Supplemental digital content is available in the text.
Key Words: Endometrial neoplasms, Practice guideline, Consensus, Treatment, Adjuvant, Surgery
Abstract
The first joint European Society for Medical Oncology (ESMO), European SocieTy for Radiotherapy & Oncology (ESTRO) and European Society of Gynaecological Oncology (ESGO) consensus conference on endometrial cancer was held on 11–13 December 2014 in Milan, Italy, and comprised a multidisciplinary panel of 40 leading experts in the management of endometrial cancer. Before the conference, the expert panel prepared three clinically-relevant questions about endometrial cancer relating to the following four areas: prevention and screening, surgery, adjuvant treatment and advanced and recurrent disease. All relevant scientific literature, as identified by the experts, was reviewed in advance. During the consensus conference, the panel developed recommendations for each specific question and a consensus was reached. Results of this consensus conference, together with a summary of evidence supporting each recommendation, are detailed in this article. All participants have approved this final article.
KEY MESSAGE
This ESMO-ESGO-ESTRO consensus conference manuscript was compiled by a multidisciplinary panel of 40 experts
It addresses clinically-relevant questions regarding prevention, screening, surgery, adjuvant therapy and management of advanced/recurrent endometrial cancer, and complements the ESMO clinical practice guidelines
Recommendations provided are accompanied by relevant supporting evidence
INTRODUCTION
Endometrial cancer is the most common gynaecological cancer in developed countries. The number of newly diagnosed cases in Europe was nearly 100,000 in 2012, with an age standardised incidence of 13.6 per 100,000 women. Cumulative risk for a diagnosis of endometrial cancer is 1.71%.1
More than 90% of cases of endometrial cancer occur in women >50 years of age, with a median age at diagnosis of 63 years. However, 4% of women with endometrial cancer are younger than 40 years old,2 many of whom still wish to retain their fertility. The majority of endometrial cancers are diagnosed early (80% in stage I), with five-year survival rates of over 95%. However, five-year survival rates are much lower if there is regional spread or distant disease (68% and 17%, respectively).3
Historically, endometrial carcinoma has been classified into two main clinicopathological and molecular types: Type I is the much more common endometrioid adenocarcinoma (80–90%) and Type II comprises non-endometrioid subtypes such as serous, clear cell and undifferentiated carcinomas, as well as carcinosarcoma/malignant mixed Müllerian tumour (MMMT) (10–20%).4 Molecular data in support of this dichotomous classification have become an integral component of pathologic evaluation, as type I carcinomas are preferentially associated with genetic alterations in PTEN, KRAS, CTNNB1 and PIK3CA and MLH1 promoter hypermethylation, whereas serous carcinomas prototypically harbour TP53 mutations. However, this dualistic model has limitations as considerable molecular heterogeneity exists; for example, 25% of high grade endometrioid carcinomas express mutated TP53 and behave like serous carcinomas.5 Extensive work performed by The Cancer Genome Atlas (TCGA) Research Network has significantly improved our understanding of the molecular landscape of endometrial cancer, introducing not 2, but 4 molecular subtypes including: 1) POLE (ultramutated) tumours, 2) microsatellite unstable tumours, 3) copy-number high tumours with mostly TP53 mutations and 4) remaining group without these alterations.6 Hereditary endometrial adenocarcinomas are mostly seen in families with hereditary non-polyposis colon cancer (HNPCC, Lynch syndrome [LS]). Although the majority of endometrial carcinomas related to LS are Type I cancers, the proportion of Type II cancers seems to be higher than in the case of sporadic endometrial carcinoma.7
Although the majority of cases of endometrial cancer are diagnosed at an early stage, differences in patient characteristics and histopathological features of the disease impact both on patient prognosis and the recommended treatment approach. Given the large body of literature available that addresses the management of endometrial cancer, the aim of this consensus conference was to produce multidisciplinary evidence-based guidelines on selected clinically-relevant questions in order to complement the already-available European Society for Medical Oncology (ESMO) Clinical Practice Guidelines (CPG) for the diagnosis, treatment and follow-up of patients with endometrial cancer.8
METHODS
In 2014, ESMO decided to update the clinical recommendations for endometrial cancer using a consensus conference approach. The consensus panel comprised 40 experts in the management of endometrial cancer, and included representation from the European SocieTy for Radiotherapy & Oncology (ESTRO), the European Society of Gynaecological Oncology (ESGO) and ESMO. Each panel member was assigned to one of four working groups, with a working group chair and co-chair appointed for each group. Three consensus conference chairs (N. Colombo, C. Creutzberg, C. Sessa) were also appointed.
Each working group was assigned a subject area as follows:
Prevention and screening of endometrial cancer (Chair: F. Amant; Co-Chair: T. Bosse)
Surgery (Chair: C Marth; Co-Chair: D. Querleu)
Adjuvant treatment (Chair: R. Nout; Co-Chair: M. Raza Mirza)
Advanced and recurrent disease (Chair: J. Ledermann; Co-Chair: A. González-Martín)
The consensus conference was held on 11–13 December 2014 in Milan, Italy. Prior to this consensus conference, three clinically-relevant questions were identified for each subject area/working group, giving a total of 12 clinically-relevant questions as follows:
Which surveillance should be used for asymptomatic women?
What work-up and management scheme should be undertaken for fertility preserving therapy in patients with atypical hyperplasia (AH)/ endometrial intraepithelial neoplasia (EIN) and grade 1 endometrioid endometrial cancer (EEC)?
Which (molecular) markers can help distinguish (pre)cancerous lesions from benign mimics?
How does the medical condition influence surgical treatment?
What are the indications for and to what extent is lymphadenectomy indicated in the surgical management of endometrial cancer?
How radical should the surgery be in different stages and pathological subtypes of endometrial cancer?
What is the current best definition of risk groups for adjuvant therapy?
What are the best evidence-based adjuvant treatment strategies for patients with low- and intermediate-risk endometrial cancer?
What are the best evidence-based adjuvant treatment strategies for patients with high-risk endometrial cancer?
Does surgery or radiotherapy (RT) have a role in advanced or recurrent endometrial cancer?
What are the optimal systemic therapies for advanced/recurrent disease?
What are the most promising targeted agents and which study designs should be used to evaluate their clinical benefit?
Each working group was responsible for reviewing relevant literature in order to draft preliminary recommendations relating to each of their assigned questions. No systematic literature search was undertaken. During the conference, in parallel sessions, the four working groups discussed and reached agreement on recommendations relating to each of their assigned questions. Recommendations from each group were then presented to the entire panel of experts, where they were discussed and modified as required. An adapted version of the ‘Infectious Diseases Society of America-United States Public Health Service Grading System’ was used (Table 19) to define the level of evidence and strength of each recommendation proposed by the group. Finally, a vote was conducted to determine the level of agreement amongst the expert panel for each of the recommendations. Panel members were allowed to abstain from voting in cases where they either had insufficient expertise to agree/disagree with the recommendation or if they had a conflict of interest that could be considered as influencing their vote.
TABLE 1.
Levels of evidence and grades of recommendation
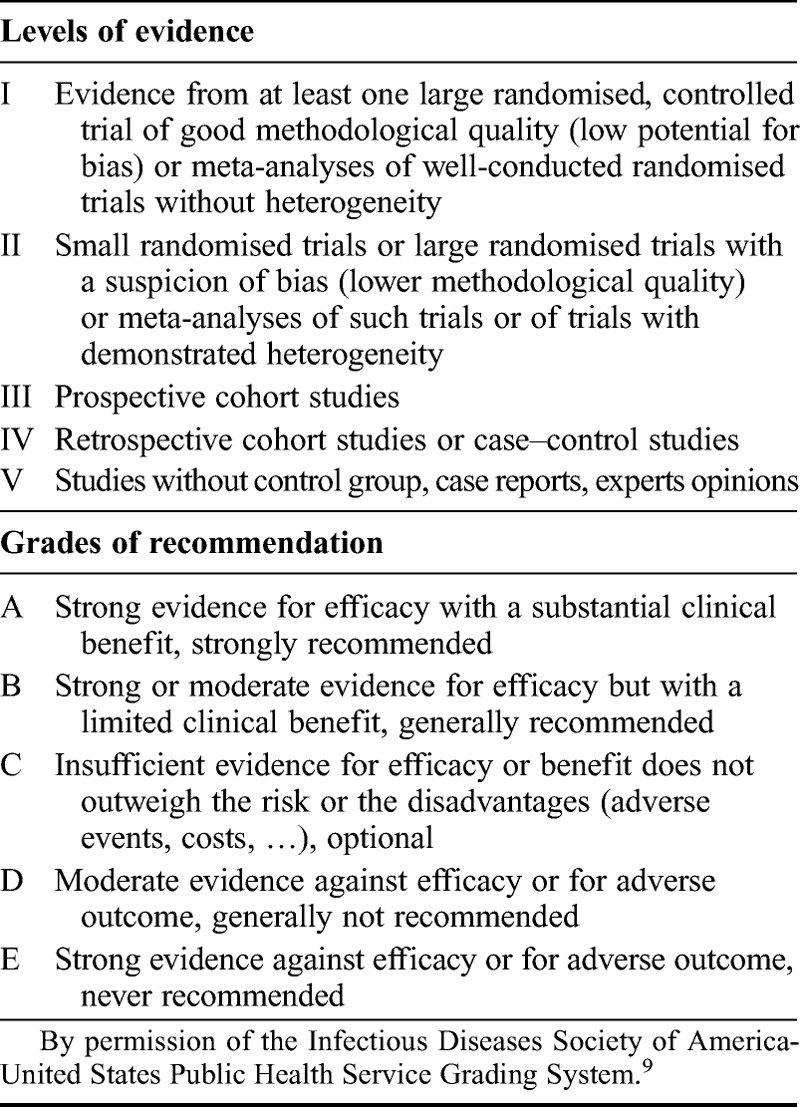
Results of this consensus conference, together with a summary of evidence supporting each recommendation, are detailed in this article, and a summary of all recommendations is included in Supplemental Digital Content, http://links.lww.com/IGC/A344. However, these additional recommendations for specific clinical situations should be read in conjunction with the ESMO CPG for the diagnosis, treatment and follow-up of patients with endometrial cancer.8
RESULTS
PREVENTION AND SCREENING OF ENDOMETRIAL CANCER
Risk Factors for Endometrial Cancer
Most patients with endometrial cancer have an identifiable source of excess oestrogen and typically display a characteristic clinical profile comprising a high body mass index (BMI) that is considered as overweight (BMI 25–30) or obese (BMI 30), often with other components of metabolic syndrome (e.g. hypertension, diabetes). The evidence that greater body fatness (reflected by BMI, measures of abdominal girth and adult weight gain) is a cause of endometrial cancer is convincing. Glycaemic load is probably a cause of endometrial cancer, while the evidence suggesting that sedentary habits (marked by sitting time) and adult attained height are causes of endometrial cancer is limited.10
High BMI correlates with good prognostic features of endometrial cancer, including low tumour grade, endometrioid histology and presentation at early stage. In a small subset of patients, the pathogenesis is related to mismatch repair abnormality and LS. Tumours associated with mismatch repair abnormalities and LS appear to be distinct, with worse prognostic factors and worse clinical outcome.11
According to a recent meta-analysis involving six studies and 3,132 cancer cases, relative risk (RR) for developing endometrial cancer in women with metabolic syndrome is 1.89 (95% confidence interval [CI] 1.34–2.67, P = <0.001). According to individual components of metabolic syndrome, obesity is associated with the greatest increase in RR of 2.21 (P = <0.001).12 The strength of association between obesity and cancer risk increases with increasing BMI: RR for overweight is 1.32 (95% CI 1.16–1.50) and for obesity is 2.54 (95% CI 2.11–3.06).13 Other components of the metabolic syndrome linked to endometrial cancer include hypertension, with a RR of 1.81 (P = 0.024)12 or an odds ratio (OR) of 1.77 (1.34–2.34).14 Hypertriglyceridaemia has a weaker but still significant association (RR 1.17, P <0.001).12
Diabetes mellitus, in particular type II, has long been held as an independent risk factor for endometrial cancer, with an approximate doubling of risk (OR 2.1; 95% CI 1.40–3.41),.14 However, the fact that people with type II diabetes mellitus (T2DM) tend to be obese is a confounding factor, and a recent epidemiological study from the United States questioned the independent role of T2DM as a risk factor for endometrial cancer.15
Nulliparity and infertility are also classical risk factors for endometrial cancer. Among the causes of infertility, polycystic ovarian syndrome (PCOS) seems to be the most important, with an almost 3-fold increase in risk (OR 2.79–2.89).16 However, as with diabetes, obesity seems to be a confounding factor, and the BMI-adjusted OR is lower (2.2; 95% CI 0.9–5.7).17
Other risk factors for endometrial cancer include unopposed oestrogen therapy, oestrogen-producing tumours and early menarche / late menopause. Unopposed oestrogen therapy increases the risk for endometrial cancer 10- to 30-fold if treatment continues 5 years or more.18 Oestrogen-producing tumours, or ovarian granulosa, and theca cell tumours carry an increased risk for endometrial cancer, with up to 20% of women with these tumours reported as having a simultaneous endometrial cancer.19 Both early menarche and late menopause are associated with a 2-fold increased risk for endometrial cancer. The RR is 2.4 for women <12 vs ≥15 years20 and is 1.8 for women ≥55 vs <50 years.21
Studies of women with breast cancer taking tamoxifen with therapeutic or preventive intent have shown that the RR of developing endometrial cancer is 2.53 times higher than that of an age matched population. This risk differs depending on menopausal status. Premenopausal women treated with tamoxifen have no known increased risk of endometrial cancer, whilst this risk in postmenopausal women is 4.0 (95% CI 1.70–10.90).22 The level of risk of endometrial cancer is also dose and time dependent.
LS or HNPCC is an autosomal dominant inherited disorder caused by germline mutations in DNA mismatch repair genes. Women with mutations in MLH1, MSH2, MSH6 or PMS2 have up to a 40–60% lifetime risk of developing both endometrial and colorectal cancers, as well as a 9–12% lifetime risk of developing ovarian cancer.23
Screening and Prevention of Endometrial Cancer
Most cases of endometrial cancer cannot be prevented, but reducing the risk factors and introducing protective factors into the lifestyle whenever possible, may lower the risk of developing this disease.
All women should be told about the risks and symptoms of endometrial cancer and be strongly encouraged to engage in regular physical activity (exercise) and adopt an active lifestyle which can help to attain and maintain a healthy weight as well as lowering the risk of other risk factors for endometrial cancer such as high blood pressure and diabetes. The use of combined oral contraceptives is significantly associated with a decrease in endometrial cancer in ever users, a benefit that is greater with increasing duration of use.
1. Which Surveillance Should be Used for Asymptomatic Women?
Women With Average Risk for Endometrial Cancer
There is no indication that population-based screening has a role in the early detection of endometrial cancer among women who are at average endometrial cancer risk and have no symptoms. There is also no standard or routine screening test for endometrial cancer. Screening of asymptomatic women for endometrial carcinoma has in general been recommended only for those with LS.24,25
There is no evidence that screening by ultrasonography (e.g. endovaginal or transvaginal ultrasound) reduces mortality from endometrial cancer. Moreover, cohort studies indicate that screening asymptomatic women will result in unnecessary additional biopsies because of false-positive test results. Risks associated with false-positive tests include anxiety and complications from biopsies.26
At the time of menopause, women should be strongly encouraged to report any vaginal bleeding, discharge or spotting to their doctor to ensure they receive appropriate treatment of any precancerous disorders of the endometrium.
Recommendation 1.1: There is no evidence for endometrial cancer screening in the general population
Level of evidence: II
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Women at Increased Risk for Endometrial Cancer
Women at increased risk for endometrial cancer due to a history of unopposed oestrogen therapy, late menopause, tamoxifen therapy, nulliparity, infertility or failure to ovulate, obesity, diabetes or hypertension should be informed of the risks and symptoms of endometrial cancer and strongly encouraged to report any unexpected bleeding or spotting to their physicians.
Asymptomatic women with risk factors for endometrial cancer who have endometrial thickening and other positive findings on ultrasound, such as increased vascularity, inhomogeneity of the endometrium, particulate fluid or thickened endometrium over 11 mm should be managed on a case-by-case basis. The potential benefits, risks and limitations of testing for early endometrial cancer should be explained in order to ensure informed decision-making about testing.
Premenopausal women treated with tamoxifen do not require additional monitoring beyond routine gynaecological care. Postmenopausal women taking tamoxifen should be informed about symptoms of endometrial hyperplasia or cancer.27
Although findings from a recently published meta-analysis have verified the efficacy of the levonorgestrel intrauterine device (LNG-IUD) in preventing de novo polyps in breast cancer patients treated with tamoxifen, there was insufficient evidence to ascertain whether the LNG-IUD was associated with any benefit in reducing the incidence of precancerous or cancerous lesions.28
Recommendation 1.2: Unopposed oestrogen treatment should not be started or should be discontinued in women with a uterus in situ
Level of evidence: III
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 1.3: Routine surveillance in asymptomatic women with obesity, PCOS, diabetes mellitus, infertility, nulliparity or late menopause is not recommended
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 1.4: For women with adult granulosa cell tumour (AGCT), if hysterectomy has not been performed, endometrial sampling is recommended. If this shows no evidence of (pre)malignancy, no further screening for endometrial malignancies is required
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 1.5: In patients with epithelial ovarian cancer undergoing fertility sparing treatment, endometrial sampling is recommended at the time of diagnosis
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 1.6: Routine screening for endometrial cancer in asymptomatic tamoxifen users is not recommended
Level of evidence: III
Strength of recommendation: B
Consensus: 94.6% (35) yes, 5.4% (2) abstain (37 voters)
Women With High Risk for Endometrial Cancer
Women with a high risk for endometrial cancer include known carriers of HNPCC-associated genetic mutations, those who have a substantial likelihood of being a mutation carrier (i.e. a mutation is known to be present in the family), and women without genetic testing results but who are from families with a suspected autosomal dominant predisposition to colon cancer.
