Abstract
The use of mind-body therapies, including Tai Chi, Qigong, yoga, and meditation, has grown steadily in recent years. These approaches have been shown to be effective in reducing symptoms and improving quality of life, and research has begun to examine the impact of these therapies on biological processes, including inflammation. A review of 26 randomized controlled trials was conducted to describe the effects of mind-body therapies (MBTs) on circulating, cellular, and genomic markers of inflammation. This qualitative evaluation showed mixed effects of MBTs on circulating inflammatory markers, including CRP and IL-6, and on measures of stimulated cytokine production. More consistent findings were seen for genomic markers, with trials showing decreased expression of inflammation-related genes and reduced signaling through the proinflammatory transcription factor NF-κB. Potential mechanisms for these effects are discussed, including alterations in neuroendocrine, neural, and psychological and behavioral processes.
Keywords: Tai Chi, Qigong, Yoga, Meditation, Inflammation, Review
Introduction
Mind-body therapies, or MBTs, have been broadly defined as a group of therapies that emphasize use of the brain in conjunction with the body to assist the healing process (Spencer, 2003). These therapies, the majority of which are based on ancient practices and traditions, are believed to have beneficial effects on mental and physical health and are widely used to manage symptoms and improve well-being. Indeed, in a nationwide survey of community-dwelling adults in the US conducted in 2007, 19% reported that they had used at least one mind-body therapy in the past year, and rates are even higher among clinical populations (Barnes, Bloom, & Nahin, 2008). Over the past two decades, the efficacy of these approaches has been subjected to empirical scrutiny through randomized controlled trials conducted in clinical and non-clinical populations. Meta-analyses of these trials suggest that MBTs are effective in reducing symptoms and improving quality of life and certain functional outcomes (Bussing, Ostermann, Ludtke, & Michalsen, 2012; Goyal et al., 2014; Wang, Collet, & Lau, 2004).
Alterations in inflammatory processes are thought to play a role in many of the symptoms and conditions that are responsive to MBTs, including fatigue, depression, and pain (Irwin & Cole, 2011). Given the importance of inflammation on these patient-reported outcomes, a growing number of trials have evaluated effects of MBTs on markers of inflammation. The goal of this review is to qualitatively evaluate the evidence that MBTs lead to changes in these markers, and to discuss potential mechanisms and issues in this emerging area of research. We include results of randomized controlled trials that assessed inflammatory cytokine activity at multiple levels, including circulating, cellular, and genomic markers of inflammation.
We focus here on four types of MBTs that have received considerable research attention and are widely available to clinical and community populations: Tai Chi, Qigong, yoga, and meditation. Tai Chi and Qigong are practices from traditional Chinese medicine that combine specific movements or postures, coordinated breathing, and mental focus. Yoga has its origins in ancient Indian philosophy; as practiced in the West, it typically includes physical postures, breathing, and meditation or relaxation, though there is considerable variability across different schools of yoga and specific interventions. Meditation refers to a broad range of practices that involve training the mind, typically to focus attention. In particular, mindfulness meditation teaches individuals to bring attention to present moment experiences with openness, curiosity, and non-judgment.
To identify studies for inclusion in this qualitative review, we searched MEDLINE (from 1946), through November 1, 2014. Searches were limited to human studies and the English language. We searched using the following terms: mind-body therapies, tai chi, qigong, meditation, mindfulness, or yoga; and inflammation, cytokines, or proinflammatory. In addition, we screened the reference lists of selected reviews and primary articles for additional publications.
Effects of MBTs on circulating markers of inflammation
CRP
The most common inflammatory marker assessed in the MBT trials reviewed here is C reactive protein (CRP), a well-established marker of inflammatory activity. We identified 14 RCTs that reported effects of a MBT on CRP, described in Table 1. The majority of these trials evaluated Tai Chi or Qigong (n=7), with additional studies of yoga (n=3) and meditation (n=4). Various control conditions were used including health education, usual care or wait-list, aerobic exercise, and cognitive behavioral therapy (CBT). All studies assessed CRP at pre- and post-treatment, and several also included follow-ups from 6 weeks to 3 months post-treatment, although one had a one-year post-treatment follow-up (Irwin et al., 2014, 2015).
Table 1.
Randomized controlled trials evaluating the effects of mind-body therapies on circulating markers of inflammation
| Study | Population | N, % Female | Mean age (range) | Intervention Duration, Type | Intervention Frequency, Style | Control Group | Finding |
|---|---|---|---|---|---|---|---|
| Circulating Markers of Inflammation | |||||||
| Bower 2014 | Breast cancer survivors with fatigue | 31(100%) | 54 (40–65) | 12 weeks, Yoga | 90 min, 2 ×/week Iyengar yoga | Health education | sTNFRII − (stable in yoga vs. increase in controls) IL-1RA ns CRP ns IL-6 ns |
| Bower 2014 | Breast cancer survivors | 71 (100%) | 46.9 (28–60) | 6 weeks, Meditation | 2 hrs, 1×/week Mindful Awareness Practices | Wait-list control | CRP ns IL-6 ns sTNFRII ns |
| Chen 2006 | Healthy middle-aged women | 87 (100%) | 45 | 12 weeks, Qi Gong | 3 ×/week, Baduanjin | No specified activities | IL-6 − |
| Chen 2010 | Adults with diagnosis of Type II diabetes and BMI 30–35 | 104 (43%) | 58 | 12 weeks, Tai Chi | 60 min, 3 ×/week, Chen-Style Tai Chi Chuan 99-form | Conventional aerobic exercise | CRP − |
| Creswell 2012 | Healthy older adults | 40 (80%) | 65 (55–85) | 8 weeks, Meditation | 2 hrs, 1 ×/week Mindfulness based stress reduction | Wait-list control | CRP − (trend) IL-6 ns |
| Irwin 2012 | Healthy older adults | 83 (63%) | 70 (59–86) | 16 weeks, Tai Chi | 40 min, 3 ×/week Tai Chi Chih | Health education | IL-6 ns (marg decrease in TCC group with high IL-6 at baseline) CRP ns IL-1ra ns sIL-6R ns sICAM ns IL-18 ns |
| Irwin 2014 | Breast cancer survivors with insomnia | 90 (100%) | 59.8 (42–83) | 12 weeks, Tai Chi | 2 hrs, 1 ×/week Tai Chi Chih | Cognitive behavioral therapy | CRP ns |
