Abstract
Persistent muscle pain is a common and disabling symptom for which available treatments have limited efficacy. Since tetrodotoxin (TTX) displays a marked antinociceptive effect in models of persistent cutaneous pain, we tested its local antinociceptive effect in rat models of muscle pain induced by inflammation, ergonomic injury and chemotherapy-induced neuropathy. While local injection of TTX (0.03-1 μg) into the gastrocnemius muscle did not affect mechanical nociceptive threshold in naïve rats, exposure to the inflammogen carrageenan produced a marked muscle mechanical hyperalgesia, which was dose-dependently inhibited by TTX. This antihyperalgesic effect was still significant at 24 hours. TTX also displayed a robust antinociceptive effect on eccentric exercise-induced mechanical hyperalgesia in the gastrocnemius muscle, a model of ergonomic pain. Finally, TTX produced a small but significant inhibition of neuropathic muscle pain induced by systemic administration of the cancer chemotherapeutic agent oxaliplatin. These results indicate that TTX-sensitive sodium currents in nociceptors play a central role in diverse states of skeletal muscle nociceptive sensitization, supporting the suggestion that therapeutic interventions based on TTX may prove useful in the treatment of muscle pain.
Keywords: Mechanical hyperalgesia, inflammation, neuropathic muscle pain, delayed-onset muscle soreness, voltage-dependent sodium channels, clinical trials
1. Introduction
For many decades tetrodotoxin (TTX), which is present in many poisonous animals including fishes from the Tetraodontidae family such as the pufferfish, has been used as a pharmacological tool to selectively block a subset of inward sodium currents (TTX-S INa) in electrophysiological recordings (Narahashi, 2008). Indeed, in vitro studies have shown that TTX is able to inhibit the conduction of action potentials in isolated nerve preparations (Muroi et al., 2011) and to block inward sodium currents in neurons from sensory ganglia (Muroi et al., 2011; Blair and Bean, 2002). The current subsets identified by TTX have been demonstrated to depend on specific voltage-gated sodium channels (VGSC): TTX-sensitive (TTX-S) sodium channels, such as Nav1.1, Nav.1.3, Nav1.6 and Nav1.7 which are blocked by TTX at nanomolar concentrations, and TTX-resistant (TTX-R) sodium channels, such as Nav1.8 and Nav1.9 which are blocked by TTX only at micromolar concentrations (Dib-Hajj et al., 2009). This potent sodium channel block can explain the classical local symptoms of exposure to this toxin (e.g., fugu poisoning), including oral numbness, tingling and anesthesia (Bane et al., 2014; You et al., 2015). These properties are consistent with the strong antinociceptive effect exhibited by TTX in a number of in vivo pre-clinical (Lyu et al., 2000; Marcil et al., 2006; Nieto et al., 2008) and clinical (Hagen et al., 2008; Hagen et al., 2011; Shi et al., 2009; Song et al., 2011) studies. Importantly, while the expression of VGSC varies between sensory neurons contributing to different pain symptoms (Minett et al., 2014), the antinociceptive effects of TTX have, however, been mainly studied in models of cutaneous pain.
While chronic muscle pain is an extremely common and disabling group of syndromes, which lack effective therapy, it has received much less attention than cutaneous pain. This is probably due to the fact that clinical entities related to chronic muscle pain, such as neuropathic muscle pain, are still not well characterized. Because of this scarcity of preclinical muscle pain models, most of the preclinical screening of new analgesic drugs is performed in models assessing cutaneous nociception. TTX-S VGSC have been reported to be present in dorsal root ganglion (DRG) nociceptors innervating skeletal muscle (Ramachandra et al., 2012), and nociceptive spinal monosynaptic reflexes are attenuated after exposure of sensory fibers innervating skeletal muscle to TTX as observed in in vivo preparations (Schomburg et al., 2012). Furthermore, large-diameter sensory neurons, likely innervating skeletal muscle, exhibit de novo expression of TTXS VGSC after spinal nerve injury (Fukuoka et al., 2015). However, whether TTX is able to produce antinociceptive effects in models of persistent muscle pain remains to be determined. Thus, given the clinical and societal importance of persistent muscle pain and the promising profile of TTX as a putative analgesic, we explored its antinociceptive effects in models of nociceptive inflammatory, ergonomic and neuropathic muscle pain.
2. Experimental Procedures
2.1 Animals
Adult male Sprague Dawley rats (initial weight 250–300 g; Charles River, Hollister, CA) were used in these experiments. They were housed in the Laboratory Animal Resource Center facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were euthanized by CO2 induced asphyxia followed by bilateral thoracotomy. Animal care and use conformed to NIH guidelines (NIH Guide for the Care and Use of Laboratory Animals) and to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. The University of California San Francisco Institutional Animal Care and Use Committee approved all experimental protocols. Concerted effort was made to minimize number and suffering of experimental animals.
2.2 Muscle inflammatory hyperalgesia
Rats were briefly anaesthetized with 2.5% isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO) in 97.5% O2. After the hair on hind limbs was clipped and disinfected with 70% isopropyl alcohol, carrageenan ( -carrageenan, 1% in NaCl 0.9%) was injected into the belly of the gastrocnemius muscle, using a ½” 27G needle attached to an 100 μl microsyringe (Gastight®, Hamilton, Reno, NV). The 100 μg/10 μl dose of carrageenan was determined in previous studies as sufficient to produce robust mechanical hyperalgesia in the rat's gastrocnemius muscle (Dina et al., 2008).
