Abstract
Phytoestrogen-rich Pueraria mirifica (PM) tuberous extract is a promising candidate for the development of anti-osteoporosis drugs for postmenopausal women, but its action has never been validated in humans or in non-human primates, which are more closely related to humans than rodents. In vitro study of non-human primate osteoblasts is thus fundamental to prepare for in vivo studies of phytoestrogen effects on primate bone. This study aimed to establish a culture system of baboon primary osteoblasts and to investigate the effects of PM extract and its phytoestrogens on these cells. Primary osteoblasts from adult baboon fibulae exhibited osteoblast characteristics in regard to proliferation, differentiation, mineralization, and estrogen receptor expression. They responded to 17β-estradiol by increased proliferation rate and mRNA levels of alkaline phosphatase (ALP), type I collagen, and osteocalcin. After being exposed for 48 h to 100 μg/ml PM extract, 1000 nM genistein, or 1000 nM puerarin, primary baboon osteoblasts markedly increased the rate of proliferation and mRNA levels of ALP and type I collagen without changes in Runx2, osterix, or osteocalcin expression. PM extract, genistein, and puerarin also decreased the RANKL/OPG ratio, suggesting that they could decrease osteoclast-mediated bone resorption. However, neither PM extract nor its phytoestrogens altered calcium deposition in osteoblast culture. In conclusion, we have established baboon primary osteoblast culture, which is a new tool for bone research and drug discovery. Furthermore, the present results provide substantial support for the potential of PM extract and its phytoestrogens to be developed as therapeutic agents against bone fragility.
Keywords: baboon, genistein, puerarin, Pueraria mirifica, osteoblasts, osteoporosis
Graphical Abstract
Introduction
Postmenopausal osteoporosis and associated fragility fractures are a major public health problem in the aging population worldwide and contribute substantially to an increasing economic healthcare burden (Lane 2006; Cole et al. 2008). Although estrogen replacement therapy is effective in fragility fracture reduction, many side effects of considerable concern surround widespread use of this treatment (Manolagas et al. 2002). Phytoestrogens, i.e., plant estrogen-like compounds, have attracted attention as potential inexpensive and effective alternatives to estrogen replacement therapy in the treatment of osteoporosis. Pueraria mirifica (PM), a member of the Leguminosae family endemic to Thailand, is an isoflavone/phytoestrogen-rich tuberous herb that is widely used as a dietary supplement and in other products in the United States, China, Japan, Korea, and Thailand. In comparison with other related and renowned plant species P. lobata, although PM had a comparable amount of puerarin, daidzin, genistin, genistein and lower amount of daidzein (Cherdshewasart et al. 2007), PM also contained miroestrol and deoxymiroestrol, species-specific chemicals, which elicited greater estrogenic activities than other phytoestrogens (Malaivijitnond 2012). Besides, PM ethanol extract did not contain kudzusaponin, kudzusapogenol, and soyasapogenol (Malaivijitnond 2012), all of which were reported in P. lobata extract (Wong et al. 2011). Estrogenic activity of PM has been studied in a variety of animal species, including humans (Malaivijitnond 2012); however, its bone-forming actions are largely unknown. Recently, our group reported that PM extract could prevent bone loss in both male and female osteoporotic rats (Urasopon et al. 2007; Urasopon et al. 2008). The mechanisms identified in research with rat bone cells in vitro were clearly shown to be induction of bone formation and suppression of bone resorption by upregulation of mRNA expression of alkaline phosphatase (ALP) and osteoprotegerin (OPG), respectively (Tiyasatkulkovit et al. 2012).
However, based on the regulatory guidelines of the US-FDA (2004), results from two animal species are required to assess safety of any new therapeutic agent for treating osteoporosis in humans (Smith et al. 2009). One of the two suggested species is rat because rat bone is well characterized, but the second species should have intracortical bone remodeling similar to humans. While no single animal model exactly mimics the human condition, nonhuman primates (NHPs; e.g., baboon) are the most closely related taxonomic group to humans and this close relationship is reflected in similarities in bone metabolism (Jerome and Peterson 2001; Black and Lane 2002; Smith et al. 2009). Although the baboon clearly serves as a good model for humans in regard to skeletal maintenance and turnover, the regulatory processes involved in bone function at the cellular level have never been established in baboons, or even in an in vitro baboon model.
NHPs—especially macaques and baboons, which are second only to apes in genetic proximity to humans—exhibit many biological, physiological and anatomical similarities to humans. Thus, much translational research, such as development of drugs and vaccines for a wide array of conditions including cardiovascular (Shen 2010), neurodegenerative (Schneider et al. 2013), infectious (Zompi and Harris 2012), and skeletal diseases (Smith et al. 2009), have been conducted with NHPs. As observed in humans, an important feature of NHP bone is the presence of osteonal or Haversian remodeling in cortical bone (i.e., intracortical bone remodeling), which is not normally present in rodent bone (Jerome and Peterson 2001; Havill et al. 2013). Baboons are also more similar to humans in regard to cellular response after fracture, microstructural and compositional properties, bone mineral density, organic density, bone volume fraction, and length of collagen-mineral bundles as compared to dogs, cows, and rabbits (Wang et al. 1998). Moreover, NHPs, particularly older females, resemble humans in age-related decrease in bone mass and menopausal osteoporosis (Aufdemorte et al. 1993; Wang et al. 1998; Havill et al. 2008).
