Abstract
Objective
Procedural volume is associated with outcomes for many surgical interventions. Little is known about the association between volume and outcomes of radiation. We examined the association between treatment center and hospital volume and outcomes for women with locally advanced cervical cancer treated with radiation.
Methods
Women with stage IIB-IVA cervical cancer treated with primary radiation from 1998-2011 and recorded in the National Cancer Database were examined. Hospital volume was estimated as the mean annualized volume, while center-specific effects on care were examined using a hospital-specific random effect. Multivariable regression models adjusted for metrics of treatment quality were used to estimate survival.
Results
20,766 patients treated at 1115 hospitals were identified. The median follow-up was 24.2 months while 5-year survival was 36.5% (95% CI, 35.6-37.4%). Higher hospital volume was associated with receipt of brachytherapy (P<0.05), but had no effect on use of chemotherapy. In a multivariable model accounting for clinical and demographic factors as well as quality of care, hospital volume was not associated with survival (P=0.25). The specific hospital in which patients received care was the strongest predictor of survival (P<0.0001) followed by stage, year of diagnosis and treatment quality (P<0.0001 for all). The hospital-specific effect on mortality expressed as a hazard ratio, ranged from 0.66-1.53 across hospitals.
Conclusion
For locally advanced cervical cancer, hospital volume has a minimal impact on outcome; however, the specific center in which care is delivered is strongly associated with survival.
Keywords: Cervical cancer, radiation, chemoradiation, chemosensitization, volume, outcomes
Introduction
Women with locally advanced cervical cancer (stages IIB-IVA) have tumors that have spread beyond the cervix to the adjacent pelvic structures. In the late 1990’s, a series of studies demonstrated that chemotherapy combined with radiation therapy was superior to radiation alone for these neoplasms.1-4 The magnitude and consistency of the survival benefit demonstrated in these studies prompted the National Cancer Institute to issue a clinical alert in 1999 recommending that chemoradiation should be considered the standard of care for patients with newly diagnosed, advanced stage cervical cancer.5
The multimodal treatment of locally advanced cervical cancer is complex. External beam radiotherapy is typically administered every day with concurrent, weekly cisplatin. In addition, curative intent therapy requires intracavitary brachytherapy delivered through an apparatus placed directly into the cervix and vagina. Appropriate radiation planning is essential to ensure delivery of an adequate therapeutic dose to the pelvis while minimizing toxicity to surrounding tissues.6 However, given the decreasing incidence of cervical cancer in the United States, many centers treat only a small number of patients each year.
For many complex medical interventions, procedural volume has been shown to have an association with treatment outcomes.7-12 This paradigm has been demonstrated for high-risk oncologic and cardiovascular surgical procedures in which outcomes are superior when the operations are performed by high-volume surgeons at high-volume centers.7-12 The improved outcomes for high-volume providers are likely due to a multitude of factors, including increased technical expertise, adherence to evidence-based treatment recommendations, and appropriate management of complications.13-15
Despite the fact that delivery of therapeutic radiation is often technically demanding, there has been little prior data exploring the influence of the treating hospital on outcomes in patients treated with primary radiotherapy. We performed a population-based analysis to examine the influence of treatment center and hospital volume on quality of care and survival for women with locally advanced cervical cancer.
Methods
Data Source and Patient Selection
Data from the National Cancer Data Base (NCDB) was utilized. NCDB is a nationwide oncology outcomes database sponsored by the American College of Surgeons and American Cancer Society.16,17 NCDB captures data on approximately 70% of all newly diagnosed invasive cancers and includes over 1500 Commission on Cancer (CoC) affiliated hospitals from across the United States. NCDB collects data on patient demographics, tumor characteristics, staging data, treatment information, and survival.16,17 Data are abstracted by trained cancer registrars and are regularly audited to ensure accuracy. The Columbia University Institutional Review Board deemed the study exempt.
Women with stage IIB-IVA cervical cancer diagnosed from 1998-2011 were included in the analysis. We included only those patients whose initial, primary treatment included radiation therapy. Patients treated with primary surgery and those who did not receive any treatment were excluded. As survival data in NCDB is only included for patients with at least five years of follow-up, we present data on the entire cohort (1998-2011) and analyzed survival outcomes in a limited survival cohort (1998-2006).
Treating Hospital and Hospital Volume
The treating hospital was defined as the hospital in which radiation was administered. The primary analysis was limited to patients who received their entire course of radiotherapy at the institution in which treatment was initiated. A sensitivity analysis including patients who received radiation at multiple facilities was also performed. Prior studies have explored modeling volume in a variety of fashions.18 The primary analysis of hospital volume was performed using annualized hospital volume.18 For each hospital, we calculated the total number of patients treated and divided this by the number of years in which a hospital treated at least one patient with locally advanced cervical cancer. Exploratory analyses were performed modeling volume in several ways. First, we classified previous year volume as the number of cases treated at a given hospital in the calendar year prior to the index patient. Second, we defined current year volume as the number of cases treated at a given hospital in the same calendar year in which an index patient was treated.
The primary analysis was performed including hospital volume as a continuous variable.18 We also explored the influence of classifying hospital volume as a categorical variable and dividing the cohort into patient-based quartiles: <2, 2-3.99, 4-5.99, and ≥6 cases per year.