Findings from a prospective observational cohort study of women with LS opting for endometrial cancer screening and who underwent annual outpatient hysteroscopy and endometrial sampling (OHES) suggest that in women with LS, annual OHES is acceptable and has high diagnostic accuracy in screening for endometrial cancer and atypical endometrial hyperplasia (AEH).29 However, larger international studies are needed for confirmation.
Women with an HNPCC-associated mutation or with a substantial likelihood of having an HNPCC-associated mutation should be informed of the potential benefits, risks and limitations of testing for early endometrial cancer; they should also be informed that the recommendation for screening is based on expert opinion in the absence of definitive scientific evidence.
Although there is insufficient evidence to endorse annual screening for endometrial cancer in this group, annual screening beginning at age 35 is recommended due to the high risk of endometrial cancer and the potentially life-threatening nature of this disease. As screening will be of limited efficacy in gynaecological cancers (endometrial and ovarian), once the family is completed, particularly by age 35–40 years, careful consideration must be given to the option of prophylactic hysterectomy and bilateral salpingo-oophorectomy.30
In women with LS, the following options are available:
Annual screening beginning at age 35 (recommended)
Regular hysteroscopy and endometrial biopsies or hysterectomy (current options)
The application of local progesterone using the LNG-IUD
Treatment of premalignant disease (AEH, EIN)
Hysterectomy and bilateral oophorectomy
Evaluating the likelihood of a patient having a gynaecological cancer predisposition syndrome enables the physician to provide individualised assessments of cancer risk, as well as the opportunity to offer tailored screening and prevention strategies such as surveillance, chemoprevention and prophylactic surgery that may reduce the morbidity and mortality associated with these syndromes.
Recommendation 1.7: Surveillance of the endometrium by gynaecological examination, transvaginal ultrasound and aspiration biopsy starting from the age of 35 years (annually until hysterectomy) should be offered to all LS mutation carriers
Level of evidence: IV
Strength of recommendation: B
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Recommendation 1.8: Prophylactic surgery (hysterectomy and bilateral salpingo-oophorectomy), preferably using a minimally invasive approach, should be discussed at the age of 40 as an option for LS mutation carriers to prevent endometrial and ovarian cancer. All pros and cons of prophylactic surgery must be discussed
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
2. What Work-up and Management Scheme Should be Undertaken for Fertility Preserving Therapy in Patients With AH/EIN and Grade 1 EEC?
Work-up for Fertility Preserving Therapy
The diagnosis of endometrial carcinoma in young women of childbearing age is rare. Indeed, only 4% of patients with endometrial carcinoma are less than 40 years of age.2 Younger and premenopausal women with endometrial carcinoma seem to have a better prognosis than older patients, with increased rates of early stage and low grade disease reported.2,31,32
The standard approach for the management of endometrial cancer in young women of childbearing age is hysterectomy and bilateral salpingo-ophorectomy with or without lymphadenectomy. Although this is a highly effective approach, carrying a 5-year survival rate of 93%, it also results in a permanent loss of reproductive potential. Conservative management of endometrial carcinoma is based on medical treatment with oral progestins. The most important issues when considering a conservative management approach are the assessment of clinical and pathological characteristics of the tumour and selection of the appropriate medical intervention.
A conservative management approach could be considered in patients with a histological diagnosis of grade 1 endometrial carcinoma (or premalignant disease such as AH).31 The optimal method to obtain these histologic characteristics is dilatation and curettage (D&C)33; this procedure is superior to pipelle biopsy in terms of accuracy of the tumour grade.34
The histological diagnosis should be reviewed by an expert pathologist to improve the accuracy of histological assessment (endometrial carcinoma or AH) and the reliability of tumour grading,35 whereas the initial stage should be confirmed by enhanced pelvic magnetic resonance imaging (MRI) to exclude overt myometrial invasion, as well as adnexal or pelvic nodes involvement.36 Patients should be informed that this is a non-standard approach and they should be willing to accept close follow-up during and after the treatment. They should also be informed of the need for future hysterectomy in case of failure of the treatment and/or after pregnancies.
Recommendation 2.1: Patients with AH/EIN or grade 1 EEC requesting fertility preserving therapy must be referred to specialised centres
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 2.2: In these patients, D&C with or without hysteroscopy must be performed
Level of evidence: IV
Strength of recommendation: A
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Recommendation 2.3: AH/EIN or grade 1 EEC must be confirmed/diagnosed by a specialist gynaecopathologist
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 2.4: Pelvic MRI should be performed to exclude overt myometrial invasion and adnexal involvement. Expert ultrasound can be considered as an alternative
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 2.5: Patients must be informed that fertility-sparing treatment is a non-standard treatment and the pros and cons must be discussed. Patients should be willing to accept close follow-up and be informed of the need for future hysterectomy
Level of evidence: V
Strength of recommendation: A
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Management Schemes for Fertility-Preserving Therapy
Conservative medical treatment for endometrial cancer is based on progestins with medroxyprogesterone acetate (MPA; 400–600 mg/day) or megestrol acetate (MA; 160–320 mg/day).33 Few papers have addressed the use of LNG-IUD but preliminary data using such treatment (added to gonadotropin-releasing hormone [GnRH] analogues) seem to demonstrate similar remission and recurrence rates as oral progestins.37 Assessment of response must be performed at 6 months with a new D&C and imaging.38
Response rates associated with the conservative management of endometrial carcinoma are around 75%,39,40 but recurrence rates are 30–40%.39,41,42 Standard surgery with hysterectomy should be proposed to non-responders while maintenance treatment for a further 6 months can be considered in responders who wish to delay pregnancy.33
Although progesterone receptor (PgR) status is a reliable predictive factor for disease remission, a routine check is not recommended since 50% of PgR negative patients will respond to treatment.43
Pregnancy is associated with a reduced risk for endometrial cancer recurrence.40 Findings from recent meta-analyses showed that the pooled live birth rate among women receiving fertility-preserving treatment for endometrial cancer was 28%, and reached 39% when assisted reproduction technology was used.39,44 Thus, for patients achieving a complete response at 6 months, conception must be encouraged and these patients should be referred to a fertility clinic.
For patients with disease recurrence after an initial response, hysterectomy should be proposed as the first option. Moreover, given the high rate of recurrence, after completion of childbearing (or after the age of potential pregnancy), standard treatment with hysterectomy and salpingo-oophorectomy is recommended. Preservation of the ovaries can be considered in selected cases, depending on the patient’s age and genetic risk factors.
Recommendation 2.6: For patients undergoing fertility-preserving therapy, MPA (400–600 mg/day) or MA (160–320 mg/day) is the recommended treatment. However, treatment with LNG-IUD with or without GnRH-analogues can also be considered
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 2.7: In order to assess response, D&C, hysteroscopy and imaging at 6 months must be performed. If no response is achieved after 6 months, standard surgical treatment should be performed
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 2.8: In case of complete response, conception must be encouraged and referral to a fertility clinic is recommended
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 2.9: Maintenance treatment should be considered in responders who wish to delay pregnancy
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 2.10: Patients not undergoing hysterectomy should be re-evaluated clinically every 6 months
Level of evidence: IV
Strength of recommendation: B
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Recommendation 2.11: After completion of childbearing, a hysterectomy and salpingo-oophorectomy should be recommended. The preservation of the ovaries can be considered depending on age and genetic risk factors
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
3. Which (molecular) Markers Can Help Distinguish (pre)Cancerous Lesions from Benign Mimics?
Differential diagnosis between benign uterine lesions and endometrial (pre)carcinomas is based mainly on morphological criteria but may be supported by additional immunohistochemical (IHC) markers and molecular alterations in problematic cases.45
Currently, AH/EIN is the preferred terminology of the precursor lesion of the most common type of endometrial carcinoma, endometrioid carcinoma, including its variants.
Recommendation 3.1: In case of uncertainty low threshold referral to a specialised gynaecopathologist is recommended
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (37 voters)
The differential diagnosis of AH/EIN includes, in particular, endometrial hyperplasia without atypia, but also includes other mimics, such as glandular and stromal breakdown, focal glandular crowding and epithelial metaplasias (e.g. hypersecretory changes). Loss of PTEN expression, mostly by mutations, and loss of PAX-2 by downregulation46–48 are the only immunohistochemical markers that have been sufficiently studied and can be used on curettage material. Loss of PTEN occurs in 40–50% of AH/EIN cases, whereas loss of PAX-2 occurs in 70% of AH/EIN, and a joint loss of PTEN and PAX-2 occurs in around 30% of AH/EIN.49–51
Recommendation 3.2: PTEN and PAX-2 IHC is recommended to distinguish AH/EIN from benign mimics. Other markers that can be used in this context are MLH1 and ARID1a by IHC
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Another histological entity that may arise in the differential diagnosis of AH/EIN is the rare atypical polypoid adenomyoma (APA), for which there are no IHC stains with practical value.
Recommendation 3.3: IHC is not recommended to distinguish APA from AH/EIN
Level of evidence: V
Strength of recommendation: B
Consensus: 100% yes (37 voters)
The putative precursor of serous carcinoma, serous endometrial intraepithelial carcinoma (SEIC), is considered a non-invasive cancer rather than a precancer since it may be associated with extensive extrauterine disease.9 Molecular alterations of serous carcinoma are already present in SEIC, which is especially true for p53 expression.52–54 A completely negative immunoreactive pattern for p53 (‘all or null’) is considered a surrogate for p53 mutation, and is present in almost all SEIC and invasive serous carcinomas.55
Recommendation 3.4: p53 by IHC is recommended to distinguish SEIC from its mimics
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
In selected cases of endometrial cancer, clinical and radiological work-up may not be conclusive about the endometrial origin of the uterine tumour. In addition, endocervical, ovarian and endometrial adenocarcinomas may show histopathological overlap. Several IHC markers have been proposed for these differential diagnoses, but these markers lack sensitivity or specificity to be used as single markers. When endocervical origin is considered, the use of a panel of markers, including carcinoembryonic antigen (CEA), vimentin, oestrogen receptor (ER) and p16 (as surrogate for human papilloma virus [HPV]), is recommended.56 In case of p16 positivity, the staining pattern should be taken into account. Diffuse p16 staining is frequently seen in serous, clear cell and mucinous carcinoma endometrial cancers.57,58 In cases of scanty tissue with serous carcinoma, an ovarian origin of the serous carcinoma should be considered. The most discriminatory marker for this differential diagnosis is Wilms tumour 1 gene (WT-1),59 which is expressed in 80–100% of high-grade serous carcinomas of the ovary60,61 compared with 7–20% in serous endometrial carcinomas.62,63 In general, the expression profile should be interpreted in the context of the morphological subtype. An individual approach, with close correlation between clinical presentation and morphological subtype, is therefore recommended.
Recommendation 3.5: A panel of markers must be used in cases where endocervical cancer is suspected. This panel should include at least ER, vimentin, CEA and p16 by IHC, and needs to be assessed in the histologic and clinical context. In addition, HPV analysis can be considered
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 3.6: WT-1 by IHC is the recommended marker to determine the origin of serous cancer
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 3.7: Morphology (and not IHC) should be used to distinguish AH/EIN from EEC
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
SURGERY
4. How Does the Medical Condition Influence Surgical Treatment?
Mandatory Pre-operative Work-up
The consensus is based on current clinical practice. Family history is usually taken to identify risk factors associated with LS including endometrial cancer, colon cancer and other cancers belonging to the Lynch spectrum. General assessment and, if appropriate, geriatric assessment is required in patients with comorbidities and elderly patients, respectively, in order to adapt the surgical strategy. Indeed, endometrial cancer is frequently associated with obesity, hypertension and diabetes, and in some patients, the extent of surgery or staging that is theoretically required may not be feasible. In such cases, a benefit-risk assessment of surgery may lead to an individualised decision to perform a ‘non-standard’ surgery or a limited staging procedure.
Pelvic examination and pelvic ultrasonography are mandatory components of clinical staging of endometrial cancer in order to establish a tentative International Federation of Gynecology and Obstetrics (FIGO) staging before definitive pathology. In addition to being the first imaging technique used to evaluate abnormal uterine bleeding, ultrasonography, preferably specialised ultrasonography,64 offers the possibility of evaluating the size of the tumour, ruling out ovarian disease, and assessing myometrial invasion and cervical stromal involvement.65
Pre-operative pathological information is crucial for establishing the surgical plan. First, all patients with a risk of cancer, particularly patients with postmenopausal bleeding and a hyperplastic endometrium at ultrasound, should be investigated with endometrial biopsy or curettage in order to (1) avoid uterine morcellation, which poses a risk of spreading unsuspected cancerous tissue, notably endometrial carcinomas or sarcomas, beyond the uterus and may make the pathological assessment of myometrial invasion extremely difficult; and (2) prevent the discovery of an unexpected malignancy after inadequate surgery (subtotal hysterectomy and/or preservation of the ovaries in a postmenopausal patient, incomplete staging). Second, as grading of EEC has a significant prognostic impact66 and various histotypes of endometrial cancer harbour different natural histories, the primary therapeutic strategy must be adapted to the information provided by a pre-operative pathological examination, despite the fact that discrepancies between pre-operative evaluation and final pathology exist.67
The final therapeutic strategy should be adapted according to the information available before surgery, taking into account the tentative stage (apparent stage I or more advanced stage), grade (of endometrioid tumours; grade 1–3 or a binary system) and histotype (endometrioid versus non-endometrioid tumour).
Recommendation 4.1: Mandatory work-up must include: Family history; general assessment and inventory of comorbidities; geriatric assessment, if appropriate; clinical examination, including pelvic examination; transvaginal or transrectal ultrasound; and complete pathology assessment (histotype and grade) of an endometrial biopsy or curettage specimen
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 4.2: Extent of surgery should be adapted to the medical condition of the patient
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Optional Pre-operative Work-up
Imaging
Additional imaging is considered according to the clinical situation. Computed tomography (CT) scan and/or positron emission tomography (PET)-CT are options in clinically advanced endometrial cancer. In apparent stage I endometrial cancer, MRI may be useful to complete information regarding myometrial invasion.65 However, this applies only in institutions where the indication for lymph node dissection (LND) is tailored according to the stratification of low-, intermediate- and high-risk groups. In this setting, specialised ultrasonography and/or intra-operative pathological examination of the uterus may also be considered.68
Recommendation 4.3: In clinical stage I, grade 1 and 2: At least one of the three following tools should be used to assess myometrial invasion if LND is considered: Expert ultrasound and/or MRI and/or intra-operative pathological examination
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 4.4: Other imaging methods (thoracic, abdominal and pelvic CT scan, MRI, PET scan or ultrasound) should be considered to assess ovarian, nodal, peritoneal or metastatic disease
Level of evidence: IV
Strength of recommendation: C
Consensus: 94.6% (35) yes, 2.7% (1) abstain, 2.7% (1) no (37 voters)
Serum Tumour Markers
There is evidence that the serum tumour markers cancer antigen 125 (CA-125) and, more recently, human epididymis protein 4 (HE-4), are significantly correlated with histological grade, stage, lymph node metastases, myometrial invasion and cervical involvement.69–71 However, the appropriate cut-off has not been established and evidence that serum marker assessment is clinically useful is lacking.
Recommendation 4.5: There is no evidence for the clinical usefulness of serum tumour markers, including CA-125
Level of evidence: IV
Strength of recommendation: B
Consensus: 91.9% (34) yes, 5.4% (2) abstain, 2.7% (1) no (37 voters)
Surgical Management of Apparent Stage I Endometrial Cancer
With the exception of patients managed conservatively, extrafascial total hysterectomy without colpectomy is the mainstay of management for patients with endometrial cancer. The rationale for the additional removal of the adnexae is to prevent ovarian cancer and rule out ovarian metastases. In premenopausal patients, however, ovarian preservation may be discussed in selected cases. Younger patients with endometrial cancer often have early stage, low grade tumours. Thus, to avoid the short-term and long-term consequences of surgical menopause, there is a rationale for ovarian preservation in young women. Several retrospective studies have recently provided evidence that ovarian preservation has no statistically significant impact on the overall survival (OS) of young patients with early-stage endometrial cancer.72 However, extreme care must be taken to rule out synchronous concomitant ovarian malignancy.
Recommendation 4.6: Standard surgery is total hysterectomy with bilateral salpingo-oophorectomy without vaginal cuff
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 4.7: Ovarian preservation can be considered in patients younger than 45 years old with grade 1 EEC with myometrial invasion <50% and no obvious ovarian or other extrauterine disease
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 4.8: In cases of ovarian preservation, salpingectomy is recommended
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 4.9: Ovarian preservation is not recommended for patients with cancer family history involving ovarian cancer risk (e.g. BRCA mutation, LS, etc.). Genetic counselling/testing should be offered
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Minimally Invasive Surgical Techniques
Hysterectomy and bilateral salpingo-oophorectomy can be carried out using the open, laparoscopic or vaginal approach.
