Abstract
Objective:
To examine the effects of oral sucrose on procedural pain, and on biochemical markers of adenosine triphosphate utilization and oxidative stress in preterm neonates with mild to moderate respiratory distress.
Study design:
Preterm neonates with a clinically required heel lance that met study criteria (n = 49) were randomized into three groups: (1) control (n = 24), (2) heel lance treated with placebo and non-nutritive sucking (n = 15) and (3) heel lance treated with sucrose and non-nutritive sucking (n = 10). Plasma markers of adenosine triphosphate degradation (hypoxanthine, xanthine and uric acid) and oxidative stress (allantoin) were measured before and after the heel lance. Pain was measured using the Premature Infant Pain Profile. Data were analyzed using repeated measures analysis of variance, chi-square and one-way analysis of variance.
Results:
We found that in preterm neonates who were intubated and/or were receiving ⩾30% FiO2, a single dose of oral sucrose given before a heel lance significantly increased markers of adenosine triphosphate use.
Conclusion:
We found that oral sucrose enhanced adenosine triphosphate use in neonates who were intubated and/or were receiving ⩾30% FiO2. Although oral sucrose decreased pain scores, our data suggest that it also increased energy use as evidenced by increased plasma markers of adenosine triphosphate utilization. These effects of sucrose, specifically the fructose component, on adenosine triphosphate metabolism warrant further investigation.
Keywords: Anesthesia/pain, nursing, critical care/emergency medicine
Introduction
We previously reported that commonly performed neonatal intensive care unit (NICU) procedures, such as heel lance1 or tape removal,2 not only caused pain but also significantly increased markers of adenosine triphosphate (ATP) utilization and oxidative stress in premature neonates. More importantly, we also reported that oral sucrose, an intervention commonly used to relieve procedural pain, also significantly increased markers of ATP depletion and oxidative stress.3 These findings were obtained during a clinically required heel lance from subjects who were clinically stable, had a low mean illness severity score (Score for Neonatal Acute Physiology, Perinatal Extension II (SNAPPE-II) score ⩽ 8)4 and had minimal oxygen requirement. The current report examined the effects of oral sucrose analgesia given before a heel lance in neonates who were intubated and/or had high oxygen requirements (FiO2 ⩾ 30%). Such information has not yet been documented. Because premature neonates have reduced energy stores5 and are at risk of ATP depletion, we hypothesized that oral sucrose analgesia before heel lance will further enhance ATP utilization in this group of neonates. Heel lance was chosen because it is the most predominant painful procedure in the NICU, as shown in 26 different clinical trials.6 Pain was quantified using the Premature Infant Pain Profile (PIPP).7 ATP metabolism was quantified by measuring plasma concentrations of purines (hypoxanthine, xanthine and uric acid), which are well-documented markers of ATP utilization and breakdown.8–10 Oxidative stress was measured through plasma concentrations of allantoin, a well-accepted in vivo free radical marker.11
Methods
