Abstract
We review here our studies on early exposure to low doses of the estrogenic endocrine-disrupting chemical bisphenol A (BPA) on behavior and metabolism in CD-1 mice. Mice were exposed in utero from gestation day (GD) 11 to delivery (prenatal exposure) or via maternal milk from birth to postnatal day 7 (postnatal exposure) to 10 µg/kg body weight/d of BPA or no BPA (controls). Bisphenol A exposure resulted in long-term disruption of sexually dimorphic behaviors. Females exposed to BPA pre- and postnatally showed increased anxiety and behavioral profiles similar to control males. We also evaluated metabolic effects in prenatally exposed adult male offspring of dams fed (from GD 9 to 18) with BPA at doses ranging from 5 to 50 000 µg/kg/d. The males showed an age-related significant change in a number of metabolic indexes ranging from food intake to glucose regulation at BPA doses below the no observed adverse effect level (5000 µg/kg/d). Consistent with prior findings, low but not high BPA doses produced significant effects for many outcomes. These findings provide further evidence of the potential risks that developmental exposure to low doses of the endocrine disrupter BPA may pose to human health, with fetuses and infants being highly vulnerable.
Keywords: endocrine disrupters, animal model, prenatal and postnatal exposure, sex differences, behavior, metabolism
Introduction
Most endocrine-disrupting chemicals (EDCs) have been in commerce for many decades. Initially, EDCs were used in products without any requirement for hazard testing. Today, it is still the case, as the majority of chemicals in products can be used in the United States without any prior toxicity testing. As information has accumulated about the hazards associated with specific compounds, EDCs have become prominent on lists of chemicals of health concern (referred to as chemicals of emerging concern).
While overall very few chemicals undergo toxicity testing for risk assessments (for the few chemicals that are tested), current assumptions used in the regulatory system’s approach to assess the risks posed by environmental chemicals classified as EDCs are based on outdated principles. Specifically, 2 main assumptions are that all dose–response relationships are monotonic and that there is always a threshold dose below which no effects of a chemical occur.1,2 Although resistance to change is hard to overcome, there is an ongoing paradigm shift. Indeed, a large number of man-made chemicals pose health risk as disruptors of signaling systems (intracellular, neural, and endocrine), which are required for normal development and subsequent functioning of organ systems that are already operating above threshold.1
A central assumption in current chemical risk assessments is that “the dose makes the poison.” This assumption led to the development of testing procedures in the 1950s that relied on only examining a few very high doses of a chemical and then estimating the effects of much lower doses based on the results observed at the few very high doses.3 Thus, in experiments to assess health effects of chemicals, the current approach used by toxicologists is to only test a few very high doses of a chemical and then extrapolate (using linear models) from high-dose effects to predict a threshold daily exposure dose below which chemicals are declared “safe.”1 Since the assumption that high-dose testing predicts low-dose effects is accepted as true by risk assessors, there is no requirement to verify that the dose of a chemical that is estimated to be “safe” for daily human exposure actually poses no health hazard. It is well recognized by endocrinologists that high doses of hormones can “downregulate” responses to hormones, while the opposite occurs (“up regulation”) in response to much lower doses.4 Therefore, the observed high-dose effects cannot predict the low-dose effects.5
Endocrine-disrupting chemicals are able to mimic the actions of endogenous hormones and thus interfere with the endocrine systems that regulate development.6,7 This disruption occurs at low doses presumed to be safe and within the range of exposure experienced by the general population (referred to as environmentally relevant doses8,9); EDC effects on the development of the reproductive system and the brain result in functional and behavioral changes later in life.10-14 Man-made EDCs may also impact metabolism (eg, permanent change in the regulation of insulin and glucose levels) both directly15 and through effects on the neuroendocrine systems that control feeding as well as other behaviors.16-18 The issue of EDCs acting as “metabolic disruptors” on developing organisms has become a high health concern.19
Adipose tissue is an endocrine organ with a substantial role in glucose and insulin homeostasis, and EDCs can disrupt hormonal signals produced in adipocytes, such as adiponectin and leptin.20,21 Endocrine-disrupting chemicals have thus been implicated as factors in the human obesity and diabetes epidemics in developed countries as well as in developing countries.15,22 Importantly, some neural and metabolic effects due to fetal exposure to EDCs are observed during specific periods during postnatal life, such as during early adolescence,23 adulthood24 or middle age25 as well as during early development.26
Research on the effects of prenatal exposure to low doses of EDCs in animal models is important to evaluate a possible role of such man-made chemicals in development of neuroendocrine, behavioral, and metabolic dysfunctions. Based on published data27-29 obtained in laboratory studies on CD-1 mice (Mus musculus) concerning effects on behavior and physiology of pre-, post-, and perinatally exposed males and females, we review here the case of estrogenic chemical bisphenol A (BPA), one of the most intensively studied EDCs. Bisphenol A has been shown to alter the development of estrogen-dependent neural circuits, related behaviors, and endocrine functions27 and is one of the highest volume chemicals in commerce among the known EDCs.30
The laboratory mouse is a good experimental model to investigate the effects of developmental exposure to xenoestrogens on physiology and certain types of behavioral systems that are differently expressed in adult males and females, such as exploration, emotionality, anxiety, activity patterns, learning, memory, and social behavior.
