Objective:
HIV testing is the entry point to access HIV care. For HIV-infected children who survive infancy undiagnosed, diagnosis usually occurs on presentation to health care services. We investigated the effectiveness of routine opt-out HIV testing (ROOT) compared with conventional opt-in provider-initiated testing and counseling (PITC) for children attending primary care clinics.
Methods:
After an evaluation of PITC services for children aged 6–15 years in 6 primary health care facilities in Harare, Zimbabwe, ROOT was introduced through a combination of interventions. The change in the proportion of eligible children offered and receiving HIV tests, reasons for not testing, and yield of HIV-positive diagnoses were compared between the 2 HIV testing strategies. Adjusted risk ratios for having an HIV test in the ROOT compared with the PITC period were calculated.
Results:
There were 2831 and 7842 children eligible for HIV testing before and after the introduction of ROOT. The proportion of eligible children offered testing increased from 76% to 93% and test uptake improved from 71% to 95% in the ROOT compared with the PITC period. The yield of HIV diagnoses increased from 2.9% to 4.5%, and a child attending the clinics post intervention had a 1.99 increased adjusted risk (95% CI: 1.85 to 2.14) of receiving an HIV test in the ROOT period compared with the preintervention period.
Conclusion:
ROOT increased the proportion of children undergoing HIV testing, resulting in an overall increased yield of positive diagnoses, compared with PITC. ROOT provides an effective approach to reduce missed HIV diagnosis in this age group.
Key Words: HIV, children, Africa, routine opt-out HIV testing, PITC
BACKGROUND
The prevalence of undiagnosed HIV in children is higher and coverage of antiretroviral therapy (ART) lower compared with adults.1 HIV testing for HIV-exposed infants is available as part of the prevention of mother-to-child transmission programs. However, the coverage of HIV testing in infancy remains poor, with only 20% of HIV-exposed infants accessing early infant diagnosis in high burden countries.2,3 Nearly a third of undiagnosed HIV-infected infants survive to late childhood.4,5 For these children who survive infancy untreated, diagnosis of their HIV infection relies predominantly on testing within health care services when they present with HIV-indicator conditions in late childhood and adolescence.6,7 Delayed diagnosis of HIV results in increased morbidity from infections and other chronic complications such as growth failure and organ damage.8 Treatment outcomes are worse among those who start HIV treatment at an advanced disease stage.9 In addition, once children enter adolescence and become sexually active, there is a high risk of onward HIV transmission.8
In the early stages of the HIV epidemic, client-initiated testing strategies termed voluntary counseling and testing were used. As antiretroviral therapy was scaled up, guidelines shifted to a model of provider-initiated testing and counseling (PITC) in high HIV burden settings, whereby testing is proactively offered to any client attending a health care facility by the health care worker (HCW), regardless of the reason for presentation.10–12 Although the goal of PITC is to provide HIV testing to a high proportion of health service users, coverage remains incomplete, particularly in older children. This approach relies on both the HCW taking the initiative to offer testing and the client agreeing to test. In practice, the proportion of individuals offered HIV testing varies, and is influenced by provider-perceived risks and benefits of testing the relationship between provider and client, client demographics, and logistical constraints.13
An alternative testing approach that limits the influence of the HCW and hence variability in testing is routine opt-out HIV testing (ROOT). In this model, rather than individual HCW deciding whether or not to test on a case-by-case basis, HIV testing is conducted routinely as part of each clinical consultation and must be actively declined by the client if not desired. To date, the use of ROOT is largely confined to specialist services such as PMTCT programs, sexually transmitted disease, and tuberculosis services.14,15 In children, ROOT has been shown to be acceptable, feasible, and effective within immunization services and pediatric inpatient wards.16–18
We have previously shown that opt-in PITC offered to older children in primary care services resulted in only 54% of those eligible being tested for HIV.19 Key barriers to the provision of PITC to children by HCW included a lack of confidence in counseling children, limited awareness of the burden of HIV in older children, and uncertainty about legal guidelines. This study aimed to compare the effectiveness of ROOT with PITC in older children aged 6–15 years in 7 primary care facilities in Harare, Zimbabwe.
METHODS
An evaluation of PITC for children aged between 6 and 15 years was performed in 6 primary health care clinics (PHCs) in high-density suburbs in Harare, Zimbabwe, between mid-January and mid-May 2013. Each clinic serves 1 suburb and provides comprehensive outpatient primary care, including acute care, maternal and child health services and HIV care services, as well as antenatal, delivery, and postnatal services. Clinical care is provided by nurses, with visits by a doctor on a weekly basis. PITC in all health care facilities has been part of the National Guidelines since 2007. HIV testing in PHCs is usually performed by lay counselors who have undergone certified training in HIV counseling and testing.
