Abstract
INSM1 is a zinc-finger protein expressed throughout the developing nervous system in late neuronal progenitors and nascent neurons. In the embryonic cortex and olfactory epithelium, Insm1 may promote the transition of progenitors from apical, proliferative, and uncommitted to basal, terminally-dividing and neuron producing. In the otocyst, delaminating and delaminated progenitors express Insm1, whereas apically-dividing progenitors do not. This expression pattern is analogous to that in embryonic olfactory epithelium and cortex (basal/subventricular progenitors). Lineage analysis confirms that auditory and vestibular neurons originate from Insm1-expressing cells. In the absence of Insm1, otic ganglia are smaller, with 40% fewer neurons. Accounting for the decrease in neurons, delaminated progenitors undergo fewer mitoses, but there is no change in apoptosis. We conclude that in the embryonic inner ear, Insm1 promotes proliferation of delaminated neuronal progenitors and hence the production of neurons, a similar function to that in other embryonic neural epithelia. Unexpectedly, we also found that differentiating, but not mature, outer hair cells express Insm1, whereas inner hair cells do not. Insm1 is the earliest known gene expressed in outer versus inner hair cells, demonstrating that nascent outer hair cells initiate a unique differentiation program in the embryo, much earlier than previously believed.
Keywords: Spiral ganglion, vestibular ganglion, outer hair cell, otocyst, basal progenitors, subventricular zone
Graphical abstract
1. Introduction
During embryogenesis, several different neural epithelia contribute to the generation of central and peripheral nervous system neurons. The primary sensory neurons (auditory and vestibular) of the ear are generated by a largely placode-derived structure known as the otocyst (Anniko and Wikstrom, 1984; Freyer et al., 2011). There is also a controversy as to whether the neural tube might contribute a small number of cells to the otocyst (Freyer et al., 2011; Sandell et al., 2014; Steventon et al., 2014). In the mouse, beginning at embryonic day 8.5 (E8.5), the otic placode begins to invaginate. It then pinches off and separates from the ectoderm to form the epithelial sac known as the otocyst or otic vesicle by E9.5 (Anniko and Wikstrom, 1984; Barald and Kelley, 2004). Within the otocyst, the anteroventral quadrant specializes to produce the neurons, as well as the sensory receptor cells (i.e. hair cells) and their support cells (Appler and Goodrich, 2011).
As in other neural epithelia, uncommitted otocyst progenitors undergo interkinetic nuclear migration whereby mitosis occurs apically within the epithelium but DNA synthesis (S-Phase) occurs basally. Beginning at E9.5, neuronal progenitors migrate basally and delaminate from the otocyst into the adjacent mesenchyme (Raft et al., 2004; Rubel and Fritzsch, 2002) (Fig. 1). There they divide to produce only auditory and vestibular primary neurons. However, it is yet to be determined the extent of proliferation after delamination, whether all delaminated cells divide or some delaminate as postmitotic nascent neurons (D’Amico-Martel and Noden, 1983). In fact, one report has shown that some cells within the otic epithelium may have already sent projections to the hindbrain before delaminating (Fritzsch, 2003; Yang et al., 2011). Delaminated progenitors exit the cell cycle from E9.5–E13.5 (Matei et al., 2005). All glia associated with these neurons derive from the neural crest and migrate into the forming ganglia around E10.5. In this article, we will refer to all cells that delaminate from the otocyst as delaminated progenitors (DPs), including those that will divide to produce neurons and those that may directly differentiate into neurons.
Fig. 1. Schematic of otocyst proliferation and differentiation.
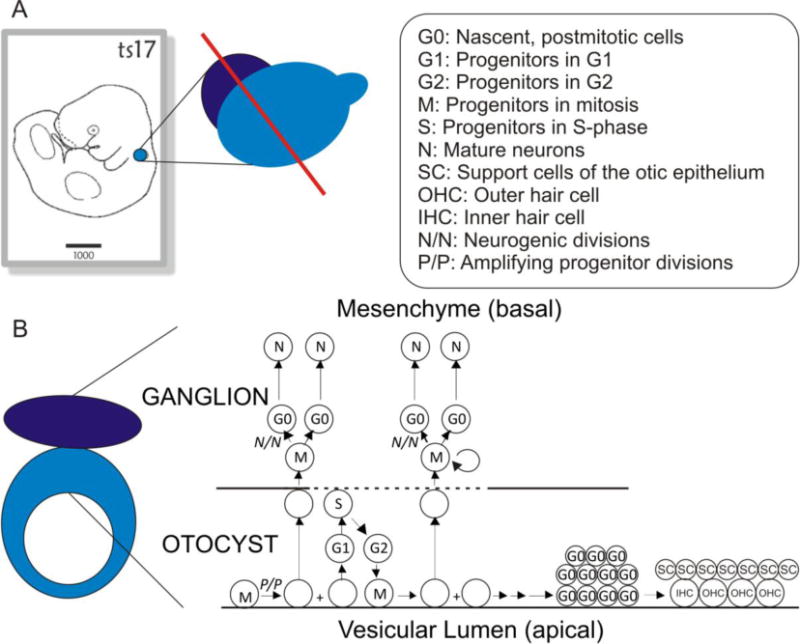
(A) Position of the otocyst in an E10.5 mouse embryo (left panel, highlighted in blue), and relationship between delaminated progenitor region (purple) to the otocyst (center panel). (B) Morphological structure through a cross section of the otocyst along the red line in (A) with its arrangement of cell nuclei shown to the right. Within the otocyst uncommitted progenitors undergo interkinetic nuclear migration and divide apically. Neuronally-committed progenitors delaminate into the mesenchyme where some or all of them will divide an undetermined number of times to produce neurons. These neurons will then coalesce to form both spiral and vestibular ganglia. Cells that remain within the otocyst will form auditory and vestibular hair cells as well as support and structural cells. The arrangement of mature cells in the organ of Corti is more complex than we have illustrated in this figure. For a more detailed and accurate representation of the arrangement, ratios and cell types present in the organ of Corti see (Jahan et al., 2015a). Left panel in (A) from emouseatalas.org. See figure for abbreviations.
The morphological changes of the developing spiral and vestibular ganglia (SVG) are accompanied by a cascade of transcription factors. First, Neurog1 defines the proneural domain and is necessary for SVG formation (Ma et al., 2000; Ma et al., 1998). After Neurog1 establishes the neuron producing region of the otocyst, it activates NeuroD in the neuronal progenitors, which promotes delamination and neuron survival (Jahan et al., 2010a; Kim et al., 2001; Liu et al., 2000). As DPs migrate to the base of the epithelium they activate Isl1, whose function is not known. Around this time, Gata3 expression is restricted in the otocyst and enriched in the ventral region that will become the organ of Corti (Lawoko-Kerali et al., 2002). DPs that express Gata3 will produce the SpG and, in its absence, no SpG neurons are generated (Duncan et al., 2011; Lawoko-Kerali et al., 2004). Gata3 has also been conditionally ablated in SpG neurons after neurogenesis and has been shown to be necessary for their axonal pathfinding and survival (Appler et al., 2013; Duncan and Fritzsch, 2013). After DPs delaminate, they express Pou4f1, which contributes to SpG neuron differentiation by regulating cell size and maintaining TrkC expression (Deng et al., 2014; Huang et al., 2001). Following cell cycle exit, differentiating nascent SVG neurons express Pou4f2, whose function has not yet been determined (Deng et al., 2014; Huang et al., 2001).
While elements of this signal cascade are well understood, there are still many aspects of SVG formation that are not. As of yet, the regulation of DP proliferation is not well understood. Deletion of N-Myc from the mouse ear results in an overall smaller ear beginning as early as E9.0, ultimately including a shortened cochlea and smaller spiral ganglion(Dominguez-Frutos et al., 2011; Kopecky et al., 2011). This phenotype is attributed to a decrease in proliferation in the otocyst, where the effect is global and not specific to delaminating progenitors (Dominguez-Frutos et al., 2011; Kopecky et al., 2011). Likewise, Foxg1 is expressed throughout the developing ear and its deletion leads to global disruptions in the ear including a shortened cochlea with shorter spiral ganglion, though the mechanism for the shortened ganglion was not investigated (Pauley et al., 2006). Tis21, a marker of cortical basal progenitors, was recently proposed to promote neurogenesis of the spiral ganglion, though the specific mechanism and timing of expression were not determined (Yamada et al., 2015). Also, experiments using chick otic explants have shown that IGF-1 can promote proliferation of DPs (Camarero et al., 2003). However, SpG size and number of neurons were unaltered in Igf1 null mice at postnatal day 5. Hence, IGF-1 has little to no effect on neurogenesis in the mouse, though it is required for neuron survival (Camarero et al., 2001).
INSM1 is a zinc-finger protein whose mRNA is expressed in all examined neurogenic regions of the developing nervous system, including areas of adult neurogenesis (Duggan et al., 2008). In many developing neural epithelia, Insm1 is expressed in a basal zone that includes neuronally-committed progenitors and nascent neurons. Insm1 is not expressed in the apical zones, where uncommitted progenitors divide, or in mature neurons (Duggan et al., 2008). In the cortex and olfactory epithelium, deletion of Insm1 results in fewer basally-dividing, neuronally-committed progenitors and consequently in fewer neurons (Farkas et al., 2008; Rosenbaum et al., 2011). Here, we examine the expression pattern and function of Insm1 in the embryonic mouse ear. We find that Insm1 is transiently expressed during neurogenesis in delaminating, neuronally-committed progenitors and nascent SVG neurons. This pattern of expression is equivalent to that in embryonic olfactory epithelium and cortex, revealing homologies in the neurodevelopment of ear, nose and cortex. Additionally, the absence of Insm1 results in fewer SVG neurons. This is not due to an increase in apoptosis, or to a decrease in delaminations, but instead it is due to a reduction in divisions of DPs. Unexpectedly, we also find expression of Insm1 in outer hair cells (OHCs) of the embryonic cochlea beginning as they initiate differentiation and subsiding as they mature.
2. Results
Since previous studies show that Insm1 is typically expressed transiently by neuronal progenitors (Duggan et al., 2008), we used a lineage tracing technique to determine which inner ear cell types are generated by Insm1 expressing progenitors (see methods). In postnatal day 7 (P7) mouse ear, all spiral ganglion (SpG) neurons, vestibular ganglion (VG) neurons, and OHCs were positive for β-gal (Fig. 2A and C). Saccular, utricular, and cochlear inner hair cells (IHCs) and support cells of the sensory epithelia were not β-gal positive. Also, no cell was Insm1GFP.Cre positive at P7, limiting Insm1 expression to prior (embryonic to early postnatal) stages of development. The geniculate ganglion and other nearby facial neurons were also positive for β-gal (data not shown).