Prenatal and Postnatal Exposure to BPA: Effects on Male and Female Neurobehavioral Development
Sexual differentiation in rats and mice begins prenatally and continues into the early postnatal period. These rodents are about equivalent to gestation week 17 human fetuses at birth, so developmental events during the neonatal period in rodents occur prenatally in humans. Differential actions of gonadal steroids during the prenatal and perinatal periods play a crucial role in permanently organizing sexual dimorphisms in behavior and its underlying neural substrates in rodents.31,32 Maternal exposure to estrogenic chemicals can thus interfere with the male- and female-typical development of brain areas that control the occurrence and pattern of a wide range of social and nonsocial behaviors in adult life. Disruption of the normal processes of masculinization of males and feminization of females may undermine the survival and reproductive success (ie, fitness) of exposed individuals.33 Many studies indicate that the developing fetus is more sensitive to estrogenic chemicals relative to the adult.34,35 Mothers can pass BPA to their offspring transplacentally and after birth to a lesser degree by breast-feeding newborns.36,37 For this reason in our experiments, we focused on the timing of the BPA exposure of offspring (prenatal or postnatal period), lactating dam condition (BPA- or oil-treated lactating dams), and age at testing (juveniles or adults) to investigate the behavioral effects of developmental exposure to a low dose of BPA in male and female mice. Exposure occurred by feeding pregnant mice either corn oil (controls) or 10 µg/kg/d BPA from the last week of pregnancy through the first postpartum week. At birth, the litters were cross-fostered between groups to discriminate between effects due to in utero exposure and those due to postnatal exposure via milk. We assessed explorative and emotional behaviors (sexually dimorphic in mice) of the maternally exposed offspring at different ages and in different experimental settings.27,9,29
Procedure, Behavioral Testing, and Results
In a previous study,9 we observed the offspring of pregnant and lactating mice fed with 10 µg/kg/d BPA. This dose is far below the US Environmental Protection Agency (US-EPA) lowest observed adverse effect level (50 mg/kg/d) that was used to calculate a reference dose (estimated to be safe for daily oral human exposure) of 50 µg/kg/d.38 Our group developed a procedure to allow the oral administration of chemicals to pregnant females without handling the animals (ie, without stressing the pregnant dams27,39); polycarbonate cages and bottles were used. However, only new bottles and cages were used, since Howdeshell and coworkers40 reported that BPA leaching at environmental temperatures is minimal under such conditions. Starting on day 6 after time mating, the females were trained to spontaneously drink a small volume of corn oil (Sigma, Milano, Italy) from a modified syringe. On day 11 of pregnancy, each female was randomly assigned to one of the following treatment groups: oil (control, n = 27) or BPA (10 μg of BPA/kg body weight/d, n = 15). From gestation day (GD) 11 to postnatal day (PND) 8, each female drank 0.1 mL/50 g body weight/d of oil, with or without BPA. The dams were weighed during the dosing period to adjust the administered dose for the body weight changes during pregnancy and lactation and to monitor their health (average body weight on gestational day 16; oil dams: 54.23 ± 0.82 g; BPA dams: 53.62 ± 1.42 g, mean ± standard error). No differences in the dams’ body weight were noted with respect to the treatment. Within 12 hours after birth, the litters were culled to 4 to 6 males and 4 to 6 females per litter and cross-fostered to discriminate between the effects of the prenatal and postnatal exposure to BPA on the offspring’s behavior. The dams were removed from their cage, and the pups were gently placed in the nest of the foster dam, which was returned 15 minutes later. All of the dams continued to receive the same treatment postnatally that they had received during pregnancy. Thus, the pups that were exposed prenatally to BPA were not exposed during lactation, whereas the pups not exposed prenatally to BPA were exposed during lactation. A total of 15 prenatal oil-exposed mothers received pups from 15 prenatal BPA mothers and vice versa. Additionally, within the additional 12 oil control dam group, the offspring from 6 prenatally oil-treated dams were cross-fostered to the other 6 prenatally oil-treated foster mothers to control for any possible effects of the fostering procedure. At 25 days of age, the offspring were weaned and group-housed with same-sex littermates (4-6 mice/group) until the behavioral tests started. The pups were weighed every 10 days, beginning from birth. No differences in offspring body weight were recorded with respect to prenatal or postnatal BPA exposure. The animals were maintained and tested under a normal 12-hour light–12-hour dark cycle. All animal experimentation was conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/EEC) and was approved by the Italian Institute of Health.