Activities were initiated in mid-May 2013 in preparation for the introduction of ROOT. These activities were supported by the municipal health authorities and clinic management teams. A 1-day meeting was held for senior nursing personnel (the nurses in charge at each clinic and the district nursing officers) to understand the challenges of providing HIV testing to children in the primary care environment and to discuss the changes that would be required to implement ROOT. This was followed by a 5-day training course at each clinic site for clinic nurses and lay counselors, who are responsible for performing the bulk of the HIV testing. The training focused specifically on issues relating to testing children, including counseling of children and guardians, frameworks for consent and guardianship, the burden of HIV among older children, and the benefits of early treatment. Specifically, HCW were trained on how to implement an opt-out testing model. Further training was not provided during the course of the study.
A mentorship program in pediatric HIV was established to provide HCW with ongoing support. An additional lay counselor was deployed at each clinic, whose main task was to perform HIV testing in children when routine clinic staff was unavailable. In addition, a buffer supply of HIV testing kits was made available to ensure an uninterrupted supply. The kits and additional staff were funded by the study for the duration of the study period. The outcomes of ROOT were evaluated over a period of 17 months.
The implementation of ROOT involved several activities over a period of 2 months (mid-May to mid-July 2013). Thus, full implementation of ROOT was only in place from mid-July 2013 onward. The period mid-January to mid-February 2013 was labeled February 2013, mid-February to mid-March 2013 was labeled March 2013 and so forth. ROOT was performed for every child aged 6–15 years, attending the PHC for any reason, unless the child had a documented HIV test result from the past 6 months, was already registered in an HIV care service, or was attending without a caregiver (unless an emancipated minor). HIV testing was performed unless the caregiver or the child specifically declined permission, as per national guidelines.20 A caregiver was defined as someone aged 18 years or older and responsible for the day-to-day care of the child. Emancipated minors were defined as those who were married, living with a sexual partner, or who had children gave independent consent. ROOT was not performed in children who were moribund or required immediate hospitalization. The standard HIV testing algorithm recommended by the national guidelines was used; a rapid HIV antibody testing kit (Abbott Determine) was used with all positive tests confirmed by a second rapid antibody test (SD Bioline). A discrepant test result was resolved using a third tie-breaker test (INSTI). Ethical approval for the study was obtained from the Medical Research Council of Zimbabwe and the Ethics Committees of Harare City Health Services, the Biomedical Research and Training Institute, and the London School of Hygiene and Tropical Medicine.
Data on socio-demographics of the child and guardian, the number of attendances of children aged 6–15 years, the number of tests offered and accepted, and reasons why testing did not occur were collected prospectively as previously described.19 Data were analyzed using STATA version 12.0 (StataCorp, College Station, TX). The proportion of children being offered and accepting testing, the yield of HIV-positive diagnoses (defined as the number of children testing positive among all children eligible for testing), and reasons for not being tested for HIV were compared before and after the introduction of ROOT. The proportions not tested due to a particular reason were calculated and stratified by PITC and ROOT period using the total number of children eligible during a period as a denominator. Modified Poisson regression was used to calculate the risk of being tested before and after the intervention, controlling for child and guardian age and sex, as well as client factors likely to raise the suspicion of HIV infection including orphanhood, skin conditions, previous hospitalization, and self-reported poor health in the last 3 months.