Fig. 2. Insm1 lineage analysis shows that Insm1 is expressed early in otic neurons and OHCs and/or in their progenitors, but is no longer expressed by P7.
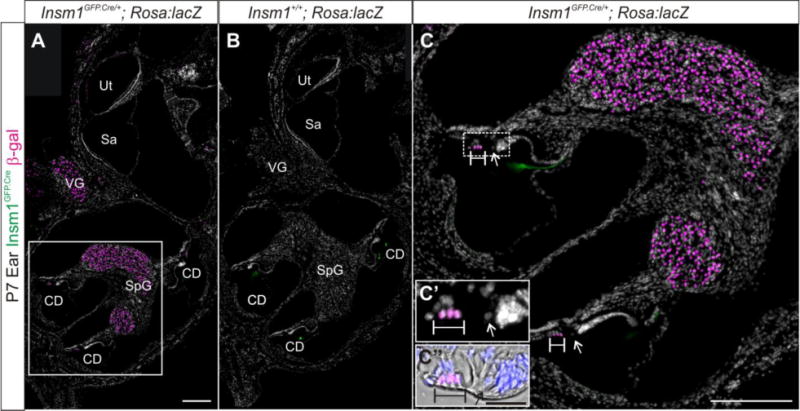
Immunohistochemistry for β-gal (magenta), GFP (green), and nuclear label with DAPI (white) in inner ear sections of P7 Insm1GFP.Cre/+; ROSA:lacZ (A,C) and Insm1+/+; ROSA:lacZ (B) littermate controls. (A) Insm1 is not expressed in the ear at P7; however, before P7 it was expressed by SpG and VG neurons or progenitors and OHCs or their progenitors. (B) In the absence of the Insm1GFP.Cre allele, lacZ is not expressed. The tectorial membrane appears green in both the presence and absence of Insm1GFP.Cre so we conclude that this is nonspecific binding of the GFP antibody or autofluorescence. (C) Close up of boxed region in (A) showing β-gal in OHCs and not IHCs. (C′ and C″) High magnification of dotted box region in (C) where C“ includes bright field to show the structure of the organ of Corti with scale bar of 20 μm. Arrows indicate IHC nucleus. Brackets designate OHC nuclei. CD: cochlear duct, Sa: saccule, SpG: spiral ganglion, VG: vestibular ganglion, Ut: utricle. Scale bar in (A) and (C): 200 μm.
To elucidate the pattern of expression of Insm1 in the ear throughout development, we used both in situ hybridization for Insm1 mRNA and immunohistochemistry for GFP in the Insm1GFP.Cre reporter line. By using both methods to resolve Insm1 expression we were also able to confirm that Insm1GFP.Cre is an accurate indicator of Insm1 expression.
2.1 Insm1 is transiently expressed by delaminating progenitors and nascent neurons in the embryonic inner ear
We examined Insm1 expression during delamination at E10.5 to determine if Insm1 is expressed in DPs (Fig. 3). Both methods of detection show Insm1 expression in the basally located cells of the anteroventral quadrant of the otocyst and in the delaminated zone. We found that Insm1GFP.Cre cells express Islet1 (Isl1) (Fig. 3E–H), a marker of DPs (Radde-Gallwitz et al., 2004). Within the epithelium, Insm1GFP.Cre cells are located along the basal lamina, and most are also Isl1 positive, indicating that these cells are also neuronally-committed DPs about to delaminate. None of the epithelial cells undergoing mitosis apically expressed Insm1, but some of the delaminated cells expressing Insm1 were in mitosis (co-expressing phospho-histone H3; Fig. 3J,K), while others had left the cell cycle (did not express Ki67, which labels all proliferating cells; Fig. 3L,M).
Fig. 3. Insm1 is expressed in delaminating and delaminated neuronal progenitors that will produce the SVG.
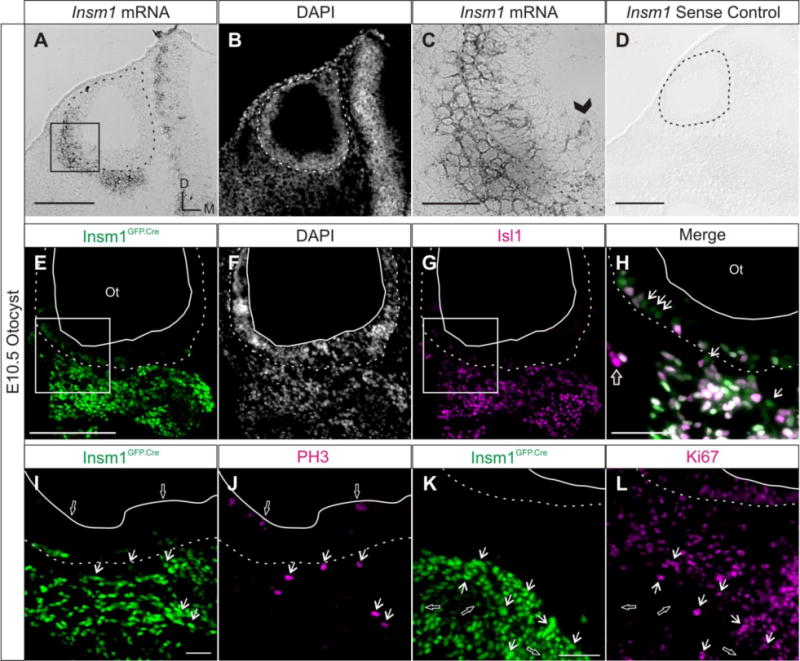
Representative horizontal sections of (A–D) wild type and (E–L) Insm1GFP.Cre/+ E10.5 embryos demonstrating the Insm1 expression pattern in the otocyst during delamination. (A) In situ hybridization with an Insm1 antisense probe on a section through the anterior portion of the otocyst. Insm1 mRNA is expressed in DPs within the anteroventral aspect of the otic epithelium and in the mesenchyme. (B) Nuclear pattern of panel (A). (C) Close up of boxed region in (A). Arrowhead indicates an Insm1 positive cell located more apically in the epithelium, presumably prior to delamination. (D) Control in situ hybridization with an Insm1 sense probe shows no signal. (E) Immunohistochemistry for GFP (green) representing Insm1GFP.Cre expression in epithelial and delaminated DPs of the anteroventral otocyst. (F) Nuclear pattern corresponding to panel (E). (G) Immunohistochemistry for DP marker Isl1 (magenta) in the same section as (E,F). (H) Close up of merge of boxed region in panels (E) and (G) shows that the GFP and Isl1 patterns overlap (white), except for a few GFP positive cells not positive for Isl1 (arrows) and even fewer Isl1 positive cells not expressing GFP (open arrow). (I,J) Immunohistochemistry co-staining for Insm1GFP.Cre expression (I, green) and PH3 (J, magenta) shows that Insm1 is not expressed in apically-dividing cells within the epithelium (examples indicated by open arrows), but is expressed by delaminated cells in mitosis (arrows). (K,L) Immunohistochemistry co-staining for Insm1GFP.Cre expression (K, green) and Ki67 (L, magenta) shows that while some Insm1 expressing cells are proliferating (Ki67+; examples indicated by arrows), many are not (examples indicated by open arrows). Dotted lines in all panels delineate the basal lamina of the otocyst. Solid lines delineate the apical edge of the otic epithelium. Boxes indicate regions magnified in panels (C) and (H). (A,D,E) Scale bar: 200 μm. (C,H,I,K) Scale bar: 50 μm. Ot: otocyst.
We detected a few Insm1-expressing cells at the apical edge of the epithelium (Fig. 3C; arrowhead). In addition, a few of the Insm1-expressing cells did not co-express Isl1, and these cells were primarily located within the otic epithelium (Fig. 3H; arrows). We wondered whether these cells would eventually delaminate and produce neurons, or whether they would remain within the epithelium and generate other cell types. In fate mapping experiments (Fig. 2) we demonstrated that OHCs were the only epithelial cells of the mature inner ear descendent from Insm1-expressing cells. However, we examined the epithelium at E14.5, when delamination is nearly complete, and found no Insm1-expressing cells in the cochlear epithelium. In the wild type mice we did not detect Insm1 mRNA (Fig. 4A), nor did the Insm1GFP.Cre/+; ROSA:lacZ mice express the Insm1 reporter GFP or the lineage tracer β-gal (Fig. 4C,D). Therefore all Insm1 expressing cells at E10.5 in the otocyst will leave the epithelium or die. Since most other Insm1-expressing cells at this stage have delaminated, the few still in the epithelium are likely DPs about to delaminate.
Fig. 4. Insm1 expression is maintained in nascent SpG and VG neurons.
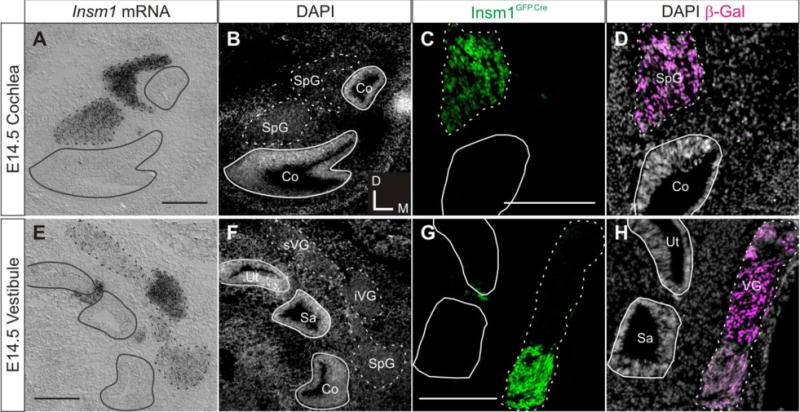
Representative coronal sections demonstrating Insm1 expression at E14.5 in the cochlea (A–D) and the vestibule (E–H) of (A,B,E,F) wild type and (C,D,G,H) Insm1GFP.Cre/+; ROSA:lacZ embryos. (A) In situ hybridization with an Insm1 antisense probe. Section is through the two turns of the cochlea and shows Insm1 mRNA expression in the SpG and not within the epithelium. (B) Nuclear pattern corresponding to (A). (C) Immunohistochemistry for GFP (green) representing Insm1GFP.Cre expression in Insm1GFP.Cre/+; ROSA:lacZ embryos. Section is through one turn of the cochlea and shows Insm1GFP.Cre expression in spiral ganglion neurons, but not within the epithelium. (D) Nuclear pattern corresponding to (C) with immunohistochemistry for lineage marker β-gal (magenta) showing no descendant cells of Insm1 progenitors within the epithelium. (E) In situ hybridization with an Insm1 antisense probe. Section is through basal cochlea, utricle and saccule and shows Insm1 mRNA expression in SpG, iVG, and faintly in sVG. (F) Nuclear pattern corresponding to panel (E). (G) Immunohistochemistry for GFP (green) representing Insm1GFP.Cre expression in Insm1GFP.Cre/+; ROSA:lacZ embryos. Section through utricle and saccule shows Insm1GFP.Cre is expressed in the iVG. (H) Nuclear pattern corresponding to panel (G) with immunohistochemistry for lineage marker β-gal (magenta) showing no descendant cells of Insm1 progenitors within the epithelium. Solid lines in all panels delineate basal lamina of all epithelia. Dotted lines in all panels delineate ganglia. Scale bar: 200 μm. Co: cochlea, Sa: saccule, SpG: spiral ganglion, iVG: inferior vestibular ganglion, sVG: superior vestibular ganglion, Ut: utricle.
