Abstract
Introduction
Alcohol dependence is characterized by a reduction in reward threshold, development of a negative affective state, and significant cognitive impairments. Dependence-induced glutamatergic neuroadaptations in the neurocircuitry mediating reward, affect and cognitive function are thought to underlie the neural mechanism for these alterations. These changes serve to promote increased craving for alcohol and facilitate the development of maladaptive behaviors that promote relapse to alcohol drinking during periods of abstinence.
Objective
To review the extant literature on the effects of chronic alcohol exposure on glutamatergic neurotransmission and its impact on reward, affect and cognition.
Results
Evidence from a diverse set of studies demonstrates significant enhancement of glutamatergic activity following chronic alcohol exposure and up-regulation of GluN2B-containing NMDA receptor expression and function is a commonly observed phenomenon that likely reflects activity-dependent adaptive homeostatic plasticity. However, changes in NMDA receptors and additional glutamatergic neuroadaptations are often circuit and cell-type specific.
Discussion
Dependence-induced alterations in glutamate signaling contribute to many of the symptoms experienced in addicted individuals and can persist well into abstinence. This suggests they play an important role in the development of behaviors that increase the probability for relapse. As our understanding of the complexity of the neurocircuitry involved in the addictive process has advanced, it has become increasingly clear that investigations of cell-type and circuit-specific effects are required to gain a more comprehensive understanding of the glutamatergic adaptations and their functional consequences in alcohol addiction.
Conclusion
While pharmacological treatments for alcohol dependence and relapse targeting the glutamatergic system have shown great promise in preclinical models, more research is needed to uncover novel, possibly circuit-specific, targets with improved efficacy and reduced side effects.
Keywords: negative affect, glutamate, plasticity, withdrawal, addiction, relapse
1. Introduction
Alcohol dependence is a chronic relapsing disorder characterized by repeated episodes of withdrawal and relapse to heavy alcohol consumption. This repetitive process promotes the development of maladaptive behaviors through neural adaptations that ultimately serve to facilitate continued drinking and hinder long-term abstinence. A substantial body of work has focused on the role of dopaminergic changes in promoting continued alcohol consumption. However, it has become apparent that adaptations in the dopamine system are not solely responsible for the increased propensity for relapse that occurs as a result of alcohol dependence. In particular, accumulating evidence indicates that aberrant glutamatergic-based plasticity plays a critical role in alcohol addiction and relapse. Early evidence for the involvement of the glutamatergic system in alcohol dependence and alcohol-related behaviors stemmed from the efficacy of NMDA receptor antagonists in alleviating seizures and other symptoms of acute withdrawal (Hoffman et al., 1990) and evidence later emerged pointing to glutamate’s role in reward regulation (Taber and Fibiger, 1995). During this same time, in vitro work examining the effects of chronic ethanol exposure in neuronal culture systems also revealed a direct effect of ethanol on the NMDA receptor (Chandler et al., 1998; Chandler, 2003) that has subsequently been confirmed in vivo (Burnett et al., 2014; Szumlinski and Woodward, 2014). Together, these findings sparked intense interest in glutamate’s involvement in the neuroadaptive processes induced by alcohol dependence. Indeed, decades of research have uncovered glutamate’s involvement in motivation and reward, affect, learning and memory, and cognitive performance, all of which are fundamentally altered in alcohol dependent individuals.
The aim of this review is to explore the role of glutamatergic neuroadaptations that arise as a result of chronic alcohol exposure and withdrawal within the overall framework of the neurocircuitry mediating the motivational, cognitive, and affective aspects of dependence that contribute to the neural dysfunction and heightened probability for relapse to alcohol drinking.
2. Overview of glutamate receptors
Glutamate serves as the brain’s main excitatory neurotransmitter and acts through two receptor types classified by their structure and function as either ionotropic or metabotropic. While a detailed review of glutamate and its receptor subtypes is beyond the scope of the present review, a brief overview is provided below (see Niswender and Conn, 2010; Traynelis et al., 2010; Szumlinski and Woodward, 2014 for more comprehensive reviews).
2.1 Ionotropic glutamate receptors
Ionotropic glutamate receptors (iGluRs) are tetrameric subunit complexes that are themselves divided into four classes of receptors with distinct pharmacology and biophysical properties: 2-amino-3(3-hydroxy-5-methyl-isoxazol-4yl)propanoic acid (AMPA), N-methl-D-asparate (NMDA), kainate and delta receptors.
AMPA receptors are fast depolarizing channels with activation and deactivation rates within the millisecond timescale and thus mediate the fast component of excitatory neurotransmission. Four different AMPA receptor subunits have been characterized (GluA1-4), which can form either homo- or heteromeric complexes. AMPA receptors are localized postsynaptically and glutamate binding results in the influx of sodium ions into the cell leading to the production of a subthreshold excitatory postsynaptic potential (EPSP) that, upon summation with other EPSPs, can result in an action potential.
Although the existence and function of presynaptic NMDA receptors is becoming increasingly appreciated, for the most part, NMDA receptors are localized postsynaptically and have a high affinity for glutamate. NMDA receptors are unique among the glutamate receptor family in that their activation requires the removal of a magnesium block. Thus, in its inactive state, magnesium binds inside the pore of the NMDA receptor facilitating a closed conformation that prohibits the influx of ions into the cell. Removal of this magnesium block is voltage-dependent and therefore occurs only after the cell begins to depolarize, which is typically associated with the release of glutamate and activation of neighboring synaptic AMPA receptors. Upon initial depolarization, the ion channel opens allowing sodium and calcium ions into the cell facilitating further depolarization and, more importantly, activation of intracellular calcium-dependent processes. The unique role of NMDA receptors as “coincidence detectors” that are activated only following glutamate release and AMPA receptor activation serves to facilitate cellular processes (i.e., long-term potentiation) and synaptic remodeling. NMDA receptor subunits are classified into three subtypes referred to as GluN1, GluN2A-D and GluN3A-B. Functional receptors are comprised of two requisite GluN1 subunits in combination with two GluN2A-D subunits. Activation of the receptor requires co-agonism with glutamate and glycine and, in contrast to AMPA receptors, NMDA receptors desensitize weakly following activation. GluN2A-receptors tend to be localized synaptically whereas GluN2B-containing receptors are found both inside and outside the synapse and likely activated by astrocytic release of glutamate. Because of the slower deactivation properties and subsequent prolonged influx of calcium of GluN2B-containing NMDA receptors, these receptors play a particularly important role in mediating both structural and functional synaptic plasticity.
Much less is known about the physiology and pharmacology of kainate receptors relative to AMPA and NMDA iGluRs. Kainate receptors appear to behave similarly to AMPA receptors, but are found both pre- and postsynaptically. Three receptor subunits have been identified as GluK1-3 and are characterized for their high affinity for kainite relative to AMPA. Like both AMPA and NMDA receptors, subunit composition of kainate receptors influences their affinity for glutamate and permeability of calcium through the ion channel pore.
The delta family of iGluRs was identified based on sequence homology with other iGluR subunits, but despite their homology, neither the GluRdelta1 (GluRD1) nor the GluRdelta2 (GluRD2) subunit responds to glutamate (Lomeli et al., 1993). Consequently, these receptors remain categorized as orphans and our understanding of them is limited. Of the two receptor subtypes, GluRD2 receptors are the most well characterized likely because of their dense expression in cerebellar purkinje neurons where they are known to play an important role in motor coordination (Kashiwabuchi et al., 1995). Interestingly, recent work has shown that GluRD2 is activated by Group 1 metabotropic glutamate receptors (Ady et al., 2014) indicating that they are indeed involved, at least indirectly, in glutamatergic signaling. In contrast to GluRD2, GluRD1 expression is diffuse but widespread throughout the brain including in the prefrontal cortex, amygdala and hippocampus (Yadav et al., 2012; Hepp et al., 2014). While the GluRD1 subunit is much less well understood than its GluRD2 counterpart, recent work suggests it is involved in mediating social and anxiety-like behavior (Yadav et al., 2012).
2.2 Metabotropic glutamate receptors
The metabotropic glutamate receptors (mGluRs) are seven transmembrane g-protein coupled receptors that regulate cell excitability via second messenger systems. There are eight mGluRs that are classified into three groups. Group 1 mGluRs include mGluR1 and mGluR5. These receptors are localized to the perisynaptic region of the postsynaptic density. This family of mGluRs is coupled to Gq/G11, and thus their activation leads to stimulation of phospholipase C second messenger pathways and calcium signaling. In contrast, Group 2 and 3 mGluRs are coupled to Gi/o and agonist binding results in inhibition of adenylyl cyclase and modulation of potassium channels. When localized presynaptically Group 2/3 mGluR activation typically results in the inhibition of neurotransmitter release, whereas activation of postsynaptic Group2/3 mGluRs typically results in cell hyperpolarization. Group 2 mGluRs include mGluR2 and mGluR3. mGluR2 is localized presynpatically, whereas mGluR3 can be found both pre- and postsynaptically. Group 3 mGluRs are localized exclusively presynaptically and include mGluR4, mGluR7 and mGluR8. Of note, both Group1 mGluR5s and Group 2 mGluR3s are also expressed on astrocytes where they play a key role in mediating neurotransmission occurring between astrocytes and neighboring neurons (Sun et al., 2013).
