Abstract
Invariant NKT (iNKT) cells, which express the invariant Vα14Jα18 TCR that recognizes lipid antigens, have the ability to rapidly respond to agonist stimulation, producing a variety of cytokines that can shape both innate and adaptive immunity. iNKT cells have been implicated in host defense against microbial infection, in anti-tumor immunity, and a multitude of diseases such as allergies, asthma, graft versus host disease, and obesity. Emerging evidence has demonstrated crucial role for mammalian target of rapamycin (mTOR) in immune cells, including iNKT. In this review we will discuss current understanding of how mTOR and its tight regulation control iNKT cell development, effector lineage differentiation, and function.
Keywords: iNKT cell, mTOR, Raptor, Rictor, TSC1/2, diacylglycerol kinases, RasGRP1, PTEN, Ras, CARMA1, iNKT1, iNKT2, iNKT17, signal transduction
Introduction
Invariant natural killer T (iNKT) cells harbor the invariant Vα14-Jα18 (iVα14) TCRα chain in mice and the invariant Vα24-Jα18 TCR in humans paired with restricted Vβ chains [1–3]. Unlike conventional TCRαβ T (cαβ T) cells that recognize the major histocompatibility complex (MHC)-peptide complex, iNKT cells selectively recognize lipid-antigens such as endogenous, microbial derived, and synthetic ligands presented by MHC class I-like CD1d molecules via the iVα14 TCR [4–6]. Engagement of iVα14TCR with endogenous ligand-CD1d complexes presented by CD4+CD8+ double positive (DP) thymocytes in the thymic cortex leads to positive selection and generation of iNKT cells. Following TCR stimulation with a synthetic ligand α-galactosylceramide (α-GalCer), mature iNKT cells rapidly release various cytokines such as IL-4, IL-17, IL-10, IL-13, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α [7–9], which enable them to play important roles in both innate and adaptive immune responses. Although iNKT cells only comprise a small portion of T cells, their roles have been described in various immune responses and diseases, including tumor surveillance, defense against microbial infection, as well as pathogenesis of autoimmune diseases, graft-versus-host disease, and obesity [10–13].
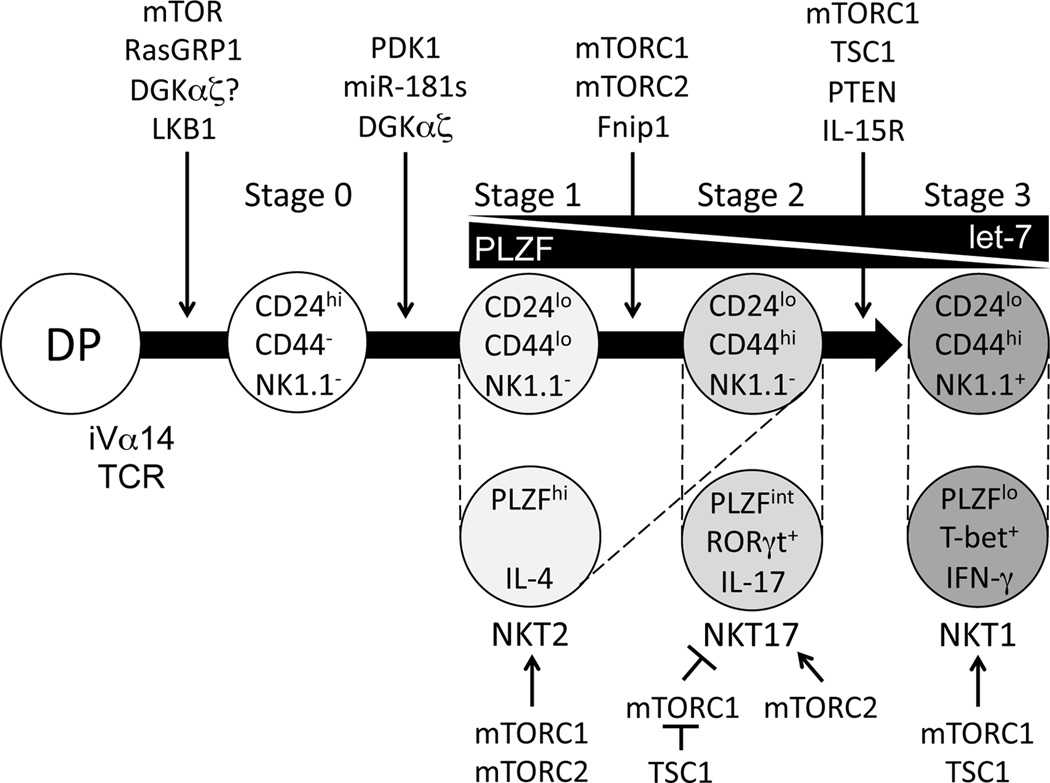
Traditionally, thymic development of iNKT cells from CD4+CD8+ DP precursor has been defined into four stages based on surface levels of CD24, CD44, and NK1.1 (Figure 1): stage 0 (CD24+CD44−NK1.1−), stage 1 (CD24−CD44−NK1.1−), stage 2 (CD24−CD44+NK1.1−), and terminally matured stage 3 (CD24−CD44+NK1.1+) [1, 2]. More recently, iNKT cells have been classified into multiple terminally differentiated effector lineages that include IFN-γ-producing iNKT1, IL-4-producing iNKT2, and IL-17-producing iNKT17 lineage [14–16]. In addition, IL-10-producing iNKT10, T follicular helper (Tfh)- and regulatory T cell (Treg)-like iNKT cells (iNKTFH) have also been recently reported [17–21]. Similar to Th lineages, iNKT effector lineages are governed by critical transcription factors including RORγt, T-bet, Gata3, and PLZF [2, 14, 22, 23]. iNKT1 cells express low levels of promyelocytic leukemia zinc-finger (PLZF) but high levels of T-bet (PLZFlowT-bet+); iNKT2 cells are PLZFhigh; while iNKT17 are PLZFintRORγt+. iNKT1 cells mostly reside in the CD44+NK1.1+ stage 3 population, iNKT2 cells reside in both stage 1 and stage 2 populations, and iNKT17 cells are restricted to the CD44+NK1.1−ICOS+ population [22–25].
Figure 1. iNKT cell development and effector lineages.
iNKT cells are originated from CD4+CD8+ DP thymocyte precursors that express the iVα14TCR. Engagement of the iVα14TCR with CD1d bearing self-lipid ligand expressed by DP thymocytes ensures the generation of iNKT cell lineages. Immature iNKT cells from DP thymocytes undergo four maturation stages. Each stage is characterized by different expression of CD24, CD44, and NK1.1 on their surface. Based on their distinctive expression of transcription factors and cytokines, iNKT cell effector subsets can be defined into iNKT1, iNKT2, and iNKT17 cells. These two categories are closely correlated as marked with dotted lines. Various receptors, signaling molecules, miRNAs, and transcription factors dictating iNKT maturation are depicted.