The present study aimed to establish primary baboon osteoblast cultures as a model for phytoestrogen testing, and to determine the effects of PM extract and its phytoestrogens (i.e., genistein and puerarin) on baboon primary osteoblasts.
Materials and Methods
PM extract
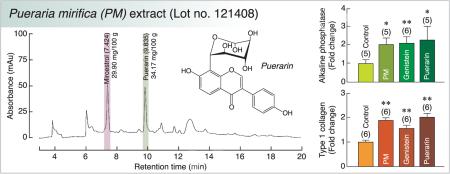
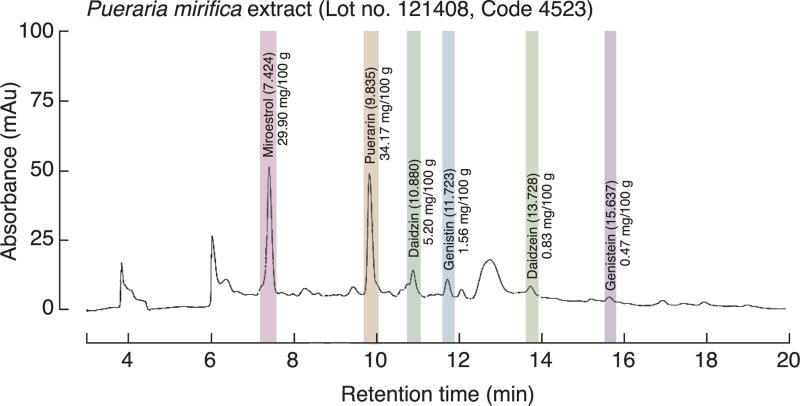
PM tuberous extract (lot no. 121408) was provided by Dr. I. Sandford Schwartz, Smith Naturals Co., Ltd., Thailand. P. mirifica was cultivated and the tuberous roots were collected from Chiang Mai Province, Northern Thailand. The tuberous roots were cut, dried, and shipped to Bio-Botanica Inc., New York, USA for extraction and standardization. The P. mirifica powder was exhaustively extracted with concentrated ethanol. The pooled extracts were distilled under reduced pressure at a temperature not exceeding 45 °C to remove alcohol before a concentrated extract was obtained. The liquid chromatography–mass spectrometry (LC/MS) profile of the extract showed three major phytoestrogens (Fig. 1). Each 100 g of PM extract contained 29.90, 0.47 and 34.17 mg of miroestrol, genistein, and puerarin, respectively. However, only puerarin (LKT Laboratories Inc., St. Paul, MN, USA) and genistein (Sigma, St. Louis, MO, USA) were studied in baboon primary osteoblasts since miroestrol was unstable when it was isolated or synthesized as pure compound (Malaivijitnond 2012).
Fig. 1.
The liquid chromatography–mass spectrometry (LC/MS) profile shows the isoflavone and phytoestrogen contents in the ethanol extract of P. mirifica tuber powder.
Animals
Female baboons (Papio hamadryas ssp.), 10–15 years old (roughly developmentally equivalent to 30 to 45 years of age in humans), were housed outdoors in social groups at the Southwest National Primate Research Center (SNPRC), Texas Biomedical Research Institute, San Antonio, Texas, USA. The animals were fed commercial monkey chow ad libitum. Animal care personnel and staff veterinarians provided daily maintenance and health care to all animals in accordance with the Guide for the Care and Use of Laboratory. Clinical records of each animal were examined to ascertain that no animal had medical conditions known to affect bone metabolism (e.g., diabetes mellitus or chronic renal disease).
Bone cell isolation and culture
Fibulae were collected during routine necropsy under sterile conditions. The extraneous soft connective tissue from the outer surface was removed using a scalpel blade. The bone was rinsed with phosphate-buffered saline pH 7.4 without calcium and magnesium (Gibco), and transferred to a Petri dish containing Dulbecco's modified Eagle's medium (DMEM) (Sigma) with 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco). The bone sample was cut into small fragments of 1–3 mm2 using bone snips and washed in 10 ml of DMEM five times or until no bone marrow remained (i.e., until the white color of the bone fragment was visible). Bone chips were transferred to a culture flask containing 30 ml DMEM and digested with 0.25% collagenase (Sigma) at 37 °C for 4 h. The digestion was stopped by removing the DMEM and collagenase and adding 30 ml DMEM with 10% fetal bovine serum (FBS; Sigma). Bone chips were then transferred to a culture flask with medium containing 30 mL DMEM supplemented with 30% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin, 50 μg/ml l-ascorbate-2-phosphate and 100 μM sodium pyruvate (Gibco). Bone cell cultures were incubated at 37 °C in 5% CO2 for 6 days. Cell growth was maintained in the same complete medium but containing only 15% FBS, and the medium was changed every 3 days. All of the above procedures were performed under sterile conditions.
