Abstract
Background:
Recent studies described an increase in acute kidney injury when high dose gentamicin was included in perioperative prophylaxis for orthopedic surgeries. To this effect, we compared the rate of nephrotoxicity for selected orthopedic surgeries where gentamicin was included (Gentamicin Group) to those where it was not included (Control Group) for perioperative prophylaxis and evaluated risk factors for nephrotoxicity.
Methods:
Spine, hip and knee surgeries performed between April 2011 and December 2013 were reviewed retrospectively. Gentamicin was given to eligible patients based on age, weight and Creatinine Clearance. Nephrotoxicity was assessed using Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) criteria.
Results:
Among selected surgeries (N = 1590 in Gentamicin Group: hip = 926, spine = 600, knee = 64; N = 2587 in Control Group: hip = 980, spine = 902, knee = 705), patients’ body weight, serum creatinine, comorbidities and surgery duration were similar in Gentamicin Group and Control Group. Gentamicin median dose was 4.5 mg/kg of dosing weight. Nephrotoxicity rate was 2.5% in Gentamicin Group and 1.8% in Control Group, p = 0.17. Most cases of nephrotoxicity were Risk category by RIFLE criteria (67% in Gentamicin Group and 72% in Control Group, p = 0.49). In logistic regression, risk factors for nephrotoxicity were hospital stay >1 day prior to surgery (odds ratio = 8.1; 95% confidence interval = 2.25–28.97, p = 0.001), knee or hip surgery (odds ratio = 4.7; 95% confidence interval = 2.9–9.48, p = 0.0005) and diabetes (odds ratio = 1.95; 95% confidence interval = 1.13–3.35, p = 0.016). Receipt of gentamicin was not an independent predictor of nephrotoxicity (odds ratio = 1.5; 95% confidence interval = 0.97–2.35, p = 0.07).
Conclusion:
In this cohort, rate of nephrotoxicity was similar between Gentamicin Group and Control Group. Single high dose gentamicin is a safe and acceptable option for perioperative prophylaxis in eligible patients undergoing orthopedic surgeries.
Keywords: Gentamicin, high dose, perioperative prophylaxis, nephrotoxicity
Introduction
Prophylactic antimicrobials have been shown to significantly reduce rate of surgical site infections (SSI) after arthroplasty and spine fusion surgeries.1–3 The most common pathogens involved in SSI after these procedures are Staphylococcus aureus, gram-negative bacilli (GNB), coagulase-negative staphylococci and β-hemolytic Streptococci.4 Since early 1960, cefazolin remains the recommended drug of choice for perioperative prophylaxis in these surgeries.1,2,4,5 An increase in the proportion of SSI following arthroplasty and spine fusion caused by GNB has been recently reported nationwide.4,6–8 Also, an increased resistance of GNB to cefazolin has been observed.6–8 Accordingly, current clinical practice guidelines for antimicrobial prophylaxis in surgery recommend using active surveillance and local resistance patterns when selecting agents for perioperative prophylaxis.4
Review of orthopedic surgeries complicated by SSI at our institution showed that GNB accounted for 30% of SSI after hip arthroplasty and 25% of SSI after spine fusion. Resistance to cefazolin among GNB isolated from SSI was high, 44% for hip arthroplasty SSI and 57% for spine fusion SSI. Gentamicin resistance among GNB was low, 7% in arthroplasty SSI and 10% in spine fusion SSI.9 As a result of this review, in July 2012, our institutional guidelines were changed and recommend an addition of a single high dose gentamicin to perioperative prophylaxis regimen in order to provide adequate coverage for GNB for thoracic/lumbar spine fusion and hip arthroplasty.
Acute kidney injury (AKI) is a serious post-operative complication associated with increased risk of re-admission, progression to chronic kidney disease and poor long-term survival.10–12 Reported AKI rate after orthopedic surgery is in the range of 0.4%–6%.13–15 When gentamicin is used for surgical prophylaxis, current clinical practice guidelines recommend a single high dose gentamicin (5 mg/kg once) to be administered before incision. This is in contrast to conventional low dose gentamicin (1.5–2 mg/kg every 8 h for 24 h) previously recommended for perioperative prophylaxis.16 Nephrotoxicity is of concern with gentamicin use. Although extensive data support safety of high dose gentamicin in hospitalized patients for therapeutic indications, safety data are limited for perioperative prophylaxis in patients undergoing major surgery.17–19 In fact, few recent studies described an increase in the rate of AKI when high dose gentamicin was included in perioperative prophylaxis in patients undergoing orthopedic surgery.13,20 The objective of this study was to compare the rate of nephrotoxicity for selected orthopedic surgeries where gentamicin was included to those where it was not included for perioperative prophylaxis and identify risk factors for nephrotoxicity.