Behavioral Tests and Results
The tests were performed at 10:00 to 12:00 and 15:00 to 18:00 hours. Specific tests conducted with each treatment group consisted of 12 to 15 animals per sex. One female and one male from each litter were randomly selected for specific behavioral tests as juveniles and during adulthood to control for litter effects. Male and female offspring (prenatal and postnatal exposure) at different ages and in different experimental settings (see9,27,29 for details) were examined in 3 experimental paradigms, which included:
Novelty preference test as juveniles before puberty (PND 28-30)
In total, 83 juvenile (28-30 days old) male (n = 41) and female (n = 42) mice were examined. A transparent Plexiglas cage (40-25-15 cm) was divided into 2 compartments (A—familiar and B—novel; 20-25 cm each) by a partition of white opaque polypropylene with a small opening in the middle that could be opened and closed by the experimenter. Male or female sibling groups were housed in compartment A of the apparatus. After 24 hours, all mice but one were randomly removed from the cage so that only 1 mouse was tested, and the door dividing the 2 compartments was opened thus allowing the mouse to enter the novel area (compartment B). This test can measure the propensity of exploration (curiosity) and preference for novelty, amount of locomotion, and also serves as an index of anxiety (see27,29).
The results in Table 1 from Gioiosa and coworkers9 showed that in the control group, female mice entered the novel compartment less quickly and spent more time in it when compared to males. Prepuberal unexposed females thus showed a behavioral profile suggestive of lower anxiety and higher propensity to explore a novel environment (which suggests that females showed lower risk assessment levels than males). Relative to controls, prepuberal BPA-exposed females entered the novel compartment more quickly, spent less time in it, and showed less risk assessment behaviors; they also spent more time exhibiting self-grooming behaviors. Remarkably, BPA-exposed males also entered the arena with shorter latencies and spent less time in risk assessment behaviors relative to control males. Overall, these results suggest a BPA effect in decreasing risk assessment behaviors in exposed animals of both sexes. Under present conditions, pre- and postnatal exposures affected similarly the direction of the behavioral changes, resulting in a disruption of the behavioral sex differences, although with a greater effect associated with postnatal exposure primarily in females.
Table 1.
Sex Differences in Behavioral Test Responses of Prepuberal and Adult Mice Prenatally or Postnatally Exposed to Vehicle (Control Oil) or to Bisphenol A (BPA 10 μg/kg body weight/day; Data from Gioiosa et al.).9
| Behavioral Test | Behavioral Response | Control oil | Prenatal BPA | Postnatal BPA |
|---|---|---|---|---|
| Novelty test (prepuberty) | Latency to novelty | F ≤ M | F = M | F = M↓ |
| Risk assessment | F ≤ M | F = M↓ | F = M↓ | |
| Exploration of novelty | F > M | ↓F = M | ↓F = M | |
| Self-grooming | F < M | F = M | ↑F > M | |
| Free-exploratory open field | Exploration | F > M | F = M | ↓F = M |
| Time center | F > M | F = M | ↓F < M | |
| Returns home | F > M | F = M | ↓F < M↑ | |
| Elevated plus maze | Entrance open arms | F > M | ↓F = M | ↓F = M |
| Time center | F > M | F > M | ↓F = M↑ | |
| Time closed arms | F < M | ↑F < M | ↑F = M |
Abbreviations: F = M, level of behavior do not significantly differ in males and females; F > M, levels of behavior significantly higher in females than in males; F < M, levels of behavior significantly lower in females than in males; ↓ and ↑ statistically significant decrease or increase of behavior following BPA exposure as analyzed by 2-way ANOVA with sex (male and female) and exposure (prenatal BPA, postnatal BPA, and oil) as factors. Each treatment group consisted of 12 to 15 animals per sex; F, female; M, male; ANOVA, analysis of variance; BPA, bisphenol A.
