Abstract
Introduction
Knee osteoarthritis (KOA) is typically a slowly progressive disorder; however, a subset of knees progress with dramatic rapidity. We aimed to describe magnetic resonance imaging (MRI) findings that are associated with accelerated KOA.
Materials and Methods
We conducted a longitudinal descriptive study in the Osteoarthritis Initiative (OAI) cohort. We selected participants who had no radiographic KOA at baseline with one of the following in the most severe knee: 1) accelerated KOA (progressed to end-stage KOA within 48 months), 2) common KOA, and 3) no KOA at all visits. We enriched the sample by selecting knees with a self-reported or suspected knee injury. A musculoskeletal radiologist blinded to group assignments but not to time sequence performed MRI readings for the visit before and after an injury.
Results
We assessed 38 participants (knees), 66% were female, mean age 61 (9) years, and mean body mass index 28.5 (4.9) kg/m2. Fifteen of 20 knees with no or common KOA, had no incident findings consistent with acute damage. Among the 18 knees with accelerated KOA most had incident findings: 13 (72%) had incident medial meniscal pathology with extrusion and 5 (28%) knees had subchondral damage.
Conclusions
Incident MRI findings that are associated with incident accelerated KOA are characterized by structural damage that compromises subchondral bone or the function of the meniscus. Recognizing meniscal extrusion and/or change in shape, lateral meniscal tear, or acute subchondral damage may be vital for identifying individuals at risk for accelerated KOA.
Keywords: osteoarthritis, meniscus, ligaments, magnetic resonance imaging
INTRODUCTION
Osteoarthritis, particularly knee osteoarthritis, is one of the most common causes of disability (Losina et al., 2012; Vos et al., 2013) and the main reason for joint replacement at a cost of over $30 billion/year (Agency for Healthcare Research and Quality, 2014). The lack of an optimal treatment strategy for osteoarthritis may be at least in part, attributable to it being a common endpoint defined by several distinct phenotypes that may require different treatment strategies (Driban et al., 2009; Felson, 2010; Hunter and Hellio Le Graverand-Gastineau, 2008; Martel-Pelletier et al., 2006). One newly described phenotype is knees that progress with dramatic rapidity (e.g. from normal appearance to end-stage disease within 4 years) compared with the more gradual progression that typically characterizes osteoarthritis. The dramatic rate of joint destruction raises questions about whether accelerated knee osteoarthritis could be a distinct disease; hence, this subsample of individuals warrants additional attention. Determining factors that differentiate or identify accelerated knee osteoarthritis could generate insights into its pathogenesis and offer therapeutic targets.
Self-reported knee injuries are predictive of accelerated knee osteoarthritis (Driban et al., 2014). While self-reported injuries are important because they represent an individual’s perception of an event that elicited knee symptoms it does not help us determine the incident structural damage (e.g., anterior cruciate ligament tear, meniscal tear) that may precipitate accelerated knee osteoarthritis. To help clinicians identify individuals at risk for accelerated knee osteoarthritis it is crucial to determine the incident structural damage that may precede accelerated knee osteoarthritis, common knee osteoarthritis, or no osteoarthritis.
To better understand the underlying etiopathogenesis of accelerated knee osteoarthritis, we conducted a descriptive study to evaluate those with accelerated knee osteoarthritis, common knee osteoarthritis, and those who did not develop knee osteoarthritis over a 48-month observation period. We aimed to describe magnetic resonance (MR) imaging findings that are associated with incident accelerated knee osteoarthritis. We enriched the sample by selecting knees with a self-reported or suspected knee injury because this increased the likelihood in all three groups that we would detect incident structural damage on magnetic resonance images. These results will help inform clinicians about key imaging findings to be concerned about among adults without knee osteoarthritis.