The largest randomised trial comparing laparoscopy to laparotomy is the LAP2 study,73 which was designed to compare laparoscopy versus laparotomy for comprehensive surgical staging and management of stage I–IIA uterine cancer, including hysterectomy, salpingo-oophorectomy, pelvic cytology and pelvic and para-aortic lymphadenectomy. In this trial, patients were randomly assigned to laparoscopy (n = 1,696) or open laparotomy (n = 920). A significantly longer operative time was reported for the laparoscopy group compared with the laparotomy group (204 vs 130 minutes, respectively). Intra-operative complication rates were similar between groups. However, laparoscopy was associated with significantly fewer moderate to severe post-operative adverse events (14% vs 21%) and a lower frequency of hospitalisations of more than 2 days (52% v 94%) than laparotomy. Although pelvic and para-aortic lymph nodes were not removed in 8% and 4% of patients in the laparoscopy and laparotomy groups, respectively (P <0.0001), there was no difference in the overall detection of advanced stage disease between the two groups. The major shortcoming of this trial is the high conversion rate related to its multicentric design. Indeed, 25.8% of patients assigned to the laparoscopic group were converted to laparotomy, with a statement of ‘poor visibility’ reported in 14.6% of cases, reflecting the learning curve of some investigators, particularly for LND. In contrast, a conversion rate of 10.8%, with poor visibility recorded as the main factor in 4.9% of cases, was reported in a Dutch randomised trial in which no lymphadenectomy was performed.74 However, as further training or the use of robotic assistance would likely have resulted in even better results with laparoscopic surgery, this high conversion rate reported in LAP2 does not weaken the authors’ conclusions, and this trial provides evidence that laparoscopic surgical staging for uterine cancer results in fewer complications and shorter hospital stay.
According to a meta-analysis of data from eight randomised controlled trials (RCTs) conducted by Zullo et al.,75 intra-operative complication rates were not different between laparoscopy and laparotomy (RR 1.25; 95% CI 0.99–1.56) with no significant heterogeneity across the studies. Estimated blood loss and haemoglobin or haematocrit changes were consistently less after laparoscopy in the six studies where this was reported. Operative time was higher by 34–74 minutes in the laparoscopy group. The authors also found a significant advantage of laparoscopy over laparotomy in terms of post-operative complications (RR 0.71; 95% CI 0.63–0.79) with significant heterogeneity across the studies.
Aortic dissection can also be achieved in obese patients using an extraperitoneal laparoscopic approach.76
Taken together, these findings provide definitive evidence of the short-term benefit and cost-effectiveness of laparoscopic hysterectomy in patients with gynaecological cancer. This includes patients with comorbidities, obesity or advanced age. Regarding comorbidity, Tozzi et al.77 found that the surgical technique is the only significant parameter associated with complication rate, regardless of risk group, stressing the fact that patients with serious comorbidities benefit most from laparoscopy. The issue of advanced age has also been addressed in the gynaecological oncology literature. Siesto et al.78 reported outcomes from a series of 48 patients aged >65 years who had undergone laparoscopic surgery for endometrial cancer. Outcomes from this group were comparable to younger patients in terms of operative time, blood loss, need for blood transfusions, nodal count and intra-operative and post-operative complications. The authors conclude that in the absence of absolute anaesthesia contraindications, laparoscopy is feasible and safe in older women with endometrial cancer. However, as cancer in older women was more frequently upstaged than in younger women, they state that comprehensive surgical staging should be offered, regardless of age, to avoid understaging and to optimise treatment strategies.
Six randomised trials comparing outcomes after laparotomy versus laparoscopy are currently available, four of which have been included in a published metanalysis.79 However, only two of these four trials reported data for OS, disease-free survival and cancer-related survival. Based on the availability of new data, this meta-analysis was subsequently updated by Palomba et al. in 200980 to include a third trial reporting these long-term outcomes, resulting in a sample of 359 patients. No significant heterogeneity was observed among these trials and there was no significant adverse effect of a laparoscopic approach on the OS, disease-free survival or cancer-related survival (OR 0.96, 0.95 and 0.91, respectively).
Long-term outcomes of the randomised controlled LAP2 trial were published in 2012.81 The primary endpoint was non-inferiority of the recurrence-free interval. Non-inferiority was defined as no more than a 40% increase in the risk of recurrence with laparoscopy compared with laparotomy. The estimated hazard ratio (HR) for recurrence-free survival with laparoscopy versus laparotomy was 1.14 (90% CI 0.92–1.46). Actual recurrence rates were substantially lower than anticipated; the estimated 3-year recurrence rate was 11.4% with laparoscopy and 10.2% with laparotomy, and the estimated 5-year OS was almost identical in both arms (89.8%).
Recommendation 4.10: Minimally invasive surgery is recommended in the surgical management of low-and intermediate-risk endometrial cancer
Level of evidence: I
Strength of recommendation: A
Consensus: 100% yes (37 voters)
In a retrospective, multi-institutional trial of patients with high grade endometrial cancer, outcomes of 191 patients who underwent laparotomy were compared with 192 patients who underwent minimal invasive surgery. In this trial, women with high grade endometrial cancer staged by minimally invasive techniques experienced fewer complications and similar survival outcomes compared with those staged by laparotomy.82
Recommendation 4.11: Minimally invasive surgery can be considered in the management of high-risk endometrial cancer
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Alternative Approaches for Patients Unsuitable for Standard Surgical Therapy
Although advances in surgical techniques, anaesthesiology and peri-operative management mean that the vast majority of patients with endometrial cancer are amenable to standard surgical therapy, a small proportion of patients are still medically unfit for laparoscopic surgery or laparotomy. However, these patients can still be managed either surgically by vaginal hysterectomy, whenever possible, with bilateral salpingo-oophorectomy, or by definitive RT, combining external beam radiation therapy (EBRT) and brachytherapy, or by hormonal treatment. In addition, vaginal hysterectomy is an acceptable minimally invasive surgical option in some low-risk patients who do not need LND (see section 4).
Recommendation 4.12: Vaginal hysterectomy with salpingo-oophorectomy can be considered in patients unfit for the recommended surgery and in selected patients with low-risk endometrial cancer
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Recommendation 4.13: In medically unfit patients, RT or hormone treatment can be considered
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
5. What are the Indications for and to What Extent is Lymphadenectomy Indicated in the Surgical Management of Endometrial Cancer?
Surgical Staging in Apparent Stage I EEC
Collection of peritoneal cytology was included as a staging procedure in earlier recommendations, but it is no longer considered mandatory. However, since retrospective studies indicate that positive peritoneal cytology has prognostic value, collection of this information could be considered, especially in patients with tumours of non-endometrioid histology.83,84
Recommendation 5.1: Peritoneal cytology is no longer considered mandatory for staging
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Lymphadenectomy
Lymphadenectomy is an integral part of the comprehensive surgical staging of endometrial cancer. However, the role of lymphadenectomy in early endometrial cancer is unclear and controversy remains regarding the indications for, the anatomic extent of, and the therapeutic value of lymphadenectomy in the management of the disease.
The definition of an adequate lymphadenectomy has not been standardised: current approaches include pelvic lymphadenectomy, para-aortic lymphadenectomy to the inferior mesenteric artery (IMA) and para-aortic lymphadenectomy up to the renal vessels. Lymph node counts have become a marker for adequacy of lymph node evaluation in a variety of solid tumour disease sites. In endometrial cancer, two retrospective reviews have shown that patients had improved survival when at least 10 to 12 lymph nodes were removed during lymphadenectomy.85,86 Lymph node counts therefore provide a surrogate way of measuring the adequacy of a LND and, as such, more than 10 nodes should be removed.87,88
Sampling of lymph nodes has a low sensitivity in endometrial cancer.89 Indeed, it has been shown that para-aortic nodes may be positive in the absence of positive pelvic nodes,90,91 suggesting that para-aortic lymph nodes should be removed in cases where a lymphadenectomy is indicated. In the Mayo Clinic experience of 281 patients with endometrial cancer who underwent lymphadenectomy, 22% of patients with high-risk disease had lymph node metastases: 51% had both positive pelvic and para-aortic nodes, 33% had positive pelvic lymph nodes only, and 16% had isolated para-aortic lymphadenopathy.92 As the majority (77%) of patients with para-aortic lymph node involvement had metastases above the IMA, para-aortic lymphadenectomy up to the renal vessels is recommended.
The concept of sentinel lymph node (SLN) dissection (SLND) was first developed in cervical cancer as a tool to select patients most suitable for surgical management. In low- and intermediate-risk endometrial cancer, the rationale is different as the need for SLND is controversial. However, SLND could represent a compromise between no dissection (leaving a small proportion of node positive patients) and full dissection (adding a useless procedure for the majority of node-negative patients). In addition, ultrastaging of the sentinel lymph nodes (SLNs) detects micrometastases otherwise undiagnosed by conventional histology, even in patients considered at low risk, on the basis of grade and depth myometrial invasion.93 However, these large series only use the cervix as the injection site. The question of alternative injection sites in the endometrium or uterine fundus, which are anatomically more logical, is still a topic for investigation. Injection under hysteroscopic, ultrasound, laparoscopic or open guidance in patients with endometrial cancer has been addressed, without evidence of benefit of the more demanding and less practical modalities. Nevertheless, evidence is accumulating that the SLND may be useful in the management of endometrial cancers.94
Recommendation 5.2: If a lymphadenectomy is performed, systematic removal of pelvic and para-aortic nodes up to the level of the renal veins should be considered
Level of evidence: IV
Strength of recommendation: B
Consensus: 91.9% (34) yes, 2.7% (1) abstain, 5.4% (2) no (37 voters)
Recommendation 5.3: SLND is still experimental, but large series suggest that it is feasible. SLND increases the detection of lymph nodes with small metastases and isolated tumour cells; however, the importance of these findings is unclear
Level of evidence: IV
Strength of recommendation: D
Consensus: 100% yes (37 voters)
Indications for Lymphadenectomy
Although the therapeutic effect of lymphadenectomy is unclear, it is an integral part of comprehensive staging. The advantages of comprehensive surgical staging are a better definition of prognosis and appropriate triage of patients for adjuvant therapy.
Data from two RCTs do not support the therapeutic benefit of lymphadenectomy in early stage endometrial cancer. Benedetti-Panici et al. randomised 514 women with clinical stage I endometrial cancer to either systematic pelvic lymphadenectomy or no LND and found no improvement in disease-free survival or OS between the two groups.95 Similarly, the ASTEC trial, which included 1408 patients with stage I endometrial cancer who were randomised to receive surgical staging with or without pelvic lymphadenectomy, failed to show a beneficial effect of lymphadenectomy.96 Although these trials represent the best data available, controversy still exists, partly due to criticisms of the ASTEC trial, in which the number of lymph nodes removed was low and systematic para-aortic lymphadenectomy was not performed. A mathematical model applied to the ASTEC trial suggested a survival difference of less than 2% between the experimental and control arms under all circumstances.97 This model suggested that even if LND was therapeutic, this trial would have been negative due to the trial design. In the Italian trial,95 median node counts were 26, or 30 for the 26% of patients who also had para-aortic lymphadenectomy, and there were no differences in relapse rates, disease-free survival and OS.
In contrast, retrospective data, which are prone to selection bias and stage migration, suggest that patients who underwent systematic lymphadenectomy had improved survival over those who had limited or no sampling performed.88 Data from 42,184 patients with endometrial cancer, obtained from the Surveillance, Epidemiology, and End Results Program of the US National Cancer Institute for the years 1988–2003, showed that the average frequency of LND was 31%, 40%, 47% and 53% for the years 1988–1991, 1992–1995, 1996–1999 and 2000–2003, respectively (P <0.0001).98 On multivariate analysis, the presence of LND was associated with overall and uterine-specific survival benefits with HRs of 0.81 (P <0.0001) and 0.78 (P <0.0001), respectively, and removal of >11 lymph nodes was associated with HRs of 0.74 (P <0.0001) and 0.69 (P <0.0001), respectively. On the basis of these findings, the authors concluded that the presence of LND and increased number of nodes dissected predicted for improved OS and uterine-specific survival in women with adenocarcinoma of the endometrium.
Retrospective single institution studies advocate lymphadenectomy for all grades of tumour.87,88 In contrast, a series using a US database supports lymphadenectomy for high-grade tumours only.99 This was confirmed by the SEPAL trial in a series of intermediate- or high-risk patients with pelvic lymphadenectomy with or without para-aortic LND.100 Patients who underwent para-aortic lymphadenectomy had a superior survival compared with those who did not.
In addition to risk factors, the number of lymph nodes removed also seems to be important, with higher node count associated with improved survival.85,101 Kim et al. recently analysed data from nine trials (two RCTs and seven observational studies) involving 16,995 patients with endometrial cancer, and showed that the efficacy of systematic lymphadenectomy, defined as removal of ≥10–11 lymph nodes, was associated with limited survival benefit in patients with low-risk endometrial cancer, but resulted in improved OS in patients with intermediate- or high-risk endometrial cancer.102 However, patients with low-risk disease (i.e., grade 1 and 2 endometrioid lesions with <50% myometrial invasion) have a very low probability of lymphadenopathy and therefore derive no benefit from a systematic lymphadenectomy.103
Recommendation 5.4: Lymphadenectomy is a staging procedure and allows tailoring of adjuvant therapy
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 5.5: Patients with low-risk endometrioid carcinoma (grade 1 or 2 and superficial myometrial invasion <50%) have a low risk of lymph node involvement, and two RCTs did not show a survival benefit. Therefore, lymphadenectomy is not recommended for these patients
Level of evidence: II
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 5.6: For patients with intermediate risk (deep myometrial invasion >50% or grade 3 superficial myometrial invasion <50%), data have not shown a survival benefit. Lymphadenectomy can be considered for staging purposes in these patients
Level of evidence: II
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Recommendation 5.7: For patients with high risk (grade 3 with deep myometrial invasion >50%), lymphadenectomy should be recommended
Level of evidence: IV
Strength of recommendation: B
Consensus: 73.0% (27) yes, 8.1% (3) abstain, 18.9% (7) no (37 voters)
Recommendation 5.8: Lymphadenectomy to complete staging could be considered in previously incompletely operated high-risk patients to tailor adjuvant therapy
Level of evidence: V
Strength of recommendation: C
Consensus: 100% yes (37 voters)
6. How Radical Should the Surgery be in Different Stages and Pathological Subtypes of Endometrial Cancer?
Surgical Management of Stage II–IV Endometrial Cancer
In a recent study from Japan, radical surgery in stage II endometrial cancer did not result in any survival benefit compared with simple hysterectomy but was associated with more peri-operative and late adverse events.104 Another recent study found that parametrial spread cannot be predicted by cervical involvement alone but may be predicted by various lymphovascular space invasion (LVSI)-related histopathological factors.105 However, radical hysterectomy is considered in cases of obvious involvement of the parametrium. Surgery should then be tailored according to the recent classification of radical hysterectomy106 in order to obtain free margins. Lymphadenectomy is recommended.
Recommendation 6.1: Radical hysterectomy is not recommended for the management of stage II endometrial cancer
Level of evidence: IV
Strength of recommendation: B
Consensus: 91.9% (34) yes, 8.1% (3) abstain (37 voters)
Recommendation 6.2: Modified (type B) or type A radical hysterectomy should be considered only if required for obtaining free margins
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 6.3: Lymphadenectomy is recommended for clinical or intra-operative stage II endometrial cancer
Level of evidence: IV
Strength of recommendation: B
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Surgical Management of Stage III–IV Endometrial Cancer
Although there is no evidence from randomised trials for stage III-IV endometrial cancer, there is consensus that multimodality therapy is required, generally starting with radical cytoreductive surgery. Several retrospective studies have shown a statistically significant advantage in progression-free survival (PFS) and OS when optimal cytoreduction can be achieved.107 However, not all patients are amenable to optimal cytoreduction as a result of poor general condition or tumour extent. In addition, the surgical management of metastatic vaginal disease may impair the vaginal function. Primary radiation therapy is therefore preferable in some cases.
Recommendation 6.4: Complete macroscopic cytoreduction and comprehensive staging is recommended in advanced endometrial cancer
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Recommendation 6.5: Multimodality management should be considered for the treatment of advanced endometrial cancer when surgery may significantly impair vaginal function
Level of evidence: IV
Strength of recommendation: B
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
Surgical Management of Non-EEC
The standard of surgical therapy in non-EEC is not different from EEC (see sections 3 and 5). Hysterectomy and bilateral salpingo-oophorectomy is the mainstay of therapy in apparent stage I disease. Radical hysterectomy is not recommended in stage II disease, whereas complete cytoreduction is required in advanced disease stages. However, there is no documentation on ovarian preservation. Bilateral salpingo-oophorectomy is mandatory.
Comprehensive surgical staging of more advanced disease stages is mandatory (see section 5). Although no data from randomised trials are available in non-EEC, the staging of apparent stage I disease is similar to high-risk EEC. Omentectomy is also considered in apparent stage I papillary serous carcinoma, in which peritoneal implants are not uncommon. However, omentectomy is not mandatory in cases of clear cell carcinoma,108 but should be considered where there is a serous component since uterine serosal spread has a negative impact on survival.109
Recommendation 6.6: In non-EEC (apparent stage I), lymphadenectomy is recommended
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
Recommendation 6.7: Staging omentectomy is not mandatory in clear cell or undifferentiated endometrial carcinoma and carcinosarcoma
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Recommendation 6.8: Staging omentectomy should be considered in serous carcinoma
Level of evidence: IV
Strength of recommendation: C
Consensus: 94.6% (35) yes, 5.4% (2) abstain (37 voters)
ADJUVANT TREATMENT
7. What is the Current Best Definition of Risk Groups for Adjuvant Therapy?
The majority of patients with endometrial cancer have a low risk of recurrence and are managed by surgery alone.110 Risk groups have been devised based on clinicopathological prognostic factors to identify patients at risk of recurrence who may benefit from adjuvant therapy.