2.3 Eccentric exercise-induced muscle hyperalgesia
The method used to eccentrically exercise the rat hind limb has been described previously (Alvarez et al., 2010; Kano et al. 2004; Taguchi et al., 2005). Briefly, isoflurane-anesthetized rats were placed in supine position on a heating pad (set to maintain core body temperature at 37°C), and ophthalmic ointment applied to prevent corneal dehydration. The right hind paw was affixed to the foot bracket of a rodent exercise apparatus (Model RU-72, NEC Medical Systems, Tokyo, Japan) with 3M Micropore® surgical paper tape, such that the angle of the knee and ankle joints was 90° (paw 30° from vertical). The gastrocnemius muscle was stimulated via subcutaneous needle-type electrodes attached to a Model DPS-07 stimulator (Dia Medical System Inc., Tokyo, Japan) that delivered trains of rectangular electric pulses (100 Hz, 700 ms, 3 V) every 3 s, to give a total of 300 contractions. During these stimulus-induced contractions of the gastrocnemius muscle, the electromotor system rotated the foot to produce extension of this muscle.
2.4 Oxaliplatin-induced neuropathic muscle pain
As previously reported (Alvarez et al., 2011; Joseph and Levine, 2009), oxaliplatin was freshly dissolved in normal saline at a concentration of 2 mg/ml and immediately administered by intravenous route (1 ml/kg) to isoflurane-anesthetized rats.
2.5 Drugs
Unless otherwise stated, all chemicals used in these experiments were obtained from Sigma-Aldrich (St. Louis, MO). The stock solution of TTX (Abcam, Cambridge, MA) was made by dissolving it in distilled water (1 μg/μl) and stored at −20°C; further dilutions were made in Dulbecco's phosphate buffered saline (D-PBS). Rats were briefly anesthetized with 2.5% isoflurane to facilitate the intramuscular injections (20 μl) of TTX or its vehicle (D-PBS) into the gastrocnemius muscle. The injection site was previously shaved and scrubbed with alcohol. Immediately after injection the skin puncture site was marked with a fine-tip indelible ink pen, so that the mechanical nociceptive threshold of the underlying injection site in the muscle could be repeatedly tested.
2.6 Testing of mechanical nociception
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL) with a custom-made 7 mm diameter probe. This width of probe allows for selective evaluation of muscle pain (vis-à-vis overlying skin pain) (Alvarez et al., 2010). Rats were lightly restrained in a cylindrical acrylic holder with lateral slats that allow for easy access to the hind limb and application of the force transducer probe to the injection site in the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in mN, required to produce a flexion withdrawal reflex in the hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5 min intervals and magnitude of hyperalgesia calculated as percentage decrease from the baseline mechanical nociceptive withdrawal threshold.
2.7 Statistics
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software, Inc., La Jolla, CA). Comparisons were made by means of one-or two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons test. A P value < 0.05 was considered statistically significant.
3. Results
3.1 Effect of TTX on muscle nociceptive threshold
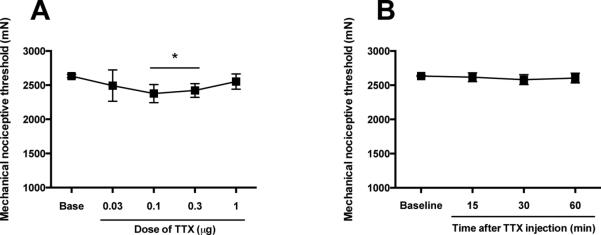
While the presence of TTX-S sodium channels in primary afferents innervating skeletal muscle has been demonstrated (Ramachandra et al., 2012), whether the local administration of TTX is able to modify the baseline mechanical nociceptive threshold in skeletal muscle remains to be established. To test this we evaluated the effect of cumulative incremental doses of TTX (0.03, 0.1, 0.3 and 1 μg/20 μl) on nociceptive threshold in normal control rats. We found that, while the TTX doses of 0.1 and 0.3 produced a small, albeit significant, decrease in mechanical nociceptive threshold (−10 ± 1.8% and −8 ± 1.5, respectively, P < 0.05, n=6/group, Fig. 1A), the 1 μg TTX dose did not produce significant change in this parameter (P > 0.05, n=6/group, Fig. 1A). Furthermore, in a time-course study in a separate group of rats in which we followed the effect of the highest dose of TTX (1 μg/20 μl) for 60 min, we again observed no significant effect of TTX on mechanical nociceptive threshold in naïve control rats (P > 0.05, n=6/group, Fig. 1B). No gross motor or behavioral side effects were observed after i.m. administration of 1 μg of TTX.
Figure 1. Effect of intramuscular TTX on mechanical nociceptive threshold in naïve rats.
(A) Sequentially increasing the i.m. doses of TTX, each cumulative dose one-half log unit greater than the previous dose, were injected at 45 min inter-injection intervals. Prior to each subsequent dose, the mechanical nociceptive threshold was again assessed. TTX failed to produce an increase in mechanical nociceptive threshold in normal control rats at any of the tested doses. Indeed, the doses of 0.1 and 0.3 μg produced a small, albeit significant, decrease in mechanical nociceptive threshold. (B) In a separate group of rats, the injection of the highest dose of TTX (1 μg) did not modify the mechanical nociceptive threshold up to 60 min after injection. *P < 0.05.
