Abstract
Objective:
Patients categorized Rutherford category IV might have different characteristics compared with Rutherford category V and VI. Our study aims were to estimate the clinical differences between Rutherford category IV and Rutherford category V and VI, for those underwent endovascular therapy for isolated infrapopliteal disease, and also to find risk factors for endovascular therapy in Rutherford category IV.
Methods:
Based on the Japanese multi-center registry data, 1091 patients with 1332 limbs (Rutherford category IV: 226 patients with 315 limbs, Rutherford category V and VI: 865 patients with 1017 limbs) were analyzed retrospectively.
Results:
Patients’ backgrounds and lesions’ characteristics had significant differences. Both freedom rate from major adverse limb event with perioperative death and amputation-free survival rate at 1 year were better in Rutherford category IV than Rutherford category V and VI (93.6% vs 78.3%, 87.7% vs 66.7%) and those maintained to 3 years (p < 0.0001). Significant predictors for major adverse limb event/perioperative death were small body mass index (<18.5 kg/m3) and initial endovascular therapy success, and those for amputation-free survival were small body mass index (<18.5 kg/m3), non-ambulatory status, high systematic inflammatory reaction (C-reactive protein > 3.0 mg/dL), chronic obstructive pulmonary disease, and coronary artery disease in Rutherford category IV.
Conclusion:
From the present results, Rutherford category IV should be recognized to have quite different backgrounds and better outcome from Rutherford category V and VI.
Keywords: Critical limb ischemia, endovascular therapy, multivariate analysis
Introduction
Patients who have critical limb ischemia (CLI) might be on the end stage of peripheral artery disease and also on systemic critical status, so their poor prognosis with a high mortality rate (19%–54% at 1 year) is quite well known.1–3 CLI does not represent a simple pathophysiologic process, but is caused by multiple pathogenetic mechanisms. Besides, the current knowledge base about CLI is considerably less established than for other major cardiovascular diseases by use of varying CLI definitions and due to incomplete knowledge regarding CLI natural history.4 On the other hand, recent reports have demonstrated the effectiveness of endovascular therapy (EVT) for CLI patients as the first-line approach5–9 and also the difference of clinical outcome between Rutherford category IV (R-4) and Rutherford category V/VI (R-5/6).10
The recent reports showed that about half of CLI was due to isolated infrapopliteal lesions,11 and CLI attributable to pure isolated infrapopliteal lesions might be thought as the most severe form of the peripheral artery disease.12 Therefore, it would be important to evaluate the outcome of EVT procedure focusing on the CLI patients with isolated infrapopliteal diseases.
Our study aims were to estimate the clinical differences between R-4 and R-5/6 with isolated infrapopliteal lesions and also to find risk factors of endovascular intervention for R-4 from Japanese registry database.
Method
Patient
We retrospectively analyzed a prospectively maintained multi-center database from April 2004 to December 2012. The patients who were considered poor candidate for revascularization because of severe comorbidities, dementia, and social impairment were excluded. Although revascularization was not attempted in patients with unsalvageable limbs, non-ambulatory patients without cognitive and social problems were challenged to be treated in expectation of their better social outcome by limb salvage. During this period, 1415 limbs of 1193 consecutive CLI patients underwent EVT in 14 cardiovascular centers in Japan. In the same period, crural bypass operations were performed in 470 CLI patients by a vascular surgeon. After excluding patients with CLI due to not only below-the-knee (BTK) lesions but also proximal lesions, such as femoro-popliteal or aorto-iliac lesions, the data set used for the study included 1332 limbs from 1091 consecutive patients who underwent angioplasty alone for de novo BTK lesions. And all of them gave consent to provide clinical follow-up during the chronic phase. This study stratified the enrollees into R-4 patients (no. 226, 315 limbs) and R-5/6 patients (no. 865, 1017 limbs) to compare clinical backgrounds and outcomes. The study protocol was developed in accordance with the Declaration of Helsinki and was approved by the ethics committee of each hospital. This study was sub-analysis based on the database of Japanese BElow-the-knee Artery Treatment (J-BEAT) registry, which was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) (No. UMIN000004917). All patients gave written informed consent for both EVT and inclusion in this study prior to procedure.