Roles of intracellular signaling pathways in iNKT development and function
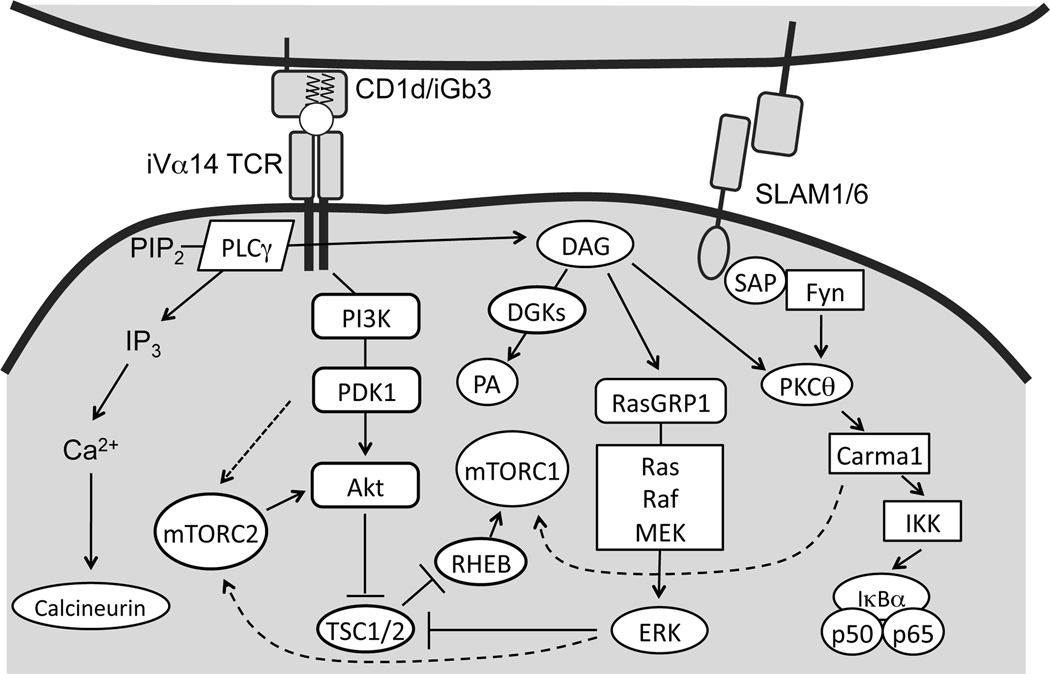
Multiple receptors, including the iVα14TCR, co-stimulatory molecules, IL-15R, IL-7R, and intracellularly located Vitamin D receptor (VDR) transduce signals that are important for iNKT cell development and/or function. Engagement of the iVα14TCR triggers the activation of proximal tyrosine kinases Lck and Zap70 and subsequently activates phospholipase Cγ1 (PLCγ1), which hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3) as second messengers [26–28] (Figure 2). Diacylglycerol (DAG) is an essential second messenger downstream of the TCR that activates several signaling pathways. The membrane-bound DAG induces activation of the Ras guanyl nucleotide-releasing protein 1 (RasGRP1)-Ras-extracellular signal regulated kinase 1/2 (Erk1/2) pathways, which is critical for proper iNKT cell development [29, 30]. IP3 induces calcium release from the endoplasmic reticulum and subsequent extracellular calcium influx into the cytosol, leading to calcineurin-mediated dephosphorylation and nuclear translocation of nuclear factor of activated T cells (NFAT). NFAT induces maximal gene expression of both early growth response 1 and 2 (Egr1 and 2). This pathway is critical for early iNKT cell development [31, 32]. Additionally, Egr2 directly binds to the Zbtb16 promoter and activates PLZF expression [31, 33]. PLZF-deficient iNKT cells in mice show developmental blockage at stage 1 and fail to differentiate to cytokine-producing cells, highlighting the importance of this molecule for iNKT cells to acquire effector function [34, 35].
Figure 2. TCR signaling and mTOR activation in T cells.
Engagement of the TCR as well as the iVα14TCR leads to activation of PLCγ1, which hydrolyzes membrane bound PIP2 into membrane bound DAG and soluble IP3. IP3 binds to its receptor on ER, leading to subsequent influx of calcium and activation of calcinuerin. Activated calcinuerin dephosphorylates NFAT to induce its nuclear localization and activation of transcription of target genes. DAG associates with and activates RasGRP1, leading to the activation of the Ras-Mek1/2-Erk1/2 pathway. In thymocytes, this pathway acts upstream of TSC1/2-mTOR as well as PI3K/Akt to induce both mTORC1 and mTORC2 activation. Together with the SLAM/SAP/Fyn pathway, DAG also associates with and activates the PKCθ-Carma1/Bcl10-IKK-NF-κB pathway. Carma1 also promotes mTORC1 activation following TCR engagement. In thymocytes, DGKs terminate DAG by converting it to phosphatidic acid (PA) and negatively control the activation of both mTORC1 and mTORC2.
Signaling through the signaling lymphocytic-activation molecule (SLAM) family is also required for early iNKT cell maturation. Homotypic interactions of SLAM molecules such as SLAMSF1 and SLAMSF6 on iNKT cells and thymocytes activate the downstream SLAM adaptor protein (SAP)-FynT pathway, which is critical for iNKT cell development and function in both human and mice [36–39]. The SLAM-SAP-FynT pathway, together with DAG, activates NF-κB signaling cascade via protein kinase θ (PKCθ) and the Bcl10 adaptor protein. The PKCθ-Bcl10-IKK-NFκB pathway plays essential roles in the ontogeny of functional iNKT cells at least in part by increasing expression of anti-apoptotic proteins such as Bcl-xL [40–43]. Interestingly, although CARMA1 and Malt1 (Mucosa-associated lymphoid tissue lymphoma translocation protein 1) are crucial for TCR induced NFκB activation, they are dispensable for iNKT cell development or survival [44], suggesting that SLAM-SAP–FynT axis activates NFκB via PKCθ-Bcl10 but bypassing CARMA1 and Malt1 to promote iNKT cell development.
Homeostasis and terminal differentiation of iNKT cells are highly dependent on IL-15R signal, which induces the expression of pro-survival molecules Bcl-xl and Bcl-2 and T-bet. Mice deficient of either IL-15 or IL15R display iNKT cell terminal maturation defect and have severely decreased stage 3 iNKT cells [45–48]. T-bet directly induces CD122 (IL-15Rβ) transcription and subsequently promotes iNKT cell survival [49]. T-bet deficiency also results in defective terminal maturation of iNKT cells [47].
Vitamin D binds to the intracellular VDR, a member of the steroid thyroid super family of nuclear receptors [50]. VDR signals to regulate T cell responses, but not T cell development. TCR induced PLCγ1 expression is dependent on Vitamin D and VDR, which is critical for T cell activation [51]. VDR deficient mice display normal T cell development but have diminished iNKT numbers in thymus and periphery. VDR deficient iNKT cells display defective terminal maturation as observed in T-bet deficient mice. Intriguingly, VDR deficient iNKT cells express normal levels of CD122 even though lack of T-bet expression [52]. The exact mechanisms by which VDR control iNKT development and function remain unclear.