Variables and Outcomes
Clinical variables analyzed included age (<40, 40-49, 50-59, 60-6, ≥70 years), race (white, black, Hispanic, other), insurance (commercial, Medicare, Medicaid, uninsured, other), Tumor characteristics included grade (1, 2, 3, or unknown), stage, and histology (squamous, adenocarcinoma, adenosquamous, or other). Hospital characteristics analyzed included region (northeast, midwest, south, or west) and location (metropolitan, urban, rural). Based on the ACS CoC criteria, hospitals are also classified as academic/research cancer centers or community cancer centers.17
A number of metrics of treatment quality were analyzed. For each patient, receipt of brachytherapy (either low or high dose rate) as well as chemotherapy was recorded. The primary outcome of the analysis was survival.16 Survival is reported as all cause mortality and includes death from cancer and other causes.
Statistical Analysis
Frequency distributions for categorical variables were analyzed across volume quartiles using χ2 tests. Median volume for each quartile is reported along with interquartile ranges (IQR). Generalized linear mixed effects models using a Poisson distribution and a log link function were developed to examine predictors of treatment. These models included all of the clinical and demographic variables as well as a hospital-specific random effect to account for hospital-level clustering.19 Multivariable models were developed to estimate factors associated with treatment at high-volume hospitals (annual volume >6 patients) and to explore factors associated with use of evidence-based treatments (brachytherapy and chemotherapy). To examine whether cluster size influenced outcomes, we performed sensitivity analyses using cluster weighted generalized estimating equations (CWGEE) that account for informative cluster size through inverse weighting.20
Survival was assessed using mixed-effects Cox proportional hazards regression models.21 Covariates for these models were chosen using purposeful selection.21,22 We first included all variables that were significant at the P<0.02 level in bivariate analysis, as well as all variables thought to be of clinical significance. We then removed variables that did not contribute to the multivariate fixed-effects model based on a P-value of >0.05 and a change in the coefficient of the remaining variables by >20%.21,23 Based on this selection, we constructed a model (clinical model) including the following patient and hospital characteristics: age, year of diagnosis, tumor histology, tumor grade, stage, race, insurance status, hospital type, and hospital region. To account for differences in quality of treatment, a treatment-adjusted model was developed that included the above covariates as well as receipt of brachytherapy and chemotherapy.
The assumption of proportionality was assessed visually by plotting scaled Schoenfeld residuals.21,24 A hospital-specific random effect was included in the Cox proportional hazards regression model to assess the center-specific effects on survival. The models assumed that the random effect followed a Gaussian distribution with a mean of 0. The proportional hazard assumption for the random effect was tested by fitting fixed-effect Cox models with the covariates of interest along with the random effect from the mixed-effects Cox model and visually inspecting the scaled Schoenfeld residuals.
The primary models included annualized hospital volume as a continuous variable. The linear relationship between annualized hospital volume and the log-hazard for death was assessed by Martingale residual plots. Sensitivity analyses were performed in which volume was modeled as a categorical variable; volume was estimated as the previous year volume, or current year volume. Additional models excluding stage IVA patients, limited to stage IIIB subjects, and models excluding the lowest volume hospitals were also developed.
The relative importance of each variable on survival was assessed using variations in the Akaike information criteria (AIC) induced by removing individual covariates from the models.21 A higher AIC in a multivariable model indicates a greater importance for the given variable omitted from the model. Separate analyses were performed using the clinical model and the treatment adjusted model.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina, USA) and the “coxph” and “coxme” routine in the R programing language version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).25 All analyses were two-tailed and a P-value of <0.05 was considered statistically significant.
Results
A total of 20,766 patients who received all their radiation treatments at 1115 hospitals were identified (Table 1, Supplemental table 1). Overall, the median annualized number of patients was 3.6 per year (IQR, 2.2-5.9). When stratified by center volume, there were 4027 patients treated in 664 hospitals with a volume of <2 cases per year, 7237 patients in 315 centers with a volume of 2-3.99 cases per year, 4447 women at 86 centers with a volume of 4-5.99 cases and 5055 patients at 50 hospitals with a volume of ≥6 cases per year (Table 1).
Table 1.
Clinical and demographic characteristics stratified by hospital volume.
