Abstract
Gastric cancer (GC) still keeps up high mortality worldwide with poor prognosis. Efficient and non-invasive prognostic biomarkers are urgently needed. MicroRNAs are non-coding RNAs playing roles in post-transcriptional gene regulation, which contribute to various biological processes such as development, differentiation and carcinogenesis. MicroRNA expression profiles have been associated with the prognosis and outcome in GC. MicroRNA prognostic biomarkers have been identified from blood or tissues samples, but with different prognostic features. Understanding the various roles of microRNAs in different sample sources of GC will provide deep insights into GC progression. In this review, we highlight the distinct prognostic roles of microRNAs biomarkers in blood and tissue according to their relationships with prognostic parameters, survival rates and target pathways. This will be useful for non-invasive biomarker development and selection in prognosis of GC.
Keywords: blood, gastric cancer, microRNA, prognostic biomarker, tissues
Introduction
Gastric cancer (GC) or stomach cancer (SC) is highly heterogeneous in histological pattern, biological behavior, outcome and biomarkers. It is the fourth common cancer and the second major contributor to cancer mortality worldwide 1, 2. Although the incidence and mortality of GC declined in the last decades because of the improvement in surgical and adjuvant multimodal treatment approaches, the overall prognosis for advanced GC remains poor and the 5-year survival rate for advanced GC is still low between 10% and 25% 3, 4. New prognostic biomarkers for GC are extremely needed.
MicroRNAs are small non-coding RNAs (18-25 nucleotides) and changes in the abundance of them reveal promising prognostic associations with major cancer outcome such as clinicopathological features and survival rates. Many works have demonstrated that microRNAs could be as potential biomarkers in different diseases, such as prostate cancer, clear cell renal cell carcinoma, sepsis, gastric cancer and so on 5-8. In GC, several reports have reviewed the microRNAs as biomarkers for GC from different perspectives. Shrestha et al. and Wang et al. focused on the systematic summarizing microRNA expression profile from 6 studies and 14 studies in gastric cancer tissues, respectively 9, 10. Li and her colleagues overviewed the epigenetic biomarkers including DNA methylation, histone modification and microRNAs in gastrointestinal cancers 11. Another review also summarized the epigenetic biomarkers, DNA methylation and microRNAs, but only paid attention to their function as diagnostic markers in body fluids 12. A meta-analysis was performed on circulating microRNAs in 22 studies and concluded that miR-21 can be a biomarker for detection of GC with AUC = 0.91 and Q = 0.8466 13. Most of the comprehensive reviews summarized the microRNAs function and the role of microRNAs as markers for GC diagnosis, prognosis or therapeutic response 14-20. But the different prognostic roles of microRNAs in blood and tissues remain poorly understood, which is much more important to the understanding of the clinical roles of these microRNAs in different sample sources.
In this review, we give an elaborate comparison of microRNAs as prognostic biomarkers in blood and tissues. MicroRNA biomarkers in tissues indicate the samples from tissues of patients while microRNA biomarkers in blood indicate the samples from serum, plasma, or blood. We selected the studies by the search criteria “(gastric cancer OR stomach cancer) AND (biomarker* OR marker*) AND (prognos*) AND (microRNA OR miRNA)” from PubMed. We considered only the researches which take the expression of microRNAs as prognostic biomarkers. Since we compared the prognostic features of microRNAs in human blood and tissues, articles about microRNA biomarkers in other body fluid such as gastric juice and other animal samples were excluded. Altogether, as prognostic markers, 14 microRNAs in blood and 36 microRNAs in tissues from 45 studies were compared according to their association with clinicopathological features of GC and survival analysis (See Tables 1 and 2). We also summarized the validated targets of given microRNAs in GC by searching databases, such as, TarBase 21, miR2Disease 22, and miRTarBase 23. In each database, we just considered the terms which studied gastric cancer of Homo sapiens. In TarBase, the terms with prediction score larger than 0.8 were included. In miRTarBase, we only selected the reports based on 'strong evidence'. This review provides complementary to the previous reviews and essential information that will help discover non-invasive biomarkers in prognosis of GC.
Table 1.
MicroRNA biomarkers in blood for gastric cancer.
| ID | Sample | Features | Poor Survival | Expression | Reference | Validated Targets |
|---|---|---|---|---|---|---|
| miR-122 | 96 GC 7 BGC 10 CG 36 HC |
Distance metastases | Down | Down | Chen et al.[68] | - |
| miR-17-5p | 79 PRE GC 30 POST GC 6 relapse GC |
Differentiation TNM stages |
Up | Up1 | Wang et al.[30] | - |
| miR-18a | 82 GC 65 HC |
LNM Pathological grade |
Up* | Up | Su et al. [66] | - |
| miR-20a | 79 PRE GC 30 POST GC 6 relapse GC |
Differentiation TNM stages |
Up | Up1 | Wang et al.[30] | - |
| miR-200c | 67 GC 15 HC |
LNM | Up | Up | Valladares-Ayerbes et al.[64] | BCL2, XIAP[99] |
| miR-203 | 154 GC 22 HC |
Gender Lymphatic invasion Venous invasion Peritoneal metastasis Distance metastasis LNM Liver metastasis TNM stage |
Down | Down | Imaoka et al. [28] | - |
| miR-21 | 69 GC | Venous invasion | Up* | - | Komatsu et al.[26] | RECK[92] PTEN[100] Serpini1[101] |
| miR-21 | 42 PRE GC 42 POST GC |
Differentiation LNM |
- | Up1 | Ma et al.[27] | RECK[92] PTEN[100] Serpini1[101] |
| miR-218 | 68 GC 56 HC |
Metastasis Tumor stage |
Down | Down | Xin et al.[69] | ECOP[102] |
| miR-221 | 82 GC 46 dysplasia 128 SG or CAG |
Differentiation | - | Up | Song et al.[31] | p27, p57 [103] PTEN[104] |
| miR-222 | 114 GC 36 CAG 56 HC |
LNM | Up | Up | Fu et al.[65] | p27, p57 [103] PTEN[104] RECK[105] |
| miR-25 | Tissue: 33 GC 33 HC Blood: 70 GC 70 HC |
LNM TNM stage |
Up | Up | Li et al.[70] | p57 [103] BCL2L11[106] FBXW7[46] |
| miR-27a | 82 GC | Metastasis Recurrent |
Up | Up | Huang et al.[67] | Prohibitin [107] APC[108] |
| miR-376c | 82 GC 46 dysplasia 128 SG or CAG |
Differentiation | - | Up | Song et al.[31] | - |
| miR-744 | 82 GC 46 dysplasia 128 SG or CAG |
Differentiation | - | Up | Song et al.[31] | - |
Abbreviations and note: BGC: benign gastric ulcer; CAG: chronic atrophic gastritis; CG: chronic gastritis; GC: Gastric cancer; HC: healthy control; LNM: Lymph node metastasis; PRE: pre-operative; POST: post-operative; SG: superficial gastritis; * Disease-specific; 1 Pre-operation.
Table 2.
