Abstract
Atypical antipsychotics (AAPs), such as olanzapine (OLZ), are associated with metabolic side effects, including hyperglycemia. Although a central mechanism of action for the acute effects on glycemia has been suggested, evidence for peripheral versus central effects of AAPs has been mixed and has not been explored for an effect of OLZ on the respiratory exchange ratio (RER). Here, we tested the hypothesis that some inconsistencies in the glycemic responses are likely a result of different doses and central sites of injection. We also compared the effects of central versus peripherally administered OLZ on the RER of unsedated rats. Third ventricle infusion of OLZ at 0.3 mg/kg caused hyperglycemia within 30 minutes, with a higher dose (1.8 mg/kg) needed to elicit a similar response in the lateral ventricles. In contrast, 3 mg/kg of OLZ was needed to raise blood glucose within 30 minutes when given intragastrically, and 10 mg/kg resulted in a prolonged hyperglycemia lasting at least 60 minutes. Third ventricle injection of OLZ significantly decreased RER after 75 minutes, whereas intragastric OLZ resulted in a faster drop in RER after 30 minutes. Since changes in glycemia were most sensitive when OLZ was infused into the third ventricle, but effects on RER were more rapidly and efficaciously observed when the drug was given peripherally, these results raise the likelihood of a dual mechanism of action involving hypothalamic and peripheral mechanisms. Some discrepancies in the literature arising from central administration appear to result from the injection site and dose.
Keywords: olanzapine, antipsychotics, glucose, diabetes, metabolism, insulin
Introduction
Atypical antipsychotics (AAPs), including olanzapine (OLZ), are commonly associated with metabolic side effects including overeating, hyperglycemia, insulin resistance, and obesity.1 These side effects may lead to decreased drug compliance with increased risk of psychosis, cardiovascular disease, diabetes, and reduced quality of life.2,3 Moreover, the incidence of AAP prescriptions is rising, increasing the number of individuals at risk.4-6 Thus, elucidating the mechanisms by which AAPs increase blood glucose and interfere with metabolism is paramount to protecting the population of individuals taking these medications.
The immediate glycemic effects of OLZ may be central or peripheral. Studies have been conducted to evaluate this previously, however, different conclusions have emerged. For instance, Martins and colleagues7 delivered OLZ (in acidified artificial cerebral spinal fluid [CSF]) into the third ventricle using an elaborate, continuous infusion protocol and found that blood glucose was significantly elevated within 10 minutes of treatment (after ∼0.4 mg/kg). In contrast, Girault and colleagues8 found no central effect of OLZ (in artificial CSF, physiological pH) on glycemia when infused into the lateral ventricle at 0.03 mg/kg/h. Hahn and colleagues9 also did not detect an effect of OLZ (in acetic acid buffered with 1 N NaOH, pH 5.5-6.0) on glycemia when infused as a single bolus of 75 mg/kg over 60 seconds into the third ventricle. These different findings for this important question may have arisen because of differences between the infusion protocols, doses used, and the vehicles (VEHs) and pH of the solutions (Notably, OLZ is highly insoluble at physiological pH in artificial CSF). Thus, in the current series of experiments, we sought to test the effects of OLZ on glycemia using the same VEH and protocols for drug infusion and whole body glucose measurement, changing only the central site of administration (lateral vs third ventricle injection sites). In parallel, we examined these dose- and time-dependent effects of peripheral administration of OLZ.
In addition to monitoring whole body glucose, we extended our measurements to include the respiratory exchange ratio (RER), a measure of major fuel selection, after central or peripheral injection of OLZ. Intragastric OLZ has been shown to alter major fuel preference, causing an irreversible switch from glucose to fatty acid oxidation in mice, reflected as a decrease in RER.10,11 We have shown that blocking fatty acid oxidation with carnitine palmitoyltransferase 1 inhibitors after intragastric OLZ treatment leads to death in mice, and mice are unable to switch back to using glucose as a metabolic fuel substrate while under OLZ treatment.10 Although no one is questioning the peripheral effects of OLZ on metabolism, it is unclear whether the peripheral effects are a result of a primary central mechanism. By comparing peripheral to central administration of OLZ and complementing measurements of glycemia with major fuel selection, it may lead us closer to understanding where and how the detrimental, metabolic effects of OLZ occur. In particular, the strong peripheral effects of OLZ on decreasing RER (reducing glucose utilization) have not been examined after central administration.
Materials and Methods
Materials
Olanzapine (PubChem CID: 4585) was a generous gift of Neuland Laboratories Ltd (Hyderabad, India). Angiotensin II (PubChem CID: 172198) and rimonabant (RIM; PubChem CID: 104850) were purchased from Tocris Bioscience (Minneapolis, Minnesota). Angiotensin II was used at the end of the experiment to verify that the intracerebroventricular (ICV) cannulas were placed correctly by performing a water drinking test.