| Irwin 2015 | Older adults with insomnia | 123 (73%) | 65 (55–85) | 16 weeks, Tai Chi | 2 hrs, 1 ×/week Tai Chi Chih | Health education; Cognitive behavioral therapy | CRP − |
| Lavretsky 2011 | Elderly adults with major depression | 73 (62%) | 71 | 10 weeks, Tai Chi Chih | 120 min, 1 ×/week Tai Chi Chih | Health education | CRP − |
| Malarkey 2013 | Adults with elevated CRP >3.0 mg/ml, cardiovascular risk | 186 (88%) | 50 | 8 weeks, Meditation | 1 hr, 1 ×/week, Mindfulness based stress reduction | Lifestyle education | CRP − (trend) IL-6 ns |
| Janelsins 2011 | Breast cancer survivors | 19 (100%) | 53 (43–78) | 12 weeks, Tai Chi | 60 min, 3 × /week Yang style Tai Chi | Psychosocial support group | IL-6 ns |
| Manzaneque 2009 | Healthy college students | 39, (87%) | 18–21 | 4 weeks, Qi Gong | 30 min, 3 ×/week Ba Duan Jin | No specified activities | TNF ns |
| Oh 2008 | Cancer patients | 30 (75%) | 54 (35–75) | 8 weeks, QiGong | 2 ×/week Medical QiGong | Normal activities | CRP − (attenuated increase vs. controls) |
| Oh 2010, 2012 | Cancer patients | 162 (57%) | 60 (31–86) | 10 weeks, Qi Gong | 90 min, 1–2 ×/week, Medical Qi Gong | Usual care | CRP − |
| Oken 2010 | Dementia caregivers | 31 (81%) | 65 (45–85) | 7 weeks, Meditation | 90 min, 1× per week Mindfulness based cognitive therapy | Education class; Respite only | CRP ns IL-6 ns TNF ns |
| Pace 2009 | Healthy college students | 61 (52%) | 19 (17–19) | 6 weeks, Meditation | 50 min, 2 ×/week Compassion meditation | Health discussion group | IL-6 ns |
| Pullen 2008 | Heart failure patients (Class I–III) | 19 (53%) | 51 | 8 weeks, Yoga | 70 min, 2 ×/week Hatha Yoga | Standard medical therapy | CRP − IL-6 − |
| Pullen 2010 | Heart failure patients (Class I–III) | 40 (43%) | 54 (31–76) | 8–10 weeks, Yoga | 60 min, 2 × /week Hatha Yoga | Standard medical therapy | CRP − IL-6 − |
| Rao 2008 | Breast cancer patients undergoing surgery | 69 (100%) | 49 | 4 weeks, Yoga | 4 sessions, individual practice Integrated Yoga | Counseling + exercise rehabilitation | TNF − (increase in controls) sIL-2R ns |
| Robins 2013 | Breast cancer patients undergoing chemotherapy | 145 (100%) | 50 (27–75) | 10 weeks, Tai Chi | 90 min, 1×/week | Spiritual growth group, usual care | Multiplex panel: IL-1β ns IL-2 ns IL-4 ns IL-5 ns IL-6 ns IL-7 ns IL-8 ns IL-10 ns IL-12 ns IL-13 ns IL-17 ns G-CSF ns GM-CSF ns IFN-γ ns MCP ns MCAF ns MIP-1β ns TNF ns |
Notes: The symbol “−” is used to indicate a significant decrease (or attenuated increase) in a particular inflammatory marker. “Trend” is used to indicate marginally significant findings (0.05 < p < .10), and “ns” is used to indicate non-significant findings.
A previous meta-analysis had reported that these various types of MBTs, typically lasting eight to twelve weeks, yielded decreases in circulating levels of CRP (Morgan, Irwin, Chung, & Wang, 2014). However, results inclusive of research published since that review show that findings for CRP were evenly split; seven of the trials yielded decreases in circulating levels of CRP, and seven yielded no significant changes in this marker. Hence, at best, it appears that MBTs have mixed effects on CRP, although this conclusion is tempered by the absence of a meta-analytic approach as compared to the prior review. There is some evidence that Tai Chi or Qigong, and possibly yoga, which incorporate physical activity components, are more likely to reduce levels of CRP, relative to meditation; the majority of studies using Tai Chi/Qigong or yoga showed decreases in CRP, whereas none of the meditation studies showed this effect (though two reported marginally significant reductions of CRP; Creswell et al., 2012; Malarkey, Jarjoura, & Klatt, 2013). Nevertheless, the number of studies categorized by treatment type is too small to make any conclusions about the relative benefit of one treatment or another.
Interestingly, patient type may be more relevant, as those studies that enrolled participants who were likely to have elevated levels of CRP at baseline due to depression, cancer diagnosis, diabetes, or cardiovascular disease appear to show decreases in CRP; such decreases in response to intervention were not found in studies that focused on healthy adults or healthy older adults for whom substantial medical co-morbidities were an exclusion criteria. The latter conclusion is supported by meta-analytic results from Morgan et al. (2014) who found that CRP was likely to be reduced following mind-body therapies in populations with disease conditions, but not in healthy persons.
However, even this conclusion is mixed. Bower et al. found no change in CRP in two trials conducted with breast cancer survivors; one used a 12-week yoga intervention for survivors with persistent fatigue (Bower et al., 2014) and the other used a 6-week mindfulness intervention for younger breast cancer survivors (Bower et al., 2015). Likewise, Irwin et al. in two different populations, breast cancer survivors (Irwin et al., 2014) and older adults with insomnia (Irwin et al., 2014; Irwin et al., 2015), found inconsistent results. Tai Chi yielded a decrease in CRP in older adults with insomnia when administered for 16 weeks (Irwin et al., 2014; Irwin et al., 2015), but not in breast cancer survivors with insomnia in which Tai Chi was administered for only 12 weeks (Irwin et al., 2014). Average levels of CRP were similar in these two independent samples suggesting that higher baseline levels in one vs. the other did not account for differential responses. Alternatively the duration of Tai Chi administration in the breast cancer survivors may have been too short to detect changes. CRP is regulated in part by IL-6, and Tai Chi-related reduction of IL-6 at 16 weeks (Irwin & Olmstead, 2012) is reportedly followed by reductions of CRP at 24 weeks (Lavretsky et al., 2011). Interestingly, we had previously reported that cognitive behavioral therapy for insomnia (CBT-I) induced a remission of insomnia in the older adults, and this remission was associated with sustained decreases in CRP during the one-year follow-up period (Irwin et al., 2014). Nevertheless, other studies have found decreases in CRP to occur as early as 8–10 weeks after administration of a mind-body treatment (e.g., Oh et al., 2008, 2010, 2012; Pullen et al., 2008, 2010). There is another issue related to the interpretation of these results. As noted in Table 1, some of the reported “decreases” in the intervention group are accounted for by increases in the control condition, either due to a confounding influence in the controls that produced the reported increase in CRP or that the intervention resulted in an attenuated age-related increase in inflammation; the latter seems a more tenuous explanation given that many of the interventions only lasted weeks to months.
