Abstract
Plant-derived antioxidants with free radical scavenging activities can be relevant as chemopreventive agents against the numerous diseases associated with free radicals and reactive oxygen species. Some phytoconstituents possess antioxidant activities in biological systems. On this basis, we evaluated the antioxidant potential, and determined the total phenolic and flavonoid contents of the ethanol extract of the stem bark of Anogeissus leiocarpus [EESAL]. Antioxidant assays carried out include: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, phosphomolybdate, β-carotene bleaching, ferric reducing, and hydroxyl radical scavenging activities. Results of DPPH assay showed no significant difference (p < 0.001) between EESAL and butylated hydroxyanisole [BHA], while EESAL exhibited a significantly (p < 0.001) higher activity than BHT [butylated hydroxytoluene]. Phosphomolybdate method recorded a total antioxidant capacity of 190.00 ± 70.53 µg butylated hydroxytoluene equivalents [BHTE]/mg dry extract, while β-carotene bleaching assay gave percent antioxidant activities of both EESAL and BHT as 81.46±1.62 and 80.90±1.39 respectively. Ferric reducing abilities of both EESAL and ascorbic acid increased in a concentration-dependent manner with EESAL displaying a significantly (p < 0.001) higher reductive activity than vitamin C. EESAL displayed a significantly higher hydroxyl radical scavenging activity as compared with BHT at the lowest concentration with no significant difference at the highest concentration. Total phenolic and flavonoid contents of EESAL were obtained as 608.10 ± 2.12 µg GAE/mg and 78.96 ± 3.37 µg QE/mg respectively. Taken together, the free radical scavenging and antioxidant activity of EESAL is likely due to its high phenolic content with complementary effects of the flavonoid components.
Keywords: Anogeissus leiocarpus, DPPH, antioxidants, free-radicals, BHT, BHA
INTRODUCTION
Free radicals can be said to be biologically essential because of their involvement in the induction of xenobiotic detoxification pathways, stimulation of signal transduction pathways, and stimulation of phagocytosis [1]. Paradoxically, the same radicals have been directly implicated in the pathogenesis of various diseases among which are atherosclerosis, aging, ischemia, and reperfusion injury of many tissues, central nervous system injury, gastritis, cancer etc. [1]. This stems from the fact that free radicals and reactive oxygen species (ROS), such as superoxide anion, hydroxyl radical, nitric oxide, peroxyl radical etc., are generally reactive with cellular macromolecules resulting in degradation/denaturation of proteins, lipid peroxidation, and oxidation of DNA due to oxidative stress [2]. These deleterious effects of oxidative stress can culminate into cell injury, cell transformation or cell death [3].
Antioxidants have the capability to scavenge free radicals and thus neutralize them from being able to reactively damage cellular lipids, proteins, enzymes, carbohydrates and DNA [4]. Plant-derived antioxidants with free radical scavenging activities can have great relevance as chemopreventive agents against the aforementioned free-radical-associated diseases [5]. Some phenolic compounds and flavonoids possess antioxidant activities because they are able to act as scavengers of singlet oxygen and free radicals in biological systems [6]. Phenolics are among the major phytochemicals that have been considered as bioactive compounds with health benefits based on clinical trials and epidemiological studies of oxidative stress related diseases.
The application of plant parts as sources of medicinal agents dates back to ancient times coupled with the corroboration by the World Health Organisation (WHO) that almost 80% of the world inhabitants depend majorly on traditional medicines [7]. Anogeissus leiocarpus (DC) Guill and Perr family Combretaceae (English/common name: Axlewood tree; Yoruba name: Ayin) is one of the medicinal plants contained in the Nigeria’s diverse flora and is found majorly in the Sahel to forest zones [8]. It has varied applications in Nigerian traditional medicine mostly in the treatment of respiratory diseases (asthma, catarrh, chronic bronchitis, cough, hay-fever, hemoptysis, pneumonia, pulmonary disorders and tuberculosis), ascaricide, gonorrhoea, general body pain, blood clots etc. [9]. Various parts (leaf, stem bark, root) of A. leiocarpus are also relevant as remedies for many ailments of livestock and specifically, the leaves are feeds for livestock [10]. However, a review of literature on A. leiocarpus showed that there is a dearth of information on the potency of extracts from the plant to serve as free radical scavengers and antioxidants. Therefore, the main objective of this study was to evaluate the in vitro antioxidant potential, and quantitatively determine the total phenolic and flavonoid contents of the ethanol extract of the stem bark of Anogeissus leiocarpus.