Although iGluRs, and in particular NMDA receptors, are frequently thought of as the primary mediators of synaptic plasticity, emerging evidence indicates that mGluRs also play a role in facilitating some forms of plasticity including long-term potentiation (LTP) and depression (LTD) (Citri and Malenka, 2008; Bellone and Mameli, 2012). Evidence for mGluR-mediated synaptic plasticity has focused primarily on Group 1 mGluRs and their ability to elicit trafficking of AMPA receptors to the membrane following agonist stimulation, as well as their role as retrograde synaptic second messengers altering endocannabinoid release and facilitating LTD via CB1 receptor activation (Abraham, 2008). In addition, while the primary focus of dopamine-mGluR interactions has been in the context of motor behavior dysfunction, the interaction between these two systems within the context of reward and motivation has become increasingly appreciated (Vezina and Kim, 1999; Rouse et al., 2000; Creed et al., 2015).
2.3 Glutamate signaling & alcohol
Dependence on alcohol produces cognitive impairments that manifest as increased risk taking, poor decision-making and loss of inhibitory control over behavior. In addition, repeated alcohol exposure results in tolerance to its rewarding properties requiring increasing doses of alcohol to produce the same euphoric effect (Gilpin and Koob, 2008). Moreover, withdrawal from alcohol is associated with a negative affective state that is characterized by increased levels of anxiety, sensitivity to stress, and dysphoria (Heilig et al., 2010). Together these symptoms hinder abstinence by promoting the transition away from controlled, causal drinking toward uncontrolled and compulsive alcohol consumption. Importantly, dependence-induced neuroadaptations in glutamate signaling are thought to contribute to the development of these symptoms. In fact, withdrawal from alcohol is associated with increased glutamate concentration in both humans and rodents (Hermann et al., 2012) and severity of alcohol dependence is positively correlated with CSF glutamate (Umhau et al., 2010). Furthermore, a growing body of literature is emerging indicating that significant glutamatergic adaptations occur in brain regions underlying reward, cognition, and affect in alcohol-dependent individuals (Jin et al., 2014a, 2014b; Enoch et al., 2014; Bhandage et al., 2014; Laukkanen et al., 2015). While human studies are critical to our understanding of the neuroadaptive processes that occur in addicted individuals, animal models have been essential to our appreciation of the cellular adaptations that occur within the neural circuits underlying dependence-induced symptomology, which may help to uncover pharmacological targets for the treatment of alcoholism.
3. Glutamatergic adaptations to neurocircuitry mediating reward
Addiction is associated with increased incentive salience, or wanting, for alcohol accompanied by decreased pleasure in response to previously rewarding stimuli (i.e., anhedonia) (Heilig et al., 2010; Robinson et al., 2013). As such, withdrawal from alcohol is associated with an increased threshold for reward (Schulteis et al., 1995) that corresponds with a reduction in dopamine that accompanies withdrawal (Weiss et al., 1996). These effects of chronic alcohol on mood and motivation are thought to play a significant role in promoting relapse and escalated alcohol consumption (Heilig et al., 2010).
A large body of research has focused on the role of the ventral tegmental area (VTA) and its dopaminergic projection onto GABAergic medium spiny neurons (MSNs) of the nucleus accumbens (NAc) in mediating dependence-induced plasticity due to its well-characterized role in signaling reward and the salience of environmental stimuli (Figure 1) (Schultz, 2013). As such, the NAc is viewed as a sensorimotor integrator that receives dopaminergic signaling from the VTA in response to stimuli and integrates that signal with other incoming sensory inputs to motor areas, such as the ventral pallidum and substantia nigra, to provide information that facilitates a behavioral response (Saddoris et al., 2013). Furthermore, reciprocal connections between the NAc and VTA act as an additional regulator of dopamine release (Xia et al., 2011).
Figure 1. Simplified neurocircuitry illustrating the primary neural substrates for reward, negative affect and cognition.
Alcohol dependence induces significant alterations in reward processing, the development of a negative affective state, and significant cognitive impairments that result in loss of behavioral flexibility and impulse control. Both dopaminergic and GABAergic projections from the VTA to the NAc play a key role in reward signaling (shown in green). This pathway is further modulated by glutamatergic input from the LHb, which in turn receives a reciprocal projection from the VTA that co-expresses both glutamate and GAB. In addition, glutamatergic projections from the LHb synapse onto GABAergic neurons of the RMTg to form a functional circuit that exerts inhibitory control over dopamine release in the VTA. Connections between nuclei of the amygdala and extended amygdala (shown in blue) mediate anxiety, anhedonia and increased sensitivity to stress. Glutamatergic inputs from the BLA impinge on neurons of both the CeA and BNST to facilitate the expression of anxiety-like behavior. Release of glutamate from BLA terminals in the BNST is further augmented by the release of corticotropin releasing factor (CRF) from neurons arising in the CeA. The PFC exerts top-down control over behavior via glutamatergic inputs to various subcortical regions including the DS, NAc, VTA and BLA (shown in red). In addition, cognitive performance is heavily controlled through network synchrony via a dense glutamatergic monosynaptic connection between the PFC and hippocampus (shown in red). Abbreviations: BLA – basolateral nucleus of the amygdala; BNST – bed nucleus of the stria terminals; CeA – central nucleus of the amygdala; DS – dorsal striatum; Hipp – hippocampus; LHb – lateral habenula; mPFC – medial prefrontal cortex; NAc – nucleus accumbens; OFC – orbitofrontal cortex; RMTg – rostrmedial tegmental nucleus; VTA – ventral tegmental area.
Based on the prominent role that VTA dopamine plays in signaling reward, it is not surprising that most of the work examining synaptic plasticity within this region has focused primarily on dopaminergic adaptations following acute and chronic ethanol. Nevertheless, the few studies that have investigated glutamatergic neuroadaptations within the VTA collectively suggest that prolonged ethanol exposure leads to significant alterations in glutamatergic neurotransmission that are likely involved in promoting escalated drinking and relapse during abstinence. For example, repeated intraperitoneal ethanol administration for seven days resulted in enhanced NMDA receptor-mediated LTP in VTA dopamine neurons (Bernier et al., 2011). Similarly, repeated ethanol exposure is associated with enhanced ethanol-induced NMDA-mediated burst firing of dopamine neurons within the VTA (Hopf et al., 2007). Prolonged ethanol self-administration also resulted in enhanced postsynaptic AMPA receptor function in VTA neurons in the absence of any change in presynaptic glutamate release (Stuber et al., 2008). This enhancement of postsynaptic glutamatergic neurotransmission may be due, at least in part, to increased expression of mGluR1 and GluN1-containing receptors following chronic ethanol (Ortiz et al., 1995). However, not all data are in agreement with this observation. For example, recent work in alcohol-preferring P rats revealed a decrease in NMDA receptor sensitivity within the VTA following prolonged alcohol consumption (Fitzgerald et al., 2012). Whether these discrepancies may be attributed to methodological differences and/or adaptations that necessitate physical dependence remains unclear and is an important avenue for future exploration.
Although behavioral correlates of the reported increase in VTA glutamatergic neurotransmission following prolonged ethanol exposure are limited, ethanol-induced enhancements in NMDA receptor-mediated activity have been associated with enhanced learning of drug-associated cues (Bernier et al., 2011) and sensitization to cocaine-induced hyperlocomotion (Hopf et al., 2007). This suggests that ethanol-induced glutamatergic plasticity within the VTA may facilitate an altered motivational state. An explicit link between these neuroadaptive changes and alterations in reward threshold, ethanol intake, and ethanol seeking is a critical gap that requires addressing before more definitive conclusions can be made regarding the role of VTA glutamatergic signaling in facilitating relapse behavior.
Another important consideration is the growing appreciation for the heterogeneity of cell types expressed within the VTA and the anatomically distinct projections exhibited by different VTA subregions. Indeed, it has become increasingly apparent that the VTA is a rather heterogeneous nucleus expressing both glutamatergic and GABAergic neurons in addition to classical dopamine neurons (Sanchez-Catalan et al., 2014; Lammel et al., 2014). Importantly, recent work has also demonstrated co-localization of glutamate with dopamine as well as co-localization of glutamate and GABA in non-dopaminergic neurons within the region (Morales and Root, 2014). Notably, both GABAergic and glutamatergic non-dopaminergic neurons of the VTA are now known to target regions outside of the VTA including several known to play a key role in reward and aversion (Lammel et al., 2014; Root et al., 2014a, 2014b). Recent work has also reported subregion specificity in the expression of Ih current – the standard physiological signature used to identify dopamine neurons. In fact, Ih is present in only a subset of dopaminergic neurons that exhibit connectivity that is distinct from non-Ih expressing dopamine neurons (Zhang et al., 2010; Lammel et al., 2012). Thus, studies measuring synaptic changes in Ih-expressing VTA dopamine neurons – like those described above – are reporting changes in an anatomically distinct subset of dopamine neurons. Investigating whether similar changes are observed in other dopaminergic cell populations will be important for a more comprehensive understanding of the effects of chronic ethanol exposure on dopamine signaling. In addition, it will be critical for future studies to discriminate between alterations in anatomically distinct circuits within the VTA, particularly since it receives dense glutamatergic input from a variety of brain regions including the prefrontal cortex (PFC), lateral habenula (LHb), periacqueductal gray (PAG), raphe nuclei, hypothalamus and ventral pallidum (Geisler and Wise, 2008).