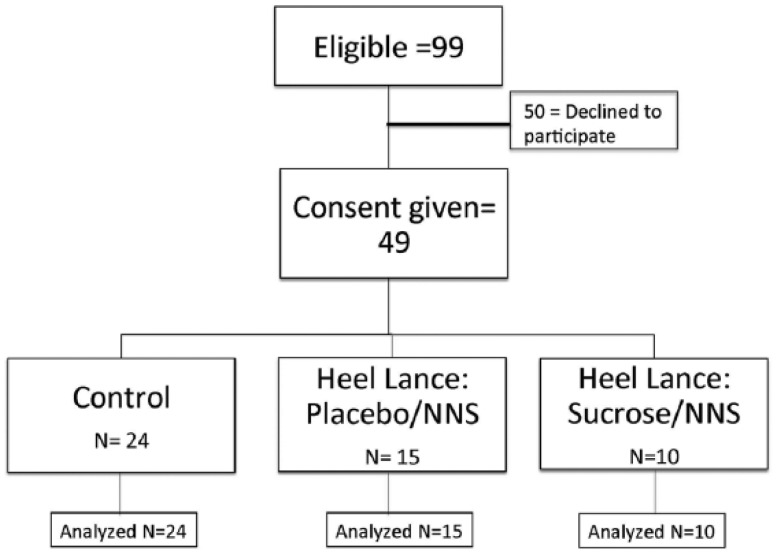
We conducted a prospective double-blind randomized controlled study at Loma Linda University Children’s Hospital NICU at Loma Linda, California. Study protocol and informed consent documents were approved by the Loma Linda University Institutional Review Board (IRB). Subject recruitment occurred from July 2009 to February 2013 and was publicly registered retrospectively. Throughout the study, ethical oversight was provided by the IRB and the study’s data and safety monitoring committee. Yearly progress reports, as well as any study-related activities, were submitted to the IRB and the funding agency. Subjects included in the study were preterm infants who (1) weighed ⩾800 g, (2) had a central catheter in place, (3) were intubated or had an FiO2 requirement ⩾30%, (4) had a clinically required heel lance and (5) had a postnatal age of less than 36 weeks’ gestation. Exclusion criteria included neonates who were (1) receiving opioids or sedatives or any anti-epileptic medications, (2) diagnosed with intraventricular hemorrhage ⩾grade 3 or (3) with unstable or severe respiratory and/or cardiovascular status, or (4) with facial or multiple congenital anomalies that might alter the apparent pain response. The clinically required heel lance was performed to obtain an accurate measure of blood glucose from neonates receiving glucose-rich total parenteral nutrition through their central catheter. Parents of preterm infants who met study criteria were approached for informed consent as soon after birth as possible. Once consent was obtained, subjects were randomized into one of three groups: (1) control, (2) placebo with non-nutritive sucking (NNS or pacifier) or (3) sucrose (Sweet-Ease) with NNS (Figure 1). Subjects randomized to the control group did not receive any heel lance or study drug, but were enrolled to control for the effect of time and the NICU environment (noise, light) on ATP utilization. Subjects randomized to the other two study groups received either NNS with sterile water 2 min before a heel lance or NNS with 24% sucrose (Sweet-Ease) 2 min before heel lance. Randomization was performed by a research pharmacist using a permuted block randomization table generated by the study statistician. A trained NICU research nurse performed the heel lance and blood sampling procedure for all enrolled subjects.
Figure 1.
Enrollment flow chart.
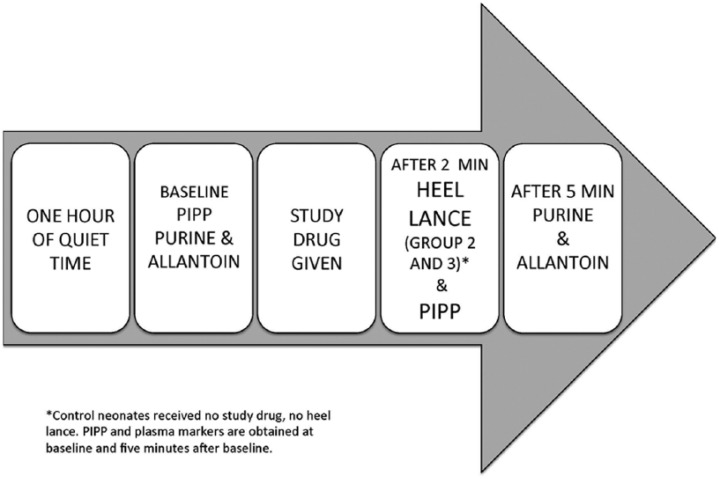
The experimental procedure is described in Figure 2. An earlier report provides detailed description of purine and allantoin analysis as well as pain measurement.3
Figure 2.
Study procedure.
Statistics
A repeated measures analysis of variance (ANOVA) with one between-subject factor (type of intervention) and one within-subject factor (time) was used to compute the minimum sample size needed for this study. The sample size was based on the following assumptions: (1) the significance level was set to 0.05, and (2) the required power was 80%. Initial calculation required 42–45 participants per group for a total of 131 subjects. However, we suspended subject recruitment when our preliminary analysis showed achievement of significance and power for ATP metabolism after the recruitment of 49 subjects (control = 24, placebo = 15 and oral sucrose = 10).