| <2 | 2-3.99 | 4-5.99 | ≥6 | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | ||
| Number of hospitals | 664 | 315 | 86 | 50 | |||||
| Patients | 4027 | 7237 | 4447 | 5055 | |||||
| Median (IQR) | 1.5 (1.3-1.7) | 2.8 (2.3-3.4) | 4.7 (4.3-5.4) | 8.7 (6.7-11.5) | |||||
| Year of diagnosis | <0.0001 | ||||||||
| 1998 | 309 | (21.8) | 492 | (34.8) | 333 | (23.5) | 281 | (19.9) | |
| 1999 | 264 | (21.2) | 410 | (32.9) | 288 | (23.1) | 284 | (22.8) | |
| 2000 | 238 | (21.7) | 393 | (35.8) | 223 | (20.3) | 243 | (22.2) | |
| 2001 | 221 | (20.6) | 397 | (36.9) | 209 | (19.4) | 248 | (23.1) | |
| 2002 | 155 | (26.2) | 201 | (34.0) | 115 | (19.4) | 121 | (20.4) | |
| 2003 | 350 | (20.8) | 575 | (34.2) | 366 | (21.8) | 391 | (23.3) | |
| 2004 | 317 | (18.6) | 614 | (36.1) | 341 | (20.0) | 430 | (25.3) | |
| 2005 | 294 | (17.9) | 582 | (35.4) | 369 | (22.5) | 399 | (24.3) | |
| 2006 | 298 | (18.5) | 567 | (35.1) | 363 | (22.5) | 387 | (24.0) | |
| 2007 | 288 | (16.5) | 608 | (34.8) | 374 | (21.4) | 478 | (27.4) | |
| 2008 | 319 | (18.6) | 582 | (33.9) | 379 | (22.1) | 437 | (25.5) | |
| 2009 | 312 | (17.9) | 598 | (34.3) | 384 | (22.0) | 452 | (25.9) | |
| 2010 | 333 | (19.1) | 589 | (33.8) | 360 | (20.6) | 463 | (26.5) | |
| 2011 | 329 | (18.9) | 629 | (36.1) | 343 | (19.7) | 441 | (25.3) | |
| Age | <0.0001 | ||||||||
| <40 | 532 | (15.4) | 1219 | (35.3) | 782 | (22.6) | 925 | (22.6) | |
| 40-49 | 884 | (16.9) | 1737 | (33.3) | 1187 | (22.7) | 1411 | (22.7) | |
| 50-59 | 991 | (19.4) | 1751 | (34.2) | 1056 | (20.7) | 1317 | (20.7) | |
| 60-69 | 663 | (19.7) | 1236 | (36.7) | 713 | (21.2) | 756 | (21.2) | |
| ≥70 | 957 | (26.5) | 1294 | (35.9) | 709 | (19.7) | 646 | (19.7) | |
| Race | <0.0001 | ||||||||
| White | 2872 | (23.2) | 4628 | (37.4) | 2381 | (19.3) | 2487 | (20.1) | |
| Black | 590 | (12.8) | 1479 | (31.8) | 1207 | (25.9) | 1377 | (29.6) | |
| Hispanic | 346 | (13.7) | 764 | (30.3) | 499 | (19.8) | 917 | (36.3) | |
| Other | 187 | (18.1) | 323 | (31.3) | 287 | (27.8) | 236 | (22.9) | |
| Unknown | 32 | (17.2) | 43 | (23.1) | 73 | (39.3) | 38 | (20.4) | |
| Insurance status | <0.0001 | ||||||||
| Commercial | 356 | (13.6) | 792 | (30.1) | 606 | (23.1) | 874 | (33.3) | |
| Medicare | 1570 | (20.8) | 2798 | (37.1) | 1619 | (21.5) | 1552 | (20.6) | |
| Medicaid | 753 | (15.6) | 1578 | (32.7) | 1064 | (22.0) | 1434 | (29.7) | |
| Uninsured | 1227 | (26.1) | 1754 | (37.2) | 886 | (18.8) | 844 | (17.9) | |
| Other | 35 | (15.8) | 82 | (36.9) | 70 | (31.5) | 35 | (15.8) | |
| Unknown | 86 | (10.3) | 233 | (27.8) | 202 | (24.1) | 316 | (37.8) | |
| Region | <0.0001 | ||||||||
| Northeast | 644 | (14.2) | 1845 | (40.8) | 1092 | (24.1) | 942 | (20.8) | |
| Midwest | 1367 | (27.7) | 1683 | (34.1) | 896 | (18.2) | 985 | (20.0) | |
| South | 1293 | (15.6) | 2892 | (34.8) | 1642 | (19.8) | 2484 | (29.9) | |
| West | 723 | (24.1) | 817 | (27.2) | 817 | (27.2) | 644 | (21.5) | |
| Location | <.0001 | ||||||||
| Metro | 2957 | (18.1) | 5560 | (34.1) | 3625 | (22.2) | 4177 | (25.6) | |
| Urban | 797 | (25.9) | 1155 | (37.5) | 475 | (15.4) | 654 | (21.2) | |
| Rural | 95 | (27.8) | 126 | (36.8) | 64 | (18.7) | 57 | (16.7) | |
| Unknown | 178 | (17.4) | 396 | (38.7) | 283 | (27.6) | 167 | (16.3 | |
| Hospital type | <.0001 | ||||||||
| Community cancer program |
3342 | (32.7) | 4470 | (43.7) | 1469 | (14.4) | 942 | (9.2) | |
| Academic | 513 | (5.1) | 2642 | (26.1) | 2880 | (28.4) | 4099 | (40.5) | |