Finally, IL-7 regulates T cell homeostasis by enhancing survival and proliferation of naive and memory T cells. Similarly, it has been documented that IL-7 also play roles in the expansion and/or survival of iNKT cells [53]. A recent report demonstrated that the survival requirements are distinct among effector NKT subsets. Tissue derived iNKT-17 cells are maintained in the absence of IL-15. However, in the absence of IL-7, their survival has been dramatically impaired compared to conventional iNKT cells. This strict dependence on IL-7 does not affect intracellular STAT or TCR signaling pathways, but significantly modulates the PI3K/Akt/mTOR pathway, suggesting that IL-7 controls tissue homeostasis and survival of iNKT17 cells by TCR-independent but mTOR-dependent mechanisms [54].
mTOR signaling complexes
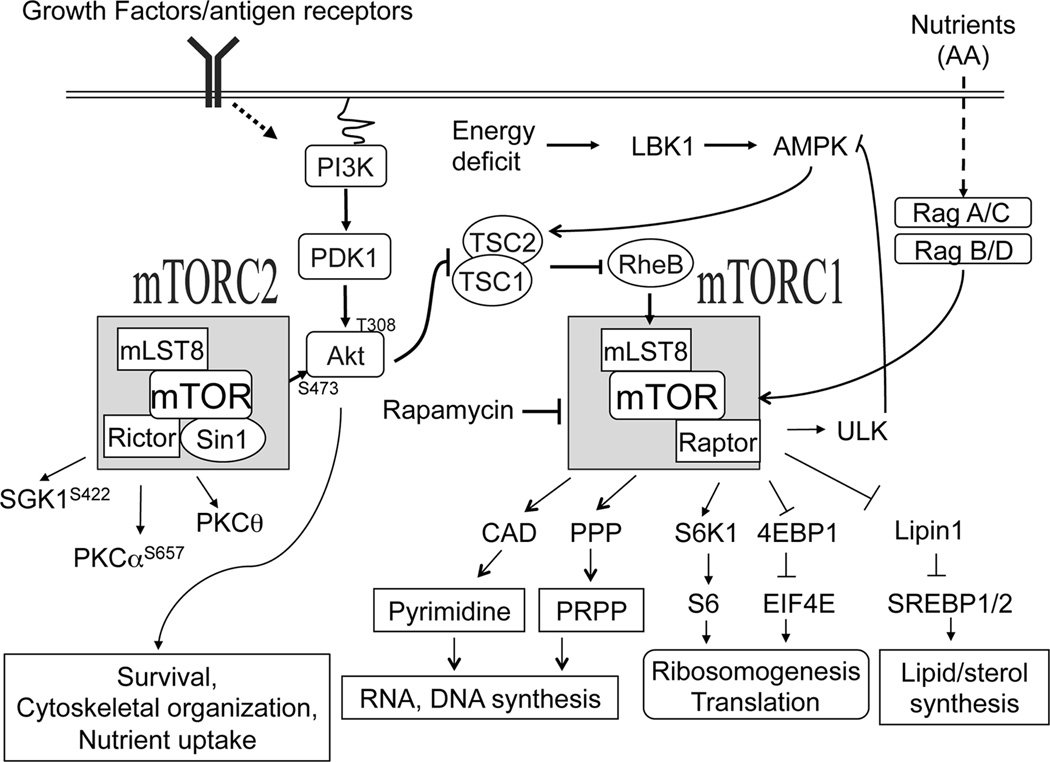
The serine/threonine kinase mTOR responds to diverse environmental cues such as nutrients, growth factor, cytokines and other stress signals to regulate metabolism, cell growth, survival, differentiation, autophagy and activation [55–62]. It forms two functionally distinct complexes: mTOR complex 1 (mTORC1) and mTORC2 (Figure 3). In mTORC1, mTOR associated with Raptor, GβL, and DEPTOR, whereas mTORC2 is composed of mTOR, Rictor, GβL, Sin1, PRR5/Protor-1, and DEPTOR [63]. While mTORC1 is sensitive to rapamycin, mTORC2 is insensitive to acute rapamycin treatment [64].
Figure 3. TSC1/2-mTOR signaling.
mTOR signals through two functionally distinct complexes: mTORC1 and mTORC2. In addition to mTOR and several other components, mTORC1 and mTORC2 contain distinct adaptor molecules raptor and rictor, respectively. mTOR senses and integrates various extracellular signals such as growth factor, cytokines, immune receptor, amino acids and intracellular signals including stress and energy levels. The Small GTPase Rheb in its GTP bound active state directly activates mTORC1. TSC1/2 complex acts as a negative regulator of mTORC1 via the GAP activity of TSC2 to RheB. Growth factors and antigen receptors signal through the PI3K-PDK-Akt pathway to activate mTORC1 and mTORC2. In contrast, nutrients activate mTORC1 through Rag GTPases. Cellular energy deficits can take a direct route to suppress mTORC1 activation through LKB1 and AMPK, which promote TSC2 activity. mTORC1 phosphorylate multiple targeting proteins such as S6K1, 4E-BP’s, Lipin1, CAD (carbamoylphosphate synthetase, aspartate transcarbamoylase, and dihydroorotase), and 5’-hosphoribosyl-1’-pyrophosphate (PRPP) that promote anabolic processes such as protein synthesis, lipid/sterol synthesis and de novo pyrimidine synthesis. mTORC1 also prevents autophagy indirectly by inhibiting AMPK through direct phosphorylation of ULK1. Although PI3K is important for mTORC2 activation, the exact mechanisms that activate mTORC2 remain elusive. mTORC2 also phosphorylates multiple intracellular signaling molecules such as Akt at the serine 473 residue, PKCα at S657, PKCθ at S660/676, and SGK at S422 to regulate cell survival, nutrient uptake, cytoskeletal organization, and Th differentiation.
The small GTPase Ras homologue enriched in the brain (RheB), is critical for growth factor-induced mTORC1 activation [65] and is inactivated by TSC2 through its GAP activity. TSC1 associates with and stabilizes TSC2 [56]. The PI3K-Akt pathway phosphorylates TSC2, leading to its degradation and subsequent mTORC1 activation [66–68]. mTORC1 promotes cell growth and proliferation through phosphorylation of many substrates: phosphorylation of the 70 kDa ribosomal S6 kinase (S6K1) and the translational repressor 4E-BP1 to increase protein synthesis [65, 69, 70]; phosphorylation and activation of CAD (Gln-dependent carbamoyl-phosphate synthase, Asp carbamoyltransferase, dihydroorotase), a key rate limiting enzyme of the de novo pyrimidine pathway [71]; activation of the pentose phosphate pathway aiding in nucleotide synthesis; phosphorylation and inactivation of LIPIN1, a phosphatidic acid phosphatase and an inhibitor of sterol regulatory element-binding proteins (SREBPs), to activate SREBPs for lipid/sterol biosynthesis[72]. Moreover, S6K1 can directly regulate lipid synthesis and pyrimidine synthesis through direct phosphorylation of SREBPs and CAD, respectively [73, 74]. Amino acids activate mTORC1 in a TSC1/2 independent manner through direct triggering the activation of RAG GTPases via a sensing cascade that include Ragulator and v-ATPase at lysosome [75].
mTORC2 phosphorylates Akt at S473 located in the hydrophobic motif which is required for maximal Akt activation and for Akt mediated phosphorylation on its downstream targets such as of FoxO1/3 proteins. Increased Akt activity promotes nutrient uptake and cell survival [70]. Besides mTORC2, the PI3K-PDK1 pathway phosphorylates Akt at threonine308 residue within its catalytic domain [76]. PKCα regulates cytoskeleton arrangement and cell polarity through direct phosphorylation of paxilin and Rho GTPase. mTORC2 controls this event by phosphorylating PKCα at S657 [64, 77]. In T cells, mTORC2 also phosphorylates PKCθ at S660/676 to promote Th2 differentiation, while Akt phosphorylation at S473 promotes Th1 differentiation [78]. In addition, mTORC2 phosphorylates the serum- and glucocorticoid-induced protein kinase 1 (SGK1) at S422 shown to be implicated in cell survival and lipid metabolism; however underlying mechanisms are not known [79]. Additionally, SGK1 serves as a salt-sensor to promote Th17 differentiation [80, 81].