RESULTS
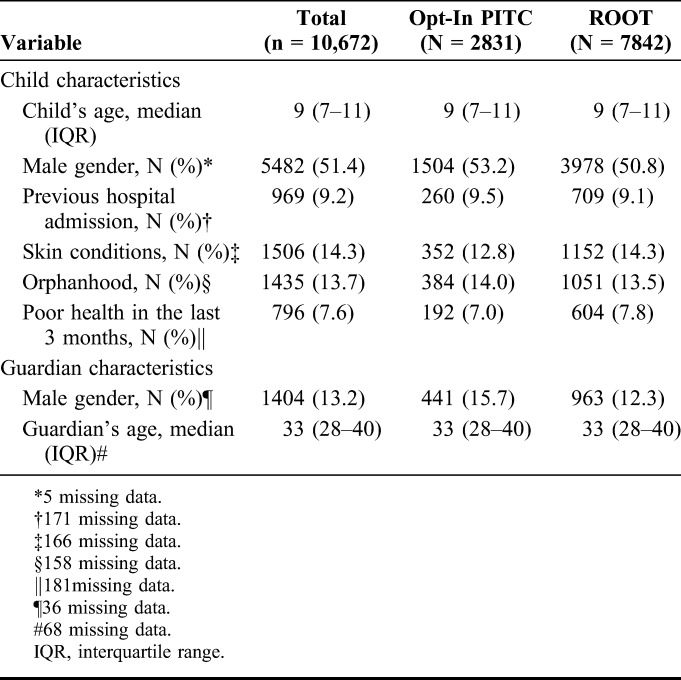
There were 3394 and 14,084 visits in the PITC and ROOT period respectively (Fig. 1). A total of 2831 and 784 children were eligible for HIV testing before and after the introduction of ROOT. The proportion of children eligible for HIV testing decreased in the PITC period to the ROOT period, mainly due to an increase in the proportion of children who had tested previously and know HIV-positive children (Fig. 1). In the PITC period, 3% of visits were due to children who had tested for HIV in the previous 6 months compared with 11% during the ROOT period. Furthermore, the proportion of visits by known HIV-infected children increased from 9% to 18%. The median age was 9 years (interquartile range 7–11), 13.7% of children were single or double orphans, 9.2% reported a previous hospital admission, and 7.6% reported poor health in the 3 months before the visit. The guardian and client characteristics of those presenting before and after the introduction of ROOT were similar (Table 1).
FIGURE 1.
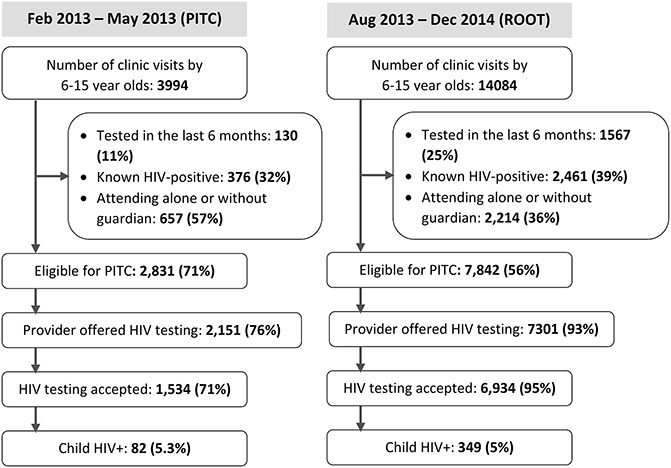
Numbers of children aged 6–15 years who attended primary care clinics, eligible, offered testing, underwent testing, and tested HIV-positive during the PITC and ROOT periods respectively.
TABLE 1.
Characteristics of Children Eligible for HIV-Testing (n = 10,672)
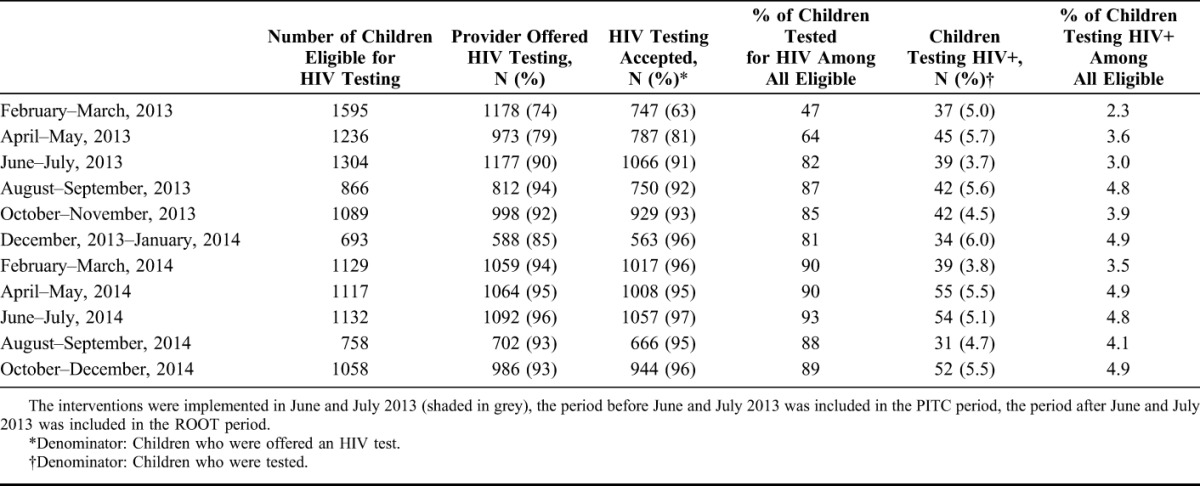
The proportion of eligible children offered testing increased from 76.0% to 93% after the introduction of ROOT (Table 2). Test uptake among children offered a test improved from 71% to 95% during the ROOT period. The percentage of children testing HIV positive among all children eligible for testing (yield) increased from 2.9% to 4.5%, comparing the period before and after introduction of ROOT. Of note, the improvement in the proportion of tests offered and accepted, and HIV yield, persisted throughout the 17 months of ROOT (Table 2).