IL-6
A total of 12 trials were identified which examined effects of a MBT on circulating concentrations of the proinflammatory cytokine IL-6, including studies using Tai Chi or Qigong (n=4), yoga (n= 3) and meditation (n=5). Again, a variety of controls were used such as wait list, education, or social support. Overall, it appears that IL-6 does not show any consistent changes following the administration of these various MBTs, with three studies showing modest decreases, and the remaining nine studies showing no change. Two of the three trials that found decreases in IL-6 were trials of yoga for patients with heart failure, a group with pronounced elevations in inflammation (Pullen et al., 2008, 2010). None of the Tai Chi/qigong or meditation trials yielded decreases in IL-6. However, it is notable that decreases in IL-6 were found among individuals with higher baseline levels of this marker in a trial of Tai Chi in healthy older adults (Irwin & Olmstead, 2012) and among individuals with more hours of meditation practice in trials of mindfulness (Bower et al., 2014) and compassion meditation (Pace et al., 2009).
TNF-α and other circulating inflammatory cytokines
Seven of the listed studies also evaluated the effects of mind-body therapies on other inflammatory markers such as TNF-α IL-1 receptor antagonist (IL-1RA), and IL-18. The limited data for any of these other circulating outcomes do not allow for any conclusions, although two of the studies that administered yoga and measured TNF or its soluble receptor found stable levels in the yoga group vs. controls (Bower et al., 2014; Rao et al., 2008). Both of these trials focused on breast cancer patients and one specifically targeted women with cancer-related fatigue, who have previously been shown to have elevated concentrations of soluble TNF receptor type II (sTNF-RII; Bower et al., 2011).
Effects of MBTs on cellular markers of inflammation
Systemic changes in circulating markers of inflammation might be due to effects of MBTs on the release of cytokines such as IL-6 from non-immune sources such as adipose tissue. To evaluate whether mind-body therapies have specific effects on immune cells, which might account for increases in circulating markers of inflammation, various cellular assays have been used to determine changes in stimulated production of IL-6, TNF, and IL-1 before and after administration of Tai Chi (n=3), yoga (n=1), and meditation (n=2). The total number of studies is small (n=6), and the findings are again mixed, which might be due to differences in assay methods as well as length of treatment administration and/or follow-up duration. For example, 4 studies evaluated the production of proinflammatory cytokines using ex vivo methods in whole blood or mixed peripheral blood mononuclear cells (Elsenbruch et al., 2005; Kiecolt-Glaser et al., 2014; McCain et al., 2008; Zautra et al., 2008), whereas 2 studies examined the intracellular production of IL-6 and TNF at the level of the monocyte, the primary source of proinflammatory cytokines (Irwin et al., 2014; Irwin et al., 2015). In addition, some studies have focused on changes immediately after 8–10 weeks of treatment (Elsenbruch et al., 2005; McCain et al., 2008), whereas other studies have examined changes at follow-ups lasting 3 months to one year post-treatment (Irwin et al., 2014; Irwin et al., 2015; Kiecolt-Glaser et al., 2014). Given the varying approaches and findings from these different studies, results from the individual trials are reviewed below, stratified by type of assay method. Studies are also summarized in Table 2.
Table 2.
Randomized controlled trials evaluating the effects of mind-body therapies on cellular production of markers of inflammation
| Study | Population | N, % Female | Mean age (range) | Intervention Duration, Type | Intervention Frequency, Style | Control Group | Finding |
|---|---|---|---|---|---|---|---|
| Cellular Markers of Inflammation | |||||||
| Elsenbruch 2005 | Adults with ulcerative colitis | 30 (67%) | 42 | 10 weeks, Meditation | 6 hrs, 1×/week Mindfulness based stress reduction program | Wait-list control | TNF ns |
| Irwin 2014 | Breast cancer survivors with insomnia | 90 (100%) | 59.8 (42–83) | 12 weeks, Tai Chi | 2 hrs, 1×/week Tai Chi Chih | Cognitive behavioral therapy | IL-6 − TNF − |
| Irwin 2015 | Older adults with insomnia | 123 (73%) | 65 (55–85) | 16 weeks, Tai Chi | 2 hrs, 1×/week Tai Chi Chih | Health education; Cognitive behavioral therapy | IL-6 − TNF − |
| Kiecolt-Glaser 2014 | Breast cancer survivors | 200 (100%) | 51.6 (27–76) | 12 weeks, Yoga | 90 min, 1×/week Hatha yoga | Wait-list | IL-6 − TNF − IL-1 − (all at 3-month FU) |
| McCain 2008 | Adults with diagnosis of HIV | 252 (40%) | 42 | 10 weeks, Tai Chi | 90 min, 1 ×/week Short form Tai Chi | Cognitive behavioral relaxation training; Spiritual growth; Wait-list control | IL-6 ns TNF ns |
| Zautra 2008 | Rheumatoid arthritis patients | 144 (68%) | 56 | 8 weeks, Meditation | 2 hrs, 1 ×/week Mindfulness based stress reduction without yoga | Cognitive behavioral therapy (CBT) for pain; Education only | IL-6 ns in MBSR; IL-6 − in CBT |
Notes: The symbol “−” is used to indicate a significant decrease (or attenuated increase) in a particular inflammatory marker. “Trend” is used to indicate marginally significant findings (0.05 < p < .10), and “ns” is used to indicate non-significant findings.
Elsenbruch et al. (2005) evaluated the effects of mindfulness-based stress reduction (MBSR) on lipopolysaccharide (LPS) stimulated production of proinflammatory cytokines using whole blood. Despite significant improvements in patient-reported outcomes of health functioning and colitis symptoms, no change in the stimulated production of TNF was found. Moreover, the intervention did not have effects on other biologic markers such as cortisol that might regulate cellular production of inflammatory cytokines. Similar negative findings were found in two other studies that showed improvements in behavioral and/or patient-reported outcomes without showing changes in cellular production of proinflammatory cytokines (McCain et al., 2008; Zautra et al., 2008). However in the Zautra et al. study, a significant reduction in the production of IL-6 was found with the CBT group, but not in the mindfulness meditation group, as compared to the education control.