MATERIALS AND METHODS
Chemicals
Chemicals used in this study are ethylenediaminetetraacetic acid, potassium ferricyanide, trichloroacetic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu reagent, gallic acid, L-ascorbic acid, 2-deoxy-D-ribose, β-carotene, linoleic acid, thiobarbituric acid, Tween 20, trichloroacetic acid, ammonium molybdate, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), sodium bicarbonate, ferric chloride hexahydrate, ethanol, hydrogen peroxide etc. All reagents used were of analytical grade and were purchased from Sigma Co. (St. Louis, USA).
Plant source and identification
The stem bark of Anogeissus leiocarpus was obtained from Lokoja, Nigeria. It was identified and authenticated at the Department of Botany and Microbiology, University of Ibadan, Ibadan, Nigeria, and a voucher specimen was deposited in the University Herbarium with a voucher number UIH 22402.
Preparation of extract
The plant part of interest was washed with distilled water (dH2O) to remove any contaminants, air-dried under shade until it attained a constant weight, grinded to powder, sieved, packed into polythene bags and stored at 4 °C. Four hundred grams (400 g) of the powered plant part was macerated in 70 % ethanol (1600 mL) for 72 hours with intermittent stirring/shaking [11]. At the end of the extraction, the extract was filtered using Whatman filter paper No.1 (Whatman Ltd., England) to remove all unextractable matters, including cellular materials and other constituents that are insoluble in the extraction solvent. The filtrate was concentrated using a rotary evaporator (RE-52A, Shanghai Ya Rong Biochemistry Instrument Factory, Shanghai) under reduced pressure (in order to speed up the process) at 40 °C and stored at 4 °C until when needed. The percentage yield of the extraction was 10.00 % w/w.
Quantitative phytochemical analysis
Determination of total phenolic content (TPC)
The amount of total phenolics in the plant extract was determined with the Folin-Ciocalteu reagent using the method of Spanos and Wrolstad [12], as modified by Lister and Wilson [13].
Determination of total flavonoid content (TFC)
The determination of the total flavonoid content (TFC) was carried out as described by Nickavar [14].
In vitro antioxidant/free-radical scavenging activity assays
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The antiradical activity of the extract was estimated according to the procedure described by Nickavar [15]. The percentage of the radical scavenging activity (RSA) was calculated based on the following equation:
Acont and Asample are the absorbance values for the control and sample respectively.
The results were also expressed as equivalent antioxidant capacity of standards i.e. mg standard equivalents/mg dry wt of extract, which was calculated as follows [16]:
Where IC50 Vit C and IC50 sample are the effective concentrations of vitamin C and sample respectively.
Total antioxidant assay by phosphomolybdate method
The total antioxidant capacity of the extract was determined by phosphomolybdate method using butylated hydroxytoluene (BHT) as the standard compound [17]. The total antioxidant capacity was expressed as µg equivalents of butylated hydroxytoluene (BHT) by using the standard BHT graph.
B-carotene–linoleate bleaching assay
The antioxidant activity of the extract was also assayed based on the β-carotene bleaching (BCB) method developed by Velioglu [18]. Degradation rate (DR) was calculated according to first order kinetics, using the following equation based on Al-Saikhan [19]:
where ln is natural log, a is the initial absorbance (@470 nm) at time 0, b is the absorbance (@470 nm) at 20, 40, 60, 80, 100 or 120 mins and t is the initial absorbance (470 nm) at time 0. Antioxidant activity (AA) was expressed as percent of inhibition relative to the control, using the following formula:
Ferric ion reducing power (FRP)
The reducing power of the extract was investigated by the Fe3+-Fe2+ transformation as described by Fejes [20]. All tests were performed in triplicate and the graph was plotted with the average of the three determinations.