MATERIALS AND METHODS
Study Design
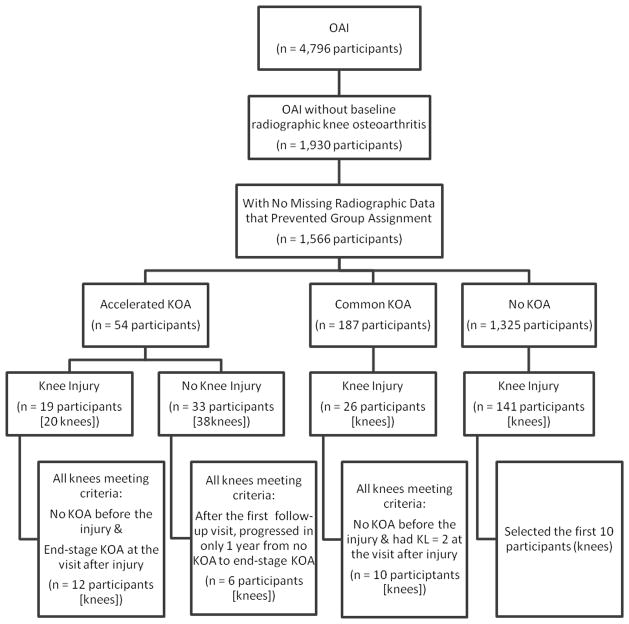
We conducted a longitudinal descriptive knee-based study using data from the Osteoarthritis Initiative (OAI) to determine the type of incident findings associated with incident accelerated knee osteoarthritis. The cases were knees with incident accelerated knee osteoarthritis. The comparison groups were knees with incident common knee osteoarthritis and knees that did not develop knee osteoarthritis over 48 months of observation. To help identify incident structural damage in all three groups we enriched the sample by including individuals with a self-reported knee injury during the observation period or a suspected injury. Specific selection criteria are defined below and in Figure 1.
Figure 1.
Selection criteria for study sample.
Sample Selection / Setting
The OAI is a longitudinal observational study of individuals with or at risk for knee osteoarthritis at four sites in the United States. The staff at the four clinical sites enrolled 4,796 men and women (45 to 79 years of age) between February 2004 and May 2006. The OAI cohort comprised three groups classified at baseline: 1) progression subcohort, including individuals with at least one knee with symptomatic radiographic knee osteoarthritis, 2) incidence subcohort, including individuals at risk for symptomatic radiographic knee osteoarthritis, and 3) non-exposed control subcohort, which included individuals with no knee osteoarthritis and no risk factors for knee osteoarthritis. Detailed descriptions of the eligibility criteria for each subcohort and the OAI protocol are publicly available at the OAI website. Institutional review boards at each OAI clinical site and the OAI coordinating center approved the OAI study. These analyses were deemed not human subject research because they used publicly available de-identified data and images.
Case and Control Definitions
Case and control definitions were based on baseline and change in radiographic osteoarthritis severity over a period of 48 months. The OAI annually collected bilateral, posterior-anterior, weight-bearing, fixed-flexion knee radiographs during the first four years. Central readers assessed each knee for radiographic osteoarthritis severity (Kellgren-Lawrence [KL] Grade). The readers had good intra-reader agreement (read-reread weighted kappa = 0.70 to 0.78). The protocol and data are publicly available at the OAI website (Files: kXR_SQ_BU##_SAS; versions 0.6, 1.6, 3.5, 5.5, 6.3) (Osteoarthritis Initiative, 2015).
Among OAI participants with no baseline radiographic knee osteoarthritis (KL < 2) in both knees (n = 1,930) we evaluated three groups based on radiographic definitions of osteoarthritis: 1) accelerated knee osteoarthritis: knee progressed to end-stage knee osteoarthritis (KL Grade 3 or 4) within 48 months, 2) common knee osteoarthritis progression: knee increased in radiographic scoring within 48 months (excluding those defined as accelerated knee osteoarthritis progression), and 3) no knee osteoarthritis: no change in KL grade in either knee at baseline and 48-month follow-up. We omitted 364 (19%) participants because missing radiographic scores precluded our ability to determine group assignment (19 possible participants with common knee osteoarthritis progression, 345 possible participants with no knee osteoarthritis). From this sample, we had 1325 individuals with no knee osteoarthritis, 187 individuals with common knee osteoarthritis, and 54 individuals with accelerated knee osteoarthritis. Clinical differences among these groups have been published (Driban et al., 2014).