T cell receptor induced mTOR activation
TCR proximal signaling events drive PI3K-PDK1-Akt signaling to induce TSC2 degradation, RheB activation, and subsequent mTORC1 activation [82]. Beside the PI3K-PDK1-Akt axis, the DAG-RasGRP1-Ras-Erk1/2 pathway also stimulates TCR-induced activation of mTORC1 and mTORC2 (Figure 2) [83]. In thymocytes, both TCR engagement and low concentrations of PMA, a functional analogue of DAG, can trigger mTORC1, mTORC2, and PI3K/Akt activation. Additionally, expression of constitutively active KRas (CAKRas) in thymocytes enhances mTORC1, mTORC2, and PI3K-PDK1-Akt activation. In contrast, TCR stimulation of RasGRP1 deficient thymocytes fails to activate not only the Ras-Erk1/2 axis but also PI3K-Akt, mTORC1 and mTORC2 [83]. Furthermore, treatment of primary T cells with a Mek1/2 inhibitor or expression of dominant negative Mek1 in a T cell line decreased PI3K-Akt, mTORC1 and mTORC2, suggesting that Mek1/2-Erk1/2 function as upstream activators for TCR-induced PI3K and mTOR activation. The ability of the RasGRP1-Ras-Mek1/2-Erk1/2 cascade to activate mTORC1 is consistent with the finding that Erk1/2 and Erk1/2-activated kinase RSK can phosphorylate and inhibit TSC2 in cell line models [68, 84]. However, whether Erk1/2 indeed directly or indirectly via Rsk1 phosphorylate TSC2 to promote mTORC1 activation and how Erk1/2 lead to mTORC2 activation in T cells remains to be demonstrated.
In addition to the RasGRP1-Ras-Erk1/2 pathway, a recent report has revealed that the PKCθ-CARMA1 pathway is also required for mTORC1 activation following TCR engagement [85]. Although IKKβ associates with TSC1 to promote TNFα-induced mTORC1 activation and CAIKKβ contributes to elevated mTOR signaling in several tumor types [86], expression of a constitutively active form of IKKβ does not elevate mTOR signaling in thymocytes or primary T cells (our unpublished observations). Thus, CARMA1 may promote mTOR signaling bypassing the IKK complex in T cells.
TCR engagement and PMA treatment increase mTORC2 activation in both thymocytes and primary T cells as well as in T cell lines using Akt phosphorylation at S473 as readout [27, 87]. Deficiency of RasGRP1 or expression of CAKRas in thymocytes dampened or enhanced Akt phosphorylation at S473, respectively, in response to TCR stimulation. While these studies revealed that both PI3K-PDK-Akt and RasGPR1-Ras-Erk1/2 pathways play critical roles in mTORC2 activation [83], the precise mechanisms that lead to mTORC2 activation remain elusive.
mTOR signaling for iNKT cell development
Very recently, several studies have provided genetic evidence demonstrating the importance of mTOR in iNKT cells. T cell specific mTOR deficient (mTORf/f-CD4Cre) mice exhibited minimal changes in cαβ T cell populations in the thymus [88, 89], but virtual absence of iNKT cells in the thymus, spleen, and liver [89]. The decrease of iNKT cells can be detected at stages 0 and 1 and dramatically exacerbates in stages 2 and 3 in mTOR deficient thymus, indicating that mTOR not only plays a critical role for early iNKT cell development but may also contribute to late stage iNKT cell development [89].
Selective ablation of mTOR in late stages of iNKT cell development will be needed to clearly understand the role of mTOR in late stage iNKT cell development. Several studies have further delineated the roles of mTORC1 and mTORC2 in iNKT cells. Studies using T cell specific Raptor deficient mice have shown that the number of iNKT cells is comparable at stages 0 and 1, and decreased at stage 2 and 3 [89, 90], suggesting that mTORC1 is required for the entry into stage 2. Chimeric mice reconstituted with mixed wild type (WT) and Raptor-deficient bone-marrow cells at 1:8 ratio showed that the ratio was maintained at about 1:8 in stage 0 and 1 iNKT cells, but increased to about 1:1 and 98:1 in stages 2 and 3, respectively [89], suggesting that mTORC1 intrinsically controls the transition of iNKT cell development not only from stage 1 to 2, but also from stage 2 to 3. Interestingly, PLZF is also crucial for iNKT cell maturation beyond stage 1 [35]. Raptor deficient iNKT cells display reduced nuclear localization of PLZF, thereby sequestering it from accessing target gene promoters [89]. These observations suggest that mTORC1 may promote PLZF nuclear localization for efficient iNKT cell maturation from stage 1 to stage 2. It will be interesting to determine the way in which mTORC1 modulates PLZF localization and function.
In addition to being regulated by mTORC1, PLZF can also control mTORC1 signaling. PLZF has been reported to activate expression of regulated in development and DNA damage responses 1 (REDD1) in human cell lines [91]. REDD1 suppresses mTORC1 activity by releasing TSC2 from its growth factor-induced association with inhibitory 14–3–3 proteins [92]. Additionally, PLZF and mTORC1 signaling are both regulated by microRNA let-7. miR-let-7 directly targets the 3’UTR of Zbtb16 mRNA, which encodes PLZF, resulting in decreased PLZF protein expression [93]. PLZF and miR-let-7 expression are inversely correlated during the iNKT cell developmental stages. Intriguingly, miR-let-7 also represses mTOR signaling through downregulation of the amino acid sensing and glucose metabolism pathways [94, 95]. These findings are suggestive there is crosstalk between mTOR signaling pathways and PLZF during iNKT cell development.
Two groups have reported that mTORC2 also promotes iNKT cell development [96, 97]. Both studies found modest decreases of total iNKT cells in the thymus. However, there are noted differences between these two reports with respect to the developmental stages affected by Rictor deficiency. While Prevot et al reported decreases of stage 0, 2, and 3 iNKT cells in Rictor deficient thymus, Wei et al found only stage 2 and 3 iNKT cells were decreased in these mice. There were also discrepancies in the contribution of mTORC2 to survival and proliferation of iNKT cells between these studies. In contrast to Raptor deficient iNKT cells, nuclear localization of PLZF was not altered by Rictor deficient iNKT cells, suggesting mTORC2 controls iNKT cell development through PLZF-independent mechanism [97].