Experimental design
After investigating the expression of estrogen receptor (ER)-α and ER-β, the responses of baboon primary osteoblasts to 17β-estradiol (E2; Sigma) were monitored by determining (i) cell proliferation using 5-bromo-2′-deoxyuridine (BrdU) enzyme-linked immunosorbent assay kit (catalog no. 11647229001; Roche), (ii) mRNA expression of osteoblast differentiation markers (i.e., ALP, type I collagen and osteocalcin), and two markers associated with osteoblast-regulated osteoclast function [i.e., receptor activator of nuclear factor-κB ligand (RANKL) and OPG], using qRT-PCR, and (iii) in vitro mineralization. To investigate the effects of PM extract, genistein, and puerarin on baboon primary osteoblasts, cells were incubated in culture media containing 100 μg/ml PM extract (PM group), 1000 nM genistein (GEN group), 1000 nM puerarin (PU group), or 0.3% dimethyl sulfoxide (vehicle; control group). The concentrations of PM extract, genistein and puerarin used in this study were optimal doses reported previously (Tiyasatkulkovit et al. 2012). Cell proliferation was also determined by BrdU assay. The mRNA levels of bone formation markers [i.e., runt-related transcription factor (Runx)-2, osterix, ALP, type I collagen and osteocalcin] and bone resorption markers (i.e., RANKL and OPG) were quantified by qRT-PCR. In vitro mineralization was determined by fluorescence analysis of calcein binding.
ALP activity
The ALP activity in baboon primary osteoblasts was determined by SigmaFast BCIP/NBT (Sigma, St. Louis, MO, USA), according to the manufacturer's instruction. In brief the second passage of baboon primary osteoblasts was seeded in 24-well plates at a density of 5 × 104 cells/well for 6 days. Cells were fixed with 70% ethanol and later incubated with BCIP/NBT solution for 10 min. Cells were washed, and the ALP positive cells were stained and visualized for dark blue-violet color.
Total RNA preparation and qRT-PCR
Osteoblasts were cultured in 6-well plates at a concentration of 2 × 105 cells/well. After 48-h incubation with E2, PM extract, or phytoestrogens, total RNA samples were prepared by QIAshredder (catalog no. 79654; Qaigen) and purified by RNeasy Mini Plus (catalog no. 74134; Qaigen). One microgram of total RNA was then reverse-transcribed with iScript Select cDNA synthesis kit (Bio-Rad) to cDNA using the Applied Biosystems GeneAmp PCR System 9700.
The baboon primers (Table 1) were designed using the Primer 3 program based on the primers for rat and human genes, as well as the baboon genome. All primers were first verified by conventional PCR and the amplicon sizes were confirmed by electrophoresis. The sequence homology between baboon and rat genes was 82.6–96.1%, and between baboon and human genes was 97.2–99.5%. Conventional PCR was performed by Bio-Rad MyCycler with GoTaq Green Master Mix (Promega). Thereafter, PCR products were visualized on 1.5% agarose gel stained with 1 μg/ml ethidium bromide (Sigma) under UV transilluminator (Eastman Kodak, Rochester, NY, USA). qRT-PCR and melting curve analyses were performed in triplicate by Bio-Rad CFX96 Real-Time PCR Detection system with SsoFast EvaGreen Supermix (catalog no. 1725203; Bio-Rad) for 40 cycles at 95 °C for 60 s, 56–60 °C annealing temperature for 30 s, and 72 °C for 30 s.
Table 1.
Baboon (Papio hamadryas) primers used in the qRT-PCR experiment.
| Genes | Primers (Forward/Reverse) | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| β-actin | 5′-CACACGCAGCTCATTGTAGA-3′ 5′-GGCATGGGTCAGAAGGATT-3′ |
153 | 56 |
| ER-α | 5′-AGGGTGGCAGAGAGAGATTG-3′ 5′-TCTTGAAGAAGGCCTTGCAG-3′ |
156 | 60 |
| ER-β | 5′-ATCAGCCCCACCATTAACAC-3′ 5′-GAGCCACCCCATGTACTGAT-3′ |
206 | 60 |
| Runx2 | 5′-CAAAATGAGCGACGTGAGC-3′ 5′-GGCGATGATCTCCACCAT-3′ |
214 | 58 |
| Osterix | 5′-TGCATCTCTTCCACACTTGC-3′ 5′-TGACGGGCAGTAGCTATGAG-3′ |
166 | 56 |
| Type I collagen | 5′-CAGAGTGGCACATCTTGAGG-3′ 5′-TGGTTTCGACTTCAGCTTCC-3′ |
283 | 56 |
| ALP | 5′-AACCACCACGAGAGTGAACC-3′ 5′-TCCCTGATGTTATGCACGAG-3′ |
150 | 56 |
| Osteocalcin | 5′-TGGACTTTAGCTCTCCATCTCTG-3′ 5′-ATCGCATGAAAGCATGGAA-3′ |
102 | 58 |
| RANKL | 5′-TCAGAAGATGGCACTCACTG-3′ 5′-AGCAAAAGGCTGAGCTTCAA-3′ |
215 | 58 |
| OPG | 5′-TGTATTTCGCTCTGGGGTTC-3′ 5′-CTGCAGTACGTCAAGCAGGA-3′ |
153 | 56 |
ER, estrogen receptor; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; RANKL, receptor activator of nuclear factor-κB ligand; OPG, osteoprotegerin.
