Background
Little research has focused on whether there are individual differences among children in their sensitivity to sweet taste and, if so, the biological correlates of such differences.
Objectives
Our goal was to understand how variations in children’s sucrose detection thresholds relate to their age and gender, taste genotype, body composition, and dietary intake of added sugars.
Methods
Sucrose detection thresholds in 7- to 14-year-old children were tested individually using a validated, two-alternative, forced-choice, paired-comparison tracking method. Five genetic variants of taste genes were assayed: TAS1R3 and GNAT3 (sweet genes; one variant each) and the bitter receptor gene TAS2R38 (three variants). All children were measured for body weight and height. A subset of these children were measured for the percentage of body fat and waist circumference and provided added sugar intake by 24-hour dietary recall.
Results
Sucrose thresholds ranged from 0.23 to 153.8 mM with most of the children completing the threshold task (216/235; 92%). Some children were biologically related (i.e., siblings), and for the genetic analysis, one sibling from each family was studied. Variants in the bitter but not the sweet genes were related to sucrose threshold and sugar intake; children with two bitter-sensitive alleles could detect sucrose at lower concentrations (F(2,165) = 4.55, p = .01; rs1726866) and reported eating more added sugar (% kcal; F(2, 62) = 3.64, p = .03) than did children with less sensitive alleles. Age, gender, and indices of obesity also were related to child-to-child differences in sucrose threshold; girls were more sensitive than boys (t(214) = 2.0, p = .05), older children were more sensitive than younger children (r(214) = −.16, p = .02), and fatter (r(84) = −.22, p = .05) or more centrally obese children (r(84) = −.26, p = .02) were more sensitive relative to others.
Discussion
Inborn differences in bitter sensitivity may affect childhood dietary sugar intake with long-term health consequences. There may be a more complex interplay between the developing bitter and sweet taste systems than previously understood.
Key Words: anthropometry, bitter, children, genotype, sensory thresholds, sweet, taste
Sweetness as a sensation starts on the tongue and engages several signaling proteins that are coded by specific genes in the human genome. Sucrose stimulates a receptor on taste cells; the resulting signal is conducted via G proteins and eventually produces a signal interpreted centrally as sweet taste (i.e., taste transduction). The sweet receptor has two parts: the gene TAS1R2 encoding the first part was discovered in 1999, and the second gene, TAS1R3, was discovered in 2001 (for a review, see Reed & McDaniel, 2006). The respective proteins from these genes are T1R2 and T1R3. Among the G proteins, the one associated with sweet signaling is gustducin (Gα protein subunit), encoded by GNAT3 (McLaughlin, McKinnon, & Margolskee, 1992).
In adult populations, variation in the TAS1R3 and GNAT3 genes relates to differences in the ability to perceive sweet tasting stimuli. For GNAT3, adults with two C alleles (CC) were better able to sort low concentrations of sucrose into the correct order than those with two T alleles (TT; rs7792845; Fushan, Simons, Slack, & Drayna, 2010). For TAS1R3, adults with one or two copies of the T nucleotide (TT) were less sensitive to the taste of sucrose than were those with two copies of the alternative C allele (CC; rs35744183; Fushan, Simons, Slack, Manichaikul, & Drayna, 2009). Adults with the TT genotype of the TAS1R3 gene also preferred higher levels of sweetness than those with the CC genotype (Mennella, Finkbeiner, Lipchock, Hwang, & Reed, 2014; Mennella, Finkbeiner, & Reed, 2012; Mennella, Reed, Mathew, Roberts, & Mansfield, 2015), possibly because they need more sucrose to obtain the same hedonic effect.
Children live in different sensory worlds than adults when it comes to sweet taste (Mennella, 2008): They prefer higher levels than do adults (Mennella, Finkbeiner, et al., 2014), with preferences declining to adult levels during adolescence, which coincides with the cessation of physical growth (Coldwell, Oswald, & Reed, 2009; Mennella, Finkbeiner, et al., 2014). To date, whether genotype-related differences in sweet taste sensitivity exist among children has not been investigated. We do know, however, that variation in the TAS1R3 gene does not relate to differences in levels of sucrose most preferred in children, like it does in adults (Mennella et al., 2012, 2015; Mennella, Finkbeiner, et al., 2014).
Unlike the sweet-associated TAS1R3 gene, variation in bitter receptor gene TAS2R38 can explain individual differences in sweet preferences among children. TAS2R38 contains three variant locations—best known for their association with the bitter perception of thioureas—such as propylthiouracil (Bufe et al., 2005; Kim et al., 2003). Foods containing thioureas are cruciferous vegetables such as kale, cabbage, and broccoli. Children with the bitter-sensitive genotypes (AP, PP; rs713598, A49P) prefer significantly higher levels of sucrose than those with the bitter-insensitive genotype (AA) both in laboratory-based measures and in reported preferences of real-world foods like cereal and beverages (Mennella et al., 2012; Mennella, Pepino, & Reed, 2005). Other investigators also report that children who are bitter sensitive consume diets higher in sugar than do bitter-insensitive children (Keller & Tepper, 2004).
With these points in mind, we examined the degree of variation in children’s sucrose detection thresholds and whether sweet- and bitter-related genotypes might partially be accounted for such variation. Genotypes that were related to sucrose threshold were examined for the propensity of children to consume part of their calories as added sugars. Thus, estimates of dietary intake of added sugars (mg) and daily caloric intake (kcal/day) were obtained for a subset of the children. We also hypothesized that if sweet taste sensitivity, diet, and obesity share a common etiology, then sweet sensitivity could potentially provide insights into obesity. To that end, we examined how sensitivity to sweet taste varies with anthropometric measures of obesity: body mass index (BMI; a ratio of height to weight with national norms by age), percentage of body fat (an index of overall adiposity), and central obesity (waist-to-height ratio [WHtR]).
METHODS
Participants
Mothers of healthy children 7–14 years of age were recruited for a “taste study” from local advertisements and from a list of past participants who asked to be notified of future studies. Only children who were healthy at the time of testing, with no major medical illness such as diabetes, heart disease, or asthma, were included. All procedures were approved by the institutional review board, which is part of the Office of Regulatory Affairs at the University of Pennsylvania. Before study participation, written informed consent was obtained from each mother and informed assent from each child. All measures were collected during one test session, with anthropometry collected at the beginning of the session.