In contrast to the above findings that assessed proinflammatory cytokine production after 8 to 12 weeks of treatment, differences may emerge when assessment occurs later during the follow-up period. For example, Kiecolt-Glaser et al. (2014) found no differences LPS stimulated production of IL-6, TNF, and IL-1 in isolated peripheral blood mononuclear cells in 200 breast cancer survivors after a 12 week yoga intervention, but significant group differences were identified at 3 month follow-up. Furthermore, such differences were associated with the frequency of yoga practice, which predicted changes in these inflammatory markers. Additionally, sleep quality improved along with fatigue. Given that sleep disturbance can activate inflammatory signaling and increase markers of inflammation, it was suggested that improvements in sleep might have accounted for the decreases in cellular inflammation in this sample of breast cancer survivors.
To evaluate a cellular source of inflammation, Irwin et al. have examined the effects of Tai Chi on monocyte production of IL-6 and TNF in two independent samples of insomnia patients (Irwin et al., 2014; Irwin et al., 2015). Assessment of cellular inflammation focused on LPS or Toll-like receptor (TLR)-4 stimulated production of IL-6 and TNF in monocytic populations, as compared to prior studies that had used mixed mononuclear cell cultures or whole blood. Tai Chi administration over 12 or 16 weeks reversed the insomnia-related increases in the percentage of monocytes expressing IL-6 alone, expressing TNF alone, and co-expressing IL-6 and TNF, with significant decreases for each of these measures (Irwin et al. 2014; Irwin et al., 2015). Interestingly, Tai Chi administration induced decreases as early as 2 months, with effects maintained over the course of a one year follow-up.
Effects of MBTs on gene expression inflammatory pathways
As demonstrated in several studies in the growing field of social genomics, significant life adversity is associated with alterations in gene transcriptional programs expressed under basal conditions in circulating immune cells (Cole, 2014). Indeed, genome wide transcriptional profiling of leukocytes from individuals experiencing a range of life adversities (e.g., bereavement, loneliness, low socioeconomic status) has shown a common pattern of increased expression of proinflammatory genes, which is often accompanied by a focal suppression of genes involved in innate antiviral responses (Irwin & Cole, 2011). Bioinformatic analyses of these gene expression data have identified specific transcription factors (TFs) as potential mediators of this transcriptional shift, with evidence of activation of proinflammatory NF-κB/Relfamily TFs and GATA-family TFs, decreased activity of interferon response factors (IRFs), and decreased activity of the glucocorticoid receptor (Cole, 2014). The glucocorticoid receptor would otherwise antagonize NF-κB/Rel factors and reduce inflammation.
Building on this work, genomics-based methods have been recently used to assess inflammation and the ability of MBTs to reverse inflammatory gene expression. Seven RCTs have examined the effects of MBTs on inflammation-related gene expression, including trials of Tai Chi (n=3), meditation (n=3), and yoga (n=1), described in Table 3. These studies have included a variety of control and comparison groups, including CBT, health education, and wait list. Results are consistent across trials and approaches; all have been shown to reverse the pattern of leukocyte transcriptional alterations including activation of genes regulated by the proinflammatory NF-κB/Rel family.
Table 3.
Randomized controlled trials evaluating the effects of mind-body therapies on inflammatory gene expression pathways
| Study | Population | N, % Female | Mean age (range) | Intervention Duration, Type | Intervention Frequency, Style | Control Group | Finding |
|---|---|---|---|---|---|---|---|
| Gene Expression Inflammatory Pathways | |||||||
| Black 2013 | Adult caregivers | 39 (92%) | 60.5 | 8 weeks, Meditation | 12 min, 7 days/week Kirtan Kriya | Relaxing music | NF-κB − IRF + |
| Black 2014 | Older adults | 26 (80.8%) | 67.1 (60–72) | 12 weeks, Tai Chi | 60 min, 1 ×/week | Health education | NF-κB − (attenuated increase vs controls) |
| Bower 2014 | Breast cancer survivors with fatigue | 31(100%) | 54 (40–65) | 12 weeks, Yoga | 90 min, 2 ×/week Iyengar yoga | Health education | NF-κB − IRF + |
| Bower 2014 | Breast cancer survivors | 71 (100%) | 46.9 (28–60) | 6 weeks, Meditation | 2 hrs, 1×/week Mindful Awareness Practices | Wait-list control | NF-κB − |
| Creswell 2012 | Healthy older adults | 40 (80%) | 65 (55–85) | 8 weeks, Meditation | 2 hrs, 1×/week Mindfulness based stress reduction | Wait-list control | NF-κB − IRF + |
| Irwin 2014 | Breast cancer survivors with insomnia | 90 (100%) | 59.8 (42–83) | 12 weeks, Tai Chi | 2 hrs, 1 ×/week Tai Chi Chih | Cognitive behavioral therapy | NF-κB − IRF + |
| Irwin 2015 | Older adults with insomnia | 123 (73%) | 65 (55–85) | 16 weeks, Tai Chi | 2 hrs, 1 ×/week Tai Chi Chih | Health education; Cognitive behavioral therapy | NF-κB − IRF + |
Abbreviations: NF-κB = nuclear factor kappa B; IRF = interferon response factor
Notes: The symbol “−” is used to indicate a significant decrease (or attenuated increase) in a particular inflammatory marker.
The effects of three different types of meditation on gene expression profiles have now been examined in various samples – lonely older adults (Creswell et al., 2012), adult caregivers (Black et al., 2014), and women with early stage breast cancer (Bower et al., 2015) – yielding consistent findings across each type of meditation. Creswell et al. (2012) identified 143 genes showing greater than 25% differential change over time with mindfulness based stress reduction (MBSR), in which the MBSR group showed a down-regulation of 69 genes and an up-regulation of 74 genes relative to the wait list controls. Using a TELiS bioinformatics analytic strategy, there was evidence of reduced activity of NF-κB target genes in the MBSR subjects that were prominently found in the monocytes consistent with prior studies on the cellular production of inflammatory cytokines. Black et al. (2014) generated a similar set of results using a yogic meditation intervention, in which the yogic meditation group showed a down-regulation of 49 genes and an up-regulation of 19 genes relative to a control group that listened to relaxing music. Down-regulated transcripts included a number of proinflammatory cytokines (e.g., IL8) as well as activation-related immediate-early genes (e.g., JUN, FOSB). Given the prior findings by Creswell et al. (2012), 3 other prototypical proinflammatory cytokines were examined with small reductions in IL-6 and IL-1 transcripts and a small increase in TNF transcripts. Nevertheless, promoter-based bioinformatic analysis again showed reduced expression of genes bearing NF-κB-response elements in the yogic meditation group as compared to controls. Finally, Bower et al. (2015) interrogated differences in a 19-transcript composite of proinflammatory genes to reveal a significantly greater decline from baseline to post-intervention in a mindfulness training group versus wait list control. Reduced activity of the proinflammatory transcription factor NF-κB and increased activity of anti-inflammatory glucocorticoid receptor was again implicated using TELiS based bioinformatics analyses.