2. Free-exploratory open field (FOL) as adults (42 males and 41 females; 70 days old)
This test was conducted in an apparatus with a home-cage area and an unfamiliar arena (an open-field—OF) of 73 × 110 cm, bordered by a 50-cm high wall, and in which a bright and a dark zone were created. One male and female per litter were individually housed in the home-cage section, and after 24 hours, the barrier between the compartments was removed allowing entrance into the OF. A cutoff of 10 minutes was used for those animals that did not enter the OF. Starting from the first entrance in the OF, behavioral observation lasted 5 minutes. The results in Table 1 showed that adult control females displayed higher exploration than males when challenged to explore a novel environment; control females spent more time exploring the arena and showed more returns between OF and home cage, and when in the OF arena, they spent more time in the bright zone and in the central area of relative to males. Relative to their controls, prenatally exposed females explored less the arena (they spent less time in the center and entered less frequently the arena) whereas exposed males were more explorative than their controls. Postnatal exposure to BPA reduced exploration in both males and females compared to controls, in particular postnatally exposed females spent less time in the center relative to controls. Compared to same-sex controls, postnatally exposed females showed decreased frequencies of returns, whereas postnatally exposed males displayed increased returns between home-cage and OF. As a result, postnatally exposed females were more similar to control males, while postnatally exposed males were more similar to control females. Thus, an interesting finding was that the behavioral sex differences observed in controls were either eliminated by the BPA prenatal exposure or reversed by the postnatal exposure.
3. Elevated plus maze (EPM) as adults with the same males and females used in the FOL test
The EPM paradigm is one of the most widely used animal tests for the study of anxiety.41 It consists of 2 open arms and 2 closed arms that extend from a common central platform. A mouse was placed in the center, and tests lasted 5 minutes. In the conventional form of the test, anxiety is assessed by measures of open-arm avoidance, while locomotor activity is most reliably measured by the frequency of closed arm entries. Additional ethological measures, which include stretched attend postures, head-dipping, and self-grooming, have been linked to risk assessment.42
Our data indicate that controls’ behavioral profile differed between males and females, with females being more explorative on the EPM (Table 1). Compared to controls, both pre- and postnatally BPA-exposed females displayed a lower frequency of open-arm entries and spent more time in the closed arms. A behavioral profile is more similar to that of control males, resulting in a disruption of the sex differences observed in controls. Even more marked was the effect of postnatally exposure to BPA on the time spent in the center. When compared to controls, exposed females spent less time in center, whereas exposed males more time in the center, eliminating the sex difference observed in controls. Consequently, both prenatal and postnatal exposures were effective in disrupting behavioral sex differences on the EPM test, with a larger effect of postnatal exposure.
As a general result, unexposed female mice, when allowed to explore a novel environment, were more reactive and explorative when compared to unexposed males, either prior to puberty or in adulthood. Therefore, control mice showed sex differences on a number of behavioral responses at both ages and in all the test paradigms. In contrast, mice pre- or postnatally exposed to BPA showed either decreased or no sex differences or even a reversal of the observed behavioral sex differences, particularly when exposure occurred postnatally.