The NAc is ideally situated to regulate behavior in response to environmental stimuli, and as such, is known to be critically involved in alcohol-seeking behavior following exposure to alcohol-related cues and context (Chaudhri et al., 2008, 2010). Similar to findings in the VTA, glutamatergic signaling is also enhanced within the NAc following chronic ethanol exposure and withdrawal. Thus, Jeanes et al. (2011) observed a transition from NMDA-receptor dependent LTD to LTP in the NAc of mice that underwent chronic intermittent ethanol vapor exposure. This effect is accompanied by increased intrinsic excitability of NAc MSNs, reduced probability of presynaptic glutamate release, and increased AMPA receptor-mediated activity (Marty and Spigelman, 2012a, 2012b; Spiga et al., 2014). This enhancement of AMPA receptor signaling may be due to increased GluA1 expression in the NAc after chronic ethanol exposure (Neasta et al., 2010). In addition, it should be noted that LTD in NAc MSNs requires GluN2B-containing NMDA receptors (Schotanus and Chergui, 2008) further suggesting that the neuroadaptations observed within the NAc following chronic ethanol are likely mediated, at least in part, by alterations in GluN2B receptor expression and/or function similar to what has been observed in other areas of the brain (see below). In support of this, increased GluN2B expression has been observed in the NAc following chronic ethanol exposure, along with increased expression of GluN2A, mGluR5 and mGluR1 (Obara et al., 2009).
Presynaptically, prolonged ethanol exposure is associated with increased basal glutamate in the NAc. For example, acute withdrawal from chronic ethanol vapor inhalation is associated with significantly elevated NAc glutamate (Dahchour and De Witte, 2000, 2003). More recently, this same finding was reported to last well into protracted abstinence (Griffin et al., 2014). In the same study, Griffin et al. (2014) also showed that pharmacological reduction in glutamate signaling in the NAc returned dependence-induced escalated ethanol consumption to control levels. Of note, chronic ethanol-induced elevation in NAc basal glutamate levels are not associated with alterations in glutamate transport (Griffin et al., 2015) suggesting that this effect is mediated instead by increased vesicular release of glutamate and/or reduced mGluR autoreceptor activity. In addition, both repeated daily ethanol exposure or binge-like ethanol exposure reportedly increased glutamate release in the NAc following an acute ethanol challenge indicating that prolonged ethanol exposure sensitizes the glutamatergic system within the NAc to ethanol’s effects (Melendez et al., 2005; Szumlinski et al., 2007; Kapasova and Szumlinski, 2008). These data as well as others support findings from clinical studies suggesting that dependence on alcohol induces a lasting hyperglutamatergic state that facilitates continued alcohol consumption and relapse during abstinence (Spanagel, 2009; Becker et al., 2013; Holmes et al., 2013; Becker and Mulholland, 2014).
Evidence suggests that these glutamatergic alterations are mediated, at least in part, by enhanced expression of Homer proteins – scaffolding proteins that play an important role in the regulation of receptor signaling and are highly enriched at the postsynaptic density. Importantly, Homer proteins can directly affect the trafficking and expression of glutamate receptors to the plasma membrane (Szumlinski et al., 2008a) and are therefore frequently examined in association with changes in glutamate receptor expression. Consequently, many of the same studies reporting enhanced glutamate receptor expression in the NAc also report increased Homer expression following chronic and binge-like alcohol exposure (Szumlinski et al., 2008b; Obara et al., 2009). In addition, viral overexpression of Homer2 in the NAc is associated with greater operant responding for ethanol, enhanced ethanol conditioned place preference, and tolerance to ethanol’s sedative effects (Szumlinski et al., 2008b). Neasta et al. (2010) extended these findings to demonstrate a role for the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway in mediating glutamatergic plasticity in the NAc. Here, the authors showed that increased GluA1 subunit expression in the NAc of binge-drinking mice was associated with greater Homer expression and increased activation of the mTORC1 signaling pathway. Furthermore, intra-NAc inhibition of mTORC1 activity decreased ethanol intake and seeking. Together, these studies have uncovered a key role for Homer and the signaling pathways, including mTORC1, that affect its translation, in mediating glutamatergic synaptic plasticity in the NAc. Further exploration of their role in alcohol reward and continued alcohol consumption may help to identify new pharmacological targets for relapse prevention.
Collectively, these data, in combination with the well-characterized role of NMDA receptors in learning drug-associated cues (Harris et al., 2004; Zweifel et al., 2008) and the involvement of the NAc in mediating behavioral responses to alcohol-associated cues specifically (Chaudhri et al., 2008, 2010), suggest that enhanced glutamatergic signaling in the NAc in response to chronic ethanol exposure may facilitate increased salience of alcohol-related cues and promote cue-induced relapse. In addition, enhanced glutamatergic signaling within the NAc may influence regulation of dopamine release via its reciprocal connections with the VTA and may play a role in the lasting effects of chronic ethanol exposure on dopamine signaling and the associated increase in reward threshold observed during withdrawal. To date, however, efforts to understand the effects of chronic ethanol exposure on NAc neuroplasticity have focused largely on MSNs throughout the region with little appreciation for circuit specificity. The NAc receives glutamatergic input from the PFC, hippocampus, thalamus and amygdala (Phillipson and Griffiths, 1985) yet our understanding of the neuroadaptive changes that take place within each of these circuits as a result of chronic ethanol exposure is still not well understood. This concept is exemplified in findings from an elegant study using in vivo optogenetics that demonstrate that stimulation of glutamatergic inputs from the basolateral nucleus of the amygdala to the NAc is both necessary and sufficient to facilitate cue-induced reward seeking behavior (Stuber et al., 2011). In contrast, it was observed that PFC inputs to the NAc are not involved in this behavioral outcome. A greater appreciation for circuit-specificity is evolving as illustrated by recent work by Meinhardt et al. (2013) who used retrograde labeling in combination with laser capture microdissection to examine projection-specific changes in glutamatergic signaling following chronic ethanol exposure. This study revealed significant down-regulation of the mGluR2 gene Grin2 in NAc-projecting neurons of the infralimbic PFC following seven weeks of chronic intermittent ethanol exposure. A similar reduction in Grm2 expression was observed in post-mortem samples of the anterior cingulate cortex of alcohol-dependent individuals (Meinhardt et al., 2013). The authors then demonstrated a direct link between mGluR2 expression in the NAc and propensity for relapse by showing that viral expression of mGluR2 in NAc-projecting infralimbic neurons of ethanol-exposed rats reduced cue-induced seeking for ethanol. These results are in contrast to an earlier study reporting no differences in mGluR2/3 activity in the medial PFC following four weeks of ethanol vapor exposure (Kufahl et al., 2011). However, length of exposure and the absence of circuit-specific examination of the PFC may have contributed to this discrepant observation. In fact, strong support for a role of NAc mGluR2 in mediating alcohol intake can be found in a more recent study reporting that genetic variation in the Grin2 gene contributes to reduced mGluR2 protein expression and decreased mGluR2-mediated signaling in the striatum of alcohol-preferring P rats compared to their non-preferring NP counterparts (Zhou et al., 2013). In addition, these authors showed that mGluR2 antagonism increased operant responding for ethanol in Wistar rats, and that Grin2 knockout mice exhibited greater voluntary ethanol intake and preference compared to wild-types, further supporting a causal role for mGluR2 in mediating alcohol consumption. Clearly, additional work aimed at parsing out the precise role of specific glutamatergic inputs to the NAc and the changes they undergo following alcohol dependence will be critical for uncovering the precise mechanisms underlying dependence-induced changes in behavior that promote relapse.
4. Glutamatergic adaptations to neurocircuitry responsible for negative affect
Withdrawal from alcohol is associated with a series of symptoms that increase the motivation for relapse including craving, heightened anxiety, anhedonia and increased sensitivity to stress (Heilig et al., 2010). These symptoms, collectively referred to as withdrawal-induced negative affect, are thought to promote drinking via negative reinforcement (i.e., alcohol consumption relieves the negative state) (Koob and Le Moal, 2008; George et al., 2014). Given the role that negative affect plays in the high propensity for relapse during periods of both acute and protracted withdrawal, a significant body of work has focused on understanding the neurobiological underpinnings of negative affect and the corresponding neuroadaptations that result from chronic ethanol exposure. By far the greatest amount of attention has been paid to components of the amygdala and extended amygdala due to their roles in processing emotion, fear and pain, as well as their shared connections with brain regions involved in reward and motivation (Gauriau and Bernard, 2002; Shin and Liberzon, 2009; Janak and Tye, 2015).