Animals
Animal studies and protocols were approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee. Male Sprague Dawley rats (230-490 g) were purchased from Charles River Laboratories (Wilmington, Massachusetts). Males were chosen because their food intake, energy metabolism, and physical activity are not confounded by hormone fluctuations associated with an estrous cycle.12 Moreover, acute OLZ has been shown to cause hyperphagia and weight gain in female, but not male, rats,13 which may have secondary effects on glycemia and metabolic fuel utilization. Rats were acclimated to our facilities at least 14 days before undergoing surgical cannulation of the lateral or third ventricle. Rats were singly-housed and maintained on a reversed 12:12 light–dark cycle (lights on at 1900 hours) with food (Harlan-Teklad Rodent Chow, no. 2018; Harlan-Teklad, Madison, Wisconsin) and water provided ad libitum unless otherwise indicated.
Intracerebroventricular Cannulation
In 6 rats, the lateral ventricle was targeted −0.8 mm anterior–posterior, ±1.3 mm medial–lateral, and −3.2 mm dorsal–ventral from bregma.14 Placements of cannula (22 gauge; Plastics One, Roanoke, Virginia) were alternated between the left and right hemispheres of the brain. In 8 separate rats, the third ventricle was targeted −2.2 mm anterior–posterior, 0 mm medial–lateral, and −8.5 mm dorsal–ventral from bregma. Cannulas were held in place using surgical stainless steel screws (Small Parts, Inc, Logansport, Indiana) and dental cement. Rats were given 7 days to recover from surgery with surgical sites cleaned daily with 3% hydrogen peroxide and triple antibiotic ointment (bacitracin, neomycin, and polymyxin). Afterward, rats were trained to receive ICV injections of sterile, artificial CSF (Harvard Apparatus, Holliston, Massachusetts) with minimal restraint (ie, rats sat in the experimenter’s lap with ICV cannula tethered to a Hamilton syringe and pump for the duration of the injection). At the completion of the experiment, cannula placements were verified by injecting the well-known dipsogenic agent angiotensin II (200 ng/kg) and measuring water consumption over 30 minutes.15 Any angiotensin II-treated rat that consumed more than 5 mL of water in 30 minutes was considered to have correct cannula placement in or very near the ventricles.
Drug Preparation
For ICV injections, OLZ or RIM powder was dissolved in dimethyl sulfoxide (DMSO). Stock solutions of OLZ and RIM (0.05 to 0.2 mg/ml) and VEH were filtered through sterile 0.45-μm syringe filters and stored at −20°C until use. The concentrations of OLZ that were infused varied depending on the body weight of the rat so that no more than 2 μL was injected at a given time, thus reducing the probability of direct contact with nuclei nonadjacent to the sites of injection. Olanzapine was injected into the lateral or third ventricle at a range of doses including 0.44 mg/kg, a dose shown to rapidly induce hyperglycemia when injected into the third ventricle of rats.7 Angiotensin II was dissolved in saline and filtered (0.45 μm) before use. Angiotensin II was injected into the lateral or third ventricle at 200 ng/kg (∼3.0 μL volume), a dose that significantly increases drinking within 30 minutes after ICV injection in rats.15
For oral gavage treatments, OLZ was prepared as described previously.13 Briefly, OLZ powder was first solubilized in 0.1 N HCl. Next, pH was adjusted to 5.2 ± 0.2 with 0.01 N NaOH to yield a desired drug concentration of 0.11 mg/mL. We started the intragastric treatments at 0.44 mg/kg (a dose given centrally in the current experiment) and increased the dose to 10 mg/kg. This high dose was chosen based on published data that demonstrate significant metabolic effects in rodents, including RER, blood glucose, and insulin.11,16 Rimonabant for oral administration was prepared similar to OLZ. Vehicle-treated rats were given water adjusted to a similar pH.
Blood Glucose
After a 4-hour fast, glucose was measured before ICV injection of OLZ or RIM (time 0) and at 30 and 60 minutes postinjection, in duplicate from tail blood using an Ascensia Contour blood glucose meter (Bayer HealthCare LLC, Mishawaka, Indiana). Members of our laboratory previously showed that acute OLZ treatment, following acute fasting, impairs glucose metabolism.11,13,17 Therefore, exposing animals to a long (14-16 hours) fast was not necessary to determine glycemic or metabolic effects after OLZ. Intracerebroventricular injections and blood collections were performed under dim red light illumination during the first 3 hours of the dark phase of the light cycle. Rats were given an average of 6 ICV injections during the course of the experiment with OLZ and VEH treatments in a random order (injections given every 3 days). One week after the last ICV treatment, rats were given OLZ (0.44-10 mg/kg), RIM (0.44 mg/kg), or VEH via oral gavage, and blood glucose was measured at the same time points.