TABLE 2.
Number of Children Eligible, Offered Testing, Accepting Testing and Testing Positive per Month
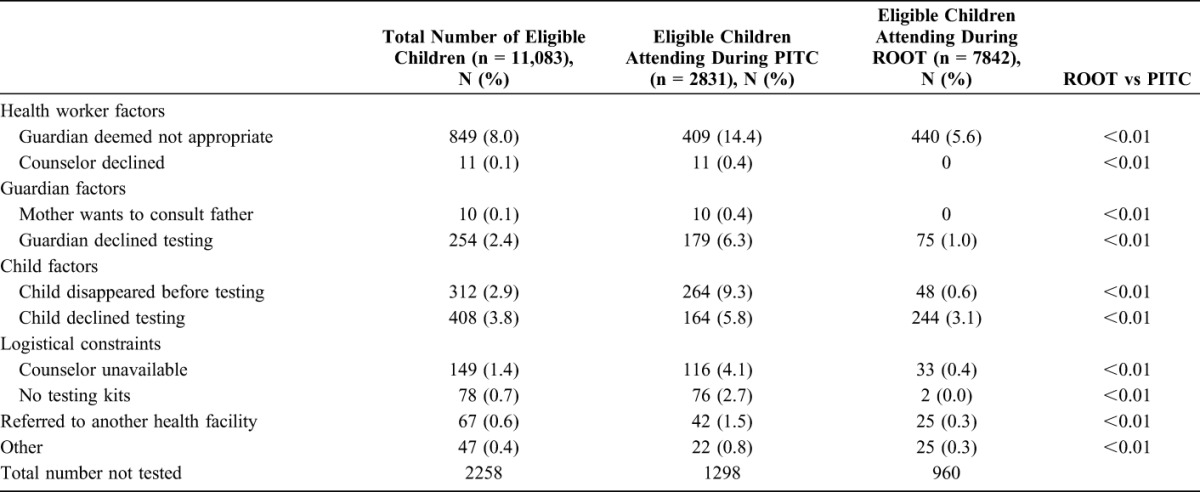
The overall proportion of children attending PHCs and eligible for HIV testing, who did not receive a test, fell from 45.8% with PITC to 11.6% with ROOT. A child attending the primary health care clinics post intervention was twice as likely (adjusted risk ratio 1.99; 95% CI: 1.85 to 2.14) to receive an HIV test compared with the preintervention period. The main reasons for not testing pre-ROOT were related to HCW concerns with the guardian (14.4%), children leaving the facility (9.3%) or declining tests (5.8%), guardian refusal of testing (6.3%) or shortage of HIV testing kits or staff (6.8%) (Table 3). A decrease was seen in all reasons for not testing. The proportion of guardians or children who declined testing dropped to 4.1% after the introduction of ROOT.
TABLE 3.
Reasons for Eligible Child Not Receiving an HIV Test
DISCUSSION
To our knowledge, this is the first study to evaluate ROOT for older children in primary health care services. It demonstrated that the use of ROOT, when accompanied by adequate training and support for HCWs, effectively increases the proportion of older children to whom testing is offered, the uptake, and the yield compared with opt-in PITC. Reassuringly, the results were sustained over a 17-month period without any evidence of testing fatigue, despite no further training.
This study was conceived after our findings that routine PITC in primary care services resulted in nearly half the eligible children not being tested for HIV. The predominant reason that HIV testing did not occur related to HCW factors. HCWs worried about whether an accompanying caregiver (if not a biological parent) was “suitable” to give consent and feared the consequences if they made an incorrect decision. After the introduction of ROOT, the proportion of guardians felt to be inappropriate diminished significantly, as did the proportion of children referred to other services for HIV testing. This may be a result of an improved understanding of the legal framework for pediatric HIV testing in Zimbabwe and might reflect greater comfort with offering testing when the decision to test is removed from the individual HCW and is instead adopted as the default approach.