Hydroxyl radical scavenging assay
Non-site-specific hydroxyl radical-mediated 2-deoxy-D-ribose degradation:
Hydroxyl radical scavenging activity was measured by the ability of the extract to scavenge the hydroxyl radicals generated by the Fe3+-ascorbate-EDTA-H2O2 system (Fenton reaction) [21]. The scavenging activity on hydroxyl radicals was expressed as:
Where A0 is the absorbance of the negative control (without sample) at 532 nm, and A is the absorbance at 532 nm of the reaction mixture containing sample.
Statistical analysis
Data were analysed using Sigma Plot (version 11.0). The results were expressed as mean ± SD and the IC50 values were obtained from the linear regression plots. Pearson’s correlation test was used to assess correlations between means. One-way ANOVA was used to assess differences between means at p<0.001 level of significance. The means were compared with control groups using Holm-Sidak Test.
RESULTS
Quantitative phytochemical analyses
Total phenolic content (TPC)
Quantitative assessment of TPC shows that EESAL contained 608.10 ± 2.12 microgram gallic acid equivalents per milligram (µg GAE/mg) of dry plant extract.
Total flavonoid content (TFC)
We recorded a TFC value of 78.96 ± 3.37 microgram quercetin equivalents per milligram (µg QE/mg) of dry extract.
In vitro antioxidant/free-radical scavenging activity assays
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging assay:
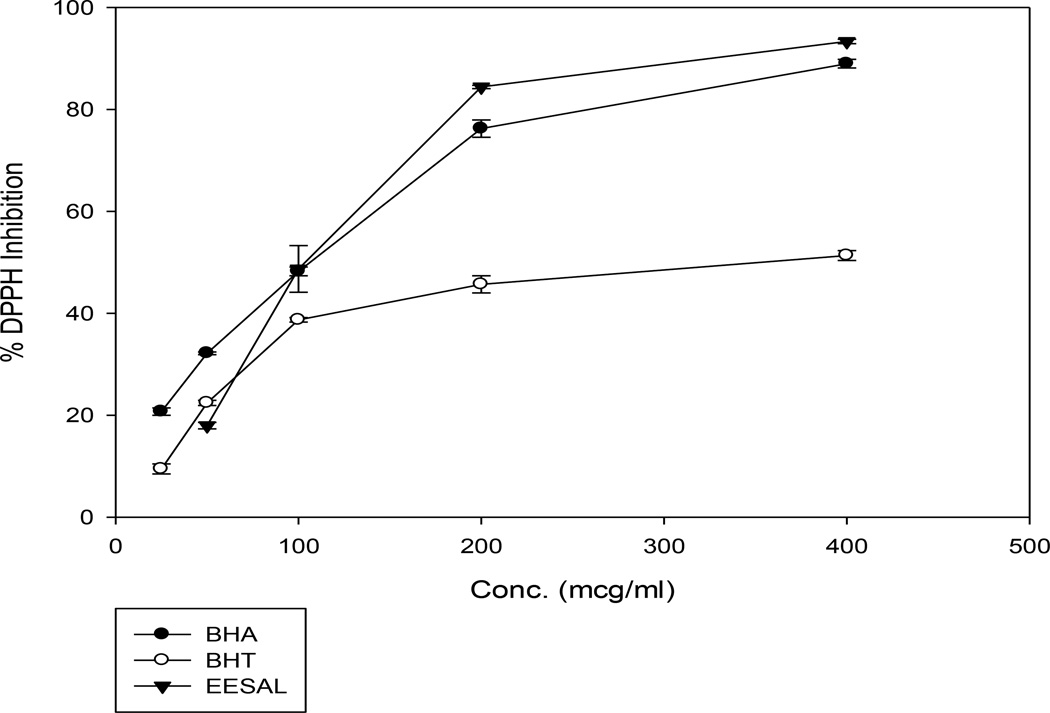
At a low concentration of 50 µg/mL, BHA recorded a statistically higher DPPH free radical scavenging activity than EESAL as shown in Figure 1, while a comparison between EESAL and BHT did not portray any statistical difference (p<0.001). On the other hand, at a higher concentration of 400 µg/mL, there was no statistical difference in the activities of both BHA and EESAL, whereas EESAL gave a statistically (p<0.001) higher activity than BHT.
Figure 1.
The percent DPPH radical inhibition of BHA, BHT and EESAL. Values are the average of triplicate experiments.