MicroRNA biomarkers in tissues for gastric cancer.
| ID | Sample | Features | Poor Survival | Expression | Reference | Validated Targets |
|---|---|---|---|---|---|---|
| miR-107 | 161 GC 161 ANTT |
Invasion LNM Tumor stage |
Up | Up | Inoue et al. [41] | CDK6[40] DICER1[41] |
| miR-1207-5p | 23 GC with LNM 23 GC without LNM |
LNM Lymphovascular invasion Stromal reaction type TNM stage |
- | Down1 | Huang et al. [53] | - |
| miR-125a-3p | 70 GC 70 ANTT |
Invasion LNM Liver metastasis Tumor stage Tumor size Peritoneal dissemination |
Down | Down | Hashiguchi et al. [48] | - |
| miR-125a-5p | 87 GC | Invasion depth Liver metastasis Tumor stage Tumor size |
Down | Down4 | Nishida et al. [47] | ERBB2[47] |
| miR-130a | 41 GC 41 ANTT |
Metastasis Invasion Proliferation |
Up | Up | Jiang et al. [45] | RUNX3[45] |
| miR-141 | 36 GC 36 ANTT |
Invasion Proliferation Metastasis |
- | Down | Zuo et al. [54] | - |
| miR-142-5p | 29 REGC 36 non-REGC |
Recurrence | Up | Down2 | Zhang et al. [76] | - |
| miR-143 | 138 GC 30 NTT |
Tumor stage Scirrhous type |
Up* | Up | Naito et al. [73] | - |
| miR-145 | 138 GC 30 NTT |
Tumor stage Scirrhous type |
Up* | Up | Naito et al. [74] | CDH2[109] |
| miR-148a | 106 GC 106 ANTT |
Distant metastasis Organ invasion Peritoneal invasion |
Down | Down | Tseng et al. [50] | DNMT1[110] p27[111] ROCK1 [63] |
| miR-153 | 80 GC 80 ANTT |
Invasion LNM Migration |
Down | Down | Zhang et al [55] | - |
| miR-181c | 103 GC | Differentiation Invasive depth Tumor stage |
Up | Up4 | Cui et al. [32] | NOTCH4, KRAS[112] BCL2[113] |
| miR-192 | 118 GC 118 ANTT |
Tumor sizes Borrmann type |
- | Down3 | Chiang et al [61] | - |
| miR-192 | 38 GC 38 ANTT |
LNM | - | Up | Xu et al. [62] | - |
| miR-193b | 48 GC 48 ANTT |
Differentiation Lauren type Tumor stage Invasion Metastasis |
Down | Down | Mu et al. [35] | - |
| miR-195 | 45 GC | Recurrence | - | Up2 | Brenner et al. [75] | - |
| miR-196a | 109 GC 20 ANTT |
Invasion depth Serosal invasion Lymphatic invasion LNM Distant metastasis TNM stage Peritoneal seeding Gross type Lauren subtype |
Up | Up | Tsai et al. [44] | radixin[44] |
| miR-196a | 48 GC 48 ANTT |
Differentiation | Up | Up | Mu et al. [35] | - |
| miR-196a-5p | 58 GC 58 ANTT |
LNM TNM stage |
Up | Up | Li et al. [58] | - |
| miR-199a-3p | 45 GC | Recurrence | - | Up2 | Brenner et al. [75] | SMARCA2 [114] |
| miR-199a-5p | 28 GC 48 GC LNM 25 NTT |
Metastasis | - | Up | Zhao et al. [60] | MAP3K11 [115] Smad4[116] SMARCA2 [114] |
| miR-196b | 109 GC 20 ANTT |
Invasion depth Serosal invasion Lymphatic invasion LNM Distant metastasis TNM stage Peritoneal seeding Gross type |
Up | Up | Tsai et al. [44] | - |
| miR-206 | 98 GC 98 ANTT |
Venous invasion LNM Hematogenous recurrence pStage |
Down | Down | Yang et al. [51] | CCND2[117] |
| miR-20b | 102 GC 102 ANTT |
LNM Distance metastasis TNM stage |
Up | Up | Xue et al. [59] | - |
| miR-21 | 56 GC without LNM 30 GC with LNM 72 ANTT |
Differentiation LNM |
Up | Up | Xu et al. [33] | RECK[92] PTEN[100] Serpini1[101] |
| miR-215 | 118 GC 118 ANTT |
Borrmann type Tumor sizes pT stage |
- | Down3 | Chiang et al [61] | - |
| miR-215 | 38 GC 38 ANTT |
- | - | Up | Xu et al. [62] | - |
| miR-217 | 83 GC 83 ANTT |
Differentiation Distant metastasis Invasion Tumor size TNM stag |
Down | Down | Chen et al. [36] | - |
| miR-22 | 98 GC 98 ANTT |
LNM Distant metastasis pStage |
Down | Down | Yang et al. [51] | SP1[118] |
| miR-23a/b | 160 GC 160 ANTT |
Invasion LNM TNM stage |
Up | Up | Ma et al. [43] | IL6R[119] |
| miR-25 | 40 GC 40ANTT |
Invasion Proliferation LNM Migration |
Up | Up | Gong et al [46] | p57 [103] BCL2L11[106] FBXW7[46] |
| miR-29c | 115 GC 115 ANTT |
Venous invasion TNM stage |
- | Down | Gong et al. [52] | - |
| miR-335 | 31 REGC 43 non-REGC |
Recurrence | Up | Up2 | Yan et al. [77] | - |
| miR-34a | 137 GC 137 |
Lymph node involvement TNM stage Differentiation Tumor recurrence |
Down | Down | Zhang et al. [34] | BCL2[120] |
| miR-375 | 29 REGC 36 non-REGC |
Recurrence | Up | Up2 | Zhang et al. [76] | PDK1, YWHAZ[121] JAK2[122] |
| miR-451 | 45 GC | Recurrence | Up* | Up2 | Brenner et al. [75] | MIF [123] |
| miR-520d-3p | 120 GC 120 ANTT |
Invasion depth LNM Tumor stage |
Down | Down | Li et al. [49] | - |
| miR-630 | 236 GC 236 ANTT |
Invasion LNM Distant metastasis TNM stage. |
Up | Up | Chu et al. [42] | - |
| miR-92a | 97 GC | Tumor growth | Up | - | Wu et al. [78] | - |
ANTT: adjacent non-tumor tissues; GC: gastric cancer; LNM: Lymph node metastasis; NTT: non-tumor tissues; REGC: gastric cancer with recurrence; non-REGC: GC without recurrence;
* Disease-specific; 1 LNM samples; 2 Recurrence; 3 GC cell line; 4 advanced GC
Clinicopathological features
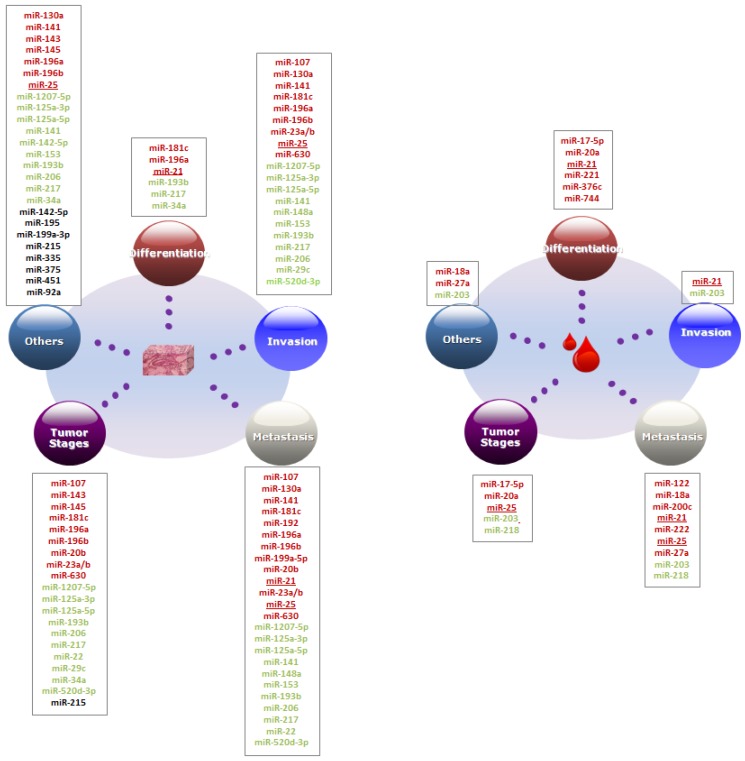
We summarized and discussed the association between GC clinicopathological features and microRNAs biomarkers in blood and tissues. The clinicopathological features were generally classified into five groups including differentiation, invasion, metastasis, tumor stages and others, these are related to the important cancer 2hallmarks 24, 25 (See Figure 1).