Summary of Behavioral Findings
Developmental exposure to the estrogenic pollutant BPA resulted in behavioral alterations mainly in females. Altogether these findings may well be seen as indexes of reduced reactivity of BPA-exposed females to novel stimuli and are consistent with an estrogenic action of BPA and possibly a combination of both “defeminization” and “masculinization” as a result of developmental exposure to BPA.32 We also found that BPA-exposed males showed female-type behavior on a few measures. The overall result was a reduction or even a reversal of sexual differences in exposed mice, relative to differences displayed by control males and females. The fact that sexual behavioral differences occur before puberty (ie, before the increase of gonadal sex hormone production is activated) and that exposure to estrogenic compounds decreased differences in response to a challenge (novelty) suggests an interference of BPA in the processes of development and organization of the central nervous system and possibly of hormone and/or neurotransmitter receptor systems of both sexes (reviewed in43). Thus, prenatal and early postnatal exposures to a low dose of BPA reduced or reversed the sex differences in emotional behaviors in response to novelty in both juvenile and adult mice, with perinatal9 and postnatal-only exposure producing greater effects than prenatal-only exposure.29
Fetal Exposure to Low but Not High Doses of BPA Disrupts Metabolic Function in Male Mice
Studies with laboratory rats and mice have reported increased body fat in animals exposed to exogenous drugs and chemicals, such as diethylstilbestrol (DES) and BPA during the fetal period of the differentiation of preadipocytes and early postnatal period of the differentiation of adipocytes.25,44-46 The finding that exposure to BPA and other estrogenic chemicals during adipogenesis can lead to an increase in body fat later in life is supported by epidemiological evidence for a relationship in humans between BPA and obesity (including children and teenagers) as well as cardiovascular disease, insulin resistance, glucose intolerance, and type 2 diabetes in adults.47-49 Metabolic diseases significantly impact the brain, behavior and fertility as well as mortality.50
The goal of this study was to conduct a comprehensive examination of the effects of developmental exposure to BPA on outcomes related to metabolic disease, using doses that ranged from 5 to 50 000 µg/kg/d. The wide BPA dose range used extended from 10-fold below the currently estimated reference dose (50 µg/kg/d) to 10-fold greater than the estimated no observed adverse effect level (NOAEL, 5000 µg/kg/d), which allowed for a more detailed assessment of variation in dose responses among the different anatomical and physiological outcomes.
Procedure, Tests, and Results
Angle and colleagues28 conducted a study in which pregnant CD-1 female mice were randomly assigned to the following oral dosing groups: 0 (negative controls), 5, 50, 500, 5000, and 50 000 µg/kg body weight/d BPA or 0.1 µg/kg body weight/d DES (the positive control for low-dose estrogenic effects). We will refer to these groups as negative controls, BPA-5, BPA-50, BPA-500, BPA-5000, BPA-50 000, and DES-0.1; the number of litters per treatment group was 14, 9, 12, 12, 11, 14, and 9, respectively. On PND 1, the sex and weight of mouse pups were determined. The pups were then not handled until weaning except to change cage bedding and then only after the pups were at least 1 week old. Offspring were weaned on PND 22 and housed 2 to 4 of the same sex in polypropylene cages with corncob bedding. At this time, animals were individually identified by an ear-notch pattern. After weaning, animals were fed soy-based Purina 5001 rodent maintenance chow (with only 4% fat) ad libitum; food was weighed every 3 to 4 days to monitor food intake per week; and body weight gain per week was also determined for the duration of the study, which ended when males were about 5-month old. Metabolic energy consumed per week was calculated (for Purina 5001 chow, this is 3.04 kcal/g feed). The following measures and test data were recorded: (1) birth weight, body weight from weeks 3 to 19, and metabolic energy consumption per week; (2) gonadal and renal fat pad weight, adipocyte number, and adipocyte volume; (3) liver and kidney weight and liver histopathology (ie, association of hepatic steatosis with obesity and metabolic disease); (4) glucose and insulin tolerance tests; and (5) serum hormones (insulin, adiponectin, and leptin).