Figure 2 shows the inhibitory concentration (IC)-50 values of the standards (BHA and BHT), and EESAL. EESAL recorded the lowest IC50 value of 104.74 µg/mL, followed by BHA which was 112.05 µg/mL and lastly BHT with a value of 202.35 µg/mL. Beside these values, the equivalents of EESAL with respect to the standards were found to be 1.07 mg BHA equivalents/ mg dry weight of extract and 1.93 mg BHT equivalents/ mg dry weight of extract.
Figure 2.
IC 50 values of EESAL, and the standards (BHA and BHT).
B-carotene bleaching (BCB) assay
Figure 3 presents the degradation curves of the negative and positive controls along with that of EESAL. Ethanol served as our negative control while BHT was the positive control. The percent antioxidant activity of EESAL was found to be significantly (p<0.001) higher than that of BHT as shown in Figure 4.
Figure 3.
Degradation rate of EESAL assayed by β-carotene bleaching method. Each point represents the average of triplicate values.
Figure 4.
Antioxidant activity (%) of EESAL assayed by β-carotene–linoleate bleaching. Values are mean ± SD for triplicate assay. *Significantly different (p< 0.001).
Total antioxidant assay by phosphomolybdate method
Based on this method, EESAL displayed a total antioxidant capacity of 190.00 ± 70.53 µg butylated hydroxytoluene equivalents [BHTE]/mg dry extract.
Ferric ion reducing power (FRP)
The result in Figure 5 shows that the ferric reducing abilities of both EESAL and ascorbic acid. Comparison of means at the highest and lowest concentrations considered in this study i.e. 50 and 800 µg/mL respectively, shows that EESAL displayed a significantly (p < 0.001) higher reductive activity than the standard (Vitamin C) at both concentrations.
Figure 5.
Antioxidant activity of EESAL measured by the reducing power method. Ascorbic acid was used as a positive control. Each absorbance value represents the average of triplicates of different samples analysed.
Hydroxyl radical scavenging activity
The capability of EESAL to scavenge hydroxyl radicals is evident from Figure 6. EESAL displayed a significantly (p<0.001) higher hydroxyl radical scavenging activity when compared with the standard, BHT at the lowest concentration of 50 µg/ml, but at the highest concentration (800 µg/ml), there was no significant difference in the activities of EESAL and BHT.
Figure 6.
Hydroxyl radical scavenging activity of EESAL and the positive control [BHT]. All values are reported as means ± SD (n = 3).
DISCUSSION
This study demonstrates that ethanol extract of the stem bark of Anogeissus leiocarpus [EESAL] possesses potent free radical-scavenging and antioxidant activities in comparison with standard/reference compounds. The antioxidant activities of plant extracts are majorly due to the presence of phytochemicals such as polyphenols/flavonoids, which have been known to have the capacity to absorb, neutralize, and to quench singlet and triplet oxygen [22]. Plants generate phenolics as secondary metabolites and these metabolites possess a wide range of medicinal properties including anticarcinogenic, antioxidant, antimutagenic, free radical-scavenging activities etc. [23]. These activities could be attributed to their redox properties, presence of conjugated ring structures and carboxylic groups [24]. Cook and Samman posited that flavonoids and phenolic compounds have considerable positive effects on human nutrition and health because of their strong antioxidant potential [25]. It was on this basis that it became necessary for us to determine the total phenolic and total flavonoid contents of EESAL since Anogeissus leiocarpus has been known to be locally used in traditional medical practices in Nigeria. The high TPC value obtained should be an indication of the potential therapeutic relevance of the plant extract based on the fact that phenolics have been associated with diverse biological properties [26]. Closely related to this value has also been reported by Annegowda for acid-hydrolysed and non-hydrolysed extracts of Terminalia catappa [27].
Flavonoids are known to exert several biological properties among which are anticancer, anticarcinogenic, antiulcer, antiallergic, antiinflammatory, antihepatotoxic, antiviral activities etc. These varied pharmacological and biological activities that they exhibit may be related to their ability to effectively scavenge or chelate reactive oxygen species [28]. Dorman reported that plant flavonoids which show an antioxidant activity in vitro correspondingly function as antioxidants in vivo [29]. Acid-hydrolysed and non-hydrolysed extracts of Terminalia catappa have been reported to have TFC values of 50.77 ± 0.93 and 70.36 ± 2.64 mg catechin equivalents (CAE)/g of extract respectively [27]; while Pourmorad reported the TFC of Adiantum capillus-veneris as 78.30 ± 4.50 mg quercetin equivalents (QE)/g of extract [29]. These values are within the range that we obtained for EESAL although Annegowda [27] used catechin as the standard flavonoid compound whereas we used quercetin.