| Other | 172 | (42.1) | 125 | (30.6) | 98 | (24.0) | 14 | (3.4) | |
| Histology | 0.7496 | ||||||||
| Squamous | 3271 | (19.3) | 5958 | (35.1) | 3620 | (21.3) | 4144 | (24.4) | |
| Adenocarcinoma | 343 | (20.0) | 597 | (34.9) | 371 | (21.7) | 401 | (23.4) | |
| Adenosquamous | 115 | (19.8) | 185 | (31.9) | 134 | (23.1) | 146 | (25.2) | |
| Other | 298 | (20.1) | 497 | (33.6) | 322 | (21.7) | 364 | (24.6) | |
| Grade | 0.0090 | ||||||||
| 1 | 180 | (20.2) | 308 | (34.6) | 201 | (22.6) | 202 | (22.7) | |
| 2 | 1212 | (19.2) | 2225 | (35.2) | 1318 | (20.8) | 1574 | (24.9) | |
| 3 | 1462 | (20.2) | 2486 | (34.3) | 1491 | (20.6) | 1816 | (25.0) | |
| Unknown | 1173 | (18.7) | 2218 | (35.3) | 1437 | (22.8) | 1463 | (23.3) | |
| Stage | <.0001 | ||||||||
| IIB | 1426 | (17.4) | 2852 | (34.9) | 1812 | (22.2) | 2085 | (25.5) | |
| IIIA | 198 | (23.1) | 304 | (35.4) | 161 | (18.7) | 196 | (22.8) | |
| IIIB | 1955 | (19.5) | 3496 | (34.8) | 2124 | (21.2) | 2459 | (24.5) | |
| IVA | 448 | (26.4) | 585 | (34.5) | 350 | (20.6) | 315 | (18.6) | |
| Brachytherapy | <.0001 | ||||||||
| No | 2284 | (25.9) | 2937 | 33.3) | 1531 | (17.3) | 2080 | (23.6) | |
| Yes | 1743 | (14.6) | 4300 | 36.0) | 2916 | (24.4) | 2975 | (24.9) | |
| Chemotherapy | <.0001 | ||||||||
| No | 1010 | (24.5) | 1426 | 34.6) | 847 | (20.5) | 840 | (20.4) | |
| Yes | 2967 | (18.1) | 5730 | 34.9) | 3554 | (21.7) | 4156 | (25.3) | |
| Unknown | 50 | (21.2) | 81 | 34.3) | 46 | (19.5) | 59 | (25.0) | |
Predictors of Treatments and Treatment Center
In a multivariable Logistic model, patients treated at academic centers were more likely to receive care at a high-volume facility (OR=7.41; 95% CI, 6.82-8.05) (Supplemental table 2). Patients with private insurance were less likely to receive treatment at a high-volume center than Medicaid/Medicare recipients and women without insurance coverage. Hispanic women were more likely to receive treatment at a high-volume center while women aged 70 years or above were less likely to choose a high volume hospital. Patients treated in the Northeast were less likely to receive care at a high volume hospital than those treated in other areas. In a series of models examining the association between volume and quality of care, we noted that there was no association between center volume and receipt of chemotherapy (RR=1.003; 95% CI, 0.998-1.008); however, increasing center volume was associated with a slightly greater likelihood of receiving brachytherapy (RR=1.026; 95% CI, 1.013-1.039) (Table2). These findings were similar in a sensitivity analyses performed using models that were based on cluster weighted GEE (Supplemental table 3).
Table 2.
Mixed effects models of receipt of evidence-based treatments.
| Chemotherapy | Brachytherapy | |
|---|---|---|
| Annualized hospital volume | 1.003 (0.998-1.008) | 1.026 (1.013-1.039)* |
| Year of diagnosis | ||
| 1998 | Referent | Referent |
| 1999 | 1.90 (1.70- 2.11)** | 0.91 (0.83- 1.01) |
| 2000 | 1.99 (1.78- 2.21)** | 0.88 (0.79- 0.98)* |
| 2001 | 2.01 (1.80- 2.24)** | 0.80 (0.72- 0.89)** |
| 2002 | 1.98 (1.75- 2.25)** | 0.33 (0.27- 0.40)** |
| 2003 | 2.05 (1.85- 2.26)** | 0.80 (0.72- 0.88)** |
| 2004 | 2.05 (1.85- 2.27)** | 0.80 (0.73- 0.89)** |
| 2005 | 2.10 (1.90- 2.32)** | 0.83 (0.75- 0.91)* |
| 2006 | 2.16 (1.95- 2.38)** | 0.82 (0.74- 0.90)* |
| 2007 | 2.11 (1.91- 2.33)** | 0.83 (0.75- 0.91)* |
| 2008 | 2.15 (1.95- 2.38)** | 0.85 (0.77- 0.93)* |
