GSCs, we determined that SPRY2 protein is definitively expressed in human GBM with or without EGFRvIII expression and that SPRY2 expression is elevated in tumors expressing EGFRvIII, which tend to be especially aggressive. Moreover, elevated SPRY2 transcript expression is associated with reduced patient survival and the classical GBM subtype. Importantly, the somewhat modest elevation of SPRY2 expression in EGFRvIII-positive tumors should not be misinterpreted to suggest that SPRY2 control of phenotypes is relegated to the EGFRvIII-positive setting. Indeed, our data suggest that SPRY2 exerts important control over cell fate decisions in glioma cells with or without EGFRvIII expression. In aggregate, our findings point to SPRY2 and key pathways it regulates as potential therapeutic targets and biomarkers in GBM.
Figure 7. SPRY2 promotes GBM cell proliferation, anchorage-independent growth, and resistance to tyrosine kinase inhibition.
The schematic highlights our study’s key findings regarding regulation of SPRY2 expression and SPRY2-mediated regulation of signaling and cellular phenotypes. In tumors expressing EGFRvIII, SPRY2 expression is increased compared to tumors lacking EGFRvIII. However, data in cell lines suggest important roles for SPRY2 whether or not EGFRvIII is expressed. SPRY2 is also promoted by ERK activity. In GBM cell lines, SPRY2 promotes anchorage-independent growth and proliferation in adherent cultures, and drives resistance to targeted inhibitors of oncogenic kinases (EGFR and c-MET). In at least some cell settings, SPRY2 regulates these phenotypes by permitting p38 activity to play a more prominent role in cell fate determination. The functional role of JNK activity may also be regulated by SPRY2, but JNK and p38 generally play opposing roles in the regulation of GBM cell phenotypes.
The finding that SPRY2 promotes growth and resistance to inhibition of receptor tyrosine kinases in the GBM cell context was initially surprising given SPRY2’s purported role as a tumor suppressor in hepatocellular carcinoma, lung cancer, breast cancer, and prostate cancer (14–16). While SPRY2 appears to negatively regulate tumor growth in those settings, an oncogenic role for SPRY2 has recently been demonstrated in colon cancer, where elevated SPRY2 expression is a marker of poor prognosis (17, 46). Because the net outcome of SPRY2 expression and its mechanism of action appear highly cell context-dependent, additional work is clearly needed to clarify the determinants of SPRY2’s function in different contexts and to develop strategies to interrupt SPRY2’s functions in GBM and other cancers where it may promote growth and resistance to therapy.
Another question raised by our results is how SPRY2 expression might be promoted in EGFRvIII-positive tumors, as suggested by our analyses of human and rat tumor transcripts and our analysis of human GBM sections by immunohistochemistry. Our data show that SPRY2 protein expression correlates well with ERK phosphorylation across many GBM cell lines and with multiple genetic and pharmacological perturbations. It has been suggested, however, that EGFRvIII-driven tumors do not activate canonical EGFR downstream signaling pathways such as ERK, STAT3, and AKT (47). It is possible that tumor microenvironment features altered by EGFRvIII expression allow for increased ERK activation, or that an alternative pathway not explored here promotes SPRY2 expression in GBM. If it is true that ERK regulates SPRY2 expression in vivo, MEK inhibition could be a useful approach to downregulate SPRY2 expression in GBM. Post-transcriptional regulation of SPRY2 by miR-21 (20, 48) could also potentially complicate the relationship between SPRY2 transcript and protein levels in some settings. Whether or not such regulation occurs in vivo as a means to suppress SPRY2 expression in gliomas relative to normal tissue, as suggested by one study (20), our aggregate results are clear that meaningful levels of SPRY2 protein are expressed in tumors with or without EGFRvIII expression.