Glycemic Effect of RIM
Since OLZ is insoluble in aqueous solutions at a neutral pH, we were concerned that the drug would precipitate when it came in contact with CSF and tissue. Precipitation could possibly have confounding actions such as either a lack of an effect that should exist or conversely an effect due to physical effects of the precipitate on neuronal pathways affecting peripheral glucose concentrations.18 To test this, we gave 0.44 mg/kg of RIM, a cannabinoid receptor inverse agonist19 that acutely induces hyperglycemia and has a similar poor aqueous solubility as OLZ (but excellent solubility in DMSO, ∼16-20 mg/mL), into the third or lateral ventricles. Like OLZ, RIM possesses a strong acute glycemic effect, but in this case, owing to K-ATP channel opening in islets.20 Thus, we would expect that if drug precipitation was influencing glycemia, central infusion of RIM at a dose that was comparable to those planned for OLZ would also result in a glycemic response.
Energy Expenditure and Locomotor Activity
Rats were acclimated to test chambers for 2 days before testing. For injections, rats were removed from the test chambers for 2 minutes and replaced immediately after treatment. Dark cycle RER (Vco 2/Vo 2) was used as a noninvasive approach to monitor changes in major fuel selection. Vo 2, RER, and locomotor activity were determined as described previously.11 Briefly, after calibration, O2 and CO2 concentrations were measured from sealed chambers every minute at 15-minute intervals (Oxymax; Columbus Instruments, Columbus, Ohio). Flow rate was set to 3.0 L/m. Locomotor activity was measured simultaneously using infrared technology (OPT-M3; Columbus Instruments). The number of light beam breaks along the x-axis (XTOT), y-axis (XAMB), and z-axis (ZTOT) was summed over 3 hours of treatment.
Statistical Analysis
All data, unless otherwise noted, are expressed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) was used to test for differences in blood glucose. When significant, a Bonferroni multiple comparison posttest was used to determine individual differences among groups. When only 2 groups were compared, an unpaired t test was used to test for differences. Differences in RER and Vo 2 were determined using 2-way ANOVA. All data analysis was performed using GraphPad Prism computer software with significance at P < .05 (GraphPad Software, San Diego, California).
Results
Glycemic Effect of RIM
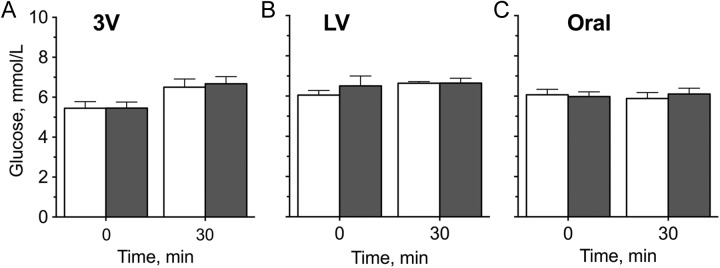
Infusion of RIM at 0.44 mg/kg had no significant effect on blood glucose when infused into the third ventricle (Figure 1A) or lateral ventricle (Figure 1B). Rimonabant (0.44 mg/kg) also had no significant effect on glycemia when given intragastrically (Figure 1C).
Figure 1.
Blood glucose concentrations of fasted rats treated with a low dose (0.44 mg/kg) of rimonabant (gray bars) or vehicle (white bars) that does not increase plasma glucose when provided by peripheral administration. This dose was identical to that planned for olanzapine studies. Rimonabant was (A) injected into the third ventricle (3V), (B) injected unilaterally into the left or right lateral ventricle (LV), or (C) administered via oral gavage. Blood glucose was measured at 0 and 30 minutes. Results were not significantly different from rats given vehicle.
Glycemic Effect of the Third Ventricle OLZ Infusion
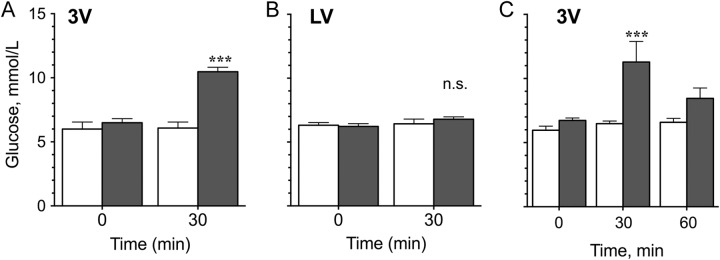
Olanzapine increased (P < .001) blood glucose when infused into the third ventricle at a dose of 0.44 mg/kg (Figure 2A and C)—a dose comparable to those used in human studies measuring dopamine receptor occupancy.21 A dose–response test revealed that OLZ had significant effects on blood glucose concentrations within 30 minutes at a dose of 0.3 mg/kg (P < .001) but not at 0.1 to 0.2 mg/kg (Figure 3A). By 60 minutes, blood glucose concentrations were still higher than animals given VEH but were no longer statistically different (Figures 2C and 3A).
Figure 2.
Blood glucose concentrations of fasted rats treated with 0.44 mg/kg olanzapine (gray bars) or vehicle (white bars). Drugs were injected into either (A) the third ventricle (3V) or (B) the lateral ventricle (LV). Blood glucose was measured before injection (time 0) and 30 minutes later. On a different day, rats were given a 3V injection of olanzapine or vehicle and were also tested at 60 minutes (C). ***Significantly different from controls at the same time point at P < .001.