We next investigated Insm1 expression in nascent neurons following the completion of delamination and neurogenesis at E14.5. At this stage, the regions within the epithelium that will form the six sensory epithelia of the ear, the cochlea, two maculae, and three cristae are also exiting the cell cycle (Ruben, 1967). We found that differentiating SpG neurons continue to express Insm1 (Fig. 4A–D). However, in the VG, superior regions have lowered levels of Insm1, while inferior regions have not (Fig. 4E–H). Indeed, the progenitors of sVG and iVG neurons delaminate from different locations of the otocyst and thus could be molecularly distinct (Yang et al., 2011). We do see some Insm1 signal in the region between utricular and saccular epithelia, which sometimes appears slightly within epithelia (Fig. 4E and G). We believe these are also migrating neurons, because lineage analysis at P7 shows there are no cells in this region that had expressed Insm1 (Fig. 2). Furthermore, we did not detect cells that had once expressed, or descended from progenitors that had previously expressed, Insm1 (β-gal positive) within the cochlear or vestibular epithelium (Fig. 4D and H). These data show that Insm1 expression is sustained in nascent SVG neurons, whereas no Insm1-expressing cells remain within the epithelium.
As SpG (Fig. 5) and VG (data not shown) neurons continue to differentiate, Insm1 expression levels slowly decrease. At E16.5 Insm1 mRNA and Insm1GFP.Cre signal strength have decreased from the levels seen at E14.5 (compare Fig. 5A–D with Figs. 3 and 4). At E18.5 the Insm1GFP.Cre expression in the SpG is almost below detection (Fig. 5E–F) and by P1 we are unable to detect Insm1 mRNA in SpG neurons (Fig. 5G–H). Unexpectedly, as Insm1 expression levels subside in the ganglia, we found Insm1 expression in the sensory epithelium in a pattern that corresponds with nascent OHCs. We investigated this expression within the sensory domain further.
Fig. 5. Insm1 expression subsides in SpG neurons as they differentiate and is undetectable at birth.
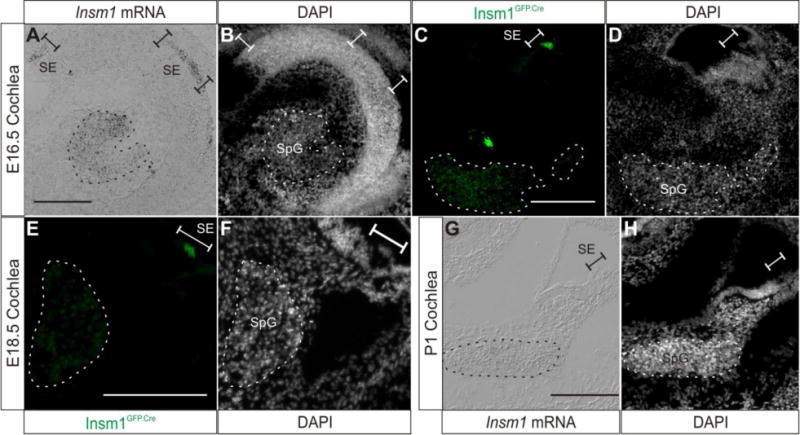
Representative sections demonstrating Insm1 expression at E16.5-P1 in the cochlea. (A) In situ hybridization with an Insm1 antisense probe on a coronal section of E16.5 head, which results in an axial (perpendicular to the modiolus) section of the cochlea. Insm1 signal appears reduced in the SpG when compared with E14.5 (Fig. 4A). Insm1 mRNA is also expressed in the sensory epithelium. (B) Nuclear pattern corresponding to (A). (C) Immunohistochemistry for GFP (green) representing Insm1GFP.Cre expression in the E16.5 cochlea. Insm1GFP.Cre signal is decreased in SpG neurons when compared to Fig. 4C, and is also detected in sensory epithelium. (D) Nuclear pattern corresponding to (C). (E) Immunohistochemistry for GFP (green) representing Insm1GFP.Cre expression in modiolar section of E18.5 cochlea. Insm1GFP.Cre signal is further decreased in SpG neurons when compared to previous stages and epithelial pattern appears to correspond to OHCs. (F) Nuclear pattern corresponding to panel (E). (G) In situ hybridization with an Insm1 antisense probe on a modiolar section of P1 cochlea (mid-basal turn) showing no Insm1 mRNA expression. (H) Nuclear pattern corresponding to panel (G). Dotted lines in all panels delineate SpG. Brackets designate sensory epithelium. Scale bar: 200 μm. SpG: spiral ganglion, SE: sensory epithelium.
2.2 Nascent embryonic OHCs also express Insm1 transiently
At E14.5, nearly all the cells of the sensory epithelia have exited the cell cycle and the presumptive organ of Corti can be identified by its expression of cell cycle regulating protein p27kip1 (Chen and Segil, 1999). After identifying the sensory epithelium in an adjacent section, we confirmed that there is no Insm1GFP.Cre or lineage marker β-gal expression in the cochlea at E14.5 (Fig. 6A–B). Therefore, Insm1 is not expressed in the progenitors of the cochlear epithelium cells.
Fig. 6. Insm1 is transiently expressed by nascent OHCs, but not IHCs.
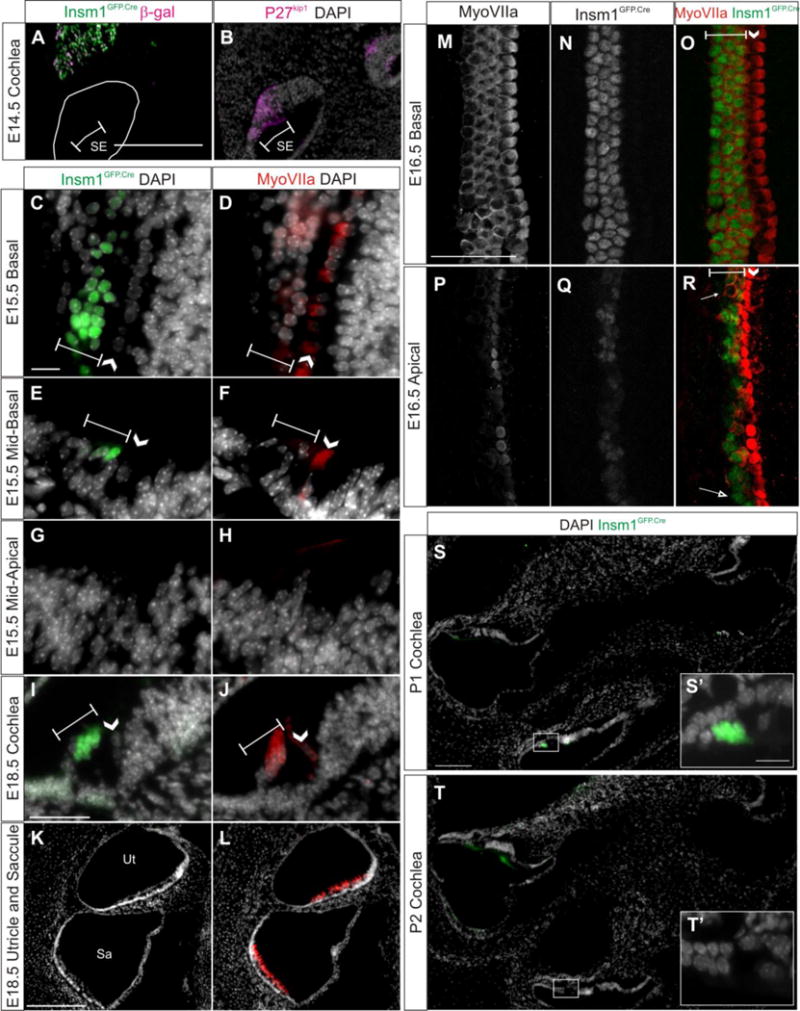
(A,C,E,G,I,K,S,T) Representative sections demonstrating Insm1 expression by immunohistochemistry for GFP (green) in cochlear sections of Insm1GFP.Cre mice at E14.5–E18.5. In (A), immunohistochemistry for lnsm1 lineage marker β-gal is represented in magenta. (A,B) At E14.5 Insm1 expressing cells or their descendants are not in the sensory epithelium (A), which is identified in an adjacent section by immunohistochemistry for P27kip1 (B, magenta). (D,F,H,J,L) Immunohistochemistry for HC marker MyoVIIa (red) in sections adjacent to C,E,G,O,Q, respectively, with nuclear pattern (white). (C,D) At E15.5 in the most basal aspect of the organ of Corti (section provides a glancing view and hence multiple hair cells per row), Insm1GFP.Cre and MyoVIIa are expressed in all rows of OHCs. (E,F) At E15.5 in the mid-basal turn of the organ of Corti, Insm1GFP.Cre is expressed in 2–3 of OHCs and MyoVIIa is only expressed in the first OHC. (G,H) At E15.5 in the mid-apical turn of the organ of Corti, Insm1GFP.Cre and MyoVIIa are not detectable. (M,N,O) Immunohistochemistry for MyoVIIa (M, white), Insm1GFP.Cre (N, white), and the merged image (O, MyoVIIa in red and Insm1GFP.Cre in green) in a whole mount show that, at E16.5 in the basal organ of Corti, Insm1 is expressed in all OHCs. (P, Q, R) Immunohistochemistry for MyoVIIa (P, white), Insm1GFP.Cre (Q, white), and the merged image (R, MyoVIIa in red and Insm1GFP.Cre in green) in a whole mount show that, at E16.5 in the apical organ of Corti, Insm1 is typically expressed in OHCs that are expressing MyoVIIa, with some exceptions where OHCs express MyoVIIa without Insm1 (filled arrow) or vise versa (open arrow). (I,J) At E18.5 throughout the cochlea, Insm1GFP.Cre and MyoVIIa are expressed in all three rows of OHCs, and Insm1 is clearly not expressed in IHCs. (K,L) At E18.5 in the utricle and saccule, Insm1GFP.Cre is not detectable in MyoVIIa expressing HCs. (S–T) Insm1GFP.Cre (green) in sections through two turns of the cochlea at P1 (S) and P2 (T) demonstrating that Insm1 expression persists in apical OHCs through P1 and has mostly subsided throughout the cochlea by P2. (S′ and T′) represent boxed regions in (S and T), respectively. Brackets designate rows of OHCs. Arrow heads indicate the single row of IHCs. Scale bar: (A,Q) 200 μm, (C, I) 50 μm. SE: sensory epithelium, Sa: saccule, Ut: utricle.
