Abstract
Background
Imiquimod is a topical cream approved by the US Food and Drug Administration for treatment of superficial basal cell carcinoma, actinic keratosis, and genital-perianal warts. Its successful use in patients with persistently positive margins of melanoma in situ (MIS) after surgical excision has been previously reported.
Case Report
A 75-year-old female presented with a primary melanoma that was removed through an elliptical excision with 1 cm margins. Pathology revealed 3 involved margins with residual MIS without an invasive component. After a second operation removed an additional 1 cm margin, pathology revealed 2 positive margins with residual MIS. Rather than undergoing a third excision, the patient decided to pursue a nonsurgical approach with topical imiquimod, and at the 4-month follow-up examination, the incision was completely healed with no clinical evidence of tumor recurrence.
Conclusion
A nonsurgical approach with 5% topical imiquimod cream applied along the incision was utilized. In specific patient populations, the use of imiquimod is a reasonable alternative approach for the management of persistently positive MIS margins. Long-term follow-up is necessary to assess for evidence of recurrence and the ultimate success of this nonsurgical approach.
Keywords: Administration–topical, imiquimod, melanoma
INTRODUCTION
Imiquimod is a topical cream approved by the US Food and Drug Administration (FDA) for treatment of superficial basal cell carcinoma, actinic keratosis, and genital-perianal warts.1 Its successful use in patients with persistently positive margins of melanoma in situ (MIS) after surgical excision has been previously reported.1-6
Lentigo maligna (LM) is the most prevalent subtype of MIS and most commonly presents on the face, head, or neck.7 The majority of patients with LM are >40 years, with a mean age at presentation of 65 years and a peak incidence in the 7th and 8th decades of life.8 Surgical intervention is the mainstay of treatment for MIS, with current National Comprehensive Cancer Network guidelines recommending surgical removal with 5 mm margins.9 However, alternative treatment options have been examined, such as topical medication, cryotherapy, and radiotherapy for patients who are not considered optimal surgical candidates. Other nonsurgical approaches may be applicable for reasons such as size and location of the MIS, poor cosmesis, and comorbidities that preclude an operative approach.1 LM is considered the precursor of its invasive counterpart, LM melanoma. While the risk of untreated MIS evolving to invasive melanoma is unknown, the risk of untreated LM evolving to LM melanoma is 5%-50%.1
We present a case demonstrating the short-term efficacy of topical imiquimod after excision of LM melanoma from the face with residual and persistent margins positive for MIS.
CASE REPORT
A 75-year-old female with a medical history significant for rheumatoid arthritis and osteoporosis presented to the dermatologist with a changing, pigmented, nonraised skin lesion on her left cheek. She had noticed that the skin lesion had slowly grown and changed in color during a period of 2-3 years. Of note, the same lesion had been previously diagnosed as an actinic keratosis and treated with cryotherapy. At presentation, the lesion measured 0.8 cm in diameter and had a slight brown pigmentation (Figure 1). A shave biopsy was performed under local anesthesia, and the pathology showed a primary melanoma with a Breslow depth of at least 0.40 mm (deep margin involved). Microstaging revealed a mitotic rate <1/mm2 with no evidence of ulceration, tumor regression, vertical growth phase, or angiolymphatic invasion.
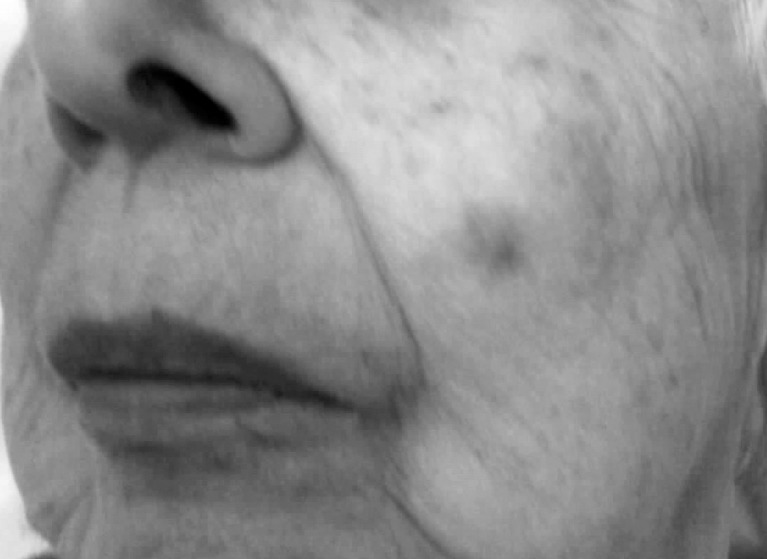
Figure 1.