Figure 3.
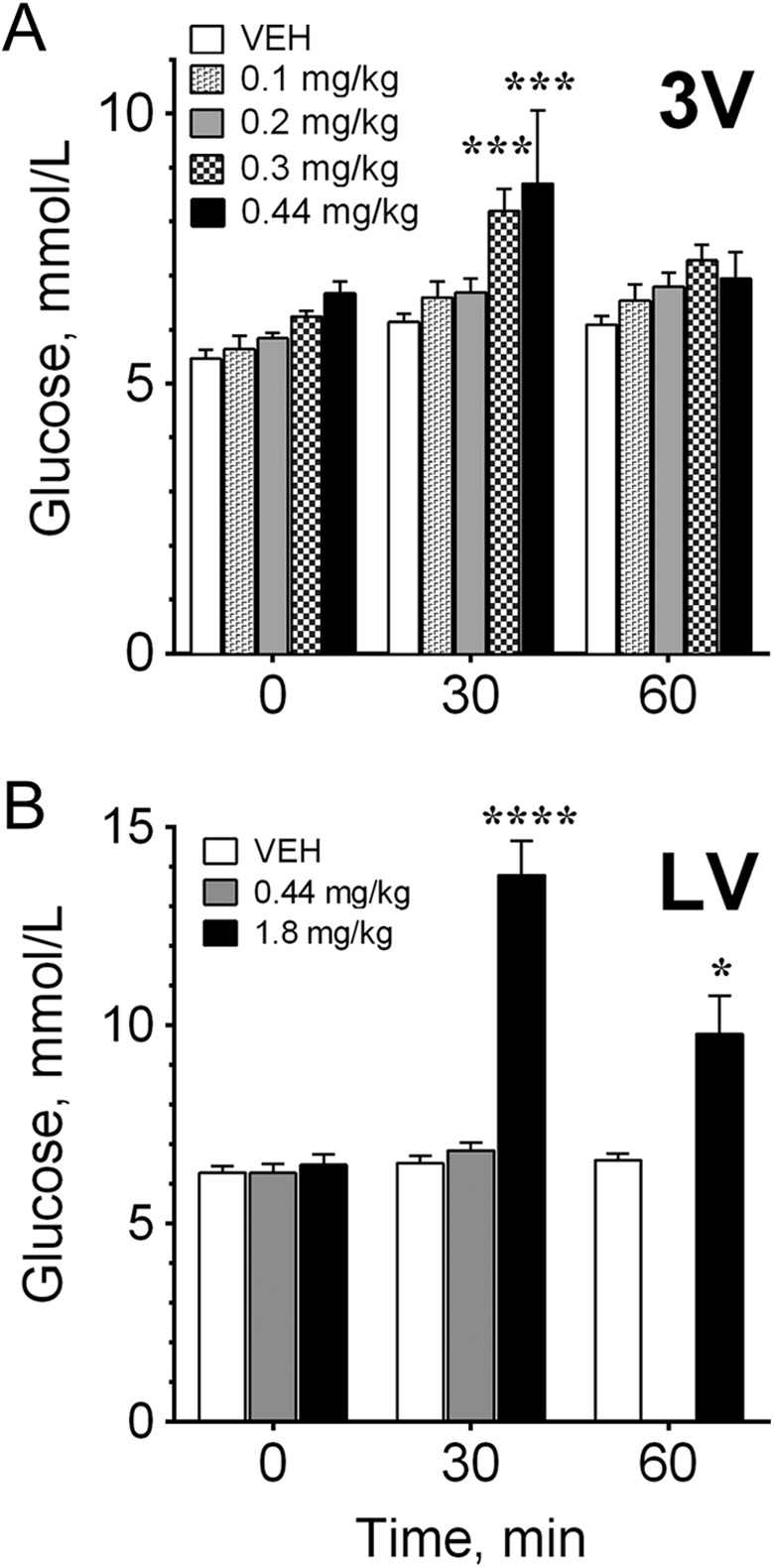
Dose-dependent effects of olanzapine on glycemia. A, Olanzapine was injected in the third ventricle (3V), and blood glucose was measured at 0, 30, and 60 minutes after treatment. B, Olanzapine was injected unilaterally into the left or right lateral ventricle (LV), and glucose was measured at the same time points. Because there was a lack of an effect of 0.44 mg/kg olanzapine into the LV at 30 minutes, blood glucose was not measured at 60 minutes for that treatment. Significantly different from controls at the same time point at *P < .05, ***P < .001, and ****P < .0001.
Glycemic Effect of the Lateral Ventricle OLZ Infusion
Olanzapine at 0.44 mg/kg was administered unilaterally into either the left or the right lateral ventricle. No significant effect on blood glucose was observed after 30 minutes compared to VEH (Figures 2B and 3B) in contrast to the third ventricle infusion (Figure 2A). When we increased the dose by over 4× to 1.8 mg/kg and administered it into the lateral ventricle (Figure 3B), we did observe a significant increase in blood glucose at 30 minutes (P < .0001) and 60 minutes (P < .05) compared to VEH, but we cannot rule out drug diffusion into other brain regions or the peripheral circulation at this dose.
