Abstract
Host cells possess the metabolic assets required for viral infection. Recent studies indicate that control of the host's metabolic resources is a core host–pathogen interaction. Viruses have evolved mechanisms to usurp the host's metabolic resources, funneling them towards the production of virion components as well as the organization of specialized compartments for replication, maturation, and dissemination. Consequently, hosts have developed a variety of metabolic countermeasures to sense and resist these viral changes. The complex interplay between virus and host over metabolic control has only just begun to be deconvoluted. However, it is clear that virally induced metabolic reprogramming can substantially impact infectious outcomes, highlighting the promise of targeting these processes for antiviral therapeutic development.
Keywords: virus, metabolism, glycolysis, citric acid cycle, energy, immunity, infection, lipid, fatty acid
Trends
Numerous viruses modulate host-cell metabolic processes to ensure successful infection.
The host-cell metabolic network contributes the energy, precursors, and specialized components necessary to produce infectious virions.
Viruses deploy host-cell metabolic activities to organize viral maturation compartments.
Metabolic control is a host–pathogen interaction that can sway the outcome of viral infection.
The Host Metabolic Network: Multifaceted Contributions to Viral Infection
Viruses are obligate parasites that depend on the host cell to provide the energy and molecular precursors necessary for successful infection. A wide variety of evolutionarily divergent viruses have evolved mechanisms that target the host cell metabolic network as part of their infectious programs, and virally induced metabolic activities are commonly exploited for therapeutic intervention. For example, numerous different nucleotide metabolic activities are targeted by a variety of pharmaceuticals to treat viral infections, including hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), human cytomegalovirus (HCMV), varicella-zoster Virus (VZV), and herpes simplex virus (HSV) (Table 1 ) 1, 2, 3, 4, 5. In recent years, the number of metabolic activities that have been found to be important for viral infection has expanded. Further, our understanding of the viral mechanisms through which viruses usurp cellular metabolic resources has increased. Many of these viral mechanisms stimulate nutrient uptake and catabolism to support the production of viral progeny. In addition to providing the energy and biomass necessary for turning cells into productive ‘virus factories’, new metabolic contributions to infection have emerged. These include small-molecule enzymatic activities that organize viral maturation compartments, synthesize specialized virion components, or regulate the immunological environment (Figure 1 ). Such virally induced metabolic changes do not go unnoticed by the host, but rather represent a major host–pathogen interaction that can sway infectious outcomes. Collectively, recent findings have made it clear that the landscape for metabolically targeted therapeutic intervention has expanded.
Table 1.
Nucleoside/Nucleotide-Based Therapeuticsa
| Virus | Nucleoside/Nucleotide Analogs |
|---|---|
| HIV | Tenovovir; Emtricitabine; Zidovudine; Abacavir; Lamivudine |
| HBV | Tenovovir; Lamivudine; Entecavir; Telbivudine |
| HCV | Sofosbuvir; Ribavirin |
| HCMV | Ganciclovir; Cidofovir |
| HSV | Acyclovir; Valacyclovir |
| VZV | Acyclovir; Valacyclovir |
HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HCMV, human cytomegalovirus; HSV, herpes simplex virus; VZV, varicella-zoster virus.
Figure 1.
Small–Molecule Metabolic Contributions to Viral Infection. Production of infectious virions requires energy and biomolecular building blocks derived from the host cell metabolic network (A). A diverse set of host metabolic activities drives the mass production of viral nucleic acids (red), structural proteins (black octagons), non-structural proteins and phospholipid envelopes (both in black circles), and glycosylated proteins (white circles). Additionally, organization of viral maturation compartments has increasingly been found to be dependent on lipid-modifying enzymes (B). Viral infection has also been found to induce specific metabolic activities to form specialized virion components that are important to infection (C). Lastly, the evidence supporting the importance of small-molecule metabolism for immune regulation is increasing, as are the findings that these processes are targeted by viral infection (D).
Viral Targeting of Core Metabolic Pathways
A wide variety of viruses activate glycolysis, which drives the production of energy in the form of ATP, NADH, and NADPH (Figure 2 ). Activated glycolysis also supplies the carbon necessary for the synthesis of numerous core biomolecules, including nucleotides, lipids, amino acids and carbohydrates (Figure 2). A number of DNA viruses induce glycolysis, including Kaposi's sarcoma-associated herpesvirus (KSHV) [6], HCMV [7], adenovirus [8], human papillomavirus (HPV) and Epstein–Barr virus (EBV) [9]. Multiple RNA viruses also activate glycolytic flux, including dengue [10], hepatitis C (HCV), [11] and influenza A [12]. Although there are some notable exceptions, such as herpes simplex virus-1 (HSV-1) and vaccinia virus 13, 14, both the number and evolutionary diversity of viruses that target glycolysis speak to the broad importance of this pathway for viral infection.