3.2 Effect of TTX on inflammatory and ergonomic hyperalgesia
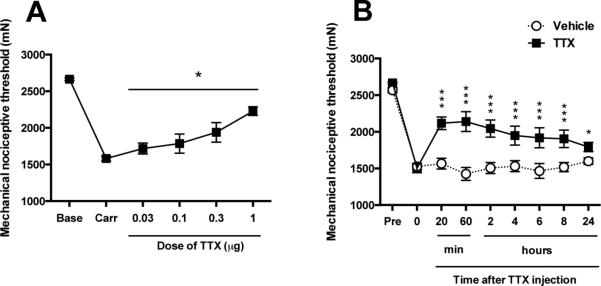
The effect of a TTX cumulative dosing protocol on mechanical hyperalgesia induced by carrageenan, an inflammogen (i.e., a model of inflammatory muscle pain), was evaluated in the gastrocnemius muscle of the adult rat. Twenty-four hours after injection of carrageenan, rats displayed marked mechanical hyperalgesia (i.e., ~40% decrease in mechanical nociceptive threshold, Fig. 2A,B), compared to baseline mechanical nociceptive threshold (i.e., 2664 ± 7.3 mN versus 1580 ± 12.9 mN, respectively, P < 0.001, n=6/group, Fig. 2A,B). Sequentially higher doses of TTX were injected every 45 min (doses administered were 0.03, 0.1, 0.3 and 1 μg/20 μl, respectively). In rats exhibiting inflammatory hyperalgesia, TTX produced a dose-dependent increase in mechanical nociceptive threshold that was statistically significant in all the doses studied (P < 0.05, n=6/group, Fig. 2A). A time course study showed that, compared to vehicle, 1 μg of TTX produced a significant increase in mechanical nociceptive threshold up to 24 h after its local injection (P < 0.01, n=6/group, Fig. 2B).
Figure 2. Effect of intramuscular TTX on inflammatory mechanical hyperalgesia.
(A) The injection of λ-carrageenan (100 μg/10 μl) into the gastrocnemius muscle produced a decrease of mechanical nociceptive threshold (Carr) measured 24 h later. At this time point sequentially increasing doses of TTX, each one-half log unit greater than the previous dose, were injected at 45 min inter-injection intervals. Prior to the injection of each higher dose of TTX, the mechanical nociceptive threshold was again assessed, to determine the effect of that dose on the carrageenan-induced mechanical hyperalgesia. TTX produced a dose-dependent increase in mechanical nociceptive threshold, in the presence of carrageenan induced mechanical hyperalgesia. Of note, not even the highest dose elevated the mechanical nociceptive threshold above the pre-carrageenan baseline. (B) After the highest intramuscular dose of TTX, or vehicle, were injected the mechanical nociceptive threshold was monitored to evaluate time course of their antihyperalgesic effect. Compared to vehicle, TTX induced a significant increase in mechanical nociceptive threshold, reversal of hyperalgesia, up to twenty-four hours after the local injection of a 1 μg dose of TTX, in carrageenan-injected rats. *P < 0.05; ***P < 0.001.
3.3 Effect of TTX on ergonomic muscle pain
Next, we explored whether TTX also displays antihyperalgesic effects in models of ergonomically induced muscle injury pain. Based on the results obtained in the inflammatory hyperalgesia model, we administered the 1 μg dose to rats previously submitted to a protocol of eccentric exercise of the hind limb, a model of delayed-onset muscle soreness. As previously reported (Alvarez et al., 2010), twenty-four hours after exposure to ergonomic injury induced by eccentric exercise animals exhibited significant muscle mechanical hyperalgesia (P < 0.001, n=6/group, Fig. 3). In these rats local injection of TTX (1 μg/20 μl), but not vehicle (D-PBS), produced a significant increase in mechanical nociceptive threshold (P < 0.001, n=6/group, Fig. 3).
Figure 3. Local effect of TTX on ergonomic injury-induced mechanical hyperalgesia.
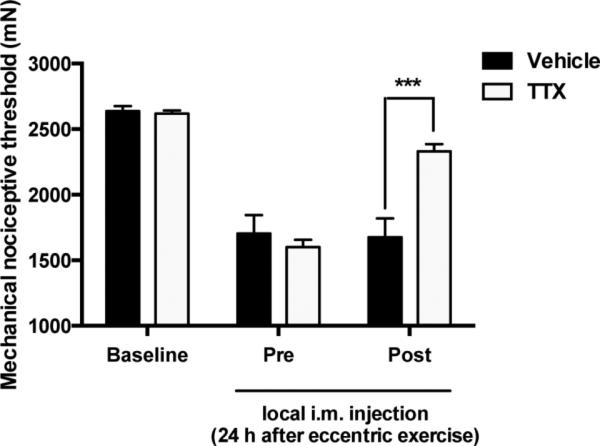
After assessment of the mechanical nociceptive threshold (Baseline), rats were submitted to an eccentric exercise protocol. Twenty-four hours later, rats exhibited significant mechanical hyperalgesia (Pre). The local treatment (Post) with a dose of 1 μg of TTX, but not of D-PBS (Vehicle), markedly attenuated the eccentric exercise-induced muscle mechanical hyperalgesia. ***P < 0.001.