The amygdala is a collection of nuclei within the mediotemporal lobe that can be divided into three main subgroups. First is the basolateral group (BLA) made up of the basal, lateral and accessory basal nuclei; second is the cortical group comprised of the cortical nuclei and nucleus of the lateral olfactory tract; and third is the centromedial group comprised of the central (CeA) and medial (MeA) amygdalar nuclei (Sah et al., 2003). This final grouping has come under dispute with some neuroanatomists arguing that the CeA and MeA make up the primary nuclei of the extended amygdala along with their connections to the nearby bed nucleus of the stria terminalis (BNST) and, more rostrally, the caudal ventral pallidum (Alheid, 2009; McDonald, 2009).
Regardless of their categorization as amygdalar or extended amygdalar, many of these nuclei, by virtue of their connections and functional output, have been implicated in the neurobiology of alcohol dependence. The BLA is neuroanatomically situated to integrate sensory information through its inputs from sensory corticies, as well as the PFC and hippocampus. Its projections to the NAc, CeA and BNST provide a path through which this sensory information can be transformed into a behavioral response (Figure 1) (Janak and Tye, 2015). Importantly, projections from the BLA to the CeA are thought to be responsible for acute responses to aversive or stressful stimuli through activation of CeA efferents to the hypothalamus and PAG. In contrast, BLA input to the BNST is thought to be the primary regulator of sustained anxiety, likely via its outputs to monoaminergic and cholinergic nuclei mediating behavioral responses including the locus coreulus, substantia nigra, VTA, raphe nuclei, and nucleus basalis (Sah et al., 2003; Davis et al., 2010). There is also a prominent reciprocal PFC-amygdalar projection that is thought to play an important role in the executive control of emotional response to fear, anxiety and aversive stimuli. Thus, the local microcircuitry as well as the broader connections associated with this region serve to influence both acute and chronic states of anxiety that may correspond respectively to acute and protracted withdrawal from alcohol.
Within the BLA, chronic ethanol exposure and acute withdrawal are associated with both pre-and postsynaptic enhancement of glutamatergic neurotransmission that may be pathway-specific. For example, BLA neurons receiving cortical input via the external capsule (EC) generally exhibit alterations in postsynaptic glutamate signaling following chronic ethanol including increases in NMDA, AMPA and kainate receptor-mediated activity (Läck et al., 2007, 2009; Christian et al., 2012). These alterations in glutamatergic neurotransmission are accompanied by sensitization of GluN2B-containing NMDA receptors (Floyd et al., 2003) and increased surface expression and phosphorylation of GluA1-3 receptors that likely facilitates long-term changes in BLA plasticity following chronic ethanol (Christian et al., 2012). Unlike EC-BLA synapses, which do not show presynaptic glutamatergic alterations following chronic ethanol, thalamic inputs to the BLA arising via the internal capsule exhibit an enhanced probability of presynaptic glutamate release that is likely associated with increases in synaptic pooling of glutamate-containing vesicles (Christian et al., 2013). Collectively these data demonstrate a significant enhancement of glutamatergic neurotransmission in the BLA following chronic ethanol. Of note, intra-BLA administration of an AMPA/kainate receptor antagonist reverses dependence-induced anxiety-like behavior using a light-dark box test (Läck et al., 2007). This is consistent with glutamatergic adaptations within the BLA facilitating withdrawal-induced negative affect. Furthermore, while the precise pathway-specificity of these effects is only beginning to be explored the current data is suggestive of differential effects of chronic ethanol on different BLA circuits.
Similar to the BLA, chronic ethanol exposure also enhances NMDA receptor function within the CeA in a manner, once again, indicative of an upregulation of GluN2B-containing NMDA receptors. This effect is accompanied by enhanced ethanol-induced presynaptic glutamate release (Roberto et al., 2004). While postsynaptic alterations in glutamatergic signaling recovered following one week of abstinence, the effect of chronic ethanol on presynaptic glutamatergic neuroadaptations was long-lasting and remained up to two weeks following cessation of ethanol vapor exposure (Roberto et al., 2006). In addition, a transient upregulation of GluN1, GluN2A and GluN2B NMDA receptor subunit expression was observed following chronic ethanol, that recovered after one week of abstinence (Roberto et al., 2006).
In agreement with the data described above, additional work exploring glutamatergic adaptations in alcohol-preferring P rats that chronically self-administered ethanol using a two-bottle choice home cage drinking procedure reported similar enhancement of GluN2B expression in the CeA (Obara et al., 2009). Unlike previous work, however, these authors reported long-lasting effects of chronic ethanol exposure on GluN2B expression with elevations lasting up to four weeks into abstinence. This same study also reported long-lasting elevations in mGluR1 and Homer 2a/b expression in the CeA. These effects were accompanied by transient increases in GluN2A and mGluR5 expression that were present during acute withdrawal but had recovered following protracted abstinence (Obara et al., 2009). Interestingly, recent work using a chronic binge model of alcohol consumption in mice reported reduced alcohol, but not sucrose, intake following intra-CeA infusion of either an mGluR1 or mGluR5 antagonist pointing to a direct role for CeA mGluR1 and mGluR5 receptors in continued drinking following chronic exposure (Cozzoli et al., 2014). Together, these data demonstrate significant pre- and postsynaptic glutamatergic adaptations within the CeA following chronic ethanol exposure, some of which may be long lasting. Discrepancies across studies are likely dependent, at least in part, on differences in species, strain, and method/extent of ethanol exposure. Of note, while the ethanol vapor exposure paradigm utilized in the earlier studies is well known to produce dependence, the data obtained using home cage and binge drinking paradigms may not be reflective of dependence-induced neuroadaptations since no clear indicators of dependence were reported in these studies. Nevertheless, up-regulated NMDA receptor function following prolonged ethanol exposure is commonly observed in the CeA across these studies.
While much less work has focused on glutamatergic adaptations in the BNST following chronic ethanol exposure, recent reports are in general agreement with the effects reported in the BLA and CeA. Thus, chronic intermittent ethanol vapor exposure produced increased GluN2B receptor activity and expression in the BNST. These postsynaptic adaptations are not accompanied by a presynaptic increase in glutamate release (Kash et al., 2009). In an extension of this work, a recent study reported enhanced LTP in the BNST of mice chronically exposed to ethanol compared to non-exposed control mice – an effect that was dependent on up-regulation of extrasynaptic GluN2B-containing NMDA receptors (Wills et al., 2012).
Collectively, these data reveal significant glutamatergic effects of chronic ethanol exposure within the amygdala and extended amygdala. While some inconsistencies have been reported, chronic ethanol exposure appears to exert similar postsynaptic effects throughout the region, the most common of which is an up-regulation of both expression and function of GluN2B-containing NMDA receptors. However, clear regionally-specific, and even projection-specific, effects exist in terms of enhanced presynaptic glutamate release following chronic ethanol exposure. Given the role of the CeA and BNST in mediating stress- and anxiety-like behavior and the prominent glutamatergic input these nuclei receive from the BLA, it is conceivable that hyperexcitability within these regions can promote the increased sensitivity to stress and prolonged anxiety associated with both acute and protracted withdrawal from alcohol. It should be noted, however, that as our understanding of the role of specific afferent and efferent connections to the region increases, it becomes increasingly clear that this idea is an oversimplified view of the neuroadaptive processes that mediate dependence-induced vulnerability for relapse. Indeed, a growing body of work has identified a distinct topography of connections between the BLA, CeA and BNST that appear to exert circuit-specific effects on fear and anxiety-like behavior (Davis et al., 2010). Future work aimed at parsing out the effects of chronic ethanol on the specific micro- and macro-circuits of the amygdalar complex will be critical to improving our understanding of the role of these nuclei in withdrawal-induced negative affect.
5. Glutamatergic adaptations involved in dependence-induced cognitive impairment
Alcohol dependent individuals exhibit significant cognitive dysfunction that is thought to facilitate continued drinking and relapse during periods of withdrawal and early abstinence. Many alcoholics exhibit impairments in psychomotor and executive functions including attention, cognitive flexibility, decision-making, working memory and problem solving during early abstinence (Sullivan et al., 2000; Bechara et al., 2001; Ratti et al., 2002). While the most severe impairments typically improve after at least one month of abstinence, substantial cognitive dysfunction remains during the first year of recovery, and significant impairments often persist during protracted abstinence (i.e., greater than one yr) (Parsons, 1998; Stavro et al., 2013). Importantly, the magnitude of cognitive impairment is predictive of the risk for relapse in abstinent individuals (Abbott and Gregson, 1981; Parsons, 1998; Bowden-Jones et al., 2005) suggesting that impaired executive function facilitates continued alcohol consumption. Consequently, the neuroadaptations that result from chronic alcohol exposure in brain regions responsible for cognitive performance are of particular importance for identifying the neurobiological mechanisms behind vulnerability for relapse.
