Abstract
Background
At-risk drinking, defined as alcohol use that is excessive or potentially harmful in combination with select comorbidities or medications, affects about 10% of older adults in the United States and is associated with higher mortality. The Project SHARE intervention, which uses patient and provider educational materials, physician counseling, and health educator support, was designed to reduce at-risk drinking among this vulnerable population. Although an earlier study showed that this intervention was successful in reducing rates of at-risk drinking, it is unknown whether these reductions translate into improved health and health-related quality of life (HRQL).
Objective
To examine changes in health and HRQL of older adult at-risk drinkers resulting from a patient-provider educational intervention.
Research Design
A randomized controlled trial to compare the health and HRQL outcomes of patients assigned to the Project SHARE intervention vs. care as usual at baseline, six- and twelve-months post assignment. Control patients received usual care, which may or may not have included alcohol counseling. Intervention group patients received a personalized Patient Report, educational materials on alcohol and aging, a brief provider intervention, and a telephone health educator intervention.
Subjects
Current drinkers age 60 years and older accessing primary care clinics around Santa Barbara, California (n=1,049).
Measurements
Data were collected from patients using baseline, six- and twelve-month mail surveys. Health and HRQL measures included mental and physical component scores (MCS and PCS) based on the Short Form-12v2 (SF-12v2), the SF-6D, which is also based on the SF-12, and the Geriatric Depression Scale (GDS). Adjusted associations of treatment assignment with these outcomes were estimated using generalized least squares regressions with random provider effects. Regressions controlled for age group, sex, race/ethnicity, marital status, education, household income, home ownership and the baseline value of the dependent variable.
Results
After regression adjustment, the intervention was associated with a 0.58 point (95% CI: −0.06, 1.21) increase in 6-month MCS and a 0.14 point (95% CI: 0.01, 0.26) improvement in 12-month GDS score, compared to the control group. The intervention also increased adjusted SF-6D scores by 0.01 points at both six and twelve months (6-month 95% CI: 0.01, 0.02; 12-month 95% CI: 0.01, 0.01).
Conclusions
Despite the previously shown effectiveness of the Project SHARE intervention to reduce at-risk drinking among older adults, this effect translated into effects on health and HRQL that were statistically but not necessarily clinically significant. Effects were most prominent for patients who received physician discussions, suggesting that provider counseling may be a critical component of primary care-based interventions targeting at-risk alcohol use.
Keywords: health-related quality of life, older adults, at-risk drinking, provider interventions
INTRODUCTION
At-risk drinking, or drinking that puts individuals at high risk for developing alcohol use disorder, is currently defined by the National Institute on Alcohol Abuse and Alcoholism for women as consuming more than 3 drinks on any single day and more than 7 drinks per week. For men, it is defined as consuming more than 4 drinks on any single day and more than 14 drinks per week (National Institute on Alcohol Abuse and Alcoholism (NIAAA), 2015). According to this threshold, approximately 3% of female and 10% of male older adults are defined as at-risk drinkers (Breslow, Faden, & Smothers, 2003; Kirchner et al., 2007; Merrick et al., 2008). However, older adults face additional risks associated with drinking because of age-related physiological changes that increase blood alcohol levels for a given dose, increased brain sensitivity to alcohol and increases in morbidity and medication use (Linnoila, Erwin, Cleveland, Logue, & Gentry, 1978; Moore, Whiteman, & Ward, 2007; Vestal et al., 1977). Using a definition of at-risk drinking that includes alcohol use that is excessive or potentially harmful in combination with select comorbidities or medications, 3% of women and 18% of men aged 60 years and older have been defined at-risk drinkers in a population-based sample of US adults (Moore et al., 2006). Further, it affects 35% of older adults who use alcohol, and is associated with higher mortality (Barnes et al., 2010; Moore et al., 2006).
At-risk drinking among older adults is also associated with health problems like hypertension, accidental injury, dementia and depression (Bakhshi & While, 2014). However, alcohol use has mixed effects on health and health-related quality of life (HRQL), which are self-reported measures of physical, social and mental well-being (Centers for Disease Control and Prevention, 2011). Some of the extant literature finds that alcohol consumption – in some cases, even heavy drinking – is associated with improved physical HRQL compared to no alcohol consumption (Valencia-Martín, Galán, Guallar-Castillón, & Rodríguez-Artalejo, 2013), while others indicate no (Martinez, Lien, Landheim, Kowal, & Clausen, 2014) or negative association between alcohol use and HRQL (Chen & Storr, 2006; Okoro et al., 2004; Wen et al., 2012). There is disagreement on how drinking patterns relate to HRQL status, though in general it seems that low-quantity is better than high-quantity drinking for HRQL outcomes (Volk, Cantor, Steinbauer, & Cass, 1997).