Nascent hair cells differentiate in a basal-to-apical and medial-to-lateral gradient, meaning that the first HCs to differentiate are basal IHCs, and then differentiation spreads radially to the first, then second, then third rows of OHCs and tangentially towards more apical HCs (Chen et al., 2002; Lim and Anniko, 1985; Sher, 1971). When we examined the Insm1GFP.Cre expression in the developing cochlea, we used expression of the early hair cell marker MyoVIIa to identify HCs. The MyoVIIa expression pattern also follows the developmental gradients of the HCs (Chen et al., 2002; Sahly et al., 1997). At E15.5, we examined Insm1GFP.Cre expression compared to MyoVIIa in adjacent sections and found both are expressed in all rows of basal OHCs (Fig. 6C–D), whereas neither is yet expressed in apical HCs (in fact, the apical organ of Corti is not developed enough to even determine which cells are the HCs anatomically; Fig. 6G–H). At an intermediate position, in a section through the mid-basal turn of the cochlea, we saw MyoVIIa expression in the IHC, and faintly in the first OHC (Fig. 6F), and Insm1GFP.Cre expression in not only the first OHC, but also faintly in the second and third OHCs (Fig. 6E). When we traced Insm1GFP.Cre and MyoVIIa expression from the base to mid-apical regions, we found that in almost every case Insm1GFP.Cre was expressed in 1–2 more OHCs than MyoVIIa in adjacent sections (both in front and behind).
To better determine the relationship between the expression of these two factors as the organ of Corti differentiates, we co-stained an E16.5 whole mount preparation for both MyoVIIa and GFP (using a chicken anti-GFP antibody and a rabbit anti-MyoVIIa). We again found that at the base, MyoVIIa was expressed in all HCs and that Insm1GFP.Cre was present in all OHCs (Fig. 6M, O). More apically, both markers were expressed in fewer cells and at lower levels. In apical regions where we saw MyoVIIa was expressed in IHCs and 1–2 OHCs, we found that often these OHCs also expressed Insm1GFP.Cre (Fig. 6P–R). Occasionally we would identify a MyoVIIa positive OHC which was not expressing Insm1GFP.Cre or an Insm1GFP.Cre expressing OHC where we could not detect MyoVIIa expression (Fig. 6R filled and open arrow, respectively).
By E18.5 Insm1 is expressed in all OHCs throughout the cochlea (Fig. 6I–J), whereas by P1 Insm1 expression was detected at the apical but not the basal turns of the cochlea (Fig. 5G and 6S) and by P2 was undetected throughout the cochlea (Fig. 6T). This indicates that Insm1 expression onset is roughly contemporaneous with MyoVIIa, but then is down regulated by postnatal stages. Insm1 was never detected in IHCs or HCs of the utricle and saccule (Fig. 6K, L), a result confirmed by the lack of the lineage tracer β-gal in postnatal vestibular and inner hair cells of Insm1GFP.Cre/+; ROSA:lacZ mice (Fig. 2A).
2.3 Insm1 ablation results in fewer SpG and VG neurons
Having established the expression of Insm1 in the developing ear, we sought to determine its function using a different Insm1−/− mouse line, in which the Insm1 coding sequence is completely deleted (Rosenbaum et al., 2011). Unfortunately, mice homozygous for this deletion are not viable beyond E18.5, so we were not able to investigate phenotypes beyond this time point. Since both the SVG neurons and OHCs continue to mature beyond E18.5, we were unable to identify all of the potential effects of Insm1 deletion. At each stage of development we examined, Insm1−/− SVG neurons appeared normal and sent processes to all appropriate sensory epithelia (saccule, utricle, cristae and cochlea) in typical patterns (axons appeared bundled, consistent with normal fasciculation, cochlear IHCs received the majority of SpG innervation when compared with OHCs, and processes extending to OHCs turned basally; Supplemental Fig. 1). However, at times the innervation of the sensory epithelia appeared slightly less dense, which we attribute to a reduction in the number of neurons (see below). Neurons also expressed markers of SVG neuronal differentiation such as Islet1, β-III Tubulin, Pou4f1, Pou4f2 and Neurofilament 200 in patterns undistinguishable from those of Insm1+/+ littermates (Supplemental Figs. 2 and 3). OHCs also appeared to be differentiating normally when compared to Insm1+/+ littermates and were arranged in three rows, expressed MyoVIIa, had begun to form stereocilia bundles, and did not express the embryonic IHC marker Fgf8 (Supplemental Fig 4).
Since the process of delamination and neurogenesis is complete days before E18.5, we were able to examine the function of Insm1 in DPs. First, we compared the sizes of the otic ganglia of Insm1+/+ and Insm1−/− littermates at several developmental stages (Fig. 7A–F). With immunohistochemistry for Isl1 we labeled DPs and nascent neurons at E10.5–E12.5, stages during which delamination and proliferation are still occurring. At E14.5–E18.5, when delamination and neuronal proliferation have ceased, we identified SVG neurons with an antibody to β-III Tubulin. We then measured the cross sectional area (X- and Y-dimensions) of representative sections of ganglion at regular intervals throughout the ear and calculated the length of the ganglion in the Z-dimension (Fig. 7A–F).
Fig. 7. Mice lacking Insm1 (Insm1−/−) have fewer SpG and VG neurons.
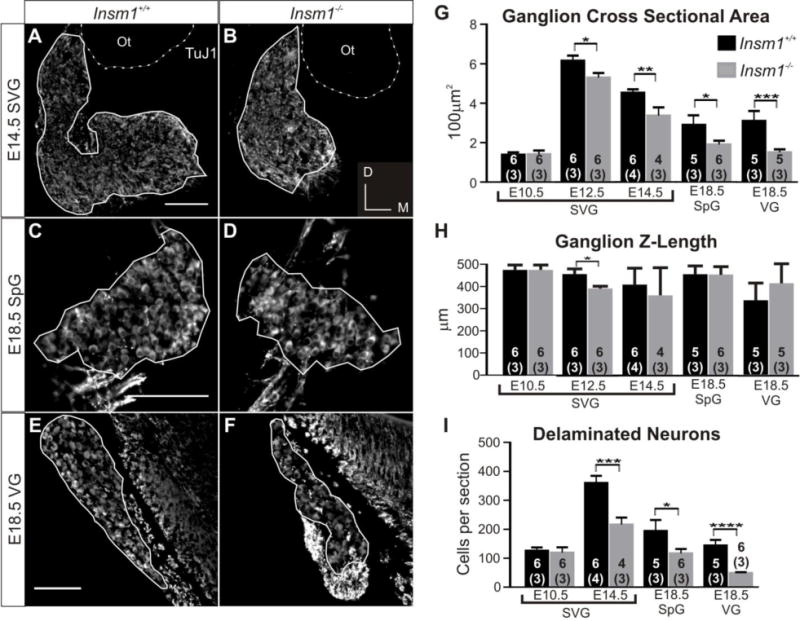
(A–F) Immunohistochemistry for nascent neuron marker β-tubulin III (labeled with the TuJ1 antibody). Representative anatomically matched sections of E14.5 SVG from Insm1+/+ (A) and Insm1−/− (B) littermate embryos, E18.5 SpG from Insm1+/+ (C) and Insm1−/− (D) littermate embryos, and E18.5 VG from Insm1+/+ (E) and Insm1−/− (F) littermate embryos. (A–F) Solid lines delineate boundaries of ganglia, defined by neuronal cell bodies. (A–B) Dotted lines delineate basal lamina of the otocyst based off of nuclear DAPI pattern (not shown). (G) Average cross sectional area (in multiples of 100 μm2) of Insm1+/+ and Insm1−/− otic ganglia from littermate pairs at E10.5, E12.5, E14.5 and E18.5. There is no significant difference in the average cross sectional area of Insm1+/+ and Insm1−/− SVGs at E10.5 (1.39;SEM=0.12 and 1.40;SEM=0.19, respectively. P=0.47), but there is a significant difference at E12.5 (6.16;SEM=0.26 and 5.29;SEM=0.23), and E14.5(4.53;SEM=0.18 and 3.36;SEM=0.42). At E18.5 there is a significant difference between the average cross sectional areas of Insm1+/+ and Insm1−/− SpGs (2.90;SEM=0.18 and 1.90;SEM=0.19) and VGs (3.10;SEM=0.51 and 1.49;SEM=0.13). (H) Average Z-length in μm of Insm1+/+ and Insm1−/− otic ganglia from littermate pairs at E10.5, E12.5, E14.5 and E18.5. There is no significant difference in the average Z-length of Insm1+/+ and Insm1−/− SVGs at E10.5 (472;SEM=21.84 and 472;SEM=21.84. P=0.5), E14.5 (405;SEM=77.02 and 357.5;SEM=123.65. P=0.3), and E18.5 SpGs (450.8;SEM=37.41 and 450;SEM=36.46. P=0.47) and VGs(333.2;SEM=78.19 and 411.3;SEM=87.83. P=0.1). The only significant difference in Z-length is at E12.5 (452;SEM=23.53 for Insm1+/+ and 388;SEM=10.12 for Insm1−/−), when Insm1−/− may transiently have a shorter ganglion. (I) Average number of delaminated neurons or progenitors per cross section of Insm1+/+ and Insm1−/− otic ganglia at E10.5, E14.5, and E18.5. There is no significant difference in the average number of neurons in Insm1+/+ and Insm1−/− SVGs at E10.5 (125.97;SEM=11.24 and 119.29;SEM=18.49, respectively. P=0.38), but there is a significant difference at E14.5 (360.00;SEM=24.33 and 216.23;SEM=24.02). At E18.5 there is a significant difference between the average number of neurons in Insm1+/+ and Insm1−/− SpGs (193.96;SEM=38.08 and 116.79;SEM=15.13) and VGs (114.05;SEM=18.93 and 48.46;SEM=3.02). Sample size in the number of ears for each condition is indicated by the number within the column representing that group. The number of mice in each sample is written in parentheses below the number of ears. Error bars indicate SEM. *P≤0.05, **P≤0.01, ***P≤0.005, ****P≤0.0005. Scale bars: 100 μm. Ot: otocyst.
