Abstract
Bradyzoite forms of Toxoplasma gondii persist in tissue cysts for the lifetime of an infected host and can reactivate to cause clinical disease. It was thought that in vivo bradyzoites within tissue cysts are biologically inactive dormant forms that rarely replicate. Apparently, consensus was wrong.
Keywords: Toxoplasma, bradyzoite, tissue cyst
The opportunistic pathogen Toxoplasma gondii is an Apicomplexan parasite that has the unique ability to persist within its host as latent bradyzoite forms that lie within tissue cysts. The rapidly growing tachyzoite form that is responsible for clinical disease is controlled by the immune system and differentiates into bradyzoites. Bradyzoites have unique antigens and metabolism that are presumed to protect them from the immune response and facilitate long-term viability in tissue [1] (Figure 1). Bradyzoites are important because they can reactivate to cause lethal disease in an immunocompromised host. Although T. gondii can infect all nucleated cells, cysts are common in the brain, and clinical toxoplasmosis often presents as encephalitis, acute inflammation of the brain. Bradyzoites are also infectious when ingested. So the biology of bradyzoites has been the subject of intense interest to scientists investigating pathogenesis of toxoplasmosis.
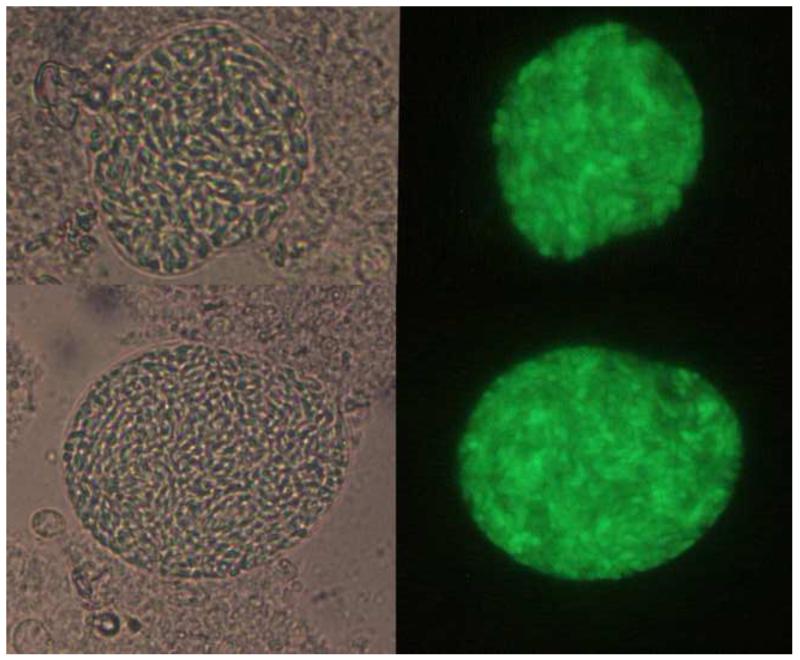
Figure 1. Toxoplasma tissue cysts harvested from mouse brains.
The PruΔku80Δhxgprt strain stably expresses GFP-positive cysts during chronic infection. The images show examples of cysts observed at 5 weeks after intraperitoneal infection of C57BL/6 mice with tachyzoites of strain PruΔku80ΔhxgprtLDH2-GFP. Left images show bright-field laser confocal microscopy and right images are GFP fluorescence driven by the bradyzoite-specific LDH2 promoter. Images courtesy of Tadakimi Tomita and Louis Weiss.
Recently, Watts et al. took a closer look at the dynamics of T. gondii bradyzoites within tissue cysts [2]. After painstakingly optimizing the classic purification protocol developed by Cornelissen [3], Watts et al. examined the size, density, and bradyzoite contents of cysts harvested at different times from mouse brains. Ninety-nine cyst preparations, 630 cysts, and two years later, they found that more goes on within tissue cysts than had been appreciated previously.
Bradyzoite biology has been difficult to study. In the laboratory, bradyzoite differentiation can be induced by various environmental stresses, but it is difficult to obtain pure populations of bradyzoites that are not contaminated with tachyzoite forms. Strains of T. gondii differ in their propensity to differentiate, and strains cultivated in the laboratory gradually lose their ability to differentiate. Epigenetic gene regulation also plays an important role in the tachyzoite-bradyzoite transition, but the exact molecular triggers for differentiation are not understood, as it has not been possible to follow the progression of bradyzoite differentiation over time. There has been controversy in the field whether laboratory induced bradyzoites are ‘real’ bradyzoites, with bradyzoites harvested from tissue cysts serving as the gold standard reference. It has been assumed that in vivo bradyzoites are homogeneous and static. The careful, standardized quantitation and characterization of cysts by Watts et al. [2] reveals unexpected heterogeneity amongst bradyzoites and tissue cysts.