| 2009 | 2.18 (1.98- 2.41)** | 0.89 (0.81- 0.98)* |
| 2010 | 2.20 (2.00- 2.43)** | 0.86 (0.78- 0.94)* |
| 2011 | 2.16 (1.95- 2.38)** | 0.86 (0.78- 0.95)* |
| Age | ||
| <40 | Referent | Referent |
| 40-49 | 0.99 (0.94- 1.03) | 0.96 (0.91- 1.02) |
| 50-59 | 0.96 (0.92- 1.01) | 0.94 (0.89- 0.99)* |
| 60-69 | 0.94 (0.89- 0.99)* | 0.94 (0.88- 1.01) |
| ≥70 | 0.67 (0.62- 0.72)** | 0.77 (0.71- 0.84)** |
| Race | ||
| White | Referent | Referent |
| Black | 0.97 (0.93- 1.01) | 0.97 (0.92- 1.02) |
| Hispanic | 1.02 (0.97- 1.07) | 1.02 (0.96- 1.09) |
| Other | 1.02 (0.95- 1.10) | 1.04 (0.95- 1.13) |
| Unknown | 0.98 (0.84- 1.15) | 0.97 (0.80- 1.18) |
| Insurance status | ||
| Commercial | Referent | Referent |
| Medicare | 0.93 (0.88- 0.98)* | 0.92 (0.86- 0.98)* |
| Medicaid | 0.96 (0.92- 1.00) | 0.92 (0.88- 0.97)* |
| Uninsured | 0.98 (0.94- 1.03) | 0.91 (0.85- 0.96)* |
| Other | 0.98 (0.85- 1.14) | 0.99 (0.83- 1.17) |
| Unknown | 0.95 (0.88- 1.04) | 0.83 (0.74- 0.93)* |
| Region | ||
| Northeast | Referent | Referent |
| Midwest | 1.01 (0.96- 1.05) | 0.99 (0.91- 1.08) |
| South | 0.96 (0.92- 1.00) | 0.92 (0.85- 1.00) |
| West | 1.00 (0.95- 1.05) | 0.83 (0.75- 0.92)* |
| Location | ||
| Metro | Referent | Referent |
| Urban | 0.99 (0.95- 1.04) | 1.01 (0.95- 1.07) |
| Rural | 1.02 (0.91- 1.15) | 1.11 (0.97- 1.28) |
| Unknown | 1.01 (0.95- 1.09) | 1.03 (0.95- 1.13) |
| Hospital type | ||
| Community cancer program | Referent | Referent |
| Academic | 1.01 (0.97- 1.04) | 1.03 (0.96- 1.11) |
| Other | 0.93 (0.81- 1.06) | 0.97 (0.82- 1.14) |
| Histology | ||
| Squamous | Referent | Referent |
| Adenocarcinoma | 0.98 (0.93- 1.04) | 0.91 (0.85- 0.98)* |
| Adenosquamous | 1.02 (0.93- 1.12) | 1.03 (0.92- 1.14) |
| Other | 0.98 (0.93- 1.05) | 0.91 (0.85- 0.99)* |
| Grade | ||
| 1 | Referent | Referent |
| 2 | 0.99 (0.92- 1.08) | 0.97 (0.89- 1.07) |
| 3 | 0.99 (0.92- 1.07) | 0.94 (0.86- 1.04) |
| Unknown | 0.99 (0.91- 1.07) | 0.97 (0.88- 1.06) |
| Stage | ||
| IIB | Referent | Referent |
| IIIA | 0.98 (0.90- 1.06) | 0.82 (0.74- 0.91)* |
| IIIB | 1.02 (0.98- 1.05) | 0.83 (0.80- 0.87)** |
| IVA | 0.98 (0.92- 1.04) | 0.45 (0.41- 0.49)** |
| Brachytherapy | ||
| No | Referent | - |
| Yes | 1.10 (1.07- 1.14)** | - |
| Chemotherapy | ||
| No | - | Referent |
| Yes | - | 1.29 (1.22- 1.36)** |
| Unknown | - | 0.92 (0.75- 1.14) |
Risk ratio (95% confidence interval).
P<0.05,
P<0.0001
Survival Outcomes
The survival cohort consisted of 12,048 patients from 1008 hospitals (Supplemental table 1). The median follow-up was 24.2 months (IQR, 11.1-61.4). At last follow-up, 7452 (61.9%) patients had died while 4596 (38.1%) were alive. The overall observed survival rate was 77.3% (95% CI, 76.5-78.0%) at 1-year, 46.6% (95% CI, 45.6-47.5%) at 3-years, and 36.5% (95% CI, 35.6-37.4%) at 5-years.
In a mixed-effects Cox model accounting for clinical and demographic variables, the specific hospital in which a patient received care was the most important predictor of survival (rank 1, P<0.0001) (Table 3). Stage (rank 2, P<0.0001) and year of diagnosis (rank 3, P<0.0001) were the most important predictors of survival following hospital effect. Other factors statistically significantly associated with survival included age at diagnosis, race, tumor grade, histology, and insurance status (P<0.0001 for all). In this model, annualized hospital volume was associated with survival (HR=0.98; 95% CI, 0.97-0.99; P=0.01, rank 9).
Table 3.