The PFC plays an integral role in cognitive functions. This region exerts top-down control over behavior by integrating input from various brain regions and delivering the corresponding information to subcortical structures to generate behavioral responses. In addition, persistent population-level activity within the PFC enables synchronous network signaling that facilitates the maintenance of information within the PFC and its associated connections. The PFC is highly interconnected with other cortical and subcortical regions sending afferents to the hippocampus, amygdala, striatum, VTA, dorsal raphe, and various other cortical regions (Figure 1) (Ongür and Price, 2000; Vertes, 2004; Gabbott et al., 2005; Petrides et al., 2012). Virtually all connections to the PFC are reciprocal with the exception of its connection to the striatum, which sends efferents back to the PFC via a polysynaptic route through the thalamus.
As the role of dependence-induced cognitive impairments in risk for relapse became apparent, studies have focused on neuroadaptive changes that take place in the PFC following alcohol exposure in an attempt to define the mechanisms behind these impairments. Early work investigated the effects of chronic alcohol in vitro using cerebral cortical neuronal cultures. Here, chronic ethanol exposure resulted in a significant enhancement of NMDA receptor-mediated activity accompanied by a transient increase in NMDA receptor density (Chandler et al., 1993; Hu and Ticku, 1995). More recent studies observed increased levels of GluN1 and GluN2B subunits specifically targeted to the synapse after chronic ethanol exposure suggesting that this effect may be mediated by increased NMDA receptor subunit expression (Carpenter-Hyland et al., 2004; Qiang et al., 2007). Studies examining the effects of in vivo alcohol exposure have yielded similar findings. For example, chronic intermittent ethanol vapor exposure resulted in increased NMDA receptor-mediated activity in the medial PFC (mPFC) accompanied by enhanced LTP, an increase in mushroom shaped spines on mPFC dendrites, and increased GluN1 and GluN2B subunit expression (Kroener et al., 2012). Interestingly, while the enhancement of NMDA receptor activity persisted, the changes in subunit expression returned to baseline levels following one week of abstinence. Importantly, these changes were also associated with deficits in cognitive flexibility, which lasted up to one week into abstinence. A subsequent study examining expression of GluN2B-containing NMDA receptors in the mPFC following three weeks of abstinence from chronic intermittent ethanol vapor exposure reported similar findings with unaltered total GluN2B levels despite the presence of significantly increased dendritic arborization of pyramidal neuron apical dendrites within this region (Navarro and Mandyam, 2015). Interestingly, while total GluN2B levels were unchanged at this time-point, the authors reported a significant reduction in the levels of phosphorylated GluN2B-containing receptors. Given that GluN2B dephosphorylation is associated with receptor endocytosis (Snyder et al., 2005), these data may be indicative of a homeostatic adaptation that presumably is a mechanism by which the brain prevents prolonged overexpression of GluN2B-containing receptors. Another recent study extended these findings to demonstrate that long-lasting enhancement of postsynaptic NMDA receptor-mediated activity in the mPFC in response to chronic ethanol exposure may be due, at least in part, to loss of dopamine receptor modulation of glutamatergic activity (Trantham-Davidson et al., 2014). Of note, these authors also reported alterations in both pyramidal neurons and fast-spiking interneurons within layer V of the mPFC suggesting that alterations in the activity of neuronal ensembles may also contribute to the deficits in executive function that were observed. In contrast to these studies, Holmes et al. (2012) reported a significant decrease in NMDA receptor-mediated activity as well as decreased expression of GluN1-containing NMDA receptors with no change in GluN2 expression in the mPFC following chronic ethanol exposure. The reason for these discrepant findings is not clear, however, the studies above restricted measurement of glutamatergic neurotransmission to deep layer V neurons, whereas Holmes et al. (2012) collected data from both superficial layers II/III and deep layer V neurons, leaving open the possibility of layer-specific alterations in glutamate signaling following chronic ethanol.
Despite the fact that the orbitofrontal cortex (OFC) plays a critical role in value-based decision-making and goal-directed behavior, very little work has examined the effects of chronic ethanol exposure on OFC glutamate. One recent study suggests that chronic ethanol exposure does indeed, induce neuroadaptive changes in the OFC, although the role of the glutamatergic system in these changes is not clear. Chronic intermittent ethanol exposure was associated with an increase in dendritic spine density in layer II/III OFC neurons with a specific increase in thin, long spines (McGuier et al., 2015). In contrast to changes in other brain regions including the mPFC, these changes were not observed immediately after chronic ethanol exposure and instead appear after one week of abstinence. Although speculative, this suggests that withdrawal-induced hyperexcitability may have contributed to synaptic remodeling and impairments of OFC-dependent executive functions that are also observed during this time frame (Badanich et al., 2011). Of note, the alterations in dendritic spine density were not accompanied by changes in NMDA receptor subunit expression immediately following exposure or during early abstinence (McGuier et al., 2015).
Because of the breadth of PFC connections to subcortical regions, it is important to consider that dependence-induced cognitive impairments may result not only from changes within the PFC, but also within regions that project to and from the PFC. The PFC receives dense monosynaptic input from the hippocampus, and synchrony between these regions is essential for efficient cognitive performance (Jones and Wilson, 2005; Hyman et al., 2011; Colgin, 2011; O’Neill et al., 2013). During performance of executive functions, the activity in the PFC is phase-locked to the hippocampal theta rhythm and disruption of this entrainment produces deficits in executive function (Uhlhaas and Singer, 2006; Burnett et al., 2014).
In the hippocampus, chronic ethanol exposure has been reported to both diminish and enhance NMDA receptor dependent LTP in the Schaffer collateral pathway (Durand and Carlen, 1984; Tremwel and Hunter, 1994; Ripley and Little, 1995; Roberto et al., 2002, 2003; Thinschmidt et al., 2003; Fujii et al., 2008). In contrast to these contradictory observations, enhanced NMDA receptor-mediated activity and NMDA receptor up-regulation following chronic ethanol have both been, for the most part, consistently reported in this brain region. This was first observed in studies carried out in hippocampal cultures and has subsequently been replicated in studies of in vivo chronic ethanol exposure (Trevisan et al., 1994; Chandler et al., 1999; Carpenter-Hyland et al., 2004; Nelson et al., 2005; Hendricson et al., 2007; Mulholland et al., 2014). While not conclusive, some evidence suggests that the inconsistency between diminished LTP in some studies and enhanced NMDA receptor activity in others is indicative of changes that occur during chronic intoxication as opposed to early abstinence. Indeed, most studies have reported recovery of LTP after 1–5 days of abstinence (Roberto et al., 2002, 2003) suggesting that the increase in NMDA receptor activity and expression observed during withdrawal may reflect a compensatory mechanism that occurs upon removal of alcohol from the system (Nelson et al., 2005; but see Tremwel and Hunter, 1994). Whether other methodological differences including species, strain, and model of dependence employed also contribute to the discrepant findings is an important consideration that could benefit from empirical testing.
Behavioral flexibility and inhibitory control are frequently lost or impaired in alcohol dependent individuals (Koob and Volkow, 2010). Causal drinkers typically exhibit goal-directed behavior as they seek out alcohol specifically for its rewarding properties. However, with repeated episodes of alcohol consumption and intoxication, an individual begins to develop an association between their alcohol seeking and taking behavior and the surrounding environmental stimuli. Over time, for those that develop an alcohol use disorder, these associations may promote habitual alcohol consumption whereby alcohol is no longer sought after for its rewarding value but instead is sought habitually due to re-exposure to alcohol-associated stimuli (Barker and Taylor, 2014). This transition away from goal-directed behavior toward habitual responding is also thought to be mediated, at least in part, via connections between the PFC and striatum. In particular, the dorsomedial striatum (DMS) and the prelimbic (PrL) subregion of the mPFC are involved in the performance of goal-directed directed behavior, whereas activity in the dorsolateral striatum (DLS) and infralimbic subregion of the mPFC play prominent roles in the formation of habits (Barker et al., 2014; Barker and Taylor, 2014). Importantly, learning of goal-directed behaviors requires NMDA receptor-mediated LTP as well as the activation of dopamine D1 receptors on MSNs of the DMS (Lovinger, 2010), which facilitate behavior via input onto neurons in the substantia nigra (direct pathway). In contrast, both LTP and LTD on dorsal striatal neurons projecting to the globus pallidus (indirect pathway) are likely involved in habit formation (Lovinger, 2010).