Pathways leading to mTOR activation for iNKT cell development
Several reports have shed light on the mechanism by which mTOR is activated for directing iNKT cell development. Treatment of iNKT cells with α-Galcer induces mTOR activation in iNKT cells, indicating that iVα14TCR, similar to TCR in cαβ T cells, is able to trigger mTOR activation [98]. Both growth factor and TCR induced mTORC1 activation requires RheB [99, 100]. Interestingly, RheB deficiency only caused 50% reduction of stage 2 and 3 thymic iNKT cells [96]. Thus developmental defects in RheB deficient mice are less severe than in Raptor deficient mice, suggesting that both RheB-dependent and -independent mTORC1 activation must occur in iNKT cells to facilitate their development. mTORC1 can sense the nutrient availability, such as amino acids through Rag A/C or Rag B/D GTPases, which serve as an alternative means to induce mTORC1 activation. It remains to determine if this alternative pathway plays an important role in iNKT cells.
As mentioned earlier, both RasGRP1 and PI3K-PDK1 are important for mTORC1 and mTORC2 activation in developing thymocytes following TCR engagement [83, 101]. Similar to the effects of mTOR deficiency, RasGRP1 deficiency also caused severe iNKT cell intrinsic developmental defects starting at stage 0 [29]. Thus mTOR may function as a downstream effector molecule of the RasGRP-Ras-Erk1/2 pathway to ensure early iNKT cell development. Mutant mice carrying inactivated PDK1 displayed severe decreases of iNKT cells and impaired maturation [101]. However, in contrast to RasGRP1 deficiency, PDK1 deficiency appears to cause defective transition of stage 0 iNKT cells to stage 1, one stage after mTOR or RasGRP1 deficiency. Expression of crucial nutrient transporters CD71 and CD98 is decreased in PDK1 deficient iNKT cells, suggesting that PDK1-Akt signaling is critical for metabolic requirement required for iNKT cell development. Although CARMA1 is important for mTORC1 activation in T cells, CARMA1 is dispensable for iNKT cell development and survival despite their critical role in TCR induced NF-κB signaling pathway [44]. Thus, CARMA1 may play a minimal role for mTORC1 activation during iNKT cell development.
Negative control of mTOR signaling for iNKT cell development: TSC1, LKB1 and and AMPK1
Due to its convergent role in metabolic pathways, mTOR signaling requires tight control during iNKT cell development. As mentioned earlier, TSC1 inhibits mTORC1 by stabilizing TSC1/2 complex [56]. In T cells as well as in many other immune cell lineages, deletion of TSC1 causes virtually absence of TSC2 [102–107]. Multiple studies have demonstrated that TSC1 is critical for T cell quiescence, survival, and anergy, proper effector lineage differentiation, CD8 T cell-mediated primary and memory responses, and Treg suppressive function [105, 107–109]. In addition, TSC1 plays critical roles in B cell maturation [110, 111], in the development and/or function of macrophages [103, 112, 113] and dendritic cells, and toll-like receptor (TLR)-medicated responses [104, 113–115] as well as survival and activation of mast cells [102].
TSC1 deficiency causes a 2 to 3 fold decrease in iNKT cells in the thymus and peripheral lymphoid organs and severe blockade of iNKT cell terminal maturation, leading to accumulation of stage 2 but drastic decreases of stage 3 iNKT cells [23, 116]. Such developmental blockade is at least in part due to increased mTORC1 signaling, decreased T-bet expression, and increased death of stage 3 iNKT cells [23].
The tumor suppressor LKB1 directly phosphorylates AMP-activated protein kinase (AMPK), a central metabolic sensor. Activated AMPK suppresses mTORC1 by phosphorylation and enhances TSC2 activity in response to low intracellular energy status [62]. LKB1 deficient mice have normal number of DP thymocytes, but decreased TCRβhigh mature thymocytes and iNKT cells. However, detailed analysis of iNKT cell developmental stages was not reported [117]. Intriguingly, AMPK deficient mice appear to have normal numbers of cαβ T and iNKT cells, suggesting that LKB1 may bypass AMPK to promote iNKT development [117]. Mice lacking AMPK-interacting Fnip1 manifest severe decreases of iNKT cells starting at stage 2, correlated with increased mTORC1 signaling and increased apoptosis [118]. However, long-term rapamycin administration in Fnip1 deficient mice did not rescue impaired iNKT cell development. Although it was concluded that Fnip1 deficiency inhibited iNKT cell development independent on mTORC1 [118], caution must be taken due to severe iNKT cell developmental defect at the same developmental stage in the absence of mTORC1 [89]; restoration of mTORC1 signaling to normal levels and severe inhibition of mTORC1 in Fnip1 deficient mice by different regimes of rapamycin treatment could lead to distinct outcomes. Thus it is still unclear if Fnip1 regulates iNKT cell development through modulating mTOR signaling.
Negative control of mTOR signaling for iNKT cell development: diacylglycerol kinases and PTEN
Several studies have also identified multiple upstream negative regulators that control mTOR signaling in T cells. As mentioned earlier, TCR stimulation induces transient accumulation of DAG in thymocytes to activate several pathways important for mTOR activation. DAG kinases (DGKs) are a family of ten enzymes that phosphorylate DAG to produce phosphatidic acid. DGKα and ζ, the predominant isoforms expressed in T cells, are important regulators in preventing TCR induced DAG signaling [119–122]. Although genetic deletion of either DGKα or ζ does not obviously affect iNKT cell numbers and deletion of DGKζ only impairs iNKT17 differentiation via cell extrinsic mechanisms [123], simultaneous ablation of both enzymes resulted in drastic decreases of iNKT cells in the thymus and in peripheral lymphoid organs [124], correlated with prolonged DAG accumulation, elevated Ras-Erk1/2 and PKCθ-IKK signaling, and enhanced activation of both mTORC1 and mTORC2 activities in DP thymocytes [83, 125]. In DGKα and ζ double knockout mice, stage 1 to stage 3 iNKT cells are decreased but stage 0 iNKT cells were not examined. The remaining iNKT cells in these mice are mostly stage 2 cells, suggesting that DGKα and ζ promote both early and terminal iNKT cell maturation [124]. Interestingly, in mice expressing CAKRas in thymocytes, iNKT cell development is selectively blocked during the transition from stage 2 to 3 and was associated with elevated mTOR signaling and decreased T-bet expression [124], which coincided with terminal maturation defect observed in TSC1 deficient mice. Together, data from TSC1, DGKα and ζ double knockout mice, and CAKRas mice suggest that DGKα and ζ inhibit the Ras-Erk-mTORC1 cascade in order to ensure iNKT cell terminal maturation.