3.4 Effect of TTX on neuropathic muscle pain
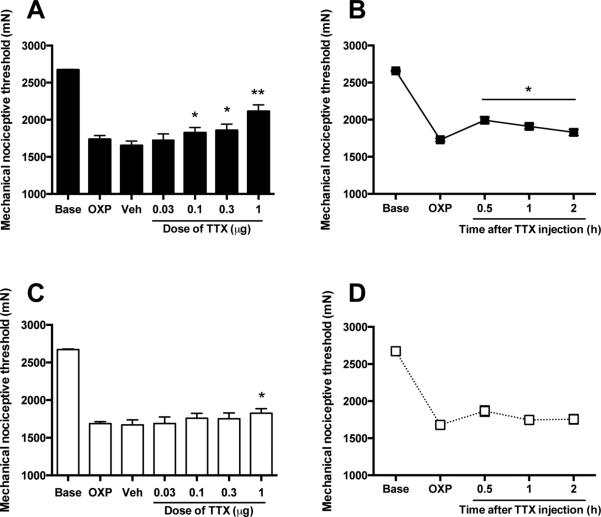
The effect of cumulative doses of TTX on mechanical hyperalgesia induced by the neurotoxic antineoplastic chemotherapy agent oxaliplatin, a model of chemotherapy induced neuropathic muscle pain (Alvarez et al., 2011), was evaluated in the gastrocnemius muscle of the adult rat. Consistent with our previous observations, rats displayed a robust muscle mechanical hyperalgesia at 4 and 15 days after intravenous injection of oxaliplatin (Alvarez et al., 2011) (P < 0.001, n=12/group, Fig. 4A,B,C,D). At these time points, cumulative higher doses of TTX (0.03, 0.1, 0.3 and 1 μg/20 μl), or vehicle, were injected locally (i.m.) every 45 min and read 15 min after each injection (Fig. 4A,C). The time course of the antinociceptive effect was further delineated by injecting a single dose of TTX (1 μg) in separate groups of rats at 4 and 15 days after injection of oxaliplatin (Fig. 4B,D).
Figure 4. Effect of intramuscular TTX on oxaliplatin-induced mechanical hyperalgesia.
After assessment of the mechanical nociceptive threshold (Base), rats received an i.v. dose of oxaliplatin (2 mg/kg) and their mechanical nociceptive thresholds were assessed 4 and 15 days later (OXP). At these time points sequentially increasing doses of TTX, each one-half log unit greater than the previous dose, were injected at 45 min inter-injection intervals. Prior to each higher dose of TTX, the mechanical nociceptive threshold was again assessed, to determine the effect of that dose on the oxaliplatin-induced mechanical hyperalgesia. (A) Four days after oxaliplatin injection TTX produced a small, albeit statistically significant, increase in mechanical nociceptive threshold compared to D-PBS vehicle (Veh). (B) Also four days after oxaliplatin injection, the higher dose of TTX (1 μg) was injected i.m., and the mechanical nociceptive threshold was measured at four different time points. TTX induced a small, albeit statistically significant, increase in mechanical nociceptive threshold after the local injection. (C) Fifteen days after oxaliplatin, only the highest dose of TTX (1 μg) produced a small, yet significant, increase in mechanical nociceptive threshold compared to vehicle. (B) Fifteen days after oxaliplatin injection, the dose of TTX (1 μg) was devoid of statistically significant effect on muscle mechanical hyperalgesia, as revealed by readings taken at 30 min to 2 hours. *P < 0.05; **P < 0.01.
Four days after oxaliplatin, rats exhibited a significant decrease in nociceptive mechanical threshold compared to baseline (−35.1 ± 1.8%, P < 0.01, n=6/group, Fig. 4A). This hyperalgesia was not significantly modified by vehicle treatment (−38.2 ± 2.2%, P > 0.05, n=6/group, Fig. 4A). However, compared to vehicle, a significant increase in mechanical nociceptive threshold was observed with TTX doses of 0.1 μg (−31.8 ± 2.6%, P < 0.05, n=6/group, Fig. 4A) or higher, the maximal antinociceptive effect reached with the dose of 1 μg (−21 ± 3.3 %, P < 0.01, n=6/group, Fig. 4A). An additional group of rats injected with oxaliplatin, 4 days before, also exhibited a reduced mechanical nociceptive threshold compared to baseline (−34.9 ± 0.7%, P < 0.001, n=6/group, Fig. 4B). In this group of rats, 1 μg of TTX produced a small, albeit statistically significant increase in mechanical nociceptive threshold 30 min after injection (-25 ± 1.5%, P < 0.001, n=6/group, Fig. 4B), which lasted for up to 2 hours after injection (−31.2 ± 0.9%, P < 0.05, n=6/group, Fig. 4B).
Rats injected with oxaliplatin 15 days before also exhibited a decrease in mechanical nociceptive threshold compared to baseline (−36.9 ± 0.4%, P < 0.05, n=6/group, Fig. 4C).
In this group, however, only the highest dose of TTX explored (1 μg) produced a modest, although statistically significant, increase in mechanical nociceptive (−31.7 ± 0.9%) respect to vehicle (-37.5 ± 1.1%, P < 0.05, n=6/group, Fig. 4C). Another group of rats injected with oxaliplatin 15 days before also exhibited reduced mechanical nociceptive threshold compared to baseline (−37.2 ± 1%, P < 0.001, n=6/group, Fig. 4D). In this group, however, a single dose of 1 μg of TTX did not produce significant antinociceptive effects at any of time points studied (P > 0.05, n=6/group, Fig. 4D).