Consistent with these homogenous effects using various types of meditation, yoga appears to generate similar differences in inflammatory gene expression. In a study of yoga for breast cancer survivors with persistent fatigue, Bower et al. (2014) identified 435 gene transcripts showing a >15% differential change over time; 282 transcripts showed up-regulation, and 153 transcripts showed down-regulation in the yoga group relative to health education controls. TELiS promoter-based bioinformatic analyses of these differentially regulated genes again demonstrated reduced activity of NF-kB, and also showed increased activity of the glucocorticoid receptor in the yoga group vs. controls.
Finally, Irwin et al. (2014, 2015) have shown that Tai Chi produced decreases in inflammatory gene expression, similar to the effects of meditation and yoga. In a study of breast cancer survivors with insomnia, analyses of an a priori-selected set of 19 proinflammatory gene transcripts showed a 9.0% (± 2.5%) greater decline over time in average expression in TCC participants relative to CBT-I participants (Irwin et al., 2014). Genome wide analysis of this sample showed a ≥1.2-fold down-regulation of 68 gene transcripts in the TCC group relative to CBT-I, of which inflammatory transcripts were prominently identified. In a study of older adults with insomnia, Tai Chi similarly induced a down-regulation of genes transcripts involved in immunological activation and inflammation (e.g., IL-6, IFNGR1, CD69, FOSB, FOS, IFNG, JUNB, IL-8, IL-1B, PTGS2) (Irwin et al., 2015). TELiS analyses in both studies implicated reduced activity of NF-κB with the primary cellular context being the monocyte population. Only one small study has directly measured changes in activation of NF-κB (as opposed to the TELiS-based inferential analyses) and found that the practice of Tai Chi relative to a health education control attenuated the rise in NF-κB found in the controls(Black et al., 2014).
One issue relevant for interpretation of results from these trials that have evaluated gene expression profiling is the use of a single social genomics lab. Cole et al. have performed the gene expression assays and associated bioinformatics analyses for all 7 studies. However, there are corroborating data from another group that found decreased NF-kB activity following acute induction of the relaxation response (Bhasin et al., 2013). Because these results were not obtained as part of an RCT, the findings were not included in the overall review. Additional studies are needed to substantiate the positive findings seen in these initial trials.
Summary of results
Over the past decade investigators have become increasingly interested in the impact of MBTs on markers of inflammation. We reviewed a total of 26 trials that examined effects of Tai Chi, Qigong, meditation, and yoga interventions on inflammatory outcomes. The majority of studies (n = 19) focused on circulating markers, particularly CRP. We found mixed evidence that the MBTs evaluated in these trials led to alterations in circulating concentrations of CRP, with half of the studies showing decreases (or attenuated increases) of CRP in the intervention group and the other half of the trials showing no significant change of CRP. The evidence for effects of MBTs on IL-6 and other inflammatory markers was also mixed, with the majority of studies showing no change. Of note, the trials that yielded non-significant results for inflammatory markers did show beneficial effects on symptoms and other outcomes, indicating that the therapies were effective in improving some aspects of health.
Studies that evaluated cellular markers of inflammation, assessed by production of proinflammatory cytokines after ex vivo stimulation, were somewhat more promising. Among these studies, 50% (3 of 6) of the trials showed that production of inflammatory cytokines was reduced following Tai Chi or yoga administration. Two of the trials that showed a reduction of inflammatory cytokines were conducted with breast cancer survivors, though trials conducted with other populations at risk for elevated inflammatory activity (HIV, ulcerative colitis) did not show such effects.
Results for trials examining genomic inflammatory markers consistently indicated a decrease in inflammatory gene expression profiles; each of the 7 trials that assessed genomic markers of inflammation showed effects on inflammatory signaling pathways, specifically reductions in NF-kB activity. These effects were seen in trials of yoga, Tai Chi, and meditation, and occurred in diverse populations. Further, effects on genomic markers were seen even in trials that did not evidence decreases in circulating inflammatory markers, suggesting that alterations in molecular signaling pathways may be more sensitive to these interventions, at least in the short term. More sustained practice may be required to alter circulating markers, such as CRP, that provide a more integrated, comprehensive measure of systemic inflammatory activity. Indeed, in the exercise literature, year-long physical activity interventions have been required to reduce circulating levels of CRP (e.g., Nicklas et al., 2008).
The limitations of the current literature are several fold. First, many of the RCTs included only a small number of subjects, and did not rely on estimates of statistical power to determine sample size needed for outcomes related to inflammation. Hence, inability of several studies to identify reductions in markers of inflammation may be a consequence of inadequate statistical power, as opposed the lack of efficacy of the MBTs. Second, subjects included within studies, and across RCTs, showed considerable heterogeneity in terms of age as well as clinical characteristics (i.e., healthy adults vs. patient populations) which might contribute to mixed results. Third, studies did not routinely evaluate levels of inflammation as an inclusion criteria for study entry; inclusion of subjects who show no or little evidence of inflammation at baseline might limit the ability to determine whether MBTs or other interventions reduce inflammation given limits of assay sensitivity and potential “floor effects”. Fourth, many of the interventions were administered over weeks and such a short time frame may not be adequate to detect changes in circulating or systemic markers of inflammation. This possibility is particularly salient given evidence of more consistent effects of MBTs to reduce inflammatory gene expression profiles and cellular markers of inflammation; such upstream changes in the inflammatory cascade would likely be occurring prior to changes in circulating markers. Fifth, the latter issue is relevant to the vast majority of RCTs that did not conduct longer term follow-up following administration of the MBTs. Sixth, many of the RCTs relied on wait list or usual care controls, and it is possible that the effects of MBTs on the inflammatory markers might be a consequences of non-specific factors such as social support due to group participation or expectation for benefit. Finally, many of the RCTs did not systematically evaluate fidelity of intervention administration and/or effects of participant adherence on changes in the inflammatory outcomes.