While the effects of chronic ethanol on dorsal striatum function have only recently been explored, recent data suggests that the consequences of chronic ethanol exposure on glutamate receptor expression and function are generally similar to that observed in other brain regions. For example, repeated ethanol exposure produced long-lasting increases in GluN2B-containing NMDA receptor activity as well as increased GluN2B phosphorylation and membrane localization in the DMS (Wang et al., 2010). Importantly, inhibition of GluN2B-containing receptors in the DMS, but not the DLS, decreased operant ethanol self-administration and ethanol-primed reinstatement suggesting that GluN2B activity in the DMS plays a role in relapse (Wang et al., 2010). In an extension of this study, Wang et al. (2012) also showed that the same exposure paradigm facilitated GluN2B-dependent LTP in the DMS. This effect was mediated by a long-lasting increase in the synaptic localization of GluA1 and GluA2-containing AMPA receptors. Similar to findings from their earlier study, this group also reported that administration of an AMPA receptor antagonist into the DMS decreases operant responding for ethanol and reinstatement of ethanol-seeking (Wang et al., 2012). In addition to the effects of repeated ethanol exposure on striatal LTP, several studies have also reported attenuated LTD in the DMS. Whether this effect recovers during early abstinence may depend on length and method of chronic ethanol exposure (Xia et al., 2006; Cui et al., 2011). A similar loss of LTD has been observed in the DLS following chronic ethanol exposure (Adermark et al., 2011; DePoy et al., 2013, 2015). This effect was accompanied by alterations in dendritic morphology and increased DLS activity during tasks that measure cognitive performance (DePoy et al., 2013) as well as changes in DLS-dependent learning (DePoy et al., 2013, 2015). In addition, a recent study utilizing a non-human primate model of chronic heavy drinking reported enhanced glutamatergic neurotransmission in the putamen (analogous to the rodent DLS) that appeared to be mediated by a presynaptic mechanism (Cuzon Carlson et al., 2011). This same study also reported increased intrinsic excitability of putamen MSNs and decreased inhibitory activity, which collectively facilitate MSN hyperexcitability in this region.
While animal models are often are better suited for examining the effect of chronic alcohol on synaptic plasticity, studies have examined changes in glutamate receptor density or gene expression in regions involved in cognitive performance including the PFC, hippocampus and caudate using postmortem tissue from humans with a history of alcohol use disorders. For the most part, these studies have generally supported the animal work demonstrating an up-regulation in glutamate receptors following chronic alcohol exposure (Breese et al., 1995; Jin et al., 2014b; Enoch et al., 2014; Bhandage et al., 2014; Laukkanen et al., 2015). Together with the animal studies described above, these data suggest that glutamatergic alterations within these regions likely plays a role in the impaired cognitive function and the transition from goal-direct to habitual behavior that is characteristic of alcohol addiction.
6. Discussion
The work described above clearly demonstrates a significant role for the glutamatergic system in the neuroadaptive processes that are associated with chronic alcohol exposure and repeated episodes of withdrawal. Together with neuropharmacological studies that have also implicated the glutamatergic system in alcohol drinking behavior (Gass and Olive, 2008), these results have led to interest in the development of novel compounds targeting the glutamate system in the treatment of alcohol addiction. While several pharmacotherapeutic strategies focused on glutamatergic signaling are currently being considered, to date, no single drug has proven effective at reducing alcohol craving and consumption without prohibitive side effects (Gass & Olive, 2008), further highlighting the need to identify novel pharmacological strategies. The significant and consistent glutamatergic changes being reported following chronic ethanol exposure suggests that modulation of this system would be a promising therapeutic direction, particularly for restoring cognitive function and reducing craving. Of particular interest will be the development of compounds that exhibit circuit-specific actions and thereby selectively target the addiction neurocircuitry.
Altered GluN2B-containing NMDA receptor function and expression is a common finding across virtually all regions that have been investigated (Figure 2). Consequently, much of the focus remains on the effects of chronic ethanol on NMDA receptor-mediated synaptic plasticity. While this is clearly an important avenue of research this focus has occurred to the near exclusion of other glutamate receptor subtypes. This is despite a well-established role for other receptor subtypes, particularly the mGluRs, in synaptic plasticity and remodeling. In particular, neuropharmacological studies have demonstrated involvement of group one mGluRs in mediating alcohol self-administration as well as extinction of responding for alcohol and cue-and stress-induced seeking for alcohol (Gass and Chandler, 2013). These data make clear the need for future investigation of the mGluR-mediated cellular processes underlying these behaviors and how these processes are altered following chronic ethanol exposure. Furthermore, the possibility of dependence-induced alterations in monoaminergic modulation of glutamatergic activity adds an additional layer of complexity. This has received little attention despite its prominent role in mediating neuronal activity and behavioral output, particularly via dopaminergic inputs to the PFC (Tseng and O’Donnell, 2004).
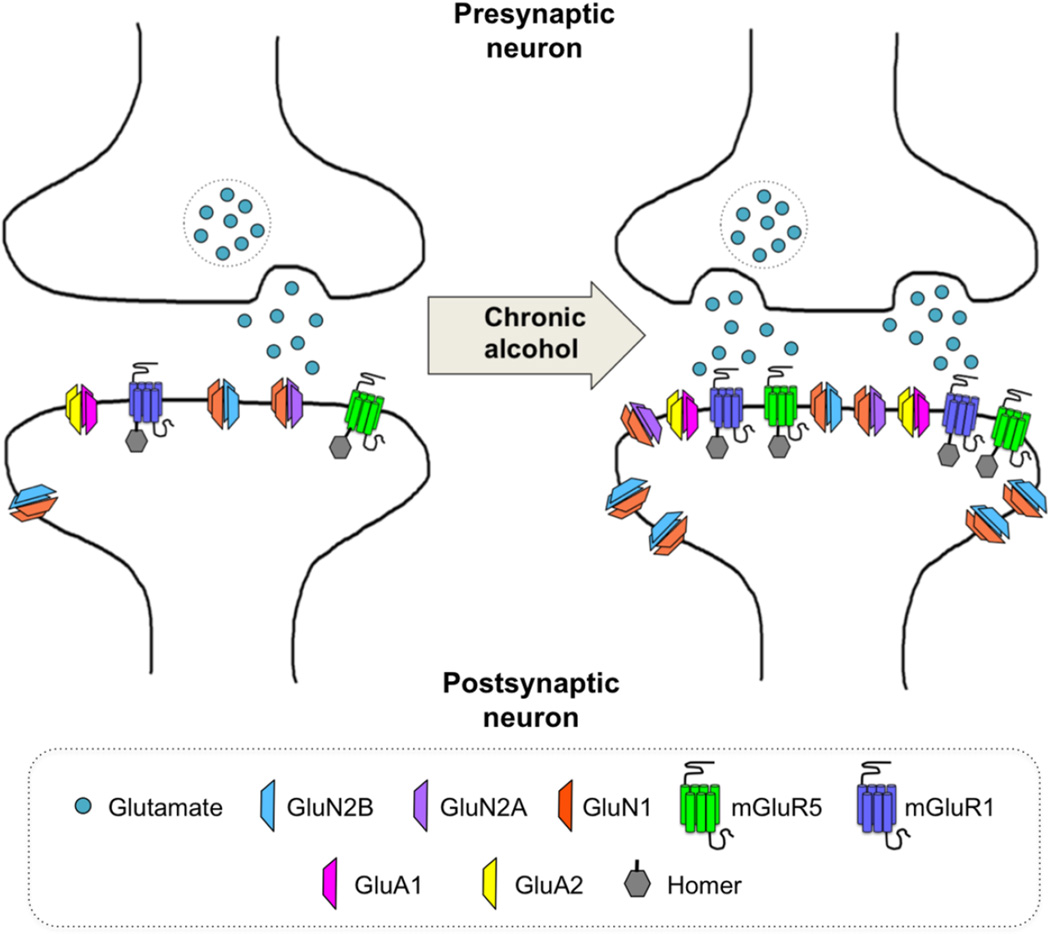
Figure 2. Commonly observed glutamatergic synaptic adaptations observed following chronic alcohol exposure.
Chronic alcohol exposure is associated with an overall enhancement of glutamatergic signaling in the brain – a so-called hyperglutamatergic state. Many, though not all, brain regions exhibit an increase in presynaptic release of glutamate accompanied by an upregulation in receptor expression. In particular, an increase in extrasynaptic GluN2B expression has been consistently observed throughout the brain. Additional increases in GluN2A, GluA1, and GluA2 are also frequently noted. Chronic alcohol exposure is also associated with greater expression of the Group 1 mGluRs. These increases in receptor expression are driven, at least in part, by increased Homer levels, which have been observed in association with all receptor subtypes but most frequently with the mGluRs.