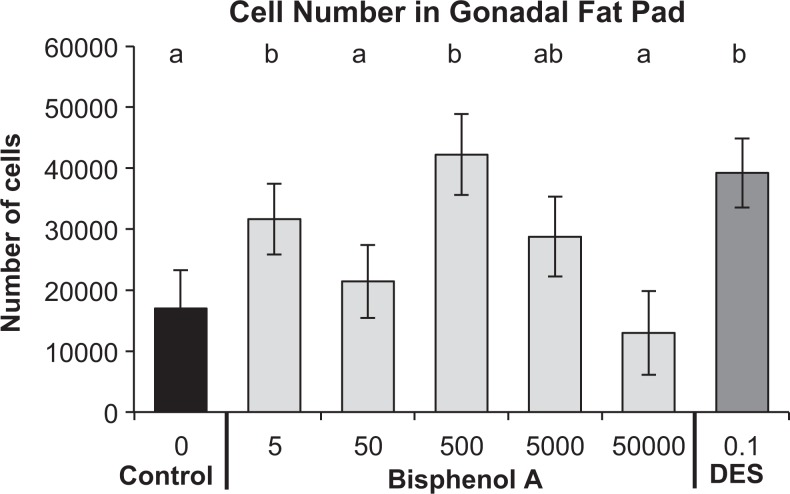
As a result of exposure of male fetuses to BPA via feeding pregnant mice doses of BPA at and below the current predicted NOAEL (5000 µg/kg/d), there was a significant increase in postnatal body weight gain, adipocyte number and volume, and the overall amount of abdominal fat, altered food intake, serum insulin, adiponectin and leptin levels, and impaired glucose tolerance (Figure 1; Table 2). These long-term effects were observed mainly in response to low doses of BPA maternal exposure (500 µg/kg/d), and it should be noted that these are components of metabolic syndrome. These findings may thus have implications for metabolic diseases that are observed to be related to BPA exposure in humans.47,15,49,51 These effects were not predicted by responses to the highest (50 000 µg/kg/d) dose of BPA that we examined (none of which differed from controls). Without testing doses below the current NOAEL, it is likely that we would have found no effect of BPA on any of these outcomes related to metabolic syndrome.
Figure 1.
Effect of fetal exposure to bisphenol A (BPA) at 5 to 50 000 µg/kg/d or diethylstilbestrol (DES) at 0.1 µg/kg/d as a result of feeding the chemicals to pregnant CD-1 mice from gestation day (GD) 9 to 18. The data are the mean (±standard error of the mean [SEM]) gonadal adipocyte number in male offspring from different prenatal treatment groups when 19 weeks old. The statistical analyses are presented in Table 2. Groups with just the letter “b” are significantly different from negative controls. The BPA 50 000 dose did not result in any significant effects. Adapted from Angle et al.28.
Table 2.
P Value Summary for Analyses of Effect of Prenatal Negative Controls and BPA Doses (0, 5, 50, 500, 5000, and 50 000 µg/kg/d), not Including the DES-0.1 Group.a
| Variable | PROC GLM ANOVA | Multilevel Regression |
|---|---|---|
| BPA Doses | Improvement in Fit: Quadratic Over Linear-Only Model | |
| Body weight | ||
| Birth weight | = 0.550 | = 0.420 |
| Week 3 weight | = 0.020 | = 0.019 |
| Week 19 weight | = 0.193 | = 0.069 |
| Energy intake, week 3-4 | = 0.358 | = 0.033 |
| Energy intake, week 4-5 | = 0.201 | = 0.028 |
| Adipocytes | ||
| Gonadal fat weight | = 0.017 | = 0.022 |
| Renal fat weight | = 0.019 | = 0.076 |
| Total abdominal fat weight | = 0.035 | = 0.047 |
| Gonadal adipocyte number | = 0.003 | = 0.002 |
| Gonadal adipocyte volume | = 0.006 | = 0.052 |
| Renal adipocyte number | = 0.011 | = 0.250 |
| Renal adipocyte volume | = 0.121 | = 0.654 |
| Liver and kidney weight | ||
| Liver weight | = 0.003 | < 0.001 |
| Kidney weight | = 0.137 | = 0.947 |
| GTT and ITT | ||
| Glucose tolerance test | = 0.062 | = 0.008 |
| Insulin tolerance test | = 0.144 | = 0.137 |
| Serum hormones | ||
| Insulin | = 0.008 | = 0.004 |
| Leptin | = 0.035 | = 0.349 |
| Adiponectin | = 0.031 | = 0.099 |
Abbreviations: ANOVA, analysis of variance; BPA, bisphenol A; DES, diethylstilbestrol; GTT, glucose tolerance test; ITT, insulin tolerance test; GD, gestation day.