Significance of each variable included in the mixed effects Cox models.
| Clinical model | Treatment-adjusted model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Covariates | Rank | AIC | LRT | P-value | Covariates | Ran k |
AIC | LRT | P-value |
| Hospital | 1 | 130132 | 65.61 | <0.0001 | Hospital | 1 | 129913.1 | 80.4 | <0.0001 |
| Stage | 2 | 129653.44 | 820.99 | <0.0001 | Stage | 2 | 129068.52 | 682.85 | <0.0001 |
| Year | 3 | 129253.6 | 425.15 | <0.0001 | Year | 3 | 128732.12 | 356.45 | <0.0001 |
| Age | 4 | 128988.4 | 151.94 | <0.0001 | Brachytherapy | 4 | 128670.9 | 281.23 | <0.0001 |
| Race | 5 | 128926.02 | 89.55 | <0.0001 | Chemotherapy | 5 | 128536.3 | 148.64 | <0.0001 |
| Insurance status | 6 | 128911.56 | 77.1 | <0.0001 | Race | 6 | 128473.24 | 89.57 | <0.0001 |
| Histology | 7 | 128890.16 | 51.7 | <0.0001 | Age | 7 | 128461.02 | 77.36 | <0.0001 |
| Grade | 8 | 128888.34 | 49.88 | <0.0001 | Grade | 8 | 128439.54 | 53.88 | <0.0001 |
| Hospital volume | 9 | 128849.1 | 6.65 | 0.01 | Insurance status | 9 | 128436.56 | 54.89 | <0.0001 |
| Hospital type | 10 | 128841.56 | 1.11 | 0.57 | Histology | 10 | 128431.18 | 45.51 | <0.0001 |
| Hospital region | 11 | 128840.86 | 2.41 | 0.49 | Hospital volume | 11 | 128391 | 1.32 | 0.25 |
| Hospital type | 12 | 128388.38 | 0.71 | 0.70 | |||||
| Hospital Region | 13 | 128386.08 | 0.41 | 0.94 | |||||
AIC, Alkaike information criterion. LRT, likelihood ratio test.
The null model has an AIC of 114468.18. AIC was calculated as minus twice log likelihood plus 2 df. The LRT compares the full model (including all variables) with a reduced model omitting 1 variable at a time. The higher the AIC, the greater importance of the omitted variable.
In a mixed-effects Cox model in which metrics of quality of treatment were added, the hospital effect (rank=1, P<0.0001) remained the most important predictor of survival, while hospital volume was no longer associated with survival (HR=0.99; 95% CI, 0.98-1.00; P=0.25). In this model, receipt of brachytherapy (rank=4), and chemotherapy (rank=5) also contributed substantially to survival (all P<0.0001). The importance of the other covariates was similar to the clinical model.
Individual Hospital Effects
Based on the mixed-effects treatment-adjusted Cox model, there was a statistically significant hospital effect on survival that followed a normal distribution with a mean of 0 and a SD of 0.22 (95% CI, 0.19 to 0.26). The association between the specific hospital in which a patient was treated and mortality expressed as an HR, ranged from 0.66 (95% CI, 0.60 to 0.72) to 1.53 (95% CI, 1.40 to 1.67) across the individual hospitals (Figure 1A). Based on the treatment-adjusted model, 1-, 3- and 5-year survival probabilities were expected to vary by center from 12.3% to 92.5%, 0.1% to 77.3%, and 0% to 70.2%, respectively. When stratified by volume quartiles, there remained significant variability between the hospitals within each quartile for mortality (Figure 1B, Supplemental Figure 1).
Figure 1.
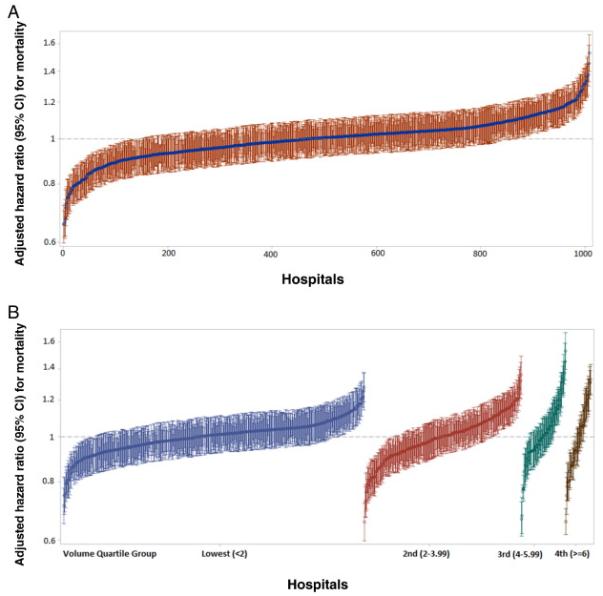
A. Adjusted hazard ratio for death for each hospital ranked by lowest (0.66) to highest (1.53) hazard ratio with 95% confidence intervals. Hazard ratios derived from treatment-adjusted models and includes all clinical and demographic variables as well as treatment metrics.
B. Adjusted hazard ratio for death for each hospital stratified by annualized volume quartiles. Within each quartile hospitals are ranked from the lowest to highest hazard ratio with 95% confidence intervals.
Sensitivity Analyses
A series of sensitivity analyses were performed in which hospital volume was modeled as a categorical variable and the measurement period for volume altered (Table 4). When annualized volume was stratified into quartiles, there was no association between volume and survival. Similarly, when hospital volume was measured as the volume in the year prior to treatment of a given patient, there was no association between volume and survival. When volume was estimated as the volume during the year in which the index patient was treated, survival was improved at the highest volume centers (HR=0.90; 95% CI, 0.82-0.97) and when volume was a continuous variable (HR=0.99; 95% CI, 0.98-1.00). Similarly, there was no association between volume and survival when patients with stage IV tumors were removed, when the analysis was limited to women with stage IIIB neoplasms or when very low volume hospitals (≤2 annualized cases) were excluded. Finally, we analyzed patients who received a portion of their treatment at multiple facilities. In this analysis, our findings were largely unchanged.