Appearance of the lesion at the initial dermatologic consultation, prior to shave biopsy.
The patient was referred to our office for surgical consultation, and an initial operation was performed under a local anesthetic for removal of an ellipse with 1 cm margins and primary wound closure. Sentinel lymph node mapping was not indicated according to current guidelines. The specimen was properly oriented for the pathologist. The final pathology revealed previous biopsy site changes and a proliferation of atypical melanocytes at the dermoepidermal junction with single cell predominance and atypia consistent with MIS overlying a solar elastotic dermis that extended to multiple margins (Figure 2).
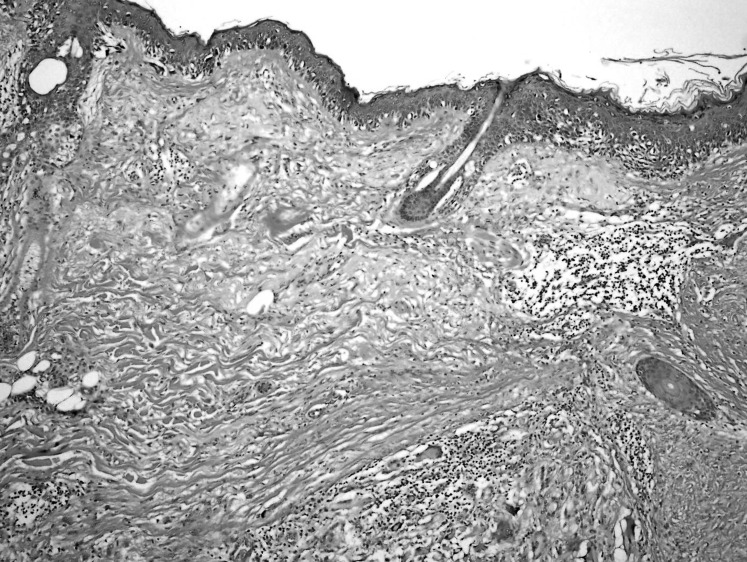
Figure 2.
Atypical melanocytes, some in nests but mainly as single cells with pagetoid spread, underlying solar elastosis, and giant cell reaction consistent with residual melanoma in situ (hematoxylin and eosin stain, 40×).
We performed a second excision of the scar with additional 1 cm margins, again under a local anesthetic. Laxity of the surrounding skin permitted closure of the residual defect primarily. The pathology revealed scar tissue, biopsy site changes, and once again residual MIS with atypical melanocytes growing in a nested pattern and as single cells with pagetoid spread (Figure 3).
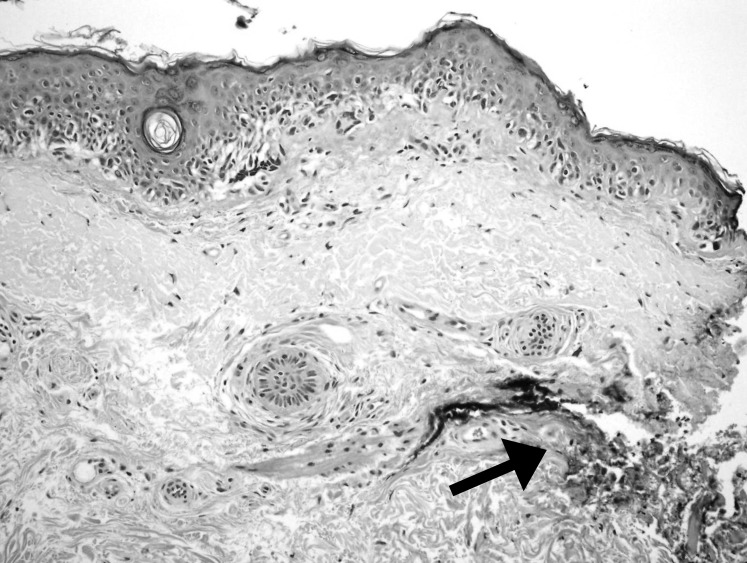
Figure 3.