We found that, when compared with Insm1+/+ littermates, Insm1−/− mice had a smaller average cross sectional area of the otic ganglia at stages after E10.5 (E12.5, E14.5, and E18.5; Fig. 7G). At E14.5 and earlier stages, the spiral and vestibular ganglia have not fully separated so the two were analyzed together (SVG), but at E18.5, after the two ganglia have visually segregated, it is clear that both are smaller (34% for SpG and 51% for VG) in Insm1−/− when compared with Insm1+/+. When we examined the length of the ganglia in the Z-dimension, we saw no difference in ganglion length, with the exception of a slightly shorter ganglion in the Insm1−/− mouse at E12.5 (Fig. 7H), but not at later stages. This result shows that the reduction in cross sectional area is not due to the ganglion lengthening or to a difference in the angle of section. As a result, we were then able to quantify the number of neurons in those sections and found that, at E14.5 and E18.5, Insm1−/− mice had fewer neurons composing their ganglia (Fig. 7I). When we looked at E10.5, we did not see a difference; hence the reduction in ganglion size and neuron number in Insm1−/− mice occurs after this time. We hypothesized two potential sources for the decrease in neuron number in Insm1−/− ganglia: first, an increase in cell death and second, a decrease in neuron production.
2.4 Insm1 ablation results in reduced proliferation of delaminated progenitors
We examined apoptosis levels at two time points, E12.5 and E14.5, within the developmental period during which the neuron reduction phenotype develops. We used immunohistochemistry for activated caspase 3 (ACC3) as an indicator of apoptotic cells, together with Isl1 for DPs at E12.5, and β-III-tubulin for immature neurons at E14.5. We counted cells that were positive for both ACC3 and the neuronal marker in representative sections throughout each developing ear (Fig. 8A,C; white cells). We found that the levels of apoptosis were low (on average 2–5 cells per section) but variable. We found no significant increase in apoptosis levels in the absence of Insm1 (Fig. 8E) and therefore no evidence that programed cell death contributed to the decrease in neuron number in Insm1−/−.
Fig. 8. A change in apoptosis does not accompany the reduction of SVG neurons in Insm1−/−.
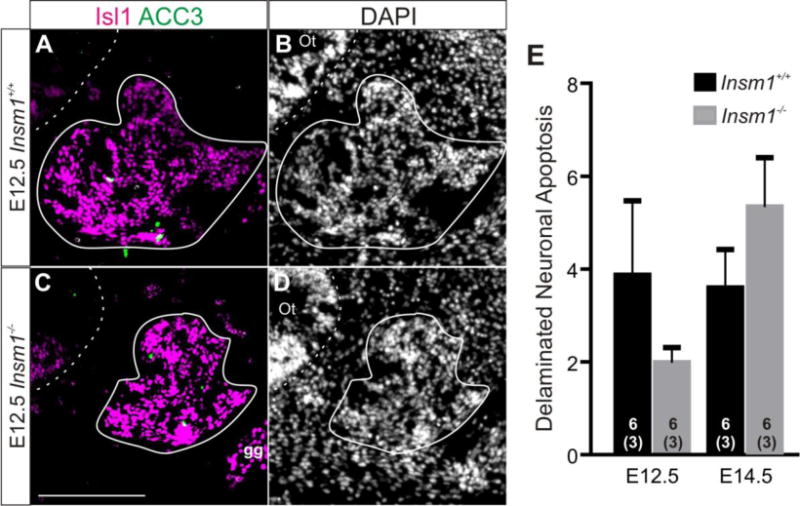
Representative anatomically matched sections of E12.5 ears from Insm1+/+ (A–B) and Insm1−/− (C–D) littermate embryos. (A,C) Immunohistochemistry for DP marker Isl1 (magenta) and apoptosis marker ACC3 (green). Colabeled cells appear white. (B,D) Nuclear pattern for A and C, respectively. Dashed lines delineate the otic epithelia. Solid lines delineate SVG and do not include nearby facial ganglion which is also expresses Isl1. (E) There is no significant difference in the number of delaminated neurons undergoing apoptosis per section between Insm1−/− and Insm1+/+ at E12.5 (3.87;SEM=1.6 and 1.98;SEM=0.32, respectively. P=0.14) or E14.5 (3.59;SEM=0.83 and 5.37;SEM=1.06, respectively. P=0.11). Sample size in the number of ears for each condition is indicated by the number within the column representing that group. The number of mice in each sample is written in parentheses below the number of ears. Error bars indicate SEM. Scale bar: 200 μm. gg: geniculate ganglion, Ot: otocyst.
We next sought to determine if Insm1−/− ganglia have lower levels of proliferation. We looked at two time points during neuronal proliferation, E10.5 (prior to the onset of phenotype) and E12.5 (when the phenotypic reduction in ganglion size is first detected) and used immunohistochemistry for PH3 as a marker of mitotic cells and Isl1 as a marker of DPs (Fig. 9A, D). Outside the epithelium (identified by the DAPI pattern) we counted cells positive for both Isl1 and PH3 as mitotic DPs (Fig. 9A,C; arrows). We also counted mitotic cells within the epithelium, because if fewer apical progenitors were becoming DPs then we would expect more apical mitosis. At E12.5 we counted PH3 positive cells within the neurosensory zone, which at this time is labeled by Isl1. All DPs originate from the shared pool of progenitors in this zone, and therefore if there was a change in apical to basal transition it would only affect this region. However, at E10.5 Isl1 does not label the neurosensory zone, so we counted all PH3 positive cells within the otic epithelium as apical progenitors.
Fig. 9. Reduction in proliferation of DPs precedes decrease of neurons in Insm1−/−.
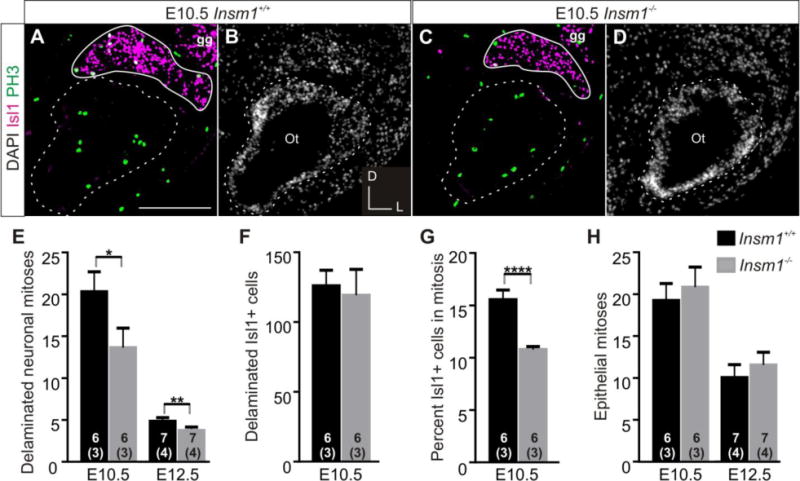
(A,C) Immunohistochemistry for DP marker Isl1 (magenta) and mitosis marker PH3 (green) in representative, anatomically matched, coronal sections of E10.5 Insm1+/+ (A) and Insm1−/− (C) heads. Colabeled cells appear white. (B,D) Nuclear pattern for (A) and (C), respectively. Dotted lines delineate the basal lamina of the otic epithelia. Solid lines delineate SVG and do not include nearby facial ganglion which is also expresses Isl1. (E) Average number of DPs in mitosis per cross section of Insm1+/+ and Insm1−/− otic ganglia at E10.5 and E12.5. There are approximately 30% fewer DPs in mitosis in Insm1−/− than in Insm1+/+ ganglia at E10.5 (13.63;SEM=2.33 and 20.32;SEM=2.37) and E12.5 (3.87;SEM=0.46 and 5.55;SEM=0.41). (F) Average number of DPs per cross section of Insm1+/+ and Insm1−/− otic ganglia at E10.5. There is no significant difference in the average number of DPs in Insm1+/+ and Insm1−/− SVGs at E10.5 (125.97;SEM=11.24 and 119.29;SEM=18.49, respectively. P=0.38). (G) Average percent of DPs that are in mitosis per cross section of Insm1+/+ and Insm1−/− otic ganglia at E10.5. Of the total number of Isl1 cells, approximately 30% fewer are in mitosis in Insm1−/− than Insm1+/+ ganglia at E10.5 (11.29%;SEM=0.28% and 16.06%;SEM=0.92%). (H) Average number of mitotic progenitors within the epithelium per cross section of Insm1+/+ and Insm1−/− otic ganglia at E10.5 and E12.5. There is no significant difference in the average number of epithelial mitotic progenitors in Insm1+/+ and Insm1−/− SVGs at E10.5 (19.25;SEM=2.01 and 20.83;SEM=2.39. P=0.31) and at E12.5 (10.05;SEM=1.54 and 11.53;SEM=1.53. P=0.25). Sample size in the number of ears for each condition is indicated by the number within the column representing that group. The number of mice in each sample is written in parentheses below the number of ears. Error bars indicate SEM. *P≤0.05, **P≤0.01, ****P≤0.0005. Scale bars: 100 μm. gg: geniculate ganglion, Ot: otocyst.
We found roughly a 30% decrease in the number of mitotic DPs in the developing ears of Insm1−/− at both E10.5 and E12.5 (Fig. 9E). We also found that at E10.5 there was no change in the number of DPs (Isl1 positive cells; Fig. 9F), but that the percentage of DPs in mitosis was decreased by roughly 30% in Insm1−/− (Fig. 9G). Finally, there was no change in the number of epithelial mitoses at either time point (Fig. 9H). Taken as a whole, these data suggest that in the absence of Insm1, epithelial progenitors are undergoing the same number of apical-to-basal transitions (delaminations) and concomitant neuronal commitments; however, Insm1−/− DPs undergo fewer divisions and hence produce fewer neurons.