Quantification of in vitro mineralization
Osteoblasts were seeded in 24-well plates at a density of 2 × 104 cells/well and cultured for 14 days in complete medium containing 50 mM β-glycerophosphate and 50 μg/ml l-ascorbate-2-phospate (Sigma). Thereafter, they were exposed to the complete medium containing 1 μg/ml calcein (Sigma) for 4 h at 37 °C. The cultures were then washed three times with PBS and overlaid with 1 ml PBS. Bound calcein fluorescence was read in a fluorescence multiwell plate reader (model 1420; Wallac) at 485-nm excitation and 530-nm emission.
Statistical analysis
The results are expressed as mean ± SE. Two-group comparisons were analyzed by Mann Whitney test using GraphPad Prism 5. Comparisons of more than two groups were performed by one-way analysis of variance followed by Dunnett's post-test. The level of significance was p < 0.05.
Results
Characterization of primary baboon osteoblasts
In the first passage of primary culture, round or polygonal cells were observed migrating from bone chips. These cells became attached to the culture dish surface on day 6 of culture. As culture time increased, the cells proliferated and became more triangular, short, spindle-shaped or polygonal. These cells formed a nearly confluent cell layer around the bone chips covering 80% of the culture dish by day 13. During differentiation cells secreted matrix protein, and ALP was visualized as dark blue-violet staining (ALP activity assay; data not shown). In addition, they became multilayered, and a thick extracellular matrix formed. On day 14 these cells exhibited mineralization capacity. The results, therefore, confirmed that our baboon cells had osteoblastic morphology and function (e.g., ALP production and mineralized nodule formation).
ER expression and responses to E2 in primary baboon osteoblasts
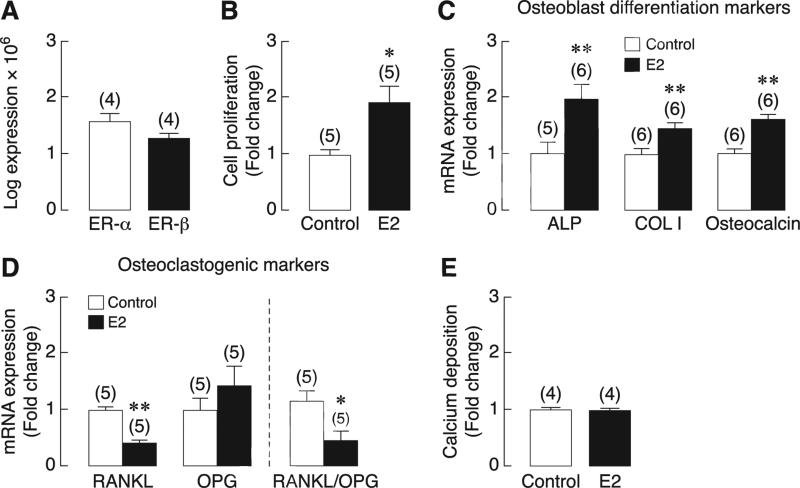
Primary baboon osteoblasts abundantly expressed ER transcripts, which suggested that they were direct targets of E2. The mRNA levels of ER-α were apparently comparable to that of ER-β (Fig. 2a). After 48-h exposure to 10 nM E2, proliferation of primary baboon osteoblasts was found to increase by 2-fold (Fig. 2b). E2 also upregulated the mRNA expression of osteoblast differentiation markers, i.e., ALP, type I collagen and osteocalcin (Fig. 2c), while decreasing RANKL expression and RANKL/OPG ratio by ~50% (Fig. 2d). Exposure to 10 nM E2 did not affect calcium deposition, as determined by calcein-labeling technique (Fig. 2e).
Fig. 2.
(A) Expression of estrogen receptors (ER-α and ER-β) in primary baboon osteoblasts as determined by qRT-PCR. (B) Proliferation, (C and D) the mRNA levels of alkaline phosphatase (ALP), type I collagen (COL I), osteocalcin, RANKL and OPG, and (E) calcium deposition in 10 nM E2-exposed primary baboon osteoblasts. The value of each control group was normalized to 1. Numbers in parentheses represent the numbers of independent samples. * p < 0.05, ** p < 0.01 compared with the corresponding control group.