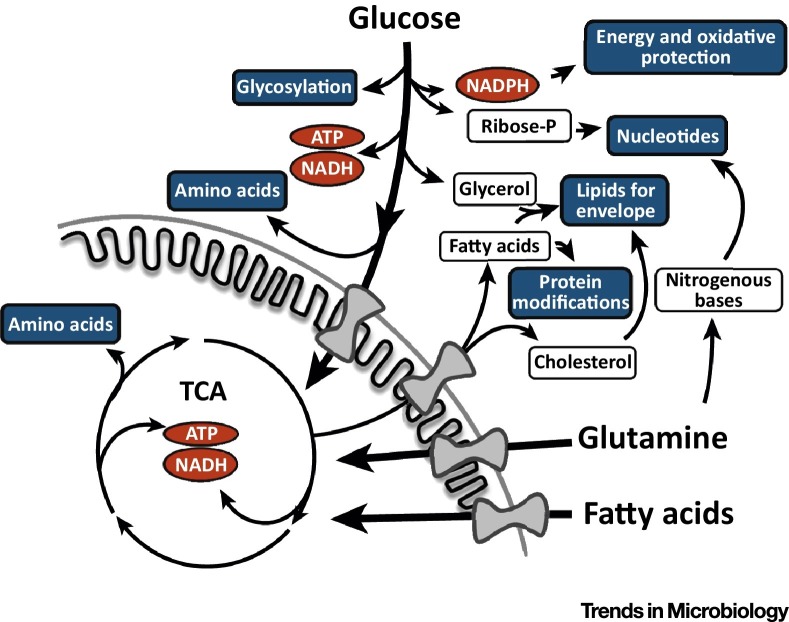
Figure 2.
The Metabolism of Glucose and Glutamine and Support of Viral Infection. Glucose and glutamine catabolism provide energy and reducing equivalents [ATP, NADH, NADPH (shown in red)] as well as the molecular precursors to synthesize virion components (shown in blue). Abbreviation: TCA, tricarboxylic acid cycle.
Recently, the specific viral mechanisms targeting glycolysis have begun to be elucidated. For instance, the HCV NS5A protein has been shown to bind and activate hexose kinase, a rate-controlling glycolytic enzyme [15]. KSHV employs specific viral microRNAs targeting known regulators of glucose metabolism and mitochondrial biogenesis to induce glycolytic activity [6]. HCMV has been shown to induce the activity of the AMP-activated kinase (AMPK) [16], which regulates numerous glycolytic activities [17]. HCMV-mediated activation of AMPK was found to be necessary for glycolytic activation and high-titer infection [16]. HCMV has also been shown to induce a pro-glycolytic transcriptional program through activation of a host transcription factor, chREBP, which induces the expression of numerous glycolytic enzymes, and is important for virally mediated activation of glycolysis [18].
Glycolytic carbon can enter the tricarboxylic acid (TCA) cycle, the reactions of which provide additional energy as well as metabolic precursors that feed biosynthesis of amino acids and fatty acids (Figure 2). Many of the viruses that activate glycolysis also induce increased concentrations of TCA cycle components during infection. HCMV activates both the TCA cycle and glycolysis simultaneously, using glycolytic carbon to feed the TCA cycle and ultimately produce fatty acids that are important for infection [7]. Similarly to HCMV, vaccinia virus induces increased glutamine catabolism [13], and both HCMV and vaccinia are dependent on increased glutamine catabolism for high-titer infection 13, 19 (Figure 2). In contrast, HSV-1 does not substantially impact glycolysis, but induces pyruvate carboxylation to anaplerotically replenish TCA cycle metabolites while diverting other TCA intermediates towards pyrimidine biosynthesis [14]. In comparison to glycolysis, less is known about how various viral infections impact specific TCA cycle metabolic fluxes. Measuring TCA cycle metabolic activity is difficult due to the number of metabolic fluxes that flow into and out of the cycle. Additionally, many TCA intermediates are compartmentalized into both cytoplasmic and mitochondrial pools, further complicating metabolic inquiry.
Mitochondrial physiology plays an important role in TCA cycle function, and while not covered in detail here, a number of viruses have been implicated in targeting mitochondrial dynamics (reviewed in [20]). As stated above, KSHV encodes targeted microRNAs that negatively impact mitochondrial biogenesis and activity, potentially through an interaction with heat shock protein HSPA9 [21].HBV and HCV promote mitochondrial fission and mitophagy through dynamin-related protein 1 (Drp1), which downregulates apoptosis and may enhance viral persistence 22, 23. The HCMV UL37 protein also disrupts the reticular mitochondrial network and blocks mitochondrial apoptotic signaling [24]. While many of these activities are anti-apoptotic, with clear pro-viral consequences, they would also be predicted to have substantial effects on mitochondrial metabolism, including TCA cycle activity and cellular energetics, which are likely also important for viral infection. However, it is largely unclear how the viral proteins that target mitochondria dynamics impact core mitochondrial metabolic function.