Figure 1.
Association between clinicopathological features and microRNA biomarkers. MicroRNAs in red and green denote the up-regulated and down-regulated expression in GC. MicroRNAs in black denote that the microRNAs were differentially expressed between two-sample groups other than GC patient and healthy controls, e.g. between recurrence and non-recurrence groups. microRNAs marked with underline present the microRNAs could be prognostic markers both in tissues and blood.
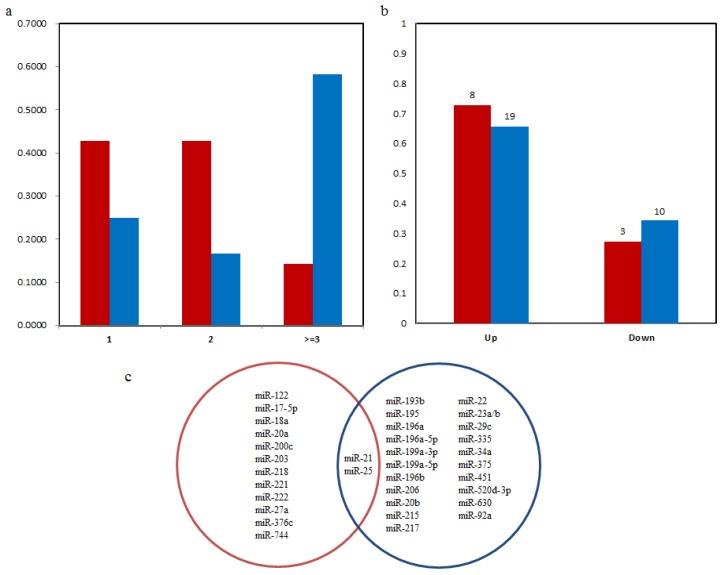
We first compared the number of clinicopathological factors that correlated with the microRNAs in blood and tissues. As shown in Figure 2(a), 42.86% of the blood microRNAs significantly correlated with one and two clinicopathological factors. Only two microRNAs (14.29%) significantly correlated with three or more than three factors: miR-21strongly correlated with venous invasion, differentiation and lymph node metastasis 26, 27 and miR-203 correlated with gender, invasion, metastasis, TNM stage 28. Conversely, tissues-based microRNAs tend to correlated with more clinicopathological factors. Nearly 60% microRNAs in tissues were significantly associated with more than three clinicopathological factors and only 25 % microRNAs were associated with one factor.
Figure 2.
(a) Distribution of the number of clinicopathological features correlated with microRNAs. X axis is the number of clinicopathological features. Y axis is the percent of microRNAs in blood or tissues that correlated with different number of features. (b) Distribution of expression pattern of microRNA biomarkers from blood and tissues with poor survival of GC patients. Red is the microRNAs from blood and blue is from tissues. Numbers above the bars are the number of microRNA biomarkers in corresponding group. (c) The Venn diagram for microRNA prognostic biomarkers in blood and tissue. Blue and red circles represent microRNAs in tissue and blood respectively.
Differentiation
In histology, tumor was classified as three degrees of differentiation: well, moderate and poor differentiation according to WHO classification 29. Patients with well-differentiated tumors usually carry a better prognosis whereas patients with poorly differentiated tumors carry a worse prognosis. There are six circulating microRNA biomarkers associated with differentiation and all of them were up-regulated in poor differentiation group, including miR-17-5p, miR-20a, miR-21, miR-221, miR-376c and miR-744 27, 30, 31, whereas in tissue samples, miR-181c, miR-196a and miR-21, were up-regulated and miR-193b, miR-217 and miR-34a, were down-regulated in poor differential groups 32-36.
Invasion
GC invasion is a process when tumor cells invade the tumor nearby tissues and vasculature. It is an elementary factor that affects patient survival rate and the most important step of tumor cells dissemination and metastasis in different types of cancer 37-39. Zhao et al. had reviewed the role of microRNAs in the GC invasion and metastasis 39. Some of the microRNAs could be as prognostic biomarkers. miR-107, for example, is a potential prognostic biomarker in tissue and inhibits the GC cells invasion by directly targeting the cyclin-dependent kinase 6 (CDK6) 40, 41. In tissue, expression levels of miR-181c, miR-630 and co-expression of miR-23a and miR-23b were strongly associated with invasion depth 32, 42, 43. Increased miR-196a/b expression was significantly correlated with serosal, vascular, lymphatic and depth of invasion but in another study miR-196a doesn't have such significantly association with depth of invasion one-way analysis of variance 35, 44 . High expression of miR-130a and miR-25 promoted the migration, invasion and proliferation of gastric cancer cells by targeting RUNX3 and FBXW7, respectively 45, 46.
Decreased expression of eight microRNAs were associated with different types of invasion, e.g. miR-125-3p/-5p and miR-520d-3p with depth of invasion 47-49, miR-148a with organ invasion and peritoneal invasion 50, miR-29c and miR-206 with venous invasion 51, 52. Patients with down-regulated miR-1207- 5p had more lymphovascular invasion 53. Down regulation of miR-141 promoted cell proliferation, invasion and migration in AGS GC cell lines 54. Suppression of miR-153 also promoted GC cell migration and invasion by inhibiting SNAI1-induced epithelial-mesenchymal transition (EMT) 55. In blood, however, only two microRNAs miR-21 and miR-203 in blood was associated with invasion 26, 28.
Metastasis
Metastasis, a complex and multi-step process, is a primary clinicopathological feature of advanced GC. In metastasis, cancer cells migrate from the primary neoplasm to a distant location and proliferate to form anther macroscopic tumors 56, 57. However, the mechanisms that regulate metastasis remain poorly understood.