Methods
Study site and design
We performed the study at a single specialty orthopedic hospital located in New York City. In July 2012, hospital guidelines for perioperative prophylaxis for orthopedic procedures were changed to recommend a single high dose of gentamicin in addition to cefazolin or clindamycin (in patients with severe penicillin (PCN) allergy). Patients with a history of methicillin-resistant S. aureus (MRSA) infection or colonization (positive nasal MRSA screen) receive single high dose gentamicin and weight-based vancomycin. For knee arthroplasty, gentamicin is recommended only for patients with severe PCN allergy.
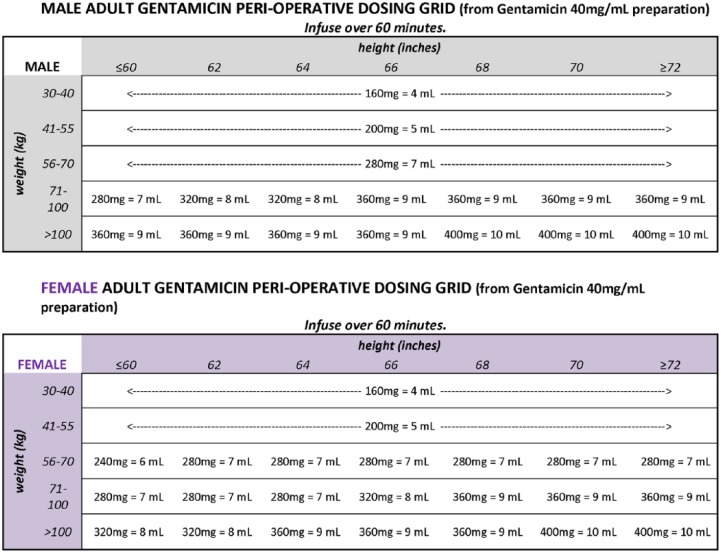
Patients are considered eligible to receive gentamicin if they met all of the following criteria: age < 75 years, CrCl > 20 mL/min and actual body weight (ABW) < 120 kg. Per our gentamicin weight-based protocol, gentamicin dose was provided for weight ranges and rounded off for ease of use and compounding in operating room. The intention was to provide a simplified calculation of a high dose gentamicin that approximated 3–5 mg/kg of dosing weight. Gentamicin dosing followed a protocol based on weight, height and gender (Figure 1). The dosing protocol was further simplified in August 2013 to be based on weight alone (Table 1). Aztreonam was recommended for patients who did not meet the gentamicin eligibility criteria.
Figure 1.
Original gentamicin weight-based dosing protocol.
Table 1.
| Actual body weight (kg) | Dose (mg) |
|---|---|
| 40–49 | 160 |
| 50–59 | 200 |
| 60–69 | 240 |
| 70–79 | 280 |
| 80–89 | 320 |
| 90–99 | 360 |
| 100–119 | 400 |
All doses to be diluted in 250 mL of normal salien and infused over 30 min.
Gentamicin doses are provided for weight ranges and rounded off for ease of compounding in operating room.
Criteria for gentamicin include age < 75 years, actual body weight < 120 kg, baseline creatinine clearance > 20 mL/min.
Thoracolumbar spine fusions/refusions, total hip replacement and total knee replacement surgeries performed over 32 months (1 April 2011–31 December 2013) were selected from hospital databases where these surgeries were listed as a primary procedure. Surgeries performed after guidelines implementation (July 2012–December 2013) when gentamicin was administered for perioperative prophylaxis were included in Gentamicin Group (GG). Surgeries performed before guidelines implementation (April 2011–June 2012) when cefazolin was administered for perioperative prophylaxis and surgeries performed after guidelines implementation when aztreonam was administered for patients not eligible to receive gentamicin were included in Control Group (CG). Patient age, comorbidities, ABW, height, serum creatinine (SCr), CrCl and surgery duration (opTime) were extracted from electronic medical records and retrospectively reviewed. Only perioperative antibiotics were reviewed. SCr from a pre-admission testing visit (<30 days prior to surgery) if available or from the day of surgery was used as a baseline value. Subsequent SCr values from the day of surgery and up to 5 days post-operatively were evaluated. Patients who did not have complete SCr data (baseline SCr and at least one subsequent SCr result post-operatively) and/or who were missing height and weight values were excluded from the study. This study was approved by NYU School of Medicine’s Institutional Review Board (IRB). In view of retrospective nature of the study, the need for informed consent was waived.