Because our particular interest was on the influence of incident structural damage in these 3 groups, we required that most participants self-report knee injury to the index knee. At each follow-up visit, participants were asked “Since your last annual visit to the OAI clinic about 12 months ago, have you injured your right knee badly enough to limit your ability to walk for at least two days?” A similar question was asked for the left knee. The injury data are publicly available (Files: allclinical##; versions 0.2.2, 1.2.1, 3.2.1, 5.2.1). For individuals with no knee osteoarthritis, bilateral common knee osteoarthritis, or bilateral accelerated knee osteoarthritis we initially considered both knees and selected one knee per person, as described below.
Among knees with accelerated osteoarthritis and a self-reported injury (n = 20 knees, 19 individuals), we selected the 12 knees that went from no osteoarthritis (KL Grade 0 or 1) before the self-reported injury to end-stage knee osteoarthritis (KL Grade 3 or 4) within 12 months after the injury. This selection criteria allowed us to focus on injuries that are associated with dramatic accelerated knee osteoarthritis.
We also identified 38 knees from 33 individuals who had accelerated knee osteoarthritis but no self-reported injury. We focused on knees that transitioned from not having radiographic evidence of knee osteoarthritis (KL 0 or 1) to end-stage knee osteoarthritis (KL 3 or 4) within one year because their progression was comparable those who reported a knee injury and we suspected this dramatic progression was triggered by incident structural damage (19 knees, 17 individuals). For those who were eligible bilaterally we only selected the right knee. We excluded individuals who had accelerated knee osteoarthritis during the first 12 months of OAI follow-up (8 knees) because there was no MR imaging prior to progression to understand the knee before accelerated progression. We also excluded two knees that changed from KL 0 to KL 1 before showing accelerated progression because it was unclear if this initial change may reflect an early stage of the progression. Three people in the accelerated knee osteoarthritis group with no self-reported injury were excluded because the contralateral knee had a self-reported injury. One individual was excluded because they had images missing at multiple visits. In total 6 knees (6 individuals) with accelerated knee osteoarthritis and no self-reported injury were included.
Knees with common osteoarthritis progression were included only if the contralateral knee had no osteoarthritis during the first 48 months of OAI follow-up. Among 26 knees (26 individuals) with common OA and a self-reported injury we selected the 10 knees that went from no knee osteoarthritis (KL Grade 0 or 1) before the self-reported injury to mild knee osteoarthritis (KL Grade 2) within 12 months after the injury. This selection criterion allowed us to focus on incident structural damage that we could attribute to the self-reported knee injury more so than progression that happens much later.
Knees with no osteoarthritis and a self-reported injury were only included if the contralateral knee also had no knee osteoarthritis during 48 months of observation. Among 141 knees with no osteoarthritis and a self-reported injury we selected the first 10 knees (sorted by identification number) for review. One knee had missing imaging data at the visit prior to their self-reported injury so we evaluated the MR images from the year before that. No individuals had a self-reported injury in the contralateral knee.
Magnetic Resonance Images
At baseline and the first four annual OAI visits, participants underwent bilateral knee MR imaging. The four OAI sites had identical Siemens Trio 3-Tesla MR systems. The protocol, quality assurance procedures, and rationale have been described in detail (Peterfy et al., 2008).
Magnetic Resonance Imaging Readings
A single experienced, fellowship-trained, musculoskeletal radiologist (RJW) who was blinded to group assignment read all relevant MR images. For knees with a self-reported injury the radiologist provided clinical readings for the MR imaging readings at the visit before the self-reported injury and at the visit when the participants reported an injury in the prior 12 months. As noted above, all knees had no osteoarthritis (KL < 2) before the injury and at the visit when participants reported an injury knees with accelerated knee osteoarthritis had end-stage osteoarthritis (KL = 3 or 4), knees with common knee osteoarthritis had moderate osteoarthritis (KL = 2), and the third group had no osteoarthritis (KL = 0 or 1). For individuals with accelerated knee osteoarthritis but no reported injury the radiologist provided clinical readings for all five visits (baseline and four follow-up visits). In this study we focused on the penultimate visit when a knee had no osteoarthritis (KL < 2) and the annual visit subsequent to that, when they presented with end-stage osteoarthritis (KL = 3 or 4). In total, the radiologist performed 84 clinical readings.