Glycemic Effect of Oral OLZ Administration
Olanzapine significantly increased blood glucose within 30 minutes when given via oral gavage at 3 mg/kg (P < .05) and 10 mg/kg (P < .0001; Figure 4). Blood glucose remained elevated for 60 minutes after oral gavage of 10 mg/kg OLZ (P < .001), but not at lower doses.
Figure 4.
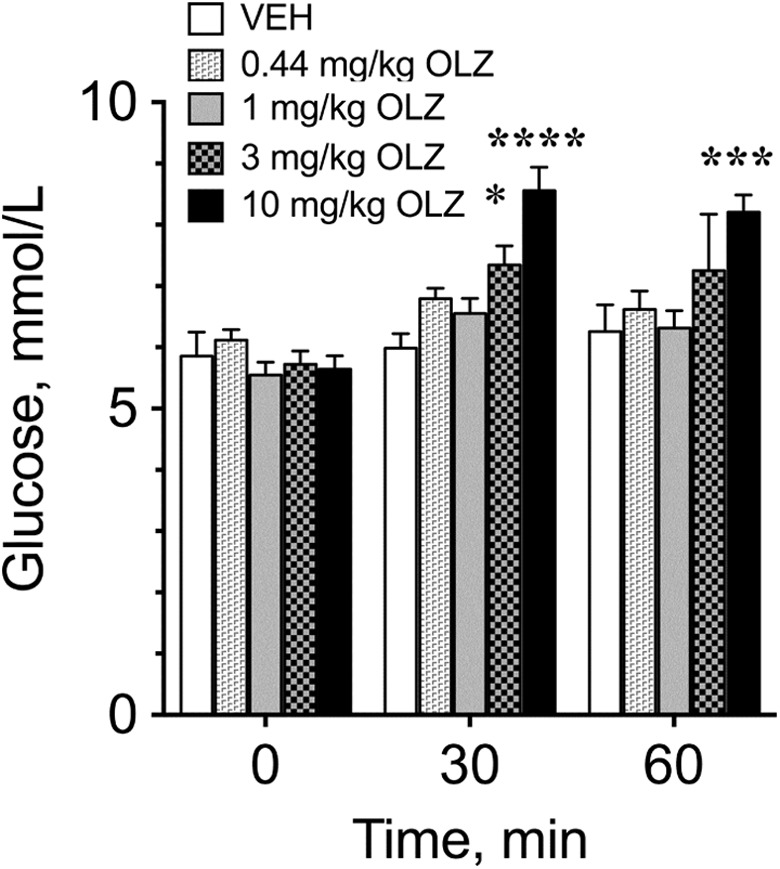
Blood glucose concentrations of fasted rats treated with 0.44 to 10 mg/kg of olanzapine via oral gavage. Blood glucose was measured at 0, 30, and 60 minutes after treatment. *Significantly different from controls at the same time point at *P < .05, ***P < .001, and ****P < .0001.
Effects of OLZ on Energy Expenditure and Locomotor Activity
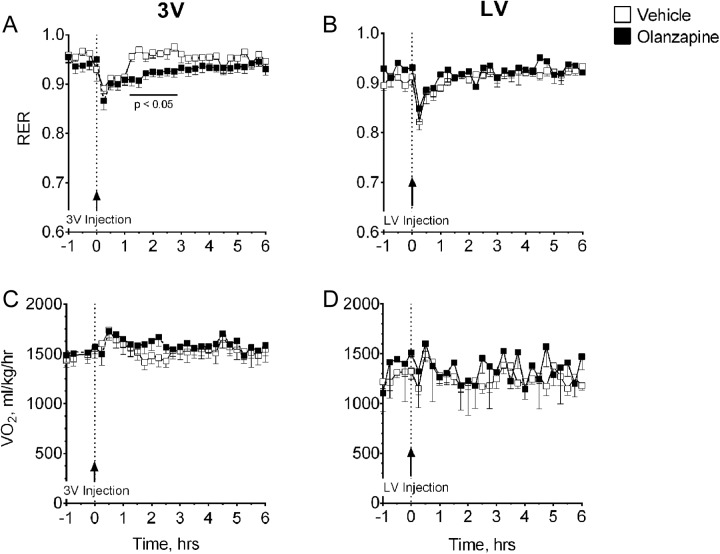
Olanzapine (0.44 mg/kg) significantly (P < .05) decreased dark cycle RER within 75 minutes when injected into the third ventricle (Figure 5A), and this effect persisted through 135 minutes postinjection. This effect was not observed when OLZ was injected at the same dose into the lateral ventricle (Figure 5B). This dose of OLZ had no measureable effect on whole body oxygen consumption, Vo 2, when injected into either brain region (Figure 5C and D).
Figure 5.