4. Discussion
The safe and effective treatment of muscle pain remains a major problem in clinical medicine, with most of the currently used analgesics lacking of adequate efficacy and safety. Since persistent pain is, in most cases, dependent on activity in primary afferent nociceptors, it has long been a goal of translational pain research to develop effective therapies that would normalize nociceptor function (Richards and McMahon, 2013). Toward this end we explored whether TTX affected the protective baseline mechanical nociceptive threshold and whether it could reduce the abnormally increased responsiveness of muscle nociceptors in the setting of tissue injury and painful peripheral neuropathy. We found that TTX in fact produced a dose-dependent reversal of mechanical hyperalgesia in preclinical models of inflammatory and ergonomic, and to a much lesser degree, neuropathic muscle pain, while leaving the protective nociception intact.
Given the well known narrow therapeutic index and reported lethal effects of TTX, its motor and sedative effects have been previously studied by means of the rotarod test in mice (Nieto et al., 2008) and the assessment of behavioral responses in rats and mice (Marcil et al., 2006; Nieto et al., 2008). For instance, it has been shown that systemic administration of TTX to rats, in doses comparable to those used here (up to 6 μg/kg), did not produce signs of respiratory distress, motor impairment or paralysis (Marcil et al., 2006). However, these symptoms can be observed after administration of higher doses, and lethality between 8-20% of treated rats have been observe when 4-5 μg (total dose, respectively) are administered in the form of sciatic nerve block (Kohane et al., 1998). Thus, compatible with our subjective observation, the doses used in the present study are likely devoid of motor effects.
4.1 Effects of TTX on baseline nociception
The present experiments shown that TTX did not increase baseline mechanical nociceptive threshold in control (naïve) rats. Instead, a small albeit statistically significant proalgesic effect was observed after the injection of lower doses of TTX, possibly produced by injection trauma. The cumulative dose-response protocol used here involves multiple injections and repeated nociceptive testing in the same area. Thus, injections of low doses of TTX, presumably devoid of antinociceptive effect, are likely to induce a decrease in nociceptive threshold. This was not observed after the injection of the highest dose of TTX studied, suggesting that this dose produced a mild antinociceptive effect, enough to counteract the decrease in threshold induced by previous injections.
The lack of effect of TTX in naïve rats is probably not due to ineffective low doses, since they are close to previously reported antinociceptive doses (Kayser et al., 2010; Marcil et al., 2006) and they displayed inhibitory effects on inflammatory and ergonomic models here. Instead, we postulate that this is likely due to an insufficient access of TTX to muscular nociceptors in noninflammed tissue. Indeed, a lack of antinociceptive effect for local TTX has been previously reported (Hackel et al., 2012). In contrast, local inflammation causes a disruption of the perineurial barrier, which enhances the local analgesia produced by drugs such as opioids (Antonijevic et al., 1995). Interestingly, matrix metalloproteinase 9 plays a major role in the opening of the perineurial barrier (Hackel et al., 2012), being up-regulated locally by interventions such as eccentric exercise (Koskinen et al., 2002) and carrageenan-induced inflammation (Vieira et al., 2013). Thus, changes in local pharmacokinetics triggered by these insults probably resulted in an increased access of TTX to peripheral terminals at the site of injection, allowing the effect exhibited by TTX under these conditions.
4.2 Effects of TTX on models of persistent muscle pain
We observed that local injection of TTX into injured inflamed gastrocnemius muscle produced a dose dependent antihyperalgesic effect. Importantly, even the highest dose of TTX did not elevate the mechanical nociceptive threshold to above the pre-carrageenan baseline mechanical nociceptive threshold, supporting the idea that TTX acted as an antihyperalgesic agent rather than as a local anesthetic. It has been previously reported that local injection of inflammogens such as carrageenan or complete Freund's adjuvant enhance the expression of TTX-S sodium channels in DRG neurons innervating the area of injury concomitant to enhanced nociceptive responses (Black et al., 2004; Strickland et al., 2008; Tanaka et al., 1998). Importantly, the changes induced by carrageenan injection have been observed only in DRG ipsilateral to the injury (Black et al., 2004; Strickland et al., 2008), indicating that the actions exhibited by TTX are due to a local effect.
While the up-regulation of TTX-S sodium channels may contribute to muscle hyperalgesia induced by carrageenan, and therefore to the effects displayed by TTX 24 h later, we cannot exclude the contribution of other mechanisms involving TTX-S sodium channels and their targeting by TTX at this time point or earlier. Indeed, in the absence of inflammogens, TTX applied topically onto acutely isolated lumbar dorsal roots attenuates the exercise-induced pressor reflex evoked by static contraction of the triceps surae muscles (Tsuchimochi et al., 2011; Stone et al., 2015), an effect that is mainly mediated by TTX-S sodium channels, namely Nav1.7 (Stone et al., 2015). In carrageenan-induced muscle inflammation, enhanced responses of group III nociceptors innervating the gastrocnemius muscle are readily blocked by TTX at time points as early as 1-1.5 hr after carrageenan injection (Schomburg et al., 2012). In this context, several inflammatory mediators signal through protein kinase C (PKC), which is known to enhance TTX-S sodium channel resurgent currents (Tan et al., 2014). Besides, tumor necrosis factor alpha, is produced at the site of carrageenan inflammation and signals through extracellular-regulated kinases (ERK) (Utreras et al., 2009), which phosphorylates Nav1.7 in DRG neurons (Stamboulian et al., 2010). Of note, ERK and the epsilon isoform of PKC are required for full expression of carrageenan-induced hyperalgesia (Han et al., 2012; Khasar et al., 1999).