Study outcomes
The primary outcome of the study was rate of nephrotoxicity. The severity of nephrotoxicity was defined by RIFLE (Risk, Injury, Failure, Loss, End-stage kidney disease) criteria (Table 2).21 Rate of nephrotoxicity was compared for surgeries where gentamicin was included in perioperative prophylaxis (GG) versus surgeries where gentamicin was not included (CG). We also identified risk factors associated with nephrotoxicity in patients who underwent these surgeries.
Table 2.
RIFLE criteria.
| Risk | Increased SCr level × 1.5 or GFR decrease >25% |
| Injury | Increased SCr level × 2 or GFR decrease >50% |
| Failure | Increased SCr level × 3 or GFR decrease >75% |
| Loss | Persistent acute renal failure or loss of function >4 weeks |
| ESRD | ESRD >3 months |
SCr: serum creatinine; GFR: glomerular filtration rate; ESRD: end-stage renal disease.
Bellomo et al.21
Assessment of SCr only and not GFR decrease was used in our study.
To evaluate gentamicin dose administered preoperatively, we used dosing weight. In nonobese patients, ideal body weight was used as dosing weight. For patients who weigh 20% above their ideal body weight, the dosing weight was calculated using the ideal body weight plus 40% of the difference between the actual and ideal weights.4
Statistical analysis
Categorical variables were compared using Chi-square or Fisher’s exact test. Continuous variables were analyzed using Mann–Whitney U test. Multivariate logistic regression was used to identify independent risk factors associated with nephrotoxicity. Any variable with a p-value ⩽ 0.2 in univariate analysis was included in the multivariate regression. P-values of <0.05 were considered significant. All analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL).
Results
A total of 4177 surgeries were evaluated. The GG (N = 1590) consisted of 926 hip replacements, 600 spine fusions and 64 knee replacements. The CG (N = 2587) consisted of 980 hip replacements, 902 spine fusions and 705 knee replacements. Only 64 knee replacements were included in GG because gentamicin in combination with clindamycin or vancomycin is preserved for patients with severe PCN allergy for this type of surgery according to our guidelines. Patient demographic and clinical characteristics are summarized in Table 3. There were more male in GG (43% vs 38% in CG, p = 0.002). Patients were older in CG (median 62 vs 57 years in GG, p = 0.0005). CrCl was higher in patients in GG (median 90.8 vs 89.3 mL/min in CG, p = 0.0005). ABW, baseline SCr and duration of surgery (opTime) were comparable between groups (median ABW = 80 kg; SCr = 0.8 g/dL; opTime = 124 min in GG and 117 min in CG, p = 0.29). Patients’ comorbidities were also comparable between the GG and CG except pulmonary disease which was more common among the patients in the CG (4.2% vs 1.9% in GG, p = 0.0005). In GG, perioperative prophylaxis in addition to gentamicin included cefazolin (84%), clindamycin (13%) and vancomycin (3%). Gentamicin median perioperative dose was 4.5 mg/kg (interquartile range (IQR) = 4.2–4.9 mg/kg) of dosing weight. In CG, perioperative antibiotics included cefazolin (87%), clindamycin (10%), aztreonam (8.6%) and vancomycin (3%).
Table 3.