Children’s Sucrose Taste Threshold Training and Testing
Participants were tested after fasting for at least 1 hour and acclimation to the study personnel and room for at least 15 minutes. Detection thresholds were measured by using a two-alternative, forced-choice staircase procedure developed at the Monell Center for adults (Cowart & Beauchamp, 1990; Mennella, Lukasewycz, Griffith, & Beauchamp, 2011; Pribitkin, Rosenthal, & Cowart, 2003) and later adapted for use among pediatric populations (Bobowski & Mennella, 2015). Testing took place in a private, comfortable room specifically designed for sensory testing that was illuminated with red light to mask any visual differences among samples. Prior to testing, all children were trained to become familiar with the method and to assess whether they understood the detection threshold task, as follows. Children were presented with a pair of 30-ml disposable medicine cups (Fisher Scientific, Pittsburgh, PA), one containing distilled water and the other containing either 0.056 mM or 18 mM sucrose solution in random order (Pepino, Finkbeiner, Beauchamp, & Mennella, 2010). Children were asked to taste both solutions in the order presented and to point to the solution that had a taste. The two pairs provided the children with the experience of tasting a pair of solutions in which they could not detect a difference (i.e., water vs. 0.056 mM sucrose) and a pair that was easily discernible (i.e., water vs. 18 mM sucrose)—conditions encountered during threshold testing. This method eliminated the need for a verbal response and has been shown to be an effective method for assessing both taste and olfaction in children (Bobowski & Mennella, 2015; Mennella, Finkbeiner, et al., 2014).
For testing and as shown in Figure 1, the 17 solutions ranged from 1000 to 0.056 mM in quarter-log steps. The first stimulus presented was near the middle of the concentration series starting at 3.2 mM. During this and each subsequent trial, participants were presented with pairs of solutions—the distilled water and the taste stimulus. Participants were instructed to taste the first solution presented within a pair, swish the solution in their mouth for 5 seconds, and expectorate and then to taste the second solution within a pair using the same protocol; participants rinsed their mouths with water once between solutions within a pair and twice between successive pairs. After tasting both solutions within a pair, participants were asked to point to the solution that had a taste, as in the training task. The concentration of the tastant in the solution presented in the subsequent pair was increased after a single incorrect response and decreased after two consecutive correct responses. A reversal occurred when the concentration sequence changed direction (i.e., an incorrect response followed by a correct response or vice versa). A tracking grid was used to record participants’ responses (Figure 1). The testing procedure ended after four reversals occurred, provided that there were no more than two dilution steps between two consecutive reversals and the reversals did not form an ascending pattern such that positive and negative reversals were achieved at successively higher concentrations. The participants’ detection threshold for sucrose was the mean of the log values of the last four reversals.
FIGURE 1.
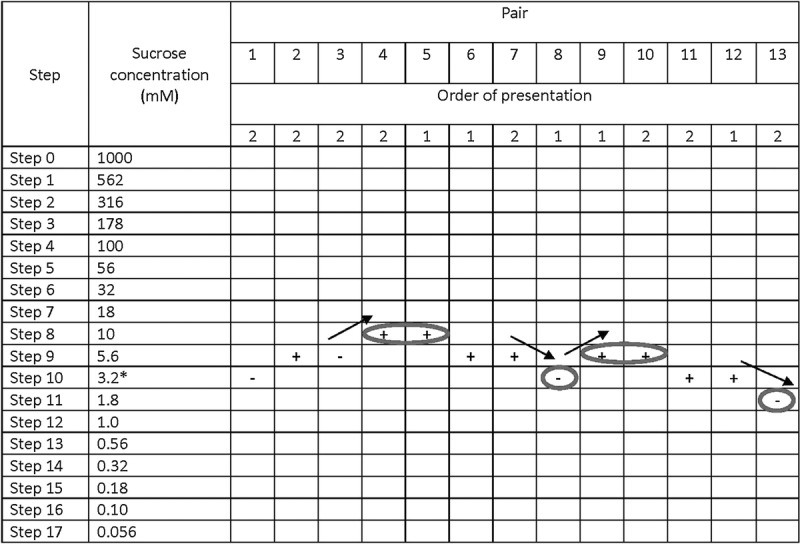
The tracking grid used in the paired comparison method to determine sucrose detection thresholds. The order of presentation of pairs of solutions was determined at random (presentation order 1 signifies water is presented first within that particular pair, whereas 2 signifies the solution with a tastant [sucrose] is presented first within that particular pair). Testing began at Step 10, which corresponded to a concentration of 3.2 mM sucrose. Participants were presented with a pair of solutions, asked to taste each solution following the test protocol, and to point to the solution that had a taste. The tracking grid was used to record whether a participant correctly (+) or incorrectly (−) identified the solution with a taste; the concentration of the tastant in the solution presented in the subsequent pair was increased after a single incorrect response and decreased after two consecutive correct responses. Testing continued until the participant attained four reversals in performance (circled and marked with arrows to indicate the direction of reversal); a participant’s threshold for a tastant was calculated as the mean of the log values of the last four reversals. In this example, the participant’s molar concentrations for each reversal were 10, 3.2, 5.6, and 1.8 mM. Thus, the calculation is [((−2.00) + (−2.49) + (−2.25) + (−2.74)/4)] = −2.37. We computed the antilog, which is ~4.2 mM.
Demographics, Anthropometry, and Diet
Demographic data were collected by maternal interview. All but three children were weighed (kg; Model 439 physical scale; Detecto, Webb City, MO) and measured for height (cm) wearing light clothing and no shoes. BMI (kg/m2) was determined, after which age- and gender-specific BMI z scores were calculated using EpiInfo 3.5 (www.cdc.gov/epiinfo). Participants were placed into one of four BMI categories (underweight, healthy weight, overweight, and obese) according to Centers for Disease Control and Prevention’s pediatric growth charts (Kuczmarski et al., 2002).