Older age can affect both alcohol use patterns (Bakhshi & While, 2014) and health and HRQL (De Luca d’Alessandro, Bonacci, & Giraldi, 2011), but the relationship between the two is unclear. Conflicting results find that older adults report lower HRQL than younger adults at intake to inpatient alcohol treatment, but report higher HRQL than younger patients after treatment (Donovan, Mattson, Cisler, Longabaugh, & Zweben, 2005). Further, few studies focus on older populations in the community (Byles, Young, Furuya, & Parkinson, 2006; Strandberg et al., 2007); those that do note that older women who drink moderately have lower HRQL (Byles et al., 2006) and that older men who prefer wine to other types of alcoholic beverages have higher HRQL (Strandberg et al., 2007). In middle-aged and older adults, regular moderate consumption was associated with the highest initial HRQL, although all types of drinkers and abstainers declined in HRQL over time (Kaplan et al., 2012).
Interventions to reduce alcohol use can improve health and HRQL. One review found that reductions in alcohol use markedly increase HRQL for alcoholics (Donovan et al., 2005), even if the HRQL for those reducing alcohol intake is still lower than HRQL for normative or abstaining populations (Donovan et al., 2005; Saarni et al., 2008). Alcohol abuse treatment programs also improve HRQL (Srivastava & Bhatia, 2013; Ugochukwu et al., 2013). Further, simple interventions like motivational aid or advice can help reduce alcohol use among heavy drinkers, which improves HRQL (Kraemer et al., 2002). Yet, whether these health and HRQL gains extend to older adults is unknown. Such evidence is essential to how best to integrate behavioral health and medical treatment among at-risk drinking older adults in order to reduce harmful alcohol use and to improve health, HRQL and longevity.
The Project Senior Health and Alcohol Risk Education (SHARE) intervention, which uses patient and provider educational materials, physician counseling, and health educator support, was designed to reduce at-risk drinking among adults ages 60 and older. Although an earlier study showed that this intervention was successful in reducing rates of at-risk drinking and health care utilization (Ettner et al., 2014), it is unknown whether this translates into improved health and HRQL.
METHODS
Setting
The study population was drawn from Sansum Clinic, a community-based group practice with seven clinics in the Santa Barbara, California area. The practice has a strong primary care base, with service lines representing all major specialties and sub-specialties appropriate for elder care (e.g., cardiology, diabetes, geriatrics, urology).
Recruitment
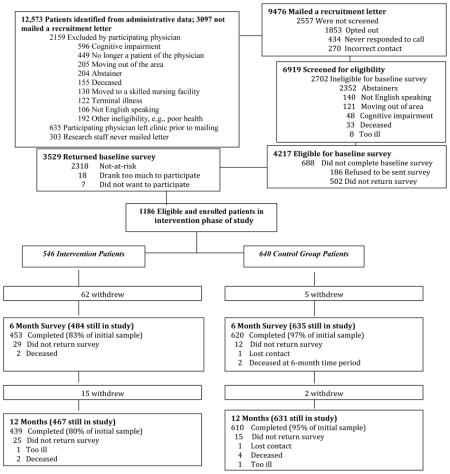
A detailed figure of participant flow through Project SHARE can be found in Appendix 1. Of the 42 primary care physicians approached, 31 agreed to participate in the study (n=20 male, 11 female, 17 internal medicine, 14 family practice). The percentages of physicians who were internal medicine vs. family practice looked almost identical among participating and non-participating physicians. However, female physicians were more likely than male physicians to participate in the study, as were younger physicians. The mean age of participating physicians was 48.3 for physicians in the intervention group and 44.4 for the control physicians, compared with a mean age of 52.5 years for non-participating physicians.
Clinic information technology personnel identified all adults 60 and older who were current patients of these providers (n=12,573). Providers initially screened out 2,159 patients who had severe cognitive impairment, were terminally ill or deceased, were moving to a skilled nursing facility or out of the area within the next year, did not speak English, were no longer a patient of the physician, or other (e.g., physician preference, personal reasons). Of the remaining patients, 9,476 were mailed recruitment letters. Of these, 2,557 were not screened either because they actively refused, never responded to a call (passively refused), or staff had the incorrect contact information. Among the 6,919 who were screened, 4,217 patients agreed to participate, met the inclusion criteria (i.e., consumed at least one drink containing alcohol in the past three months, planning to live in the area for 12 months, not cognitively impaired, spoke English, not deceased, not too ill), and were mailed a baseline survey. Of the 3,529 subjects who returned baseline surveys, 1,186 were identified as at-risk drinkers and eligible for the intervention phase of the study.