Thus far, much of the work aimed at understanding the neurobiology of alcohol addiction has focused on brain regions that play a role in the maladaptive behaviors produced by chronic alcohol exposure and withdrawal. The well-deserved focus on the VTA and NAc stemmed in large part from their known role in motivation and reward. The amygdala and extended amygdala have been of great interest based on their involvement in behavioral measures of anxiety, while the PFC and hippocampus have been studied largely because of their prominent roles in learning, memory and cognitive function. While these studies have greatly advanced our current understanding of the neurobiological effects of chronic alcohol exposure, the past decade has witnessed a move away from the theoretical framework that assigns specific behaviors to discrete brain regions and instead, toward the investigation of specific neural circuits and their distinct functional roles --- often despite close neuroanatomical proximity. Moreover, it has become increasingly apparent that integration of signals from multiple projection-specific pathways and synchronized network activity across brain regions frequently serves to mediate behavioral output. Thus, the region specific functions described above are likely an oversimplified view of the neurocircuitry of addiction. Indeed, while the VTA and NAc have traditionally been associated with reward and reinforcement, there are in fact data indicating that some dopamine neurons of the VTA fire during exposure to aversive stimuli (Brischoux et al., 2009). Furthermore, the reciprocal connections with the LHb --- a region involved in negative reward prediction error (Hikosaka, 2010) --- may play a role in response to aversive stimuli. In addition, the NAc can be divided into core and shell subregions that exhibit marked differences in connectivity and function. Though not conclusive, the NAc core appears to play a significant role in goal-directed action whereas the NAc shell appears to play a larger role in signaling the value of rewards (Saddoris et al., 2013). Likewise, while the BNST and CeA have been well characterized for their involvement in anxiety-like behavior, there are clear subregional differences within each nucleus. For example, the CeA can be divided functionally, neurochemically, and neuroanatomically into medial and lateral subdivisions. While both subregions receive input from the BLA, the lateral CeA also receives a prominent, stress-sensitive, efferent projection from the hypothalamus that, in combination with input from the insular cortex, is thought to play a role in facilitating sustained anxiety via its projection to the lateral subdivision of the BNST. In contrast, BLA inputs to the medial CeA are thought to facilitate immediate fear responses to aversive stimuli (Davis et al., 2010). Interestingly, the BNST can also be subdivided into dorsal and ventral components (separated by the anterior commissure) based on the topography of glutamatergic efferents to the VTA (Georges and Aston-Jones, 2001, 2002). Similarly, it is now well understood that the hippocampus can be divided into ventral and dorsal subregions (anterior and posterior in primates), which exhibit striking differences in function and connectivity (Fanselow and Dong, 2010). Finally, despite a large body of work ascribing different functions to discrete subregions of the PFC, recent work has reported conflicting functions within the same subregion that may reflect projection-specific top-down control over behavior (Warden et al., 2012). As our understanding of the complexity of this neurocircuitry and its functions has improved, it has become increasingly evident that studies investigating pathway-specific glutamatergic adaptations following chronic ethanol exposure will be critical to a comprehensive understanding of the neurobiology of alcohol dependence.
While much of the research to date has focused on the reinforcing aspects (both positive and negative) of dependence that promote continued alcohol use, the aversive aspects of alcohol and their role in limiting use have been relatively understudied. Interestingly, recent work has characterized a functional circuit made up of the recently identified GABAergic nucleus known as the rostromedial tegmental nucleus (RMTg), which receives glutamatergic input from the LHb, and projects to the VTA exerting inhibitory modulation over dopaminergic neurotransmission (Figure 1) (Kaufling et al., 2009; Jhou et al., 2009a, 2009b). This circuit has been recently characterized for its involvement in mediating the aversive properties of psychostimulants (Kaufling et al., 2010; Jhou et al., 2013) and it may also be involved in alcohol-related aversion. In addition, the RMTg, which receives prominent monosynaptic input from the BNST, hypothalamus and PAG, is reportedly involved in mediating expression of anxiety-like behavior (Jhou et al., 2009a). Thus, the heightened anxiety associated with withdrawal from alcohol coupled with reports of tolerance to the aversive properties of alcohol following chronic exposure suggests that dependence-induced neuroadaptive processes within this circuit may facilitate some of the maladaptive behaviors associated with alcohol addiction. Recent work has also identified a role for glutamatergic projections from both the mPFC and insular cortex to the NAc core in mediating aversion-resistant alcohol intake (Seif et al., 2013). Determining whether or not these neural circuits interact with the LHb-RMTg-VTA circuitry and the effects of chronic ethanol exposure on their function will facilitate a more comprehensive understanding of the role this neural circuit plays in mediating aversive signaling in addiction.
7. Conclusion
Dependence and withdrawal from alcohol are associated with significant hyperexcitability that is likely mediated by a hyperglutamatergic state stemming from increased levels of extracellular glutamate and an up-regulation of GluN2B-containing NMDA receptor activity throughout the brain (Figure 2). Nevertheless, chronic alcohol exposure exerts region-specific effects on glutamatergic activity and the neurophysiological consequences of chronic ethanol exposure on mGluR-mediated synaptic plasticity has been largely overlooked. In addition, the complexity of connections between regions that mediate the maladaptive behaviors characteristic of addiction makes clear the need for work examining the effects of chronic ethanol exposure on glutamatergic neurotransmission in specific neural circuits as opposed to simply focusing on functional nuclei. New technological advancements including optogenetics, pharmacogenetics and the development of Cre-expressing transgenic animals allow for the investigation of cell-type and circuit-specific neuronal populations. Studies employing these technologies in models of alcohol dependence will allow for the investigation of the effects of chronic ethanol exposure on both neurophysiology and behavior of discrete neural circuits. Approaches such as these are critical for a more complete understanding of the glutamatergic neuroadaptations that contribute to maladaptive behaviors to aid in the development of more appropriate and precise drug targets for the treatment of alcohol use disorders.
Highlights.
Alcohol dependence is associated with alterations in glutamatergic neuroplasticity.
Alterations in NMDA receptor mediated activity are common across brain regions.
Dependence-induced changes occur in regions mediating reward, affect and cognition.
Circuit-specific changes may present new targets for the treatment of alcoholism.
Acknowledgements
This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism: AA010983, AA019967 and AA010761 (LJC), AA022475 (HTD), AA022836 (EJB).
Abbreviations
- AMPA
2-amino-3(3-hydroxy-5-methyl-isoxazol-4yl)propanoic acid
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amgydala
- CSF
cerebrospinal fluid
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- EC
external capsule
- EPSP
excitatory postsynaptic potential
- iGluR
ionotropic glutamate receptor
- GluA1-4
AMPA receptor subunits
- GluK1-3
kainate receptor subunits
- GluN1-3
NMDA receptor subunits
- LHb
lateral habenula
- LTD
long-term depression
- LTP
long-term potentiation
- MeA
medial nucleus of the amygdala
- mGluR
metabotropic glutamate receptor
- mPFC
medial prefrontal cortex
- MSN
medium spiny neuron
- NAc
nucleus accumbens
- NMDA
N-methyl-D-asparate
- OFC
orbitofrontal cortex
- PAG
periacqueductal gray
- PFC
prefrontal cortex
- PrL
prelimbic cortex
- RMTg
rostromedial tegmental nucleus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MW, Gregson RA. Cognitive dysfunction in the prediction of relapse in alcoholics. J. Stud. Alcohol. 1981;42:230–243. doi: 10.15288/jsa.1981.42.230. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Ericson M, Söderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, Levenes C. Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors. EMBO Rep. 2014;15:103–109. doi: 10.1002/embr.201337371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. In: Extended Amygdala. Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience Springer Berlin Heidelberg; 2009. pp. 1501–1506. [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav. Neurosci. 2011;125:879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR. Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neurosci. Biobehav. Rev. 2014;47:281–294. doi: 10.1016/j.neubiorev.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, Chandler LJ. A unifying model of the role of the infralimbic cortex in extinction and habits. Learn. Mem. Cold Spring Harb. N. 2014;21:441–448. doi: 10.1101/lm.035501.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Griffin WC, Lopez MF. Neuroadaptive changes that result from chronic drug exposure. In: Blume AW, Kavanagh DJ, Kampman KM, Bates ME, Larimer ME, Petry NM, De Witte P, Ball SA, editors. Biological Research on Addiction: Comprehensive Addictive Behaviors and Disorders. Vol. 2. Amsterdam; New York: Academic Press; 2013. pp. 169–178. [Google Scholar]
- Becker HC, Mulholland PJ. Neurochemical mechanisms of alcohol withdrawal. In: Sullivan EV, Pfefferbaum A, editors. Alcohol and the Nervous System: Handbook of Clinical Neurology. 3rd. Amsterdam: The Netherlands: Elsevier; 2014. pp. 133–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Mameli M. mGluR-Dependent Synaptic Plasticity in Drug-Seeking. Front. Pharmacol. 2012;3:159. doi: 10.3389/fphar.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandage AK, Jin Z, Bazov I, Kononenko O, Bakalkin G, Korpi ER, Birnir B. GABA-A and NMDA receptor subunit mRNA expression is altered in the caudate but not the putamen of the postmortem brains of alcoholics. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J. Neuropsychiatry Clin. Neurosci. 2005;17:417–420. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. 1995;674:82–90. doi: 10.1016/0006-8993(94)01384-t. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett EJ, Barker JM, Glen WB, Chandler LJ. Impact of alcohol abuse and dependence on the structure and function of the prefrontal cortex. In: Noronha AB, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of Alcohol Dependence. London: Academic Press; 2014. pp. 291–320. [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J. Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol. Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol. Sci. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Newsom H, Sumners C, Crews F. Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J. Neurochem. 1993;60:1578–1581. doi: 10.1111/j.1471-4159.1993.tb03326.x. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol. Clin. Exp. Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur. J. Neurosci. 2008;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacol. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr. Opin. Neurobiol. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacol. 2014;39:435–444. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Cui S, Wang S, Li J, Xie G, Zhou R, Chen L, Yuan X. Alteration of synaptic plasticity in rat dorsal striatum induced by chronic ethanol intake and withdrawal via ERK pathway. Acta Pharmacol. Sin. 2011;32:175–181. doi: 10.1038/aps.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and Morphological Neuroadaptations in the Putamen Associated with Long-Term, Relapsing Alcohol Drinking in Primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Taurine blocks the glutamate increase in the nucleus accumbens microdialysate of ethanol-dependent rats. Pharmacol. Biochem. Behav. 2000;65:345–350. doi: 10.1016/s0091-3057(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur. J. Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, Lovinger D, Holmes A. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict. Biol. 2015;20:345–348. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Res. 1984;308:325–332. doi: 10.1016/0006-8993(84)91072-2. [DOI] [PubMed] [Google Scholar]