TCA-cycle-derived citrate is transported out of the mitochondria to supply carbon for fatty acid biosynthesis, which ultimately supports lipid biosynthesis (Figure 2). A number of DNA viruses, including HCMV and vaccinia, induce lipid biosynthesis, which is necessary for their high-titer infections 7, 25, 26. HCMV activates this pathway in part by inducing the expression and activity of acetyl-CoA carboxylase (ACC1) [25], a rate-controlling enzyme of fatty acid biosynthesis. Inhibition of ACC1 or fatty acid synthase (FASN) attenuates HCMV replication at a late stage of infection without impacting viral protein accumulation 7, 25. The late timing of this defect is consistent with a role for fatty acid biosynthesis in viral assembly or envelopment. During HCMV infection, the induction of fatty acid biosynthetic enzymes is mediated by viral activation of the sterol regulatory element binding proteins (SREBPs) 1 and 2 25, 27, transcription factors that control the expression of fatty acid metabolic genes. Vaccinia infection is also sensitive to ACC1 or FASN inhibition. Pharmacological inhibition of either ACC or FASN strongly reduces viral titers and inhibits virion envelopment in a manner that could be partially rescued by the addition of exogenous palmitate, the key product of fatty acid biosynthesis [26].
Various RNA viruses also target fatty acid biosynthesis. Many studies have implicated the importance of lipid metabolism during HCV infection, which are summarized in more detail in the following reviews 28, 29. Rotavirus (RV) replication has also been shown to be susceptible to inhibitors targeting various lipid synthetic enzymes, and studies in the context of dengue virus infection show that the viral nonstructural protein 3 preferentially recruits and activates FASN at sites of viral replication 30, 31. De novo fatty acid biosynthesis and formation of lipid droplets have been found to promote tombusvirus and rhinovirus replication [32].
In addition to targeting fatty acid biosynthesis, viruses have been found to target the reverse catabolic process, fatty acid oxidation. HCMV and the Japanese encephalitis virus (JEV) inhibit fatty acid oxidation by targeting the mitochondrial trifunctional protein (MTP) which catalyzes fatty acid oxidation 33, 34. JEV inhibits MTP through its NS5 protein [33], while HCMV redirects the cellular viperin protein to inhibit MTP [34]. In contrast to these viruses that inhibit fatty acid oxidation, HCV infection requires fatty acid oxidation for its replication, which again highlights that certain aspects of viral metabolic manipulation are virus specific [35]. Combined, the data indicate that virally mediated manipulation of lipid metabolism is broadly important to viral infection, and further, that viruses have evolved diverse mechanisms to ensure that the host-cell lipid metabolic machinery is co-opted to support viral infection.
Small-Molecule Enzymatic Activities and Viral Replication, Maturation, and Assembly
As indicated above, a number of viruses induce and rely on host-cell lipid metabolism. In many cases, the exact contributions that specific lipid metabolic enzymes make towards viral infection are unclear; however, certain themes are emerging. Increasingly, it appears that viruses are targeting host-cell lipid-modifying enzymes as a means to organize viral assembly and maturation compartments (Figure 1). This theme is readily apparent in recent work on the replication complexes formed by plus-strand RNA viruses. Dengue virus, HCV, and rhinovirus all remodel the endoplasmic reticulum (ER) to generate sites for their replication using mechanisms that rely on host lipid biosynthesis. The dengue virus NSP3 protein specifically recruits fatty acid synthase to viral replication sites, which is critical for dengue replication 31, 36. For HCV, phosphatidylinositol 4-kinase III alpha (PI4KA) is an important host factor for the formation of replication compartments [37]. HCV stimulates PI4KA activity, likely through its interaction with the HCV NS5A, which induces accumulation of phosphatidylinositol 4-phosphate (PI4P) in the ER and allows for productive replication [37]. Similarly, rhinovirus replication requires redistribution of PI4P and cholesterol in ER and Golgi membranes [38]. Despite the similar lipid requirements of these related viruses, their respective ER-derived replication compartments are each unique in both conformation and size [39]. Further investigation may reveal virus-specific lipid requirements driving the formation of these structures that could be therapeutically exploitable 40, 41.
Modulation of host-cell lipid metabolic activities also appears to be important for virion assembly. HCV relies on triglyceride (TAG) and cholesterol ester biosynthesis for viral assembly, as pharmaceutical inhibition of these pathways impairs this process, reducing virus infectivity [42]. TAG mediates the interaction of HCV nucleocapsid protein with lipid droplets and plays a critical role in the stability of HCV nucleocapsids [42]. Phosphatidylserine, a core phospholipid membrane constituent, has also been implicated as being important for viral infection. Ebola particles preferentially incorporate phosphatidylserine-rich membranes into their envelope [43], and during enterovirus infection, specialized phosphatidylserine-rich vesicles facilitate mass transmission of multiple genomes to uninfected cells [44]. It is apparent that virally mediated alteration of lipid-modifying metabolic activities to shape lipid compartments has emerged as an important component of diverse viral life cycles.