Protocols
The study protocol was already reported.13 On admission, physical findings including pulsation of peripheral arteries were examined and the ankle-brachial index (ABI) and the skin perfusion pressure (SPP) were measured to assess the hemodynamic status of lower limb ischemia. Lower limb arteries were routinely evaluated by duplex ultrasound. All patients also underwent digital subtraction angiography (DSA) before the procedure.
All EVT procedures were performed by cardiovascular interventional specialists, and indication of EVT was decided when the lesion showed >75% diameter stenosis on DSA. Generally, a 4-Fr sheath was inserted from the ipsi-lateral common femoral artery as the ante-grade approach. After administration of un-fractionated heparin (5000 units), manipulation of a 0.014-in guide wire initiated the procedure. The appropriate balloon diameter was visually assessed and introduced. Any stent deployment and atherectomy were not approved in BTK intervention in Japan. We generally attempted angiosome-based intervention of the target lesion. And in a case of failed angiosome-based recanalization, achieving one straight-line flow to the ischemic area was attempted. Dual anti-platelet therapy (aspirin at 100 mg per day and ticlopidine at 200 mg per day or cilostazol at 200 mg per day) was prescribed at least 1 week prior to procedure and continued as long as possible.
Follow-up evaluation was planned at 1 week; 1, 3, and 6 months after revascularization; and thereafter every 3 months by hospital visits or interview on a telephone in all patients. The patients with wounds were individually followed up by a plastic surgeon. For severity of wounds, a plastic surgeon carefully assessed involving the indication for antibiotic therapy and amputation timing. Ulcer healing was defined on the basis of the Tissue, Infection or Inflammation, Moisture imbalance and Edge (TIME) of wound (non-advancing or undermined)) concept, and time to complete healing was recorded. Indication for repeat intervention was left to each operator or plastic surgeon based on worsening clinical symptoms and delayed wound healing.
Definitions
Ischemic tissue loss was defined in accordance with TransAtlantic Inter-Society Consensus (TASC) as tissue loss associated with an ankle pressure <70 mmHg or a toe pressure <50 mmHg. An SPP <40 mmHg was defined as indicating ischemic tissue loss.14 Atherosclerosis risk factors have been reported previously.15 Chronic obstructive pulmonary disease (COPD) was defined as history of estimated forced expiratory volume in 1 s (FEV1)/the forced vital capacity (FVC) ratio less than 70%. Coronary artery disease (CAD) was defined as having a history of coronary interventions or coronary artery bypass grafting. Cerebral vascular disease (CVD) was defined as having a history of strokes or cerebral bleedings. Non-ambulatory status was defined as wheelchair-bound or bedridden status. EVT procedural success in R-5/6 was defined as successful angiosome-based recanalization or obtaining one straight-line flow to the wound region without occurrence of any flow-limiting dissection. It was difficult for R-4 patients to clearly separate painful region because of their fussy complaints compared with R-5/6 patients in whom it was easy to recognize ischemic area by existence of wounds. So that, obtaining not only one straight-line flow but also increasing blood flow to the whole resting pain region was defined as EVT procedure success in R-4 group. EVT with angioplasty was considered hemodynamically successful when post-procedural SPP level increased more than 40 mmHg, which indicated high limb salvage rate. Perioperative death (POD) was defined as death occurring within 30 days. Major adverse limb event (MALE) was defined as major amputation or any major re-intervention (repeat angioplasty or bypass graft procedures) during the study period. Major amputation was defined as surgical excision of the limb above the ankle, and any amputation at or distal to the Lisfranc level was not considered a limb salvage failure.
Statistical analysis
The unpaired t-test or Mann–Whitney U-test was used to compare continuous variables with or without normal distributions between groups, respectively. Variables with a normal distribution were shown as mean values ± standard deviation (SD), while median and interquartile range were used for asymmetrically distributed data. Chi-square test was used to compare proportions between groups. Statistical significance level was defined as p < 0.05. Independent predictors for outcome were identified by the Cox proportional hazard ratio in multivariable analysis including all statistically significant variables in univariable analysis. Each outcome was estimated using the Kaplan–Meier method and compared using the log-rank test. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) (SPSS Inc., Chicago, IL, USA) software.