Effects of PM extract and phytoestrogens on primary baboon osteoblasts
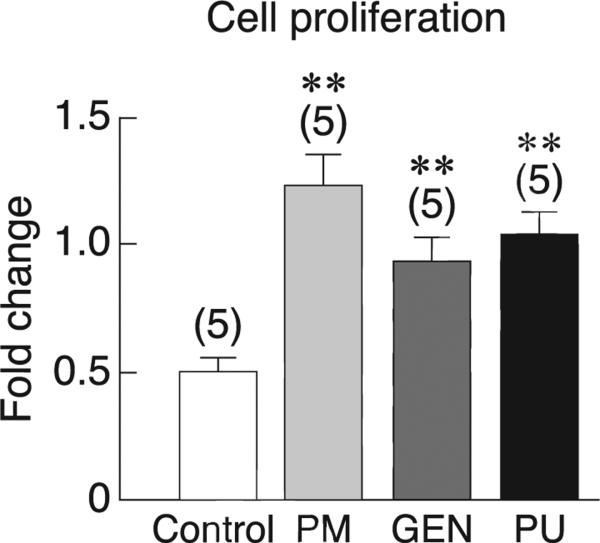
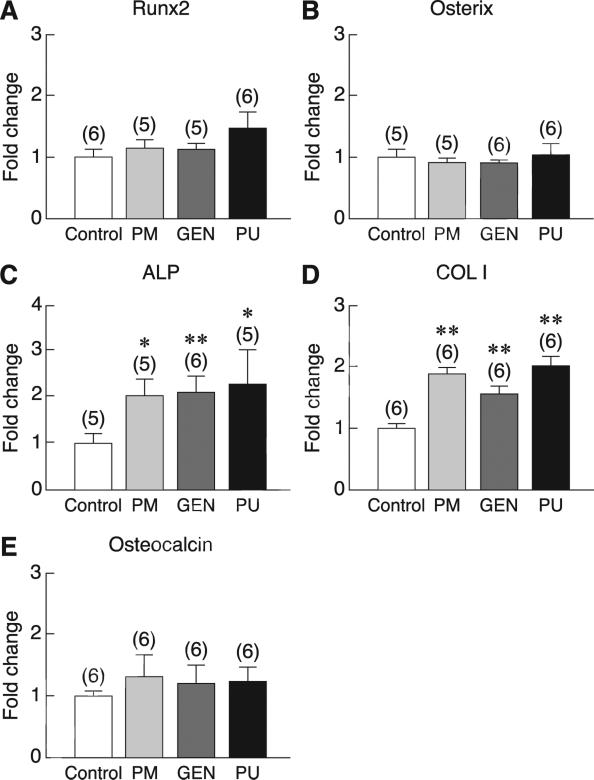
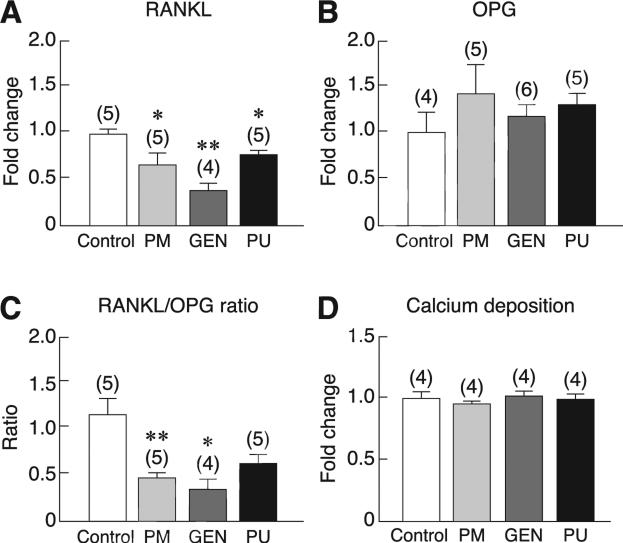
Consistent with the responses to E2, exposure of primary baboon osteoblasts to 100 μg/ml PM extract, 1000 nM genistein, or 1000 nM puerarin for 48 h markedly increased cell proliferation, as indicated by BrdU incorporation assay (Fig. 3). Moreover, PM extract, genistein, and puerarin increased the mRNA levels of some osteoblast differentiation markers, i.e., ALP and type I collagen, but not Runx2, osterix, or osteocalcin (Fig. 4). Primary baboon osteoblasts also expressed RANKL and OPG, both of which are commonly used as markers for assessment of osteoblast-regulated osteoclast function and bone resorption. As shown in Fig. 5a, after 48-h incubation with PM extract, genistein, or puerarin, the levels of RANKL mRNA expression were significantly decreased, whereas the OPG expression remained unchanged (Fig. 5b). The RANKL/OPG ratios were significantly decreased only in the PM and genistein groups (Fig. 5c), suggesting that both PM extract and genistein had potential to suppress osteoclast function and bone resorption. After 14-day exposure to PM extract, genistein, or puerarin, there was no significant difference in calcium deposition among treatment groups (Fig. 5d).
Fig. 3.
Proliferation of primary baboon osteoblasts after direct exposure for 48 h to vehicle (control), 100 μg/ml PM extract, 1000 nM genistein (GEN), or 1000 nM puerarin (PU) as determined by BrdU assay. The value of control group was normalized to 1. Numbers in parentheses represent the numbers of independent samples. ** p < 0.01 compared with the control group.
Fig. 4.
The mRNA levels of osteoblast differentiation markers, i.e., (A) Runx2, (B) osterix, (C) alkaline phosphatase (ALP), (D) type I collagen (COL I), and (E) osteocalcin in primary baboon osteoblasts after 48-h exposure to vehicle (control), 100 μg/ml PM extract, 1000 nM genistein (GEN), or 1000 nM puerarin (PU). The expression level was quantified by qRT-PCR. The value of each control group was normalized to 1. Numbers in parentheses represent the numbers of independent samples. * p < 0.05, ** p < 0.01 compared with the corresponding control group.