To analyze the data, assumptions of normality and equal variance were assessed. Demographic data for categorical variables were analyzed using chi-square test. Repeated measures ANOVA for one between-subject factor (group) and one within-subject factor (time) were assessed to evaluate the effect of the heel lance on plasma purines and allantoin concentrations over time. Interaction terms in the general linear model were used for this purpose. The interaction terms assess the differences between the groups over time. All statistical analyses were performed using SPSS Statistics for Windows Version 22. Differences were considered significant at p < 0.05.
Results
Subject demographics
As shown in Figure 1, we obtained parental consent for 49 newborn subjects who met study criteria between the months of July 2009 and August 2012. An additional 50 preterm neonates met criteria, but parents of these babies did not give consent, either without giving any specific reason or by stating that they did not want to expose their baby to additional blood draws. From the consented group, subjects were randomized into one of three groups: control (n = 24), heel lance and placebo (n = 15), or heel lance and 24% sucrose (Sweet-Ease) (n = 10). All subjects randomized to the heel lance groups were given a pacifier (NNS) immediately before, during and after study drug administration. As shown in Table 1, there were no significant differences in birth weight (control: 1021 ± 395 g; placebo/NNS 857 ± 295 g; sucrose/NNS: 1098 ± 603 g), estimated gestational age (control: 27.7 ± 2.5 weeks; placebo/NNS: 26.3 ± 3.2 weeks; sucrose/NNS: 30.1 ± 3.1 weeks), acuity scores (control: 19.6 ± 11.8; placebo/NNS: 26.4 ± 17.8; sucrose/NNS: 15.9 ± 16.1) and oxygen (FiO2) requirement (control: 0.35 ± 0.12%; placebo/NNS: 0.39 ± 0.11%; sucrose/NNS: 0.37 ± 0.14%) between the three groups.
Table 1.
Subject demographics.
| Control (n = 20) | Heel lance Placebo–NNS (n = 15) | Heel lance Sweet-Ease–NNS (n = 9) | F value | p valuea | |
|---|---|---|---|---|---|
| EGA (weeks) | 27.7 ± 2.5 | 26.3 ± 3.2 | 27.3 ± 2.9 | 1.480 | 0.238 |
| Birth weight (g) | 1021.2 ± 395 | 857.2 ± 295 | 1097.6 ± 603 | 1.146 | 0.327 |
| Apgar (1 min) | 4 ± 2 | 4 ± 3 | 5 ± 2 | 0.482 | 0.620 |
| Apgar (5 min) | 6 ± 2 | 6 ± 2 | 7 ± 2 | 0.046 | 0.955 |
| Sex | Male 11 (46%) | Male 6 (40%) | Male 5 (50%) | 0.878b | |
| Female 13 (54%) | Female 9 (60%) | Female 5 (50%) | |||
| Race | 0.560b | ||||
| Caucasian | 10 (42%) | 7 (47%) | 5 (50%) | ||
| Hispanic | 10 (42%) | 8 (53%) | 4 (40%) | ||
| African American | 4 (16%) | 0 (0%) | 1 (10%) | ||
| Condition at time of sampling | |||||
| FiO2 (%) | 0.35 ± 0.12 | 0.39 ± 0.11 | 0.37 ± 0.14 | 0.526 | 0.595 |
| EGA, weeks | 30.4 ± 2.2 | 30.4 ± 2.2 | 30.4 ± 2.8 | 0.004 | 0.996 |
| SNAPPE-II | 19.6 ± 11.8 | 26.4 ± 17.8 | 15.9 ± 16.1 | 1.710 | 0.192 |
| Mode of O2 delivery | 0.784b | ||||
| Nasal cannula | 3 (12.5%) | 3 (20%) | 3 (30%) | ||
| NCPAP | 2 (8.3%) | 3 (20%) | 0 | ||
| NIPPV | 6 (25%) | 5 (33.3%) | 3 (30%) | ||
| SIMV | 5 (20.8%) | 2 (13.3%) | 2 (20%) | ||
| HFV/HFO | 8 (33.4%) | 2 (13.3%) | 2 (20%) | ||
| Hemoglobin | 11.8 ± 2.1 | 11.6 ± 2.4 | 11.6 ± 1.4 | 0.073 | 0.930 |
| Hematocrit | 35.5 ± 5.7 | 35.2 ± 6.5 | 34.7 ± 4.2 | 0.061 | 0.941 |
EGA: estimated gestational age; SNAPPE-II: Score for Neonatal Acute Physiology, Perinatal Extension II; NNS: non-nutritive sucking; NCPAP: nasal continuous positive airway pressure; NIPPV: non-invasive intermittent positive pressure ventilation; SIMV: synchronized intermittent mandatory ventilation; HFV; high-frequency ventilation; HFO: high-frequency oscillation.