Results
Table 1 shows patients’ characteristics. For patients’ backgrounds, there were significant differences between R-4 and R-5/6 groups, in age, body mass index, ambulatory status, serum albumin level, C-reactive protein (CRP) level, dyslipidemia, diabetes mellitus, end stage of renal disease, heart failure history, and baseline SPP. For lesions’ characteristics, TASC proportion, lesion length, and a number of patent arteries below the ankle had significant differences between the groups (Table 2).
Table 1.
Patient characteristics (n = 1091).
| R-4 group (n = 226) | R-5/6 group (n = 865) | p Value | |
|---|---|---|---|
| Age, years (mean ± SD) | 74 ± 9 | 71 ± 11 | <0.0001 |
| Male, n (%) | 155 (66) | 609 (71) | 0.1519 |
| BMI, kg/m3 (mean ± SD) | 22.4 ± 4.5 | 21.6 ± 3.8 | 0.0016 |
| Ambulatory, n (%) | 187 (80) | 460 (54) | <0.0001 |
| Albumin, g/dL (mean ± SD) | 3.8 ± 0.5 | 3.5 ± 0.6 | <0.0001 |
| CRP, mg/dL (mean ± SD) | 1.1 ± 2.4 | 3.2 ± 4.7 | <0.0001 |
| Hypertension, n (%) | 183 (78) | 625 (73) | 0.051 |
| Dyslipidemia, n (%) | 92 (39) | 260 (30) | 0.0026 |
| DM, n (%) | 144 (61) | 646 (75) | <0.0001 |
| End stage of renal disease, n (%) | 127 (54) | 567 (66) | <0.0001 |
| Current smoking, n (%) | 89 (38) | 301 (35) | 0.4231 |
| COPD, n (%) | 19 (8) | 76 (9) | 0.5239 |
| HF history, n (%) | 27 (11) | 199 (23) | <0.0001 |
| CAD, n (%) | 116 (50) | 439 (51) | 0.6891 |
| CVD, n (%) | 49 (21) | 203 (24) | 0.25 |
| Aspirin, n (%) | 185 (79) | 682 (80) | 0.7539 |
| Thienopiridine, n (%) | 79 (34) | 309 (36) | 0.1699 |
| Cilostazol, n (%) | 115 (49) | 426 (50) | 0.8891 |
| Warfarin, n (%) | 46 (20) | 185 (22) | 0.4592 |
| Statin, n (%) | 57 (24) | 182 (21) | 0.2453 |
| Insulin, n (%) | 45 (19) | 267 (31) | 0.0035 |
| Beta-blocker, n (%) | 33 (14) | 123 (14) | 0.8291 |
| ABI (mean ± SD) | 0.80 ± 0.23 | 0.82 ± 0.26 | 0.234 |
| SPP dorsal, mmHg (mean ± SD) | 35 ± 17 | 31 ± 17 | 0.0035 |
| SPP plantar, mmHg (mean ± SD) | 39 ± 19 | 34 ± 18 | 0.0004 |
R-4: Rutherford category IV; R-5/6: Rutherford category V and VI; SD: standard deviation; BMI: body mass index; CRP: C-reactive protein; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; HF: heart failure; CAD: coronary artery disease; CVD: cerebral vascular disease; ABI: ankle-brachial index; SPP: skin perfusion pressure.
Table 2.
Lesion characteristics (n = 1332).
| R-4 group (n = 315) | R-5/6 group (n = 1017) | p Value | |
|---|---|---|---|
| TASC A/B/C/D, n | 14/7/26/266 | 18/10/65/909 | 0.0044 |
| Lesion length, mm (mean ± SD) | 177 ± 98 | 194 ± 97 | 0.0096 |
| Lesion distribution | |||
| ATA, n (%) | 300 (95) | 973 (96) | 0.9201 |
| PTA, n (%) | 296 (94) | 950 (93) | 0.7684 |
| PA, n (%) | 245 (78) | 750 (74) | 0.1504 |
| CTO, n (%) | 215 (68) | 712 (70) | 0.1776 |
| Below the ankle | |||
| Patent dorsal artery, n (%) | 232 (74) | 561 (55) | <0.0001 |
| Patent plantar artery, n (%) | 209 (67) | 523 (51) | <0.0001 |
| Number of patent arteries, n (mean ± SD) | 1.0 ± 0.8 | 0.8 ± 0.7 | 0.0001 |
R-4: Rutherford category IV; R-5/6: Rutherford category V and VI; TASC: TransAtlantic Inter-Society Consensus; SD: standard deviation; ATA: anterior tibial artery; PTA: posterior tibial artery; PA: peroneal artery; CTO: chronic total occlusion.