Effects of 0.44 mg/kg of centrally administered olanzapine (OLZ) on energy expenditure. Respiratory exchange ratio (RER) was determined after the administration of OLZ (injection shown at arrow) into (A) the third ventricle (3V) or (B) the lateral ventricle (LV), and whole body oxygen consumption (Vo 2) was measured after treatment into (C) the third ventricle or (D) the lateral ventricle. *Olanzapine significantly decreased RER 75 to 135 minutes after injection into the third ventricle at P < .05.
To compare the timing effects of central to peripheral treatment with OLZ on RER, we also gave the drug via oral gavage (4 mg/kg). Within 30 minutes, OLZ caused a rapid drop in RER that was significantly different (P < .05) from animals given VEH (Figure 6). This decrease in RER remained significant for 180 minutes posttreatment.
Figure 6.
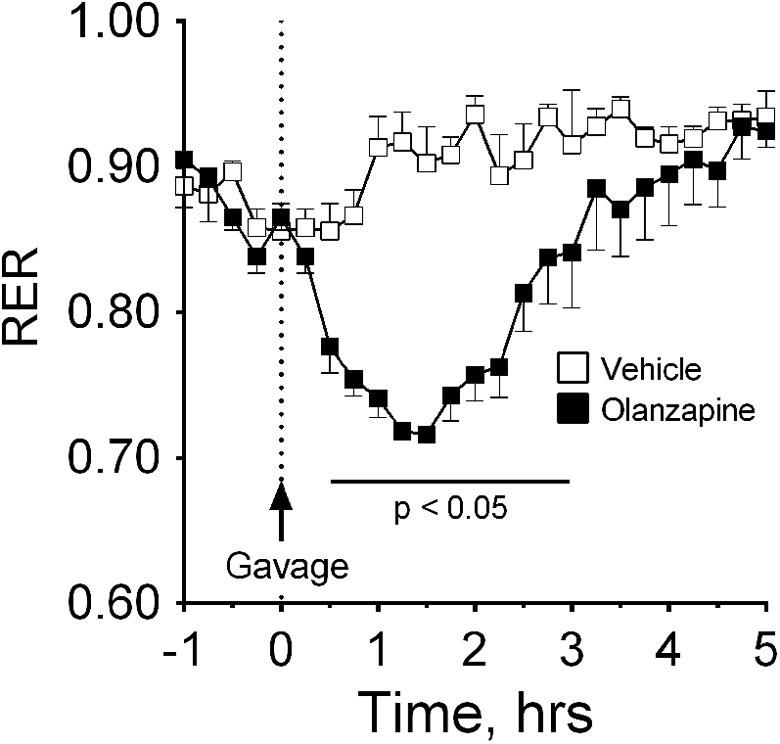
Effects of (4 mg/kg) olanzapine (OLZ) given intragastrically on energy expenditure. Respiratory exchange ratio (RER) was determined after the administration of olanzapine (n = 4) or vehicle (n = 4) via oral gavage. *Olanzapine significantly decreased RER 30 to 180 minutes after oral gavage at P < .05.
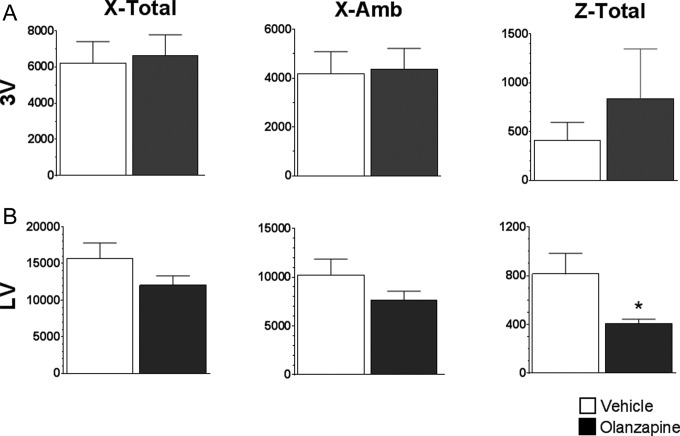
Since others have reported a sedative effect when OLZ was given peripherally,22,23 we tested whether centrally administered OLZ would cause sedation using the number of infrared beam breaks across the x, y, and z axes over 3 hours after treatment. Both lateral and third ventricle injection of OLZ (0.44 mg/kg) had little effect on activity levels (Figure 7). The only movement affected by OLZ was along the z-axis (up–down) in animals injected into the lateral ventricle (Figure 7B).
Figure 7.
Effects of 0.44 mg/kg of olanzapine (OLZ) on activity levels. The number of infrared beam breaks along the x-axis (x-total), y-axis (x-amb), and z-axis (z-total) was summed over 3 hours after injection of OLZ into (A) the third ventricle (3V) or (B) the lateral ventricle (LV). *Significantly different from controls at P < .05.