One-way analysis of variance (ANOVA).
Chi-square.
Effects of oral sucrose on behavioral and physiological markers of pain
There were no significant differences in baseline and procedural pain scores between the three groups (Table 2). However, we found that sucrose attenuated the increase in the pain score in response to heel lance, compared to placebo, although the reduction was not significant (Table 2), which may be due to a small sample size.
Table 2.
Pain score, heart rate and oxygen saturation.
| Control (n = 20) | Heel lance Placebo–NNS (n = 15) | Heel lance Sweet-Ease–NNS (n = 9) | p value* | |
|---|---|---|---|---|
| Pain score | ||||
| Mean (min–max) | ||||
| Baseline | 4.6 (2–7) | 4.3 (2–8) | 4.3 (2–7) | 0.611 |
| Procedural | 7.3 (2–12) | 6.7 (3–12) | 5.7 (4–11) | 0.337 |
| Heart rate | ||||
| Baseline | 156.7 (12.0) | 159.8 (11.8) | 151.6 (11.9) | 0.244 |
| Procedural | 156.8 (14.2) | 168.4 (11.5) | 164.6 (11.9) | 0.025a |
| Oxygen saturation | ||||
| Baseline | 96.5 (0.5) | 96.2 (0.5) | 96.2 (0.5) | 0.885 |
| Procedural | 96.2 (0.6) | 95.8 (0.6) | 96.4 (0.6) | 0.791 |
NNS: non-nutritive sucking.
Control group procedural pain score was significantly lower than both heel lance groups.
Kruskall–Wallis test.
More importantly, we observed that the heart rate response to heel lance was highest in the sucrose group (p < 0.025). Heart rate increased by 7.4% in the sucrose group, compared to 4.9% in the placebo group. These data support our previous findings and suggest that the reduced pain scores in the oral sucrose group may be due to lower scores in the behavioral/facial components of the PIPP scoring tool and not from changes in the physiological markers of pain such as heart rate or oxygen saturation. We observed no significant changes in mean oxygen saturation in response to heel lance in any of the three groups (Table 2).
Effects of oral sucrose on purine markers of ATP metabolism (purines)
There were no significant differences in baseline and 5-min purine levels in any of the groups. However, although plasma purine concentration decreased over time in subjects randomized to the control and placebo groups, we observed a significant increase over time in plasma hypoxanthine, xanthine and uric acid in neonates who received sucrose before the heel lance (Figure 3).
Figure 3.
Plasma hypoxanthine, xanthine and uric acid concentration increased significantly over time in preterm neonates who received oral sucrose before a clinically required heel lance.
Effects of oral sucrose on plasma allantoin in neonates with a minimal pain response to heel lance
There were no significant differences in baseline allantoin (control: 2.88 µM, placebo/NNS: 3.30 µM, sucrose/NNS: 2.85 µM) or 5-min allantoin levels (control: 2.91 µM, placebo/NNS: 3.05 µM, sucrose/NNS: 3.14 µM) in any of the groups. Similar to our previously published data, we found that in preterm neonates in the sucrose group with minimal pain response (PIPP score increased by less than 33% in response to heel lance), mean plasma allantoin concentration increased over time. This increase, however, was not statistically significant (p = 0.147).