Table 3 shows the rate of EVT procedure success and perioperative complications. In R-4 group, EVT procedural success rate was significantly higher, and perioperative bypass conversion and amputation rate were significantly lower. The rate of POD was low in both groups.
Table 3.
Procedure success rate and perioperative complications.
| R-4 group (n = 315) | R-5/6 group (n = 1017) | p Value | |
|---|---|---|---|
| Procedure success, n (%) | 308 (98) | 919 (90) | <0.0001 |
| Bypass conversion, n (%) | 0 (0) | 19 (2) | 0.0146 |
| Perioperative death, n (%) | 6 (2) | 25 (2) | 0.5692 |
| Perioperative amputation, n (%) | 1 (0.3) | 34 (3) | 0.0034 |
R-4: Rutherford category IV; R-5/6: Rutherford category V and VI.
Seven cases had failed initial EVT in R-4 group. Of them, four cases had MALEs (three cases were converted to distal bypass and one case had major amputation); another one case was dead due to cardiac event during follow-up period.
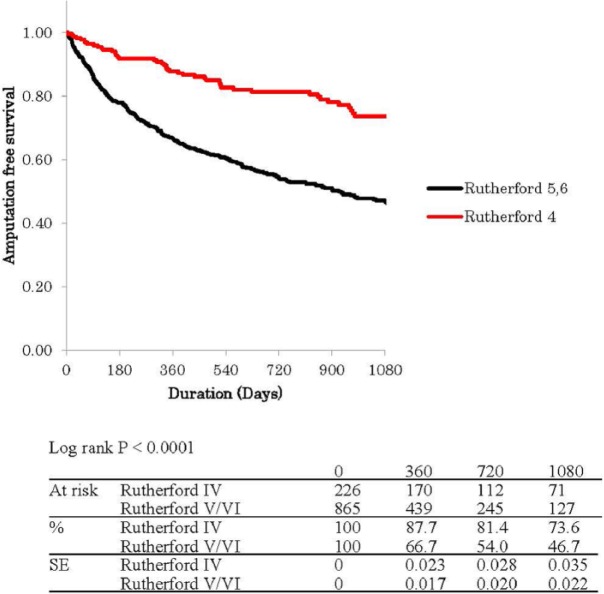
Figure 1 shows Kaplan–Meier curves for free rate from MALEs with POD (MALE + POD). The estimated MALE + POD free percentage and standard error was 93.6% ± 1% in R-4 versus 78.3% ± 1% in R-5/6 at 1 year, 91.1% ± 2% versus 74.5% ± 2% at 2 years, and 91.1% ± 2% versus 73.9% ± 2% at 3 years (p < 0.0001). Figure 2 shows Kaplan–Meier curves for amputation-free survival (AFS) rate. The estimated AFS percentage and standard error was 87.7% ± 2% in R-4 versus 66.7% ± 2% in R-5/6 at 1 year, 81.4% ± 3% versus 54.0% ± 2% at 2 years, and 73.6% ± 4% versus 46.7% ± 2% at 3 years (p < 0.0001). Both freedom rate from MALE/POD and AFS rate had been better in R-4 group than R-5/6 group all the time during the follow-up period.
Figure 1.