In order to have clinical value, a definition of risk groups should have both prognostic value and consequences for the indication of adjuvant therapy. Well defined clinicopathological prognostic factors include: Age, FIGO stage, depth of myometrial invasion, tumour differentiation grade, tumour type (endometrioid versus serous and clear cell) and LVSI.89 Compared with the ESMO risk group classification,8 the adverse prognostic role of both LVSI and tumour grade 3 within the intermediate risk group (stage IA grade 3 or stage IB grade 1–2) has been recognised.111–115 This has led to a new subdivision of low risk, intermediate risk and high-intermediate risk in the current classification, which is different from the risk classification used in many clinical trials. Historically, low-risk endometrial cancer was defined as endometrioid adenocarcinoma FIGO stage I and grade 1 with superficial invasion or grade 2 without invasion, and high-risk as stage I, grade 3 with deep myometrial invasion, with other combinations of grade and invasion defined as intermediate risk. Against this background, the large trials evaluating the role of RT for intermediate-risk endometrial cancer (PORTEC-1, GOG99, ASTEC/EN5, described below116–118) were conducted, and based on the results of these trials and a subsequent meta-analysis,119 a refined classification of low risk, intermediate risk and high-intermediate risk has been introduced.
Factors such as tumour size and several molecular factors (e.g. TP53, L1CAM) have been reported as having prognostic value in observational studies but have not been incorporated into this classification since they are still under investigation and currently not in clinical use.120–124 A definition of risk groups to identify patients at risk of recurrence who may benefit from adjuvant therapy has been devised by the consensus panel and is shown in Table 2.
TABLE 2.
New risk groups to guide adjuvant therapy use
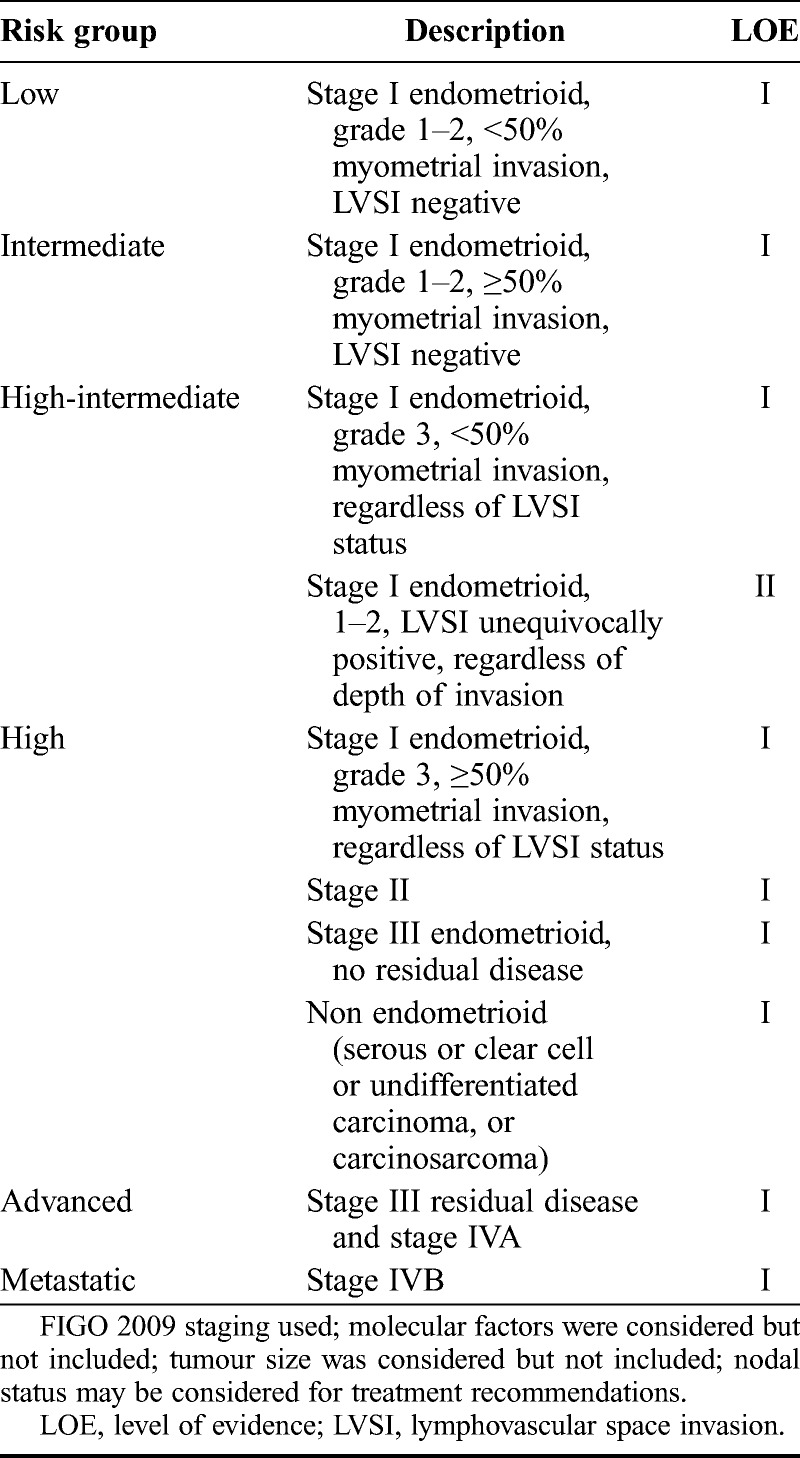
8. What are the Best Evidence-Based Adjuvant Treatment Strategies for Patients With Low- and Intermediate-Risk Endometrial Cancer?
Although the 1988 FIGO staging system included surgical staging, two large randomised trials have since found no benefit of routine lymphadenectomy for nodal staging purposes in low- and intermediate-risk endometrial cancer.95,96 Given the absence of a survival benefit and its associated side effects, routine lymphadenectomy is not recommended for low- and (high)intermediate-risk disease in most national and international guidelines for these patients. The value of lymphadenectomy in high-risk endometrial cancer is the subject of ongoing investigations. Recommendations regarding what defines adequate (lymph node) staging are detailed in the chapter on surgery.
Compared with the ESMO CPG on endometrial cancer,8 the current recommendations are specified to address both scenarios that surgical nodal staging is performed and is not performed, and to specifically address non-endometrioid histological subtypes. In addition, the roles of vaginal brachytherapy, EBRT and chemotherapy, or combinations of these treatments, have been specified in more detail for each of these situations.
Low-Risk Endometrial Cancer
Some patients now considered as low-risk were included in the large randomised trials of adjuvant RT and no benefit of RT was found in this subgroup.116–119 A randomised trial of 645 patients with low-risk endometrial cancer treated with vaginal brachytherapy also showed no advantage for the use of adjuvant brachytherapy, likely because the risk of recurrence after surgery alone is <5%.125 Therefore, no adjuvant treatment is indicated for patients with low-risk endometrial cancer.
Recommendation 8.1: In patients with low-risk endometrial cancer (stage I endometrioid, grade 1–2, <50% myometrial invasion, LVSI negative), no adjuvant treatment is recommended
Level of evidence: I
Strength of recommendation: A
Consensus: 100% yes (37 voters)
Intermediate-Risk Endometrial Cancer
Patients considered intermediate-risk risk in the current classification were included in the large randomised trials evaluating the role of adjuvant RT in early-stage endometrial cancer.116–119 In these trials, patients were randomised after total hysterectomy with bilateral salping-oophorectomy to pelvic EBRT or observation after surgery. All three trials and a meta-analysis by Kong et al.119 found that EBRT reduced the risk of pelvic recurrence by three-fold (from 14% to 4%), but did not lead to an OS benefit and came at the cost of increased risk of (predominantly gastro-intestinal) toxicity.
In contrast to the PORTEC-1 trial,117 surgical staging lymphadenectomy was mandatory in the GOG99 trial,118 showing that for node-negative disease, EBRT still reduced the risk of recurrence. This risk reduction was mainly caused by prevention of local (vaginal) recurrence. Both PORTEC-1 and GOG99 defined a subgroup of patients who derived the greatest benefit of adjuvant EBRT, a so-called high-intermediate-risk group. In the PORTEC-1 trial, the definition of risk groups was based on risk factors for locoregional recurrence (age >60 years, deep [≥50%] myometrial invasion, grade 3), with high-intermediate-risk patients defined as having two out of three of these risk factors. In this subgroup, the 5-year risk of locoregional recurrence was 20% for observation versus 5% for adjuvant RT, and only in this subgroup was the risk of relapse deemed high enough to consider adjuvant RT.117 In the GOG99 trial, the definition of risk groups was based on risk factors for overall recurrence identified in previous Gynecologic Oncology Group (GOG) studies, with high-intermediate-risk patients defined as: Age <50 years and 1 risk factor, age 50–70 years and 2 risk factors, and age >70 and all 3 risk factors. Similar results were found in the ASTEC trial, which reported a lower risk of vaginal and pelvic relapse in the no-EBRT group (7% vs 4% in the EBRT arm). In the ASTEC trial, vaginal brachytherapy was allowed in both study arms, and more than 50% of patients in the observation arm received vaginal brachytherapy.
The randomised PORTEC-2 trial included only patients with the high-intermediate-risk factors defined in PORTEC-1, and showed that vaginal brachytherapy provided excellent vaginal control compared with EBRT, and had a more favourable toxicity and quality of life profile.126 These results have been confirmed in a Swedish trial in which vaginal brachytherapy was compared with combined EBRT and a vaginal brachytherapy boost.127
Multiple cohort studies have identified LVSI and grade 3 as risk factors for disease recurrence.111–115 This finding was confirmed in a recent pooled analysis of data from the PORTEC-1 and −2 trials, which showed that both LVSI and grade 3 are risk factors for regional nodal recurrence and for distant metastasis.128 EBRT decreased the risk of regional nodal recurrence in this small subgroup (5%) of patients, while vaginal brachytherapy did not. Since the vast majority of patients in PORTEC-2 had grade 1–2 tumours with deep (≥50%) myometrial invasion and without LVSI, this population is now considered intermediate risk in the current consensus classification. These patients have a low risk of regional and distant recurrence, while their risk of local (vaginal) recurrence is significantly decreased with adjuvant vaginal brachytherapy. In addition, others have validated the added prognostic value of the incorporation of LVSI in the ESMO risk classification.129
Since adjuvant RT does not improve OS and combined EBRT and brachytherapy for recurrent disease is associated with a high chance of complete remission, not performing routine adjuvant RT is also an option.130 However, combined EBRT and brachytherapy for recurrent disease is associated with a higher rate of side effects compared with adjuvant vaginal brachytherapy alone.
Recommendation 8.2: In patients with intermediate-risk endometrial cancer (stage I endometrioid, grade 1–2, ≥50% myometrial invasion, LVSI negative):
-
1: Adjuvant brachytherapy is recommended to decrease vaginal recurrence
Level of evidence: I
Strength of recommendation: B
-
2: No adjuvant treatment is an option, especially for patients aged <60 years
Level of evidence: II
Strength of recommendation: C
Consensus: 100% yes (37 voters)
High-Intermediate-Risk Endometrial Cancer
Patients with grade 1–2 tumours with deep (≥50%) myometrial invasion and unequivocally positive (substantial, not focal) LVSI, and those with grade 3 tumours with <50% myometrial invasion regardless of LVSI status, are referred to as high-intermediate-risk in the current classification.
In the GOG249 study, both high-intermediate- and high-risk patients were randomised between pelvic EBRT and vaginal brachytherapy followed by chemotherapy (3 cycles of carboplatin and paclitaxel). Results have been presented (abstract only) that showed no PFS benefit of adjuvant chemotherapy over the standard EBRT.131
Recommendation 8.3: In patients with high-intermediate-risk endometrial cancer (stage I endometrioid, grade 3, <50% myometrial invasion, regardless of LVSI status; or stage I endometrioid, grade 1–2, LVSI unequivocally positive, regardless of depth of invasion):
-
1: Surgical nodal staging performed, node negative:
-
Adjuvant brachytherapy is recommended to decrease vaginal recurrence
Level of evidence: III
Strength of recommendation: B
-
No adjuvant therapy is an option
Level of evidence: III
Strength of recommendation: C
Consensus: 100% yes (37 voters)
-
-
2: No surgical nodal staging:
-
Adjuvant EBRT recommended for LVSI unequivocally positive to decrease pelvic recurrence
Level of evidence: III
Strength of recommendation: B
-
Adjuvant brachytherapy alone is recommended for grade 3 and LVSI negative to decrease vaginal recurrence
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (37 voters)
-
-
3: Systemic therapy is of uncertain benefit; clinical studies are encouraged
Level of evidence: III
Strength of recommendation: C
Consensus: 94.6% (35) yes, 5.4% (2) abstain (37 voters)
9. What are the Best Evidence-Based Adjuvant Treatment Strategies for Patients With High-Risk Endometrial Cancer?
In general, high-risk endometrial cancer is characterised by an increased risk of pelvic recurrence and distant metastases that contribute to the inferior outcomes of this group. However, high-risk endometrial cancer represents a heterogeneous group of patients, including both endometrioid and non-endometroid tumour types such as serous and clear cell, and ranges from stage IB grade 3 (with or without LVSI and with or without nodal staging) to more advanced FIGO stages. Regardless of tumour type, the estimated 5-year OS according to the 26th FIGO annual report is 85–90% for stage I, 75–85% for stage II, 50–65% for stage III and 20–25% for stage IV.132 Among FIGO stage I patients, those with deep myometrial invasion and grade 3 histology are at increased risk of pelvic and distant relapse.133–135 Estimated 5-year OS rates in patients with ≥50% myometrial invasion and grade 3 tumours (without nodal staging) were only 58%. Regarding non endometrioid tumour types, approximately 60–70% of patients with uterine serous cancer have disease outside the uterus at the time of presentation. The 5-year OS rate for patients with uterine serous cancer is 20–25% versus 80% for all patients with endometrial cancer.136 For these reasons, recommendations were made for the following subgroups: Endometrioid stage I, grade 3 and >50% myometrial invasion; endometrioid stage II; endometrioid stage III without residual disease and non-endometrioid tumour types. Recommendations for patients with advanced non-resectable or residual disease are provided separately in the ‘Advanced and Recurrent Endometrial Cancer’ section of this article (see pages 17–21).
External beam pelvic RT is the standard therapy for high-risk patients and is indicated to maximise pelvic control. The addition of chemotherapy, or replacement of RT by chemotherapy, has been studied in several randomised trials. A historic GOG randomised trial that included patients with high-risk stage I and occult stage II disease found no benefit of adjuvant doxorubicin after surgery and post-operative pelvic EBRT.137 A Japanese (JGOG 2033) and an Italian trial randomised patients with high-risk endometrial cancer between pelvic EBRT and adjuvant cyclophosphamide, doxorubicin, cisplatin (CAP) chemotherapy (3 and 5 cycles, respectively), and both trials found no difference in OS or disease-free survival (5-year OS: 85% vs 87% and 69% vs 66%, respectively).138,139
Results of a combined analysis of the NSGO 9501/EORTC 55991 and MaNGO-ILIADE III randomised trials have been published.140 In this pooled analysis, the addition of adjuvant chemotherapy (4 cycles of platinum-based chemotherapy given either before or after RT) to adjuvant EBRT was associated with a significant improvement in 5-year PFS (78% vs 69%, P = 0.009), and a trend towards improved OS (82% vs 75%, P = 0.07). Findings from a subgroup analysis suggested that the benefit of adjuvant chemotherapy was restricted to patients with endometrioid tumours rather than the 36% with serous or clear cell tumours. However, as this was an unplanned and small subgroup analysis, no definite conclusions can be drawn on the efficacy of adjuvant chemotherapy for serous or clear cell cancers.
Promising results were found in the RTOG 9708 phase II study in 46 patients using concurrent pelvic RT and two cycles of Cisplatin (50 mg/m2 days 1 and 28) followed by four additional courses at 28 day intervals of cisplatin (50 mg/m2) and paclitaxel (175 mg/m2) as a 24 hour infusion.141 Reported 4-year OS rates were 85% for the whole group and 77% for stage III patients. This concurrent and adjuvant chemotherapy schedule formed the rationale for the treatment arms included in recently completed trials that investigated the role of combined cisplatin-based chemoradiation plus adjuvant chemotherapy compared with either RT alone (PORTEC-3) or chemotherapy alone (GOG258) for patients with high-risk and advanced stage endometrial cancer. The ongoing ENGOT-EN2-DGCG/EORTC55102 trial is evaluating the role of chemotherapy versus observation in patients with high-risk, node-negative endometrial cancer.