In tissues, lymph node metastasis (LNM) was significantly related with higher expression of miR-107, miR-181c, miR-196a/b, miR-20b, miR-23a/b, miR-25 and miR-630 32, 41-44, 46, 58, 59. Increased miR-196a/b, miR-20b and miR-630 expression were also more detected in GC with distant metastasis. miR-196a/b simulates cell metastasis through direct negative regulation of radixin in GC 42, 44. High-level of miR-199a-5p expression could promote cell metastasis in GC cells since suppression of miR-199a-5p decreased the metastatic ability in GC cells in vitro and in vivo 60. Moreover, there are controversial results about miR-196a, -215 and -192. As mentioned above, miR-196a was reported significantly up-regulated in distance metastasis 44 and correlated with lymph node metastasis 58 while no such correlation was found in the subsequent study.35 . Chiang et al. found there were no significant difference in the expression levels of these miR-215 and -192 between GC tissue and non-tumor tissue and both of them were decreased in the GC cell lines 61. But Xu and Fan found that miR-215 and -192 levels were increased in GC tissue and related with lymph node metastasis 62.
With regard to down-regulated microRNAs in GC tissues, reduced expression of miR-125-3p, miR-153, miR-206, miR-22 and miR-520d-3p were strongly correlated with lymph node metastasis 48, 49, 51, 55. MiR-1207-5p was significantly down-regulated in samples with LNM compared with those without LNM 53. Additionally, expression levels of miR-125-3p, miR-5p, miR-148a and miR-22 were associated with distance metastasis, especially the correlation between liver metastasis and miR-125-3p/-5p 47, 48, 50, 51. miR-217 was significantly down-regulated in patients with liver metastasis and lung metastasis and promoted tumor progression and metastasis in vivo experiment 36. miR-148a inhabits the GC cell metastasis by reducing the mRNA and protein levels of ROCK1 in GC 63. miR-141expression level was found to be decreased in primary tumors that subsequently metastasized compared with those that did not metastasize 54.
Several circulating microRNA biomarkers also displayed significantly correlation with metastasis. The expression level of miR-18a, miR-203, miR-200c and miR-222 was significantly correlated with the number of lymph node metastases 28, 64-66. Increased expression levels of miR-27a in plasma were significantly correlated with poor overall survival for metastatic or recurrent GC 67. miR-122 was significantly lower in GC with distant metastasis than healthy controls and GC with no distant metastasis 68. miR-218 was found to be associated with tumor metastasis and decreased in metastasis than non-metastasis and normal serum 69.
Two microRNAs, miR-25 and miR-21 have the same expression pattern in tissue and blood. The miR-25 expression was elevated both in plasma and tissues of GC patients with tumor node metastasis stage or lymph node metastasis 70. Although miR-21 in plasma of Japan GC patients was not associated with metastasis 26, its expression in plasma of post-operative patients in China was highly associated with lymph node metastasis rate 27 and was higher in tissues of GC patients with lymph node metastasis than those without lymph node metastasis 33.
Tumor Stages
The TNM (tumor-node-metastasis) classification is a widely used cancer staging systems based on the size and extension of the primary tumor (T), nearby lymph nodes involvement (N), and the presence of or otherwise of distant metastatic spread (M). Recently, the seventh edition of the TNM classification was published which introduced many changes for gastric cancer, especially the N stage reclassification 71, 72.
In tissues, high expression of miR-107, -181c, -196a/b, -20b, -23a/b and -630 was more frequently to be detected in GC with advanced tumor stage 32, 41-44, 59. In particular, as a potential prognostic biomarker of scirrhous type GC miR-143 and -145 expression levels were higher in scirrhous type GC than non- scirrhous type GC and strongly correlated with tumor stage and scirrhous type histology 73, 74. GC patients with low expression of miR-125-3p, -125-5p, -193b, -206, -217, -22,-29c, -34a, -520d-3p were more often found at advanced tumor stage 34-36, 47-49, 51, 52.
In blood, miR-17-5p, -20a, -203, -25 and -28 were significantly associated with TNM staging classification system. Expression levels of miR-17-5p and miR-20a were only significantly higher in TNM III stage group than I and II group 30 and the level of miR-25 was higher both in TNM III and IV than I and II 70, while miR-218 and miR-203 were decreased in the TNM later stages III and IV 28, 69.
Other clinicopathological features
Beyond the above four main clinicopathological features, microRNA biomarkers are also related with other clinicopathological features, such as GC histological classification, recurrence, tumor growth, tumor size, etc. miR-143 and miR-145 were associated with scirrhous type histology 73, 74. miR-196a was more frequently detected in diffuse and infiltrative GC subtype 44. Brenner et al. found that miR-451, -199a-3p and -195 expression were increased in GC patients with recurrence than patients without recurrence after all the patients received tumor resected surgery 75. Zhang and colleagues identified that miR-375 and miR-142-5p were differentially expressed between recurrence groups and non-recurrence groups and the combination of these two microRNAs could recognize the above groups both in the training and test samples as a classifier 76. Recently, high expression level of miR-335 was also detected in high recurrence groups and it was involved in several oncogenic pathways such as TP53, TGF-β and Wnt 77. Improving miR-90a expression promoted tumor growth in vitro and in vivo 78.
Survival analysis
Prediction of survival is one of the main functions of the prognostic biomarkers. As shown in Figure 2(b), Table 1 and Table 2, we summarize the correlation between patients' survival and expression levels of microRNA biomarkers from blood and tissues. Up-regulated microRNAs were more significantly correlated with poor survival than down-regulated ones, both in blood and tissues. In blood, high concentration of 6 microRNAs (miR-17-5p, -20a, -200c, -222, -25 and -27a) and low concentration of 3 microRNAs (miR-122, miR-203 and -218) were significantly associated with worse overall survival 28, 30, 64, 65, 67-70. High expression of miR-18a in plasma was associated with shorter both disease-free and disease-specific survival of GC patients 66. Post-operative patients with increased miR-21 levels had a significantly worse prognosis (disease-specific survival) than those with decreased expression levels 26. In tissues, increased levels of 13 (miR-107, -130a, -142-5p, -181c, -196a, -196b, -20b, -21, -25, -335, -375, -630 and -92a) and 3 (miR-143, -145 and -451) microRNAs were correlated with overall poor survival and disease specific survival rates separately while decreased expression of 10 microRNAs (miR-125a-3p, -125a-5p, -153, -193b, -148a, -206, -217, -22, -34a and -520d-3p) were correlated significantly with overall poorer survival rates 32-36, 41, 42, 44-51, 55, 59, 73-78 .