3. Discussion
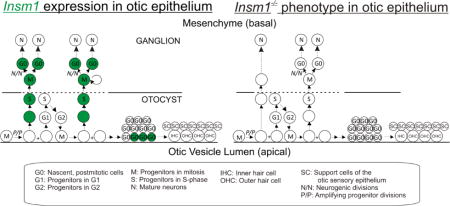
We found that, in the embryonic ear, Insm1 is expressed in the progenitors of SVG neurons and promotes their proliferation (Fig. 10A,B). Insm1 is not absolutely required for neurogenic proliferation of delaminated progenitors, but it adjusts the extent to which it occurs. This fine tuning role substantially affects the number of spiral and vestibular ganglion neurons produced. As early as E10.5, we observed Insm1 in the neurogenic DPs at the basal edge of the otocyst and in the mesenchyme. This would indicate that Insm1 is likely expressed after NeuroD, which is downstream of Neurog1 and is expressed in early neuronal progenitors (apical and delaminating) of the otic epithelium (Deng et al., 2014; Kim et al., 2001; Liu et al., 2000; Ma et al., 2000). Several potential functions have been proposed for NeuroD. Among them are promoting delamination of neuronal progenitors and neuron survival, neither of which is affected in the Insm1 null mouse (Jahan et al., 2010b; Kim et al., 2001; Liu et al., 2000). Insm1 does appear roughly at the same time as Isl1, and does not regulate its expression. Nor does Insm1 regulate the expression of factors known to come on after Isl1, namely Pou4f1 or Pou4f2 (Supplemental Figs. 2 and 3) (Deng et al., 2014). Following the cessation of neurogenesis we found that Insm1 expression was maintained in the nascent neurons of the SpG and the VG. As these neurons differentiated, Insm1 expression was reduced until P0, when no Insm1 expression was detected in the otic ganglion neurons.
Fig. 10. Insm1 expression in the developing inner ear, the effects of Insm1 ablation in otic neurogenesis, and a suggested common mechanism of neurogenesis among neural epithelia.
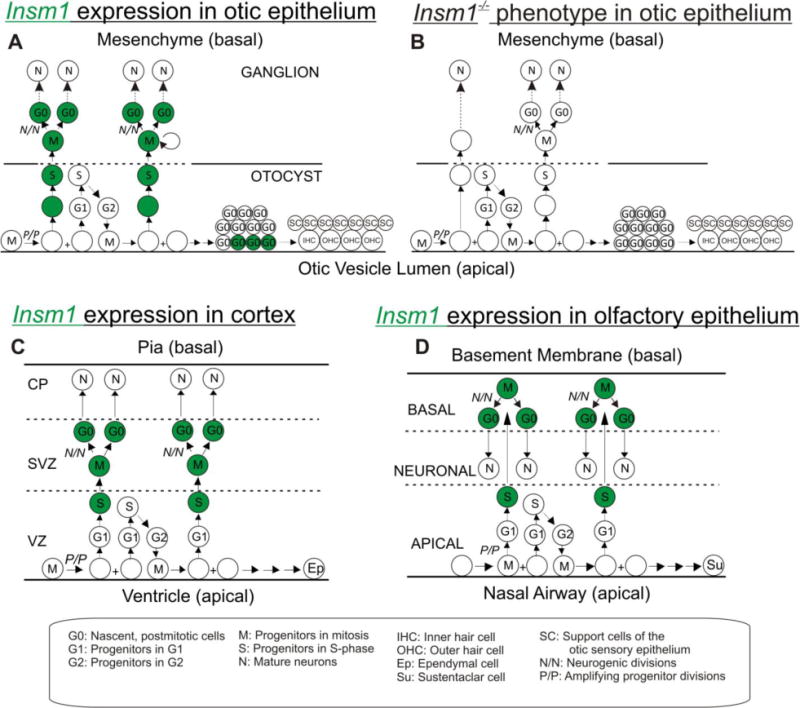
(A) During the embryonic development of the ear, uncommitted, amplifying progenitors within the otocyst undergo interkinetic nuclear migration whereby mitosis occurs apically within the epithelium and synthesis basally. From E9.5–E12.5 neuronally-committed progenitors are generated by amplifying progenitors, express Insm1 (green), and delaminate from the otocyst. Once they have delaminated, these progenitors undergo an unknown number of divisions to produce SVG neurons. Nascent neurons maintain expression of Insm1. As they differentiate, Insm1 levels subside, and ultimately Insm1 is undetectable mature neurons. Following neurogenesis and the cessation of amplification in the organ of Corti, the OHCs initiate Insm1 expression in accordance with the wave of hair cell differentiation. As in neurons, OHCs decrease Insm1 expression as they mature. (B) In the absence of Insm1, the DPs undergo fewer divisions, and therefore, produce fewer neurons resulting in smaller spiral and vestibular ganglia. (C, D) The expression pattern of Insm1 in otic neurogenesis (A) is very similar to Insm1 expression in the embryonic cortex (Duggan et al., 2008)(C) and olfactory epithelium (Rosenbaum et al., 2011) (D). In each case, uncommitted progenitors undergo interkinetic nuclear migration. When a progenitor commits to neuronal cell fate it expresses Insm1, migrates basally, either staying within the epithelium or delaminating into the mesenchyme, and divides to produce neurons. This schematic also highlights differences between epithelia. The neurons migrate to different regions within or outside of the epithelium, interacting differently with the basal lamina as they do so. Also, those cells that continue to divide apically produce different types of epithelial cells specific to their organ. Circles represent cell nuclei. Abbreviations are defined in figure key.
The expression pattern of Insm1 in the DPs is consistent with our observation that Insm1 promotes neurogenic divisions. Insm1 ablation did not cause a decrease in the number of delaminations, but a decrease in proliferation of the progenitors that have delaminated. As a result, when compared with a non-mutant littermate, mice lacking Insm1 produced roughly half the number of SVG neurons. Thus far, many SVG transcription factors have been implicated in determining ganglion size, either by promoting cell fate, delamination, or neuron survival, but none have been shown to regulate mitosis in the living mouse in the way that we have demonstrated for Insm1 (Camarero et al., 2001; Huang et al., 2001; Kim et al., 2001; Liu et al., 2000; Ma et al., 2000; Yamada et al., 2015). Embryonic SVG neurons also express neurotrophins (BDNF and NT-3) and their receptors (TrkB and TrkC) (Durruthy-Durruthy et al., 2014; Farinas et al., 2001; Fritzsch et al., 2002; Green et al., 2012). The absence of NT-3 results in a reduction of neurons, but this differs from the reduction due to Insm1 ablation because: (1) it is much more severe (~84% vs 41% for SpG); (2) it develops after E13.5, following neurogenesis and (3) it has been attributed to increased apoptosis, not decreased proliferation (Farinas et al., 2001).
It is possible that Insm1 is also playing a functional role in the differentiation of the nascent neurons. However, we have been unable to identify any abnormalities in the differentiation of SVG neurons in the absence of Insm1. This may be, in part, due to the embryonic lethality of the mutant.
3.1 Insm1 expression reveals a common mechanism of neurogenesis among different neuroepithelia that, with few variations, generates organs as diverse as ear, olfactory epithelium and cortex
The expression of Insm1 in DPs and differentiating neurons supports previous claims that Insm1 is expressed transiently by late neuronal progenitors and nascent neurons throughout the developing nervous system (Duggan et al., 2008; Farkas et al., 2008). The expression pattern and function of Insm1 has been characterized in depth in three different neural epithelia, the cortex (Duggan et al., 2008; Farkas et al., 2008) the olfactory epithelium (Rosenbaum et al., 2011) and now the otocyst (Fig. 10).
Comparing these expression patterns side-by-side reveals a common neurogenic morphology amongst these (and potentially all) developing neural epithelia. In each epithelium, uncommitted, amplifying progenitors extend processes to both the apical and basal lamina and undergo interkinetic nuclear migration (Taverna and Huttner, 2010). Once an amplifying progenitor transitions to becoming neuronally-committed, it migrates in the basal direction and begins to express Insm1 (Farkas et al., 2008; Haubensak et al., 2004; Rosenbaum et al., 2011). There, it can further divide to produce neurons. Those progenitors that continue to divide apically will produce non-neuronal epithelial cells (Guerout et al., 2014; Murdoch and Roskams, 2007; Raft et al., 2007). We believe that these morphogenic similarities among neural epithelia have not been widely recognized. Furthermore, the conserved pattern of Insm1 expression across various neural epithelia suggests a common molecular mechanism as well.
Of course, each epithelium displays unique structural variations from the others that allow that epithelium to generate a different organ. Most apparent is the interaction of the nascent neurons with the basal lamina. In the cortex, nascent neurons displace the basal lamina radially as they migrate outward to build new layers (Taverna and Huttner, 2010) (Fig. 10C). In the olfactory epithelium, the basal lamina is unchanged as nascent neurons migrate back apically to produce a neuronal layer in the middle of the epithelium (Murdoch and Roskams, 2007) (Fig. 10D). And in the otocyst, the basal lamina breaks down to allow basally migrating progenitors to delaminate and form ganglia (Raft et al., 2004; Rubel and Fritzsch, 2002) (Fig. 10A). Another important difference is the fate of the apically-dividing progenitors which in all cases generate epithelial cells, though of very different types: ependymal cells in cortex, sustentacular cells in olfactory epithelium, and various types of epithelial cells - including sensory hair cells - in inner ear (Guerout et al., 2014; Murdoch and Roskams, 2007; Raft et al., 2007) (Fig. 10A,C,D).
Functionally, we found that Insm1 promotes neurogenesis in the otocyst as it does in the developing cortex and olfactory epithelium. In the embryonic cortex and olfactory epithelium, knockout of Insm1 resulted in fewer basal neurogenic progenitors and neurons (Farkas et al., 2008; Rosenbaum et al., 2011). The favored interpretation was that this resulted from a reduction in the number of apical to basal transitions of progenitors, and therefore the number of progenitors committing to neurogenesis (Farkas et al., 2008; Rosenbaum et al., 2011). However, our results are not consistent with this interpretation of Insm1 function in the otocyst. Instead, our data suggest that at least in the inner ear Insm1 is not involved in promoting delamination (apical to basal transitions), but instead promotes proliferation of delaminated (i.e. basal) progenitors.