Recommendation 9.1: In patients with high-risk endometrial cancer (stage I endometrioid, grade 3, ≥50% myometrial invasion, regardless of LVSI status):
-
1: Surgical nodal staging performed, node negative:
-
Adjuvant EBRT with limited fields should be considered to decrease locoregional recurrence
Level of evidence: I
Strength of recommendation: B
-
Adjuvant brachytherapy may be considered as an alternative to decrease vaginal recurrence
Level of evidence: III
Strength of recommendation: B
-
Adjuvant systemic therapy is under investigation
Level of evidence: II
Strength of recommendation: C
Consensus: 100% yes (37 voters)
-
-
2: No surgical nodal staging:
-
Adjuvant EBRT is generally recommended for pelvic control and relapse-free survival
Level of evidence: III
Strength of recommendation: B
-
Sequential adjuvant chemotherapy may be considered to improve PFS and cancer specific survival (CSS)
Level of evidence: II
Strength of recommendation: C
-
There is more evidence to support giving chemotherapy and EBRT in combination rather than either treatment modality alone
Level of evidence: II
Strength of recommendation: B
Consensus: 100% yes (37 voters)
-
High-Risk, Stage II Endometrial Cancer
The definition of stage II endometrial cancer was changed in the most recent FIGO 2009 staging system; tumours with endocervical glandular involvement (previously stage IIA) were moved to stage I as this has no prognostic impact.142,143 As a result, stage II now only includes tumours with cervical stromal invasion.144
Stage II tumours have been associated with an increased frequency of deep myometrial invasion and grade 3 histology, making it difficult to conclude if cervical invasion alone is the reason for the observed higher risk of recurrence and lower OS compared with stage I disease.145 In a SEER analysis that included 1577 patients with stage II endometrial cancer, of which half had stromal invasion, a multivariate analysis demonstrated no OS benefit for radical compared with simple hysterectomy, while RT (independently of the type of surgery) was associated with a survival benefit.146
Controversy exists regarding the role of additional vaginal brachytherapy boost in combination with EBRT.147 The indication for a brachytherapy boost is clear in the rare situation of a tumour with positive vaginal margin. However, in the adjuvant setting, it has historically been performed largely for stage II disease. In randomised trials conducted in patients with intermediate-risk stage I endometrial cancer, there is no clear benefit in terms of vaginal control among trials that included a vaginal brachytherapy boost compared with those that did not, with low recurrence rates of approximately 2% at 5-years after EBRT or vaginal brachytherapy alone.126,127 A SEER analysis conducted in patients with stage IIIC endometrial cancer suggested a survival benefit for patients with ‘direct extension’ of the tumour, but no vaginal recurrence rates are available.148 Other studies have found no difference in local recurrence or OS rates among patients with stage II endometrial cancer treated with or without vaginal brachytherapy in addition to EBRT, but it was associated with increased risk of side effects.149–153
Recommendation 9.2: In patients with high-risk, stage II endometrial cancer:
-
1: Simple hysterectomy, surgical nodal staging performed, node negative:
-
Grade 1–2, LVSI negative: Recommend vaginal brachytherapy to improve local control
Level of evidence: III
Strength of recommendation: B
-
Grade 3 or LVSI unequivocally positive:
-
Recommend limited field EBRT
Level of evidence: III
Strength of recommendation: B
-
Consider brachytherapy boost
Level of evidence: IV
Strength of recommendation: C
-
Chemotherapy is under investigation
Level of evidence: III
Strength of recommendation: C
-
Consensus: 97.3% (36) yes, 2.7% (1) abstain (37 voters)
-
-
2: Simple hysterectomy, no surgical nodal staging:
-
EBRT is recommended
Level of evidence: III
Strength of recommendation: B
-
Consider brachytherapy boost
Level of evidence: IV
Strength of recommendation: C
-
Grade 3 or LVSI unequivocally positive: Sequential adjuvant chemotherapy should be considered
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (37 voters)
-
High-Risk, Stage III Endometrial Cancer
In patients with stage IIIC endometrial cancer, pelvic and/or extended field RT have been associated with increased OS and locoregional control rates, while a higher rate of pelvic recurrence was found after adjuvant chemotherapy alone.154,155 In the GOG122 trial,156 women with advanced stage III/IV endometrial cancer were randomised between whole abdominal irradiation and 8 cycles of doxorubicin/cisplatin chemotherapy. Both adjusted PFS and OS were higher in the group who received chemotherapy (predicted 5-year rates of 50% vs 38% and 55% vs 42%, respectively). However, event rates were high in both arms (50% and 54%). Patients with up to 2 cm residual disease were included in this trial, suggesting that the dose delivered with whole abdominal irradiation is not effective for macroscopic disease and is toxic. In view of findings from the pooled NSGO/EORTC/Iliace trials140 as well as results from prospective and retrospective trials,141,154,155,157,158 the use of combined RT and chemotherapy is recommended as opposed to either alone. Results of the recently completed GOG258 for stage III-IV endometrial cancer are awaited to see if the combination of EBRT and chemotherapy, as also evaluated in PORTEC-3, does indeed improve PFS and OS compared with chemotherapy alone.
Recommendation 9.3: In patients with high-risk, stage III endometrial cancer and no residual disease:
-
1: EBRT is recommended to:
-
Decrease pelvic recurrence
Level of evidence: I
Strength of recommendation: B
-
Improve PFS
Level of evidence: I
Strength of recommendation: B
-
Improve survival
Level of evidence: IV
Strength of recommendation: B
-
-
2: Chemotherapy is recommended to improve PFS and CSS
Level of evidence: II
Strength of recommendation: B
-
3: There is more evidence to give chemotherapy and EBRT in combination than either alone in stage III disease:
IIIA: Chemotherapy AND EBRT to be considered
IIIB: Chemotherapy AND EBRT to be considered
IIIC1: Chemotherapy AND EBRT to be considered
-
IIIC2: Chemotherapy AND extended field EBRT to be considered
Level of evidence: II
Strength of recommendation: B
Consensus: 94.6% (35) yes, 5.4% (2) abstain (37 voters)
High-Risk, Non Endometrioid Cancers
For the purpose of these recommendations, serous, clear cell, carcinosarcoma, undifferentiated and mixed (>10%) tumours are regarded as high-risk non-endometrioid type cancers. These tumours represent an infrequent subset of patients; hence most studies are retrospective and have included a limited number of patients. The largest retrospective study conducted to date suggested a survival benefit for the combination of chemotherapy and RT in uterine serous cancer.159 However, a subgroup analysis of the NSGO 9501/EORTC 55991 and MaNGO-ILIADE III trials did not show a survival benefit for patients with serous or clear cell tumours.140 Given the high rates of distant metastasis observed in patients with uterine serous and clear cell tumours, adjuvant chemotherapy can be considered and clinical trials addressing these rare subtypes are encouraged.136,160 One retrospective study investigated the role of vaginal brachytherapy for stage I serous or clear cell cancers. The majority were either non-invasive (26%) or had <50% myometrial invasion (58%), and 34% received adjuvant chemotherapy. The 5-year rate of isolated pelvic recurrence was 4% and locoregional recurrence was 7%; the 5-year OS rate was 84%, suggesting that vaginal brachytherapy alone is sufficient in patients with stage IA disease.161
Carcinosarcomas are regarded as metaplastic carcinomas containing both sarcomatous and carcinomatous elements.162 They are rare and aggressive tumours with more than 35% of patients presenting with extra-uterine disease at diagnosis and are associated with a 5-year OS rate of 50% for patients with stage I disease.163 In the EORTC-55874 trial, patients with stage I–II uterine sarcomas were randomised to receive adjuvant RT after surgery. Of the 224 patients included, 91 had carcinosarcoma. In both groups, RT significantly reduced the risk of local relapse but there was no difference in the rate of distant metastasis and OS.164 Three analyses of SEER data have been reported in this setting, which initially showed a survival benefit for patients who received RT but who did not undergo lymphadenectomy.165,166 However, in a subsequent analysis, this survival benefit was not maintained,167 thus limiting the conclusions that can be drawn from these analyses. Finally, the GOG performed a trial in which whole abdominal irradiation was compared with three courses of ifosfamide and cisplatin after complete resection. In this trial, chemotherapy was associated with a numerically lower risk of recurrence and better survival but the differences were not statistically significant.168
Recommendation 9.4: In patients with high-risk, non-endometrioid cancers:
-
1: Serous and clear cell after comprehensive staging:
-
Consider chemotherapy; clinical trials are encouraged
Level of evidence: III
Strength of recommendation: B
-
Stage IA, LVSI negative: Consider vaginal brachytherapy only without chemotherapy
Level of evidence: IV
Strength of recommendation: C
-
Stage ≥ IB: EBRT may be considered in addition to chemotherapy, especially for node positive disease
Level of evidence: III
Strength of recommendation: C
Consensus: 100% yes (37 voters)
-
-
2: Carcinosarcoma and undifferentiated tumours:
-
Chemotherapy is recommended
Level of evidence: II
Strength of recommendation: B
-
Consider EBRT; clinical trials are encouraged
Level of evidence: III
Strength of recommendation: C
-
Consensus: 94.6% (35) yes, 5.4% (2) abstain (37 voters)
ADVANCED AND RECURRENT ENDOMETRIAL CANCER
10. Does Surgery or RT Have a Role in Advanced or Recurrent Endometrial Cancer?
Surgical Cytoreduction
Patients with advanced disease (defined as bulky FIGO stage IIIA-IV), or recurrent disease should only be considered for surgery if it is anticipated that cytoreduction with no macroscopic residual disease can be achieved. Cytoreduction also includes removal of enlarged lymph nodes, but as there is no evidence that a systematic pelvic and para-aortal lymphadenectomy will influence PFS or OS, it should not be routinely performed. In a meta-analysis of 14 publications containing retrospective data from 672 patients, median OS time was positively associated with an increasing proportion of patients with no residual disease (each 10% increase improved survival by 9.3 months, P = 0.04); the change in survival for patients with between 0 and ≤2 cm of disease after surgery was not significant.169 Exenteration may be considered for FIGO stage IIIA and central local relapse. In selected cases, palliative surgery can be performed to alleviate symptoms (e.g. bleeding or bowel obstruction).
For patients with oligometastases or isolated retroperitoneal lymph node metastases, surgical resection is an option that can be considered but the evidence of its benefit is limited.
Recommendation 10.1: For patients with advanced or recurrent disease, surgery is recommended only if optimal cytoreduction (no residual disease) can be achieved. In selected cases, palliative surgery is recommended to alleviate specific symptoms
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Recommendation 10.2: Exenteration can be considered in selected patients with locally advanced tumours, and for isolated central local relapse after radiation, if clear margins are expected
Level of evidence: IV
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Recommendation 10.3: Complete resection of distant oligometastases and pelvic or retroperitoneal lymph node relapse can be considered if technically possible according to localisation of disease
Level of evidence: V
Strength of recommendation: C
Consensus: 100% yes (37 voters)
Histology
Uterine serous cancer and clear cell cancer account for approximately 10% and 3% of advanced endometrial cancer cases, respectively.170 Patients with advanced disease have a worse prognosis than those with endometrioid type, but there is no evidence that histology should influence the decision regarding surgery.
Recommendation 10.4: Histological type should not influence the decision whether or not to proceed with surgery
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (37 voters)
RT for Isolated Vaginal Relapse in Early-Stage Endometrial Cancer
RT is an effective therapeutic modality for improving local disease control. However, fewer patients now receive adjuvant RT for early disease. Observation after surgery particularly applies to those with early stage, grade 1–2 disease without LVSI, as salvage RT in those who have a localised vaginal relapse is associated with good local control.171 In the PORTEC1 trial, 35 of 39 patients with a vaginal recurrence after surgery alone were treated with radical intent, mostly with combinations of EBRT and brachytherapy, and in some cases with surgery. The complete remission rate was 89%, and 77 % remained disease free with a median follow-up of 44 months.130 Survival after relapse was better in patients who did not receive primary adjuvant RT; among patients who had received adjuvant RT, most relapses were at distant sites. There is currently no evidence to suggest that modern techniques of image-guided brachytherapy and intensity-modulated radiotherapy (IMRT) are superior to conventional approaches, although a single institution retrospective study of RT (EBRT predominantly using an IMRT technique followed by image-guided high dose rate [HDR] brachytherapy) for vaginal recurrence has also reported high tumour control rates.172
Recommendation 10.5: RT with curative intent is indicated in patients with isolated vaginal relapse after surgery
Level of evidence: III
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Chemotherapy With RT for Recurrence
RT can be considered for patients with vaginal or pelvic nodal recurrence. Improvements in RT techniques allow for better means of localised treatment, or possibly retreatment of patients who have previously received RT. Whether chemotherapy has an additional benefit is unclear. The ongoing randomised phase II GOG0238 (NCT00492778) trial is comparing pelvic irradiation of 45 Gy in 25 fractions plus either brachytherapy or external beam boost with the same schedule plus concomitant cisplatin (40 mg/m2 weekly) in women with vaginal/pelvic relapse who have not received prior RT.
Recommendation 10.6: For vaginal or pelvic nodal recurrence, chemotherapy with RT could be considered in patients with high-risk features for systemic relapse
Level of evidence: IV
Strength of recommendation: C
Consensus: 97.1% (33) yes, 2.9% (1) abstain (34 voters)
Combined Approaches to Recurrence and Re-Irradiation
The use of systemic therapy or surgery prior to RT for vaginal or pelvic node recurrence could be considered in certain patients with more bulky disease. As the techniques for image-guided RT have improved, there are situations where re-irradiation can be considered, although evidence from clinical trials is lacking.
Recommendation 10.7: Use of systemic therapy or surgery prior to RT for vaginal or pelvic node recurrence could be considered in certain patients
Level of evidence: V
Strength of recommendation: C
Consensus: 100% yes (34 voters)
Recommendation 10.8: Re-irradiation could be considered in highly selected patients using specialised techniques
Level of evidence: V
Strength of recommendation: C
Consensus: 100% yes (34 voters)
Palliative RT
RT can be effectively used to palliate symptoms such as bleeding, bone metastases or painful nodal recurrence. No randomised trials have been conducted comparing RT with palliative chemotherapy.
Recommendation 10.9: RT is indicated for palliation of symptoms related to local recurrence or systemic disease
Level of evidence: IV
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Radical RT for Primary Endometrial Cancer
RT can be used as a primary treatment in patients with unresectable disease, or where there are medical contraindications to surgery.173,174 Treatment involves intrauterine brachytherapy alone or in combination with EBRT. Image guided brachytherapy may improve outcomes.175 Two year local control rates of more than 90% can be achieved for medically inoperable stage I disease.
Recommendation 10.10: RT may be indicated for primary tumours that are unresectable, or where surgery cannot be performed or is contraindicated for medical reasons
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (34 voters)
11. What are the Optimal Systemic Therapies for Advanced/Recurrent Disease?
The majority of patients with advanced or recurrent disease will be candidates for systemic palliative therapy. The choice between hormonal treatment and chemotherapy relies on several factors, including histopathological and clinical features of the individual patient.
Hormonal Therapy: Which Patient and When?
Hormonal therapy is indicated for patients with advanced or recurrent endometrial cancer and endometrioid histology. This statement is based on several clinical trials that have shown clinical activity with a favourable toxicity profile.176,177
Recommendation 11.1: Hormone therapy is indicated in advanced or recurrent EEC
Level of evidence: II
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Response to hormonal therapy is quite variable, and a number of pathological factors contributing to this variation have been identified. For example, hormonal therapy is more likely to be effective in grade 1 or 2 endometrioid tumours. In a large clinical trial of MPA, the response rate was 37% for grade 1, 23% for grade 2 and 9% for grade 3 tumours.176 Others have reported similar findings.177 Patients with hormone receptor positive disease have also been shown to have a higher chance of responding to endocrine therapy. In a randomised trial, the response rate observed in patients with ER and PgR positive disease was around 25% and 37%, respectively, but was only 7–8 % in patients with ER/PgR negative disease.176,177 Based on these results, it seems that positivity of ER and/or PgR could be a predictive factor of response to endocrine therapy and so should be determined before initiating hormonal therapy.
Recommendation 11.2: Hormone therapy is more likely to be effective in grade 1 or 2 endometrioid tumours
Level of evidence: IV
Strength of recommendation: B
Consensus: 100% yes (34 voters)
Recommendation 11.3: Hormone receptor status should be determined before hormone therapy is initiated, as it is more likely to be effective in patients with PgR and ER status
Level of evidence: III
Strength of recommendation: B
Consensus: 97.1% (33) yes, 2.9% (1) abstain (34 voters)
Biopsy of recurrent disease can be considered, since there may be differences in hormone receptor status in the primary and metastatic tumour. In a prospective collection of 686 primary endometrial tumours and 171 metastatic lesions, loss of PgR expression increased with disease progression, with 23% of primary tumours and 76% of metastatic lesions demonstrating PgR loss.178
Recommendation 11.4: Biopsy of recurrent disease could be considered as there may be differences in hormone receptor status in the primary and metastatic tumour
Level of evidence: III
Strength of recommendation: C
Consensus: 100% yes (34 voters)
Hormone therapy is the preferred front-line systemic therapy for patients with hormone receptor positive grade I or 2 tumours in the absence of rapidly progressive disease, as it provides an excellent benefit/risk ratio and convenient toxicity profile. However, patients with visceral involvement and rapidly progressive disease are not candidates for hormone therapy as it is not usually associated with a rapid response.
Recommendation 11.5: Hormone therapy is the preferred front-line systemic therapy for patients with hormone receptor positive tumours – grade 1 or 2 and without rapidly progressive disease
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (34 voters)
The progestogens, MPA 200 mg or MA 160 mg, are generally recommended. They have shown clear activity for the front-line treatment of non-selected patients with recurrent or persistent endometrioid tumours not suitable for surgery or RT, with response rates of around 25% and PFS times of 3 months.176,179 Data from a randomised trial comparing low (200 mg/day) versus high (1000 mg/day) dose MPA in 299 patients with advanced or recurrent endometrial carcinoma showed that low-dose MPA was more active than the high dose in terms of response rate (25% vs 15%, respectively) and OS (11.0 vs 7.0 months, respectively).176
Recommendation 11.6: Progestogens (e.g. MPA 200 mg or MA 160 mg) are generally recommended
Level of evidence: III
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Other endocrine therapies have also demonstrated activity in phase II trials among patients with advanced or recurrent endometrial cancer, with tamoxifen, anastrozole and fulvestrant all associated with response rates of approximately 10%.180–182 Interestingly, patients included in the anastrozole trial had not received prior progestin therapy.182 The combination of tamoxifen and MPA is associated with response rates and PFS similar to MPA alone.183,184
Recommendation 11.7: Other hormonal agents to consider after progestins include tamoxifen, fulvestrant and aromatase inhibitors
Level of evidence: III
Strength of recommendation: C
Consensus: 100% yes (34 voters)
Chemotherapy: Is There any Standard of Care?