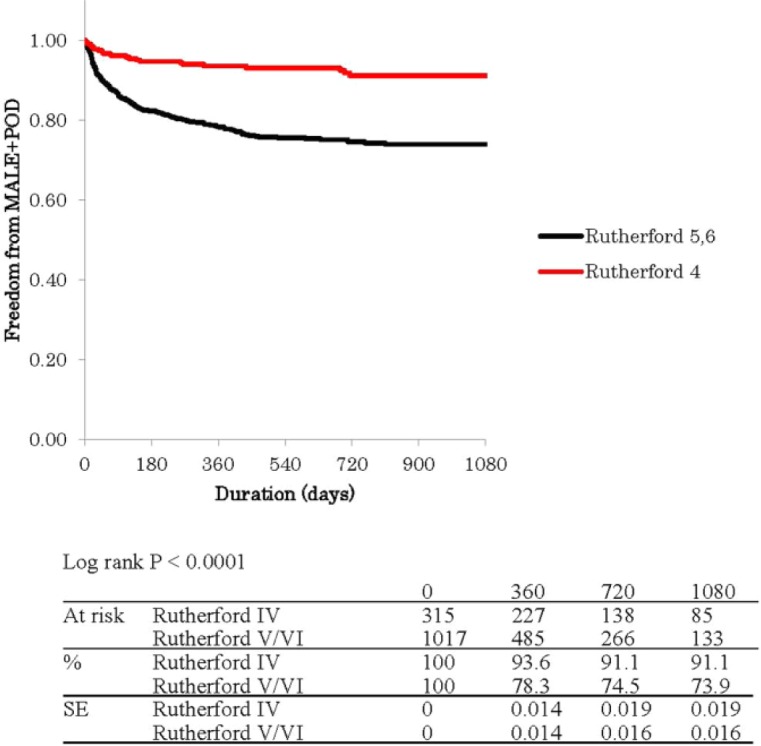
Kaplan–Meier curves show free rate from major adverse limb events with perioperative death (MALE + POD) in Rutherford category IV and V/VI.
Figure 2.
Kaplan–Meier curves show amputation-free survival (AFS) rate in Rutherford category IV and V/VI.
In multivariate Cox hazard regression analysis, significant predictors for MALE/POD were small body mass index (<18.5 kg/m3) and EVT failure (Table 4), and those for AFS were small body mass index (<18.5 kg/m3), non-ambulatory status, high systematic inflammatory reaction (CRP > 3.0 mg/dL), COPD, and CAD in R-4 group (Table 5).
Table 4.
Association of baseline characteristics with MALE and POD in Rutherford category IV.
| Univariate analysis | Multivariate analysis | |
|---|---|---|
| Patient characteristics | ||
| Age | 1.0 (0.9, 1.0)* | – |
| >80 years | 0.6 (0.2, 1.6) | – |
| <70 years | 2.3 (1.1, 5.1)* | 2.1 (0.9, 4.7) |
| <60 years | 2.3 (0.7, 7.8) | – |
| Male gender | 0.7 (0.3, 1.5) | – |
| BMI < 18.5 kg/m3 | 2.6 (1.1, 6.6)* | 3.3 (1.3, 8.6)* |
| Non-ambulatory | 1.4 (0.6, 3.4) | – |
| Albumin < 3.0 g/dL | 1.4 (0.3, 6.0) | – |
| CRP > 3.0 mg/dL | 1.9 (0.6, 6.3) | – |
| Hypertension | 0.5 (0.2, 1.1) | – |
| Dyslipidemia | 0.9 (0.4, 1.9) | – |
| Diabetes mellitus | 0.5 (0.2, 1.1) | – |
| End stage of renal disease | 2.0 (0.9, 4.7) | – |
| Current smoking | 0.6 (0.3, 1.5) | – |
| COPD | 0.6 (0.1, 4.7) | – |
| CAD | 1.1 (0.5, 2.4) | – |
| CVD | 1.0 (0.4, 2.6) | – |
| Cilostazol | 1.0 (0.5, 2.2) | – |
| Statin | 0.5 (0.2, 1.5) | – |
| Lesion characteristics | ||
| BTK lesion length | 1.0 (1.0, 1.0) | – |
| CTO | 0.9 (0.4, 2.0) | – |
| BTK run-off vessels | 1.0 (0.6, 1.6) | – |
| BA disease | 0.9 (0.6, 1.5) | – |
| Procedure success | ||
| Success | 0.1 (0.0, 0.3)**** | 0.1 (0.0, 0.3)**** |
Data are hazard ratios and 95% confidence intervals.