3.2 Insm1 is the earliest known gene expressed in outer and not inner hair cells
We also found an unexpected expression pattern of Insm1 in nascent OHCs but not in IHCs, vestibular HCs or support cells. Our results show that these OHCs are not derived from the Insm1-expressing progenitors that give rise to DPs. Instead, OHCs do not express Insm1 until after exiting the cell cycle. While this might seem surprising at first, our finding is consistent with the literature showing that there are no progenitors that produce solely SVG neurons and OHCs. Firstly, fate mapping of early SVG progenitors that express Neurog1 from E8.5–E13.5 shows that they also can produce epithelial cells of the utricle and saccule including sensory hair cells, but not those in the cochlea (Koundakjian et al., 2007; Raft et al., 2007). At later stages, retroviral lineage tracing of otic progenitors injected at E11.5, showed that cochlear hair cells only shared common progenitors with cochlear support cells, not with SVG neurons (Jiang et al., 2013). Finally, hair cell versus support cell fate within the organ of Corti is determined by Notch signaling pathways, not by cell type specific progenitors (Fekete et al., 1998; Kiernan et al., 2005). Therefore, even if an Insm1 expressing progenitor cell was able to produce SVG neurons and cochlear epithelial cells, it would not solely produce OHCs, but also IHCs and support cells.
Interestingly, other neurogenic transcription factors that regulate ganglion formation (Neurog1 and NeuroD) have also been implicated in the development of cochlear hair cells, including potentially the spatial arrangement and/or differentiation of IHCs and OHCs (Fritzsch et al., 2002; Fritzsch et al., 2011; Jahan et al., 2010a; Jahan et al., 2010b; Jahan et al., 2015b; Ma et al., 1998; Matei et al., 2005), although some believe this is due to their effects in the neurons, not to a direct action in cells of the organ or Corti (Basch et al., 2015; Bok et al., 2013; Tateya et al., 2013). NeuroD, which like Insm1 is expressed in delaminating progenitors, also appears to be expressed in nascent hair cells, although this expression is not restricted to OHCs (Fritzsch et al., 2006; Matei et al., 2005; Scheffer and Shen, 2015), as is the expression of Insm1.
Due to the embryonic lethality of our Insm1 mutant and the late stages at which OHCs mature, we were unable to determine the functional role of Insm1 in OHCs. At the latest stages we were able to obtain viable Insm1−/− mice, OHC arrangement, shape, innervation pattern, and expression of marker genes did not appear different from littermate controls. Evaluating the effect of Insm1 in OHCs will probably necessitate a conditional ablation strategy, currently underway.
We find the expression in OHCs particularly interesting for several reasons. First, Insm1 is the earliest known gene expressed by OHCs but not IHCs. This will make it an excellent tool for identifying and isolating OHCs early in the process of differentiation. Stat3 has also been found to be enriched in OHCs during this developmental window (Hertzano et al., 2004); however, we found it is also expressed at lower levels in IHCs and support cells (data not shown).
Second, Insm1 appears to be turned on shortly after HCs initiate differentiation and concomitant with the early hair cell marker, MyoVIIa. Until now, the only genes known to be expressed by OHCs and not IHCs were not expressed until around or after birth (Abe et al., 2007; He et al., 1994). Our discovery that OHCs uniquely express Insm1 as early as E15.5 significantly advances the time point at which we know OHCs initiate their own specific program of differentiation. Though we do know that IHCs express Fgf8 as early as E16.5, this finding only demonstrates that IHCs are actively differentiating differently from OHCs, not vise versa (Jacques et al., 2007).
Finally, the field of HC regeneration is rapidly expanding (for recent reviews see (Geleoc and Holt, 2014; Groves et al., 2013; Liu et al., 2014; Santaolalla et al., 2013). Since OHCs incur more damage than IHCs in noise induced hearing loss and age related hearing loss, it would be valuable to regenerate these HC types specifically. As we mentioned above, in these experiments Insm1 can be used as a marker for earlier stages of OHC specific differentiation instead of prestin which is expressed much later in OHC differentiation (Abe et al., 2007; He et al., 1994). Furthermore, since INSM1 is a zinc-finger protein and can act as a transcription factor (Breslin et al., 2002), we speculate that it could be a good candidate for driving OHC specific differentiation in these induced HCs.
4. Materials and Methods
Mice
All wild type mice for in situ hybridization were CD1 outbred strain (Charles River). Insm1−/− allele mice were maintained in a CD1 background and were genotyped as described previously (Rosenbaum et al., 2011). Rosa:lacZ reporter mice (FVB.Cg.Gt(Rosa)26Sortm1(CAG- lacZ,-EGFP)Glh) were obtained from The Jackson Laboratory (Sacramento, CA) and PCR genotyping was performed with the following primers: forward 5′-CGTCATCTGCAACTCCAGTC-3′ and reverse 5′-GGAGCGGGAGAAATGGATATG-3′ for the wild type allele; forward 5′-GATCAGCAGCCTCTGTTCCACA-3′ and reverse 5′-TCTTTGGAGCCATGATCGAAGT-3′ for the mutant allele. The Insm1GFP.Cre mice were generated as previously described (Osipovich et al., 2014). Mice were genotyped using PCR amplification using forward primer 5′-ATCCTCAGATTGTACTCAATACCTA-3′ and reverse primer 5′-CCTGCATGTCCACACTGCGAT-3′ so that the product of the wild type allele (362bp) was shorter than that of the Insm1GFP.Cre allele (423bp). For lineage tracing, we bred Insm1GFP.Cre mice to Rosa:lacZ reporter mice.
For embryo dissection, time pregnant dams were sacrificed by isoflurane overdose followed by cervical dislocation. Their abdomens were opened to expose the uterus which was dissected and transferred to cold 1xPBS. Embryos were dissected from the uterus, tails were removed for genotyping, and embryos were further processed depending upon future use. For DNA extraction, tails were incubated in 50 mM NaOH at 95°C for 20 min or until dissolved. Base was then neutralized with 1 M Tris pH8 and digested tissue was separated from DNA by centrifuging at max speed for 6 min.
All procedures and housing were approved by Northwestern University Animal Care and Use Committee on animal protocols 2012-1570 and IS00001281.
Lineage tracing
We used mice bearing the Insm1GFP.Cre allele, which replaces the Insm1 coding sequence with that of the green fluorescent protein fused to the Cre-recombinase (GFP.Cre); it is therefore a null allele (Osipovich et al., 2014). We crossed the Insm1GFP.Cre mouse with a ROSA:lacZ reporter mouse which uses a ubiquitous promoter to express β-galactosidase (β-gal) with a nuclear localization sequence following Cre-mediated deletion of a floxed stop cassette. In this way, all cells that have expressed Insm1GFP.Cre or descend from progenitors that have expressed Insm1GFP.Cre will express nuclear localized β-gal.
In situ hybridization and immunohistochemistry
In situ hybridization was performed on cryosections (10–12 μm thick) of unifixed and snap frozen embryos or ears collected at E10.5, E14.5, E16.5, and P1, as previously described (Duggan et al., 2008).
For immunohistochemistry, whole embryos (E10.5–E12.5) or decapitated embryo heads (E14.5–E18.5) were fixed in fresh 4% PFA overnight. The next day, in some cases E14.5–E18.5 ears were further dissected from heads. Tissues were washed in 1xPBS three times for 15 min each and were then dehydrated using a 10%, 20%, 30% sucrose gradient. Tissues were then mounted in Optimal Cutting Temperature (OCT) medium and frozen on dry ice. Frozen tissues were kept at −80°C until sectioned 10–12 μm thick on a cryostat and directly mounted onto slides. Slides were kept at −80°C until staining. Whole mount cochleas were fixed in fresh 4% PFA for 1 hour.
Slides were thawed at room temperature for 10 min then vacuum dried for another 10 min. Then slides were post-fixed in fresh 2% PFA in 1xPBS for 10 min. Slides or whole cochleas were washed and blocked in 3% serum with 0.1% Triton X-100 for 1–2 hours. Samples were incubated overnight at 4°C with primary antibodies in blocking solution. The following primary antibodies were used: rabbit anti-GFP (1:1000; Life Technologies, Carlsbad, CA), chicken anti-GFP (1:5000; Abcam, Cambridge, MA), chicken anti-β-gal (1:2500; Abcam, Cambridge, MA), mouse anti-Isl1 (1:1000; Developmental Studies Hybridoma Bank, Iowa City, IA), mouse anti-p27kip1 (1:250; Thermo Fisher Scientific, Freemont, CA), rabbit anti-MyoVIIa (1:100; Proteus Bioscience, Ramona, CA), mouse anti-β-Tubulin III (TuJ1; 1:500; Covance, Princeton, NJ), rabbit anti-ACC3 (1:1000; Cell Signaling, Beverly, MA), rabbit anti-phospho-Histone H3 (PH3; 1:100; Millipore, Billerica, MA), mouse anti-Ki67 (1:200; BD Pharmingen, San Diego, CA), mouse anti-Pou4f1 (1:100; Santa Cruz Biotechnology, Dallas, Tx), and goat anti-Pou4f2 (1:100; Santa Cruz Biotechnology, Dallas, Tx). Unbound primary antibody was removed with 3 washes of 1xPBS with 0.1% Triton X-100 (PBST). Slides were incubated with Alexa Fluor-conjugated secondary antibodies (Life Technologies) for 1 hour in blocking solution. Unbound secondary antibody was removed with 3 washes of PBST, sections were DAPI stained, and mounted with Prolong Gold (Life Technologies). Antigen retrieval before primary antibody incubation was necessary for TuJ1 and anti-p27kip1. Slides were incubated with 10 mM sodium citrate in 0.25% Triton X-100 at 92°C for 20 min and allowed to cool for 30 min before washing 3 times in 1xPBS.
Cell quantification and statistics
In all cell quantification studies, every 2nd or 4th section throughout the developing ears of paired littermates were stained and evaluated, while skipping the rest to avoid double counting cells. Total number of sections from beginning to end (including those not stained) were then counted and multiplied by section thickness to determine Z-length. Tissues were visualized and photographed either on a Nikon Eclipse E600 microscope with an RT Slider Spot camera (verson2.3.1; Diagnostic Instruments, Sterling Heights, MI) or on a Nikon Eclipse Ti microscope with a Photometrics Cool Snap HQ2 camera (Tucson, AZ).