Discussion
We found that in neonates who were intubated and/or receiving ⩾30% FiO2, a single dose of oral sucrose given before a heel lance significantly increased markers of ATP utilization, as evidenced by significant increases over time in plasma hypoxanthine, xanthine and uric acid concentration (Figure 3). The percent change in purine concentrations over time was higher than what we previously observed in clinically stable premature neonates.3 Because preterm neonates with acute respiratory distress were shown to have oxygen consumption rates that are 60% above normal or recovery levels,4 the increase in purine concentration over time suggests that sucrose administration may have further altered the ATP utilization to ATP synthesis ratio, resulting in significantly higher products of ATP breakdown. These findings suggest that oral sucrose is not a harmless solution and may have potentially adverse metabolic effects. These data suggest that oral sucrose should be treated as a medication, with both risks and benefits, with dosage carefully controlled and monitored. These effects of sucrose, specifically the fructose component, on ATP metabolism warrant further investigation. Unlike glucose, fructose metabolism in the liver largely bypasses a major allosterically regulated step (phosphofructokinase), which can result in the depletion of cellular inorganic phosphate levels and a reduction in ATP synthesis.12,13 This finding may have significant clinical repercussions on high-risk preterm neonates, who are already at risk of insufficient energy stores. In this cohort, plasma allantoin concentration also increased over time, although the difference was not significant.
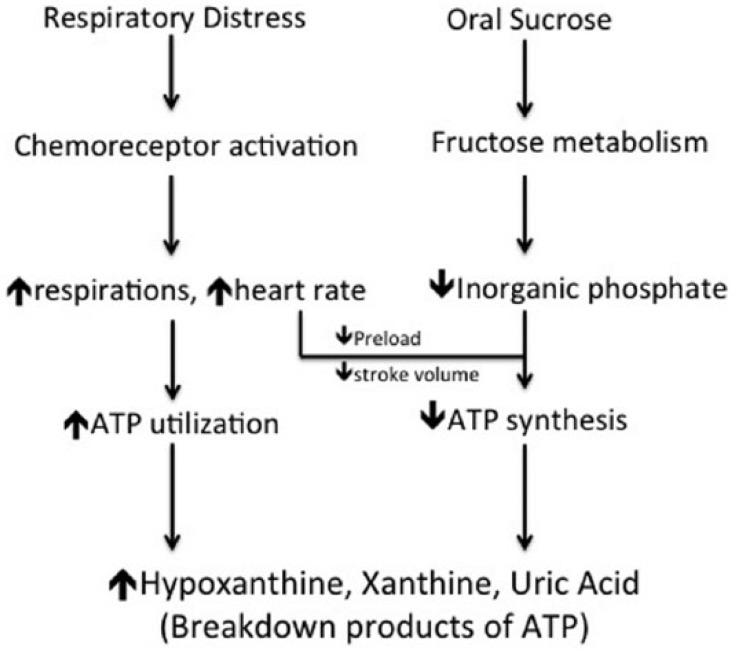
It was interesting to note the significant increases in heart rate in preterm neonates who received oral sucrose. A reduction in pain should not result in tachycardia. This finding supports Wilkinson et al. who suggested that sugar may not be an analgesic and may not remove or relieve pain, but may act as a “compensating pleasure.”14 It is not known whether oral sucrose is pleasurable to neonates, but it is well documented that pleasure or reward increases heart rate in adults.15,16 In addition, the consumption of sucrose was shown to activate the sympathetic nervous system as evidenced by an increase in heart rate and a decrease in skin blood flow in healthy adult volunteers.17,18 These changes may decrease stroke volume, increase vascular resistance and reduce tissue perfusion and oxygenation, ultimately altering ATP synthesis.19 The combined effect of respiratory distress and sucrose administration will be an alteration in ATP metabolism, which then may lead to an increase in plasma concentrations of hypoxanthine, xanthine and uric acid (Figure 4).
Figure 4.
Possible mechanism by which respiratory distress and oral sucrose increase plasma concentration of hypoxanthine, xanthine and uric acid.
This study has limitations. Although statistical power was over 80% for hypoxanthine, xanthine and uric acid, power was small for allantoin and pain scores. Because the main purpose of the study was to determine the combined effects of respiratory distress and oral sucrose administration on ATP metabolism, the study was stopped once significance and statistical power were achieved for markers of ATP metabolism. Additional subjects will most likely increase the significance in plasma allantoin levels and pain scores, as shown by our previously published data on non-intubated neonates.