MALE: major adverse limb events; POD: perioperative death; BMI: body mass index; CRP: C-reactive protein; COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease; CVD: cerebral vascular disease; BTK: below-the-knee; CTO: chronic total occlusion; BA: below-the-ankle.
p < 0.05; ****p < 0.0005.
Table 5.
Association of baseline characteristics with major amputation and mortality in Rutherford category IV.
| Univariate analysis | Multivariate analysis | |
|---|---|---|
| Patient characteristics | ||
| Age | 1.0 (1.0, 1.0) | – |
| >80 years | 1.0 (0.6, 1.9) | – |
| <70 years | 1.2 (0.7, 2.2) | – |
| <60 years | 1.0 (0.3, 3.1) | – |
| Male gender | 1.1 (0.6, 1.9) | – |
| BMI < 18.5 kg/m3 | 3.4 (1.8, 6.3)**** | 2.7 (1.4, 5.4)*** |
| Non-ambulatory | 2.1 (1.1, 3.9)* | 3.0 (1.5, 5.9)*** |
| Albumin < 3.0 g/dL | 3.1 (1.6, 6.3)*** | 2.0 (0.9, 4.4) |
| CRP > 3.0 mg/dL | 3.1 (1.5, 6.3)*** | 2.8 (1.2, 6.5)* |
| Hypertension | 1.4 (0.7, 2.8) | – |
| Dyslipidemia | 0.6 (0.3, 1.0) | – |
| Diabetes mellitus | 0.9 (0.5, 1.6) | – |
| End stage of renal disease | 1.8 (1.0, 3.1)* | 1.3 (0.7, 2.4) |
| Current smoking | 1.2 (0.7, 2.1) | – |
| COPD | 2.9 (1.2, 6.8)* | 2.9 (1.2, 7.1)* |
| CAD | 2.1 (1.2, 3.6)** | 2.6 (1.5, 4.7)*** |
| CVD | 1.5 (0.8, 2.7) | – |
| Statin | 0.5 (0.2, 1.0)* | 0.5 (0.2, 1.1) |
| Lesion characteristics | ||
| BTK lesion length | 1.0 (1.0, 1.0) | – |
| CTO | 1.7 (0.9, 3.2) | – |
| BTK run-off vessels | 1.0 (0.8, 1.4) | – |
| BA disease | 0.8 (0.6, 1.1) | – |
| Procedure success | ||
| Success | 2.4 (0.3, 17.4) | – |
Data are hazard ratios and 95% confidence intervals.
BMI: body mass index; CRP: C-reactive protein; COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease; CVD: cerebral vascular disease; BTK: below-the-knee; CTO: chronic total occlusion; BA: below-the-ankle.
p < 0.05; **p < 0.01; ***p < 0.005; ****p < 0.0005.
Table 6 shows all cause of death in both groups during follow-up period. Distribution of causes was not significantly different between groups, but interval to death from EVT in R-4 was significantly longer than R-5/6 group.
Table 6.
Cause of death (n = 470).
| R-4 group (n = 226) | R-5/6 group (n = 865) | p Value | |
|---|---|---|---|
| All cause death, n | 79 | 391 | 0.7332 |
| Cardiac death, n (%) | 30 (38) | 145 (37) | |
| Vascular death, n (%) | 4 (5) | 23 (6) | |
| Infection, n (%) | 19 (24) | 128 (33) | |
| Cancer, n (%) | 6 (8) | 18 (5) | |
| Unknown and others, n (%) | 20 (25) | 77 (20) | |
| Days to death from initial EVT, mean ± SD | 628 ± 589 | 432 ± 423 | <0.0001 |
R-4: Rutherford category IV; R-5/6: Rutherford category V and VI; EVT: endovascular therapy; SD: standard deviation.
Discussion
There were some reports that estimated the differences in clinical characteristics and outcome between the CLI patients with and without wound, but most of the patients included in their reports had infra-inguinal multiple lesions.10,16 As far as we checked, this study might be the first report that estimated the difference between two categories in CLI patients with isolated BTK lesions.