PTEN counteracts PI3K by hydrolyzing PI3K product, phosphatidyl-inositol-3,4,5-triphosphate (PIP3), and inhibits both mTORC1 and mTORC2 activation. In PTEN deficient mice, thymic iNKT cells accumulate but are developmentally blocked during terminal maturation from stage 2 to 3 [96, 126]. Such developmental blockade mimics TSC1 deficiency, suggesting the possibility of elevated mTORC1 signaling as a causal factor for the developmental blockade in PTEN deficient mice. However, different from TSC1 deficiency, total iNKT cell numbers are increased in PTEN deficient mice, which is likely due to increased mTORC2 and subsequent Akt activities. Tight control of PTEN activity also appears critical for iNKT cell development. Two recent reports have demonstrated that miR-181 is essential for early iNKT cell development and homeostasis by targeting PTEN [127, 128]. Deficiency of miR-181s leads to increased PTEN, decreased PI3K signaling and altered metabolism. Similar to RasGRP1 and mTOR deficiency, miR-181s deficiency also causes severe decreases of iNKT cells starting at stage 0 [127, 128]. Together, these studies suggested that PI3K-PTEN axis is central for iNKT cell development possibly by controlling mTOR signaling.
mTOR and its tight regulation for iNKT cell activation and effector lineage differentiation
Following agonist stimulation, iNKT cells rapidly produce various cytokines and expand drastically in vitro and in vivo. Deletion of Raptor in mature iNKT cells in Raptorf/f-CreER mice following tamoxifen treatment results in decreased production of IL-4, IFN-γ and TNF-α in vitro and in vivo following α-GalCer treatment [89]. Additionally, α-GalCer-induced in vivo iNKT cell expansion is abolished in the absence of Raptor [89]. An important consequences following α-GalCer injection is the activation of hepatic iNKT cells, which results in acute hepatitis in part due to TNFα-induced liver injury [129, 130]. Raptor deficient mice are resistant to α-GalCer-induced autoimmune hepatitis, further supporting that mTORC1 is important for iNKT cell activation [89].
As mentioned above, iNKT cells are programmed to different effector lineages during their maturation in the thymus. Raptor deficient mice were reported to have reduced iNKT1, increased iNKT2, and normal iNKT17 cell ratios, based on expression of their corresponding signature transcription factors [96]. Due to severe decreases of iNKT cells in these mice [89, 90, 96], total iNKT2 and iNKT17 numbers would be expected to be drastically decreased. Thus, the importance of Raptor/mTORC1 in the iNKT2/iNKT17 differentiation remains to be clearly defined. Two studies have also implicated mTORC2 in iNKT effector lineage differentiation [96, 97]. Both studies revealed decreased frequencies of iNKT2 but normal frequencies of iNKT1 cells in Rictor deficient mice, suggesting that mTORC2 is required for iNKT2 differentiation but dispensable for iNKT1 differentiation. However, the effect of mTORC2 deficiency on iNKT17 effector lineage differentiation appeared different between these studies [96, 97]. While one study demonstrated decreased iNKT17 ratios in Rictor deficient mice [96], the other study did not reveal significant differences in iNKT17 ratios [97]. The reason for such discrepancy remains unclear.
The ratio between iNKT1 and iNKT17 cells is influenced by genetic background [22]. In C57BL/6J mice, most iNKT cells are iNKT1 cells and iNKT17 cells are extremely rare, leading to iNKT1 predominance over iNKT17 [22, 23]. Multiple transcription factors including PLZF, RORγt, and T-bet participate in iNKT1/17 lineage differentiation and contribute to the iNKT1/17 balance [2, 14, 22, 23]. Interestingly, TSC1 deficient iNKT cells express elevated RORγt and ICOS but decreased T-bet and PLZF. In contrast to normal iNKT cells, TSC1 deficient iNKT cells are predominantly iNKT17 cells and iNKT1 cells become a minor population. Such reversal of iNKT1/iNKT17 predominance of TSC1 deficient iNKT cells is at least partially resulted from elevated mTORC1 signaling, decreased T-bet expression, and increased ICOS expression [23]. How TSC1 controls the expression of these molecules remains to be investigated.
mTOR in iNKT-mediated antitumor immunity
Numerous studies have implicated iNKT cells in tumor surveillance. iNKT cells are required for IL-12-induced tumor rejection and for protection from chemical-induced spontaneous tumors [131, 132]. Upon antigenic stimulation, iNKT cells not only directly attacks tumors through direct cytotoxicity, but also indirect activation of other immune cells such as DCs, NK cells, and cytotoxic T cells to mount effective anti-tumor immune responses. Considerable effort has been spent to exploit the immunoregulatory functions of iNKT cells for cancer immunotherapy via iNKT cell transfer and/or repetitive stimulation of iNKT cells using iVα14TCR ligands. iNKT cell immunotherapies have shown promising outcomes in several tumor settings [133–136]. However, iNKT cell anergy is a significant challenge to the success of iNKT cell immunotherapy. Following in vivo activation by α-GalCer, iNKT cells undergo dynamic changes, which are characterized by robust cytokine secretion, clonal expansion, homeostatic contraction, and then acquisition of an anergic phenotype [137, 138]. Similar to cαβ T cells, iNKT cell anergy is a long-lasting unresponsive or hyporesponsive state to secondary antigen stimulation following initial TCR stimulation. iNKT cell anergy thwarts the efficacy of repeated administration of α-GalCer for cancer immunotherapy [139] and is at least in part caused by up regulation of the inhibitor receptor PD-1 on anergic iNKT cells [139, 140].
A recent study has revealed that mTORC1 activity is decreased while both protein and mRNA levels of TSC1 and TSC2 are elevated in anergic iNKT cells [98]. In contrast, Erk1/2 phosphorylation is not obviously different between anergic versus antigen-naive iNKT cells [98, 141]. Furthermore, deletion of TSC1 in mature iNKT cells results in resistance to anergy induction from prolonged exposure to α-GalCer, manifested by increased cytokine production and enhanced expansion of TSC1 deficient iNKT cells in response to secondary α-GalCer stimulation [98], which is in reminiscent of the response by TSC1 deficient cαβ T cells [108]. In a melanoma lung metastasis model, adoptively transferred TSC1 deficient iNKT cells into iNKT null Jα18−/− mice followed by repeated injections of α-GalCer elicited more efficient antitumor immunity than wild-type counterparts. The ability of TSC1 deficient iNKT cells to resist anergy induction is correlated with decreased upregulation of anergy-promoting molecules such as PD-1 and the E3 ubiquitin ligase Grail [98]. Interestingly, the E3 ubiquitin ligase Cbl-b also contributes to iNKT cell anergy by monoubiquitination of CARMA1 and subsequent disruption of CARMA1/Bcl10 complex formation [141]. Since CARMA1 positively contributes to mTOR activation in T cells [85], it remains to be determined if Cbl-b may also negatively control mTOR activation in anergic iNKT cells via inhibiting CARMA1 function.
Multiple strategies have been developed to overcome iNKT cell anergy, such as changing the route of α-GalCer administration or using α-GalCer-loaded dendritic cells [142]. Several iNKT agonists, including phenyl-derivatives of α-GalCer, have been found to induce iNKT cell activation without induction of anergy [143]. Since it has been proposed that TCR signaling strength is involved in the regulation of iNKT cell anergy [142, 144], it would be interesting to investigate ways in which phenyl-glycolipids trigger signals in iNKT cells differently from α-GalCer and their effects on TSC1/2-mTOR signaling in iNKT cells following repetitive injection.