Endometrial cancer is a relatively chemo-sensitive disease, with anthracyclines, platinum-based drugs and taxanes shown to be the most active agents. Two clinical trials showed that the combination of cisplatin and doxorubicin was more active than doxorubicin alone in terms of response rate (43–41% vs 17–25%) but with no benefit in terms of OS.185,186 The combination also resulted in a higher incidence of grade 3–4 myelotoxicity and nausea/vomiting.
In another GOG trial, conducted in patients with measurable FIGO III-IV endometrial cancer, the addition of paclitaxel to cisplatin and doxorubicin was associated with a higher response rate and PFS than cisplatin and doxorubicin alone (objective response rate [ORR]: 57% vs 34%, respectively, P <0.01; median PFS: 8.3 vs 5.3 months, respectively, P <0.01), and a small but significant improvement in OS (median 15.3 vs 12.3 months, respectively, P = 0.037).187 However, toxicity, especially peripheral neuropathy, was significantly higher (grade 2–3: 39% vs 5%, respectively). For this reason, it has not been widely adopted as a standard of care.
Finally, GOG209 was a randomised, non-inferiority trial that compared the combination of paclitaxel 160 mg/m2, cisplatin 60 mg/m2 and doxorubicin 50 mg/m2 (TAP) with paclitaxel 175 mg/m2 and carboplatin AUC 6 (TC), both administered every 3 weeks. A total of 1305 patients were included in this trial, and preliminary data (not yet fully published) indicate a similar response rate (51.3% vs 51.2%) and PFS (median 13.5 vs 13.3 months).188 The median OS (primary study endpoint) was 40.3 months for TAP and 36.5 months for TC, which met the criteria of non-inferiority. TC had a more favourable toxicity profile than TAP in this trial, with fewer patients discontinuing therapy due to toxicity (12% vs 18%). In addition, TC can be administered in the outpatient setting whereas TAP is given in the inpatient setting in most countries. This aspect may be important in terms of logistical, financial and quality of life considerations in the palliative setting.
Recommendation 11.8: The standard of care is 6 cycles of three-weekly carboplatin and paclitaxel. This is based on the preliminary communication of a randomised trial showing similar efficacy and less toxicity compared to cisplatin/doxorubicin/paclitaxel
Level of evidence: I
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Evidence supporting the use of second line chemotherapy after platinum-containing therapy in patients with endometrial cancer is limited, especially in cases where the treatment-free interval following first line chemotherapy is less than 6–12 months. Although various regimens have been evaluated in this setting,189–192 no randomised trials have been published. Therefore, no specific regimen can be recommended as a standard of care for second line chemotherapy.
Recommendation 11.9: There is no standard of care for second line chemotherapy
Level of evidence: V
Strength of recommendation: C
Consensus: 100% yes (34 voters)
12. What are the Most Promising Targeted Agents and Which Study Designs Should be Used to Evaluate Their Clinical Benefit?
Potentially ‘Druggable’ Molecular Alterations in Endometrial Cancer
According to the WHO classification of endometrial carcinoma, there are seven different types of tumours; however, endometrioid carcinoma, grade 3 and serous carcinomas account for the vast majority of aggressive tumours. Molecular genetic alterations involved in the development of endometrioid cancers differ from those of serous tumours and this must be taken into account when designing clinical trials to evaluate the efficacy of molecular targeted agents.
Over the last fifteen years, it has been demonstrated that endometrial cancer shows microsatellite instability (MSI) and mutations in PTEN, PIK3CA and KRAS, and that beta-catenin genes are the most common molecular abnormalities in endometrioid carcinomas, whereas serous tumours have alterations of p53 and loss of heterozygosity (LOH) on several chromosomes, as well as other molecular alterations (STK15, p16, E-cadherin, and C-erbB2).193 Recently, the TCGA Research Network performed an integrated genomic characterisation of endometrial carcinoma.5
The PI3K/AKT pathway is one of the most frequently altered signalling pathways in endometrioid tumours, often resulting from mutations in PTEN, PIK3CA and PIK3RI.194 Of particular interest is the downstream effector, mammalian target of rapamycin (mTOR), and inhibitors of mTOR are now undergoing evaluation in clinical trials. The RAS-RAF-MEK-ERK signalling pathway also plays an important role in these tumours, with frequent mutations in KRAS, but also inactivation of tumour suppressors such as RASF1A.195,196 Fibroblast growth factor-2 (FGFR2) is mutated in 10–14% of endometrioid tumours and is a target for receptor tyrosine kinase inhibitors.197 Angiogenesis also plays a role in endometrial tumourigenesis.198 In addition, tumour homologous recombination and missmatch repair deficiencies are seen in endometrioid tumours, the latter of which is particularly associated with LS, and these pathways could be interesting targets.
Although there are a large number of specific gene abnormalities and aberrant signalling pathways that appear to be promising targets, the frequency of each abnormality is small and this presents a challenge to evaluating therapies in clinical trials.199 Examples include known tumour markers such L1CAM, Anexin 2, other tyrosine kinase receptors (insulin-like growth factor receptor [IGFR], epidermal growth factor receptor [EGFR]), and signalling pathways involved in epithelial to mesenchymal transition (transforming growth factor-beta [TGF-β], wnt) or stem cell-ness (Notch). PI3K/PTEN/AKT/mTOR pathway, PTEN, MAPK-KRAS, angiogenesis (especially FGFR2 and vascular endothelial growth factor [VEGF]/VEGF receptor [VEGFR]), ER/PgR and homologous recombination deficiency (HRD)/MSI are altered in endometrial cancer, and the relevance of these potential targets should be studied in clinical trials with targeted agents.
Recommendation 12.1: PI3K/PTEN/AKT/mTOR pathway, PTEN, RAS-MAPK, angiogenesis (especially FGFR2 and VEGF/VEGFR), ER/PgR and HRD/MSI are altered in endometrial cancer and their relevance should be studied in clinical trials with targeted agents
Level of evidence: III
Strength of recommendation: B
Consensus: 100% yes (34 voters)
New Agents in Recurrent or Metastatic Endometrial Cancer
The benefit of standard chemotherapy and hormonal therapies is usually modest and of short duration. Currently, several different targeted therapies are undergoing clinical evaluation but none are currently licensed for use. EGFR, human epidermal growth factor receptor-2 (HER2), mTOR and VEGFR inhibitors have been tested in phase I and II trials, with modest response rates.200–203 However, since this consensus conference was held, findings from two randomised phase II trials evaluating the addition of bevacizumab to TC in advanced or recurrent endometrial cancer suggest that this might be a promising approach worthy of further evaluation in phase III clinical trials.204,205 GOG-86P was a 3-arm trial evaluating the addition of bevacizumab, temsirolimus or ixabepilone to first line TC in 349 patients with advanced or recurrent endometrial cancer.204 No differences in PFS were seen when the three arms were compared with historical data for TC from GOG 209.188 However, bevacizumab appeared superior when the median OS results were compared with these historical control data (34.0 vs 22.7 months, P <0.039). In the MITO END-2 trial, which included 108 patients with advanced or recurrent endometrial cancer who had received 0 or 1 prior lines of chemotherapy, bevacizumab was added to 6–8 cycles of TC and then continued as maintenance therapy. This approach resulted in a significant improvement in median PFS (13 vs 8.7 months, P = 0.036) and a numerical increase in median OS (23.5 vs 18 months, P = 0.24), although these OS data are not yet mature.205
Despite these promising results, few clinical trials of new targeted therapies are molecularly driven206 and the prevalence of potential targets in metastatic lesions has been studied less than in primary tumours.178
Taken together, these findings suggest that PI3Kinase, mTOR and angiogenesis inhibitors are the most promising classes of drugs to investigate in endometrial cancer,207 and progress in this area is likely to be faster if studies are biomarker driven with biopsy at entry.
Recommendation 12.2: Drugs targeting PI3K/mTOR pathway signalling and angiogenesis have shown modest activity but no agent has been approved for clinical use, and further biomarker driven studies are warranted
Level of evidence: III
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Clinical Trial Design
While clinical trial endpoints such as OS and PFS are desirable, it may not be possible to make progress unless novel trial design and endpoints are used. There should be better selection of patients, using a more systematic approach to integration of biomarkers as well as earlier characterisation and standardisation of diagnostic imaging and biomarker assessments. Tumour response to biological agents may not occur to the same degree as with chemotherapy and alternative early endpoints, such as the percentage of patients free from progression at 18 weeks,208 have been used. Trial designs that include different gynaecological cancers of the same histotype should also be considered, an approach that is being taken in in the ongoing phase III GOG0261 trial of paclitaxel plus carboplatin versus paclitaxel plus ifosfamide in patients with different types of gynaecological carcinosarcomas (NCT00954174), and a randomised phase II trial of nintedanib versus chemotherapy in patients with recurrent clear cell carcinoma of the ovary or endometrium (EudraCT 2013-002109-73). There is also an argument for not being too selective, as the presence of a specific biomarker target may not be reflective of the probability of response. In a recent analysis of phase II studies of mTOR inhibitors, there was no correlation between response and the presence of mutations in the PI3K-AKT pathway,209 a result that could be explained by a variety of reasons, including the presence of multiple mutations, cross-talk in the signalling pathways involved, and the lack of re-biopsy samples to discount discordance between the tumour mutation profile at diagnosis versus recurrence.
Setting up individual trials is both costly and time-consuming, although adaptive phase II/III trials may offer some advantages.210 Alternative strategies such as the ‘basket’ approach, which includes all patients subdivided by specific histological or molecular cohorts under the umbrella of a single trial, may be the most efficient way forward.211 Such trials should also incorporate novel endpoints and the design would be strengthened by the inclusion of sequential and repeated assessments of biomarkers.
Recommendation 12.3: Clinical trial designs for new, targeted therapy:
Basket studies with multiple cohorts related to histological subtypes and/or molecular alterations are considered a priority
Biomarker driven clinical trials with biopsy at entry and sequential biopsies in trials with molecular endpoints are recommended
PFS or PFS at a defined time-point are the preferred primary endpoints for early phase trials
OS is the preferred primary endpoint in phase III trials, unless crossover is planned or expected
Level of evidence: V
Strength of recommendation: A
Consensus: 100% yes (34 voters)
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Jennifer Lamarre, Claire Bramley, Matthew Wallace, Aude Galli and all ESMO staff for their support throughout the whole consensus process.
Angela Corstorphine of Kstorfin Medical Communications Ltd provided medical writing support with the preparation of this manuscript. This support was funded by ESMO.
APPENDIX
ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group
Miguel Abal, Translational Medical Oncology (IDIS), Complexo Hospitalario Universitario de Santiago de Compostela (SERGAS), Santiago de Compostela, Spain; Ozden Altundag, Department of Medical Oncology, Başkent University Hospital, Ankara, Turkey; Frederic Amant, Department of Gynecological Oncology, University Hospital Leuven, Leuven, Belgium and Center for Gynecological Oncology Amsterdam (CGOA), Antoni van Leeuwenhoek, Amsterdam, The Netherlands; Susana Banerjee, Gynaecology Unit, The Royal Marsden NHS Foundation Trust, London, United Kingdom; Tjalling Bosse, Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands; Antonio Casado, EORTC Gynecological tumor group, Hospital Universitario San Carlos, Madrid, Spain; Luis Chiva de Agustín, MD Anderson Cancer Center, Madrid, Spain and University of Texas, USA; David Cibula, Department of Obstetrics and Gynecology, Charles University, Prague, Czech Republic; Nicoletta Colombo, Division of Medical Gynecologic Oncology, European Institute of Oncology and University of Milan-Bicocca, Milan, Italy; Carien Creutzberg, Department of Radiation Oncology, Leiden University Medical Center, Leiden, The Netherlands; Josep-María del Campo, Division of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain; Günter Emons, Department of Obstetrics & Gynecology, Georg-August-Universität Göttingen, Frauenklinik, Göttingen, Germany; Frédéric Goffin, Department of Gynecologic Oncology, CHU Liège, Site Hôpital de la Citadelle, Liège, Belgium; Antonio González-Martín, Medical Oncology Department, GEICO and MD Anderson Cancer Center, Madrid, Spain; Stefano Greggi, Department of Gynecologic Oncology, National Cancer Institute of Naples, Naples, Italy; Christine Haie-Meder, Radiation Oncology Department, Brachytherapy Service, Gustave Roussy Hospital, Villejuif, France; Dionyssios Katsaros, Department of Gynecologic Oncology, Azienda Ospedaliero-Universitaria Città della Salute, Sant’Anna Hospital and University of Turin, Turin, Italy; Vesna Kesic, Medical Faculty, University of Belgrade and Department of Obstetrics and Gynecology, Clinical Center of Serbia, Belgrade, Serbia; Christian Kurzeder, Department of Gynaecology and Gynaecologic Oncology, Kliniken Essen-Mitte, Essen, Germany; Sigurd Lax, Department of Pathology, Hospital Graz West, Graz, Austria; Fabrice Lécuru, Service de Chirurgie Gynécologique et Cancérologique, Hôpital Européen Georges Pompidou, Paris, France; Jonathan Ledermann, Department of Oncology and Cancer Trials, UCL Cancer Institute, London, United Kingdom; Tally Levy, Division of Gynecologic Oncology, Wolfson Medical Center, Tel-Aviv University, Holon, Israel; Domenica Lorusso, Department of Gynecologic Oncology, Fondazione “IRCCS” National Cancer Institute of Milan, Milan, Italy; Johanna Mäenpää, Department of Obstetrics and Gynecology, University of Tampere and Tampere University Hospital, Tampere, Finland; Christian Marth, Department of Obstetrics and Gynecology, Innsbruck Medical University, Innsbruck, Austria; Xavier Matias-Guiu, Department of Pathology and Molecular Genetics and Research Laboratory, Hospital Universitari Arnau de Vilanova, University of Lleida, Lleida, Spain; Philippe Morice, Gynaecological Surgery Department, Institut Gustave Roussy, Villejuif, France; Hans W. Nijman, Department of Gynaecologic Oncology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; Remi Nout, Department of Radiotherapy, Leiden University Medical Center, Leiden, The Netherlands; Melanie Powell, Department of Clinical Oncology, Barts Health NHS Trust, St Bartholomew’s Hospital, West Smithfield, London, United Kingdom; Denis Querleu, Department of Surgery, Institut Bergonié, Bordeaux, France and Gynecology and Obstetrics Department, McGill University Health Centre, Montreal, Quebec, Canada; Mansoor Raza Mirza, Department of Oncology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; Nick Reed, Department of Clinical Oncology, Beatson Oncology Centre, Gartnavel General Hospital, Glasgow, United Kingdom; Alexandros Rodolakis, First Department of Obstetrics and Gynecology, Athens University, Alexandra Hospital, Athens, Greece; Helga Salvesen, Department of Clinical Science, Haukeland University Hospital, Bergen, Norway; Jalid Sehouli, Department of Gynecology, Charité - Universitätsmedizin Berlin, Berlin, Germany; Cristiana Sessa, Department of Medical Oncology, Oncology Institute of Southern Switzerland, Ospedale San Giovanni, Bellinzona, Switzerland; Alexandra Taylor, Gynaecology Unit and Radiotherapy Department, The Royal Marsden NHS Foundation Trust, London, United Kingdom; Anneke Westermann, Department of Medical Oncology, Academic Medical Center, Amsterdam, The Netherlands; Alain G. Zeimet, Department of Obstetrics and Gynecology, Innsbruck Medical University, Innsbruck, Austria.
Footnotes
See appendix for members of the ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group.
These Guidelines were developed by the European SocieTy for Medical Oncology (ESMO), the European Society of Gynaecological Oncology (ESGO), and the European SocieTy of Radiotherapy and Oncology (ESTRO), and are published jointly in the Annals of Oncology, the International Journal of Gynecological Cancer and Radiotherapy & Oncology. The three societies nominated participants who attended the consensus conference and co-authored the final manuscript.
Funding: All costs relating to the consensus conference were covered from the European Society for Medical Oncology central funds. There was no external funding of the event or manuscript production.
Disclosures: Frederic Amant (senior investigator for the Research Fund Flanders [FWO]), Nicoletta Colombo (consultancy - Roche, Astra Zeneca), Luis Chiva de Agustín (speaker for Roche and Takeda), Günter Emons (research grants from Aeterna Zentaris and Astra Zeneca), Christian Kurzeder (research funding from Roche, speaker for Roche), Fabrice Lécuru (research grants from Intuitive Surgicals, advisory board for Roche), Jonathan Ledermann (advisory boards for AstraZeneca, Clovis Oncology, Merck/MSD, Bayer, Oxigene), Helga Salvesen (pending intellectual property rights for some aspects relating to STMN/pSTMN1 as a prognostic marker for endometrial cancer [US 127962,946(HS) and US 147155,412(HS)]). All remaining authors have declared no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
Contributor Information
Collaborators: the ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group
REFERENCES
- 1. WHO. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012; http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (3 April 2015, date last accessed).
- 2. Lee NK, Cheung MK, Shin JY, et al. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007; 109: 655– 662. [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Institute. Endometrial cancer treatment Physician Data Query (PDQ). 2015; http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/healthprofessional (1 April 2015, date last accessed).