Patient demographic and clinical characteristics.
| All (N = 4177) | Gentamicin Group (n = 1590) | Control Group (n = 2587) | p-value | |
|---|---|---|---|---|
| Median age, years (IQR) | 61 (51–69) | 57 (50–66) | 59 (51–71) | 0.0005 |
| Age >65 years | 1486 (35.6) | 467 (29.4) | 1019 (39.4) | 0.0005 |
| Male | 1659 (40.0) | 680 (43.0) | 979 (38.0) | 0.002 |
| Type of surgery | ||||
| Hip | 1906 (45.6) | 926 (58.0) | 980 (37.9) | 0.0005 |
| Knee | 769 (18.4) | 64 (4.0) | 705 (27.0) | 0.0005 |
| Spine | 1502 (36.0) | 600 (38.0) | 902 (34.9) | 0.065 |
| Median opTime, min (IQR) | 119 (73–240) | 124 (73–274) | 117 (74–224) | 0.29 |
| Median length of stay, days (IQR) | 3 (3–5) | 3 (3–4) | 3 (3–5) | 0.0005 |
| >1 day in hospital before surgery | 27 (0.6) | 12 (0.8) | 15 (0.6) | 0.627 |
| Median ABW, kg (IQR) | 80 (66.0–94.0) | 80 (66.0–93.0) | 80 (66.0–94.0) | 0.98 |
| ABW >20% over IBW (obese patients) | 2636 (63.1) | 989 (62.2) | 1647 (63.7) | 0.358 |
| ABW < IBW | 359 (9.0) | 130 (8.2) | 229 (8.9) | 0.484 |
| Median baseline SCr, g/dL (IQR) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | 0.337 |
| Median baseline CrCl, mL/min (IQR) | 86.8 (87.0–110.0) | 90.8 (72.4–113.0) | 89.3 (64.9–108.6) | 0.0005 |
| Comorbidities | ||||
| Diabetes mellitus | 457 (10.9) | 169 (10.6) | 288 (11.1) | 0.649 |
| Gastrointestinal | 77 (1.8) | 33 (2.1) | 44 (1.7) | 0.45 |
| Hepatobiliary | 26 (0.6) | 8 (0.5) | 18 (0.7) | 0.571 |
| Malignancy | 153 (3.7) | 68 (4.3) | 85 (3.3) | 0.116 |
| Neurological | 150 (3.6) | 55 (3.5) | 95 (3.7) | 0.784 |
| Pulmonary | 139 (3.3) | 30 (1.9) | 109 (4.2) | 0.0005 |
| Tobacco usea | 304/2016 (15.1) | 77/488 (15.8) | 227/1528 (14.9) | 0.672 |
| Received blood productsa | 331/3846 (7.9) | 117/1473 (7.4) | 214/2373 (8.3) | 0.316 |
OpTime: duration of surgery; ABW: actual body weight; IBW: ideal body weight; IQR: interquartile range; SCr: serum creatinine.
All values shown as n (%) unless otherwise specified.
Not all patients had data available.
Overall rate of nephrotoxicity was 2.5% (39/1950) in GG vs 1.8% (46/2587) in CG, p = 0.166 (Table 4). Rate of nephrotoxicity for hip and knee arthroplasty was similar in GG and CG (3.2% vs 2.6%, p = 0.661). Rate of nephrotoxicity was higher in GG for spine surgeries (1.2% vs 0.2% in CG, p = 0.034). Among patients who developed nephrotoxicity, most patients were in Risk category (1.5 times increase in SCr) by RIFLE criteria (66.7% in GG and 71.7% in CG, p = 0.485) as compared to Injury and Failure (Table 4). Elevation of SCr within 5 days post-surgery was transient, and on discharge SCr was ⩽1 g/dL (within normal range) in 76.9% (30/39) of patients with nephrotoxicity in GG versus 82.6% (38/46) in CG, p = 0.703. No patients required hemodialysis or continuous renal replacement post-surgery.
Table 4.
Nephrotoxicity.
| All (N = 4177) | Gentamicin Group (n = 1590) | Control Group (n = 2587) | p-value | |
|---|---|---|---|---|
| Overall nephrotoxicity rate | 85 (2.0) | 39 (2.5) | 46 (1.8) | 0.166 |
| Nephrotoxicity rate by type of surgery | ||||
| Hip and knee arthroplasty | 76/2675 (2.8) | 32/990 (3.2) | 44/1685 (2.6) | 0.661 |
| Spine fusion | 9/1502 (0.6) | 7/600 (1.2) | 2/902 (0.2) | 0.034 |
| Severity of nephrotoxicity by RIFLE criteria | ||||
| Risk | 59/85 (69.4) | 26/39 (66.7) | 33/46 (71.7) | 0.485 |
| Injury | 22/85 (25.9) | 12/39 (30.8) | 10/46 (21.7) | |
| Failure | 4/85 (4.7) | 1/39 (2.6) | 3/46 (6.5) | |
| Median time to nephrotoxicity, days (IQR) | 3 (2–3) | 2 (1–3) | 3 (2–3) | 0.0005 |
| Peak SCr, g/dL (IQR) | 0.8 (0.7–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–1.0) | 0.002 |
RIFLE: Risk, Injury, Failure, Loss, End-stage kidney disease; IQR: interquartile range; SCr: serum creatinine.