Future perspective
Recent studies have established the importance of mTOR, mTORC1, and mTORC2 for iNKT cell development, effector lineage differentiation, and function. Multiple signaling pathways that lead to mTOR activation and negative regulators that control mTOR signaling in T cells have been found to play differential roles in iNKT cells. While the importance of mTOR and its tight regulation in iNKT cells has become clear, mechanisms by which mTOR, mTORC1 and mTORC2 as well as their regulators exert their functions on iNKT cells remain to be elucidated. Because mTOR is a crucial sensor of signals from the environment and intracellular stress to control metabolism, how iNKT cell metabolism is shaped by mTOR signaling and how dysregulated mTOR signaling may influence iNKT cells via metabolic reprogramming should be addressed in the future. Of note, current understanding of mTOR signaling in iNKT cells is mostly limited to animal models; how mTOR signaling and its regulators impact on human iNKT cells is an important question to be addressed. The broad usage of rapamycin and other mTOR inhibitors in different clinical setting such as treatment of cancer and transplantation patients is likely impact on iNKT cells, which may itself influence disease progression. Given the ability of mTOR and its regulators to shape iNKT cell effector lineage differentiation, anergy, and function, it is foreseeable that modulation of mTOR signaling is a viable strategy to improve iNKT cell-mediated immunotherapy for various diseases including cancers.
Highlights.
mTOR is crucial for early iNKT cell development and function.
The RasGRP1-Ras-Erk1/2 pathway activates mTOR signaling and diacylglycerol kinases negatively control mTOR signaling.
Tight control of mTOR signaling ensures proper iNKT cell development and effector lineage differentiation.
Acknowledgements
This work was supported by INHA UNIVERSITY Research Grant INHA-51365-1 for J.S. and by NIAID, NIH (R01AI079088 and R01AI101206) to XPZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 3.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 5.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 6.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 7.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 8.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1-NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milpied P, Massot B, Renand A, Diem S, Herbelin A, Leite-de-Moraes M, Rubio MT, Hermine O. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood. 2011;118:2993–3002. doi: 10.1182/blood-2011-01-329268. [DOI] [PubMed] [Google Scholar]
- 10.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013;34:50–58. doi: 10.1016/j.it.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berzins SP, Ritchie DS. Natural killer T cells: drivers or passengers in preventing human disease? Nat Rev Immunol. 2014;14:640–646. doi: 10.1038/nri3725. [DOI] [PubMed] [Google Scholar]
- 12.Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, Nutt SL, Brink R, Godfrey DI, Batista FD, Vinuesa CG. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 18.Rampuria P, Lang ML. CD1d-dependent expansion of NKT follicular helper cells in vivo and in vitro is a product of cellular proliferation and differentiation. Int Immunol. 2015;27:253–263. doi: 10.1093/intimm/dxv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012;188:3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, Godfrey DI, Leadbetter EA, Sant'Angelo DB, von Andrian U, Brenner MB. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, Qiu Y, Que LG, Foster WM, Xia Z, Chi H, Zhong XP. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest. 2014;124:1685–1698. doi: 10.1172/JCI69780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey DI, Stankovic S, Baxter AG. Developing NKT cells need their calcium. Nat Immunol. 2009;10:231–233. doi: 10.1038/ni0309-231. [DOI] [PubMed] [Google Scholar]
- 27.Gorentla BK, Zhong XP. T cell Receptor Signal Transduction in T lymphocytes. J Clin Cell Immunol. 2012;2012:5. doi: 10.4172/2155-9899.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishna S, Zhong X. Role of diacylglycerol kinases in T cell development and function. Crit Rev Immunol. 2013;33:97–118. doi: 10.1615/critrevimmunol.2013006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC, Zhong XP. Critical roles of RasGRP1 for invariant NKT cell development. J Immunol. 2011;187:4467–4473. doi: 10.4049/jimmunol.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu T, Gimferrer I, Simmons A, Wiest D, Alberola-Ila J. The Ras/MAPK pathway is required for generation of iNKT cells. PloS one. 2011;6:e19890. doi: 10.1371/journal.pone.0019890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Miao T, Sebastian M, Bhullar P, Ghaffari E, Liu M, Symonds AL, Wang P. The transcription factors Egr2 and Egr3 are essential for the control of inflammation and antigen-induced proliferation of B and T cells. Immunity. 2012;37:685–696. doi: 10.1016/j.immuni.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, Singh H, Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant'Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 37.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanic AK, Bezbradica JS, Park JJ, Van Kaer L, Boothby MR, Joyce S. Cutting edge: the ontogeny and function of Va14Ja18 natural T lymphocytes require signal processing by protein kinase C theta and NF-kappa B. J Immunol. 2004;172:4667–4671. doi: 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanic AK, Bezbradica JS, Park JJ, Matsuki N, Mora AL, Van Kaer L, Boothby MR, Joyce S. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004;172:2265–2273. doi: 10.4049/jimmunol.172.4.2265. [DOI] [PubMed] [Google Scholar]
- 43.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, Luster AD, Xavier RJ. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 48.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z, Van Kaer L, Joyce S. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187:6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantorna MT, Zhao J, Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc. 2012;71:62–66. doi: 10.1017/S0029665111003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 52.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105:5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 54.Webster KE, Kim HO, Kyparissoudis K, Corpuz TM, Pinget GV, Uldrich AP, Brink R, Belz GT, Cho JH, Godfrey DI, Sprent J. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014;7:1058–1067. doi: 10.1038/mi.2013.122. [DOI] [PubMed] [Google Scholar]
- 55.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, Perreault C, Roux PP, Kitano H. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 61.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 62.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 65.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 67.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- 75.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 77.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 83.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamilton KS, Phong B, Corey C, Cheng J, Gorentla B, Zhong X, Shiva S, Kane LP. T cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci Signal. 2014;7:ra55. doi: 10.1126/scisignal.2005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 87.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36:13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin J, Wang S, Deng W, Wu J, Gao J, Zhong XP. Mechanistic target of rapamycin complex 1 is critical for invariant natural killer T-cell development and effector function. Proc Natl Acad Sci U S A. 2014;111:E776–E783. doi: 10.1073/pnas.1315435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Tschumi BO, Corgnac S, Ruegg MA, Hall MN, Mach JP, Romero P, Donda A. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol. 2014;193:1759–1765. doi: 10.4049/jimmunol.1400769. [DOI] [PubMed] [Google Scholar]