- 4. ACOG. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005; 106: 413– 425. [DOI] [PubMed] [Google Scholar]
- 5. Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013; 497: 67– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reid-Nicholson M, Iyengar P, Hummer AJ, et al. Immunophenotypic diversity of endometrial adenocarcinomas: implications for differential diagnosis. Mod Pathol. 2006; 19: 1091– 1100. [DOI] [PubMed] [Google Scholar]
- 7. Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006; 106: 87– 94. [DOI] [PubMed] [Google Scholar]
- 8. Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 (suppl 6): vi33– vi38. [DOI] [PubMed] [Google Scholar]
- 9. Dykewicz CA. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001; 33: 139– 144. [DOI] [PubMed] [Google Scholar]
- 10. World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Endometrial Cancer. 2013; http://www.dietandcancerreport.org (2 April 2015, date last accessed).
- 11. Garg K, Soslow RA. Endometrial carcinoma in women aged 40 years and younger. Arch Pathol Lab Med. 2014; 138: 335– 342. [DOI] [PubMed] [Google Scholar]
- 12. Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014; 45: 28– 36. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Liu H, Yang S, et al. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers. 2014; 29: e21– e29. [DOI] [PubMed] [Google Scholar]
- 14. Rosato V, Zucchetto A, Bosetti C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011; 22: 884– 889. [DOI] [PubMed] [Google Scholar]
- 15. Luo J, Beresford S, Chen C, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014; 111: 1432– 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014; 20: 748– 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009; 114: 121– 127. [DOI] [PubMed] [Google Scholar]
- 18. Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014; 24: 384– 393. [DOI] [PubMed] [Google Scholar]
- 19. Peiretti M, Colombo N. Sex cord-stromal tumors of the ovary. In: Textbook of Gynaecological Oncology, Ankara and Istanbul: Günes Publishing, 2012: 453– 456. [Google Scholar]
- 20. Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992; 167: 1317– 1325. [DOI] [PubMed] [Google Scholar]
- 21. Zucchetto A, Serraino D, Polesel J, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev. 2009; 18: 316– 321. [DOI] [PubMed] [Google Scholar]
- 22. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998; 90: 1371– 1388. [DOI] [PubMed] [Google Scholar]
- 23. Lancaster JM, Powell CB, Chen LM, Richardson DL. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015; 136: 3– 7. [DOI] [PubMed] [Google Scholar]
- 24. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009; 59: 27– 41. [DOI] [PubMed] [Google Scholar]
- 25. Jacobs I, Gentry-Maharaj A, Burnell M, et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011; 12: 38– 48. [DOI] [PubMed] [Google Scholar]
- 26. National Cancer Institute. Endometrial Cancer Screening Physician Data Query (PDQ). 2015; www.cancer.gov/cancertopics/pdq/screening/endometrial/HealthProfessional/ (2 April 2015, date last accessed).
- 27. ACOG. Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet Gynecol. 2014; 123: 1394– 1397. [DOI] [PubMed] [Google Scholar]
- 28. Fu Y, Zhuang Z. Long-term effects of levonorgestrel-releasing intrauterine system on tamoxifen-treated breast cancer patients: a meta-analysis. Int J Clin Exp Pathol. 2014; 7: 6419– 6429. [PMC free article] [PubMed] [Google Scholar]
- 29. Manchanda R, Saridogan E, Abdelraheim A, et al. Annual outpatient hysteroscopy and endometrial sampling (OHES) in HNPCC/Lynch syndrome (LS). Arch Gynecol Obstet. 2012; 286: 1555– 1562. [DOI] [PubMed] [Google Scholar]
- 30. Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013; 62: 812– 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duska LR, Garrett A, Rueda BR, et al. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001; 83: 388– 393. [DOI] [PubMed] [Google Scholar]
- 32. Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP. Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol. 1998; 91: 349– 354. [DOI] [PubMed] [Google Scholar]
- 33. Rodolakis A, Biliatis I, Morice P, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: Clinical Recommendations for Fertility-Sparing Management in Young Endometrial Cancer Patients. Int J Gynecol Cancer. 2015; 25: 1258– 1265. [DOI] [PubMed] [Google Scholar]
- 34. Leitao MM, Jr, Kehoe S, Barakat RR, et al. Comparison of D&C and office endometrial biopsy accuracy in patients with FIGO grade 1 endometrial adenocarcinoma. Gynecol Oncol. 2009; 113: 105– 108. [DOI] [PubMed] [Google Scholar]
- 35. Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007; 25: 2798– 2803. [DOI] [PubMed] [Google Scholar]
- 36. Kinkel K, Kaji Y, Yu KK, et al. Radiologic staging in patients with endometrial cancer: a meta-analysis. Radiology. 1999; 212: 711– 718. [DOI] [PubMed] [Google Scholar]
- 37. Minig L, Franchi D, Boveri S, et al. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011; 22: 643– 649. [DOI] [PubMed] [Google Scholar]
- 38. Kim MK, Seong SJ, Song T, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecol Oncol. 2013; 130: 470– 473. [DOI] [PubMed] [Google Scholar]
- 39. Gallos ID, Yap J, Rajkhowa M, et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012; 207:266 e1– e12. [DOI] [PubMed] [Google Scholar]
- 40. Park JY, Kim DY, Kim JH, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer. 2013; 49: 868– 874. [DOI] [PubMed] [Google Scholar]
- 41. Tangjitgamol S, Manusirivithaya S, Hanprasertpong J. Fertility-sparing in endometrial cancer. Gynecol Obstet Invest. 2009; 67: 250– 268. [DOI] [PubMed] [Google Scholar]
- 42. Erkanli S, Ayhan A. Fertility-sparing therapy in young women with endometrial cancer: 2010 update. Int J Gynecol Cancer. 2010; 20: 1170– 1187. [DOI] [PubMed] [Google Scholar]
- 43. Yamazawa K, Hirai M, Fujito A, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007; 22: 1953– 1958. [DOI] [PubMed] [Google Scholar]
- 44. Koskas M, Uzan J, Luton D, et al. Prognostic factors of oncologic and reproductive outcomes in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma: systematic review and meta-analysis. Fertil Steril. 2014; 101: 785– 794. [DOI] [PubMed] [Google Scholar]
- 45. Mittal K, Soslow R, McCluggage WG. Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med. 2008; 132: 402– 423. [DOI] [PubMed] [Google Scholar]
- 46. Sun H, Enomoto T, Fujita M, et al. Mutational analysis of the PTEN gene in endometrial carcinoma and hyperplasia. Am J Clin Pathol. 2001; 115: 32– 38. [DOI] [PubMed] [Google Scholar]
- 47. Orbo A, Nilsen MN, Arnes MS, et al. Loss of expression of MLH1, MSH2, MSH6, and PTEN related to endometrial cancer in 68 patients with endometrial hyperplasia. Int J Gynecol Pathol. 2003; 22: 141– 148. [DOI] [PubMed] [Google Scholar]
- 48. Hecht JL, Pinkus JL, Pinkus GS. Enhanced detection of atypical hyperplasia in endometrial polyps by PTEN expression. Appl Immunohistochem Mol Morphol. 2004; 12: 36– 39. [DOI] [PubMed] [Google Scholar]
- 49. Monte NM, Webster KA, Neuberg D, et al. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010; 70: 6225– 6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Allison KH, Upson K, Reed SD, et al. PAX2 loss by immunohistochemistry occurs early and often in endometrial hyperplasia. Int J Gynecol Pathol. 2012; 31: 151– 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Quick CM, Laury AR, Monte NM, Mutter GL. Utility of PAX2 as a marker for diagnosis of endometrial intraepithelial neoplasia. Am J Clin Pathol. 2012; 138: 678– 684. [DOI] [PubMed] [Google Scholar]
- 52. Tashiro H, Isacson C, Levine R, et al. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997; 150: 177– 185. [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng W, Khurana R, Farahmand S, et al. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998; 22: 1463– 1473. [DOI] [PubMed] [Google Scholar]
- 54. Jia L, Liu Y, Yi X, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res. 2008; 14: 2263– 2269. [DOI] [PubMed] [Google Scholar]
- 55. Lax SF, Kendall B, Tashiro H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000; 88: 814– 824. [PubMed] [Google Scholar]
- 56. Castrillon DH, Lee KR, Nucci MR. Distinction between endometrial and endocervical adenocarcinoma: an immunohistochemical study. Int J Gynecol Pathol. 2002; 21: 4– 10. [DOI] [PubMed] [Google Scholar]
- 57. Saad RS, Mashhour M, Noftech-Mozes S, et al. P16INK4a expression in undifferentiated carcinoma of the uterus does not exclude its endometrial origin. Int J Gynecol Pathol. 2012; 31: 57– 65. [DOI] [PubMed] [Google Scholar]
- 58. Netzer IM, Kerner H, Litwin L, et al. Diagnostic implications of p16 expression in serous papillary endometrial cancer. Int J Gynecol Cancer. 2011; 21: 1441– 1445. [DOI] [PubMed] [Google Scholar]
- 59. Bagby C, Ronnett BM, Yemelyanova A, et al. Clinically occult tubal and ovarian high-grade serous carcinomas presenting in uterine samples: diagnostic pitfalls and clues to improve recognition of tumor origin. Int J Gynecol Pathol. 2013; 32: 433– 443. [DOI] [PubMed] [Google Scholar]
- 60. Hashi A, Yuminamochi T, Murata S, et al. Wilms tumor gene immunoreactivity in primary serous carcinomas of the fallopian tube, ovary, endometrium, and peritoneum. Int J Gynecol Pathol. 2003; 22: 374– 377. [DOI] [PubMed] [Google Scholar]
- 61. Köbel M, Kalloger SE, Carrick J, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol. 2009; 33: 14– 21. [DOI] [PubMed] [Google Scholar]
- 62. Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004; 44: 109– 115. [DOI] [PubMed] [Google Scholar]
- 63. Egan JA, Ionescu MC, Eapen E, et al. Differential expression of WT1 and p53 in serous and endometrioid carcinomas of the endometrium. Int J Gynecol Pathol. 2004; 23: 119– 122. [DOI] [PubMed] [Google Scholar]
- 64. Eriksson LS, Lindqvist PG, Flöter Rådestad A, et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol. 2015; 45: 476– 482. [DOI] [PubMed] [Google Scholar]
- 65. Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2014; 28: 721– 739. [DOI] [PubMed] [Google Scholar]
- 66. Scholten AN, Smit VT, Beerman H, et al. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004; 100: 764– 772. [DOI] [PubMed] [Google Scholar]
- 67. Helpman L, Kupets R, Covens A, et al. Assessment of endometrial sampling as a predictor of final surgical pathology in endometrial cancer. Br J Cancer. 2014; 110: 609– 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stephan JM, Hansen J, Samuelson M, et al. Intra-operative frozen section results reliably predict final pathology in endometrial cancer. Gynecol Oncol. 2014; 133: 499– 505. [DOI] [PubMed] [Google Scholar]
- 69. Antonsen SL, Høgdall E, Christensen IJ, et al. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: a prospective multicenter study (ENDOMET). Acta Obstet Gynecol Scand. 2013; 92: 1313– 1322. [DOI] [PubMed] [Google Scholar]
- 70. Yildiz A, Yetimalar H, Kasap B, et al. Preoperative serum CA 125 level in the prediction of the stage of disease in endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2012; 164: 191– 195. [DOI] [PubMed] [Google Scholar]
- 71. Mutz-Dehbalaie I, Egle D, Fessler S, et al. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecol Oncol. 2012; 126: 186– 191. [DOI] [PubMed] [Google Scholar]
- 72. Sun C, Chen G, Yang Z, et al. Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis. Fertil Steril. 2013; 100: 782– 787. [DOI] [PubMed] [Google Scholar]
- 73. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009; 27: 5331– 5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mourits MJ, Bijen CB, Arts HJ, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol. 2010; 11: 763– 771. [DOI] [PubMed] [Google Scholar]
- 75. Zullo F, Falbo A, Palomba S. Safety of laparoscopy vs laparotomy in the surgical staging of endometrial cancer: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2012; 207: 94– 100. [DOI] [PubMed] [Google Scholar]
- 76. Pakish J, Soliman PT, Frumovitz M, et al. A comparison of extraperitoneal versus transperitoneal laparoscopic or robotic para-aortic lymphadenectomy for staging of endometrial carcinoma. Gynecol Oncol. 2014; 132: 366– 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tozzi R, Malur S, Koehler C, Schneider A. Analysis of morbidity in patients with endometrial cancer: is there a commitment to offer laparoscopy? Gynecol Oncol. 2005; 97: 4– 9. [DOI] [PubMed] [Google Scholar]
- 78. Siesto G, Uccella S, Ghezzi F, et al. Surgical and survival outcomes in older women with endometrial cancer treated by laparoscopy. Menopause. 2010; 17: 539– 544. [DOI] [PubMed] [Google Scholar]
- 79. Palomba S, Falbo A, Mocciaro R, et al. Laparoscopic treatment for endometrial cancer: a meta-analysis of randomized controlled trials (RCTs). Gynecol Oncol. 2009; 112: 415– 421. [DOI] [PubMed] [Google Scholar]
- 80. Palomba S, Falbo A, Russo T, Zullo F. Updating of a recent meta-analysis of randomized controlled trials to assess the safety and the efficacy of the laparoscopic surgery for treating early stage endometrial cancer. Gynecol Oncol. 2009; 114: 135– 136. [DOI] [PubMed] [Google Scholar]
- 81. Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012; 30: 695– 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fader AN, Seamon LG, Escobar PF, et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: a multi-site study performed at high volume cancer centers. Gynecol Oncol. 2012; 126: 180– 185. [DOI] [PubMed] [Google Scholar]
- 83. Han KH, Park NH, Kim HS, et al. Peritoneal cytology: a risk factor of recurrence for non-endometrioid endometrial cancer. Gynecol Oncol. 2014; 134: 293– 296. [DOI] [PubMed] [Google Scholar]
- 84. Garg G, Gao F, Wright JD, et al. Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013; 128: 77– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lutman CV, Havrilesky LJ, Cragun JM, et al. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol Oncol. 2006; 102: 92– 97. [DOI] [PubMed] [Google Scholar]
- 86. Abu-Rustum NR, Iasonos A, Zhou Q, et al. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am J Obstet Gynecol. 2008; 198: 457.e1– e5; discussion 457.e5–e6. [DOI] [PubMed] [Google Scholar]
- 87. Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005; 23: 3668– 3675. [DOI] [PubMed] [Google Scholar]
- 88. Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995; 56: 29– 33. [DOI] [PubMed] [Google Scholar]
- 89. Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987; 60 (suppl 8): 2035– 2041. [DOI] [PubMed] [Google Scholar]
- 90. Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009; 115: 236– 238. [DOI] [PubMed] [Google Scholar]
- 91. Dowdy SC, Aletti G, Cliby WA, et al. Extra-peritoneal laparoscopic para-aortic lymphadenectomy–a prospective cohort study of 293 patients with endometrial cancer. Gynecol Oncol. 2008; 111: 418– 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008; 109: 11– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ballester M, Dubernard G, Lécuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011; 12: 469– 476. [DOI] [PubMed] [Google Scholar]
- 94. Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging. J Natl Compr Canc Netw. 2014; 12: 288– 297. [DOI] [PubMed] [Google Scholar]
- 95. Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100: 1707– 1716. [DOI] [PubMed] [Google Scholar]
- 96. Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373: 125– 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Naumann RW. The role of lymphadenectomy in endometrial cancer: was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol Oncol. 2012; 126: 5– 11. [DOI] [PubMed] [Google Scholar]
- 98. Smith DC, Macdonald OK, Lee CM, Gaffney DK. Survival impact of lymph node dissection in endometrial adenocarcinoma: a surveillance, epidemiology, and end results analysis. Int J Gynecol Cancer. 2008; 18: 255– 261. [DOI] [PubMed] [Google Scholar]
- 99. Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998; 71: 340– 343. [DOI] [PubMed] [Google Scholar]
- 100. Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010; 375: 1165– 1172. [DOI] [PubMed] [Google Scholar]
- 101. Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006; 107: 1823– 1830. [DOI] [PubMed] [Google Scholar]
- 102. Kim HS, Suh DH, Kim MK, et al. Systematic lymphadenectomy for survival in patients with endometrial cancer: a meta-analysis. Jpn J Clin Oncol. 2012; 42: 405– 412. [DOI] [PubMed] [Google Scholar]
- 103. Vargas R, Rauh-Hain JA, Clemmer J, et al. Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: a SEER analysis. Gynecol Oncol. 2014; 133: 216– 220. [DOI] [PubMed] [Google Scholar]
- 104. Takano M, Ochi H, Takei Y, et al. Surgery for endometrial cancers with suspected cervical involvement: is radical hysterectomy needed (a GOTIC study)? Br J Cancer. 2013; 109: 1760– 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Watanabe Y, Satou T, Nakai H, et al. Evaluation of parametrial spread in endometrial carcinoma. Obstet Gynecol. 2010; 116: 1027– 1034. [DOI] [PubMed] [Google Scholar]
- 106. Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008; 9: 297– 303. [DOI] [PubMed] [Google Scholar]
- 107. Shih KK, Yun E, Gardner GJ, et al. Surgical cytoreduction in stage IV endometrioid endometrial carcinoma. Gynecol Oncol. 2011; 122: 608– 611. [DOI] [PubMed] [Google Scholar]
- 108. Vandenput I, Trovik J, Vergote I, et al. The role of adjuvant chemotherapy in surgical stages I-II serous and clear cell carcinomas and carcinosarcoma of the endometrium: a collaborative study. Int J Gynecol Cancer. 2011; 21: 332– 336. [DOI] [PubMed] [Google Scholar]
- 109. Gokce ZK, Turan T, Karalok A, et al. Clinical outcomes of uterine carcinosarcoma: results of 94 patients. Int J Gynecol Cancer. 2015; 25: 279– 287. [DOI] [PubMed] [Google Scholar]
- 110. Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005; 366: 491– 505. [DOI] [PubMed] [Google Scholar]