An important feature of the effect displayed by TTX on the carrageenan model was the prolonged duration of its antihyperalgesic effects. Previous studies have reported a long-lasting local blocking effect for TTX when injected perineurally, intracortically, intrathecally or even topically onto the cornea. Indeed, sciatic nerve block with TTX completely blocks carrageenan-induced thermal and mechanical cutaneous hyperalgesia for at least 9 h (Beloeil et al., 2006). A single intracortical injection of TTX also blocks axonal activity in the cerebral cortex of rats for at least 6 h (Carmignoto and Vicini, 1992). Also, a single intrathecal injection of TTX produces a complete paralysis of hindlimbs in otherwise naïve rats, which extends for over 40 h (Teng and Wrathall, 1997). Importantly, TTX completely blocks the enhanced nociceptive responses in type III nociceptors after intramuscular injection of carrageenan in the gastrocnemius muscle, during at least 10 h (Schomburg et al., 2012). In de-epithelialized cornea, TTX displays a local anesthetic effect, which is significant for at least 8 h (Schwartz et al., 1998). Finally, a direct effect of TTX on inflammation and its long-term consequences cannot be ruled out. Indeed, local TTX displays a protective long-term effect in a model of acute spinal cord injury (Rosenberg et al., 1999; Teng and Wrathall, 1997).
In the case of ergonomic injury induced muscle hyperalgesia, eccentric exercise has been shown to induce local tissue damage in the muscle and to release several pro-inflammatory cytokines, including interleukin 6 (IL-6) (Rosendal et al., 2005; Su et al., 2010; Tomiya et al., 2004; Willoughby et al., 2003), which is pronociceptive (Summer et al., 2008), including in muscle (Dina et al., 2008; Loram et al., 2007; Manjavachi et al., 2010). It has been previously shown that the exposure to IL-6 increases the number of action potentials, decreases the latency to response to ramp stimuli applied to meningeal nociceptors and enhances nociceptive responses to mechanical stimulation, in a manner consistent with ERK-dependent Nav1.7 phosphorylation (Yan et al., 2012). Furthermore, muscle mechanical hyperalgesia induced by injection of IL-6 is sensitive to local selective inhibitors of ERK (Manjavachi et al., 2010) and intrathecal antisense treatment directed against the subunit gp130 of the IL-6 receptor inhibits eccentric exercise induced mechanical hyperalgesia (Alvarez et al., 2010), indicating that this signaling pathway also contributes to ergonomic injury-induced muscle hyperalgesia. Thus, phosphorylation of the TTX-S sodium channel Nav1.7 in sensitized muscle nociceptors is likely to participate in the mechanical hyperalgesia induced by eccentric exercise underlying the antihyperalgesic effects exhibited by TTX.
In our hands TTX produced only a small effect on oxaliplatin-induced muscle mechanical hyperalgesia, which was mainly observed at early stages of the neuropathy. Given the reported in vitro inhibitory effect of TTX on persistent sodium currents produced by the exposure of rat isolated nerves or DRGs to oxaliplatin (Adelsberger et al., 2000), the antiallodynic effect of TTX boluses injected onto DRG of rats submitted to spinal nerve ligation (Lyu et al., 2000), and the contribution of the TTX-S sodium channel Nav1.6 to the neuropathic pain symptoms induced by oxaliplatin in mice (Deuis et al., 2013; Sittl et al., 2012) our results are somewhat unexpected. While we do not have an explanation for this discrepancy, we can hypothesize that the local doses of TTX used in our experiments might have been in a range that could produce a ceiling for its antihyperalgesic effect. Indeed, it has been reported that high concentrations of TTX can activate TTX-R VGSC, such as Nav1.8 (Farmer et al., 2008). Of note, a ceiling antihyperalgesic/antiallodynic effect of TTX at high doses has been observed in rats previously submitted to surgical models of neuropathic pain (Kayser et al., 2010; Lyu et al., 2000).
5. Conclusions
In summary, we provide evidence that TTX displays important antihyperalgesic effects on rat models of persistent muscle pain, without interfering with the nociceptor function to signal for further potentially harmful stimuli. Given its safety and efficacy profiles exhibited in patients affected by cancer pain (Hagen et al., 2008; Hagen et al., 2011) or withdrawal symptoms in heroin addicts (Shi et al., 2009; Song et al., 2011) and the present data, TTX might be an useful therapeutic agent for the treatment of persistent muscle pain.
Highlights.
While persistent muscle pain is very common and disabling it lacks effective therapy.
Tetrodotoxin is a promising analgesic but has not been evaluated for muscle pain.
Tetrodotoxin did not increase muscle nociceptive threshold in control naïve rats.
Tetrodotoxin attenuated mechanical hyperalgesia in models of persistent inflammatory and ergonomic muscle pain.
Acknowledgements
Authors thank Lindsay Conner for excellent technical assistance. This work was support by a grant from the National Institutes of Health (NIH) AR063312 and a grant from the UCSF Academic Senate Committee on Research.
Abreviations
- D-PBS
Dulbecco's phosphate buffered saline
- DRG
dorsal root ganglion
- ERK
extracellular regulated kinase
- IL-6
interleukin 6
- INa
inward sodium currents
- LD50
lethal dose 50
- mN
milli Newton
- Nav
α-subunit of mammalian voltage-gated sodium channels
- TTX
Tetrodotoxin
- TTX-R
Tetrodotoxin resistant
- TTX-S
Tetrodotoxin sensitive
- VGSC
voltage-gated sodium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors report no conflicts of interest.