Angiotensin II Cannula Placement Test
All rats had correct cannula placement as verified by injecting 200 ng/kg of angiotensin II into the ventricles (data not shown). Initially, 1 rat did not meet our criteria for verifying correct cannula placement (consumed <5 mL of water in 30 minutes), but upon a second angiotensin test, it was determined that his sipper tube had malfunctioned and actually 12 mL of water was consumed during the second test. Rats were given both angiotensin II and VEH on different days. Angiotensin II-treated rats consumed 11.67 ± 2.38 mL of water in 30 minutes compared to 0.58 ± 0.42 mL for VEH-treated rats (P < .001).
Discussion
Olanzapine caused a rapid, pronounced increase in blood glucose when infused into the third ventricle, lateral ventricle, or intragastrically. Changes in whole body glucose were most sensitive when infused into the third ventricle. Olanzapine induced hyperglycemia within 30 minutes after the third ventricle infusion at 0.3 mg/kg. This effect was transient, with blood glucose approaching baseline after 60 minutes. Lateral ventricle infusion of OLZ also induced hyperglycemia, albeit at a dose 6× greater than that needed to produce similar results obtained by the third ventricle infusion. Unlike third ventricle infusion of OLZ at 0.44 mg/kg, blood glucose remained elevated when measured at 60 minutes when given at 1.8 mg/kg, but with such a high dose, we cannot eliminate the possibility of drug diffusion into other ventricles or the periphery. Changes in whole body blood glucose were least sensitive when OLZ was administered via oral gavage. A dose of 3 mg/kg was necessary to increase blood glucose within 30 minutes, and 10 mg/kg was necessary to prevent the transient decrease in glucose concentrations within 60 minutes. Although there appears to be an immediate glycemic effect occurring at the level of the third ventricle, a longer-lasting effect of OLZ is being maintained in the periphery.
Some attribute this rapid rise in glycemia to an increase in hepatic glucose output, in this case, an effect mediated by the hypothalamus after centralized, third ventricle infusion. Olanzapine treatment has resulted in an increase in blood glucose in humans24,25 as well as animal models.10,26,27 We chose to measure fasting glucose levels after the administration of OLZ, whereas others have performed glucose tolerance tests.13,17,27,28 Regardless of the test, the increase in glycemia indicated here and by others may be a result of an increase in endogenous glucose production from the liver29,30 and/or a decrease in the rate of glucose disposal.31,32
Although the glycemic effects of OLZ took longer to manifest when given peripherally, its effect on metabolic fuel preference (RER) occurred more than 2× as fast compared to when the drug was given centrally (30 minutes for oral vs 75 minutes for third ventricle) and lasted slightly longer (180 minutes after oral and 135 minutes after third ventricle infusion). This decrease in the RER is due to a switch in metabolic substrate from glucose to fatty acids. This preference for fatty acid fuel utilization may be attributed to a rapidly developing insulin resistance.27,28,30,31,33 We predict that although OLZ and other AAPs cause hyperglycemia through an increase in hypothalamic-mediated increase in glucose rate of appearance (Ra; eg, hepatic glucose output), individuals are unable to utilize the glucose in the blood due to a decrease in glucose rate of disappearance (Rd). This presents as an insulin resistance leading to a shift in metabolic fuel preference from glucose to fatty acids. The present findings indicate that this is happening through a peripheral mechanism.
In summary, a very low dose of OLZ (0.3 mg/kg) infused into the third ventricle resulted in a rapid, pronounced hyperglycemia, whereas intragastric treatment caused a rapid drop in RER, indicating a switch from glucose to fatty acid fuel preference. These combined results indicate that OLZ may be influencing blood glucose through a dual mechanism, with a centralized component located near the third ventricle that may have direct effects on hepatic glucose output and a peripheral component that causes a rapid switch in metabolic fuel preference through other mechanisms (see Discussion). Identifying specifically how OLZ and other AAPs are influencing the action(s) of the brain and peripheral mechanisms associated with insulin and glucose regulation is paramount to understanding the metabolic disturbances associated with second-generation antipsychotic use.
Acknowledgments
The authors would like to thank Dr Carolyn Pritchett for her expertise in rodent anatomy and surgical techniques as well as Yuping Xu and Anne Pruznack for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant, DK084428. The NIDDK had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication.