At-risk patients were assigned to the intervention or control group based on the random assignment of their primary care physician. Of the 546 patients assigned to the intervention group and completing the baseline survey, 79 did not complete either the 6- or 12-month survey, 28 completed only the 6-month survey, 14 completed only the 12-month survey, and 425 completed both the 6- and 12-month surveys. The control group was comprised of 640 patients. Among patients assigned to the control group and completing the baseline survey, 15 did not complete either the 6- or 12-month survey, 15 completed only the 6-month survey, 5 completed only the 12-month survey, and 605 completed both the 6- and 12-month surveys. Patients who screened as likely dependent drinkers at baseline (7 or more drinks daily) were excluded from the study and their physicians were notified. Patients who met this criterion at follow-up were not dropped from the study but their physicians were notified, regardless of whether or not the patient was in the experimental or control group (further information on enrollment and retention can be found in Ettner et al. (2014))
Intervention
Older participants’ risk status was ascertained using the Comorbidity AlcoholRisk Evaluation Tool, or CARET (Barnes et al., 2010). The CARET, an updated and revised version of the short Alcohol-Related Problems Survey (Fink et al., 2002), uses information on amount of alcohol use, comorbidity, symptoms and medications to assess drinking risks among older adults. The face, content, and criterion validity for the CARET has been previously established (Moore, Hays, Reuben, & Beck, 2000; Moore, Beck, Babor, Hays, & Reuben, 2002; Oishi et al., 2001).
Control patients received usual care, which may or may not have included alcohol counseling. All intervention group patients received a personalized Patient Report that included educational information on alcohol and aging, a drinking diary, and tips based on the patient’s alcohol risks identified in the CARET at baseline and 6 months. Provider reports at baseline and 6 months based on results from an intervention patient’s CARET were also generated. Providers were given the reports immediately before each upcoming visit throughout the 12-month follow-up and asked to discuss the risk factors identified in the report with their patients. Among the intervention patients, 300 received at least one provider discussion (Duru et al., 2015). In addition, telephone health educators contacted intervention patients two weeks after sending the baseline Patient Report, 3 months after sending the baseline Patient Report and two weeks after sending the 6-month Patient Report (more details on the intervention can be found in Ettner et al. (2014)).
The SHARE intervention used PRECEDE-PROCEED, a coordinated approach to program development and evaluation (Gelen & MacDonald, 1997), as the conceptual framework for evaluating the effectiveness of our intervention. PRECEDE (Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation) is an educational diagnosis model developed in the 1970’s. PROCEED (Policy, Regulatory, and Organizational Constructs in Educational and Environmental Development) was added in 1991. Although not a theory itself, PRECEDE-PROCEED provides a framework for applying theories to program development and evaluation (Gelen & MacDonald, 1997; Glanz et al., 1997). The theories adapted in this research are diffusion of innovations theory, with its focus on characteristics of innovations (Green & Lewis, 1986; Green et al., 1987) and the Behavioral Model for Vulnerable Populations (Gelberg et al., 2000). The PRECEDE-PROCEED framework has proven to be useful and effective over a relatively long time in a variety of studies to change risky health behavior (Howat et al. 1997, Keith et al, 1997).
Measures
Health and HRQL outcomes included those measuring mental and physical health, as well as a global measure of HRQL. Mental and physical component scores (MCS and PCS) were based on the Short Form-12v2 (SF-12v2) (Ware, Kosinski, Turner-Bowker, & Gandek, 2002), as was the measure of overall HRQL, the SF-6D (Makai, Brouwer, Koopmanschap, Stolk, & Nieboer, 2014). The SF-12 is a validated metric in which higher scores represent better HRQL (Ware, Kosinski, & Keller, 1996). MCS and PCS scores range from 0, the worst possible health state, to 100, the best possible health state. Unlike the MCS and PCS, the SF-6D represents preference-weighted HRQL (i.e., utility scores) and ranges from 0 to 1 where 0 represents the utility associated with death and 1 represents the utility associated with perfect health. Also included was the Geriatric Depression Scale (GDS) (Yesavage et al., 1982–1983). The GDS assesses how participants have been feeling recently and was included in the 12 month follow up. The GDS was reverse coded, and ranged from 0 to 5 with higher scores indicating fewer depressive symptoms.