Based on DPPH assay the overall activities of both the standard antioxidants and EESAL were concentration dependent. The activities of both BHA and BHT used as standard compounds in this study conformed to the results earlier reported in our laboratories [30]. The principle of this assay is that antioxidants can reduce the purple-coloured solution of DPPH to the yellow-coloured non-radical form DPPH-H in a concentration-dependent manner [31]. This colour change can be quantified spectrophotometrically at 518 nm as a measure of the ability of the antioxidant-containing substance to scavenge DPPH free radicals [32]. The potency of the extract in scavenging the artificial free radical (DPPH) seems to be more effective at higher concentrations. This is obvious from the higher radical inhibition exhibited by EESAL in comparison to both BHA and BHT at higher concentrations. It directly implies that the bioactive component(s) correspondingly increased with increasing concentrations and the bioactive component(s) may even be more potent than the standard compounds if the pure form is isolated. This standpoint is further corroborated by the IC50 values and equivalents of standard compounds that were obtained. The IC50 is the concentration of an antioxidant-containing substance required to scavenge 50% of the initial DPPH radicals. The lower the IC50 value, the more potent is the substance at scavenging DPPH and this implies a higher antioxidant activity. Our results therefore depict that EESAL is more potent than both standard compounds (BHA and BHT) and it can be inferred that EESAL acts as a primary antioxidant based on its capability to scavenge DPPH free radicals. This activity of EESAL may account for its application in treating various kinds of ailments especially those that are associated with free radical generation [33].
The total antioxidant capacity of EESAL was quantitatively determined based on phosphomolybdate assay and the result was reported as microgram BHT equivalents per mg (µg BHTE/mg) of dry extract [34]. This assay relies on the ability of an extract containing antioxidant(s) to donate an electron to Mo (VI) and reducing same to Mo (V) with the corresponding formation of green coloured phosphomolybdenum V complex which displays maximum absorbance at 695 nm. Zengin reported the total antioxidant capacity of methanolic extract of Centaurea urvillei as 143.53 mgTE/g i.e. on the basis of trolox equivalents [35]. The value of the total antioxidant capacity of EESAL reported in this work may point to the fact that EESAL is very rich in antioxidant content.
The BCB method quantifies the antioxidant activity of plant extracts based on their ability to inhibit the oxidation of β-carotene in the presence of linoleic acid free radicals. It involves the incubation of linoleic acid and β-carotene with the extract at 50 °C. Under this condition, free radicals are generated from linoleic acid by the abstraction of hydrogen atoms from its methylene groups; β-carotene is extremely sensitive to these free radicals and it becomes decolorized as a result of the oxidation of its double bonds [36]. The extent of decolorization (otherwise referred to as bleaching) of β-carotene is a measure of the amount of free radicals in the system. The presence of an extract with antioxidant activity reduces or prevents the initial oxidation of linoleic acid and therefore preserves the yellow/orange colour of β-carotene from bleaching. The rate of bleaching of the β-carotene solution is referred to as the degradation rate [37]. EESAL exhibited the lowest degradation rates as a confirmation of its ability to inhibit the formation of linoleate free radicals better than the standard (BHT) since the lower the degradation rates, the higher the antioxidant activity. As expected, BCB assay graphically displayed statistical difference between the control and the samples. The data here suggest that EESAL is more potent than BHT in preventing lipid peroxidation as can be seen from its significantly lower degradation rate.
In ferric ion reducing power (FRP) assay, the Fe3+/ferricyanide complex in the reaction mixture is reduced to the ferrous form (Fe2+) due to the presence of reductants in the antioxidant-containing samples/standard [38]. The reaction basically involves the conversion of the yellow colour of the test solution to various shades of green and blue colour depending on the reducing power of the samples. The Fe2+ so formed can be monitored by measuring the formation of Perl�s Prussian blue at 700 nm [39]. Based on this assay, the reducing capacity of a compound correlates well with its potential antioxidant activity [40]. The ferric reducing abilities of both EESAL and ascorbic acid increased in a concentration-dependent manner; this observation is in consonance with the report of Huda-Faujan [38]. Since the reductive capacity is a measure of the ability of a compound to donate electrons [41], it follows that EESAL should be a better free radical scavenger than vitamin C.