Table 4.
Hazard ratios for death based on different modeling techniques for hospital volume.
| Annualized volume |
Previous year volume |
Current year volume | |
|---|---|---|---|
| Continuous volume | 0.99 (0.98-1.00) | 1.00 (0.99-1.01) | 0.99 (0.98-1.00)* |
| Volume cut-points | |||
| Lowest | Referent | Referent | Referent |
| Second | 1.01 (0.92-1.11) | 0.97 (0.90-1.06) | 0.96 (0.89-1.05) |
| Third | 0.97 (0.90-1.05) | 0.94 (0.85-1.04) | 0.95 (0.88-1.02) |
| Highest | 0.91 (0.82 -1.02) | 0.94 (0.85-1.03) | 0.90 (0.82-0.97)* |
Hazard ratio (95% confidence interval).
P<0.05.
Adjusted for clinical and demographic characteristics and quality of treatment.
Discussion
For women with locally advanced cervical cancer, our findings suggest that the center in which treatment is delivered is highly associated with survival; however, hospital volume has little influence on outcomes. While adherence to evidence-based treatment recommendations is associated with improved survival, this alone does not explain the hospital variability in survival rates.
We noted substantial center-specific variation in survival for cervical cancer patients treated with primary radiotherapy. These findings imply that outcome is highly dependent upon the particular hospital in which a woman receives care, yet it is difficult to define specific hospital characteristics that are associated with improved outcomes. There is growing recognition of the importance of between-hospital variation in medicine.21,26-28 An analysis of U.S. lung transplant centers found a substantial center-specific effect on survival that was not explained by volume or other characteristics.21 Similarly, a study examining radioactive iodine use for thyroid cancer noted that 29% of the variation in treatment allocation was unexplained by measurable patient, tumor, and hospital factors.26 The center-specific effects we noted were independent of volume and remained even after adjustment for metrics of quality of care for advanced stage cervical cancer.
Hospital volume had a minimal impact on outcomes for stage IIB-IVA cervical cancer. The association between higher physician and hospital volume and improved outcomes has been shown for a number of high-risk surgical procedures.7-12 An analysis of 2.5 million patients who underwent one of 14 cancer resections or cardiovascular procedures noted that mortality for each procedure decreased with increasing surgical volume.9 Similar finding have been noted for surgeon volume, which may also account for some of the influence of hospital volume on outcome.10 The association with volume and outcomes is most pronounced for high-risk procedures that are associated with substantial morbidity, but appears to be more modest for lower risk operations.29,30
To date there have been few studies that have explicitly examined the influence of center volume on outcomes in radiation oncology. Given that primary radiotherapy is technically demanding and requires specialized expertise in planning and delivery, there is a strong rationale for why hospital volume would influence outcomes. Further, the incidence of invasive cervical cancer has declined in the U.S. and many centers treat a very small number of women annually for this disease. Despite this rationale, we found no association between center volume and survival.
While studies of procedural volume are associated with a number of methodological challenges, we attempted to rigorously address these limitations.18,31 First, to account for hospital-level clustering, our primary analysis relied on mixed-effects Cox regression models that included a hospital-specific random effect.19,21 Second, we modeled volume in a number of ways including as both a continuous and categorical variables and we estimated volume using a number of alternative methods including annualized volume, prior year volume and current year volume.18,32 Finally, a number of sensitivity analyses were performed examining various subsets of patients. Consistently, we noted that volume had a minimal impact on survival either with or without adjustment for quality of treatment while there remained a strong center-specific association with survival. These analyses may explain in part why our findings differ from prior analyses.33
Encouragingly, the use of chemotherapy increased over time, however, there was a pronounced decline in the use of brachytherapy in the more recent years of study. While higher hospital volume was associated with receipt of brachytherapy, there was no relationship between volume and use of chemotherapy. Prior studies have often shown an association between physician and hospital volume and compliance with evidence-based treatment recommendations.14,34,35 More importantly, in a number of clinical scenarios, adherence to best practice guidelines can mitigate center-specific and volume associated influences on outcomes.36,37 While we noted that the use of evidence-based treatments was associated with survival, adherence did not blunt the strong center-specific influence on survival.
Our findings should be interpreted in light of a number of limitations. While NCDB data capture is a highly structured process and subject to rigorous quality control, some aspects of treatment, particularly use of brachytherapy, may be under captured.38,39 However, even if some aspects of care are under coded, this is likely to be balanced across hospitals and unlikely to impact our findings. We were unable to capture a number of details of the radiation delivered including fields and total fractions delivered. However, a priori we hypothesized that if high volume centers had improved survival that it was likely mediated through higher quality radiation (appropriate doses, fields, timing, and delivery). It would be of great interest to further examine whether both volume and between-hospital variation are associated with specific attributes of radiation delivery. Likewise, other factors that may have influenced treatment and outcome, such as comorbidity, were not available for the entire study period. While our primary analysis focused on survival, examining variation in short and long-term toxicities would be of great interest. Given the large sample size small differences that are statistically significant may not be clinically meaningful. Finally, as with any study of administrative data, we are unable to capture individual patient and physician preferences that influenced treatment selection.