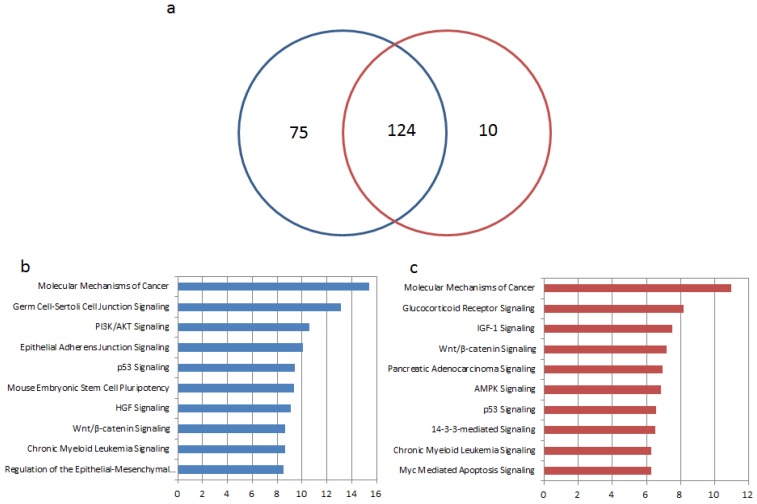
Enrichment analysis of targets of prognostic microRNA biomarkers
To further explore different functions of the above microRNA biomarkers in tissue and blood, we performed enrichment analysis of their target genes by Ingenuity Pathways Analysis (IPA®). The targets of microRNAs were collected from experimentally validated database miRecords 79, TarBase 21, miR2Disease 22, and miRTarBase 23 or predicted by computational software tools HOCTAR 80, ExprTargetDB 81, and starBase 82 as reported in our previous research 5. For the 36 microRNA biomarkers in GC tissue, 3735 target genes were predicted and 199 pathways were significantly enriched by their targets (p < 0.01). 2093 targets were predicted for 14 microRNA biomarkers in blood and significantly enriched in 134 pathways (p < 0.01). More than half of the enriched pathways by targets of microRNAs in tissue and in blood are overlapped, as see in Figure 3 (a). As shown in Table 3 and Figure 3 (b) and (c), the top 10 significantly enriched pathways by the targets of the given microRNAs in blood and tissue were listed. Among the top 10 enriched pathways, four of them are same as molecular mechanisms of cancer, p53 signaling, chronic myeloid leukemia signaling and Wnt/β-catenin Signaling. In the case of tissue, several in the top ten pathways are related with epithelial cell or tissue such as regulation of the epithelial-mesenchymal transition pathway 83, epithelial adherens junction signaling and germ cell-sertoli cell junction signaling. The function is different for the top 10 pathways that enriched by target genes of blood based microRNA markers. There are not so many pathways related to epithelial cell or tissue, but most of them play important roles in GC such as AMPK signaling, which was reported to induce apoptosis through the mitochondrial apoptotic pathway 84, 85, Wnt signaling, the one is well-known for promoting the development of hematoendothelial cell 86-89, and IGF-1 Signaling which induces epithelial-mesenchymal transition in gastric cancer 90, 91.
Figure 3.
Enrichment analyses of target genes of microRNA biomarkers in tissue and blood. (a) The Venn diagram for numbers of significantly enriched pathways. The blue and red circles represent pathways enriched by targets of microRNAs in tissue and blood respectively. (b) Top 10 significantly enriched pathways by targets of microRNA biomarker from GC tissue. (c) Top 10 significantly enriched pathways by targets of microRNA biomarker from GC blood.
Table 3.
Top 10 significantly enriched pathways by targets of microRNA biomarker from GC blood and tissue.
| Source | Ingenuity Canonical Pathways | p-value | Ratio | miRNA |
|---|---|---|---|---|
| Blood | Molecular Mechanisms of Cancer | 1.00E-11 | 0.39 | miR-200c, miR-21, miR-25 |
| Glucocorticoid Receptor Signaling | 6.76E-09 | 0.37 | miR-200c, miR-21, miR-25 | |
| IGF-1 Signaling | 2.95E-08 | 0.48 | miR-200c, miR-21, miR-221 | |
| Wnt/β-catenin Signaling | 6.46E-08 | 0.43 | miR-25, miR-21, miR-200c | |
| Pancreatic Adenocarcinoma Signaling | 1.12E-07 | 0.48 | miR-21, miR-200c, miR-20a | |
| AMPK Signaling | 1.35E-07 | 0.40 | miR-200c, miR-21, miR-25 | |
| p53 Signaling | 2.82E-07 | 0.46 | miR-21, miR-200c, miR-20a | |
| 14-3-3-mediated Signaling | 3.09E-07 | 0.44 | miR-200c, miR-221, miR-21 | |
| Chronic Myeloid Leukemia Signaling | 5.13E-07 | 0.46 | miR-20a, miR-200c, miR-21 | |
| Myc Mediated Apoptosis Signaling | 5.50E-07 | 0.56 | miR-20a, miR-200c, miR-21 | |
| Tissue | Molecular Mechanisms of Cancer | 3.98E-16 | 0.59 | miR-21, miR-25, miR-23b |
| Germ Cell-Sertoli Cell Junction Signaling | 7.94E-14 | 0.69 | miR-141, miR-23b, miR-21 | |
| PI3K/AKT Signaling | 2.51E-11 | 0.70 | miR-22, miR-23b, miR-195 | |
| Epithelial Adherens Junction Signaling | 7.94E-11 | 0.65 | miR-23b, miR-21, miR-141 | |
| p53 Signaling | 3.89E-10 | 0.70 | miR-23b, miR-21, miR-141 | |
| Mouse Embryonic Stem Cell Pluripotency | 4.17E-10 | 0.73 | miR-143, miR-451a, miR-21 | |
| HGF Signaling | 8.32E-10 | 0.68 | miR-21, miR-23b, miR-196b | |
| Wnt/β-catenin Signaling | 2.14E-09 | 0.61 | miR-141, miR-25, miR-34a | |
| Chronic Myeloid Leukemia Signaling | 2.29E-09 | 0.69 | miR-21, miR-34a, miR-22 | |
| Regulation of the Epithelial-Mesenchymal Transition Pathway | 2.88E-09 | 0.62 | miR-141, miR-21, miR-22 |
The overlapped pathways are marked by underline. The ratio is the percentage of the mapped genes divided by the number of total genes in the pathway.
We then calculated the percentage of mapped targets of the microRNA in the top ten pathways and listed the top three microRNAs in Table 3. In the case of blood, miR-200c and miR-21 are in the top three in every pathway whereas in tissue, miR-21 is in the top three in eight pathways. This indicates that miR-21 plays a pivotal role in the development of gastric cancer 92, 93. Additionally, in each of the four overlapped pathways, the top three microRNAs are not the same. Take molecular mechanisms of cancer as an example, miR-200c and miR-23b are in the top three ones in blood and tissue respectively besides miR-21 and miR-25.
Conclusions
In this review, we made a comparison of the prognostic abilities for microRNA in blood and tissues. There are almost twice as many prognostic microRNA biomarkers in tissues as in blood. This may be due to more studies investigating microRNAs from tissues. Another important reason is that microRNAs may be released into the blood selectively 94, 95. Although some microRNAs display the same expression pattern in blood and tissues, they correlate with different clinicopathological features. miR-21 for example, is associated with invasion in blood but not in tissue. Most of microRNAs in blood were significantly correlated with no more than two clinicopathological features while microRNAs in tissues were associated with more features. Both in blood and tissues, microRNAs could be strongly associated with survival and most of the microRNAs with high expression level were detected in the poor survival groups.
Gastric cancer with a very poor prognosis remains to account for considerable amount of morbidity and mortality in the world. MicroRNAs in blood are promising biomarkers since they are non-invasive and could have a possible clinical application in GC. As well-known that GC is heterogeneous and personalized, the understanding of the roles of microRNAs in GC progression needs further exploration at systems biological level 96-98.