Statistical Analysis
We provided a descriptive summary of the incident MR imaging findings among knees with incident accelerated osteoarthritis, incident common osteoarthritis, or no osteoarthritis.
RESULTS
We included 38 participants (knees), 66% were female, mean age 61 (9) years, and mean body mass index 28.5 (4.9) kg/m2 (see Table 1). At the visit prior to a suspected injury, most knees, regardless of group, had damage commonly associated with osteoarthritis despite having no radiographic knee osteoarthritis (e.g., anterior cruciate ligament degeneration, fat pad edema, patellofemoral chondropathy).
Table 1.
Baseline Characteristics of Study Sample
| Variable | No Knee OA (Self-Reported Injury) N = 10 |
Common Knee OA (Self-Reported Injury) N = 10 |
Accelerated Knee OA (Self-Reported Injury) N = 12 |
Accelerated Knee OA (No Self-reported Injury) N = 6 |
|---|---|---|---|---|
| Female, n (%) | 5 (50%) | 6 (60%) | 11 (92%) | 3 (50%) |
| Age (years), median (IQR) | 66 (52, 67) | 54 (50, 56) | 66 (61, 69) | 59 (52, 72) |
| BMI (kg/m2), median (IQR) | 29 (26, 31) | 28 (24, 33) | 29 (25, 32) | 27 (23, 29) |
Note: OA = osteoarthritis, BMI = body mass index, IQR = inter-quartile range (25th percentile, 75th percentile).
Among the 10 knees with no knee osteoarthritis, the only incident finding on MR images was a tibial (medial) collateral ligament sprain in 1/10 of the participants (see Table 2).
Table 2.
Common incident findings among knees with and without incident accelerated knee osteoarthritis (KOA).
| Group | Incident Findings (Frequency of Finding)* |
|---|---|
| No KOA (n = 10) | MCL Sprain (1/10) No changes (e.g., no change in effusion/synovitis or signs of degenerative changes) (3/10) |
| Common KOA (n = 10) | Medial meniscal pathology (posterior horn) with no-mild extrusion (3/10) MCL Sprain (1/10) No Changes (e.g., no change in effusion/synovitis or signs of degenerative changes) (4/10) |
| Accelerated KOA with Reported Injury (n = 12) | Medial meniscal pathology (posterior horn) with moderate extrusion (7/12) Subchondral Fracture (4/12) Medial meniscal pathology no-mild extrusion (3/12) Lateral meniscal pathology with mild extrusion (2/12) Evidence of post-traumatic avascular necrosis (1/12) |
| Accelerated KOA with no Reported Injury** (n = 6) | Medial meniscal pathology with mild-severe extrusion (4/6) Lateral meniscal pathology with no extrusion (2/6) No change (e.g., no change in effusion/synovitis or signs of degenerative changes) (1/6) |
Some knees had more than one incident finding.
Among those with no self-reported injury we focused on the last visit when the knee had no radiographic OA and the next visit when they presented with moderate-severe radiographic KOA.cruciate ligament
ACL – anterior cruciate ligament, MCL – tibial (medial) collateral ligament, PCL – posterior
Three out of 10 knees with common knee osteoarthritis had incident medial meniscal pathology in the posterior horn with no-mild extrusion: one knee had a complex tear and two knees had a horizontal tear with a flap component (see Table 2). Among these three knees with incident medial meniscal pathology there was no changes in the meniscal shape (e.g., morphologic deformity). A fourth knee with common knee osteoarthritis had an incident tibial collateral ligament sprain.
In total, among the 20 knees with no or common knee osteoarthritis 15 (75%) had no incident findings of damage and 7 of these knees (35%) had stable findings between visits (e.g., no incident findings, no cartilage loss, no change in effusion).