Viral Infection and Specialized Virion Components
It is becoming increasingly evident that, in addition to simply elevating the production of molecular precursors to support infection, viruses are targeting specific metabolic activities to individually tailor specialized virion components (Figure 1). For example, HCMV infection induces the expression of fatty acid elongases, which increase the concentrations of saturated very-long-chain fatty acids (VLCFAs). These VLCFAs are concentrated in the envelope of HCMV virions 45, 46. Pharmaceutical inhibition or RNAi-mediated knockdown of long-chain acyl-CoA synthetase or fatty acid elongase 7, key enzymes for biosynthesis of VLCFAs, attenuates HCMV replication 45, 46. While the exact contributions that VLFCAs make towards viral replication are unclear, it is likely that specific biophysical properties of VLCFAs contribute to aspects of virion maturation, stability, or transmission. Regardless, the findings that viral infection can selectively induce specific host-cell metabolic activities that are important for infection raise the possibility that such activities would make attractive targets for therapeutic intervention.
In addition to tailoring fatty acid metabolic activities for the viral envelope, viruses target small-molecule metabolic activities to post-translationally modify viral proteins. Such modifications are diverse and include fatty-acid-based modifications such as myristoylation and palmitoylation. Myristate modification of HIV gag plays a crucial role in gag binding to the plasma membrane, an integral step that allows HIV to enter the cell 47, 48, 49. Myristoylation of virion proteins is also important for HCMV infection. The addition of a myristoyl group to pp28, a tegument protein required for productive replication, has been shown to be crucial for its proper localization and function 50, 51, 52. Another fatty-acid-based protein modification, palmitoylation, has been shown to be important for influenza, coronavirus, and HCV replication by participating in processes ranging from protein trafficking to virion assembly 53, 54, 55, 56.
The infectivity of many viruses also depends on protein glycosylation. Glycosylation of dengue viral proteins is important for viral genome replication, and mutations that abolish the glycosylation sites decrease dengue RNA replication and production of infectious virions [57]. HBV infectivity depends on a specific glycosylation of its envelope protein [58]. Further, the infectivity of both HSV and HCMV relies on specific glycosylation of their envelope glycoproteins 59, 60. HCMV has recently been found to increase the biosynthesis of various UDP-sugars, the molecular subunits that supply glycosylation reactions, by funneling increased pyrimidine biosynthesis towards UDP-sugar biosynthesis [61]. Inhibition of de novo pyrimidine biosynthesis attenuates envelope protein glycosylation, depletes UDP-sugar pools in an infection-specific manner and reduces viral titers [61]. Protein glycosylation modifications are varied and complex, so while it is clear that viral protein glycosylation is targeted by, and critical for, viral infection, numerous questions remain about how specific glycosylation patterns contribute to virus infectivity, and whether specific UDP-glycosyl transferases are recruited for modification of viral proteins.
Viral Metabolic Modulation of the Inflammatory Environment
A growing body of literature indicates that viral infection modulates small-molecule metabolism to modulate inflammation and immune function. For example, diverse viruses, including HCV, HCMV, and West Nile virus, have been observed to target sphingolipid metabolism 62, 63, 64. Sphingolipids are a diverse family of bioactive lipid species that play a role in a variety of immune processes such as lymphocyte migration, mast cell regulation, and apoptotic control (reviewed in [65]). The levels of bioactive sphingolipids, such as sphingosine-1-phosphate (S1P) and ceramide, are induced at early time points during the HCMV life cycle and were found to be important for infection [62]. HCV infection also induces expression of sphingomyelin synthases and increases sphingolipid synthesis, which, if blocked, attenuates infection [63]. Sphingolipids represent a nexus point of cellular immunological processes, with their individual small molecules participating in a variety of context-dependent signaling events. Induction of sphingolipid pools during viral infection can potentially aid infection in a variety of ways, from enhancing cell proliferation and survival to providing lipid components for replication compartments.
Prostaglandins are another family of bioactive lipids with various immunomodulatory signaling activities. As a family, prostaglandins are diverse, and their effects on viral infection have been shown to be context-dependent, with either pro- or antiviral effects observed depending on the prostaglandin and the specific virus studied. Prostaglandins have been shown to inhibit a number of viral families, including poxviruses, some herpesviruses, and retroviruses 66, 67. However, HCMV induces the expression of the rate-limiting biosynthetic enzyme of prostaglandin biosynthesis, cyclooxygenase-2 (COX2) [68]. The induction of COX2 activity increases the levels of prostaglandin E2 (PGE2), which is important for high-titer infection [69]. Further, other primate cytomegaloviruses have acquired COX2 genes from their host, and these virally expressed homologs are important for viral replication in specific contexts [70]. Influenza A virus also upregulates PGE2 during infection, which leads to an inhibition of interferon production and decreases in antigen presentation and T cell-mediated immunity [71]. While it is clear that bioactive lipid metabolism and signaling can impact viral infection in diverse ways, their specific contributions to viral infection and host immunity are only just emerging.