References
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. PMID: 11011028. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Ferrari LF, Levine JD. Muscle pain in models of chemotherapy-induced and alcohol-induced peripheral neuropathy. Ann Neurol. 2011;70:101–9. doi: 10.1002/ana.22382. PMID: 21786301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–25. doi: 10.1111/j.1460-9568.2010.07359.x. PMID: 20726881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonijevic I, Mousa SA, Schäfer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–72. doi: 10.1523/JNEUROSCI.15-01-00165.1995. PMID: 7823127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A. Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins (Basel) 2014;6(2):693–755. doi: 10.3390/toxins6020693. PMID: 24566728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloeil H, Ababneh Z, Chung R, Zurakowski D, Mulkern RV, Berde CB. Effects of bupivacaine and tetrodotoxin on carrageenan-induced hind paw inflammation in rats (Part 1): hyperalgesia, edema, and systemic cytokines. Anesthesiology. 2006;105:128–38. doi: 10.1097/00000542-200607000-00022. PMID: 16810004. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–47. doi: 10.1016/j.pain.2003.12.035. PMID: 15030943. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–90. doi: 10.1523/JNEUROSCI.22-23-10277.2002. PMID: 12451128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, Vetter I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain. 2013;154:1749–57. doi: 10.1016/j.pain.2013.05.032. PMID: 23711479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Waxman SG. Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 2009;10:1260–9. doi: 10.1111/j.1526-4637.2009.00719.x. PMID: 19818036. [DOI] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–62. doi: 10.1016/j.jpain.2008.01.328. PMID: 18342576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. PMID: 18280048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer C, Smith K, Docherty R. Low concentrations of tetrodotoxin interact with tetrodotoxin-resistant voltage-gated sodium channels. Br J Pharmacol. 2008;155:34–43. doi: 10.1038/bjp.2008.235. PMID: 18552876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Miyoshi K, Noguchi K. De novo expression of Nav1.7 in injured putative proprioceptive afferents: Multiple tetrodotoxin-sensitive sodium channels are retained in the rat dorsal root after spinal nerve ligation. Neuroscience. 2015;284:693–706. doi: 10.1016/j.neuroscience.2014.10.027. PMID: 25453779. [DOI] [PubMed] [Google Scholar]
- Hackel D, Krug SM, Sauer RS, Mousa SA, Böcker A, Pflücke D, Wrede EJ, Kistner K, Hoffmann T, Niedermirtl B, Sommer C, Bloch L, Huber O, Blasig IE, Amasheh S, Reeh PW, Fromm M, Brack A, Rittner HL. Transient opening of the perineurial barrier for analgesic drug delivery. Proc Natl Acad Sci USA. 2012;109:E2018–27. doi: 10.1073/pnas.1120800109. PMID: 22733753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen NA, du Souich P, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Love R, Ngoc AH, Canadian Tetrodotoxin Study Group Tetrodotoxin for moderate to severe cancer pain: a randomized, double blind, parallel design multicenter study. J Pain Symptom Manage. 2008;35:420–9. doi: 10.1016/j.jpainsymman.2007.05.011. PMID: 18243639. [DOI] [PubMed] [Google Scholar]
- Hagen NA, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Gagnon B, Love R, Goel R, Hawley P, Ngoc AH, du Souich P. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr Oncol. 2011;18:e109–16. doi: 10.3747/co.v18i3.732. PMID: 21655148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Lee KS, Rong W, Zhang G. Different roles of peripheral mitogen-activated protein kinases in carrageenan-induced arthritic pain and arthritis in rats. Anesth Analg. 2012;115:1221–7. doi: 10.1213/ANE.0b013e318266c1ee. PMID: 22822193. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J Pain. 2009;10:534–41. doi: 10.1016/j.jpain.2008.12.003. PMID: 19231296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser V, Viguier F, Ioannidi M, Bernard JF, Latrémolière A, Michot B, Vela JM, Buschmann H, Hamon M, Bourgoin S. Differential anti-neuropathic pain effects of tetrodotoxin in sciatic nerve-versus infraorbital nerve-ligated rats--behavioral, pharmacological and immunohistochemical investigations. Neuropharmacology. 2010;58:474–87. doi: 10.1016/j.neuropharm.2009.09.003. PMID: 19747496. [DOI] [PubMed] [Google Scholar]
- Kano Y, Sampei K, Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand. 2004;180:291–9. doi: 10.1111/j.0001-6772.2003.01250.x. PMID: 14962011. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. PMID: 10677042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–31. doi: 10.1097/00000542-199807000-00019. PMID: 9667302. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Ahtikoski AM, Komulainen J, Hesselink MK, Drost MR, Takala TE. Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch. 2002;444:59–72. doi: 10.1007/s00424-002-0792-2. PMID: 11976917. [DOI] [PubMed] [Google Scholar]
- Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8:127–36. doi: 10.1016/j.jpain.2006.06.010. PMID: 16949880. [DOI] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. PMID: 10882788. [DOI] [PubMed] [Google Scholar]
- Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain. 2010;151:345–55. doi: 10.1016/j.pain.2010.07.018. PMID: 2070945. [DOI] [PubMed] [Google Scholar]
- Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br J Anaesth. 2006;96:761–8. doi: 10.1093/bja/ael096. PMID: 16675510. [DOI] [PubMed] [Google Scholar]
- Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard AM, Wood JN. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014;6:301–12. doi: 10.1016/j.celrep.2013.12.033. PMID: 24440715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Ru F, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ. Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol. 2011;589:5663–76. doi: 10.1113/jphysiol.2011.215384. PMID: 22005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Tetrodotoxin: a brief history. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:147–54. doi: 10.2183/pjab.84.147. PMID: 18941294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137:520–31. doi: 10.1016/j.pain.2007.10.012. PMID: 18037242. [DOI] [PubMed] [Google Scholar]
- Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol. 2012;108:2230–41. doi: 10.1152/jn.00219.2012. PMID: 22855776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra R, Elmslie KS. Society for Neuroscience. Washington, DC.: 2014. Ion channels/receptors expressed along group IV sensory afferent fibers within rat skeletal muscle. (Abstract 245.05/GG2) [Google Scholar]
- Richards N, McMahon SB. Targeting novel peripheral mediators for the treatment of chronic pain. Br J Anaesth. 2013;111:46–51. doi: 10.1093/bja/aet216. PMID: 23794644. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci. 1999;19:6122–33. doi: 10.1523/JNEUROSCI.19-14-06122.1999. PMID: 10407048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal L, Søgaard K, Kjaer M, Sjøgaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2005;98:477–81. doi: 10.1152/japplphysiol.00130.2004. PMID: 15448117. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H, Dibaj P, Sears TA. Major contribution of Aδ-fibres to increased reflex transmission in the feline spinal cord during acute muscle inflammation. Neurosci Res. 2012;72:155–62. doi: 10.1016/j.neures.2011.10.006. PMID: 22056284. [DOI] [PubMed] [Google Scholar]
- Schwartz DM, Duncan KG, Fields HL, Jones MR. Tetrodotoxin: anesthetic activity in the de-epithelialized cornea. Graefes Arch Clin Exp Ophthalmol. 1998;236:790–4. doi: 10.1007/s004170050160. PMID: 9801896. [DOI] [PubMed] [Google Scholar]
- Shi J, Liu TT, Wang X, Epstein DH, Zhao LY, Zhang XL, Lu L. Tetrodotoxin reduces cue-induced drug craving and anxiety in abstinent heroin addicts. Pharmacol Biochem Behav. 2009;92:603–7. doi: 10.1016/j.pbb.2009.02.013. PMID: 19268686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci USA. 2012;109:6704–9. doi: 10.1073/pnas.1118058109. PMID: 22493249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Li J, Lu CL, Kang L, Xie L, Zhang YY, Zhou XB, Zhong S. Tetrodotoxin alleviates acute heroin withdrawal syndrome: a multicentre, randomized, double-blind, placebo-controlled study. Clin Exp Pharmacol Physiol. 2011;38:510–4. doi: 10.1111/j.1440-1681.2011.05539.x. PMID: 21575032. [DOI] [PubMed] [Google Scholar]
- Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30:1637–47. doi: 10.1523/JNEUROSCI.4872-09.2010. PMID: 20130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, Kaufman MP. Role played by NaV 1.7 channels on thin fiber muscle afferents in transmitting the exercise pressor reflex. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00246.2015. in press. PMID: 26310938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2008;12:564–72. doi: 10.1016/j.ejpain.2007.09.001. PMID: 17950013. [DOI] [PubMed] [Google Scholar]
- Su QS, Zhang JG, Dong R, Hua B, Sun JZ. Comparison of changes in markers of muscle damage induced by eccentric exercise and ischemia/reperfusion. Scand J Med Sci Sports. 2010;20:748–56. doi: 10.1111/j.1600-0838.2009.01015.x. PMID: 19804580. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. PMID: 17590515. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol. 2005;564:259–68. doi: 10.1113/jphysiol.2004.079483. PMID: 15677691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZY, Priest BT, Krajewski JL, Knopp KL, Nisenbaum ES, Cummins TR. Protein kinase C enhances human sodium channel hNav1.7 resurgent currents via a serine residue in the domain III-IV linker. FEBS Lett. 2014;588:3964–9. doi: 10.1016/j.febslet.2014.09.011. PMID: 25240195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998;9:967–72. doi: 10.1097/00001756-199804200-00003. PMID: 9601651. [DOI] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci. 1997;17:4359–66. doi: 10.1523/JNEUROSCI.17-11-04359.1997. PMID: 9151752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiya A, Aizawa T, Nagatomi R, Sensui H, Kokubun S. Myofibers express IL-6 after eccentric exercise. Am J Sports Med. 2004;32:503–8. doi: 10.1177/0095399703258788. PMID: 14977681. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300(2):H652–63. doi: 10.1152/ajpheart.00859.2010. PMID: 21076028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem. 2009;284:2275–84. doi: 10.1074/jbc.M805052200. PMID: 19049962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira CP, De Aro AA, Da Ré Guerra F, De Oliveira LP, De Almeida Mdos S, Pimentel ER. Inflammatory process induced by carrageenan in adjacent tissue triggers the acute inflammation in deep digital flexor tendon of rats. Anat Rec (Hoboken) 2013;296:1187–95. doi: 10.1002/ar.22729. PMID: 23775880. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, McFarlin B, Bois C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24:15–21. doi: 10.1055/s-2003-37197. PMID: 12582947. [DOI] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. PMID: 22273495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Yue Y, Xing F, Xia W, Lai S, Zhang F. Tetrodotoxin poisoning caused by Goby fish consumption in southeast China: a retrospective case series analysis. Clinics (Sao Paulo) 2015;70:24–9. doi: 10.6061/clinics/2015(01)05. PMID: 25672425. [DOI] [PMC free article] [PubMed] [Google Scholar]