Photos were then analyzed in ImageJ with genotype blinded. Ganglion cross section areas were measured by circling the areas containing the neuronal cell bodies, which we labelled at E10.5–E12.5 with anti-Isl1, and at E14.5–E18.5 with TuJ1 (being careful not to include TuJ1 positive areas with neuronal processes but lacking cell bodies). Care was also taken not to include the nearby geniculate ganglion. Although a small number of facial neurons might intercalate within the vestibular ganglion (based on (Fritzsch et al., 1997) we estimate 1 to 2 per section), these are too few to significantly affect our quantifications. At each stage all Isl1 (E10.5–E12.5) or TuJ1 (E14.5–18.5) positive cells with DAPI nuclei were counted by eye using the Cell Counter plugin of ImageJ. For neuronal proliferation analysis, only cells in the mesenchyme that were both Isl1 and PH3 positive with DAPI+ nuclei were counted. PH3 positive cells with DAPI+ nuclei were also counted within the entire epithelium at E10.5 and, at E12.5, only within the neurogenic zone of the epithelium (defined by Isl1 expression). For cell death analysis, only cells that were ACC3, Isl1 or TuJ1, and DAPI positive in the mesenchyme were counted. All ganglion size and cell counting data were stored and managed in Microsoft Excel. For each ear analyzed, the average of each metric (cross section area, number of neurons, proliferation, or apoptosis) was calculated and then all ears were averaged and compared using one-tailed, unpaired, Student’s t-test. Statistics and graphs were generated using Prism Software (Graphpad; La Jolla, CA). For all graphs error bars represent the standard error of the mean (SEM) and *P≤0.05, **P≤0.01, ***P≤0.005, ****P≤0.0005.
Supplementary Material
Highlights.
Delaminating otic progenitors and nascent neurons transiently express Insm1.
Auditory and vestibular neuron progenitors proliferate less in Insm1 null mice.
Insm1 expression reveals shared morphogenesis among various neurogenic epithelia.
Embryonic outer, but not inner, hair cells express Insm1 early in differentiation.
Acknowledgments
The authors would like to thank Northwestern University Transgenic and Targeted Mutagenesis Laboratory for their help with generating the Insm1−/− mouse and reconstituting the Insm1GFP.Cre mouse line from frozen sperm. We thank the labs of Hande Ozdinler and Raj Awatramani for reagents. Ruth Anne Eatock provided her thoughts the vestibular innervation in the Insm1 mutant. We would also like to thank Jim Bartles for his critical reading of the manuscript. We would also like to thank Ana Gonzalo for her technical support during a summer internship. This work was funded by NIH grants F31-DC012483 (to S.M.L.) and R01-NS044363 (to J.G.A.), and by the Knowles Hearing Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H. Developmental expression of the outer hair cell motor prestin in the mouse. Journal of Membrane Biology. 2007;215:49–56. doi: 10.1007/s00232-007-9004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anniko M, Wikstrom SO. Pattern-Formation of the Otic Placode and Morphogenesis of the Otocyst. American Journal of Otolaryngology. 1984;5:373–381. doi: 10.1016/s0196-0709(84)80051-4. [DOI] [PubMed] [Google Scholar]
- Appler JM, Goodrich LV. Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Prog Neurobiol. 2011;93:488–508. doi: 10.1016/j.pneurobio.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appler JM, Lu CC, Druckenbrod NR, Yu WM, Koundakjian EJ, Goodrich LV. Gata3 is a critical regulator of cochlear wiring. J Neurosci. 2013;33:3679–91. doi: 10.1523/JNEUROSCI.4703-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–30. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Basch ML, Brown RM, Jen HI, Groves AK. Where hearing starts: the development of the mammalian cochlea. Journal of Anatomy. 2015 doi: 10.1111/joa.12314. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Zenczak C, Hwang CH, Wu DK. Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc Natl Acad Sci U S A. 2013;110:13869–74. doi: 10.1073/pnas.1222341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Zhu M, Notkins AL, Lan MS. Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: identification of consensus IA-1 binding sequence. Nucleic Acids Res. 2002;30:1038–45. doi: 10.1093/nar/30.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero G, Avendano C, Fernandez-Moreno C, Villar A, Contreras J, de Pablo F, Pichel JG, Varela-Nieto I. Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J Neurosci. 2001;21:7630–41. doi: 10.1523/JNEUROSCI.21-19-07630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero G, Leon Y, Gorospe I, De Pablo F, Alsina B, Giraldez F, Varela-Nieto I. Insulin-like growth factor 1 is required for survival of transit-amplifying neuroblasts and differentiation of otic neurons. Dev Biol. 2003;262:242–53. doi: 10.1016/s0012-1606(03)00387-7. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Deng M, Yang H, Xie X, Liang G, Gan L. Comparative expression analysis of POU4F1, POU4F2 and ISL1 in developing mouse cochleovestibular ganglion neurons. Gene Expr Patterns. 2014;15:31–7. doi: 10.1016/j.gep.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Frutos E, Lopez-Hernandez I, Vendrell V, Neves J, Gallozzi M, Gutsche K, Quintana L, Sharpe J, Knoepfler PS, Eisenman RN, Trumpp A, Giraldez F, Schimmang T. N-myc controls proliferation, morphogenesis, and patterning of the inner ear. J Neurosci. 2011;31:7178–89. doi: 10.1523/JNEUROSCI.0785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A, Madathany T, de Castro SC, Gerrelli D, Guddati K, Garcia-Anoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol. 2008;507:1497–520. doi: 10.1002/cne.21629. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS One. 2013;8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Lim KC, Engel JD, Fritzsch B. Limited inner ear morphogenesis and neurosensory development are possible in the absence of GATA3. Int J Dev Biol. 2011;55:297–303. doi: 10.1387/ijdb.103178jd. [DOI] [PubMed] [Google Scholar]
- Durruthy-Durruthy R, Gottlieb A, Hartman BH, Waldhaus J, Laske RD, Altman R, Heller S. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell. 2014;157:964–78. doi: 10.1016/j.cell.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LM, Haffner C, Giger T, Khaitovich P, Nowick K, Birchmeier C, Paabo S, Huttner WB. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–21. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–14. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60:423–33. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–93. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–56. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hearing Research. 2011;276:16–26. doi: 10.1016/j.heares.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997;15:563–76. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344:1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SH, Bailey E, Wang Q, Davis RL. The Trk A, B, C’s of neurotrophins in the cochlea. Anat Rec (Hoboken) 2012;295:1877–95. doi: 10.1002/ar.22587. [DOI] [PubMed] [Google Scholar]
- Groves AK, Zhang KD, Fekete DM. The genetics of hair cell development and regeneration. Annu Rev Neurosci. 2013;36:361–81. doi: 10.1146/annurev-neuro-062012-170309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerout N, Li X, Barnabe-Heider F. Cell fate control in the developing central nervous system. Exp Cell Res. 2014;321:77–83. doi: 10.1016/j.yexcr.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Evans BN, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear Res. 1994;78:77–90. doi: 10.1016/0378-5955(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Hertzano R, Montcouquiol M, Rashi-Elkeles S, Elkon R, Yucel R, Frankel WN, Rechavi G, Moroy T, Friedman TB, Kelley MW, Avraham KB. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum Mol Genet. 2004;13:2143–53. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–32. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–9. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010a;341:95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Elliott KL, Fritzsch B. The quest for restoring hearing: Understanding ear development more completely. BioEssays. 2015a;37:1016–1027. doi: 10.1002/bies.201500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010b;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurog1 can partially substitute for Atoh1 function in hair cell differentiation and maintenance during organ of Corti development. Development. 2015b;142:2810–21. doi: 10.1242/dev.123091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang L, Beier KT, Cepko CL, Fekete DM, Brigande JV. Lineage analysis of the late otocyst stage mouse inner ear by transuterine microinjection of a retroviral vector encoding alkaline phosphatase and an oligonucleotide library. PLoS One. 2013;8:e69314. doi: 10.1371/journal.pone.0069314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–26. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn. 2011;240:1373–90. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. J Neurosci. 2007;27:14078–88. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–91. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Lawlor P, Cacciabue-Rivolta DI, Langton-Hewer C, van Doorninck JH, Holley MC. GATA3 and NeuroD distinguish auditory and vestibular neurons during development of the mammalian inner ear. Mech Dev. 2004;121:287–99. doi: 10.1016/j.mod.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol Suppl. 1985;422:1–69. [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–54. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen P, Wang J. Molecular mechanisms and potentials for differentiating inner ear stem cells into sensory hair cells. Dev Biol. 2014;390:93–101. doi: 10.1016/j.ydbio.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–43. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–82. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–99. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Osipovich AB, Long Q, Manduchi E, Gangula R, Hipkens SB, Schneider J, Okubo T, Stoeckert CJ, Jr, Takada S, Magnuson MA. Insm1 promotes endocrine cell differentiation by modulating the expression of a network of genes that includes Neurog3 and Ripply3. Development. 2014;141:2939–49. doi: 10.1242/dev.104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–82. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–21. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–12. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JN, Duggan A, Garcia-Anoveros J. Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev. 2011;6:6. doi: 10.1186/1749-8104-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):1–44. [PubMed] [Google Scholar]
- Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Expression of myosin VIIA during mouse embryogenesis. Anat Embryol (Berl) 1997;196:159–70. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Butler Tjaden NE, Barlow AJ, Trainor PA. Cochleovestibular nerve development is integrated with migratory neural crest cells. Dev Biol. 2014;385:200–10. doi: 10.1016/j.ydbio.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaolalla F, Salvador C, Martinez A, Sanchez JM, Del Rey AS. Inner ear hair cell regeneration: A look from the past to the future. Neural Regen Res. 2013;8:2284–9. doi: 10.3969/j.issn.1673-5374.2013.24.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer DI, Shen J. Gene Expression by Mouse Inner Ear Hair Cells during Development. 2015;35:6366–80. doi: 10.1523/JNEUROSCI.5126-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- Steventon B, Mayor R, Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol. 2014;389:28–38. doi: 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateya T, Imayoshi I, Tateya I, Hamaguchi K, Torii H, Ito J, Kageyama R. Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development. 2013;140:3848–57. doi: 10.1242/dev.095398. [DOI] [PubMed] [Google Scholar]
- Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–14. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Yamada T, Minoda R, Miwa T, Ise M, Takeda H, Yumoto E. Neurogenesis of the spiral ganglion cells in the cochlea requires the transcriptional cofactor TIS21. Neurosci Lett. 2015;584:265–9. doi: 10.1016/j.neulet.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res. 2011;278:21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