Some oxidation reactions involving metals lead to the generation of free radicals; hydroxyl radical is a typical example of such free radicals and it can attack cellular biomolecules such as proteins, lipids, deoxyribonucleic acid (DNA), carbohydrates etc. [42]. Hydroxyl radicals are therefore one of the major causative factors involved in the irreversible damage inflicted by oxidative stress. As a matter of fact, the damaging effect of hydroxyl radicals is known to be the strongest among free radicals by causing peroxidation of lipid components of biological membranes and other biological damage [43]. The typical mechanism of action of hydroxyl radical is through the abstraction of hydrogen atoms from biological molecules having hydrogen-containing groups such as thiols, leading, for example, to the formation of sulphur radicals which is capable of combining with oxygen to generate oxysulphur radicals. Oxysulphur radicals are known to damage biological molecules [44]. It can be deduced from our results in this assay that EESAL fits perfectly into the definition of an antioxidant as being “any substance that, when present at low concentration compared with those of an oxidisable substrate, significantly delays or prevents oxidation of that substrate” [43]. The biochemical relevance of this is that the presence of EESAL in a biological system has the tendency to mop up hydroxyl radicals generated as a result of normal metabolism and thus protect biomolecules against the damaging effects of the radicals.
CONCLUSION
Taken together, we have been able to show through this study that EESAL possesses potent free radical and antioxidant activities in comparison with well-known standard compounds. These activities of EESAL may be majorly attributed to its high phenolic content and the possible presence of multiple hydroxyl groups in the chemical structure of the bioactive principles contained in the extract. The next focus is on the in vivo activities of EESAL as a way to corroborate or refute the results obtained here.
ACKNOWLEDGEMENTS
Data analysis and writing of this paper was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Centre, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed by the authors.
AUTHORS’ CONTRIBUTIONS
OAO, MAG and JOO conceived and designed the experiments. JOO performed the experiments and analyzed the data. OAO and MAG supervised the study and revised the manuscript.
REFERENCES
- 1.Costa R, Magalhães A, Pereira J, Andrade P, Valentão P, Carvalho M, Silva B. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis) Food and Chem. Tox. 2009;47:860–865. doi: 10.1016/j.fct.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Riley Pa. Free-radicals in biology-oxidative stress and the effects of ionizing-radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 3.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 5.Cesquini M, Torsoni MA, Stoppa GR, Ogo SH. Induced Oxidative Damage in Sickle Red Blood Cells and the Role of Flavonoids. Biomed. and Pharmacother. 2003;57:124–129. doi: 10.1016/s0753-3322(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 6.Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 7.Saxena MJ. Relevance of herbs in improving health index of livestock animals. Proceedings of 38th congress of Nigeria. Vet. Med. Assoc. 2001:14–16. [Google Scholar]
- 8.Dalziel JM. The useful plants of West tropical Africa Crown agents for overseas Governments. Milbank; London, UK: 1937. pp. 202–204. [Google Scholar]
- 9.Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K. An Ethnobotanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger State. Nigeria. J. Phytomed. Therap. 2007;12:1–12. [Google Scholar]
- 10.Burkill HM. Useful plants of West Tropical Africa. 2nd edition. Vol. 1. Families A-D; Royal Botanic: 1985. [Google Scholar]
- 11.Othman A, Ismail A, Ghani NA, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chemistry. 2007;100:1523–1530. [Google Scholar]
- 12.Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agric. Food Chem. 1990;38:1565–1571. [Google Scholar]
- 13.Lister E, Wilson P. Lincoln, New Zealand: Crop Research Institute; 2001. Measurement of total phenolics and ABTS assay for antioxidant activity (personal communication) [Google Scholar]
- 14.Nickavar B, Kamalinejad M, Mohandesi S. Comparison of the components of the essential oils from leaves and fruits of Grammosciadium platycarpum. Chem. Nat. Compd. 2006;42:686–688. [Google Scholar]