Fig. 5.
The mRNA levels of (A) RANKL and (B) OPG, and (C) RANKL/OPG ratio in the primary baboon osteoblasts after 48-h exposure to vehicle (control), 100 μg/ml PM extract, 1000 nM genistein (GEN), or 1000 nM puerarin (PU). The expression level was quantified by qRTPCR. The value of each control group was normalized to 1. * p < 0.05, ** p < 0.01 compared with the corresponding control group. (D) Quantitative analysis of calcium deposition in primary baboon osteoblast cultures after direct exposure to vehicle (control), 100 μg/ml PM extract, 1000 nM GEN, or 1000 nM PU for 21 days. Fluorescence analysis of the calcein bound to calcium phosphate was used in the present study. The relative fluorescent level of the control group was normalized to 1. Numbers in parentheses represent the numbers of independent samples.
Discussion
The baboon is one of the closest NHPs, in terms of evolution and physiology, to humans, and the genetic linkage map and basic information on bone biology have been established (Havill et al. 2008). In the present study, we have isolated and cultured osteoblasts derived from baboon fibulae. These cells exhibited the same osteoblast characteristics, including proliferation, differentiation and mineralization during development, as those reported in rat osteosarcoma UMR-106 cells (Forrest et al. 1985; Tiyasatkulkovit et al. 2012), rat primary osteoblasts (Heim et al. 2004), human MG-63, Saos-2 and U-2 OS cell lines (Pautke et al. 2004), and human primary osteoblasts (Heim et al. 2004). Primary baboon osteoblasts strongly expressed the genes associated with bone formation (i.e., Runx2, osterix, ALP, type I collagen, and osteocalcin) during the differentiation stage. Furthermore, calcium deposition during the mineralization phase was also observed in culture, reflecting a functional in vitro endpoint of advanced osteoblast cell differentiation. The calcium deposition observed in these baboon bone cells is amorphous hydroxyapatite similar to that seen in human bone (Neve et al. 2011). Likewise, these primary baboon osteoblasts expressed both ER-α and ER-β mRNA and could respond to E2 by increasing proliferation and expression of ALP, type I collagen, and osteocalcin genes, consistent with those found in primary human osteoblasts (O'shaughnessy et al. 2000; Heim et al. 2004). Considering all of these findings, primary baboon osteoblast cultures appear to be a good in vitro model for studying cellular and molecular mechanisms of bone metabolism as well as for translational research, phytomedical research, and drug discovery.
In women, osteoporosis can develop rapidly during the postmenopausal period after ovarian function has ceased and plasma estrogen levels become extremely low (Erben et al. 2000). Although estrogen replacement therapy (ERT) is effective in reducing bone loss, ERT is associated with a high risk of breast, endometrial and ovarian cancers (Manolagas et al. 2002). Recently, phytoestrogens and phytoestrogen-containing plants, e.g., P. mirifica, have attracted attention as alternatives to ERT (Malaivijitnond 2012). P. mirifica has been widely used and is commercially sold in the United States, China, Japan, Korea and Thailand as dietary supplements, cosmetics, and pharmaceutical products. In rodents, PM extract has been demonstrated to prevent bone loss in both male and female gonadectomized rats (Urasopon et al. 2007; Urasopon et al. 2008). Recently, PM extract and certain phytoestrogens, i.e., puerarin and genistein, were reported to significantly increase the expression of genes associated with osteogenic differentiation and bone formation, particularly ALP and OPG, in rat osteoblast-like UMR-106 cells (Tiyasatkulkovit et al. 2012). However, as mentioned previously, rat bone is different from human bone due to the absence of intracortical remodeling, which is an important characteristic of human bone remodeling (Jerome and Peterson 2001). Moreover, the results from rat cell lines may not reflect normal biological activity of animal or human osteoblasts in vivo. Therefore, in developing PM as an alternative drug for osteoporosis treatment, the investigation of its effects in an animal model that is closely related and highly similar to humans in regard to bone physiology is preferred. The establishment of baboon primary osteoblast culture was the first step required for developing this animal model.
During differentiation, the osteoblasts sequentially express different markers specific to each stage of maturation, i.e., Runx2, osterix, ALP, type I collagen, and osteocalcin (Komori 2006). Runx 2 is required for mesenchymal cell differentiation into pre-osteoblasts (Zhang 2010), whereas osterix—the osteoblast-specific transcription factor—is essential for the differentiation of pre-osteoblasts into mature osteoblasts (Komori 2006). Runx2 and osterix are expressed during the early stage of differentiation, followed by the expression of ALP, osteocalcin and type I collagen. Generally, differentiated osteoblasts express ALP, type I collagen, and osteocalcin when they commence to produce extracellular matrix and accrete calcium during mid and late stages of differentiation (Zhang 2010). In the present study, PM extract, genistein, and puerarin were found to enhance osteoblast proliferation and the expression of markers related to mid and late stages of differentiation (i.e., ALP and type I collagen), while having no effect on the markers of early differentiation (i.e., Runx2 and osterix) or matrix mineralization (i.e., calcium deposition). These results were somewhat similar to those previously reported in rat osteoblast-like UMR-106 cells treated with PM tuberous extract (Tiyasatkulkovit et al. 2012) and in rat calvarial osteoblasts treated with Chinese P. lobata-derived puerarin (Zhang et al. 2007), which could explain, in part, how PM extract induced bone gain in gonadectomized rats.