- Enoch M-A, Rosser AA, Zhou Z, Mash DC, Yuan Q, Goldman D. Expression of glutamatergic genes in healthy humans across 16 brain regions; altered expression in the hippocampus after chronic exposure to alcohol or cocaine. Genes Brain Behav. 2014;13:758–768. doi: 10.1111/gbb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald GJ, Liu H, Morzorati SL. Decreased sensitivity of NMDA receptors on dopaminergic neurons from the posterior ventral tegmental area following chronic nondependent alcohol consumption. Alcohol. Clin. Exp. Res. 2012;36:1710–1719. doi: 10.1111/j.1530-0277.2012.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Jung K-Y, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J. Pharmacol. Exp. Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res. 2008;1211:13–21. doi: 10.1016/j.brainres.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front. Psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev. Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology (Berl.) 2014;231:3911–3917. doi: 10.1007/s00213-014-3623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J. Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J. Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res. Health J. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Neuropharmacology. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-methyl-D-aspartate receptors underlies ethanol withdrawal hyperexcitability. J. Pharmacol. Exp. Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Hepp R, Hay YA, Aguado C, Lujan R, Dauphinot L, Potier MC, Nomura S, Poirel O, El Mestikawy S, Lambolez B, Tricoire L. Glutamate receptors of the delta family are widely expressed in the adult brain. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0827-4. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol. Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol. 1990;7:229–231. doi: 10.1016/0741-8329(90)90010-a. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat. Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl.) 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J. Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Brain Res. Mol. Brain Res. 1995;30:347–356. doi: 10.1016/0169-328x(95)00019-o. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Hasselmo ME, Seamans JK. What is the Functional Relevance of Prefrontal Cortex Entrainment to Hippocampal Theta Rhythms? Front. Neurosci. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J. Pharmacol. Exp. Ther. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley C, Xu S, Wang H, Burnham N, Hoffman A, Lupica CR, Ikemoto S. Cocaine Drives Aversive Conditioning via Delayed Activation of Dopamine-Responsive Habenular and Midbrain Pathways. J. Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, Birnir B. Expression of specific ionotropic glutamate and GABA-A receptor subunits is decreased in central amygdala of alcoholics. Front. Cell. Neurosci. 2014a;8:288. doi: 10.3389/fncel.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, Birnir B. Selective increases of AMPA, NMDA, and kainate receptor subunit mRNAs in the hippocampus and orbitofrontal cortex but not in prefrontal cortex of human alcoholics. Front. Cell. Neurosci. 2014b;8:11. doi: 10.3389/fncel.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol. Clin. Exp. Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacol. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier M-J, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Comp. Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Waltisperger E, Bourdy R, Valera A, Veinante P, Freund-Mercier M-J, Barrot M. Pharmacological recruitment of the GABAergic tail of the ventral tegmental area by acute drug exposure. Br. J. Pharmacol. 2010;161:1677–1691. doi: 10.1111/j.1476-5381.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:1051. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PloS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacol. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J. Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen V, Kärkkäinen O, Kupila J, Kautiainen H, Tiihonen J, Storvik M. Increased metabotropic glutamate 2/3 receptor binding in the perigenual anterior cingulate cortex of cloninger type 2 alcoholics: a whole-hemisphere autoradiography study. Alcohol Alcohol. Oxf. Oxfs. 2015;50:62–67. doi: 10.1093/alcalc/agu081. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Köhr G, Herb A, Seeburg PH, Wisden W. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter Roles in Synaptic Modulation, Plasticity and Learning in the Dorsal Striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front. Neurosci. 2012a;6:86. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Effects of alcohol on the membrane excitability and synaptic transmission of medium spiny neurons in the nucleus accumbens. Alcohol. 2012b;46:317–327. doi: 10.1016/j.alcohol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. In: Amygdala. Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience Springer Berlin Heidelberg; 2009. pp. 1501–1506. [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49:21–27. doi: 10.1016/j.alcohol.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J. Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282C:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Spencer KB, Hu W, Kroener S, Chandler LJ. Neuroplasticity of A-type potassium channel complexes induced by chronic alcohol exposure enhances dendritic calcium transients in hippocampus. Psychopharmacology (Berl.) 2014;232:1995–2006. doi: 10.1007/s00213-014-3835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015;7:35–44. doi: 10.1016/j.neuroscience.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005;1048:69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill P-K, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J. Neurosci. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 1991. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcohol. Clin. Exp. Res. 1998;22:954–961. [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex J. Devoted Study Nerv. Syst. Behav. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol. Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are imparied? Acta Neurol. Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Little HJ. Nitrendipine prevents the decrease caused by chronic ethanol intake in the maintenance of tetanic long-term potentiation. Exp. Brain Res. 1995;103:1–8. doi: 10.1007/BF00241959. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Brunelli M, Sanna PP, Gruol DL. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur. J. Neurosci. 2003;17:1646–1654. doi: 10.1046/j.1460-9568.2003.02614.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J. Neurophysiol. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: an In Vitro and In Vivo Analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Robinson T, Berridge KC. Incentive salience and the transition to addiction. In: Miller PR, editor. Biological Research on Addiction. San Diego, CA: Academic Press; 2013. pp. 391–399. [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci. 2014a;34:13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang H-L, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci. 2014b;17:1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson’s disease and related disorders. Pharmacol. Ther. 2000;88:427–435. doi: 10.1016/s0163-7258(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front. Biosci. Elite Ed. 2013;5:273–288. doi: 10.2741/e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, Barrot M. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282C:198–216. doi: 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors. Eur. J. Neurosci. 2008;27:1957–1964. doi: 10.1111/j.1460-9568.2008.06173.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2009;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Masala N, Cannizzaro C, Biggio G, Sanna E, Diana M. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3745–E3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict. Biol. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol. Clin. Exp. Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol. Clin. Exp. Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare J-F, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem. Pharmacol. 2008a;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008b;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl.) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski K, Woodward JJ. Glutamate signaling in alcohol abuse and dependence. In: Noronha AB, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of Alcohol Dependence. London: Academic Press; 2014. pp. 173–206. [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J. Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinschmidt JS, Walker DW, King MA. Chronic ethanol treatment reduces the magnitude of hippocampal LTD in the adult rat. Synapse. 2003;48:189–197. doi: 10.1002/syn.10203. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J. Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremwel MF, Hunter BE. Effects of chronic ethanol ingestion on long-term potentiation remain even after a prolonged recovery from ethanol exposure. Synapse. 1994;17:141–148. doi: 10.1002/syn.890170210. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J. Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J. Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch. Gen. Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kim JH. Metabotropic glutamate receptors and the generation of locomotor activity: interactions with midbrain dopamine. Neurosci. Biobehav. Rev. 1999;23:577–589. doi: 10.1016/s0149-7634(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J. Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J. Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E278–E287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol. Clin. Exp. Res. 2006;30:819–824. doi: 10.1111/j.1530-0277.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus Accumbens Medium Spiny Neurons Target Non-Dopaminergic Neurons in the Ventral Tegmental Area. J. Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Gupta SC, Hillman BG, Bhatt JM, Stairs DJ, Dravid SM. Deletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviors. PloS One. 2012;7:e32969. doi: 10.1371/journal.pone.0032969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch M-A, Hodgkinson CA, Shen P-H, Lovinger DM, Edenberg HJ, Heilig M, Goldman D. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16963–16968. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]