Among the 18 knees with accelerated knee osteoarthritis all but one knee had at least one incident finding (see Table 2). Thirteen (72%) knees had incident medial meniscal pathology with extrusion, 3 knees had incident meniscal pathology without extrusion, 4 knees had subchondral fractures (all had concurrent medial meniscal pathology, see an example in Fig. 2), and 1 had evidence of possible post-traumatic avascular necrosis (with lateral meniscal pathology). All of the knees with subchondral bone damage were among knees with self-reported injuries. The most common incident meniscal findings among knees with accelerated knee osteoarthritis were complex (4 knees), horizontal (6 knees), or radial tears (5 knees). Among the three meniscal pathologies with no extrusion, two occurred in the lateral meniscus (horizontal tears) and the other occurred in a knee with a subchondral fracture. Among the four meniscal pathologies with minimal extrusion 3 were characterized by changes in the meniscal size or shape and the fourth had a radial defect with a horizontal tear. One knee with accelerated knee osteoarthritis but no self-reported injury had no evidence of incident changes that would reflect a traumatic injury during the year they progressed from no knee osteoarthritis to end-stage knee osteoarthritis.
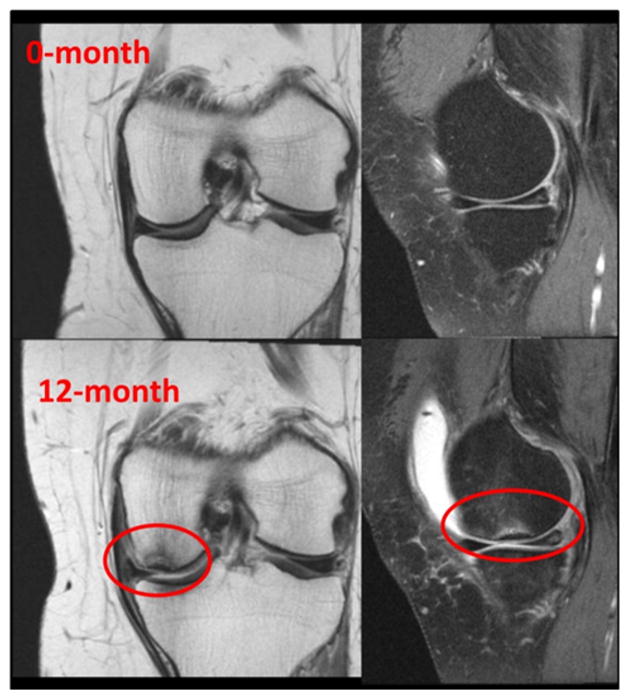
Figure 2.
Example of a knee with accelerated knee osteoarthritis and a self-reported injury between the 0-month and 12-month visit. The knee developed a subchondral medial femoral fracture, medial radial tear with moderate extrusion, and moderate effusion.
DISCUSSION
Knee injuries are a key risk factor for knee osteoarthritis, especially accelerated knee osteoarthritis. However, clinicians cannot determine which patients may be at risk for accelerated knee osteoarthritis without knowing the incident structural damage that predisposes a knee to accelerated osteoarthritis. We found that structural damage that is associated with accelerated knee osteoarthritis destabilizes and compromises the function of the meniscus or compromises the subchondral bone. Unique meniscal findings among individuals with accelerated knee osteoarthritis were lateral meniscus tears and medial meniscal tears with moderate-severe extrusion or changes in meniscal size or shape. Early detection of these lesions may be vital for identifying individuals at risk for accelerated knee osteoarthritis.
The majority of cases with accelerated knee osteoarthritis had incident meniscal pathology. Meniscal pathology is associated with an increased risk of osteoarthritis (Claes et al., 2012; Englund et al., 2009). The current study may help us identify key meniscal findings that are associated with incident accelerated knee osteoarthritis. Most knees with accelerated knee osteoarthritis had incident meniscal extrusion. In contrast, knees with no osteoarthritis were never characterized by meniscal extrusion and among knees with common osteoarthritis, only a few knees had mild extrusion. Meniscal extrusion may be a key risk factor for fast cartilage loss (Berthiaume et al., 2005; Hunter et al., 2006; Raynauld et al., 2006; Roemer et al., 2009).