Metabolic Regulation and Viral Tropism
A normal cell possess a specific metabolic program based on its functional role within an organism. Given the importance of the metabolic network to infection, it follows that viral tropism could be shaped by underlying tissue-specific metabolic differences. The tropic differences displayed by retrovirus family members present one such example. All retroviruses are capable of replicating in dividing cells, whereas only lentiviruses, for example, HIV-1, are capable of replicating in non-dividing cells, such as macrophages. The levels of dNTPs are a major metabolic distinction between quiescent non-dividing cells, such as macrophages, and rapidly dividing cells, with macrophages exhibiting ∼100–200-fold reduced concentrations of dNTP precursors relative to CD4 T cells [72]. Comparative biochemical analysis of reverse transcriptases (RTs) expressed by HIV-1 versus those expressed by a variety of retroviruses unable to replicate in macrophages, demonstrate that HIV-1 RT has a much higher dNTP-binding affinity than those of non-macrophage-tropic retroviruses [72]. This elevated dNTP binding was found to be functionally important for macrophage tropism, as HIV-1 mutants that possessed RT with the reduced retroviral enzymatic kinetics were unable to replicate in macrophages but retained the ability to replicate in dividing CD4 T cells [72]. A more comprehensive review of retroviral tropism and nucleotide pools can be found in [73]. As we broaden our understanding of how host cell metabolism contributes to viral infection, the impact of tissue-specific metabolic differences on the ability of viruses to grow in different cell types will likely become increasingly apparent.
Metabolic Regulation as a Host–Pathogen Interaction
While viruses target many aspects of host-cell metabolic regulation and activity, it is clear that the host has evolved mechanisms to maintain metabolic control upon infection. SAMHD1 is one such restriction factor that limits lentivirus infection in various myeloid and dendritic lineages 74, 75, 76. SAMHD1 is a triphosphohydrolase that controls dNTP pool sizes through its dephosphorylation activity, preventing their utilization by lentiviral reverse transcriptases. [77]. Some lentiviruses have evolved mechanisms to block SAMHD1 activity, such as the Vpx accessory protein encoded by HIV-2 (but not HIV-1). Vpx binds and inhibits SAMHD1, resulting in increased dNTP concentrations and restored viral replication [76].
Various sirtuin family members have also been found to have broad antiviral effects [78]. Sirtuins are evolutionarily conserved NAD-dependent enzymes whose family members enzymatically regulate a number of protein post-translational modifications, including ADP-ribosylation, acetylation, and acylation (reviewed in [79]). As a class, sirtuins are major metabolic regulators that, upon activation, typically inhibit metabolic pathways induced by viral infection, including glycolysis [80] and fatty acid biosynthesis [81]. While the specific antiviral effects of various sirtuin family members require further study, the reversion of common virally induced metabolic phenotypes known to be important for infection likely contributes to the sirtuin family's antiviral effects.
As mentioned above, prostaglandins have long been known to have immunomodulatory activities. New studies continue to identify novel ways in which other small-molecule-based signaling contributes to immunity. One such example is the discovery of the interferon-induced enzyme cholesterol-25-hydroxylase (CH25H), which produces oxysterols such as 25-hydroxycholesterol that regulate immune responses 82, 83. Increased CH25H activity disrupts HCV genome replication by interfering with the SREBP lipid biosynthetic transcription factors and attenuating host lipid synthesis 84, 85. Other examples of small-molecule immunoregulatory activities include recent findings that intracellular metabolites signal to immune cells upon extracellular release, for example, during apoptosis or necrosis. Release of extracellular nucleotides serves as a ‘find-me’ signal that recruits monocytes and macrophages [86]. Examples such as these strongly argue that the intracellular and extracellular small-molecule environment can shape immunity. However, the field is still at a very early stage; many questions remain about the specific mechanisms involved (see Outstanding Questions).
Metabolic Similarities between Viral Infection and Oncogenesis
A number of the virally induced metabolic changes mentioned above mirror metabolic changes that occur during oncogenesis. Despite arising from diverse tissue types, cancerous cells display relatively similar metabolic programs that include activation of glycolysis, induction of nucleotide biosynthesis, and activation of fatty acid biosynthesis 87, 88, 89. Many viruses, including HBV [90], HPV 91, 92, 93, HCV 11, 15, 94, and KSHV 6, 95, 96, which play direct causal roles in human tumorigenesis, induce these various metabolic activities. The contributions that these metabolic changes make towards oncogenesis, regardless of any infectious etiology, have become a major focus in cancer biology research (reviewed in [97]). To some extent, the shared metabolic phenotypes associated with oncogenesis and viral infection likely reflect common proliferative goals between cancer cells and viruses, that is, creation of large amounts of energy and biomass for the production of progeny. However, common metabolic phenotypes between infected cells and cancer-derived cell lines also present challenges. The study of viral infection occurs predominately in the context of cancer-derived cell lines. Given their metabolic similarities, it is perhaps unsurprising that transformed cell lines produce higher viral titers relative to non-transformed tissue. However, in studying viral replication in these cells, viral metabolic phenotypes become difficult to discern from the underlying metabolic contributions associated with oncogenesis. Further, important contributions of specific viral factors responsible for driving viral metabolic reprogramming are likely be missed when studied in a cancer-cell background which already exhibits these metabolic changes. In more physiologically relevant systems, the contributions made by these factors would be evident, and could be studied with an eye towards therapeutic intervention. To avoid complications such as these, ideally, analysis of viral metabolic modulation should occur in the most physiologically relevant system possible, thereby moving away from study solely in transformed cells.