- 15.Nickavar B, Kamalinejad M, Haj-Yahya M, Shafaghi B. Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm. Biol. 2006;44:208–212. [Google Scholar]
- 16.Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chemistry. 2002;76:69–75. [Google Scholar]
- 17.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 18.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998;46:4113–4117. [Google Scholar]
- 19.Al-Saikhan MS, Howard LR, Miller JC., Jr Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.) J. Food Sci. 1995;60(2):341–343. [Google Scholar]
- 20.Fejes S, Blázovics A, Lugasi A, Lemberkovics E, Petri G, Kéry A. In vitro antioxidant activity of Anthriscus cerefolium L. (Hoffm.) extracts. J. Ethnopharmacol. 2000;69:259–265. doi: 10.1016/s0378-8741(99)00171-3. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method - a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 22.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chyrsanthemum morifolium Ramat) LWT-Food Sci. Technol. 1999;32:269–277. [Google Scholar]
- 23.Yen GC, Duh PD, Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993;41:67–70. [Google Scholar]
- 24.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative activities of plant-derived polyphenolic flavonoid. Free Radic. Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 25.Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996;7:66–76. [Google Scholar]
- 26.Holasova M, Fiedlerova V, Smrcinova H, Orsak M, Lachman J, Vavreinova S. Buckwheat – the source of antioxidant activity in functional foods. Food Res. Int. 2002;35:207–211. [Google Scholar]
- 27.Annegowda HV, Ween NC, Mordi MN, Ramanathan S, Mansor SM. Evaluation of phenolic content and antioxidant property of hydrolysed extracts of Terminalia catappa L. leaf. Asian. J. Plant Sci. 2010;9 (8):479–485. [Google Scholar]
- 28.Cao G, Sofic E, Prior RL. Antioxidant and pro-oxidant behaviour of flavonoids: structure activity relationships. Free Radic. Biol. Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 29.Dorman HJ, Kosar M, Karlos K, Holm Y, Hittuner R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars. J. Agric. Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- 30.Gbadegesin MA, Odunola OA. In vitro antioxidant/radical scavenging activities and hepatoprotective roles of ethanolic extract of Cassia occidentalis leaves in sodium arsenite-treated male Wistar rats. British. J. of Med. Med. Res. 2013;3(4):2141–2156. [Google Scholar]
- 31.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. [Google Scholar]
- 32.Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 33.Vimala S, Adenan MI. Malaysian tropical forest medicinal plants: a source of natural antioxidants. J. Trop. Forest Prod. 1999;5:32–38. [Google Scholar]
- 34.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 35.Zengin G, Aktumsek A, Guler GO, Cakmak YS, Yildiztugay E. Antioxidant Properties of Methanolic Extract and Fatty Acid Composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec. Nat. Prod. 2011;5(2):123–132. [Google Scholar]
- 36.Gutierrez RMP, Luna HH and Garrido SH. Antioxidant activity of Tagetes erecta essential oil. J. Chil. Chem. Soc. 2006;51(2):883–886. [Google Scholar]
- 37.Juntachote T, Berghofer E. Antioxidative, properties and stability of ethanolic extracts of Holy Basil and Galangal. Food Chemistry. 2005;92:193–202. [Google Scholar]
- 38.Huda-Faujan N, Noriham A, Norrakiah AS, Babji AS. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009;8(3):484–489. [Google Scholar]
- 39.Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002;50:2454–2458. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- 40.Shiddhuraju P, Mohan PS, Becker K. Studies on antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem back leaves, flower and fruit pulp. Food Chem. 2002;79:61–67. [Google Scholar]
- 41.Sanchez-Moreno C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002;8:121–137. [Google Scholar]
- 42.Aruoma OI. Free radicals and international nutrition. Asia Pacific. J. Clin. Nutr. 1999;8:53–63. [PubMed] [Google Scholar]
- 43.Halliwell B, Gutteridge JMC. Free radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; 1999. pp. 23–27. [Google Scholar]
- 44.Aurand LW, Boone NH, Giddings GG. Superoxide and singlet oxygen in milk lipid peroxidation. J. Dairy Sci. 1977;60:363–369. [Google Scholar]