Taking advantage of advances in three-dimensional digital confocal imaging, the authors developed the BradyCount software that enabled them to accurately and efficiently determine bradyzoite number within cysts. In general, as expected, as cyst size increased, the numbers of bradyzoites within cysts increased. They also carefully measured cyst size and used digital imaging approaches to quantify parasite division within cysts.
There was a surprisingly amount of variability in cyst density and bradyzoite burden within individual cysts, suggesting that cyst size varies independently of bradyzoite number over time. Bradyzoites are surrounded by a glycan-rich cyst wall, and the Dolichos biflorus agglutinin (DBA) lectin is a commonly used cyst wall marker. CST1, the major protein recognized by DBA lectin, is a glycoprotein that is extensively modified by O-linked sugars [4], and the glycosylation of CST1 confers rigidity to the cyst wall [4]. Since the size of cysts expands without replication of bradyzoites, the work by Watts et al. [2] suggests that the contents of the cyst matrix and components of the cyst wall are continuously secreted as cyst volume expands. How the cyst wall and cyst matrix are assembled is not yet clear, but one mechanism might be via ongoing secretion of glycoproteins analogous to the constitutive secretion of dense granule contents that occurs during tachyzoite development [5].
Numerous studies have shown the importance of parasite secreted molecules in interaction with the infected host cell and modulation of host signaling and transcription [6]. In addition, immunological studies show that bradyzoites within cysts are not as immunologically silent as originally thought, and are killed by both cytotoxic CD8+ T cells [7] and macrophages [8]. Intriguingly, it appears that alternatively activated macrophages recognize chitin in the cyst walls and produce chitinase that destroys cysts [8]. Therefore further understanding of the composition and dynamics of bradyzoite secretory pathways should be an area of fruitful investigation, and the glycoproteins induced during bradyzoite development may be new targets for vaccine or drug development.
Using IMC3, an intracellular marker whose expression is most intense in newly divided parasites [9], Watts et al. [2] also show that parasite replication does occur within cysts, and that this replication is not due to reversion of bradyzoites to tachyzoites. Bradyzoite replication was often asynchronous, but the authors also observed synchronous division, which is typically associated with the pathogenic tachyzoite form. Newly divided bradyzoites were found in clusters, uniformly through the cyst, as well as at the cyst periphery. The differing patterns of replication suggest that commitment to replication can be influenced by both local and external signals such as nutrients or small signaling molecules, as described in other microbial communities.
Since bradyzoites within tissue cysts are dynamic, we need to rethink our concepts about the biology of bradyzoites and strategies that might eradicate bradyzoites. Recent studies have reported compounds that reduce cyst numbers in brains [10]. While cyst number is an important parameter to monitor, quantitation of bradyzoite burden may be more important. Cyst number was relatively stable over the course of the experiments by Watts et al. [2], indicating that cysts that rupture or degenerate are replaced by an equal number of new cysts, and reinforcing the idea that ongoing immune surveillance is likely to keep chronic T. gondii infection in check. If agents are able to reduce the average number of bradyzoites, but reduce cyst number more slowly, we may need to reconsider the metrics used to assess efficacy of treatments to eliminate bradyzoites and cysts or modify dosing regimens to account for the biology and dynamics of cyst evolution. Further investigation is needed to understand the cyst maturation process and the associated metabolic and molecular changes. But it is clear that we can no longer think of T. gondii bradyzoites as a homogeneous quiescent population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front. Biosci. 2000;5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts E, et al. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. mBio. 2015;6:e01155–15. doi: 10.1128/mBio.01155-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelissen AW, et al. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981;83:103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- 4.Tomita T, et al. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 6.Hakimi MA, Bougdour A. Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr. Opin. Microbiol. 2015;26:24–31. doi: 10.1016/j.mib.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 2010;176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nance JP, et al. Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog. 2012;8:e1002990. doi: 10.1371/journal.ppat.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson-White B, et al. Cytoskeleton assembly in Toxoplasma gondii cell division. Int. Rev. Cell Mol. Biol. 298:1–31. doi: 10.1016/B978-0-12-394309-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doggett JS, et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]