Melanoma in situ demonstrating single cell predominance and atypia present at the inked margin (arrow) with underlying solar elastosis (hematoxylin and eosin stain, 100×).
At the postoperative visit, we discussed surgical and nonsurgical options for management with the patient: a third excision that would require a local rotational skin flap under a general anesthetic or the off-label use of topical imiquimod as a nonsurgical approach. We contrasted the nonsurgical approach with the morbidity of a major surgical procedure.
Because of her multiple comorbidities and the patient's desire to attempt a nonsurgical approach, topical imiquimod therapy was initiated, prescribed 5 days per week, once daily. After 1 month, the skin around the incision was erythematous with a large central eschar (Figure 4). We asked the patient to decrease use to 3 times per week. At the 2-month follow-up visit, we observed resolving erythema and 2 small areas of scabbing peripherally. At the 4-month follow-up visit, surrounding erythema was minimal. At the 9-month follow-up visit, there was no erythema and no evidence of local recurrence (Figure 5).
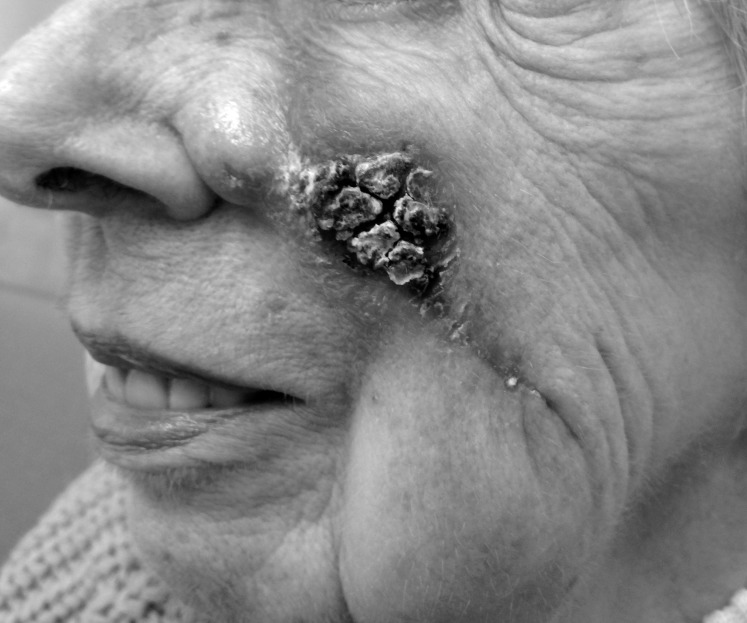
Figure 4.
After 1 month of treatment, the skin around the incision was erythematous with a large central eschar.
Figure 5.
After 9 months of treatment, the incision was completely healed with no evidence of tumor recurrence.