For a subset of the subjects tested (n = 96), we obtained additional anthropometric measures and dietary intake data. First, we determined WHtR: Participants stood with their weight evenly distributed on both feet, with their feet about 25–30 cm apart, and their abdominal circumference was measured to the nearest 0.1 cm. Children with WHtR ≥ 0.5 were considered to have central obesity based on criteria previously established (Mokha et al., 2010). We also determined the percentage of body fat on all but one of these children (n = 95) by bioelectrical impedance analysis (Chumlea et al., 2002) using the Quantum X instrument (RJL Systems, Clinton Township, MI).
Dietary intake data using the Automated Self-Administered 24-Hour Recall system (ASA24), developed by the National Cancer Institute (2014). On testing day, mothers and children sat side by side as the mother reported 24-hour dietary recall for her child to a trained researcher. Children were asked to report on snacks or foods eaten outside the home (e.g., school; Kirkpatrick et al., 2014). After a participant reported a specific food or beverage, ASA24 provided visual depiction of the item in an appropriate dish, which allowed participants to accurately estimate portion sizes. From the data collected, we focused on daily added sugar intake relative to daily total caloric intake (kcal/total kcal). The Goldberg cutoff was applied to eliminate low-energy reporters prior to analyses (Goldberg et al., 1991). Low-energy reporters are those individuals who report less calories consumed than is plausible based on their body size.
Taste Genotyping
Participants provided DNA samples extracted from cheek swabs or saliva (BuccalAmp, Epicenter, Madison, WI, or Genotek, Kanata, Canada), which were used as templates in Taqman assays (Applied Biosystems, Foster City, CA) and assayed in duplicate using previously established methods (Mennella et al., 2012). The genes and their variants selected for study were TAS1R3 (rs35744813; CC, CT, and TT), GNAT3 (rs7792845; CC, CT, and TT), and TAS2R38 (rs713598 AA, AP, and PP; rs1726866 VV, AV, and AA; and rs10246939 II, IV, and VV). For TAS1R3, of three genotypes at this locus, TT is associated with a poorer ability to distinguish among low concentrations of sucrose than is the CC genotype. For GNAT3, the CC genotype is associated with the poorest taste ability compared with the TT genotype. For TAS2R38, the variant sites were expected to change amino acids in the proteins as follows: A49P, V262A, and I296V. For A49P, the PP genotype is associated with higher sucrose preference in children than is the AA genotype (Mennella et al., 2005). Because of the tendency for closely linked alleles to be coinherited, the V allele of rs1726866 and the I allele of rs10246939 would be similar in effect to the A allele of A49P.
For the polymerase chain reaction, the extracted DNA samples were diluted to 5 ng/μl. For the two variant sites within regulatory regions TAS1R3 (rs35744813) and GNAT3 (rs7792845), genotyping results are shown as nucleotides (i.e., CC, CT or TT). For variant sites within protein-coding regions, genotyping results are shown as single amino acid codes (e.g., AA for rs713598 A49P). As quality assurance, the allele frequencies were compared against earlier studies from similar populations of children (Mennella et al., 2005, 2012; Mennella, Reed, Roberts, Mathew, & Mansfield, 2014), and we established that the observed genotypes were in Hardy–Weinberg equilibrium.
Data Analyses
The primary outcome measures were sucrose detection thresholds and taste genotypes of five variant sites. Prior to statistical analyses, the distribution of sucrose detection threshold values was evaluated by the Kolmogorov–Smirnov test for normality (d = 0.187, p < .01), and the values were square root-transformed to approximate a normal distribution. In line with study hypotheses, t-tests were used to examine the relationship between sucrose detection thresholds and gender. Pearson’s correlations were used to examine the relationship between thresholds and age, anthropometric measures (z-scores for BMI, weight for age, height for age, percentage of body fat, WHtR), and dietary intake (total daily kcal, and added sugars as kcal/total kcal and kcal/kg body weight). To explore the relationship sucrose thresholds with diet and anthropometry, we examined its correlation with age, added sugar (mg and as a percentage of total calories), percentage of body fat, and WHtR using Pearson’s correlations and whether there were gender differences in these measures using the t test.
Separate one-way ANOVAs using genotype (TAS1R3, one variant site; GNAT3, one variant site; and TAS2R38, three variant sites) were conducted. If more than one child from a family participated in the study, only one child (n = 175) was picked at random to be included in the genetic analyses (Mennella et al., 2012). Each genotype was assayed in duplicate, and values agreed in each case. All alleles were in Hardy–Weinberg equilibrium (p > .05). Genotypes that were associated with sucrose threshold were evaluated for their relationship with the percentage of added sugar (kcal) in the child’s diet.
Drawing on the conclusions from the separate analyses described above, those outcome measures related to sucrose detection thresholds were included in a general linear (multivariate) model to establish the effect size of each. The criterion for statistical significance for the omnibus statistical tests and the least square difference post hoc tests was p < .05. All analyses were conducted with Statistica, Version 12 (StatSoft, Tulsa, OK), with p-values significant at alpha ≤ .05.
RESULTS
Participant Characteristics
As shown in Table 1, the study population consisted of 235 children whose race/ethnicity, family income, maternal education, and prevalence of obesity reflected the diversity of the City of Philadelphia (Pew Charitable Trust, 2011, 2014). More than half (57.8%) of the children were Black (n = 136), 19.6% were White (n = 46), and 21.7% were of more than one race. The vast majority of children (93.2%, n = 219) were non-Hispanic. Female participants made up 52.8% (n = 124), and male participants accounted for 47.2% (n = 111) of the study population. Most children were unrelated (n = 122), but the sample included 46 two-sibling pairs (n = 92) and seven families of three siblings (n = 21).
TABLE 1.
