Abstract
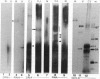
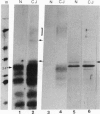
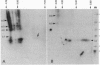
The nature of the infectious agent causing human Creutzfeldt-Jakob disease (CJD), a slowly progressive dementia, is controversial. As in scrapie, no agent-specific proteins or nucleic acids have been identified. However, biological features of exponential replication and agent strain variation, as well as physical size and density data, are most consistent with a viral structure--i.e., a nucleic acid-protein complex. It is often assumed that nuclease treatment, which does not reduce infectious titer, leaves no nucleic acids of > 50 bp. However, nucleic acids of 500-6000 bp can be extracted from highly purified infectious complexes with a mass of approximately 1.5 x 10(7) daltons. It was therefore germane to search for nucleic acid binding proteins that might protect an agent genome. We here use Northwestern blotting to show that there are low levels of nonhistone nucleic acid binding proteins in highly purified infectious 120S gradient fractions. Several nucleic acid binding proteins were clearly host encoded, whereas others were apparent only in CJD, but not in parallel preparations from uninfected brain. Small amounts of residual host Gp34 (prion protein) did not bind any 32P-labeled nucleic acid probes. Most of the minor "CJD-specific" proteins had an acidic pI, a characteristic of many viral core proteins. Such proteins deserve further study, as they probably contribute to unique properties of resistance described for these agents. It remains to be seen if any of these proteins are agent encoded.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., Williamson J. L., Borchardt L. M., Marsh R. F. Presence of mitochondrial D-loop DNA in scrapie-infected brain preparations enriched for the prion protein. J Virol. 1990 Jul;64(7):3265–3268. doi: 10.1128/jvi.64.7.3265-3268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akowitz A., Manuelidis L. A novel cDNA/PCR strategy for efficient cloning of small amounts of undefined RNA. Gene. 1989 Sep 30;81(2):295–306. doi: 10.1016/0378-1119(89)90190-x. [DOI] [PubMed] [Google Scholar]
- Akowitz A., Sklaviadis T., Manuelidis E. E., Manuelidis L. Nuclease-resistant polyadenylated RNAs of significant size are detected by PCR in highly purified Creutzfeldt-Jakob disease preparations. Microb Pathog. 1990 Jul;9(1):33–45. doi: 10.1016/0882-4010(90)90038-r. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Zapp M. L., Green M. R., Szostak J. W. HIV-1 Rev regulation involves recognition of non-Watson-Crick base pairs in viral RNA. Cell. 1991 Nov 1;67(3):529–536. doi: 10.1016/0092-8674(91)90527-6. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Diener T. O., McKinley M. P., Groth D. F., Smith D. R., Prusiner S. B. Purified scrapie prions resist inactivation by procedures that hydrolyze, modify, or shear nucleic acids. Virology. 1987 Sep;160(1):271–274. doi: 10.1016/0042-6822(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. E., McConnell I., Fraser H., Dickinson A. G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol. 1991 Mar;72(Pt 3):595–603. doi: 10.1099/0022-1317-72-3-595. [DOI] [PubMed] [Google Scholar]
- Czub M., Braig H. R., Diringer H. Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titres and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J Gen Virol. 1986 Sep;67(Pt 9):2005–2009. doi: 10.1099/0022-1317-67-9-2005. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Fraser H., McConnell I., Outram G. W., Sales D. I., Taylor D. M. Extraneural competition between different scrapie agents leading to loss of infectivity. Nature. 1975 Feb 13;253(5492):556–556. doi: 10.1038/253556a0. [DOI] [PubMed] [Google Scholar]
- Diringer H. Sustained viremia in experimental hamster scrapie. Brief report. Arch Virol. 1984;82(1-2):105–109. doi: 10.1007/BF01309373. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A., Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol. 1989 Aug;70(Pt 8):2017–2025. doi: 10.1099/0022-1317-70-8-2017. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacs E. J., Manuelidis L. Viremia in experimental Creutzfeldt-Jakob disease. Science. 1978 Jun 2;200(4345):1069–1071. doi: 10.1126/science.349691. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Interspecies transmission of Creutzfeldt-Jakob disease to Syrian hamsters with reference to clinical syndromes and strains of agent. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3432–3436. doi: 10.1073/pnas.75.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Kim J. H., Mericangas J. R., Manuelidis L. Transmission to animals of Creutzfeldt-Jakob disease from human blood. Lancet. 1985 Oct 19;2(8460):896–897. doi: 10.1016/s0140-6736(85)90165-5. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E. Transmission of Creutzfeldt-Jakob disease from man to the guinea pig. Science. 1975 Nov 7;190(4214):571–572. [PubMed] [Google Scholar]
- Manuelidis L., Murdoch G., Manuelidis E. E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Sklaviadis T., Manuelidis E. E. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987 Feb;6(2):341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N., Rosenbaum V., Schmidt B., Gilles K., Mirenda C., Groth D., Prusiner S. B., Riesner D. Search for a putative scrapie genome in purified prion fractions reveals a paucity of nucleic acids. J Gen Virol. 1991 Jan;72(Pt 1):37–49. doi: 10.1099/0022-1317-72-1-37. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Sklaviadis T., Manuelidis E. E., Manuelidis L. Potential retroviral RNAs in Creutzfeldt-Jakob disease. J Virol. 1990 Apr;64(4):1477–1486. doi: 10.1128/jvi.64.4.1477-1486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Groth D. F., Prusiner S. B., Weissmann C. Search for a scrapie-specific nucleic acid: a progress report. Ciba Found Symp. 1988;135:209–223. doi: 10.1002/9780470513613.ch14. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Scrapie prions. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Merz P. A., Kascsak R. J., Scalici C. L., Papini M. C., Carp R. I., Kimberlin R. H. Scrapie-infected spleens: analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J Infect Dis. 1991 Jul;164(1):29–35. doi: 10.1093/infdis/164.1.29. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T. K., Manuelidis L., Manuelidis E. E. Physical properties of the Creutzfeldt-Jakob disease agent. J Virol. 1989 Mar;63(3):1212–1222. doi: 10.1128/jvi.63.3.1212-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T., Akowitz A., Manuelidis E. E., Manuelidis L. Nuclease treatment results in high specific purification of Creutzfeldt-Jakob disease infectivity with a density characteristic of nucleic acid-protein complexes. Arch Virol. 1990;112(3-4):215–228. doi: 10.1007/BF01323166. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T., Dreyer R., Manuelidis L. Analysis of Creutzfeldt-Jakob disease infectious fractions by gel permeation chromatography and sedimentation field flow fractionation. Virus Res. 1992 Dec;26(3):241–254. doi: 10.1016/0168-1702(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T., Manuelidis L., Manuelidis E. E. Characterization of major peptides in Creutzfeldt-Jakob disease and scrapie. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6146–6150. doi: 10.1073/pnas.83.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y. G., Ingrosso L., Ladogana A., Masullo C., Pocchiari M. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature. 1992 Apr 16;356(6370):598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]