Moreover, differentiated baboon osteoblasts were also found to express RANKL and OPG, both of which were markers of the osteoblast-regulated osteoclast function and hence bone resorption. RANKL is an essential humoral factor for recruitment, differentiation, activation and survival of osteoclasts after binding to its specific receptor RANK in the plasma membrane of osteoclast precursors and mature osteoclasts. On the other hand, OPG is a decoy receptor of RANKL, thereby inhibiting bone resorption. Herein, PM extract and its phytoestrogens (especially genistein) were capable of decreasing RANKL expression and RANKL/OPG ratio, indicating that they had potential to suppress osteoclast-mediated bone resorption in the baboon. In contrast, rat osteoblast-like UMR-106 cells responded to PM extract by increasing the mRNA levels of OPG (Tiyasatkulkovit et al. 2012).
It was apparent that E2, PM extract, and its phytoestrogens enhanced baboon osteoblast functions similar to those found in the phytoestrogen-exposed UMR-106 cells and human osteosarcoma Saos-2 cells (Forrest et al. 1985; Karieb and Fox 2012; Tiyasatkulkovit et al. 2012). However, different mechanisms involved in the inhibition of osteoclast differentiation and subsequent bone resorption after E2 and phytoestrogen treatments were observed between rat osteoblasts and baboon osteoblasts (i.e., changes in OPG vs. RANKL). Furthermore, E2, PM extract and its phytoestrogens increased proliferation of primary baboon osteoblasts as indicated by BrdU incorporation, consistent with what occurred in genistein-exposed human osteoblast-like Saos-2 cells, whereas proliferation was decreased in estrogen-exposed rat osteoblast-like UMR-106 cells (Tiyasatkulkovit et al. 2012) and primary rat osteoblasts (Ma et al. 2011). This discrepancy could be explained by the fact that baboon gene sequence is much more similar to human gene sequence (97.2–99.5%) than is true of the rat (82.6–96.1%), and baboons are more similar to humans in term of bone remodeling and posture.
In conclusion, we have provided strong support for the potential of tuberous extract of PM from the Northern Thailand and its phytoestrogens (genistein and puerarin) to be developed as therapeutic agents against bone loss in postmenopausal women. PM extract, genistein, and puerarin could enhance proliferation and expression of ALP and type I collagen in primary baboon osteoblasts, which might, in turn, promote bone formation. A decrease in the mRNA level of RANKL further suggested that PM extract was capable of suppressing osteoclast-mediated bone resorption. Further investigation is, however, required to compare the osteogenic efficacy of PM extract with extracts from other related species, such as Chinese P. lobata and P. thomsonii. The establishment of primary baboon osteoblast culture has also provided a new tool for studying osteoblast biology as well as for phytomedical and translational research. Thus, the baboon osteoblasts can be useful in the evaluation of candidate therapeutic agents intended for treatment of metabolic bone diseases in humans, such as postmenopausal osteoporosis and age-related osteoporosis.
Acknowledgments
We thank Dr. Laura A. Cox, Dr. Robert A. Davey, Dr. Heather B. Coan, Shayna M. Levine, Ahsan Choudary and members of Dr. John L. VandeBerg's laboratory for excellent advice and technical support. We thank Dr. I. Sandford Schwartz of Smith Naturals Co., Ltd., Thailand for providing the P. mirifica tuberous extract. This work was supported by grants from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (PHD/0218/2551 to W. Tiyasatkulkovit), a Chulalongkorn University Graduate School Thesis Grant, the Ratchadaphisek Somphot Endowment Fund, Chulalongkorn University (RES 560530191-AS, Aging Cluster to S. Malaivijitnond), and funds from Texas Biomedical Research Institute (to J. L. VandeBerg). Baboons were made available to this project by NIH grants P01 HL028972 (to J. L. VandeBerg) and P51 OD011133 (to the Southwest National Primate Research Center). The baboons were housed in facilities constructed with support from NIH grants C06 RR014578 and C06 RR015456.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No conflicts to disclose.