Three knees with common osteoarthritis and four knees with accelerated osteoarthritis had mild medial meniscal extrusion. A key meniscal finding that may help distinguish injuries among these knees was changes in meniscal shape. Three out of four knees with accelerated knee osteoarthritis and mild meniscal extrusion had incident morphologic deformities (change in meniscal shape) or attenuated size, findings not present after injuries among those with common knee osteoarthritis. The combination of meniscal extrusion with changes in meniscal size or shape likely increases contact pressures in the tibiofemoral compartment, which hastens additional joint damage.
We found that two out of the three incident meniscal pathology without extrusion among knees with accelerated osteoarthritis occurred in the lateral meniscus. This is not surprising since the lateral meniscus is less likely to have extrusion compared with the medial meniscus among adults with or at risk for knee osteoarthritis (9.4% versus 44.2%, respectively) (Crema et al., 2012).
A few studies have raised concerns that partial lateral meniscectomy may be related to rapid chondrolysis (Alford et al., 2005; Ishida et al., 2006; Mariani et al., 2008). Recently, the first case report was published of rapid chondrolysis following a deep radial tear in the lateral meniscus that was conservatively treated (Thaunat et al., 2014). In the current study, only the knees that developed accelerated knee osteoarthritis had any evidence of incident lateral meniscal tears. These findings support a hypothesis that a lateral meniscal pathology that alters the contact pressures in the compartment may lead to accelerated knee osteoarthritis and that rapid changes are not just limited to knees treated with partial lateral meniscectomy. Interestingly, two out of four knees with an incident lateral meniscal tear were among individuals who failed to report a knee injury. This may make it challenging to detect this at risk population and highlights the need to carefully evaluate a patient who reports incident lateral joint line pain.
Two other unique findings among knees with accelerated osteoarthritis were subchondral fractures and a suspected post-traumatic avascular necrosis. All three knees with incident subchondral bone damage were among individuals who reported a knee injury. Three subchondral fractures were associated with a radial tear in the same tibiofemoral compartment. The other subchondral fracture was associated with a radial tear and a tibial collateral ligament sprain. The case of avascular necrosis was associated with a severely attenuated posterior horn of the lateral meniscus, large areas of full thickness cartilage loss, bone attrition, subchondral cyst formation, osteochondral lesions and acute tendinitis. All these cases demonstrate the complexity of these suspected injuries, which compromised the structural integrity of multiple tissues.
While this study offers novel insights into the etiopathogenesis of accelerated knee osteoarthritis it was limited because we could not acquire images immediately after an injury. Since the current study was nested in a longitudinal cohort that collected imaging each year it is unclear if some of these findings are related to progression rather than reflecting the damage originally caused by the injury. On the other hand, the OAI’s rich imaging dataset provided an ideal opportunity to identify knees with accelerated osteoarthritis, common osteoarthritis, and no osteoarthritis as well as to characterize incident structural changes related with each group. The OAI will also facilitate a more comprehensive analysis of accelerated knee osteoarthritis and how it relates to different types of incident structural damage but in the current study we were limited in sample size due to the burden of doing readings. We opted to use clinical readings instead of semiquantitative scoring systems because clinical readings allowed us to detect findings that are often not scored (e.g., subchondral fractures, avascular necrosis). These thorough readings and our focus on knees with well-defined change from before to after a suspected injury enabled us to offer strong evidence despite the limited sample size. It will be informative for future studies to build on these novel findings with larger sample sizes as well as semi-quantitative and quantitative measures of cartilage, bone, and meniscal changes over time. While we acknowledge the limitations of not having imaging immediately after an injury, the limited sample size, and the homogenous sample we believe these findings are noteworthy because they may have an immediate impact on how we handle adults without osteoarthritis who report a recent injury.
Our study highlights that among adults without knee osteoarthritis we must be concerned when a patient reports an injury. These patients should be carefully screened for evidence of meniscal pathology with extrusion and/or change in shape, lateral meniscal tear, or subchondral damage. Unfortunately, a disease-modifying therapy remains elusive for osteoarthritis, let alone for accelerated knee osteoarthritis, but this may highlight the importance of therapeutic approaches that target meniscal pathology or subchondral bone damage. In the meantime, patients that present with these high-risk findings should be advised to reduce their other risk factors for osteoarthritis (e.g., high body weight).