Acknowledgments
National Natural Science Foundation of China grants (31470821, 31170795, 31400712, 91230117), Special Funding for Life and Health Researches from Science and Technology Department of Jiangsu Province (BL2014046), Natural Science Foundation for Colleges and Universities in Jiangsu Province (13KJB180021) and Natural Science Foundation of USTS (XKQ201315), Technology R&D Program of Suzhou (SYN201409).
References
- 1.Hamilton JP, Meltzer SJ. A review of the genomics of gastric cancer. Clin Gastroenterol Hepatol. 2006;4:416–25. doi: 10.1016/j.cgh.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698–705. doi: 10.3238/arztebl.2011.0698. quiz 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wohrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585–95. doi: 10.1093/annonc/mdh422. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Zang J, Jing X, Sun Z, Yan W, Yang D. et al. Identification of candidate miRNA biomarkers from miRNA regulatory network with application to prostate cancer. J Transl Med. 2014;12:66. doi: 10.1186/1479-5876-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Zhang D, Zhang W, Tang Y, Yan W, Guo L. et al. Clear cell renal cell carcinoma associated microRNA expression signatures identified by an integrated bioinformatics analysis. J Transl Med. 2013;11:169. doi: 10.1186/1479-5876-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Sun Z, Yan W, Zhu Y, Lin Y, Chen J. et al. Identification of microRNA as sepsis biomarker based on miRNAs regulatory network analysis. Biomed Res Int. 2014;2014:594350. doi: 10.1155/2014/594350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan W, Xu L, Sun Z, Lin Y, Zhang W, Chen J, MicroRNA biomarker identification for pediatric acute myeloid leukemia based on a novel bioinformatics model. Oncotarget; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JL, Hu Y, Kong X, Wang ZH, Chen HY, Xu J. et al. Candidate microRNA biomarkers in human gastric cancer: a systematic review and validation study. PLoS One. 2013;8:e73683. doi: 10.1371/journal.pone.0073683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha S, Hsu SD, Huang WY, Huang HY, Chen W, Weng SL. et al. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014;3:878–88. doi: 10.1002/cam4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Jin H, Wang X. Epigenetic biomarkers: potential applications in gastrointestinal cancers. ISRN Gastroenterol. 2014;2014:464015. doi: 10.1155/2014/464015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toiyama Y, Okugawa Y, Goel A. DNA methylation and microRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem Biophys Res Commun. 2014;455:43–57. doi: 10.1016/j.bbrc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Lv M, Wang H, Guan W. Identification of circulating microRNAs as novel potential biomarkers for gastric cancer detection: a systematic review and meta-analysis. Dig Dis Sci. 2014;59:911–9. doi: 10.1007/s10620-013-2970-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259–67. doi: 10.3233/CBM-2012-00284. [DOI] [PubMed] [Google Scholar]
- 15.Tong F, Cao P, Yin Y, Xia S, Lai R, Liu S. MicroRNAs in gastric cancer: from benchtop to bedside. Dig Dis Sci. 2014;59:24–30. doi: 10.1007/s10620-013-2887-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu HH, Lin WC, Tsai KW. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16:e1. doi: 10.1017/erm.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20:10432–9. doi: 10.3748/wjg.v20.i30.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 19.Liu HS, Xiao HS. MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol. 2014;20:12007–17. doi: 10.3748/wjg.v20.i34.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang QX, Zhu YQ, Zhang H, Xiao J. Altered MiRNA expression in gastric cancer: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35:933–44. doi: 10.1159/000369750. [DOI] [PubMed] [Google Scholar]
- 21.Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA. 2006;12:192–7. doi: 10.1261/rna.2239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X. et al. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL. et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39:D163–9. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Floor SL, Dumont JE, Maenhaut C, Raspe E. Hallmarks of cancer: of all cancer cells, all the time? Trends Mol Med. 2012;18:509–15. doi: 10.1016/j.molmed.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu S, Ichikawa D, Tsujiura M, Konishi H, Takeshita H, Nagata H. et al. Prognostic impact of circulating miR-21 in the plasma of patients with gastric carcinoma. Anticancer Res. 2013;33:271–6. [PubMed] [Google Scholar]
- 27.Ma GJ, Gu RM, Zhu M, Wen X, Li JT, Zhang YY. et al. Plasma post-operative miR-21 expression in the prognosis of gastric cancers. Asian Pac J Cancer Prev. 2013;14:7551–4. doi: 10.7314/apjcp.2013.14.12.7551. [DOI] [PubMed] [Google Scholar]
- 28.Imaoka H, Toiyama Y, Okigami M, Yasuda H, Saigusa S, Ohi M, Circulating microRNA-203 predicts metastases, early recurrence, and poor prognosis in human gastric cancer. Gastric Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 29.Bosman FT, Organization WH, Carneiro F, Hruban RH. WHO Classification of Tumours of the Digestive System: International Agency for Research on Cancer (I A R C) (UN) 2010.
- 30.Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang L. et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep. 2012;5:1514–20. doi: 10.3892/mmr.2012.828. [DOI] [PubMed] [Google Scholar]
- 31.Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY. et al. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7:e33608. doi: 10.1371/journal.pone.0033608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui M, Yue L, Fu Y, Yu W, Hou X, Zhang X. Association of microRNA-181c expression with the progression and prognosis of human gastric carcinoma. Hepatogastroenterology. 2013;60:961–4. doi: 10.5754/hge121333. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Sun J, Xu J, Li Q, Guo Y, Zhang Q. miR-21 Is a Promising Novel Biomarker for Lymph Node Metastasis in Patients with Gastric Cancer. Gastroenterol Res Pract. 2012;2012:640168. doi: 10.1155/2012/640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Li S, Yang J, Liu S, Gong X, Yu X. The prognostic value of miR-34a expression in completely resected gastric cancer: tumor recurrence and overall survival. Int J Clin Exp Med. 2015;8:2635–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Mu YP, Tang S, Sun WJ, Gao WM, Wang M, Su XL. Association of miR-193b down-regulation and miR-196a up-regulation with clinicopathological features and prognosis in gastric cancer. Asian Pac J Cancer Prev. 2014;15:8893–900. doi: 10.7314/apjcp.2014.15.20.8893. [DOI] [PubMed] [Google Scholar]
- 36.Chen DL, Zhang DS, Lu YX, Chen LZ, Zeng ZL, He MM. et al. microRNA-217 inhibits tumor progression and metastasis by downregulating EZH2 and predicts favorable prognosis in gastric cancer. Oncotarget. 2015;6:10868–79. doi: 10.18632/oncotarget.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K. et al. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–95. [PubMed] [Google Scholar]