Participant Demographics
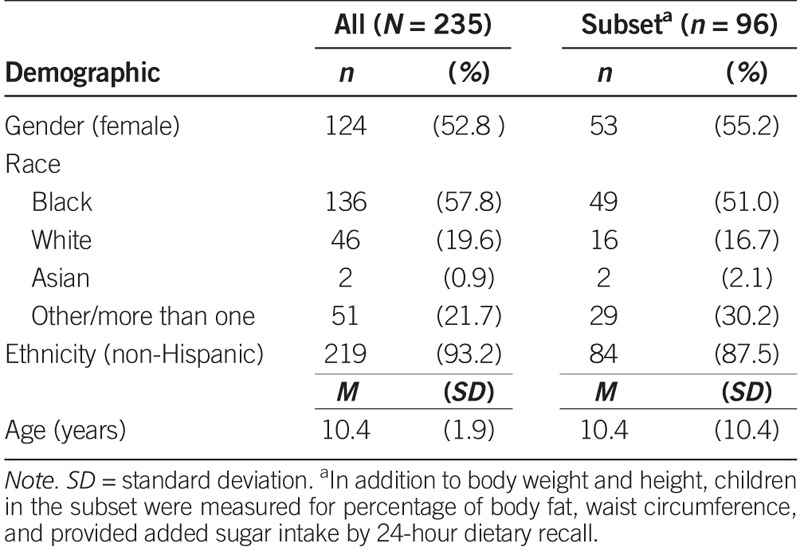
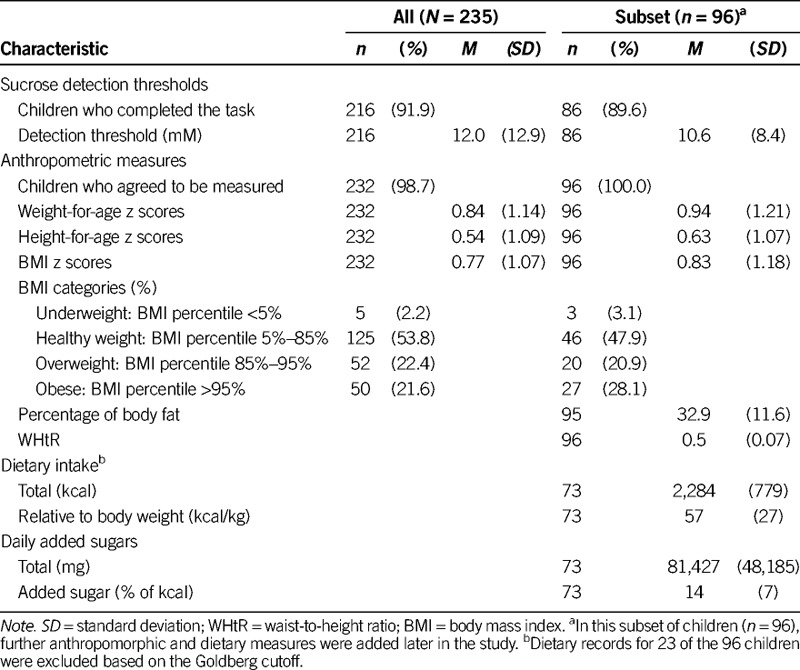
Regarding anthropometric measures, 21.6% of children were obese, 22.4% were overweight, 53.8% were normal weight, and 2.2% were underweight. In the subset of children for whom we had data on percentage of body fat (n = 95) and WHtR (n = 96; Table 2), 38.5% were classified as having central obesity; percentage of body fat averaged 32.9% (range, 9.8–60.8%).
TABLE 2.
Sucrose Detection Thresholds, Anthropometry, and Dietary Intake for Children
Sucrose Detection Threshold by Age and Gender
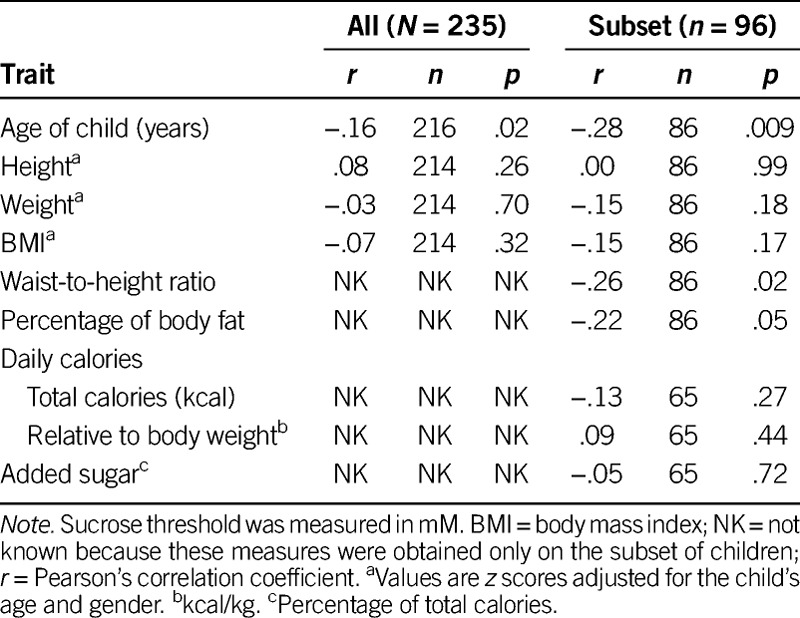
Most of the children (n = 216/235, 91.9%) completed the psychophysical taste task. The remaining children did not comply with the procedures, got tired, or refused to continue to participate. Sucrose detection thresholds were, on average, 12.0 mM and ranged from 0.23 mM to 153.8 mM sucrose. As children got older, they had significantly lower thresholds (were more sensitive) than did younger children (r(214) = −.16, p = .02; Table 3), and girls had significantly lower thresholds than boys (M = 10.5, SD = 8.6 mM vs. M =13.9, SD = 16.6 mM; t(214) = 2.0, p = .05).
TABLE 3.
Correlations Between Sucrose Detection Thresholds and Age, Anthropometric Measures, and Dietary Measures
Sucrose Detection Threshold and Added Sugar Intake by Genotype
Of the 175 unrelated children, 168 had valid sucrose detection thresholds and were included in genotype analyses. A few genomic DNA samples were refractory to genotyping: 6 for TAS1R3 and 10 for GNAT3. Of the subset of unrelated children (n = 96) for whom we had dietary records, 23 were identified as low-energy reporters by the Goldberg cutoff and, thus, were excluded from genotype–dietary analysis.
As shown in Table 4, sucrose detection thresholds were not related to the genotype of the sweet-related genes TAS1R3 (F(2,159) = 1.01, p = .36) and GNAT3(F (2,155) = 1.04, p = .36) or to the bitter receptor gene TAS2R38 variant rs713598 (F(2,165) = 2.45, p = .09). However, TAS2R38 variants rs1726866 and rs10246939 were related to sucrose threshold (F(2,165) = 4.55, p = .01; F(2,165) = 3.14, p = .05). Children with one or two bitter-sensitive alleles (the A allele of rs1726866 V262A and/or the V allele of rs10246939 I296V) had lower detection thresholds (i.e., were more sensitive to the taste of sucrose). After age and gender adjustment, the TAS2R38 variant rs713598 also met statistical threshold (F(2,163) = 3.18, p = .04), with the bitter-sensitive allele (P allele of A49P) being more common in children with lower sucrose thresholds (Figure 2).