Mechanisms
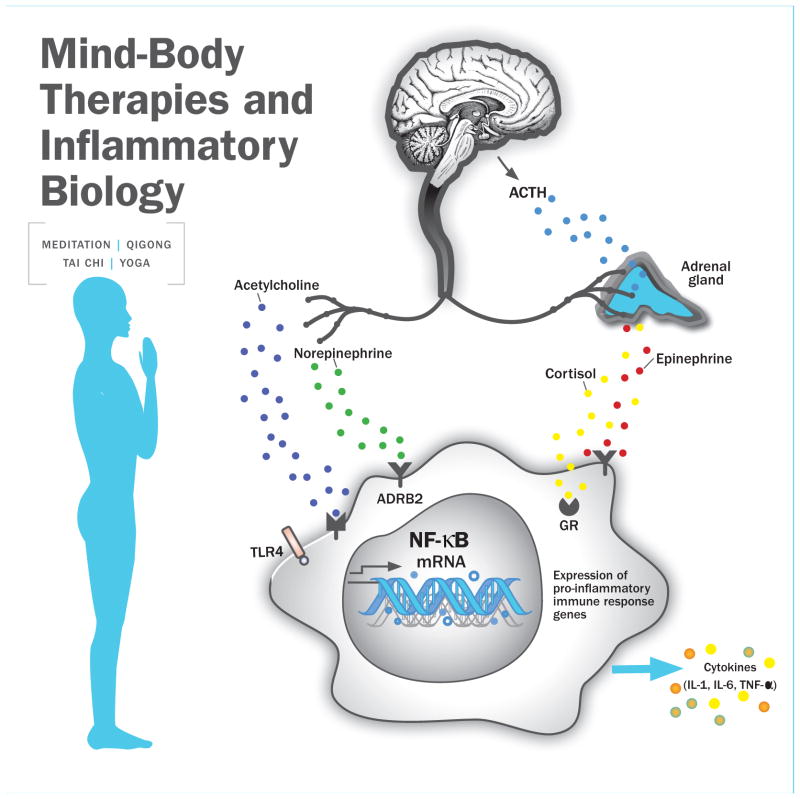
What are the mechanisms through which these diverse approaches might lead to changes in inflammation, particularly changes in cellular and genomic markers? A number of conceptual models have been proposed to explain the effects of MBTs on a broad range of outcomes (Creswell & Lindsay, 2014; Gard, Noggle, Park, Vago, & Wilson, 2014; Holzel et al., 2011; Taylor, Goehler, Galper, Innes, & Bourguignon, 2010), all of which emphasize the important of self-regulation as a central goal of MBTs. We draw from these models, and from the empirical literature, to identify pathways that are most relevant to inflammation, including neuroendocrine, neural, and psychological/behavioral mechanisms. These pathways are illustrated in Figure 1. Of note, although we focus here on “top down” pathways, MBTs may also have more direct physiological effects, as discussed in the section on active ingredients.
Figure 1.
Potential pathways linking mind-body therapies and inflammatory biology, focusing on neuroendocrine mechanisms. Mind-body interventions may influence neural regions that regulate downstream stress response pathways, including the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis. The ANS and the HPA axis may, in turn, influence the production of proinflammatory cytokines via effects of their ligands on adrenergic, cholinergic, and glucocorticoid receptors on immune cells. Stimulation of adrenergic receptors by epinephrine and norepinephrine can activate proinflammatory transcription factors such as NF-kB, whereas stimulation of GC receptors can inhibit proinflammatory cytokine production. Randomized controlled trials of mind-body therapies have demonstrated reduced pro-inflammatory signaling through NF-kB and increased anti-inflammatory GR signaling, suggesting that these approaches may modulate gene expression to reduce inflammation. However, effects on stimulated production of proinflammatory cytokines and circulating cytokine concentrations are mixed.
Neuroendocrine mechanisms
At the most proximal level, alterations in the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis likely play an important role in structuring intervention-related changes in inflammatory activity. These systems are key regulators of inflammatory gene expression (Irwin & Cole, 2011) and, as mediators of the stress response, are targeted by mind-body interventions.
Autonomic nervous system
Focusing first on the ANS, preliminary evidence indicates that MBTs are associated with decreases in sympathetic activity and increases in parasympathetic activity (Audette et al., 2006; Creswell & Lindsay, 2014; Motivala, Sollers, Thayer, & Irwin, 2006), reflecting greater sympathovagal balance. These alterations have been observed at rest, following practice of the technique, and in response to acute stress, although results may depend on the nature of the stimulus. Sympathovagal balance is thought to reduce inflammation via decreased adrenergic signaling. Indeed, Irwin et al. found that TCC led to reduced activity of cAMP response element-binding protein (CREB) family transcription factors, which is consistent with reduced sympathetic nervous system signaling through β-adrenergic receptors, in tandem with decreases in NF-kB activity (Irwin et al., 2015). Similar changes in CREB signaling were found in a yoga trial that also showed reduced NF-kB activity (Bower et al., 2014). Increases in parasympathetic nervous system activity may also reduce inflammation via the anti-inflammatory cholinergic pathway (Tracey, 2009). However, the mediating role of the ANS in intervention-induced reductions in inflammation has not been formally tested. Future studies should incorporate sophisticated measures of ANS activity along with inflammatory signaling to explicate these links.
HPA axis
Cortisol, the end product of HPA axis activity, has potent anti-inflammatory effects. Thus, alterations in cortisol production or in glucocorticoid receptor sensitivity have the potential to modulate inflammatory processes. In terms of cortisol production, there is mixed evidence that MBTs influence cortisol reactivity to acute stress; for example, a study of compassion meditation found no effect on cortisol reactivity to the Trier Social Stress Task (TSST), a standardized psychosocial stressor (Pace et al., 2009), whereas a study of brief mindfulness training found increased reactivity to the TSST (Creswell, Pacilio, Lindsay, & Brown, 2014). Effects on measures of diurnal cortisol output are similarly mixed. However, several trials have shown that MBTs lead to changes in glucocorticoid receptor signaling, which may in turn influence inflammatory processes. Specifically, studies of Tai Chi, mindfulness, and yoga have shown increases in anti-inflammatory GR signaling in the intervention group vs. control, which co-occur with decreases in NF-κB (Bower et al., 2014; Bower et al., 2015; Irwin et al., 2014; Irwin et al., 2015). This pattern of results – namely, that MBTs influence GR signaling without affecting cortisol production – suggests that receptor sensitivity may be modulated by these interventions. This is important given that the increased inflammatory activity seen in the context of chronic stress is hypothesized to be due to alterations in GR sensitivity (Miller et al., 2008). Thus, targeting GC signaling mechanisms may have substantial benefit for altering these dynamics.
Neural mechanisms
Activity in the ANS and HPA axis is modulated by threat-related areas of the brain, including the amygdala, dorsal anterior cingulate cortex, anterior insula, and periaqueductal gray. These areas have been described as a “neural alarm system” that detects and elicits adaptive responses to impending danger or harm (Eisenberger & Cole, 2012). Given that many MBTs either implicitly or explicitly involve a focus on promoting relaxation and decreasing threat reactivity, it plausible that activity in these “neural alarm” regions may be responsive to mind-body approaches and may mediate downstream effects on inflammation. Most of the research on neural correlates of MBTs has focused on meditative practices, and there is preliminary evidence that mindfulness is associated with alterations in threat-related brain regions, including decreased amygdala reactivity (Goldin & Gross, 2010) and reduced functional connectivity of the amygdala with other threat-related regions (Taren et al., 2014).