Concluding Remarks and Future Perspectives
It is clear that viruses have evolved mechanisms to target host cell metabolism to ensure their persistence. In doing so, viruses usurp the host's small-molecule resources to provide energy and molecular building blocks to support infection. Viruses also target small-molecule metabolism to organize viral maturation compartments and to modulate immune responses. The host, however, is not a passive bystander with respect to viral metabolic manipulation. Rather, the host responds at various levels, including innate mechanisms to limit viral metabolic modulation in individually infected cells as well as metabolic immunosurveillance by the immune system. As such, control of the small-molecule network continues to emerge as a core host–pathogen interaction that can determine the outcome of viral infection, making it a worthwhile goal to target viral-host metabolic regulation as a means to limit viral infection. Achieving this goal will be aided by a number of factors. First, continued elucidation of small-molecule metabolic activities that are important for viral infection, as well as the mechanisms through which they contribute to infection, will broaden the number of candidate therapeutic targets. Further, successful therapeutic development requires a sufficiently wide therapeutic window to limit viral replication without inducing toxicity. Targeting virally encoded metabolic activities has been a successful clinical strategy, for example various nucleotide analogs. However, viral enzymes can quickly evolve resistance to these targeted therapeutics, which necessitates cocktail-type therapies, increasing concerns about toxicity. Targeting host-cell metabolic activities would largely limit the ability for viruses to evolutionarily acquire resistance, but targeting host enzymes also raises concerns about potential toxicity. With these concerns in mind, virally induced host-lipid metabolic activities appear particularly attractive as candidates for therapeutic targeting, as a number of inhibitors of this type are already FDA-approved and are widely prescribed, such as the cholesterol-lowering statins.
In conclusion, we are at the very initial stages of understanding the mechanisms of virus–host metabolic interplay and how they contribute to infectious outcomes. However, the importance of these mechanisms to both viral infection and immunity continues to emerge, and therefore the area appears increasingly fertile for development of novel therapeutic strategies.
Outstanding Questions.
How does viral infection modulate host-cell metabolism in the most physiologically relevant cell types (i.e., not cancer-derived tissue)?
Is tissue-specific metabolic function a major determinant of viral tropism in vivo?
What additional mechanisms do viruses employ to direct host-cell metabolic activities towards viral infection? Are these mechanisms conserved between viral families?
Can newly identified virally induced metabolic activities be targeted to attenuate viral infection in vivo?
What additional host innate and adaptive immunity-based counter measures are employed to limit viral metabolic manipulation?
References
- 1.Das K., Arnold E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 1. Curr. Opin. Virol. 2013;3:111–118. doi: 10.1016/j.coviro.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menendez-Arias L. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr. Opin. Virol. 2014;8:1–9. doi: 10.1016/j.coviro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Lee W.A., Martin J.C. Perspectives on the development of acyclic nucleotide analogs as antiviral drugs. Antivir. Res. 2006;71:254–259. doi: 10.1016/j.antiviral.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Andrei G. Novel inhibitors of human CMV. Curr. Opin. Investig. Drugs. 2008;9:132–145. [PubMed] [Google Scholar]
- 5.Li H.C., Lo S.Y. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015;7:1377–1389. doi: 10.4254/wjh.v7.i10.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yogev O. Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathog. 2014;10:e1004400. doi: 10.1371/journal.ppat.1004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munger J. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thai M. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao L. Targeting Epstein–Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine K.A. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015;89:2358–2366. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond D.L. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6:e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritter J. Metabolic effects of influenza virus infection in cultured animal cells: Intra- and extracellular metabolite profiling. BMC Syst. Biol. 2010;4:61. doi: 10.1186/1752-0509-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontaine K.A. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014;88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vastag L. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011;7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramière C. Hexokinase activity is increased by its interaction with Hepatitis C virus protein NS5A. J. Virol. 2014;88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArdle J. HCMV Targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8:e1002502. doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towler M.C., Hardie D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1951–1956. doi: 10.1073/pnas.1310779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers J.W. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010;84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan M. Mitochondrial dynamics and viral infections: A close nexus. Biochim. Biophys. Acta. 2015 doi: 10.1016/j.bbamcr.2014.12.040. Published online January 13 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yogev O. Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. Plos Pathog. 2014;10:e1004400. doi: 10.1371/journal.ppat.1004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.J. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. U.S.A. 2014;111:6413–6418. doi: 10.1073/pnas.1321114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.J. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathog. 