Although PITC is meant to be offered to all health care facility attendees, HCW practiced “symptom-based PITC” and offering HIV testing selectively to those whom they considered at risk of being HIV-infected. Before implementation of ROOT, offering HIV testing to children by HCW was associated with a child having stigmata of HIV infection, namely orphanhood and chronic ill-health.19 No associations between offering HIV testing and any of these variables were found during the ROOT period (data not shown). The increase in yield of HIV-positive test results after introduction of ROOT indicates that HCW do not correctly estimate the HIV risk when choosing whom to offer testing to in an opt-in system.
HIV testing in children relies not only on HCWs offering the test but on guardians consenting to their child having a test. Other studies have shown that routine opt-out testing improves acceptability and uptake of HIV testing by clients.21,22 Similarly, in this study, the proportion of guardians declining to have their child tested decreased from 6.0% to 0.5% after introduction of ROOT. Decreased stigma in the context of routine practice, and again, removal of the decision of whether to test from the guardian might explain this result.
An opt-out strategy transforms an intervention into a routine, default clinical action, which removes some decision making from the HCW and guardians. A potential concern is whether an opt-out approach may result in coercion of the client and reduce client autonomy.23 This has been the subject of debate, particularly in the context of PMTCT programs.24–26 HIV diagnosis is a prerequisite to accessing HIV care. The concern about autonomy and potential coercion must be weighed against a child's right to access effective and life-saving treatment, and the limited alternative options for children to obtain HIV testing. This is particularly important among children who rely on others to give consent on their behalf. In this study, the proportion of children who declined testing remained similar in before and after implementation of ROOT. This is reassuring as it indicates that children remained empowered to refuse if they wished, and were no more pressured into HIV testing with ROOT compared with PITC. The lack of change in child refusal is perhaps unsurprising, as a child's assent for testing may relate less to the type of testing model used, but rather to the nature of the test itself.
A strength of this study is that it enrolled large numbers of children, and compared both models in the same clinical settings representative of primary care clinics. We acknowledge some limitations to our study. Before-and-after comparisons are vulnerable to coincidental effects. However, there were no differences in the demographic characteristics of the guardians or children in the PITC compared with the ROOT period. In addition, the large difference in the likelihood of HIV testing observed after adjustment for potential confounders gives confidence in the results. In addition, changes were implemented over a relatively short period and the changes are therefore unlikely to be explained by secular trends. The implementation of ROOT was accompanied by HCW training and addressing structural barriers to HIV testing, including shortage of HCW and of HIV testing kits.
The buffer supply of kits resulted in an extraneous difference between the ROOT and the PITC approach. The investment in training and human resource capacity likely contributed to improvements in HIV testing and the opt-out approach. However, the proportion of missed tests attributed to logistical constraints before the introduction of ROOT (6.8%) was relatively low compared with other reasons for HIV testing not having occurred, and the substantial improvement in HIV testing rates is unlikely to be entirely a reflection of improved logistical support. Moreover, successful implementation of any HIV testing strategy in children will require training of HCW and addressing structural issues, and these are integral to and not alternative to provision of any HIV testing strategy.
This study shows that a ROOT strategy is a feasible and effective approach to increase diagnosis among children: a group for whom opportunities to receive HIV testing have been limited. Notably, whereas other studies have shown that opt-out testing has an impact on client acceptability of HIV testing, this study demonstrates that ROOT results in an improvement in proportion of clients being offered HIV testing by HCW. This is important as policies to scale-up and improve PITC must take into account not only client acceptability but also address barriers faced by HCWs. This study was performed in a generalized HIV epidemic setting. A potential concern is that ROOT is a 'blanket testing' strategy and may be inefficient. Our findings show that ROOT does increase yield, and may be a particularly cost-effective strategy in high HIV prevalence settings, as well as in other settings where the expected yield of HIV is higher, e.g., malnutrition and tuberculosis services and inpatient wards.
If implemented, a routine opt-out testing strategy must be partnered with counselor training, engagement with local health providers and government bodies, and provision of logistical support to maximize impact. If an approach such as this is to be scaled up, fundamental structural barriers such as long clinic waiting times, limited staffing levels, health care worker training, and management of supply chains for testing kits need to be addressed. In addition, measures to avoid retesting in children will need to be put in place.