TABLE 4.
Sucrose Detection Thresholds in Children Grouped by Taste Genotype
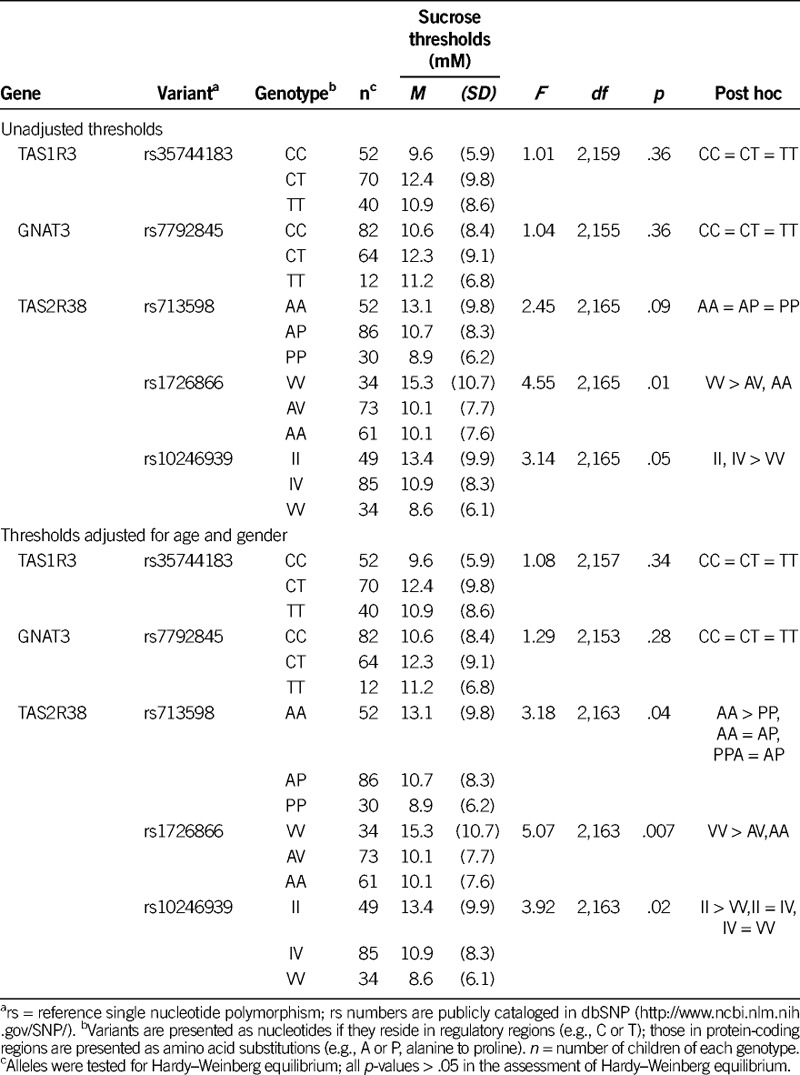
FIGURE 2.
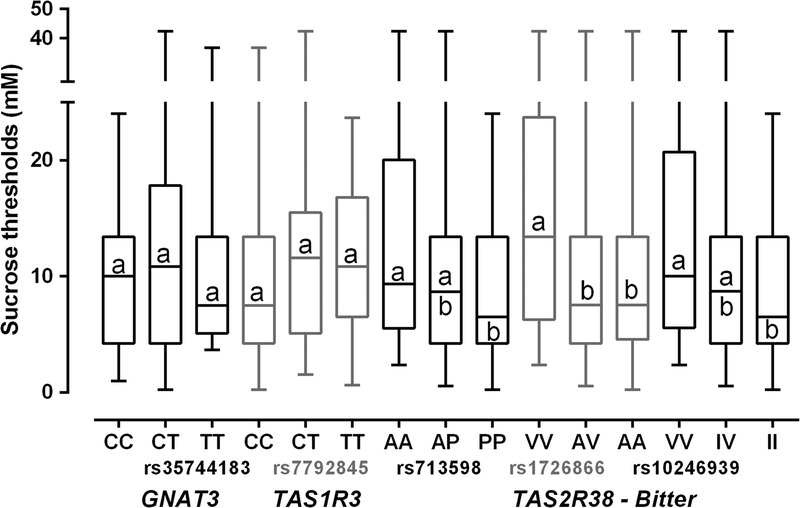
Box plots of sucrose detection threshold by genotype group (median, upper and lower quartile, and minimum and maximum values). For each variant, groups that differ significantly by post hoc testing do not share a superscript.
TAS2R38 genotypes were also related to added sugars as a percentage of total calories consumed by the children. There was no relationship of genotype with total calories (F(2,65) = 0.80, p = .45), but the diet of children with the bitter-sensitive genotype (AA, rs1726866, A262V) contained more added sugar per kcal (M = 16, SD = 6 % of kcal as added sugars) than did children with one or two copies of the insensitive allele (AV, M = 11, SD = 6 and VV, M = 13, SD = 8%; F(2,62) = 3.64, p = .03). The results were specific to TAS2R38 variant sites of this gene, and this effect was not apparent for the remaining two variants, rs713598 (F(2, 62) = 0.40, p = .67) and rs10246939 (F(2, 62) = 0.85, p = .43). Overall, added sugar intake was high, and only 5% of children (n = 4) who provided valid dietary data met World Health Organization guidelines.
Sucrose Detection Thresholds by Anthropometry
We found no significant relationships between sucrose detection thresholds and z scores for height-for-age (r(214) = .08, p = .26), weight-for-age (r(214) = −.03, p = .70), or BMI (r(214) = −.07, p = .32). However, the greater the percentage of body fat (as measured by bioelectrical impedance) or WHtR, the lower the sucrose thresholds (r(84) = −.22, p = .05 and r(84) = −.26, p = .02, respectively; Table 3). Correlation coefficients among all variables, not only sucrose thresholds, are shown (see Table, Supplemental Digital Content 1, http://links.lww.com/NRES/A165).