2013;9:e1003722. doi: 10.1371/journal.ppat.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnoult D. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer C.M. Human cytomegalovirus induces the activity and expression of acetyl-coenzyme a carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J. Virol. 2011;85:5814–5824. doi: 10.1128/JVI.02630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greseth M.D., Traktman P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014;10:e1004021. doi: 10.1371/journal.ppat.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y. Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. J. Virol. 2012;86:2942–2949. doi: 10.1128/JVI.06467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herker E., Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol. Metab. 2011;22:241–248. doi: 10.1016/j.tem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syed G.H. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol. Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur. J. Med. Chem. 2012;50:311–318. doi: 10.1016/j.ejmech.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaton N.S. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu K., Nagy P.D. Expanding use of multi-origin subcellular membranes by positive-strand RNA viruses during replication. Curr. Opin. Virol. 2014;9:119–126. doi: 10.1016/j.coviro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Kao Y.T. Japanese encephalitis virus nonstructural protein NS5 interacts with mitochondrial trifunctional protein and impairs fatty acid beta-oxidation. PLoS Pathog. 2015;11:e1004750. doi: 10.1371/journal.ppat.1004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo J.Y., Cresswell P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog. 2013;9:e1003497. doi: 10.1371/journal.ppat.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen A.L. Systems virology identifies a mitochondrial fatty acid oxidation enzyme, dodecenoyl coenzyme A delta isomerase, required for hepatitis C virus replication and likely pathogenesis. J. Virol. 2011;85:11646–11654. doi: 10.1128/JVI.05605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang W.C. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J. Virol. 2014;88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger K.L. Hepatitis C virus stimulates the phosphatidylinositol 4-kinase III alpha-dependent phosphatidylinositol 4-phosphate production that is essential for its replication. J. Virol. 2011;85:8870–8883. doi: 10.1128/JVI.00059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roulin P.S. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Chatel-Chaix L., Bartenschlager R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: the enemy inside--caught in the web. J. Virol. 2014;88:5907–5911. doi: 10.1128/JVI.03404-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu K., Nagy P.D. RNA virus replication depends on enrichment of phosphatidylethanolamine at replication sites in subcellular membranes. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E1782–E1791. doi: 10.1073/pnas.1418971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barajas D. Co-opted oxysterol-binding ORP and VAP proteins channel sterols to RNA virus replication sites via membrane contact sites. PLoS Pathog. 2014;10:e1004388. doi: 10.1371/journal.ppat.1004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liefhebber J.M. Modulation of triglyceride and cholesterol ester synthesis impairs assembly of infectious hepatitis C virus. J. Biol. Chem. 2014;289:21276–21288. doi: 10.1074/jbc.M114.582999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soni S.P., Stahelin R.V. The Ebola virus matrix protein VP40 selectively induces vesiculation from phosphatidylserine-enriched membranes. J. Biol. Chem. 2014;289:33590–33597. doi: 10.1074/jbc.M114.586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y.H. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyuncu E. Saturated very long chain fatty acids are required for the production of infectious human cytomegalovirus progeny. PLoS Pathog. 2013;9:e1003333. doi: 10.1371/journal.ppat.1003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purdy J.G. Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication. Cell Rep. 2015;10:1375–1385. doi: 10.1016/j.celrep.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalonde M.S., Sundquist W.I. How HIV finds the door. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18631–18632. doi: 10.1073/pnas.1215940109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saad J.S. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang C. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. U.S.A. 2004;101:517–522. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva M.C. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 2003;77:10594–10605. doi: 10.1128/JVI.77.19.10594-10605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seo J.Y., Britt W.J. Cytoplasmic envelopment of human cytomegalovirus requires the postlocalization function of tegument protein pp28 within the assembly compartment. J. Virol. 2007;81:6536–6547. doi: 10.1128/JVI.02852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones T.R., Lee S.W. An acidic cluster of human cytomegalovirus UL99 tegument protein is required for trafficking and function. J. Virol. 2004;78:1488–1502. doi: 10.1128/JVI.78.3.1488-1502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boscarino J.A. Envelope protein palmitoylations are crucial for murine coronavirus assembly. J. Virol. 2008;82:2989–2999. doi: 10.1128/JVI.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demers A. Palmitoylation is required for intracellular trafficking of influenza B virus NB protein and efficient influenza B virus growth in vitro. J. Gen. Virol. 2014;95:1211–1220. doi: 10.1099/vir.0.063511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grantham M.L. Palmitoylation of the influenza A virus M2 protein is not required for virus replication in vitro but contributes to virus virulence. J. Virol. 2009;83:8655–8661. doi: 10.1128/JVI.01129-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majeau N. Palmitoylation of hepatitis C virus core protein is important for virion production. J. Biol. Chem. 2009;284:33915–33925. doi: 10.1074/jbc.M109.018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naik N.G., Wu H.N. Mutation of putative N-glycosylation sites on dengue NS4B decreases RNA replication. J. Virol. 