References
- Aufdemorte TB, Fox WC, Miller D, Buffum K, Holt GR, Carey KD. A non-human primate model for the study of osteoporosis and oral bone loss. Bone. 1993;14:581–586. doi: 10.1016/8756-3282(93)90197-i. [DOI] [PubMed] [Google Scholar]
- Black A, Lane MA. Nonhuman primate models of skeletal and reproductive aging. Gerontology. 2002;48:72–80. doi: 10.1159/000048930. [DOI] [PubMed] [Google Scholar]
- Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. Journal of Pharmaceutical and Biomedical Analysis. 2007;43:428–434. doi: 10.1016/j.jpba.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Cole ZA, Dennison EM, Cooper C. Osteoporosis epidemiology update. Current Rheumatology Reports. 2008;10:92–96. doi: 10.1007/s11926-008-0017-6. [DOI] [PubMed] [Google Scholar]
- Erben RG, Eberle J, Stahr K, Goldberg M. Androgen deficiency induces high turnover osteopenia in aged male rats: a sequential histomorphometric study. Journal of Bone and Mineral Research. 2000;15:1085–1098. doi: 10.1359/jbmr.2000.15.6.1085. [DOI] [PubMed] [Google Scholar]
- Forrest SM, Ng KW, Findlay DM, Michelangeli VP, Livesey SA, Partridge NC, Zajac JD, Martin TJ. Characterization of an osteoblast-like clonal cell line which responds to both parathyroid hormone and calcitonin. Calcified Tissue International. 1985;37:51–56. doi: 10.1007/BF02557679. [DOI] [PubMed] [Google Scholar]
- Havill LM, Allen MR, Harris JA, Levine SM, Coan HB, Mahaney MC, Nicolella DP. Intracortical bone remodeling variation shows strong genetic effects. Calcified Tissue International. 2013;93:472–480. doi: 10.1007/s00223-013-9775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havill LM, Levine SM, Newman DE, Mahaney MC. Osteopenia and osteoporosis in adult baboons (Papio hamadryas). Journal of Medical Primatology. 2008;37:146–153. doi: 10.1111/j.1600-0684.2007.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, Hunziker W, Weber P, Martin I, Bendik I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology. 2004;145:848–859. doi: 10.1210/en.2003-1014. [DOI] [PubMed] [Google Scholar]
- Jerome CP, Peterson PE. Nonhuman primate models in skeletal research. Bone. 2001;29:1–6. doi: 10.1016/s8756-3282(01)00477-x. [DOI] [PubMed] [Google Scholar]
- Karieb S, Fox SW. Zinc modifies the effect of phyto-oestrogens on osteoblast and osteoclast differentiation in vitro. British Journal of Nutrition. 2012;108:1736–1745. doi: 10.1017/S0007114511007355. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by transcription factors. Journal of Cellular Biochemistry. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. American Journal of Obstetrics and Gynecology. 2006;194:S3–S11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ, Chen KM. Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. Journal of Cellular Biochemistry. 2011;112:916–923. doi: 10.1002/jcb.23007. [DOI] [PubMed] [Google Scholar]
- Malaivijitnond S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Frontiers of Medicine. 2012;6:8–21. doi: 10.1007/s11684-012-0184-8. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Progress in Hormone Research. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell and Tissue Research. 2011;343:289–302. doi: 10.1007/s00441-010-1086-1. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy MC, Polak JM, Afzal F, Hukkanen MV, Huang P, MacIntyre I, Buttery LD. Nitric oxide mediates 17β-estradiol-stimulated human and rodent osteoblast proliferation and differentiation. Biochemical and Biophysical Research Communications. 2000;277:604–610. doi: 10.1006/bbrc.2000.3714. [DOI] [PubMed] [Google Scholar]
- Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Research. 2004;24:3743–3748. [PubMed] [Google Scholar]
- Schneider JS, Pioli EY, Jianzhong Y, Li Q, Bezard E. Levodopa improves motor deficits but can further disrupt cognition in a macaque Parkinson model. Movement Disorders. 2013;28:663–667. doi: 10.1002/mds.25258. [DOI] [PubMed] [Google Scholar]
- Shen YT. Primate models for cardiovascular drug research and development. Current Opinion in Investigational Drugs. 2010;11:1025–1029. [PubMed] [Google Scholar]
- Smith SY, Jolette J, Turner CH. Skeletal health: primate model of postmenopausal osteoporosis. American Journal of Primatology. 2009;71:752–765. doi: 10.1002/ajp.20715. [DOI] [PubMed] [Google Scholar]
- Tiyasatkulkovit W, Charoenphandhu N, Wongdee K, Thongbunchoo J, Krishnamra N, Malaivijitnond S. Upregulation of osteoblastic differentiation marker mRNA expression in osteoblast-like UMR106 cells by puerarin and phytoestrogens from Pueraria mirifica. Phytomedicine. 2012;19:1147–1155. doi: 10.1016/j.phymed.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Urasopon N, Hamada Y, Asaoka K, Cherdshewasart W, Malaivijitnond S. Pueraria mirifica, a phytoestrogen-rich herb, prevents bone loss in orchidectomized rats. Maturitas. 2007;56:322–331. doi: 10.1016/j.maturitas.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Urasopon N, Hamada Y, Cherdshewasart W, Malaivijitnond S. Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas. 2008;59:137–148. doi: 10.1016/j.maturitas.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wang XD, Masilamani NS, Mabrey JD, Alder ME, Agrawal CM. Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity, and tensile properties. Bone. 1998;23:67–72. doi: 10.1016/s8756-3282(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. Journal of Ethnopharmacology. 2011;134:584–607. doi: 10.1016/j.jep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. Journal of Orthopaedic Surgery and Research. 2010;5:37. doi: 10.1186/1749-799X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zeng X, Zhang L, Zheng X. Stimulatory effect of puerarin on bone formation through activation of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Medica. 2007;73:341–347. doi: 10.1055/s-2007-967168. [DOI] [PubMed] [Google Scholar]
- Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012;4:62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]