- 38.Yoshida K, Yamaguchi K, Okumura N, Osada S, Takahashi T, Tanaka Y. et al. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343–52. doi: 10.1159/000328197. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Li X, Yuan H. microRNAs in gastric cancer invasion and metastasis. Front Biosci (Landmark Ed) 2013;18:803–10. doi: 10.2741/4144. [DOI] [PubMed] [Google Scholar]
- 40.Feng L, Xie Y, Zhang H, Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29:856–63. doi: 10.1007/s12032-011-9823-1. [DOI] [PubMed] [Google Scholar]
- 41.Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R. Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep. 2012;27:1759–64. doi: 10.3892/or.2012.1709. [DOI] [PubMed] [Google Scholar]
- 42.Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang W. et al. Increased microRNA-630 expression in gastric cancer is associated with poor overall survival. PLoS One. 2014;9:e90526. doi: 10.1371/journal.pone.0090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma G, Dai W, Sang A, Yang X, Gao C. Upregulation of microRNA-23a/b promotes tumor progression and confers poor prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2014;7:8833–40. [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai MM, Wang CS, Tsai CY, Chen CY, Chi HC, Tseng YH. et al. MicroRNA-196a/-196b promote cell metastasis via negative regulation of radixin in human gastric cancer. Cancer Lett. 2014;351:222–31. doi: 10.1016/j.canlet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Yu WW, Wang LL, Peng Y. miR-130a acts as a potential diagnostic biomarker and promotes gastric cancer migration, invasion and proliferation by targeting RUNX3. Oncol Rep; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong J, Cui Z, Li L, Ma Q, Wang Q, Gao Y, MicroRNA-25 promotes gastric cancer proliferation, invasion, and migration by directly targeting F-box and WD-40 Domain Protein 7, FBXW7. Tumour Biol; 2015. [DOI] [PubMed] [Google Scholar]
- 47.Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F. et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–33. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 48.Hashiguchi Y, Nishida N, Mimori K, Sudo T, Tanaka F, Shibata K. et al. Down-regulation of miR-125a-3p in human gastric cancer and its clinicopathological significance. Int J Oncol. 2012;40:1477–82. doi: 10.3892/ijo.2012.1363. [DOI] [PubMed] [Google Scholar]
- 49.Li R, Yuan W, Mei W, Yang K, Chen Z. MicroRNA 520d-3p inhibits gastric cancer cell proliferation, migration, and invasion by downregulating EphA2 expression. Mol Cell Biochem. 2014;396:295–305. doi: 10.1007/s11010-014-2164-6. [DOI] [PubMed] [Google Scholar]
- 50.Tseng CW, Lin CC, Chen CN, Huang HC, Juan HF. Integrative network analysis reveals active microRNAs and their functions in gastric cancer. BMC Syst Biol. 2011;5:99. doi: 10.1186/1752-0509-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Q, Zhang C, Huang B, Li H, Zhang R, Huang Y. et al. Downregulation of microRNA-206 is a potent prognostic marker for patients with gastric cancer. Eur J Gastroenterol Hepatol. 2013;25:953–7. doi: 10.1097/MEG.0b013e32835ed691. [DOI] [PubMed] [Google Scholar]
- 52.Gong J, Li J, Wang Y, Liu C, Jia H, Jiang C. et al. Characterization of microRNA-29 family expression and investigation of their mechanistic roles in gastric cancer. Carcinogenesis. 2014;35:497–506. doi: 10.1093/carcin/bgt337. [DOI] [PubMed] [Google Scholar]
- 53.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Li AF. et al. The correlation between miRNA and lymph node metastasis in gastric cancer. Biomed Res Int. 2015;2015:543163. doi: 10.1155/2015/543163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y, Yu T. et al. MicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZ. Cell Death Dis. 2015;6:e1623. doi: 10.1038/cddis.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Sun J, Bai Z, Li H, He S, Chen R. et al. MicroRNA-153 acts as a prognostic marker in gastric cancer and its role in cell migration and invasion. Onco Targets Ther. 2015;8:357–64. doi: 10.2147/OTT.S78236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 57.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Li HL, Xie SP, Yang YL, Cheng YX, Zhang Y, Wang J. et al. Clinical significance of upregulation of mir-196a-5p in gastric cancer and enriched KEGG pathway analysis of target genes. Asian Pac J Cancer Prev. 2015;16:1781–7. doi: 10.7314/apjcp.2015.16.5.1781. [DOI] [PubMed] [Google Scholar]
- 59.Xue TM, Tao LD, Zhang M, Xu GC, Zhang J, Zhang PJ. miR-20b overexpression is predictive of poor prognosis in gastric cancer. Onco Targets Ther. 2015;8:1871–6. doi: 10.2147/OTT.S85236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X, He L, Li T, Lu Y, Miao Y, Liang S. et al. SRF expedites metastasis and modulates the epithelial to mesenchymal transition by regulating miR-199a-5p expression in human gastric cancer. Cell Death Differ. 2014;21:1900–13. doi: 10.1038/cdd.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiang Y, Zhou X, Wang Z, Song Y, Liu Z, Zhao F. et al. Expression levels of microRNA-192 and -215 in gastric carcinoma. Pathol Oncol Res. 2012;18:585–91. doi: 10.1007/s12253-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 62.Xu YJ, Fan Y. MiR-215/192 participates in gastric cancer progression. Clin Transl Oncol; 2014. [DOI] [PubMed] [Google Scholar]
- 63.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R. et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–83. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 64.Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Diaz P, Lorenzo-Patino MJ, Haz M. et al. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu Z, Qian F, Yang X, Jiang H, Chen Y, Liu S. Circulating miR-222 in plasma and its potential diagnostic and prognostic value in gastric cancer. Med Oncol. 2014;31:164. doi: 10.1007/s12032-014-0164-8. [DOI] [PubMed] [Google Scholar]
- 66.Su ZX, Zhao J, Rong ZH, Wu YG, Geng WM, Qin CK. Diagnostic and prognostic value of circulating miR-18a in the plasma of patients with gastric cancer. Tumour Biol. 2014;35:12119–25. doi: 10.1007/s13277-014-2516-6. [DOI] [PubMed] [Google Scholar]
- 67.Huang D, Wang H, Liu R, Li H, Ge S, Bai M. et al. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115:549–56. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 68.Chen Q, Ge X, Zhang Y, Xia H, Yuan D, Tang Q. et al. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep. 2014;31:1863–70. doi: 10.3892/or.2014.3004. [DOI] [PubMed] [Google Scholar]
- 69.Xin SY, Feng XS, Zhou LQ, Sun JJ, Gao XL, Yao GL. Reduced expression of circulating microRNA-218 in gastric cancer and correlation with tumor invasion and prognosis. World J Gastroenterol. 2014;20:6906–11. doi: 10.3748/wjg.v20.i22.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li BS Zuo QF Zhao YL Xiao B Zhuang Y Mao XH et al. MicroRNA-25 promotes gastric cancer migration, invasion and proliferation by directly targeting transducer of ERBB2, 1 and correlates with poor survival Oncogene; 2014. 0 [DOI] [PubMed] [Google Scholar]
- 71.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours: Wiley. 2011.