DISCUSSION
Imiquimod is a synthetic imidazoquinoline amine, a type of immune response modifier that binds to toll-like receptors 7 and 8 on neutrophils, macrophages, and dendritic cells to upregulate an inflammatory response.1,4,10 This action, in turn, results in the subsequent downstream activation of the proinflammatory cytokine cascade, including interleukin-2, -6, -8, and -12, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-α (TNF-α), interferon-α, and interferon-γ.1 Furthermore, by increasing the cell surface receptor expression of CD4, CD8 on T-cells and the production of TNF-α, imiquimod activates helper T-cells 1 (Th1 cells) that mediate adaptive immunity. All these factors have been shown to result in a significant antitumor effect.11,12 Imiquimod is currently FDA approved for use in patients with superficial basal cell carcinoma, genital human papillomavirus infection, and actinic keratoses.10,13-15 Multiple studies have reported on the utility of imiquimod for the treatment of MIS, and some have described its use as an alternative to surgical intervention.1-3,5,16 Other studies describe imiquimod's use in the setting of persistently positive margins.1,17 A case report by Cotter et al describes its use before a planned staged surgical excision.11
In 2000, Ahmed and Berth-Jones published one of the first case reports using 5% imiquimod cream to treat LM.2 This alternative therapy was offered to an 88-year-old male who preferred conservative treatment to surgical intervention. He initially started using imiquimod 5% cream 250 mg 3 times weekly. After 4 weeks of treatment, there was no clinical response, so the frequency was increased to daily application. Similar to our case, he developed inflammation and crusting of the area, so application was decreased to 3 times weekly. After 7 months of therapy, an incisional biopsy of the area revealed no evidence of LM.2 Since then, numerous other case reports and small series have demonstrated the effective use of imiquimod alone or in combination with other therapies for the treatment of MIS.1-6,11,18-20
However, studies have also shown that imiquimod was not effective in clearing margin-positive MIS and it subsequently progressed to invasive melanoma.3,21 Van Meurs et al treated 10 patients with LM primarily with imiquimod, with a median follow-up of 31 months.3 Nine of 10 patients (90%) demonstrated a clinical response to the treatment and were also cleared by pathology. Of the 9 patients, 4 recurred 9-27 months after completing the treatment. One patient did not show any response to treatment with imiquimod. Treatment success was considered 50%, and the authors discussed the factors that may have led to treatment failure. First, 5 patients had been previously treated with cryotherapy, potentially creating a bias for treatment-resistant LM. Second, the duration of treatment was different for each patient; some patients only received 8 weeks of treatment compared to the standard 3-month regimen.
The current standard of care for treatment of MIS is surgical excision with 5 mm margins in all directions. The primary goal remains the wide excision of malignancy to minimize the local regional recurrence of disease. However, studies have shown that 5 mm margins may not be adequate and are associated with high rates of local recurrence.13-15 In a study by Agarwal-Antal et al, 58% of MIS lesions required margins >5 mm to achieve clear surgical margins.22 Additionally, Zalla et al treated head and neck MIS with Mohs micrographic surgery (MMS) and found that 50% of patients required >6 mm to obtain clear margins, with an 8.3 mm average margin for clearance.23
A prospective study by Kunishige et al showed that 5 mm margins only cleared 86% of MIS margins, while excisions with 9 mm margins were 98.9% successful. Additionally, 3 patients presented with local recurrences near the previous incision.24 The need for increased margins and the presentation of local recurrences can be explained by the phenomenon known as the field change effect. Atypical melanocytes can extend beyond the realm of the clinically obvious LM. These atypical melanocytes can blend with surrounding normal skin, making it difficult to delineate the lines of the lesion and distinguish atypical melanocytes from simply melanocytic hyperplasia in sun-damaged skin.
The field change effect poses a conundrum because the patient is more prone to develop a local recurrence without adequate margins. The literature has many examples of wider and wider excisions being required to remove much smaller tumors clinically.8 One study postulated that the field change effect at the perimeters of the melanoma is probably related to chronic sun exposure alone and is not actually atypical melanocytes from melanoma.25 On the other hand, melanocytes 5 cm from a melanoma on non–sun-exposed areas displayed “bizarre, pleomorphic melanocytes” in 7 of 12 cases in another study.26 Therefore, the field change effect is not only related to sun exposure but also to changes in melanocytes surrounding a tumor.27 LM melanomas are more likely to show P53 mutations compared to other subtypes that are not associated with chronic sun damage. P53 mutations also appear to have a positive correlation with solar elastosis and chronic sun damage,28 raising an interesting question: whether these recurrences actually represent multifocal disease in a field with associated molecular alterations.8
The 2 controversial surgical alternatives to ensuring adequate clearing of surgical margins are staged excisions and MMS.