aPregnant CD-1 mice were fed these doses of BPA (and DES as a positive control) in oil from GD 9 to 18, and male offspring were examined for these outcomes. The number of litters per treatment group was 14, 9, 12, 12, 11, 14, and 9, respectively. Two males per litter were examined for all outcomes except serum hormones (5-7 males from different litters). Column 1 shows results of standard linear ANOVAs using PROC GLM in SAS (controlling for litter membership). Column 2 gives P values for comparison of two nested linear regression models, with and without a [log(dose)]2 term using the R Statistical System. A significant result (indicated in bold) provides evidence that a U or inverted-U curve fits the data better than a simple linear fit, and indicates a non-monotonic dose–response function. Males were evaluated when 4 to 5 months old unless otherwise indicated. For details about the statistics, see Supplemental Materials from Angle et al.28
Conclusions
One of the most contentious issues relating to EDCs has been that current methods of risk assessment are based on the assumption that the shape of the dose–response curve is always monotonic. This is identified in toxicology as “the dose makes the poison,” which refers to the prediction that the greater the exposure, the greater the response.2 However, in experiments with hormones, drugs, and other chemicals that act via hormonal mechanisms, it is very common for the dose–response curve to be nonmonotonic and form an inverted U, which in endocrinology is called a “biphasic” dose–response curve (these curves have ascending and descending phases).5 Our studies show convincing evidence that responses to high doses of BPA are not predictive of responses to much lower doses that are within the range of human exposure.52,53
Another major conclusion from our research is that nonreproductive, sexually dimorphic behaviors are sensitive to endocrine disruption during critical periods of life and that sex differences represent a relevant issue when designing risk assessment studies.10,27,9,29,11,13,54,55 Furthermore, our and other findings demonstrate that besides the period of prenatal “in utero” development, the early postnatal environment, including offspring lactational exposure to BPA, is a period during which there is high vulnerability to long-lasting effects on physiology (metabolic disruption and disease development), brain development, and behavior. These findings provide further evidence for the potential risks that environmentally relevant low doses of EDCs may pose to animals and human health during both prenatal and early postnatal life periods.
There appears to be a lack of awareness of this low vs. high-dose phenomenon in the toxicological risk assessment community, as many toxicological studies, in which effects only occur in a restricted “low dose” range while the effect is not seen at higher doses, conclude that there is no relationship between dose and response rather than that the relationship is nonmonotonic. While not all dose–response relationships are nonmonotonic, the fact that nonmonotonic dose–response relationships do commonly occur in endocrinology8,5 has not been incorporated into the process of assessing the risks of exposure to environmental chemicals. Thus, the assessment of risks in response to chronic low-dose exposure to chemicals is determined by examining acute toxic effects of only a few very high doses. Indeed, important cell receptor-mediated activity can occur far below a high dose range for EDCs such as BPA due to prenatal and/or perinatal exposure.8 Such evidence invalidates the crucial assumption of the current risk assessment paradigm that all dose–response relationships are monotonic and “the dose make the poison.” In summary, our findings demonstrate the urgent need for development of strategies for assessing the hazards posed by EDCs that are consistent with current endocrinological principles, as recommended in a statement of principles by the Endocrine Society.4 Finally, our findings provide further evidence of the risk posed to human health by developmental exposure to BPA at environmentally relevant low doses, which lead to blood levels within the range reported in human fetuses.56,53
Footnotes
Authors’ Note: All authors equally contributed to this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support to FvS is from NIEHS, ES021394; Support to PP, LG and SP is from University of Parma and MIUR, PRIN 20107MSMA4_005.