Although the best-studied role of SPRY2 is regulation of the ERK pathway, our results suggest an important ability of SPRY2 to regulate GBM cell phenotypes by regulating p38 and JNK activities and/or the effects of those activities on cellular behaviors. We interpret the results of Figure 2 to indicate that JNK activity is required for normal GBM cell proliferation and anchorage-independent growth, but that p38 activity antagonizes anchorage-independent growth and promotes cellular response to EGFR and c-MET co-inhibition. Since p38 inhibition mitigated the effects of SPRY2 knockdown on death response to EGFR and c-MET co-inhibition even in a cell line where SPRY2 knockdown did not generate basal increases in p38 phosphorylation (U251) p38 activation may only be a necessary step along the path leading to apoptosis. SPRY2’s absence (or reduced expression) may potentiate this effect of p38 activity through specific mechanisms yet to be identified. In that sense, our findings of altered MKP1 and MKP5 expression with SPRY2 knockdown in U87MG cell lines may not represent a broadly relevant mechanism for regulating the impact of p38 activity in glioma cell lines and tumors.
Looking forward, SPRY2 and the pathways it regulates should be assessed for their value as therapeutic targets or as prognostic markers for response to therapy in GBM. While it is not presently possible to target SPRY2 with small molecule inhibitors, it may eventually be possible to target SPRY2 through RNAi-based therapies, whose delivery to the brain may be enabled through nanocarrier delivery methodologies that are in ongoing development. Through the more thorough dissection of the signaling pathways that are broadly regulated by SPRY2 to enable its ability to govern glioma cell and tumor phenotypes, it may also eventually be possible to identify druggable targets whose pharmacological inhibition phenocopies the effects of SPRY2 depletion.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Frank Furnari, Dr. Tyler Jacks, Dr. Marilyn Farquhar, Dr. Margaret Chou, Dr. Dafna Bar-Sagi, Dr. Gary Kuo, Dr. Craig Thompson, and Dr. Sylvain Meloche for generously providing reagents and to Ms. Amy Ziober and Dr. Christopher Furcht for technical assistance. The authors also acknowledge The Cancer Genome Atlas pilot project established by the NCI and NHGRI (http://cancergenome.nih.gov/).
Financial support was provided by the University of Pennsylvania, the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania, the Howard Hughes Medical Institute, and NSF Graduate Research Fellowships to AMW and JMB. MJL was supported by a Research Scholar Grant, RSG-15-010-01-CDD from the American Cancer Society.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110:13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 4.De Witt Hamer PC. Small molecule kinase inhibitors in glioblastoma: a systematic review of clinical studies. Neuro Oncol. 2010;12:304–316. doi: 10.1093/neuonc/nop068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathologica. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, et al. Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002;277:3195–3201. doi: 10.1074/jbc.M108368200. [DOI] [PubMed] [Google Scholar]
- 8.Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 9.Egan JE, Hall AB, Yatsula BA, Bar-Sagi D. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc Natl Acad Sci U S A. 2002;99:6041–6046. doi: 10.1073/pnas.052090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 11.Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- 12.Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, et al. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma B, Joshi S, Sassano A, Majchrzak B, Kaur S, Aggarwal P, et al. Sprouty proteins are negative regulators of interferon (IFN) signaling and IFN-inducible biological responses. J Biol Chem. 2012;287:42352–42360. doi: 10.1074/jbc.M112.400721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo TL, Fong CW, Yusoff P, McKie AB, Chua MS, Leung HY, et al. Sprouty and cancer: the first terms report. Cancer Lett. 2006;242:141–150. doi: 10.1016/j.canlet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Fong CW, Chua MS, McKie AB, Ling SHM, Mason L, Li R, et al. Sprouty 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048–2058. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- 16.Sutterluty H, Mayer CE, Setinek U, Attems J, Ovtcharov S, Mikula M, et al. Down-regulation of Sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms. Mol Cancer Res. 2007;5:509–520. doi: 10.1158/1541-7786.MCR-06-0273. [DOI] [PubMed] [Google Scholar]