Abdelmalek et al described staged excision.29 They examined the lesion with a Wood's lamp to delineate the clinical margins—the central portion or debulk specimen—at initial presentation. The next step is excision of the peripheral margins separately in the form of a geometric shape with at least 3 sides. These margins are measured 5-10 mm from the lesion. The defect is not closed, but the area is dressed, and the patient is sent home. The specimen is sent to a dermatopathologist for paraffin-embedded permanent sections. Once the margins have been evaluated, the patient returns to the office for either additional staged margins or for wound closure. Abdelmalek et al found that the mean margin for clearance was 6.6 mm for LM. In 11.6% of cases, LM was cleared with <5 mm margins, 62.2% of cases were cleared with 5 mm margins, and 26.2% of cases required margins >5 mm.29
Johnson et al also discussed staged excision.26 They used a square procedure in which the peripheral margins were examined prior to excision of the central portion. Once the margins were cleared, the central portion was excised, and the defect was closed. This approach prevents granulation tissue from forming and facilitates an easier closure.26 Staged excision is a viable alternative to standard excision with 5 mm margins, especially because studies have demonstrated that many cases of LM extend beyond the standard 5 mm. However, staged excision can be a costly, time consuming, and tedious process for the patient compared to a single wide local excision of the same lesion.9
MMS is similar to staged excision in that the defect is not closed until the margins are clear. However, MMS excises the lesion with minimal margins, and frozen specimen sections are evaluated to allow for tissue sparing and closure of the defect on the same day. Additional margins are obtained based on the histology.9 The main controversy with this technique is the use of frozen section analysis of the margins of resection.7,30 When examining the frozen sections, vacuolated keratinocytes may be mistaken for melanocytes, and melanocytes may be concealed by dermal inflammatory cells.16 Cohen et al found that the specificity and sensitivity of frozen sections for melanoma were 73% and 68%, respectively.31 Recurrence rates after MMS have ranged from 0%-33%.9 Therefore, although MMS may provide a better cosmetic outcome compared to a standard elliptical excision, frozen sections of melanoma are not entirely reliable in confirming clear margins.
Although several literature reviews and case studies have demonstrated the efficacy of imiquimod in the treatment of LM and MIS, surgical excision is still considered the gold standard treatment to obtain clear margins and prevent recurrence of the disease. However, depending on the location of the lesion and the age and comorbidities of some patient populations, surgery is not always the most viable option. Missall and Fosko reported 2 cases of extensive LM treated using a combination of surgical excision and 5% imiquimod.19 The lesions for these 2 patients were located on the scalp and cheek. Several months after the completion of therapy, they had no evidence of residual LM.