MBTs may also influence regulatory areas of the brain that modulate activity in threat-related regions. In particular, mindfulness training interventions have been shown to increase recruitment of the prefrontal cortex in the context of emotion-regulation tasks (Creswell & Lindsay, 2014). These findings are consistent with the focus of mindful training on enhancing emotion regulation. Further, it is possible that mindfulness and other MBTs may influence activity in reward-related regions, such as the ventromedial prefrontal cortex (VMPFC), ventral striatum, and septal area, which also have inhibitory effects on threat-related physiological responding (Eisenberger & Cole, 2012).
Although we have focused primarily on the stress-reducing effects of MBTs, other dimensions of these approaches may also be relevant for inflammation. In particular, a key component of all of the MBTs reviewed here is focused bodily awareness. The insular cortex receives afferent signals from the body via viscerosensory pathways and is involved in the representation and integration of this information; it also modulates autonomic output. Thus, to the extent that MBTs lead to alterations in these signals, or to their neural representation and integration by the insula and other neural regions, this may drive changes in downstream autonomic processing and inflammatory activity. Indeed, there is evidence that mindfulness training leads to alterations in insula activity as well as changes in connectivity with prefrontal areas (Farb, Segal, & Anderson, 2013). Yoga is also hypothesized to facilitate integration of “top down” and “bottom up” neural processes, although this has not been evaluated (Gard et al., 2014).
Psychological and behavioral mechanisms
At the psychological level, MBTs target psychological processes that have been directly and indirectly linked to threat-, reward-, and regulatory brain regions and to stress-related physiological systems. For example, MBTs lead to decreases in perceived stress, depression, and anxiety, along with increases in control, self-efficacy, emotion regulation, and peace and meaning life (Gard et al., 2014; Goyal et al., 2014; Holzel et al., 2011). Changes in these domains have been shown to be associated with MBSR effects on telomerase activity (Jacobs et al., 2011), but such pathways have not been tested for inflammatory outcomes. Certain MBTs may also have effects on processes that are not directly targeted by the intervention, but still have relevance for inflammation. For example, Creswell et al. found that MBSR led to decreases in loneliness, which has been associated with elevated inflammatory activity (Creswell et al., 2012). Further, MBTs may influence health behaviors relevant for inflammation, such as sleep (Irwin et al., 2014). Of course, therapies that involve physical activity may have
Active ingredients
Mind-body treatments include multiple components, including breathing, focused attention, meditation, and physical movements. The different MBTs place different emphasis on these components. For example, mindfulness and other meditative practices focus on conscious and intentional mental activities. Physical movements, if they are included as part of the intervention, are typically performed in the service of cultivating awareness of one’s body and its interaction with the environment. On the other hand, Tai Chi is a more physical practice; indeed, the flowing physical movements that are performed during Tai Chi can achieve 50% to 74% of the maximal heart rate, depending on the age of the individual and the intensity of practice. Yoga is also a more physical practice that emphasizes the importance of postures (asanas) for treatment effects. Although our review did not identify systematic differences between these approaches for inflammatory outcomes, it is certainly plausible that different MBTs may work through different mechanisms, which may modulate effects on inflammatory biology. Certainly effects on other outcomes (functional, behavioral, psychological) may differ by approach.
Another important issue in the literature on MBTs is the degree to which intervention effects are due to what are thought to be the “active ingredients” of the intervention, or to non-specific factors, such as expectations, attention from an experienced instructor, and/or group context. Carefully designed control groups are required to test these alternative hypotheses. In conducting this review, it is encouraging to find that many of the trials assessing inflammatory outcomes did use active control groups, including health education, support/counseling, and exercise. The optimal control group will, of course, depend on the question under investigation. For example, to determine whether the effects of Tai Chi are primarily due to physical activity, an exercise control group would be optimal. Or, to determine whether the effects of yoga are primarily due to stretching and associated changes in muscle-derived inflammatory markers, a stretching control would be preferred. Of note, research comparing yoga to stretching has shown mixed effects on psychological and physiological measures (e.g., Chandwani et al., 2014; Corey et al., 2014), though differential effects on inflammatory processes have not been examined.
One potential complication in the selection of control groups is the possibility that they may control for what is considered to be an essential part of the intervention. For example, we have elected not to use relaxation as a control condition in our yoga research, as relaxation is an integral part of yoga. Given that many of these practices have been developed over thousands of years, it is unclear whether dismantling them to identify active ingredients will be particularly useful. An alternative approach is to use control groups that control for non-specific elements of the intervention, such as meeting in a group and learning something you consider useful; in this case, health education might be optimal. Indeed, health education has been recommended for use in yoga trials (Sherman, 2012). In general, decisions about control groups will depend on the question under investigation in a particular study.
Typically, investigators have examined MBTs separately from other intervention approaches. However, it is possible that combining MBTs with other approaches may have additive or synergistic effects on inflammation. In particular, combining MBTs with pharmacologic therapies that target inflammation may enhance the anti-inflammatory effects of each. For example, in regards to anti-viral immune response (as opposed to inflammation), Irwin et al. (2007) found that Tai Chi together with varicella zoster virus vaccination augmented the immune response in older adults to a greater extent than either Tai Chi or vaccination alone. MBTs might also be combined with dietary approaches (e.g., omega-3s) and other interventions that are thought to reduce inflammation for maximal benefit. As a related issue, MBTs are rarely compared with each other, which would provide information about specificity of effects. Further, few trials examine combined MBT approaches (e.g., yoga and TCC), although in practice many patients may use these therapies simultaneously.
Conclusions
There is a growing literature examining effects of MBTs on inflammatory processes, reflecting increasing use of these approaches and recognition of the importance of inflammation for physical and mental health. Results from this review suggest that effects of these approaches may initially be reflected in alterations in gene expression profiles and indicators of proinflammatory signaling; indeed, alterations in inflammatory gene expression were identified even after relatively short (6 week) interventions (Bower et al., 2015) and generally appeared to be driven by reduced proinflammatory gene expression in the monocyte population. Studies examining cellular markers of inflammation also suggest that monocytes may be particularly sensitive to effects of MBTs, with effects on monocyte production of proinflammatory cytokines observed as early as 8 weeks after intervention onset (Irwin et al., 2015). Effects on non-specific markers, including circulating inflammatory markers, may take longer to emerge, and may require more intensive practice of the treatment (e.g., Bower et al., 2014; Kiecolt-Glaser et al., 2014; Pace et al., 2009). One of the compelling features of MBTs is the ability to integrate these practices into one’s daily life, with the potential for long-term benefit. A critical next step in research on MBTs is to determine how to maintain these practices and to examine longer-term effects on inflammation and health.