- 111. Briët JM, Hollema H, Reesink N, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005; 96: 799– 804. [DOI] [PubMed] [Google Scholar]
- 112. Cohn DE, Horowitz NS, Mutch DG, et al. Should the presence of lymphvascular space involvement be used to assign patients to adjuvant therapy following hysterectomy for unstaged endometrial cancer? Gynecol Oncol. 2002; 87: 243– 246. [DOI] [PubMed] [Google Scholar]
- 113. Gadducci A, Cavazzana A, Cosio S, et al. Lymph-vascular space involvement and outer one-third myometrial invasion are strong predictors of distant haematogeneous failures in patients with stage I-II endometrioid-type endometrial cancer. Anticancer Res. 2009; 29: 1715– 1720. [PubMed] [Google Scholar]
- 114. Gemer O, Arie AB, Levy T, et al. Lymphvascular space involvement compromises the survival of patients with stage I endometrial cancer: results of a multicenter study. Eur J Surg Oncol. 2007; 33: 644– 647. [DOI] [PubMed] [Google Scholar]
- 115. Guntupalli SR, Zighelboim I, Kizer NT, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012; 124: 31– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009; 373: 137– 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000; 355: 1404– 1411. [DOI] [PubMed] [Google Scholar]
- 118. Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004; 92: 744– 751. [DOI] [PubMed] [Google Scholar]
- 119. Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012; 104: 1625– 1634. [DOI] [PubMed] [Google Scholar]
- 120. Soong R, Knowles S, Williams KE, et al. Overexpression of p53 protein is an independent prognostic indicator in human endometrial carcinoma. Br J Cancer. 1996; 74: 562– 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol. 1999; 17: 1382– 1390. [DOI] [PubMed] [Google Scholar]
- 122. AlHilli MM, Mariani A, Bakkum-Gamez JN, et al. Risk-scoring models for individualized prediction of overall survival in low-grade and high-grade endometrial cancer. Gynecol Oncol. 2014; 133: 485– 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zeimet AG, Reimer D, Huszar M, et al. L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. J Natl Cancer Inst. 2013; 105: 1142– 1150. [DOI] [PubMed] [Google Scholar]
- 124. Bosse T, Nout RA, Stelloo E, et al. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur J Cancer. 2014; 50: 2602– 2610. [DOI] [PubMed] [Google Scholar]
- 125. Sorbe B, Nordström B, Mäenpää J, et al. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int J Gynecol Cancer. 2009; 19: 873– 878. [DOI] [PubMed] [Google Scholar]
- 126. Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010; 375: 816– 823. [DOI] [PubMed] [Google Scholar]
- 127. Sorbe B, Horvath G, Andersson H, et al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma–a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012; 82: 1249– 1255. [DOI] [PubMed] [Google Scholar]
- 128. Bosse T, Peters EE, Creutzberg CL, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer - A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer. 2015; 51: 1742– 1750. [DOI] [PubMed] [Google Scholar]
- 129. Bendifallah S, Canlorbe G, Raimond E, et al. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer. 2014; 110: 2640– 2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Creutzberg CL, van Putten WL, Koper PC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol Oncol. 2003; 89: 201– 209. [DOI] [PubMed] [Google Scholar]
- 131. McMeekin DS, Filiaci VL, Aghajanian C, et al. A randomized phase III trial of pelvic radiation therapy (PXRT) versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy (VCB/C) in patients with high risk (HR), early stage endometrial cancer (EC): A Gynecologic Oncology Group trial. Gynecol Oncol. 2014; 134:438 (abstract LBA 431). [Google Scholar]
- 132. Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95 (suppl 1): S105– S143. [DOI] [PubMed] [Google Scholar]
- 133. Greven KM, Randall M, Fanning J, et al. Patterns of failure in patients with stage I, grade 3 carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 1990; 19: 529– 534. [DOI] [PubMed] [Google Scholar]
- 134. Straughn JM, Huh WK, Orr JW, Jr, et al. Stage IC adenocarcinoma of the endometrium: survival comparisons of surgically staged patients with and without adjuvant radiation therapy. Gynecol Oncol. 2003; 89: 295– 300. [DOI] [PubMed] [Google Scholar]
- 135. Creutzberg CL, van Putten WL, Wárlám-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004; 22: 1234– 1241. [DOI] [PubMed] [Google Scholar]
- 136. Sagae S, Susumu N, Viswanathan AN, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine serous carcinoma. Int J Gynecol Cancer. 2014; 24 (9 suppl 3): S83– S89. [DOI] [PubMed] [Google Scholar]
- 137. Morrow CP, Bundy BN, Homesley HD, et al. Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high-risk endometrial carcinoma, stage I and occult stage II: a Gynecologic Oncology Group Study. Gynecol Oncol. 1990; 36: 166– 171. [DOI] [PubMed] [Google Scholar]
- 138. Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008; 108: 226– 233. [DOI] [PubMed] [Google Scholar]
- 139. Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006; 95: 266– 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010; 46: 2422– 2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Greven K, Winter K, Underhill K, et al. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006; 103: 155– 159. [DOI] [PubMed] [Google Scholar]
- 142. Cooke EW, Pappas L, Gaffney DK. Does the revised International Federation of Gynecology and Obstetrics staging system for endometrial cancer lead to increased discrimination in patient outcomes? Cancer. 2011; 117: 4231– 4237. [DOI] [PubMed] [Google Scholar]
- 143. Page BR, Pappas L, Cooke EW, Gaffney DK. Does the FIGO 2009 endometrial cancer staging system more accurately correlate with clinical outcome in different histologies? Revised staging, endometrial cancer, histology. Int J Gynecol Cancer. 2012; 22: 593– 598. [DOI] [PubMed] [Google Scholar]
- 144. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105: 103– 104. [DOI] [PubMed] [Google Scholar]
- 145. Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991; 40: 55– 65. [DOI] [PubMed] [Google Scholar]
- 146. Wright JD, Fiorelli J, Kansler AL, et al. Optimizing the management of stage II endometrial cancer: the role of radical hysterectomy and radiation. Am J Obstet Gynecol. 2009; 200:419 e1– e7. [DOI] [PubMed] [Google Scholar]
- 147. Klopp A, Smith BD, Alektiar K, et al. The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2014; 4: 137– 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rossi PJ, Jani AB, Horowitz IR, Johnstone PA. Adjuvant brachytherapy removes survival disadvantage of local disease extension in stage IIIC endometrial cancer: a SEER registry analysis. Int J Radiat Oncol Biol Phys. 2008; 70: 134– 138. [DOI] [PubMed] [Google Scholar]
- 149. Randall ME, Wilder J, Greven K, Raben M. Role of intracavitary cuff boost after adjuvant external irradiation in early endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1990; 19: 49– 54. [DOI] [PubMed] [Google Scholar]
- 150. Greven K, Winter K, Underhill K, et al. Preliminary analysis of RTOG 9708: Adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer. Int J Radiat Oncol Biol Phys. 2004; 59: 168– 173. [DOI] [PubMed] [Google Scholar]
- 151. Scotti V, Borghesi S, Meattini I, et al. Postoperative radiotherapy in stage I/II endometrial cancer: retrospective analysis of 883 patients treated at the University of Florence. Int J Gynecol Cancer. 2010; 20: 1540– 1548. [PubMed] [Google Scholar]
- 152. Jobsen JJ, Lybeert ML, van der Steen-Banasik EM, et al. Multicenter cohort study on treatment results and risk factors in stage II endometrial carcinoma. Int J Gynecol Cancer. 2008; 18: 1071– 1078. [DOI] [PubMed] [Google Scholar]
- 153. Crosby MA, Tward JD, Szabo A, et al. Does brachytherapy improve survival in addition to external beam radiation therapy in patients with high risk stage I and II endometrial carcinoma? Am J Clin Oncol. 2010; 33: 364– 369. [DOI] [PubMed] [Google Scholar]
- 154. Secord AA, Geller MA, Broadwater G, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013; 128: 65– 70. [DOI] [PubMed] [Google Scholar]
- 155. Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009; 115: 6– 11. [DOI] [PubMed] [Google Scholar]
- 156. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24: 36– 44. [DOI] [PubMed] [Google Scholar]
- 157. Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012; 127: 32– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mundt AJ, McBride R, Rotmensch J, et al. Significant pelvic recurrence in high-risk pathologic stage I–IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001; 50: 1145– 1153. [DOI] [PubMed] [Google Scholar]
- 159. Viswanathan AN, Macklin EA, Berkowitz R, Matulonis U. The importance of chemotherapy and radiation in uterine papillary serous carcinoma. Gynecol Oncol. 2011; 123: 542– 547. [DOI] [PubMed] [Google Scholar]
- 160. Hasegawa K, Nagao S, Yasuda M, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the uterine corpus and cervix. Int J Gynecol Cancer. 2014; 24 (9 suppl 3): S90– S95. [DOI] [PubMed] [Google Scholar]
- 161. Barney BM, Petersen IA, Mariani A, et al. The role of vaginal brachytherapy in the treatment of surgical stage I papillary serous or clear cell endometrial cancer. Int J Radiat Oncol Biol Phys. 2013; 85: 109– 115. [DOI] [PubMed] [Google Scholar]
- 162. Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Uterine and Ovarian Carcinosarcoma. Int J Gynecol Cancer. 2014; 24 (9 suppl 3): S55– S60. [DOI] [PubMed] [Google Scholar]
- 163. Amant F. The rationale for comprehensive surgical staging in endometrial carcinosarcoma. Gynecol Oncol. 2005; 99: 521– 522; author reply 522–523. [DOI] [PubMed] [Google Scholar]
- 164. Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer. 2008; 44: 808– 818. [DOI] [PubMed] [Google Scholar]
- 165. Wright JD, Seshan VE, Shah M, et al. The role of radiation in improving survival for early-stage carcinosarcoma and leiomyosarcoma. Am J Obstet Gynecol. 2008; 199: 536.e1– e8. [DOI] [PubMed] [Google Scholar]
- 166. Clayton Smith D, Kenneth Macdonald O, Gaffney DK. The impact of adjuvant radiation therapy on survival in women with uterine carcinosarcoma. Radiother Oncol. 2008; 88: 227– 232. [DOI] [PubMed] [Google Scholar]
- 167. Nemani D, Mitra N, Guo M, Lin L. Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol. 2008; 111: 82– 88. [DOI] [PubMed] [Google Scholar]
- 168. Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007; 107: 177– 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Barlin JN, Puri I, Bristow RE. Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol Oncol. 2010; 118: 14– 18. [DOI] [PubMed] [Google Scholar]
- 170. Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004; 95: 593– 596. [DOI] [PubMed] [Google Scholar]
- 171. Jhingran A, Burke TW, Eifel PJ. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int J Radiat Oncol Biol Phys. 2003; 56: 1366– 1372. [DOI] [PubMed] [Google Scholar]
- 172. Vargo JA, Kim H, Houser CJ, et al. Definitive salvage for vaginal recurrence of endometrial cancer: the impact of modern intensity-modulated-radiotherapy with image-based HDR brachytherapy and the interplay of the PORTEC 1 risk stratification. Radiother Oncol. 2014; 113: 126– 131. [DOI] [PubMed] [Google Scholar]
- 173. Podzielinski I, Randall ME, Breheny PJ, et al. Primary radiation therapy for medically inoperable patients with clinical stage I and II endometrial carcinoma. Gynecol Oncol. 2012; 124: 36– 41. [DOI] [PubMed] [Google Scholar]
- 174. Fishman DA, Roberts KB, Chambers JT, et al. Radiation therapy as exclusive treatment for medically inoperable patients with stage I and II endometrioid carcinoma with endometrium. Gynecol Oncol. 1996; 61: 189– 196. [DOI] [PubMed] [Google Scholar]
- 175. Gill BS, Kim H, Houser C, et al. Image-based three-dimensional conformal brachytherapy for medically inoperable endometrial carcinoma. Brachytherapy. 2014; 13: 542– 547. [DOI] [PubMed] [Google Scholar]
- 176. Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999; 17: 1736– 1744. [DOI] [PubMed] [Google Scholar]
- 177. Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007; 17: 964– 978. [DOI] [PubMed] [Google Scholar]
- 178. Tangen IL, Werner HM, Berg A, et al. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer. 2014; 50: 3003– 3010. [DOI] [PubMed] [Google Scholar]
- 179. Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 1996; 14: 357– 361. [DOI] [PubMed] [Google Scholar]
- 180. Thigpen T, Brady MF, Homesley HD, et al. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001; 19: 364– 367. [DOI] [PubMed] [Google Scholar]
- 181. Ma BB, Oza A, Eisenhauer E, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers–a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer. 2004; 14: 650– 658. [DOI] [PubMed] [Google Scholar]
- 182. Rose PG, Brunetto VL, VanLe L, et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000; 78: 212– 216. [DOI] [PubMed] [Google Scholar]
- 183. Whitney CW, Brunetto VL, Zaino RJ, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004; 92: 4– 9. [DOI] [PubMed] [Google Scholar]
- 184. Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004; 92: 10– 14. [DOI] [PubMed] [Google Scholar]
- 185. van Wijk FH, Aapro MS, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003; 14: 441– 448. [DOI] [PubMed] [Google Scholar]
- 186. Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004; 22: 3902– 3908. [DOI] [PubMed] [Google Scholar]
- 187. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004; 22: 2159– 2166. [DOI] [PubMed] [Google Scholar]
- 188. Miller D, Filiaci V, Fleming G, et al. Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012; 125: 771. [Google Scholar]
- 189. Makker V, Hensley ML, Zhou Q, et al. Treatment of advanced or recurrent endometrial carcinoma with doxorubicin in patients progressing after paclitaxel/carboplatin: Memorial Sloan-Kettering Cancer Center experience from 1995 to 2009. Int J Gynecol Cancer. 2013; 23: 929– 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Grisham RN, Adaniel C, Hyman DM, et al. Gemcitabine for advanced endometrial cancer: a retrospective study of the Memorial Sloan-Kettering Cancer Center experience. Int J Gynecol Cancer. 2012; 22: 807– 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nagao S, Nishio S, Michimae H, et al. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: the SGSG-012/GOTIC-004/Intergroup study. Gynecol Oncol. 2013; 131: 567– 573. [DOI] [PubMed] [Google Scholar]
- 192. Moore KN, Tian C, McMeekin DS, et al. Does the progression-free interval after primary chemotherapy predict survival after salvage chemotherapy in advanced and recurrent endometrial cancer?: a Gynecologic Oncology Group ancillary data analysis. Cancer. 2010; 116: 5407– 5414. [DOI] [PubMed] [Google Scholar]
- 193. Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013; 62: 111– 123. [DOI] [PubMed] [Google Scholar]
- 194. Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009; 106: 4834– 4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Myers AP. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway–the devil is in the details. Clin Cancer Res. 2013; 19: 5264– 5274. [DOI] [PubMed] [Google Scholar]
- 196. Yeramian A, Moreno-Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013; 32: 403– 413. [DOI] [PubMed] [Google Scholar]
- 197. Dutt A, Salvesen HB, Chen TH, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008; 105: 8713– 8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007; 13: 7487– 7495. [DOI] [PubMed] [Google Scholar]
- 199. Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012; 13: e353– e361. [DOI] [PubMed] [Google Scholar]
- 200. Dedes KJ, Wetterskog D, Ashworth A, et al. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011; 8: 261– 271. [DOI] [PubMed] [Google Scholar]
- 201. Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011; 29: 2259– 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer. 2013; 108: 1021– 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Tsoref D, Welch S, Lau S, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. 2014; 135: 184– 189. [DOI] [PubMed] [Google Scholar]
- 204. Aghajanian C, Filiaci VL, Dizon DS, et al. A randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus and ixabepilone/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG86P. J Clin Oncol. 2015; 33 (suppl):abstract 5500. [Google Scholar]
- 205. Lorusso D, Ferrandina G, Colombo N, et al. Randomized phase II trial of carboplatin-paclitaxel (CP) compared to carboplatin-paclitaxel-bevacizumab (CP-B) in advanced (stage III–IV) or recurrent endometrial cancer: The MITO END-2 trial. J Clin Oncol. 2015; 33 (suppl):abstract 5502. [DOI] [PubMed] [Google Scholar]
- 206. Werner HM, Salvesen HB. Current status of molecular biomarkers in endometrial cancer. Curr Oncol Rep. 2014; 16: 403. [DOI] [PubMed] [Google Scholar]
- 207. Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Curr Opin Oncol. 2012; 24: 554– 563. [DOI] [PubMed] [Google Scholar]
- 208. Konecny GE, Finkler N, Garcia AA, et al. Phase 2 study of second-line dovitinib (TKI258) in patients with fibroblast growth factor receptor 2 (FGFR2)-mutated or -nonmutated advanced and/or metastatic endometrial cancer. Ann Oncol. 2014; 25 (suppl 4): LBA27. [DOI] [PubMed] [Google Scholar]
- 209. Mackay HJ, Eisenhauer EA, Kamel-Reid S, et al. Molecular determinants of outcome with mammalian target of rapamycin inhibition in endometrial cancer. Cancer. 2014; 120: 603– 610. [DOI] [PubMed] [Google Scholar]
- 210. Korn EL, Freidlin B, Abrams JS, Halabi S. Design issues in randomized phase II/III trials. J Clin Oncol. 2012; 30: 667– 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Redig AJ, Jänne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J Clin Oncol. 2015; 33: 975– 977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