Another case study by Ellis et al presented a successful treatment of MIS using imiquimod after nonclearance with 4 surgeries.1 The patient was an 82-year-old male with MIS on the right cheek. He underwent an initial surgical excision, followed by 3 subsequent excisions with resulting positive surgical margins and was subsequently treated with 5% imiquimod for 12 weeks. The patient was followed for clinical recurrence, and 3 scouting biopsies were performed 6 months after treatment that showed histologic clearance.1 An inherent weakness of this case report is the short follow-up period that limits the interpretation and application of imiquimod therapy for patients with MIS with positive margins. Because of the slow growth pattern of LM, patients may develop recurrences after the designated follow-up time. Five-year follow-up data will help determine the long-term efficacy of imiquimod.11 Furthermore, no histologic examination verified clearance of LM after treatment with imiquimod. Clinical examination of the area for signs or evidence of recurrence was performed at each follow-up visit for 4 months. One possible way to overcome this limitation would be to perform several small punch biopsies adjacent to the residual scar to determine the persistence or recurrence of MIS or invasive melanoma.4,17
CONCLUSION
A wide surgical excision obtaining 5 mm margins remains the accepted initial treatment option for MIS. However, a nonsurgical approach, such as utilizing an immunomodulator such as imiquimod, may be justified in select cases. As with all case reports thus far, we need longer follow-up to definitively confirm that this therapy is effective in treating the MIS-positive margins.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Ellis LZ, Cohen JL, High W, Stewart L. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012 Jun;38(6):937–946. doi: 10.1111/j.1524-4725.2012.02362.x. doi: 10.1111/j.1524-4725.2012.02362.x. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed I, Berth-Jones J. Imiquimod: a novel treatment for lentigo maligna. Br J Dermatol. 2000 Oct;143(4):843–845. doi: 10.1046/j.1365-2133.2000.03787.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Meurs T, Van Doorn R, Kirtschig G. Treatment of lentigo maligna with imiquimod cream: a long-term follow-up study of 10 patients. Dermatol Surg. 2010 Jun;36(6):853–858. doi: 10.1111/j.1524-4725.2010.01560.x. doi: 10.1111/j.1524-4725.2010.01560.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolf IH, Cerroni L, Kodama K, Kerl H. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol. 2005 Apr;141(4):510–514. doi: 10.1001/archderm.141.4.510. [DOI] [PubMed] [Google Scholar]
- 5.Naylor MF, Crowson N, Kuwahara R, et al. Treatment of lentigo maligna with topical imiquimod. Br J Dermatol. 2003 Nov;149(Suppl 66):66–70. doi: 10.1046/j.0366-077x.2003.05637.x. [DOI] [PubMed] [Google Scholar]
- 6.Powell AM, Russell-Jones R, Barlow RJ. Topical imiquimod immunotherapy in the management of lentigo maligna. Clin Exp Dermatol. 2004 Jan;29(1):15–21. doi: 10.1111/j.1365-2230.2004.01452.x. [DOI] [PubMed] [Google Scholar]
- 7.Erickson C, Miller SJ. Treatment options in melanoma in situ: topical and radiation therapy, excision and Mohs surgery. Int J Dermatol. 2010 May;49(5):482–491. doi: 10.1111/j.1365-4632.2010.04423.x. doi: 10.1111/j.1365-4632.2010.04423.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LM. Lentigo maligna and lentigo maligna melanoma. Erratum. J Am Acad Dermatol. J Am Acad Dermatol. 1995 1997 Dec;Jun;3336(6)(6 Pt 1):923–936. 937–940, 913. doi: 10.1016/0190-9622(95)90282-1. quiz. Review. in. [DOI] [PubMed] [Google Scholar]
- 9.McLeod M, Choudhary S, Giannakakis G, Nouri K. Surgical treatments for lentigo maligna: a review. Dermatol Surg. 2011 Sep;37(9):1210–1228. doi: 10.1111/j.1524-4725.2011.02042.x. doi: 10.1111/j.1524-4725.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002 Feb;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 11.Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008 Feb;34(2):147–151. doi: 10.1111/j.1524-4725.2007.34031.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Cherman AM, Tyring SK. Viral and nonviral uses of imiquimod: a review. J Cutan Med Surg. 2004 Sep-Oct;8(5):338–352. doi: 10.1007/s10227-005-0023-5. [DOI] [PubMed] [Google Scholar]