These findings have a number of important policy implications for the treatment of locally advanced cervical cancer. First, hospital volume does not appear to be a useful structural metric of quality. Second, given the strong center-specific variation in survival, efforts should be focused on elucidating why these differences exist. At present, strategies to inform patient choice and guide referral to high performing centers are limited. More structured quality initiatives to report hospital outcomes for cervical cancer and, perhaps, public reporting of risk-adjusted data may improve informed decision making of patients and drive quality improvement at low performing hospitals.40 Lastly, the highly disparate outcomes across hospitals suggest that standardizing the care of women with locally advanced cervical cancer may have a meaningful impact on survival.
Supplementary Material
Highlights.
-
-
For locally advanced cervical cancer, hospital volume has a minimal impact on outcome.
-
-
The specific center in which care is delivered is strongly associated with survival.
Acknowledgements
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 4.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 5.NCI Issues Clinical Announcement on Cervical Cancer [Accessed October, 12, 2014];Chemotherapy Plus Radiation Improves Survival. at http://www.nih.gov/news/pr/feb99/nci-22.htm.
- 6.NCCN . Clinical Practice Guidelines in Oncology: Cervical Cancer. Version 2.2015 2014. [Google Scholar]
- 7.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 8.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 11.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. Jama. 2000;284:3028–35. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. Effect of surgical volume on morbidity and mortality of abdominal hysterectomy for endometrial cancer. Obstet Gynecol. 2011;117:1051–9. doi: 10.1097/AOG.0b013e31821647a0. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Herzog TJ, Siddiq Z, et al. Failure to rescue as a source of variation in hospital mortality for ovarian cancer. J Clin Oncol. 2012;30:3976–82. doi: 10.1200/JCO.2012.43.2906. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Lewin SN, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Ann Surg. 2011;253:1140–6. doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 15.Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin. 2009;59:192–211. doi: 10.3322/caac.20018. [DOI] [PubMed] [Google Scholar]
- 16.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston EH, Cao J. Procedure volume as a predictor of surgical outcomes. Jama. 2010;304:95–7. doi: 10.1001/jama.2010.905. [DOI] [PubMed] [Google Scholar]
- 19.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139:658–65. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 20.Williamson JM, Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics. 2003;59:36–42. doi: 10.1111/1541-0420.00005. [DOI] [PubMed] [Google Scholar]
- 21.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. Jama. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 22.Hosmer D, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley & Sons; New York, NY: 1999. [Google Scholar]
- 23.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 24.Grambsch P, Therneau ™ . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 25. [Accessed October, 18, 2014];Mixed effects Cox models. at http://cran.r-project.org/web/packages/coxme/vignettes/coxme.pdf.
- 26.Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. Jama. 2011;306:721–8. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121:717–26. doi: 10.1097/AOG.0b013e3182887a47. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker SG, Friedman AM, Cleary KL, et al. Patterns of use and predictors of receipt of antibiotics in women undergoing cesarean delivery. Obstet Gynecol. 2014;124:338–44. doi: 10.1097/AOG.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MM, Ng SC, Simons JP, Csikesz NG, Shah SA, Tseng JF. Predictors of major complications after laparoscopic cholecystectomy: surgeon, hospital, or patient? J Am Coll Surg. 2010;211:73–80. doi: 10.1016/j.jamcollsurg.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 30.Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341–7. doi: 10.1097/AOG.0b013e3181fca8c5. [DOI] [PubMed] [Google Scholar]
- 31.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 32.Livingston EH, Elliott AC, Hynan LS, Engel E. When policy meets statistics: the very real effect that questionable statistical analysis has on limiting health care access for bariatric surgery. Arch Surg. 2007;142:979–87. doi: 10.1001/archsurg.142.10.979. [DOI] [PubMed] [Google Scholar]
- 33.Lin JF, Berger JL, Krivak TC, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol. 2014;132:416–22. doi: 10.1016/j.ygyno.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med. 2013;173:559–68. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JD, Hershman DL, Shah M, et al. Quality of perioperative venous thromboembolism prophylaxis in gynecologic surgery. Obstet Gynecol. 2011;118:978–86. doi: 10.1097/AOG.0b013e31822c952a. [DOI] [PubMed] [Google Scholar]
- 36.Auerbach AD, Hilton JF, Maselli J, Pekow PS, Rothberg MB, Lindenauer PK. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696–704. doi: 10.7326/0003-4819-150-10-200905190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92:2643–52. doi: 10.2106/JBJS.I.01477. [DOI] [PubMed] [Google Scholar]
- 38.Smith GL, Eifel PJ. Trends in the utilization of brachytherapy in cervical cancer in the United States. In regard to Han et al. Int J Radiat Oncol Biol Phys. 2014;88:459–60. doi: 10.1016/j.ijrobp.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Han K, Milosevic M, Fyles A, Viswanathan AN. In reply to Smith and Eifel. Int J Radiat Oncol Biol Phys. 2014;88:460–1. doi: 10.1016/j.ijrobp.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–96. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