References
- 1. Ananth J, Parameswaran S, Gunatilake S. Side effects of atypical antipsychotic drugs. Curr Pharm Des. 2004;10(18):2219–2229. [DOI] [PubMed] [Google Scholar]
- 2. Arana GW. An overview of side effects caused by typical antipsychotics. J Clin Psychiatry. 2000;61(suppl 8):5–11; discussion 12-213. [PubMed] [Google Scholar]
- 3. Citrome L, Holt RI, Walker DJ, Hoffmann VP. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455–482. [DOI] [PubMed] [Google Scholar]
- 4. Politte LC, McDougle CJ. Atypical antipsychotics in the treatment of children and adolescents with pervasive developmental disorders. Psychopharmacology (Berl). 2014;231(6):1023–1036. [DOI] [PubMed] [Google Scholar]
- 5. Camsari U, Viguera AC, Ralston L, Baldessarini RJ, Cohen LS. Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Arch Womens Ment Health. 2014;17(6):583–586. [DOI] [PubMed] [Google Scholar]
- 6. Han C, Pae CU, Wang SM, et al. The potential role of atypical antipsychotics for the treatment of posttraumatic stress disorder. J Psychiatr Res. 2014;56:72–81. [DOI] [PubMed] [Google Scholar]
- 7. Martins PJ, Haas M, Obici S. Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes. 2010;59(10):2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Girault EM, Alkemade A, Foppen E, Ackermans MT, Fliers E, Kalsbeek A. Acute peripheral but not central administration of olanzapine induces hyperglycemia associated with hepatic and extra-hepatic insulin resistance. PLoS One. 2012;7(8):e43244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hahn MK, Chintoh A, Remington G, et al. Effects of intracerebroventricular (ICV) olanzapine on insulin sensitivity and secretion in vivo: an animal model. Eur Neuropsychopharmacol. 2014;24(3):448–458. [DOI] [PubMed] [Google Scholar]
- 10. Klingerman CM, Stipanovic ME, Bader M, Lynch CJ. Second-generation antipsychotics cause a rapid switch to fat oxidation that is required for survival in C57BL/6J mice. Schizophr Bull. 2014;40(2):327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albaugh VL, Vary TC, Ilkayeva O, et al. Atypical antipsychotics rapidly and inappropriately switch peripheral fuel utilization to lipids, impairing metabolic flexibility in rodents. Schizophr Bull. 2012;38(1):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8(3):523–534. [DOI] [PubMed] [Google Scholar]
- 13. Albaugh VL, Henry CR, Bello NT, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity (Silver Spring). 2006;14(1):36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 6th ed: Oxford, UK: Academic Press; 2008. [Google Scholar]
- 15. Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 pt 2):r848–r856. [DOI] [PubMed] [Google Scholar]
- 16. Savoy YE, Ashton MA, Miller MW, et al. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr Bull. 2010;36(2):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albaugh VL, Judson JG, She P, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16(5):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benzo CA. Minireview. The hypothalamus and blood glucose regulation. Life sci. 1983;32(22):2509–2515. [DOI] [PubMed] [Google Scholar]
- 19. Xie S, Furjanic MA, Ferrara JJ, et al. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism—or inverse agonism—as potential obesity treatment and other therapeutic use. J Clin Pharm Ther. 2007;32(3):209–231. [DOI] [PubMed] [Google Scholar]
- 20. Lynch CJ, Zhou Q, Shyng SL, et al. Some cannabinoid receptor ligands and their distomers are direct-acting openers of SUR1 K(ATP) channels. Am J Physiol Endocrinol Metab. 2012;302(5):e540–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bishara D, Olofinjana O, Sparshatt A, Kapur S, Taylor D, Patel MX. Olanzapine: a systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol. 2013;33(3):329–335. [DOI] [PubMed] [Google Scholar]
- 22. Ahnaou A, Megens AA, Drinkenburg WH. The atypical antipsychotics risperidone, clozapine and olanzapine differ regarding their sedative potency in rats. Neuropsychobiology. 2003;48(1):47–54. [DOI] [PubMed] [Google Scholar]
- 23. Cooper GD, Goudie AJ, Halford JC. Acute effects of olanzapine on behavioural expression including the behavioural satiety sequence in female rats. J Psychopharmacol. 2010;24(7):1069–1078. [DOI] [PubMed] [Google Scholar]
- 24. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1–93. [DOI] [PubMed] [Google Scholar]
- 25. Lindenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160(2):290–296. [DOI] [PubMed] [Google Scholar]
- 26. Assie MB, Carilla-Durand E, Bardin L, et al. The antipsychotics clozapine and olanzapine increase plasma glucose and corticosterone levels in rats: comparison with aripiprazole, ziprasidone, bifeprunox and F15063. Eur J Pharmacol. 2008;592(1-3):160–166. [DOI] [PubMed] [Google Scholar]
- 27. Boyda HN, Procyshyn RM, Tse L, et al. Intermittent treatment with olanzapine causes sensitization of the metabolic side-effects in rats. Neuropharmacology. 2012;62(3):1391–1400. [DOI] [PubMed] [Google Scholar]
- 28. Boyda HN, Procyshyn RM, Tse L, et al. Differential effects of 3 classes of antidiabetic drugs on olanzapine-induced glucose dysregulation and insulin resistance in female rats. J Psychiatry Neurosci. 2012;37(6):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32(2):289–297. [DOI] [PubMed] [Google Scholar]
- 30. Chintoh AF, Mann SW, Lam L, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28(5):494–499. [DOI] [PubMed] [Google Scholar]
- 31. Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS One. 2011;6(8):e22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chintoh AF, Mann SW, Lam TK, Giacca A, Remington G. Insulin resistance following continuous, chronic olanzapine treatment: an animal model. Schizophr Res. 2008;104(1–3):23–30. [DOI] [PubMed] [Google Scholar]