This study showed that the patients categorized Rutherford IV had different characteristics in their background and lesion morphology compared with Rutherford V and VI. O’Brien-Irr et al.16 reported that patients categorized Rutherford IV were significantly less likely than Rutherford V/VI to have diabetes mellitus and end stage of renal disease. In addition to those, our data could reveal significant differences in age, body mass index, ambulatory status, serum albumin level, CRP level, dyslipidemia, heart failure history, baseline SPP, TASC proportion, lesion length, and a number of patent arteries below the ankle. If the patients categorized Rutherford IV had a similar characteristics to patients with ischemic wound, they would be recognized on early stage of CLI and also it could be a promising strategy, “early intervention,” to perform revascularization for preventing a patient from having wound. But according to the present data, the patients with and without wound might have each different pathophysiologic process.
Overall high EVT procedural success rate (92%, 1227 success/1332 limbs) was the same as previous report.10 The reason why overall perioperative major complication rate (6%, 85 complications/1332 limbs) and POD rate (3%, 31 death/1091 patients) was not low might be attributed to the fact that this study included many high-risk patients, such as hypertension (74%, 808/1091 patients), diabetes mellitus (72%, 790/1091 patients), end stage of renal disease (64%, 694/1091 patients), and heart failure (21%, 226/1091 patients). Significant higher success rate in Rutherford IV than V and VI might be due to less complex lesion morphology, such as TASC proportion, lesion length, and number of patent below-the-ankle arteries.
The previous reports indicated that CLI patients without wounds had better prognosis after initial catheter intervention than those with wounds.10,16 This study also showed the significant different outcome between Rutherford IV and V/VI all the time during the follow-up period. For the predictors for MALE in the CLI patients who underwent catheter intervention, younger age, hypo-albuminemia, high systemic inflammatory reaction, diabetes mellitus, end stage of renal disease, heart failure, and below-the-ankle disease have been reported.9,12,13,16 However, in this study where risk factors only for the patients categorized Rutherford IV were evaluated, only low body mass index (<18.5 kg/m3) and initial EVT success were estimated as multivariate significant predictors for MALE in the patients without any wounds. Compared with patients with wound, who tend to need repeat intervention due to delayed wound healing, patients without wound seem not to recognize recurrence of symptom. This might also contribute to the better free rate from MALE and less predictors in Rutherford IV as well as advantage of their backgrounds. It might be possible of simpler lesions, even in the below-the-knee segment, to have relatively higher patency rate after the initial intervention, which conducted to less MALE. But it could not be discussed in this study because patency of treated vessel was not estimated. About age, in an attempt to find a cut-off line which might separate their prognosis, each parameters, such as over 80, under 70, and under 60 years old, were estimated. But none of them could have statistical power as a significant predictor for prognosis.
As the predictors for mortality in the CLI patients who underwent catheter intervention, over 80 years, low body mass index, non-ambulatory status, hypo-albuminemia, high systemic inflammatory reaction, diabetes mellitus, end stage of renal disease, heart failure, and presence of tissue loss have been reported.9,12,13,16,17 In this study, low body mass index (<18.5 kg/m3), non-ambulatory status, high systemic inflammatory reaction (CRP > 3.0 mg/dL), COPD, and CAD were also estimated as multivariate significant predictors for AFS in the patients without any wounds. Although the interval to death from initial intervention was longer in the patients without wounds than with wounds, the distribution of causes of death was the same and the rate of death related to peripheral vascular events was low (5%–6%). These findings might suggest that the initial EVT procedure success could lead to high rate of limb salvage and free from re-intervention, but better long-term prognosis had to have careful systemic maintenance of risk factors kept even in the patients without wounds as the same as the patients with wounds.
Study limitations
This study was a retrospective and non-randomized study despite use of a prospectively maintained database with a large number of consecutive CLI patients with isolated infrapopliteal lesions. Patients considered unsuitable for revascularization or treated with bypass surgery were not managed by the physicians involved in the study. In addition, especially in indication of EVT, selection of strategy was left to physicians’ discretion which lead to possible selection bias.