Data analysis
Descriptive and bivariate analyses were based on data from patients participating in the intervention phase of the study and completing the baseline, 3, 6, and 9 month follow-up surveys (n=1,049). Adjusted associations of treatment assignment with health and HRQL outcomes were estimated using generalized least squares regressions with random provider effects. Regressions controlled either for baseline health and HRQL only or for age group, sex, race/ethnicity, marital status, education, household income, home ownership and the baseline value of the dependent variable, except for the 12 month GDS regression, which controlled instead for baseline MCS. Our analytic sample in our adjusted analyses ranges from 953 to 1,015 depending on the completeness of the outcome data. Multiple imputation was used to test the sensitivity of our main results to missing data. All statistical analyses were computed using Stata Version 10.1 (StataCorp, 2007).
RESULTS
Sample characteristics
Just under half (46.0%) of the participants were in the intervention group (Table 1). Similar proportions of participants were at risk due to alcohol behaviors (61.2%), alcohol plus medications (60.7%), and alcohol plus symptoms (61.3%). Our sample was nearly two-thirds male (65.7%), predominantly non-Latino (94.1%) and white (97.3%). Most owned their homes (88.3%) and were married (76.2%). More than half completed college or graduate school (59.4%), 50.0% were 60–69 years old, and nearly half (47.1%) had household incomes of $80,000 or more per year.
Table 1.
Baseline characteristics of older at-risk drinkers in the Project SHARE study
| Overall (N=1,049) | Intervention (N=439) | Control (N=610) | ||
|---|---|---|---|---|
|
| ||||
| Percent | Percent | Percent | p-value | |
| Intervention group | ||||
| Intervention | 46.0% | 100% | 0% | NA |
| Control | 54.0% | 0% | 100% | |
|
| ||||
| Risk factors | ||||
| Alcohol & Medications | 60.7% | 60.6% | 60.8% | 0.95 |
| Alcohol & Symptoms | 61.3% | 59.7% | 62.7% | 0.30 |
| Alcohol Behaviors | 61.2% | 61.5% | 64.5% | 0.29 |
|
| ||||
| Demographics | ||||
| Male | 65.7% | 62.5% | 68.4% | 0.03 |
| Race/ethnicity | ||||
| Latino | 5.9% | 5.4% | 6.3% | 0.50 |
| White | 97.3% | 96.9% | 97.6% | 0.34 |
| Black | 0.3% | 0.4% | 0.3% | |
| American Indian | 1.5% | 1.3% | 1.6% | |
| Asian | 0.9% | 1.5% | 0.5% | |
| Marital status | ||||
| Married | 76.2% | 72.2% | 79.7% | 0.01 |
| Widowed | 11.4% | 13.5% | 9.6% | |
| Divorced/Separated | 10.1% | 12.2% | 8.2% | |
| Never Married | 2.3% | 2.0% | 2.5% | |
| Education | ||||
| Less than HS | 3.2% | 3.2% | 3.2% | 0.28 |
| HS Grad | 10.5% | 11.6% | 9.6% | |
| Some College | 27.0% | 28.9% | 25.4% | |
| College Grad | 24.8% | 25.0% | 24.7% | |
| Graduate School | 34.6% | 31.5% | 37.2% | |
| Household income ($) | ||||
| Less than 30,000 | 10.8% | 13.5% | 8.4% | 0.02 |
| 30,000–40,000 | 8.4% | 9.4% | 7.6% | |
| 40,001–60,000 | 16.8% | 16.2% | 17.3% | |
| 60,001–80,000 | 16.9% | 18.8% | 15.2% | |
| 80,001–100,000 | 16.1% | 13.9% | 18.0% | |
| 100,001–200,000 | 21.1% | 19.2% | 22.7% | |
| over 200,000 | 9.9% | 9.0% | 10.7% | |
| Age (yrs) | ||||
| 60 to 64 | 21.6% | 19.6% | 23.3% | 0.08 |
| 65 to 69 | 28.4% | 29.3% | 27.7% | |
| 70 to 74 | 19.1% | 16.9% | 20.9% | |
| 75 to 79 | 16.2% | 18.3% | 14.4% | |
| 80 and older | 14.8% | 15.9% | 13.8% | |
| Own home | 88.3% | 88.6% | 88.1% | 0.77 |
Significant baseline differences between treatment groups were found only for gender (p=0.03), marital status (p=0.01) and income (p=0.02). No significant differences were found between intervention and comparison groups for alcohol risk factors, race, ethnicity, education, age, or home ownership. Importantly, the baseline values of the outcome measures also did not vary significantly between the intervention and control group.