Acknowledgments
These analyses were financially supported by grants from the National Institute of Health (268201000020C-1-0-1, R01AR065977-01A1). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. One investigator is supported by K23 AR062127, an NIH/NIAMS funded mentored award. This work is also supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413). This manuscript does not reflect the views of the US government or the Veterans Administration.
Footnotes
The authors have no other conflicts of interest with regard to this work.
References
- Agency for Healthcare Research and Quality. [Accessed 06/16/2014];H*CUPnet National and Regional Estimates on Hospital Use for All Patients from the HCUP Nationwide Inpatient Sample (NIS) 2014 http://goo.gl/sZKq1X.
- Alford JW, Lewis P, Kang RW, Cole BJ. Rapid progression of chondral disease in the lateral compartment of the knee following meniscectomy. Arthroscopy. 2005;21:1505–1509. doi: 10.1016/j.arthro.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, Choquette D, Haraoui B, Altman RD, Hochberg M, Meyer JM, Cline GA, Pelletier JP. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;21:1967–1976. doi: 10.1007/s00167-012-2251-8. [DOI] [PubMed] [Google Scholar]
- Crema MD, Roemer FW, Felson DT, Englund M, Wang K, Jarraya M, Nevitt MC, Marra MD, Torner JC, Lewis CE, Guermazi A. Factors associated with meniscal extrusion in knees with or at risk for osteoarthritis: the Multicenter Osteoarthritis study. Radiology. 2012;264:494–503. doi: 10.1148/radiol.12110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2014;66:1673–1679. doi: 10.1002/acr.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban JB, Sitler MR, Barbe MF, Balasubramanian E. Is osteoarthritis a heterogeneous disease that can be stratified into subsets? Clin Rheumatol. 2009;29:123–131. doi: 10.1007/s10067-009-1301-1. [DOI] [PubMed] [Google Scholar]
- Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, Torner J, Nevitt MC, Sack B, Felson DT. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthritis Cartilage. 2010;18:601–604. doi: 10.1016/j.joca.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Hellio Le Graverand-Gastineau MP. How Close are We to Having Structure-Modifying Drugs Available? Rheum Dis Clin North Am. 2008;34:789–802. doi: 10.1016/j.rdc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, Guermazi A, Grigorian M, Gale D, Felson DT. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- Ishida K, Kuroda R, Sakai H, Doita M, Kurosaka M, Yoshiya S. Rapid chondrolysis after arthroscopic partial lateral meniscectomy in athletes: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14:1266–1269. doi: 10.1007/s00167-006-0091-0. [DOI] [PubMed] [Google Scholar]
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg. 2012;94:201–207. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani PP, Garofalo R, Margheritini F. Chondrolysis after partial lateral meniscectomy in athletes. Knee Surg Sports Traumatol Arthrosc. 2008;16:574–580. doi: 10.1007/s00167-008-0508-z. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, Lajeunesse D, Fahmi H, Tardif G, Pelletier JP. New thoughts on the pathophysiology of osteoarthritis: one more step toward new therapeutic targets. Curr Rheumatol Rep. 2006;8:30–36. doi: 10.1007/s11926-006-0022-6. [DOI] [PubMed] [Google Scholar]
- The Osteoarthritis Initiative. [Accessed 06/03/2015];2015 https://oai.epi-ucsf.org.
- Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, Tannenbaum H, Meyer JM, Beary JF, Cline GA, Pelletier JP. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, Nevitt MC, Felson DT, Hughes LB, El-Khoury GY, Englund M, Guermazi A. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–780. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaunat M, Archbold P, Conteduca J, Chatellard R, Sonnery-Cottet B. Rapid chondrolysis following an unoperated lateral meniscus tear in a young professional rugby player. Orthop Traumatol Surg Res. 2014;100:445–448. doi: 10.1016/j.otsr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]