Highlights.
We conducted a qualitative review of randomized controlled trials that examined effects of mind-body therapies (MBTs) on markers of inflammation
26 trials were identified, including studies of Tai Chi, Qigong, yoga, and meditation
Studies that examined genomic markers of inflammation showed consistent reductions in inflammatory signaling; more mixed effects were seen for circulating and cellular markers of inflammation
Acknowledgments
Supported in part by R01 CA160427 and funding from the Breast Cancer Research Foundation to JEB; by R01 CA160245, R01 AG034588, R01 AG026364, R01 CA119159, R01 HL095799, R01 DA032922, and P30 AG028748 to MRI; and by UCLA CTSI UL1TR000124, the Cousins Center for Psychoneuroimmunology, and P30-AG028748 UCLA Claude D. Pepper Older Americans Independence Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audette JF, Jin YS, Newcomer R, Stein L, Duncan G, Frontera WR. Tai Chi versus brisk walking in elderly women. Age Ageing. 2006;35(4):388–393. doi: 10.1093/ageing/afl006. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL, et al. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One. 2013;8(5):e62817. doi: 10.1371/journal.pone.0062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Irwin MR, Olmstead R, Ji E, Crabb Breen E, Motivala SJ. Tai chi meditation effects on nuclear factor-kappaB signaling in lonely older adults: a randomized controlled trial. Psychother Psychosom. 2014;83(5):315–317. doi: 10.1159/000359956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winson D, Arevalo J, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121:1231–40. doi: 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing A, Ostermann T, Ludtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1–9. doi: 10.1016/j.jpain.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Chandwani KD, Perkins G, Nagendra HR, Raghuram NV, Spelman A, Nagaranthna R, Johnson K, Fortier A, Arun B, Wei Q, Kirschbaum C, Haddad R, Morris GS, Scheetz J, Chaoul A, Cohen L. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058–65. doi: 10.1200/JCO.2012.48.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yeh ML, Lee FY. The effects of Baduanjin qigong in the prevention of bone loss for middle-aged women. Am J Chin Med. 2006;34(5):741–747. doi: 10.1142/S0192415X06004259. [DOI] [PubMed] [Google Scholar]
- Chen SC, Ueng KC, Lee SH, Sun KT, Lee MC. Effect of t’ai chi exercise on biochemical profiles and oxidative stress indicators in obese patients with type 2 diabetes. J Altern Complement Med. 2010;16(11):1153–1159. doi: 10.1089/acm.2009.0560. [DOI] [PubMed] [Google Scholar]
- Cole SW. Human social genomics. PLoS Genet. 2014;10(8):e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey SM, Epel E, Schembri M, Pawlowsky SB, Cole RJ, Araneta MR, Barrett-Connor E, Kanaya AM. Effect of restorative yoga vs. stretching on diurnal cortisol dynamics and psychosocial outcomes in individuals with the metabolic syndrome: The PRYSMS randomized controlled trial. Psychoneuroendocrinology. 2014;49:260–71. doi: 10.1016/j.psyneuen.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Lindsay EK. How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science 2014 [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Langhorst J, Popkirowa K, Muller T, Luedtke R, Franken U, et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom. 2005;74(5):277–287. doi: 10.1159/000086318. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci. 2013;8(1):15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Noggle JJ, Park CL, Vago DR, Wilson A. Potential self-regulatory mechanisms of yoga for psychological health. Front Hum Neurosci. 2014;8:770. doi: 10.3389/fnhum.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al. Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20(9):764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–7. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Tai Chi, Cellular Inflammation, and Transcriptome Dynamics in Breast Cancer Survivors With Insomnia: A Randomized Controlled Trial. JNCI Monographs. 2014;2014(50):295–301. doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive Behavioral Therapy and Tai Chi Reverses Cellular and Genomic Markers of Inflammation in Late Life Insomnia: A Randomized Controlled Trial. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–170. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32(10):1040–1049. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain Behav Immun. 2013;27(1):145–154. doi: 10.1016/j.bbi.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzaneque JM, Vera FM, Rodriguez FM, Garcia GJ, Leyva L, Blanca MJ. Serum cytokines, mood and sleep after a qigong program: is qigong an effective psychobiological tool? J Health Psychol. 2009;14(1):60–67. doi: 10.1177/1359105308097946. [DOI] [PubMed] [Google Scholar]
- McCain NL, Gray DP, Elswick RK, Robins JW, Tuck I, Walter JM, et al. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. J Consult Clin Psychol. 2008;76(3):431–441. doi: 10.1037/0022-006X.76.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One. 2014;9(7):e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. 2006;61(11):1177–1180. doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Butow P, Mullan B, Clarke S. Medical Qigong for cancer patients: pilot study of impact on quality of life, side effects of treatment and inflammation. Am J Chin Med. 2008;36(3):459–472. doi: 10.1142/S0192415X08005904. [DOI] [PubMed] [Google Scholar]
- Oh B, Butow P, Mullan B, Clarke S, Beale P, Pavlakis N, et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol. 2010;21(3):608–614. doi: 10.1093/annonc/mdp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2012;20(6):1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel D, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J Altern Complement Med. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14(5):407–413. doi: 10.1016/j.cardfail.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Pullen PR, Thompson WR, Benardot D, Brandon LJ, Mehta PK, Rifai L, et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010;42(4):651–657. doi: 10.1249/MSS.0b013e3181bf24c4. [DOI] [PubMed] [Google Scholar]
- Rao RM, Nagendra HR, Raghuram N, Vinay C, Chandrashekara S, Gopinath KS, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. Int J Yoga. 2008;1(1):11–20. doi: 10.4103/0973-6131.36789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JL, McCain NL, Elswick RK, Jr, Walter JM, Gray DP, Tuck I. Psychoneuroimmunology-Based Stress Management during Adjuvant Chemotherapy for Early Breast Cancer. Evid Based Complement Alternat Med. 2013;2013:372908. doi: 10.1155/2013/372908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman KJ. Guidelines for developing yoga interventions for randomized trials. Evid Based Complement Alternat Med. 2012;2012:143271. doi: 10.1155/2012/143271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JW, Jacobs JJ. Complentary and Alternative Medicine: an Evidence Based Approach. St. Louis, MO: Mosby; 2003. [Google Scholar]
- Taylor AG, Goehler LE, Galper DI, Innes KE, Bourguignon C. Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 2010;6(1):29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164(5):493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76(3):408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]