All values shown as n (%) unless otherwise specified.
In logistic regression, patients with hospital stay of >1 day before the surgery (odds ratio (OR) = 8; 95% confidence interval (CI) = 2.25–28.97, p = 0.001), surgery type being knee or hip (OR = 4.7; 95% CI = 2.3–9.48, p = 0.0005) or patients with diabetes (OR = 1.9; 95% CI = 1.13–3.35, p = 0.016) had higher probability to develop nephrotoxicity (Table 5). We adjusted for receipt of gentamicin, concurrent vancomycin, age >65 years and ABW >20% of ideal.
Table 5.
Risk factors associated with nephrotoxicity.
| Characteristics | Nephrotoxicity (n = 85) | No nephrotoxicity (n = 4092) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| p-value | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | |||
| Age >65 yrs | 41 (48.2) | 1445 (35.3) | 0.019 | 1.7 (1.11–2.63) | 0.091 | 1.5 (0.94–2.28) |
| ABW >20% over IBW | 65 (76.5) | 2571 (62.8) | 0.014 | 1.9 (1.160–3.186) | 0.06 | 1.6 (0.98–2.75) |
| Hospital stay >1 day before surgery | 3 (3.5) | 24 (0.6) | 0.017 | 6.2 (1.831–21.004) | 0.001 | 8.1 (2.25–28.97) |
| Diabetes mellitus | 18 (21.2) | 439 (10.7) | 0.004 | 2.2 (1.316–3.797) | 0.016 | 1.95 (1.13–3.35) |
| Surgery type hip or knee | 76 (89.4) | 2599 (63.5) | 0.005 | 4.9 (2.42–9.71) | 0.0005 | 4.7 (2.3–9.48) |
| Perioperative gentamicin | 39 (45.9) | 1551 (37.9) | 0.166 | 1.4 (0.90–2.14) | 0.071 | 1.5 (0.97–2.35) |
| Perioperative gentamicin and vancomycin | 2 (2.4) | 39 (1.0) | 0.203 | 2.5 (0.6–10.54) | 0.232 | 2.5 (0.56–10.92) |
CI: confidence interval; ABW: actual body weight; IBW: ideal body weight.
All values shown as n (%) unless otherwise specified.
Discussion
We reported the results of a large, retrospective study (N = 4177) that evaluated rate of nephrotoxicity in orthopedic surgeries (spine fusion and arthroplasty) where single high dose gentamicin was included in perioperative prophylaxis for eligible patients in addition to cefazolin, clindamycin or vancomycin. As a CG, we used surgeries where gentamicin was not included in the perioperative prophylaxis. In our study, the overall rate of nephrotoxicity based on RIFLE criteria was 2.5% in GG and 1.8% in CG, p = 0.166. This is comparable to the reported rate of nephrotoxicity in orthopedic surgeries by other studies where range of AKI was 0.4%–6%.13–15
The rate of nephrotoxicity for arthroplasty (hip and knee) in our study was 3.2% (32/990) in GG versus 2.6% (44/1685) in CG, p = 0.661. Jafari et al. conducted a retrospective case–control study to evaluate renal impairment following total joint arthroplasty (hip and knee; N = 17,938 patients; n = 98 cases that developed AKI matched to n = 196 CG) using RIFLE criteria.14 Perioperative antibiotics were not specified by the authors. The reported rate of nephrotoxicity excluding Risk and including only Injury and Failure categories by RIFLE in this study was 0.55%. The rationale for excluding Risk category in this study was to maintain higher specificity for true renal impairment. After exclusion of the Risk category from our study, the nephrotoxicity rate for hip and knee combined was 0.8% (13/1685) in CG versus 0.9% (9/990) in GG in our study, which is comparable to the results from Jafari et al.’s study.