- 91.Jin Y, Qu S, Tesikova M, Wang L, Kristian A, Maelandsmo GM, Kong H, Zhang T, Jeronimo C, Teixeira MR, Yuca E, Tekedereli I, Gorgulu K, Alpay N, Sood AK, Lopez-Berestein G, Danielsen HE, Ozpolat B, Saatcioglu F. Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc Natl Acad Sci U S A. 2013;110:E2572–E2581. doi: 10.1073/pnas.1304318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pobezinsky LA, Etzensperger R, Jeurling S, Alag A, Kadakia T, McCaughtry TM, Kimura MY, Sharrow SO, Guinter TI, Feigenbaum L, Singer A. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat Immunol. 2015 doi: 10.1038/ni.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubinsky AN, Dastidar SG, Hsu CL, Zahra R, Djakovic SN, Duarte S, Esau CC, Spencer B, Ashe TD, Fischer KM, MacKenna DA, Sopher BL, Masliah E, Gaasterland T, Chau BN, Pereira de Almeida L, Morrison BE, La Spada AR. Let-7 coordinately suppresses components of the amino acid sensing pathway to repress mTORC1 and induce autophagy. Cell Metab. 2014;20:626–638. doi: 10.1016/j.cmet.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Consortium D, Investigators M, Altshuler D, Daley GQ. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei J, Yang K, Chi H. Cutting edge: Discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J Immunol. 2014;193:4297–4301. doi: 10.4049/jimmunol.1402042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prevot N, Pyaram K, Bischoff E, Sen JM, Powell JD, Chang CH. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J Immunol. 2015;194:223–230. doi: 10.4049/jimmunol.1401985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu J, Shin J, Xie D, Wang H, Gao J, Zhong XP. Tuberous sclerosis 1 promotes invariant NKT cell anergy and inhibits invariant NKT cell-mediated antitumor immunity. J Immunol. 2014;192:2643–2650. doi: 10.4049/jimmunol.1302076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 101.Finlay DK, Kelly AP, Clarke R, Sinclair LV, Deak M, Alessi DR, Cantrell DA. Temporal differences in the dependency on phosphoinositide-dependent kinase 1 distinguish the development of invariant Valpha14 NKT cells and conventional T cells. J Immunol. 2010;185:5973–5982. doi: 10.4049/jimmunol.1000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306–3314. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan H, O'Brien TF, Wright G, Yang J, Shin J, Wright KL, Zhong XP. Critical role of the tumor suppressor tuberous sclerosis complex 1 in dendritic cell activation of CD4 T cells by promoting MHC class II expression via IRF4 and CIITA. J Immunol. 2013;191:699–707. doi: 10.4049/jimmunol.1201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O'Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011;41:3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bateup HS, Takasaki KT, Saulnier JL, Denefrio CL, Sabatini BL. Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J Neurosci. 2011;31:8862–8869. doi: 10.1523/JNEUROSCI.1617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci U S A. 2012;109:14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu Q, Liu Y, Chen C, Ikenoue T, Qiao Y, Li CS, Li W, Guan KL, Liu Y, Zheng P. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J Immunol. 2011;187:1106–1112. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benhamron S, Tirosh B. Direct activation of mTOR in B lymphocytes confers impairment in B-cell maturation andloss of marginal zone B cells. Eur J Immunol. 2011;41:2390–2396. doi: 10.1002/eji.201041336. [DOI] [PubMed] [Google Scholar]
- 111.Ci X, Kuraoka M, Wang H, Carico Z, Hopper K, Shin J, Deng X, Qiu Y, Unniraman S, Kelsoe G, Zhong XP. TSC1 Promotes B Cell Maturation but Is Dispensable for Germinal Center Formation. PloS one. 2015;10:e0127527. doi: 10.1371/journal.pone.0127527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu L, Yang T, Li L, Sun L, Hou Y, Hu X, Zhang L, Tian H, Zhao Q, Peng J, Zhang H, Wang R, Yang Z, Zhang L, Zhao Y. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 114.Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Huang G, Zeng H, Yang K, Lamb RF, Chi H. Tuberous sclerosis 1 (Tsc1)-dependent metabolic checkpoint controls development of dendritic cells. Proc Natl Acad Sci U S A. 2013;110:E4894–E4903. doi: 10.1073/pnas.1308905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, Guertin DA, Lamb RF, Chi H. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zarrouk M, Rolf J, Cantrell DA. LKB1 mediates the development of conventional and innate T cells via AMP-dependent kinase autonomous pathways. PloS one. 2013;8:e60217. doi: 10.1371/journal.pone.0060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park H, Tsang M, Iritani BM, Bevan MJ. Metabolic regulator Fnip1 is crucial for iNKT lymphocyte development. Proc Natl Acad Sci U S A. 2014;111:7066–7071. doi: 10.1073/pnas.1406473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 120.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 122.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 123.Wu J, Shen S, Yang J, Xia Z, Zhong XP. Diacylglycerol kinase zeta positively controls the development of iNKT-17 cells. PloS one. 2013;8:e75202. doi: 10.1371/journal.pone.0075202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shen S, Wu J, Srivatsan S, Gorentla BK, Shin J, Xu L, Zhong XP. Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. J Immunol. 2011;187:2122–2129. doi: 10.4049/jimmunol.1100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kishimoto H, Ohteki T, Yajima N, Kawahara K, Natsui M, Kawarasaki S, Hamada K, Horie Y, Kubo Y, Arase S, Taniguchi M, Vanhaesebroeck B, Mak TW, Nakano T, Koyasu S, Sasaki T, Suzuki A. The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood. 2007;109:3316–3324. doi: 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- 127.Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The MicroRNA miR-181 Is a Critical Cellular Metabolic Rheostat Essential for NKT Cell Ontogenesis and Lymphocyte Development and Homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zietara N, Lyszkiewicz M, Witzlau K, Naumann R, Hurwitz R, Langemeier J, Bohne J, Sandrock I, Ballmaier M, Weiss S, Prinz I, Krueger A. Critical role for miR-181a/b-1 in agonist selection of invariant natural killer T cells. Proc Natl Acad Sci U S A. 2013;110:7407–7412. doi: 10.1073/pnas.1221984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inui T, Nakashima H, Habu Y, Nakagawa R, Fukasawa M, Kinoshita M, Shinomiya N, Seki S. Neutralization of tumor necrosis factor abrogates hepatic failure induced by alpha-galactosylceramide without attenuating its antitumor effect in aged mice. J Hepatol. 2005;43:670–678. doi: 10.1016/j.jhep.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 130.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 131.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 133.Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, Shimizu N, Ueno N, Yamamoto S, Taniguchi M, Motohashi S, Nakayama T, Okamoto Y. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 134.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 136.Hong C, Park SH. Application of natural killer T cells in antitumor immunotherapy. Crit Rev Immunol. 2007;27:511–525. doi: 10.1615/critrevimmunol.v27.i6.20. [DOI] [PubMed] [Google Scholar]
- 137.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, Yagita H, Kang CY. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 141.Kojo S, Elly C, Harada Y, Langdon WY, Kronenberg M, Liu YC. Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1. Proc Natl Acad Sci U S A. 2009;106:17847–17851. doi: 10.1073/pnas.0904078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McEwen-Smith RM, Salio M, Cerundolo V. The Regulatory Role of Invariant NKT Cells in Tumor Immunity. Cancer Immunol Res. 2015;3:425–435. doi: 10.1158/2326-6066.CIR-15-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang JR, Tsai YC, Chang YJ, Wu JC, Hung JT, Lin KH, Wong CH, Yu AL. alpha-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3. J Immunol. 2014;192:1972–1981. doi: 10.4049/jimmunol.1302623. [DOI] [PubMed] [Google Scholar]
- 144.Iyoda T, Ushida M, Kimura Y, Minamino K, Hayuka A, Yokohata S, Ehara H, Inaba K. Invariant NKT cell anergy is induced by a strong TCR-mediated signal plus costimulation. Int Immunol. 2010;22:905–913. doi: 10.1093/intimm/dxq444. [DOI] [PubMed] [Google Scholar]