Moving forward, a number of questions need to be explored. Cost-effectiveness studies need to be performed, and ethical and human rights implications of opt-out HIV testing in a vulnerable group such as children need to be investigated.27,28 Qualitative studies exploring child and guardian perceptions are needed to inform service delivery. Finally, HIV testing must be accompanied by effective strategies to ensure linkage to care.29
ACKNOWLEDGMENTS
The authors thank the nurses and counselors at the 6 study clinics for their assistance with the study. They also thank Dr Saeed Ahmed for helpful discussions and constructive comments on the article.
Footnotes
Supported by the Wellcome Trust through an Intermediate Fellowship awarded to RAF (Grant no 095878/Z/11Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
R.A.F. designed the study. E.D. and R.A.F. supervised data collection. T.B. and K.K. analyzed the data. J.M. and K.K. wrote the first draft of the article. All authors contributed to the writing of the article.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2.UNAIDS. Progress Report on the Global Plan towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland; UNAIDS: 2013. [Google Scholar]
- 3.Wettstein C, Mugglin C, Egger M, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26:2361–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrand RA, Bandason T, Musvaire P, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med. 2010;7:e1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroufi A, Gunguwo H, Dixon M, et al. HIV-infected adolescents in southern Africa can achieve good treatment outcomes: results from a retrospective cohort study. AIDS. 2013;27:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. [DOI] [PubMed] [Google Scholar]
- 10.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 11.Vermund SH, Wilson CM, Rogers AS, et al. Sexually transmitted infections among HIV infected and HIV uninfected high-risk youth in the REACH study. Reaching for excellence in adolescent care and health. J Adolesc Health. 2001;29(3 suppl l):49–56. [DOI] [PubMed] [Google Scholar]
- 12.Bayer R, Edington C. HIV testing, human rights, and global AIDS policy: exceptionalism and its discontents. J Health Polit Policy Law. 2009;34:301–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roura M, Watson-Jones D, Kahawita TM, et al. Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS. 2013;27:617–626. [DOI] [PubMed] [Google Scholar]
- 14.Njeru MK, Blystad A, Shayo EH, et al. Practicing provider-initiated HIV testing in high prevalence settings: consent concerns and missed preventive opportunities. BMC Health Serv Res. 2011;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leon N, Naidoo P, Mathews C, et al. The impact of provider-initiated (opt-out) HIV testing and counseling of patients with sexually transmitted infection in Cape Town, South Africa: a controlled trial. Implement Sci. 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. [DOI] [PubMed] [Google Scholar]
- 17.McCollum ED, Preidis GA, Golitko CL, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimbabwe National Guidelines on HIV Testing and Counselling in Children. Zimbabwe, Africa: Ministry of Health and Child Welfare; 2008. [Google Scholar]
- 21.Moses A, Zimba C, Kamanga E, et al. Prevention of mother-to-child transmission: program changes and the effect on uptake of the HIVNET 012 regimen in Malawi. AIDS. 2008;22:83–87. [DOI] [PubMed] [Google Scholar]
- 22.Baisley K, Doyle AM, Changalucha J, et al. Uptake of voluntary counselling and testing among young people participating in an HIV prevention trial: comparison of opt-out and opt-in strategies. PLoS One. 2012;7:e42108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennie S, Behets F. Desperately seeking targets: the ethics of routine HIV testing in low-income countries. Bull World Health Organ. 2006;84:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson KA, Pedersen KB, Andersson AK. HIV testing of pregnant women: an ethical analysis. Dev World Bioeth. 2011;11:109–119. [DOI] [PubMed] [Google Scholar]
- 25.Angotti N, Dionne KY, Gaydosh L. An offer you can't refuse? Provider-initiated HIV testing in antenatal clinics in rural Malawi. Health Policy Plan. 2011;26:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardon A, Vernooij E, Bongololo-Mbera G, et al. Women's views on consent, counseling and confidentiality in PMTCT: a mixed-methods study in four African countries. BMC Public Health. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csete J, Schleifer R, Cohen J. “Opt-out” testing for HIV in Africa: a caution. Lancet. 2004;363:493–494. [DOI] [PubMed] [Google Scholar]
- 28.Weiser SD, Heisler M, Leiter K, et al. Routine HIV testing in Botswana: a population-based study on attitudes, practices, and human rights concerns. PLoS Med. 2006;3:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clouse K, Hanrahan CF, Bassett J, et al. Impact of systematic HIV testing on case finding and retention in care at a primary care clinic in South Africa. Trop Med Int Health. 2014;19:1411–1419. [DOI] [PubMed] [Google Scholar]