- 72.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 73.Naito Y, Sakamoto N, Oue N, Yashiro M, Sentani K, Yanagihara K. et al. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci. 2014;105:228–35. doi: 10.1111/cas.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naito Y, Yasuno K, Tagawa H, Sakamoto N, Oue N, Yashiro M. et al. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol Rep. 2014;32:1720–6. doi: 10.3892/or.2014.3333. [DOI] [PubMed] [Google Scholar]
- 75.Brenner B, Hoshen MB, Purim O, David MB, Ashkenazi K, Marshak G. et al. MicroRNAs as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2011;17:3976–85. doi: 10.3748/wjg.v17.i35.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J. et al. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257–66. doi: 10.1093/annonc/mdq758. [DOI] [PubMed] [Google Scholar]
- 77.Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z. et al. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. doi: 10.1371/journal.pone.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y. et al. MiR-19b/20a/92a regulates the self-renewal and proliferation of gastric cancer stem cells. J Cell Sci. 2013;126:4220–9. doi: 10.1242/jcs.127944. [DOI] [PubMed] [Google Scholar]
- 79.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–10. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gennarino VA, Sardiello M, Avellino R, Meola N, Maselli V, Anand S. et al. MicroRNA target prediction by expression analysis of host genes. Genome Res. 2009;19:481–90. doi: 10.1101/gr.084129.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gamazon ER, Im HK, Duan S, Lussier YA, Cox NJ, Dolan ME. et al. Exprtarget: an integrative approach to predicting human microRNA targets. PLoS One. 2010;5:e13534. doi: 10.1371/journal.pone.0013534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39:D202–9. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu YW, Sun M, Xia R, Zhang EB, Liu XH, Zhang ZH. et al. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6:e1802. doi: 10.1038/cddis.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67:2005–11. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 85.Yi B, Liu D, He M, Li Q, Liu T, Shao J. Role of the ROS/AMPK signaling pathway in tetramethylpyrazine-induced apoptosis in gastric cancer cells. Oncol Lett. 2013;6:583–9. doi: 10.3892/ol.2013.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL. et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–31. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J. et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oguma K, Oshima H, Oshima M. Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol. 2010;6:515–26. doi: 10.2217/fon.10.13. [DOI] [PubMed] [Google Scholar]
- 89.Chiurillo MA. Role of the Wnt/beta-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med. 2015;5:84–102. doi: 10.5493/wjem.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Min Y, Adachi Y, Yamamoto H, Imsumran A, Arimura Y, Endo T. et al. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut. 2005;54:591–600. doi: 10.1136/gut.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Lei X, Zhang J, Yang H, Liu J, Xu C. Insulin-like growth factor 1 promotes growth of gastric cancer by inhibiting foxo1 nuclear retention. Tumour Biol; 2015. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W. et al. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358–66. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 93.Wan X, Ding X, Chen S, Song H, Jiang H, Fang Y. et al. The functional sites of miRNAs and lncRNAs in gastric carcinogenesis. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:521–32. doi: 10.1007/s13277-015-3136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D. et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Wang Y, Shen B, Zhang D. Molecular signature of cancer at gene level or pathway level? Case studies of colorectal cancer and prostate cancer microarray data. Comput Math Methods Med. 2013;2013:909525. doi: 10.1155/2013/909525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Chen J, Li Q, Wang H, Liu G, Jing Q. et al. Identifying novel prostate cancer associated pathways based on integrative microarray data analysis. Comput Biol Chem. 2011;35:151–8. doi: 10.1016/j.compbiolchem.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Chen J, Sun M, Shen B. Deciphering oncogenic drivers: from single genes to integrated pathways. Brief Bioinform. 2015;16:413–28. doi: 10.1093/bib/bbu039. [DOI] [PubMed] [Google Scholar]
- 99.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J. et al. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–31. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 100.Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019–26. doi: 10.3892/or.2012.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamanaka S, Olaru AV, An F, Luvsanjav D, Jin Z, Agarwal R. et al. MicroRNA-21 inhibits Serpini1, a gene with novel tumour suppressive effects in gastric cancer. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2012;44:589–96. doi: 10.1016/j.dld.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao C, Zhang Z, Liu W, Xiao S, Gu W, Lu H. Reduced microRNA-218 expression is associated with high nuclear factor kappa B activation in gastric cancer. Cancer. 2010;116:41–9. doi: 10.1002/cncr.24743. [DOI] [PubMed] [Google Scholar]
- 103.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH. et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–81. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W. et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010;10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li N, Tang B, Zhu ED, Li BS, Zhuang Y, Yu S. et al. Increased miR-222 in H. pylori-associated gastric cancer correlated with tumor progression by promoting cancer cell proliferation and targeting RECK. FEBS Lett. 2012;586:722–8. doi: 10.1016/j.febslet.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I. et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–42. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Z, Liu S, Shi R, Zhao G. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genet. 2011;204:486–91. doi: 10.1016/j.cancergen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 109.Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C. et al. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2013;32:491–501. doi: 10.1038/onc.2012.61. [DOI] [PubMed] [Google Scholar]
- 110.Zhu A, Xia J, Zuo J, Jin S, Zhou H, Yao L. et al. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol. 2012;29:2701–9. doi: 10.1007/s12032-011-0134-3. [DOI] [PubMed] [Google Scholar]
- 111.Guo SL, Peng Z, Yang X, Fan KJ, Ye H, Li ZH. et al. miR-148a promoted cell proliferation by targeting p27 in gastric cancer cells. Int J Biol Sci. 2011;7:567–74. doi: 10.7150/ijbs.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hashimoto Y, Akiyama Y, Otsubo T, Shimada S, Yuasa Y. Involvement of epigenetically silenced microRNA-181c in gastric carcinogenesis. Carcinogenesis. 2010;31:777–84. doi: 10.1093/carcin/bgq013. [DOI] [PubMed] [Google Scholar]
- 113.Zhu W, Shan X, Wang T, Shu Y, Liu P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer. 2010;127:2520–9. doi: 10.1002/ijc.25260. [DOI] [PubMed] [Google Scholar]
- 114.Sakurai K, Furukawa C, Haraguchi T, Inada K, Shiogama K, Tagawa T. et al. MicroRNAs miR-199a-5p and -3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 2011;71:1680–9. doi: 10.1158/0008-5472.CAN-10-2345. [DOI] [PubMed] [Google Scholar]
- 115.Song G, Zeng H, Li J, Xiao L, He Y, Tang Y. et al. miR-199a regulates the tumor suppressor mitogen-activated protein kinase kinase kinase 11 in gastric cancer. Biol Pharm Bull. 2010;33:1822–7. doi: 10.1248/bpb.33.1822. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Fan KJ, Sun Q, Chen AZ, Shen WL, Zhao ZH. et al. Functional screening for miRNAs targeting Smad4 identified miR-199a as a negative regulator of TGF-beta signalling pathway. Nucleic Acids Res. 2012;40:9286–97. doi: 10.1093/nar/gks667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L, Liu X, Jin H, Guo X, Xia L, Chen Z. et al. miR-206 inhibits gastric cancer proliferation in part by repressing cyclinD2. Cancer Lett. 2013;332:94–101. doi: 10.1016/j.canlet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 118.Guo MM, Hu LH, Wang YQ, Chen P, Huang JG, Lu N. et al. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med Oncol. 2013;30:542. doi: 10.1007/s12032-013-0542-7. [DOI] [PubMed] [Google Scholar]
- 119.Zhu LH, Liu T, Tang H, Tian RQ, Su C, Liu M. et al. MicroRNA-23a promotes the growth of gastric adenocarcinoma cell line MGC803 and downregulates interleukin-6 receptor. FEBS J. 2010;277:3726–34. doi: 10.1111/j.1742-4658.2010.07773.x. [DOI] [PubMed] [Google Scholar]
- 120.Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D. et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T. et al. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339–49. doi: 10.1158/0008-5472.CAN-09-2777. [DOI] [PubMed] [Google Scholar]
- 122.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y. et al. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–93. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 123.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X. et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–90. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]