- 13.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002 Oct;27(7):571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith KJ, Hamza S, Skelton H. The imidazoquinolines and their place in the therapy of cutaneous disease. Expert Opin Pharmacother. 2003 Jul;4(7):1105–1119. doi: 10.1517/14656566.4.7.1105. [DOI] [PubMed] [Google Scholar]
- 15.Tyring S, Conant M, Marini M, Van Der Meijden W, Washenik K. Imiquimod; an international update on therapeutic uses in dermatology. Int J Dermatol. 2002 Nov;41(11):810–816. doi: 10.1046/j.1365-4362.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson O, Ahmed I. Lentigo maligna: prognosis and treatment options. Am J Clin Dermatol. 2005;6(3):151–164. doi: 10.2165/00128071-200506030-00002. [DOI] [PubMed] [Google Scholar]
- 17.Spenny ML, Walford J, Werchniak AE, et al. Lentigo maligna (melanoma in situ) treated with imiquimod cream 5%: 12 case reports. Cutis. 2007 Feb;79(2):149–152. [PubMed] [Google Scholar]
- 18.Rajpar SF, Marsden JR. Imiquimod in the treatment of lentigo maligna. Br J Dermatol. 2006 Oct;155(4):653–656. doi: 10.1111/j.1365-2133.2006.07476.x. [DOI] [PubMed] [Google Scholar]
- 19.Missall TA, Fosko SW. The use of imiquimod to minimize the surgical defect when excising invasive malignant melanoma surrounded by extensive melanoma in situ, lentiginous type. Dermatol Surg. 2009 May;35(5):868–874. doi: 10.1111/j.1524-4725.2009.01146.x. doi: 10.1111/j.1524-4725.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- 20.Ray CM, Kluk M, Grin CM, Grant-Kels JM. Successful treatment of malignant melanoma in situ with topical 5% imiquimod cream. Int J Dermatol. 2005 May;44(5):428–434. doi: 10.1111/j.1365-4632.2005.02582.x. [DOI] [PubMed] [Google Scholar]
- 21.Fisher GH, Lang PG. Treatment of melanoma in situ on sun-damaged skin with topical 5% imiquimod cream complicated by the development of invasive disease. Arch Dermatol. 2003 Jul;139(7):945–947. doi: 10.1001/archderm.139.7.945. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002 Nov;47(5):743–748. doi: 10.1067/mjd.2002.124085. [DOI] [PubMed] [Google Scholar]
- 23.Zalla MJ, Lim KK, Dicaudo DJ, Gagnot MM. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000 Aug;26(8):771–784. doi: 10.1046/j.1524-4725.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012 Mar;66(3):438–444. doi: 10.1016/j.jaad.2011.06.019. doi: 10.1016/j.jaad.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Fallowfield ME, Cook MG. Epidermal melanocytes adjacent to melanoma and the field change effect. Histopathology. 1990 Nov;17(5):397–400. doi: 10.1111/j.1365-2559.1990.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TM, Headington JT, Baker SR, Lowe L. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol. 1997 Nov;37(5 Pt 1):758–764. doi: 10.1016/s0190-9622(97)70114-2. [DOI] [PubMed] [Google Scholar]
- 27.Wong CK. A study of melanocytes in the normal skin surrounding malignant melanomata. Dermatologica. 1970;141(3):215–225. doi: 10.1159/000252469. [DOI] [PubMed] [Google Scholar]
- 28.Purdue MP, From L, Kahn HJ, et al. Etiologic factors associated with p53 immunostaining in cutaneousmalignant melanoma. Int J Cancer. 2005 Nov 10;117(3):486–493. doi: 10.1002/ijc.21196. [DOI] [PubMed] [Google Scholar]
- 29.Abdelmalek M, Loosemore MP, Hurt MA, Hruza G. Geometric staged excision for the treatment of lentigo maligna and lentigo maligna melanoma: a long-term experience with literature review. Arch Dermatol. 2012 May;148(5):599–604. doi: 10.1001/archdermatol.2011.2155. doi: 10.1001/archdermatol.2011.2155. [DOI] [PubMed] [Google Scholar]
- 30.Dawn ME, Dawn AG, Miller SJ. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007 Apr;33(4):395–402. doi: 10.1111/j.1524-4725.2007.33085.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen LM, McCall MW, Hodge SJ, Freedman JD, Callen JP, Zax RH. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs' micrographic surgery aided by rush permanent sections. Cancer. 1994 Jun 15;73(12):2964–2970. doi: 10.1002/1097-0142(19940615)73:12<2964::aid-cncr2820731213>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]