Model
We created a statistical model to explore the independent determinants of sucrose detection threshold, with variables that were influential in the univariate analysis: age, gender, and TAS2R38 genotype. The general linear model included gender (male vs. female) and genotype (rs10246939, II, IV, or VV) as categorical factors and age as a continuous (quantitative) covariate. This model explained 7% of the total variance among children in their sucrose detection thresholds [F(2,163) = 3.9, p = .02]. Genotype (p = .02, η2 = .05), age (p = .07, η2 = .02) and sex (p = .12, η2 = .01) independently contributed to variance in sucrose thresholds.
DISCUSSION
Like adults, children between the ages of 7 and 14 differed markedly in their ability to detect sucrose at low concentrations. Thresholds for sucrose detection ranged from 0.23 to 153.8 mM, with an average of 12.0 mM, which approximates thresholds previously reported for both adults (Pepino et al., 2010; Pepino & Mennella, 2007; Pribitkin et al., 2003) and children (James, Laing, & Oram, 1997; Overberg, Hummel, Krude, & Wiegand, 2012).
Age and gender were determinants of sucrose detection threshold—on a continuum even within this narrow age range—with younger children less sensitive than older children and boys less sensitive than girls; the latter finding is consistent with prior work (James et al., 1997). To further establish the dynamics of changes with age, research that assesses detection thresholds of children and adults of varying ages within the same study and using identical methodologies is needed (Pepino et al., 2010; Pepino & Mennella, 2007).
The age- and gender-related effects during childhood and early adolescence may be specific to sweet taste, as suggested by a recent study that found no such relationships for detection thresholds for two other basic tastes (the saltiness of sodium chloride and the umami or savory taste of monosodium glutamate; Bobowski & Mennella, 2015). That study used the same psychophysical taste method used here in children of the same age range, so we can conclude that the higher detection thresholds for sucrose we found among younger children and boys were not due to differences in cognition or the ability to complete the task. Both age and gender of the child reflect underlying hormonal and developmental processes that may shape this sensory system (Posner, Rothbart, Sheese, & Voelker, 2012). Gender-related differences may be due to girls undergoing puberty at earlier ages than boys.
Children are born with different taste genotypes, and our results suggest that some, but not all, genetic variants affect the sensory experience of the child. Unlike adults (Fushan et al., 2009), we found no relationship between a variant in the TAS1R3 gene and sucrose detection thresholds in these children. Similarly, a variant in GNAT3 is related to taste sensitivity in adults (Fushan et al., 2010) but had no measurable effect in the children studied here. One explanation is that the psychophysical methods to measure sucrose detection thresholds used in the Fushan studies of GNAT3 and TAS1R3 (Fushan et al., 2009, 2010) were slightly different than those we used here, which may account for the difference in the results. Another explanation may be age-related effects: This particular genetic variant is in a regulatory region. Regulatory regions affect how much messenger ribonucleic acid is produced, which often affects the abundance of function proteins in a given cell, and in this specific case, the result suggests that this regulatory effect may be more potent in adults than in children. Race may also play a role because one of the alleles (T) is rare in Whites. Overall, this lack of genetic association with these two taste genes is reminiscent of its effect on sweet preference, detectable in adults, but less apparent in children (Mennella et al., 2012, 2015).
Although there was no relationship with sweet taste genotypes, sucrose detection thresholds were related to variation in the bitter taste receptor gene TAS2R38 among children. Children differ in their ability to perceive the bitter compound propylthiouracil, and these differences are due in large part to alleles of this gene (Mennella et al., 2005; Mennella, Pepino, Duke, & Reed, 2010b; Mennella, Reed, et al., 2014). As discussed earlier, alleles of this bitter taste receptor TAS2R38 gene also partially explained individual differences in children’s sweet preferences (Mennella et al., 2005). Moreover, several studies in adults have linked the perception of the bitter ligands of this receptor to sucrose or sweet thresholds in adults (Chang, Chung, Kim, Chung, & Kho, 2006; Hong et al., 2005). Taken together, these studies point to a role of this bitter taste receptor gene in sweet perception and suggest that the sweet and bitter taste systems are more tightly linked than previously understood (Mennella et al., 2015).
Four hypotheses, not mutually exclusive, might account for the observed relationship between variation in the TAS2R38 gene and heightened sweet preferences and reduced sweet sensitivity among children. First, TAS2R38 alleles could lead to proteins with different capacities to bind sucrose directly. Other sweet substances, like saccharin, bind members of the bitter receptor family (Pronin et al., 2007), so this hypothesis has some experimental support. The results were mostly marked for one variant within the protein, which might point to the place in the receptor that binds sucrose (i.e., V262A). Second, the TAS2R38 genes and its alleles could be in linkage disequilibrium with nearby genes that might influence sweet taste perception and sensitivity (referring to the tendency for genes physically close on the chromosome to be coinherited during meiosis). Third, TAS2R38 allele frequency may be an especially sensitive genetic marker of racial ancestry (Guo & Reed, 2001), a variable with large and reliable effect on sweet preference (Mennella et al., 2005) and bitter taste thresholds (Guo & Reed, 2001; Mennella et al., 2010b; Mennella, Pepino, Duke, & Reed, 2010a). Fourth, differences in diet may affect sucrose threshold via changes in gene expression (Lipchock, Mennella, Spielman, & Reed, 2013).
In this study, children with a bitter-sensitive allele of TAS2R38 also reported consuming more added sugar than did those with the less sensitive allele, a finding consistent with previous reports that children with a bitter-sensitive allele preferred cereal and beverages with high-sugar content than those without (Mennella et al., 2005). These results are similar to a recent study that measured added sugar (e.g., candy) consumption in children (Hoppu, Laitinen, Jaakkola, & Sandell, 2015). Children in the study herein consumed, on average, 14% of total calories as added sugar, almost three times the 5% recommendation of international experts in public health (World Health Organization, 2015), and over the recommended 10% from the 2015 Dietary Guidelines for Americans (Dietary Guidelines Advisory Committee, 2015). In fact, of the 73 children in this study that provided valid dietary data, only four did not exceed the dietary recommendation. These reports of added sugar in this study, while not in compliance with public health recommendations, are typical and remarkably consistent with intake data obtained from larger-scale epidemiological studies (Ervin, Kit, Carroll, & Ogden, 2012).