In our study, the rate of nephrotoxicity for spine fusion was 1.2% (7/600) in GG versus 0.2% (2/902) in CG, p = 0.03. A retrospective study by Tang et al. evaluated patients (N = 236) with degeneration lumbar scoliosis who underwent spinal fusion and instrumentation and received cefazolin 1 g for perioperative prophylaxis and reported the rate of nephrotoxicity of 0.85% (progressive renal failure: n = 1, acute renal failure: n = 1).22 The rate of nephrotoxicity in CG in our study was lower than in the study by Tang et al. However, the definition of nephrotoxicity used by Tang et al. was not specified. In our review of the literature, we did not identify other studies reporting rates of nephrotoxicity in spine fusion, for comparison with that in our study.
In contrast to our study, Challagundla et al.20 (N = 98) reported a significant increase in renal impairment by RIFLE criteria after single-dose gentamicin and high-dose flucloxacillin, versus cefuroxime alone, used for perioperative prophylaxis in patients undergoing elective hip and knee replacement. Three patients who received high-dose flucloxacillin and gentamicin required temporarily hemodialysis. Lower doses of gentamicin were used in this study (160 mg for patients weighing >60 kg and 120 mg when weight was <60 kg) as compared to our study. In addition, more than half of the patients in the study by Challagundla et al. had their implants cemented with gentamicin or tobramycin. Aminoglycosides eluted from cement has been proposed as a probable link with AKI in a few studies.23,24 In our study, patients did not have implants cemented with aminoglycosides. Bell et al.13 (n = 7666) also reported an increase in AKI with high dose gentamicin for perioperative prophylaxis in patients undergoing orthopedic implant surgery. The primary definition of post-operative renal impairment used in this study was the Kidney Disease Improving Global Outcomes (KDIGO) criteria.25 Gentamicin dose in this study was 4 mg/kg, similar to our study.
Older age is considered a risk factor for AKI with aminoglycosides. In fact, the mean age of the patients in recent studies that reported increased nephrotoxicity after including gentamicin for perioperative prophylaxis was 70 years, much older than mean of 57 years for patients in GG in our study.13,20 Obesity and baseline renal insufficiency are also potential risk factors for nephrotoxicity.14,26–28 In our study, we used specific criteria of age, weight and CrCl to identify ineligible patients for gentamicin perioperative prophylaxis. The use of such criteria allowed us to exclude patients at higher risk for nephrotoxicity from receiving gentamicin. This may explain the relative absence of nephrotoxicity with gentamicin in our study.
In logistic regression, hospital stay >1 day prior to surgery, knee or hip surgery, diabetes and not receiving gentamicin for perioperative prophylaxis were identified as independent predictors of nephrotoxicity in our study. This is similar to risk factors for perioperative AKI reported in other studies. Elevated body mass index, elevated preoperative SC, history of chronic obstructive pulmonary disease, liver disease, congestive heart failure, hypertension and underlying heart disease were independent risk factors for renal impairment following total joint arthroplasty reported by Jafari et al.14 Operation time, American Society of Anesthesiologists (ASA) class, insulin-dependent diabetes and steroid use for chronic conditions were predictors for surgical complications after spinal fusion and instrumentation in the retrospective study by Tang et al.22 Data on the role of intraoperative risk factors in developing perioperative AKI are conflicting. Several intraoperative management variables (total vasopressor dose administered, use of a vasopressor infusion, diuretic administration) were independent predictors of AKI in the study by Kheterpal et al.27, whereas intraoperative risk factors were not shown to be independent predictors of AKI in the study by Jafari et al.14
A number of limitations are appreciable in our study. We did not evaluate receipt of concurrent nephrotoxic medications (nonsteroidal anti-inflammatory drugs, diuretics, etc.), did not calculate Charlson comorbidity score or ASA score, which can contribute to nephrotoxicity.14,26–28 We also were unable to adjust for intraoperative risk factors (i.e. vasopressors, fluid resuscitation, need for intensive care admission post-surgery and type of anesthesia) because these data were not available electronically for all patients included in our study.
In summary, in this cohort of patients who underwent spine fusion or arthroplasty the rate of nephrotoxicity using a robust assessment by RIFLE criteria was similar between CG and GG. The nephrotoxicity that did occur was multifactorial, and serum creatinine elevations were mild and transient. Our findings suggest that single high dose gentamicin is a safe and acceptable option for perioperative prophylaxis in eligible patients based on age, CrCl and weight.