References
- 1. vom Saal FS, Sheehan DM. Challenging risk assessment. Forum Appl Res Publ Pol. 1998;13(3):11–18. [Google Scholar]
- 2. Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117(11):1652–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113(8):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101(5):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low doses of environmental estrogens alters sex differences in exploration and emotional behavior in mice. Horm Behav. 2007;52(3):307–316. [DOI] [PubMed] [Google Scholar]
- 10. Palanza P, Morellini F, Parmigiani S, vom Saal FS. Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neurosci Biobehav Rev. 1999;23(7):1011–1027. [DOI] [PubMed] [Google Scholar]
- 11. Panzica GC, Viglietti-Panzica C, Mura E, et al. Effects of xenoestrogens on the differentiation of behaviorally-relevant neural circuits. Front Neuroendocrinol. 2007;28(4):179–200. [DOI] [PubMed] [Google Scholar]
- 12. Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31(4):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6(9):e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peretz J1, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ Health Perspect. 2014;122(8):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346–353. [DOI] [PubMed] [Google Scholar]
- 16. Wei J, Lin Y, Li Y, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–3061. [DOI] [PubMed] [Google Scholar]
- 17. Cabaton NJ, Canlet C, Wadia PR, et al. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect. 2013;121(5):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao J, Rebuli ME, Rogers J, et al. Prenatal bisphenol A (BPA) exposure alters sex specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133(1):157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heindel JJ, Vom Saal FS, Blumberg B, et al. Parma consensus statement on metabolic disruptors. Environ Health. 2015;14(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116(12):1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marroqui L, Gonzalez A, Neco P, et al. Role of leptin in the pancreatic beta-cell: effects and signaling pathways. J Mol Endocrinol. 2012;49(1):r9–r17. [DOI] [PubMed] [Google Scholar]
- 22. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- 23. Patisaul HB, Sullivan AW, Radford ME, et al. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9):e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol. 2005;73(7):478–480. [DOI] [PubMed] [Google Scholar]
- 26. Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102(19):7014–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol-A on brain and behavior in mice. Environ Res. 2008;108(2):150–157. [DOI] [PubMed] [Google Scholar]
- 28. Angle BM, Do RP, Ponzi D, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. Effects of bisphenol A on emotional behavior depend upon timing of exposure, age at testing and gender in mice. Horm Behav. 2013;63(4):598–605. [DOI] [PubMed] [Google Scholar]
- 30. GrandViewResearch. Global bisphenol A (BPA) market by application (appliances, automotive, consumer, construction, electrical & electronics) expected to reach USD 20.03 billion by 2020. Web site http://www.digitaljournal.com/pr/2009287 2014; Accessed October 14, 2015.
- 31. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. [DOI] [PubMed] [Google Scholar]
- 32. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parmigiani S, Palanza P, vom Saal FS. Ethotoxicology: an evolutionary approach to the study of environmental endocrine-disrupting chemicals. Toxicol Ind Health. 1998;14(1-2):333–339. [DOI] [PubMed] [Google Scholar]
- 34. Bern HA. The development of the role of hormones in development--a double remembrance. Endocrinology. 1992;131(5):2037–2038. [DOI] [PubMed] [Google Scholar]
- 35. vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177. [DOI] [PubMed] [Google Scholar]
- 37. Cao XL, Popovic S, Arbuckle TE, et al. Determination of free and total bisphenol A in human milk samples from Canadian women using a sensitive and selective GC-MS method. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(1):120–125. [DOI] [PubMed] [Google Scholar]
- 38. Integrated Risk Information System. File First On-Line; 1988.
- 39. Vandenberg LN, Welshons WV, Vom Saal FS, Toutain PL, Myers JP. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ Health. 2014;13(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howdeshell KL, Peterman PH, Judy BM, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111(9):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8(6-7):477–496. [DOI] [PubMed] [Google Scholar]
- 42. Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52(2):297–303. [DOI] [PubMed] [Google Scholar]
- 43. Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Somm E, Schwitzgebel VM, Vauthay DM, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149(12):6289–6299. [DOI] [PubMed] [Google Scholar]
- 45. Somm E, Schwitzgebel VM, Toulotte A, et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Perspect. 2009;117(10):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int J Androl. 2012;35(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults: evidence from NHANES 2003/4. JAMA. 2008;300(11):1303–1310. [DOI] [PubMed] [Google Scholar]
- 48. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melzer D, Osborne NJ, Henley WE, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125(12):1482–1490. [DOI] [PubMed] [Google Scholar]
- 50. Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29(3):251–259. [DOI] [PubMed] [Google Scholar]
- 51. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–1121. [DOI] [PubMed] [Google Scholar]
- 52. Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28(1):37–58. [DOI] [PubMed] [Google Scholar]
- 53. vom Saal FS, Welshons WV. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol Cell Endocrinol. 2014;398(1-2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenfeld CS. Effects of maternal diet and exposure to bisphenol A on sexually dimorphic responses in conceptuses and offspring. Reprod Domest Anim. 2012;47(suppl 4):23–30. [DOI] [PubMed] [Google Scholar]
- 56. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]