- 17.Holgren C, Dougherty U, Edwin F, Cerasi D, Taylor I, Fichera A, et al. Sprouty-2 controls c-Met expression and metastatic potential of colon cancer cells: sprouty/c-Met upregulation in human colonic adenocarcinomas. Oncogene. 2010;29:5241–5253. doi: 10.1038/onc.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng YH, Tsao CJ, Wu CL, Chang JG, Lu PJ, Yeh KT, et al. Sprouty2 protein enhances the response to gefitinib through epidermal growth factor receptor in colon cancer cells. Cancer Sci. 2010;101:2033–2038. doi: 10.1111/j.1349-7006.2010.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh AM, Lazzara MJ. Regulation of EGFR Trafficking and Cell Signaling by Sprouty2 and MIG6 in Lung Cancer Cells. J Cell Sci. 2013;126 doi: 10.1242/jcs.123208. [DOI] [PubMed] [Google Scholar]
- 20.Kwak HJ, Kim YJ, Chun KR, Woo YM, Park SJ, Jeong JA, et al. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene. 2011;30:2433–2442. doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 21.Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 22.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 23.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 24.Koochekpour S, Jeffers M, Rulong S, Taylor G, Klineberg E, Hudson EA, et al. Met and hepatocyte growth factor scatter factor expression in human gliomas. Cancer Res. 1997;57:5391–5398. [PubMed] [Google Scholar]
- 25.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 27.Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang HJS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 29.Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 32.Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007;254:165–177. doi: 10.1016/j.canlet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schaiquevich P, Mason W, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 34.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2:458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furcht CM, Buonato JM, Skuli N, Mathew LK, Munoz Rojas A, Simon MC, et al. Multivariate signaling regulation by SHP2 differentially controls proliferation and therapeutic response in glioma cells. J Cell Sci. 2014 doi: 10.1242/jcs.150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 37.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yigzaw Y, Poppleton HM, Sreejayan N, Hassid A, Patel TB. Protein-tyrosine phosphatase-1B (PTP1B) mediates the anti-migratory actions of Sprouty. J Biol Chem. 2003;278:284–288. doi: 10.1074/jbc.M210359200. [DOI] [PubMed] [Google Scholar]
- 39.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 40.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-reviews3009. REVIEWS3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tykocinski ES, Grant RA, Kapoor GS, Krejza J, Bohman LE, Gocke TA, et al. Use of magnetic perfusion-weighted imaging to determine epidermal growth factor receptor variant III expression in glioblastoma. Neuro Oncol. 2012;14:613–623. doi: 10.1093/neuonc/nos073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li A, Walling J, Kotliarov Y, Center A, Steed ME, Ahn SJ, et al. Genomic changes and gene expression profiles reveal that established glioma cell lines are poorly representative of primary human gliomas. Mol Cancer Res. 2008;6:21–30. doi: 10.1158/1541-7786.MCR-07-0280. [DOI] [PubMed] [Google Scholar]
- 43.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Scheck AC, Cloughesy TF, Lai A, Dong J, Farooqi HK, et al. Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genomics. 2008;1:52. doi: 10.1186/1755-8794-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siker ML, Wang M, Porter K, Nelson DF, Curran WJ, Michalski JM, et al. Age as an independent prognostic factor in patients with glioblastoma: a Radiation Therapy Oncology Group and American College of Surgeons National Cancer Data Base comparison. J Neurooncol. 2011;104:351–356. doi: 10.1007/s11060-010-0500-6. [DOI] [PubMed] [Google Scholar]
- 46.Ordonez-Moran P, Irmisch A, Barbachano A, Chicote I, Tenbaum S, Landolfi S, et al. SPROUTY2 is a beta-catenin and FOXO3a target gene indicative of poor prognosis in colon cancer. Oncogene. 2014;33:1975–1985. doi: 10.1038/onc.2013.140. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A. 2009;106:2712–2716. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19:3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.