Acknowledgments
This study was presented in an abstract and poster form at 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: There was no pharmaceutical grant support for this study or outside influence on study concept, design, data analysis and preparation of article.
References
- 1. Brown EM, Pople IK, de Louvois J, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine 2004; 29(8): 938–945. [DOI] [PubMed] [Google Scholar]
- 2. AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7): 915–919. [DOI] [PubMed] [Google Scholar]
- 3. Southwell-Keely JP, Russo RR, March L, et al. Antibiotic prophylaxis in hip fracture surgery: a metaanalysis. Clin Orthop Relat Res 2004; 419: 179–184. [DOI] [PubMed] [Google Scholar]
- 4. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013; 70(3): 195–283. [DOI] [PubMed] [Google Scholar]
- 5. Walters R, Moore R, Fraser R. Penetration of cephazolin in human lumbar intervertebral disc. Spine 2006; 31(5): 567–570. [DOI] [PubMed] [Google Scholar]
- 6. Peel TN, Cheng AC, Buising KL, et al. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother 2012; 56(5): 2386–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine 2013; 38(22): E1425–E1431. [DOI] [PubMed] [Google Scholar]
- 8. Benito N, Franco M, Coll P, et al. Etiology of surgical site infections after primary total joint arthroplasties. J Orthop Res 2014; 32(5): 633–637. [DOI] [PubMed] [Google Scholar]
- 9. Norton TD, Skeete F, Dubrovskaya Y, et al. Orthopedic surgical site infections: analysis of causative bacteria and implications for antibiotic stewardship. Am J Orthop 2014; 43(5): E89–E92. [PubMed] [Google Scholar]
- 10. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis 2013; 20(1): 67–75. [DOI] [PubMed] [Google Scholar]
- 11. Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010; 21(2): 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welten GM, Schouten O, Chonchol M, et al. Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis 2007; 50(2): 219–228. [DOI] [PubMed] [Google Scholar]
- 13. Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25: 2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jafari SM, Huang R, Joshi A, et al. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 Suppl.): 49–53, 53.e1–53.e2. [DOI] [PubMed] [Google Scholar]
- 15. Pavone V, Johnson T, Saulog PS, et al. Perioperative morbidity in bilateral one-stage total knee replacements. Clin Orthop Relat Res 2004; 421: 155–161. [DOI] [PubMed] [Google Scholar]
- 16. ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. American society of health-system pharmacists. Am J Health Syst Pharm 1999; 56(18): 1839–1888. [DOI] [PubMed] [Google Scholar]
- 17. Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39(3): 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bailey TC, Little JR, Littenberg B, et al. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis 1997; 24(5): 786–795. [DOI] [PubMed] [Google Scholar]
- 19. Zelenitsky SA, Silverman RE, Duckworth H, et al. A prospective, randomized, double-blind study of single high dose versus multiple standard dose gentamicin both in combination with metronidazole for colorectal surgical prophylaxis. J Hosp Infect 2000; 46(2): 135–140. [DOI] [PubMed] [Google Scholar]
- 20. Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3): 612–619. [DOI] [PubMed] [Google Scholar]
- 21. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4): R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang H, Zhu J, Ji F, et al. Risk factors for postoperative complication after spinal fusion and instrumentation in degenerative lumbar scoliosis patients. J Orthop Surg Res. 2014; 9(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6): 876–880. [DOI] [PubMed] [Google Scholar]
- 24. Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11): 2037–2042. [DOI] [PubMed] [Google Scholar]
- 25. Loeffler J, Stevens DA. Antifungal drug resistance. Clin Infect Dis 2003; 36(Suppl. 1): S31–S41. [DOI] [PubMed] [Google Scholar]
- 26. Marquez-Lara A, Nandyala SV, Sankaranarayanan S, et al. Body mass index as a predictor of complications and mortality after lumbar spine surgery. Spine 2014; 39(10): 798–804. [DOI] [PubMed] [Google Scholar]
- 27. Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6): 892–902. [DOI] [PubMed] [Google Scholar]
- 28. Abelha FJ, Botelho M, Fernandes V, et al. Determinants of postoperative acute kidney injury. Crit Care 2009; 13(3): R79. [DOI] [PMC free article] [PubMed] [Google Scholar]