2015;89:6746–6760. doi: 10.1128/JVI.00423-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Julithe R. Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies. J. Virol. 2014;88:9049–9059. doi: 10.1128/JVI.01161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K., Compans R.W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978;84:303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- 60.Luo S. Contribution of N-linked glycans on HSV-2 gB to cell-cell fusion and viral entry. Virology. 2015;483:72–82. doi: 10.1016/j.virol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 61.DeVito S.R. Cytomegalovirus-mediated activation of pyrimidine biosynthesis drives UDP-sugar synthesis to support viral protein glycosylation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:18019–18024. doi: 10.1073/pnas.1415864111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machesky N.J. Human Cytomegalovirus Regulates Bioactive Sphingolipids. J. Biol. Chem. 2008;283:26148–26160. doi: 10.1074/jbc.M710181200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirata Y. Self-enhancement of hepatitis C virus replication by promotion of specific sphingolipid biosynthesis. PLoS Pathog. 2012;8:e1002860. doi: 10.1371/journal.ppat.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martín-Acebes M.A. The composition of West Nile Virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J. Virol. 2014;88:12041–12054. doi: 10.1128/JVI.02061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maceyka M., Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santoro M.G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997;5:276–281. doi: 10.1016/S0966-842X(97)01066-4. [DOI] [PubMed] [Google Scholar]
- 67.Clemente M.I. Prostaglandin E2 reduces the release and infectivity of new cell-free virions and cell-to-cell HIV-1 transfer. PLoS ONE. 2014;9:e85230. doi: 10.1371/journal.pone.0085230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Speir E. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 1998;83:210–216. doi: 10.1161/01.res.83.2.210. [DOI] [PubMed] [Google Scholar]
- 69.Zhu H. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3932–3937. doi: 10.1073/pnas.052713799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rue C.A. A cyclooxygenase-2 homologue encoded by rhesus cytomegalovirus is a determinant for endothelial cell tropism. J. Virol. 2004;78:12529–12536. doi: 10.1128/JVI.78.22.12529-12536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coulombe F. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Diamond T.L. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amie S.M. Intracellular nucleotide levels and the control of retroviral infections. Virology. 2013;436:247–254. doi: 10.1016/j.virol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goujon C. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J. Virol. 2008;82:12335–12345. doi: 10.1128/JVI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goujon C. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology. 2007;4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laguette N. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lahouassa H. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 2012;13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koyuncu E. Sirtuins are evolutionarily conserved viral restriction factors. mBio. 2014;5 doi: 10.1128/mBio.02249-14. e02249-02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto H. Sirtuin functions in health and disease. Mol. Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 80.Hallows W.C. Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation. J. Biol. Chem. 2012;287:3850–3858. doi: 10.1074/jbc.M111.317404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purushotham A. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chalmin F. Oxysterols regulate encephalitogenic CD4(+) T cell trafficking during central nervous system autoimmunity. J. Autoimmun. 2015;56:45–55. doi: 10.1016/j.jaut.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Gold E.S. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. U.S.A. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiang Y. Identification of cholesterol 25-hydroxylase as a Novel Host restriction factor and a part of the primary innate immune responses against hepatitis C virus infection. J. Virol. 2015;89:6805–6816. doi: 10.1128/JVI.00587-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y. Interferon-inducible cholesterol-25-hydroxylase inhibits hepatitis C virus replication via distinct mechanisms. Sci. Rep. 2014;4:7242. doi: 10.1038/srep07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elliott M.R. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 88.Tong X. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr. Opin. Genet. Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhajda F.P. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition (Burbank Los Angeles County Calif.) 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 90.Teng C.F. Activation of ATP citrate lyase by mTOR signal induces disturbed lipid metabolism in hepatitis B virus pre-S2 mutant tumorigenesis. J. Virol. 2015;89:605–614. doi: 10.1128/JVI.02363-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai D. Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and modulates host cell metabolism. PLoS ONE. 2013;8:e75625. doi: 10.1371/journal.pone.0075625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazurek S. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem. J. 2001;356:247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mazurek S. Metabolic cooperation between different oncogenes during cell transformation: interaction between activated ras and HPV-16 E7. Oncogene. 2001;20:6891–6898. doi: 10.1038/sj.onc.1204792. [DOI] [PubMed] [Google Scholar]
- 94.Ripoli M. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 2010;84:647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delgado T. Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delgado T. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012;8:e1002866. doi: 10.1371/journal.ppat.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boroughs L.K., DeBerardinis R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]