Conclusions
In this study, we examined the relationships between sweet taste threshold and body weight, central adiposity, and percentage of body fat. We found that BMI was not related to sucrose thresholds, but when more direct measures of obesity were examined, children who were fatter and those with larger waistlines relative to their height had lower thresholds. This result is consistent with those of another study which measured sucrose threshold in obese and lean adolescents (Pasquet, Frelut, Simmen, Hladik, & Monneuse, 2007) but differs from another study which reports that obese adolescents, as measured by BMI, are less sensitive to low-sucrose concentrations than lean adolescents (Overberg et al., 2012). Differences in methods especially the reliance on insensitive measures of childhood obesity like BMI (Demerath et al., 2006) rather than more direct measures like percentage of body fat or WHtR may account for the inconsistencies across studies. New knowledge about the age-related and molecular basis of individual differences in taste and the use of methodologies that are validated and appropriate for children (Mennella, Spector, Reed, & Coldwell, 2013)—a generation who will struggle with obesity and diabetes—may suggest strategies to overcome diet-induced disease.
Supplementary Material
Figure.

No caption available.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s web site (www.nursingresearchonline.com).
The authors acknowledge that this work was supported by the National Institute of Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) Grant No. R01 DC011287 and an investigator-initiated grant from Ajinomoto Co., Inc. The first author was supported by Training Grant No. T32NR007100 from the National Institute of Nursing Research of the NIH to the University of Pennsylvania School of Nursing (awarded to M. Sommers) and the International Society of Nurses in Genetics. Genotyping was supported by NIH P30DC011735 to the second author. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDCD or NIH. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation or contents of the manuscript. The second and third authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors also acknowledge the valuable discussions with Drs. Charlene Compher, Marilyn Sommers, and Nuala Bobowski, and they thank Susana Finkbeiner, Loma Inamdar, and Corrine Mansfield for expert technical assistance.
The authors have no conflicts of interest to report.
References
- Bobowski N. K., Mennella J. A. ( 2015). Disruption in the relationship between blood pressure and salty taste thresholds among overweight and obese children. Journal of the Academy of Nutrition and Dietetics, 115, 1272– 1282. doi:10.1016/j.jand.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B., Breslin P. A., Kuhn C., Reed D. R., Tharp C. D., Slack J. P., Meyerhof W. ( 2005). The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Current Biology, 15, 322– 327. doi:10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. I., Chung J. W., Kim Y. K., Chung S. C., Kho H. S. ( 2006). The relationship between phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Archives of Oral Biology, 51, 427– 432. doi:10.1016/j.archoralbio.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Chumlea W. C., Guo S. S., Kuczmarski R. J., Flegal K. M., Johnson C. L., Heymsfield S. B., Hubbard V. S. ( 2002). Body composition estimates from NHANES III bioelectrical impedance data. International Journal of Obesity and Related Metabolic Disorders, 26, 1596– 1609. doi:10.1038/sj.ijo.0802167 [DOI] [PubMed] [Google Scholar]
- Coldwell S. E., Oswald T. K., Reed D. R. ( 2009). A marker of growth differs between adolescents with high vs. low sugar preference. Physiology & Behavior, 96, 574– 580. doi:10.1016/j.physbeh.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart B. J., Beauchamp G. K. ( 1990). Early development of taste perception. In R. L. McBride, H. J. H. MacFie. (Eds.), Psychological basis of sensory evaluation (pp. 1– 17). London, UK: Elsevier. [Google Scholar]
- Demerath E. W., Schubert C. M., Maynard L. M., Sun S. S., Chumlea W. C., Pickfoff A., Sievogel R. M. ( 2006). Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics, 117, e487– e495. doi:10.1542/peds.2005-0572 [DOI] [PubMed] [Google Scholar]
- Dietary Guidelines Advisory Committee ( 2015). Scientific report of the 2015 Dietary Guidelines Advisory Committee. Retrieved from http://health.gov/dietaryguidelines/2015-scientific-report/11-chapter-6/d6-3.asp [Google Scholar]
- Ervin R. B., Kit B. K., Carroll M. D., Ogden C. L. ( 2012). Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief. No. 87. Hyattsville, MD: National Center for Health Statistics; doi:10.3945/an.112.002279 [PubMed] [Google Scholar]
- Fushan A. A., Simons C. T., Slack J. P., Drayna D. ( 2010). Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chemical Senses, 35, 579– 592. doi:10.1093/chemse/bjq063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushan A. A., Simons C. T., Slack J. P., Manichaikul A., Drayna D. ( 2009). Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Current Biology, 19, 1288– 1293. doi:10.1016/j.cub.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. R., Black A. E., Jebb S. A., Cole T. J., Murgatroyd P. R., Coward W. A., Prentice A. M. ( 1991). Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. European Journal of Clinical Nutrition, 45, 569– 581. [PubMed] [Google Scholar]
- Guo S. W., Reed D. R. ( 2001). The genetics of phenylthiocarbamide perception. Annals of Human Biology, 28, 111– 142. doi:10.1080/03014460151056310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. H., Chung J. W., Kim Y. K., Chung S. C., Lee S. W., Kho H. S. ( 2005). The relationship between PTC taster status and taste thresholds in young adults. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 99, 711– 715. doi:10.1016/j.tripleo.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Hoppu U., Laitinen K., Jaakkola J., Sandell M. ( 2015). The hTAS2R38 genotype is associated with sugar and candy consumption in preschool boys. Journal of Human Nutrition and Dietetics, 28, 45– 51. doi:10.1111/jhn.12249 [DOI] [PubMed] [Google Scholar]
- James C. E., Laing D. G., Oram N. ( 1997). A comparison of the ability of 8–9-year-old children and adults to detect taste stimuli. Physiology & Behavior, 62, 193– 197. doi:10.1016/S0031-9384(97)00030-9 [DOI] [PubMed] [Google Scholar]