It has been well-known of plain balloon angioplasty for BTK lesions in CLI patients to have low patency rate even in short term. And hypertension, administration of cilostazol, statin, lesion length, and chronic total occlusion were listed as multivariate predictors for angiographic restenosis at 3 months.18,19 Actual restenosis rate of treated vessel was not estimated in this study; however, initial procedural success could conduct to high rate of freedom from MALE in the patients categorized Rutherford IV. Further study would be expected to estimate the relation between MALE and restenosis of the treated vessels in this category.
Conclusion
From the present results, patients categorized Rutherford IV should be recognized to have quite different backgrounds from those categorized Rutherford V/VI. There might be operators’ bias in indication of EVT for Rutherford IV, but their clinical outcome was better in spite of that they are categorized in CLI.
Acknowledgments
This article was orally presented at ESC Congress 2014, on 2 September 2014 at Barcelona, Spain.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Critical limb ischaemia: management and outcome. Report of a national survey. The Vascular Surgical Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg 1995; 10: 108–113. [DOI] [PubMed] [Google Scholar]
- 2. Arain SA, White CJ. Endovascular therapy for critical limb ischemia. Vasc Med 2008; 13: 267–279. [DOI] [PubMed] [Google Scholar]
- 3. Bailey CM, Saha S, Magee TR, et al. A 1 year prospective study of management and outcome of patients presenting with critical lower limb ischaemia. Eur J Vasc Endovasc Surg 2003; 25: 131–134. [DOI] [PubMed] [Google Scholar]
- 4. Alan T, Hirsch MD, Duval S. Effective vascular therapeutics for critical limb ischemia: a role for registry-based clinical investigation. Circ Cardiovasc Interv 2013; 6: 8–11. [DOI] [PubMed] [Google Scholar]
- 5. Norgren L, Hiatt WR, Dormandy JA, et al. ; TASC II Working Group. Inter-Society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33(Suppl. 1): S1–S75. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463–e654. [DOI] [PubMed] [Google Scholar]
- 7. DeRubertis BG, Faries PL, McKinsey JF, et al. Shifting paradigms in the treatment of lower extremity vascular disease: a report of 1000 percutaneous interventions. Ann Surg 2007; 246: 415–422; discussion 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conrad MF, Crawford RS, Hackney LA, et al. Endovascular management of patients with critical limb ischemia. J Vasc Surg 2011; 53: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 9. Iida O, Nakamura M, Yamauchi Y, et al. ; on behalf of the OLIVE investigators. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv 2013; 6: 68–76. [DOI] [PubMed] [Google Scholar]
- 10. Dorros G, Jaff MR, Dorros AM, et al. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia five-year follow-up. Circulation 2001; 104: 2057–2062. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez N, McEnaney R, Marone LK, et al. Multilevel versus isolated endovascular tibial interventions for critical limb ischemia. J Vasc Surg 2011; 54: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iida O, Soga Y, Yamauchi Y, et al. Long term clinical efficacy of endovascular therapy for patients with critical limb ischemia attributable to pure isolated infrapopliteal lesions. J Vasc Surg 2013; 57: 974–981. [DOI] [PubMed] [Google Scholar]
- 13. Iida O, Soga Y, Hirano K, et al. Midterm outcome and risk stratification after endovascular therapy for patients with critical limb ischemia due to isolated below-the-knee lesions. Eur J Vasc Endovasc Surg 2012; 43: 313–321. [DOI] [PubMed] [Google Scholar]
- 14. Castronuovo JJ, Jr, Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629–637. [DOI] [PubMed] [Google Scholar]
- 15. Takahara M, Kaneto H, Iida O, et al. The influence of glycemic control on the prognosis of Japanese patients undergoing percutaneous transluminal angioplasty for critical limb ischemia. Diabetes Care 2010; 33: 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Brien-Irr MS, Dosluiglu HH, Harris LM, et al. Outcomes after endovascular intervention for chronic critical limb ischemia. J Vasc Surg 2011; 53: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 17. Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg 2009; 50: 1462–1473. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt A, Ulrich M, Winker B, et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal arterial disease. Catheter Cardiovasc Interv 2010; 76: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 19. Iida O, Soga Y, Kawasaki D, et al. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg 2012; 44: 425–431. [DOI] [PubMed] [Google Scholar]