In summary, oral sucrose administration enhanced ATP use in preterm neonates with mild to moderate respiratory distress. Further studies are required to elucidate the mechanism of this effect.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grant NR011209 (D.M.A.).
References
- 1. Plank MS, Boskovic DS, Tagge E, et al. An animal model for measuring the effect of common NICU procedures on ATP metabolism. Biol Res Nurs 2011; 13(3): 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slater L, Asmerom Y, Boskovic DS, et al. Procedural pain and oxidative stress in premature neonates. J Pain 2012; 13(6): 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asmerom Y, Slater L, Boskovic DS, et al. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J Pediatr 2013; 163(1): 29.e21–35.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson P, Bose CL, Bucciarelli RL, et al. Oxygen consumption of infants with respiratory distress syndrome. Biol Neonate 1984; 46(2): 53–56. [DOI] [PubMed] [Google Scholar]
- 5. Symonds ME, Pearce S, Bispham J, et al. Timing of nutrient restriction and programming of fetal adipose tissue development. Proc Nutr Soc 2004; 63(3): 397–403. [DOI] [PubMed] [Google Scholar]
- 6. Stevens B, Yamada J, Lee GY, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2013; 1: CD001069. [DOI] [PubMed] [Google Scholar]
- 7. Stevens B, Johnston C, Petryshen P, et al. Premature infant pain profile: development and initial validation. Clin J Pain 1996; 12(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 8. Plank MS, Boskovic DS, Sowers LC, et al. Biochemical markers of neonatal hypoxia. Pediatr Health 2008; 2(4): 485–501. [Google Scholar]
- 9. Hicks M, Wong LS, Day RO. Identification of products from oxidation of uric acid induced by hydroxyl radicals. Free Radic Res Commun 1993; 18(6): 337–351. [DOI] [PubMed] [Google Scholar]
- 10. Lazzarino G, Vagnozzi R, Tavazzi B, et al. MDA, oxypurines, and nucleosides relate to reperfusion in short-term incomplete cerebral ischemia in the rat. Free Radic Biol Med 1992; 13(5): 489–498. [DOI] [PubMed] [Google Scholar]
- 11. Pavitt DV, de Fonseka S, Al-Khalaf N, et al. Assay of serum allantoin in humans by gas chromatography-mass spectrometry. Clin Chim Acta 2002; 318(1–2): 63–70. [DOI] [PubMed] [Google Scholar]
- 12. Raivio KO, Kekomaki MP, Maenpaa PH. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol 1969; 18(10): 2615–2624. [DOI] [PubMed] [Google Scholar]
- 13. Johnson MA, Tekkanat K, Schmaltz SP, et al. Adenosine trisphosphate turnover in humans. J Clin Invest 84: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilkinson DJ, Savulescu J, Slater R. Sugaring the pill: ethics and uncertainties in the use of sucrose for newborn infants. Arch Pediatr Adolesc Med 2012; 166(7): 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fowles DC, Fisher AE, Tranel DT. The heart beats to reward: the effect of monetary incentive on heart rate. Psychophysiology 1982; 19(5): 506–513. [DOI] [PubMed] [Google Scholar]
- 16. Tranel DT, Fisher AE, Fowles DC. Magnitude of incentive effects on heart rate. Psychophysiology 1982; 19(5): 514–519. [DOI] [PubMed] [Google Scholar]
- 17. Rousmans S, Robin O, Dittmar A, et al. Autonomic nervous system responses associated with primary tastes. Chem Senses 2000; 25(6): 709–718. [DOI] [PubMed] [Google Scholar]
- 18. Leterme A, Brun L, Dittmar A, et al. Autonomic nervous system responses to sweet taste: evidence for habituation rather than pleasure. Physiol Behav 2008; 93(4–5): 994–999. [DOI] [PubMed] [Google Scholar]
- 19. Boron WF, Boulpaep EL. Medical physiology. Saunders 2011; 544–550. [Google Scholar]