- Keller K. L., Tepper B. J. ( 2004). Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obesity Research, 12, 904– 912 doi:10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- Kim U. K., Jorgenson E., Coon H., Leppert M., Risch N., Drayna D. ( 2003). Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science, 299, 1221– 1225. doi:10.1126/science.1080190 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick S. I., Subar A. F., Douglass D., Zimmerman T. P., Thompson F. E., Kahle L. L., Potischman N. ( 2014). Performance of the Automated Self-Administered 24-Hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. American Journal of Clinical Nutrition, 100, 233– 240. doi:10.3945/ajcn.114.083238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R. J., Ogden C. L., Guo S. S., Grummer-Strawn L. M., Flegal K. M., Mei Z., Johnson C. L. ( 2002). 2000 CDC growth charts for the United States: Methods and development. Vital and Health Statistics. Series 11, Data from the National Health Survey, 1– 190. [PubMed] [Google Scholar]
- Lipchock S. V., Mennella J. A., Spielman A. I., Reed D. R. ( 2013). Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. American Journal of Clinical Nutrition, 98, 1136– 1143. doi:10.3945/ajcn.113.066688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. K., McKinnon P. J., Margolskee R. F. ( 1992). Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature, 357, 563– 569. doi:10.1038/357563a0 [DOI] [PubMed] [Google Scholar]
- Mennella J. A. ( 2008). The sweet taste of childhood. In S. Firestein, G. K. Beauchamp. (Eds.), The senses: A comprehensive reference (Vol 4., Olfaction and Taste, pp. 183– 188). San Diego, CA: Academic Press. [Google Scholar]
- Mennella J. A., Finkbeiner S., Lipchock S. V., Hwang L. D., Reed D. R. ( 2014). Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLOS ONE, 9, e92201. doi:10.1371/journal.pone.0092201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Finkbeiner S., Reed D. R. ( 2012). The proof is in the pudding: Children prefer lower fat but higher sugar than do mothers. International Journal of Obesity, 36, 1285– 1291. doi:10.1038/ijo.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Lukasewycz L. D., Griffith J. W., Beauchamp G. K. ( 2011). Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chemical Senses, 36, 345– 355. doi:10.1093/chemse/bjq134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., Duke F. F., Reed D. R. ( 2010a). Psychophysical dissection of genotype effects on human bitter perception. Chemical Senses, 36, 161– 167. doi:10.1093/chemse/bjq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., Duke F. F., Reed D. R. ( 2010b). Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genetics, 11, 60 doi:10.1186/1471-2156-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., Reed D. R. ( 2005). Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics, 115, e216– e222. doi:10.1542/peds.2004-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Reed D. R., Mathew P. S., Roberts K. M., Mansfield C. J. ( 2015). “A spoonful of sugar helps the medicine go down”: Bitter masking by sucrose among children and adults. Chemical Senses, 40, 17– 25. doi:10.1093/chemse/bju053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Reed D. R., Roberts K. M., Mathew P. S., Mansfield C. J. ( 2014). Age-related differences in bitter taste and efficacy of bitter blockers. PLOS ONE, 9, e103107 doi:10.1371/journal.pone.0103107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Spector A. C., Reed D. R., Coldwell S. E. ( 2013). The bad taste of medicines: Overview of basic research on bitter taste. Clinical Therapeutics, 35, 1225– 1246. doi:10.1016/j.clinthera.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokha J. S., Srinivasan S. R., Dasmahapatra P., Fernandez C., Chen W., Xu J., Berenson G. S. ( 2010). Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: The Bogalusa Heart Study. BMC Pediatrics, 10, 73 doi:10.1186/1471-2431-10-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute ( 2014). ASA24™: ASA24 Automated Self-Administered 24-Hour Recall. Retrieved from http://appliedresearch.cancer.gov/asa24/
- Overberg J., Hummel T., Krude H., Wiegand S. ( 2012). Differences in taste sensitivity between obese and non-obese children and adolescents. Archives of Disease in Childhood, 97, 1048– 1052. doi:10.1136/archdischild-2011-301189 [DOI] [PubMed] [Google Scholar]
- Pasquet P., Frelut M. L., Simmen B., Hladik C. M., Monneuse M. O. ( 2007). Taste perception in massively obese and in non-obese adolescents. International Journal of Pediatric Obesity, 2, 242– 248. doi:10.1080/17477160701440521 [DOI] [PubMed] [Google Scholar]
- Pepino M. Y., Finkbeiner S., Beauchamp G. K., Mennella J. A. ( 2010). Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity, 18, 959– 965. 10.1038/oby.2009.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino M. Y., Mennella J. A. ( 2007). Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcoholism, Clinical and Experimental Research, 31, 1891– 1899. doi:10.1111/j.1530-0277.2007.00519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Charitable Trust ( 2011). A city transformed: The racial and ethnic changes in Philadelphia over the last 20 years. Retrieved from http://www.pewtrusts.org/en/research-and-analysis/reports/2011/06/01/a-city-transformed-the-racial-and-ethnic-changes-in-philadelphia-over-the-last-20-years
- Pew Charitable Trust ( 2014). Philadelphia: The state of the city, a 2014 update. Retrieved from http://www.pewtrusts.org/en/research-and-analysis/reports/2014/04/05/philadelphia-the-state-of-the-city-a-2014-update
- Posner M. I., Rothbart M. K., Sheese B. E., Voelker P. ( 2012). Control networks and neuromodulators of early development. Developmental Psychology, 48, 827– 835. doi:10.1037/a0025530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribitkin E., Rosenthal M. D., Cowart B. J. ( 2003). Prevalence and causes of severe taste loss in a chemosensory clinic population. Annals of Otology, Rhinology, and Laryngology, 112, 971– 978. doi:10.1177/000348940311201110 [DOI] [PubMed] [Google Scholar]
- Pronin A. N., Xu H., Tang H., Zhang L., Li Q., Li X. ( 2007). Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Current Biology, 17, 1403– 1408. doi:10.1016/j.cub.2007.07.046 [DOI] [PubMed] [Google Scholar]
- Reed D. R., McDaniel A. H. ( 2006). The human sweet tooth. BMC Oral Health, 6, S17 doi:10.1186/1472-6831-6-S1-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization ( 2015). Guideline: Sugars intake for adults and children. Retrieved from http://apps.who.int/iris/bitstream/10665/149782/1/9789241549028_eng.pdf [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