Among participants, the average baseline PCS score was 48.9 (standard deviation 9.5) (Table 2). The mean MCS scores for this group at baseline was 44.4 (6.7). PCS and MCS scores for the general U.S. adult population have a mean at 50 and a standard deviation of 10 (Barnes, Robert, & Bradley, 2014), so these scores among our sample of older at-risk drinkers are not atypical. The average SF-6D score at baseline was 0.66 (0.11), suggesting our sample was below the U.S. average score of approximately 0.77 for adults over age 65 (Fryback et al., 2007). Participants scored a 4.4 (1.1) out of 5 on the GDS returned with the 12-month survey indicating that the average participant had few, if any, depressive symptoms. We found no unadjusted differences between the intervention and control group in measures of health and HRQL at baseline.
Table 2.
Baseline, 6- and 12-month outcomes of older at-risk drinkers in the Project SHARE study
| Overall | Intervention | Control | ||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | |
| Outcomes | ||||
| Physical component score | ||||
| Baseline | 48.9 (9.5) | 48.9 (9.7) | 48.8 (9.3) | 0.93 |
| 6 month | 50.1 (8.7) | 50.3 (9.0) | 50.0 (8.4) | 0.54 |
| 12 month | 49.8 (8.8) | 49.8 (8.8) | 49.9 (8.8) | 0.88 |
| Mental component score | ||||
| Baseline | 44.4 (6.7) | 44.5 (6.8) | 44.3 (6.7) | 0.68 |
| 6 month | 43.9 (6.9) | 44.2 (7.2) | 43.6 (6.6) | 0.14 |
| 12 month | 43.9 (6.8) | 44.0 (6.7) | 43.8 (6.9) | 0.61 |
| SF-6D | ||||
| Baseline | 0.66 (0.11) | 0.66 (0.11) | 0.66 (0.11) | 0.35 |
| 6 month | 0.66 (0.11) | 0.66 (0.11) | 0.66 (0.11) | 0.15 |
| 12 month | 0.66 (0.11) | 0.66 (0.11) | 0.66 (0.11) | 0.45 |
| Geriatric Depression Scale | ||||
| 12 month | 4.4 (1.1) | 4.4 (1.0) | 4.3 (1.1) | 0.36 |
Associations of the at-risk drinking intervention with the outcomes controlling for baseline health and HRQL only
After adjusting for the baseline value of the outcome variable only, the Project SHARE intervention was not significantly associated with 6-month PCS scores (Table 3). The at-risk drinking intervention was associated with a 0.56 point (95% CI: 0.06, 1.06) increase in 6-month MCS scores and a 0.01 point increase in 6-month SF-6D scores (95% CI: 0.01, 0.01). No significant associations were found between the Project SHARE intervention and changes in health and HRQL between baseline and the 12 month follow-up.
Table 3.
Adjusted intervention effects on older at-risk drinkers’ health and health-related quality of life (HRQL)
| Health and HRQL outcomes | 6 month intervention effect (95% CI) | 12 month intervention effect (95% CI) |
|---|---|---|
| Physical Component Score (PCS) | ||
| Control for baseline PCS only | 0.25 (−0.67, 1.17) | −0.13 (−0.94,0.68) |
| N | 1,066 | 1,042 |
| Control for baseline PCS, risk factors, and demographics | 0.33 (−0.51, 1.17) | 0.06 (−0.61, 0.72) |
| N | 1,011 | 990 |
|
| ||
| Mental Component Score (MCS) | ||
| Control for baseline MCS only | 0.56** (0.06, 1.06) | 0.15 (−0.60, 0.90) |
| N | 1,066 | 1,042 |
| Control for baseline MCS, risk factors, and demographics | 0.58* (−0.06, 1.21) | 0.16 (−0.56, 0.88) |
| N | 1,011 | 990 |
|
| ||
| SF-6D | ||
| Control for baseline SF-6D only | 0.01*** (0.01, 0.01) | 0.01 (−0.01, 0.01) |
| N | 1,070 | 1,047 |
| Control for baseline SF-6D, risk factors, and demographics | 0.01*** (0.01, 0.02) | 0.01** (0.01, 0.01) |
| N | 1,015 | 995 |
|
| ||
| Geriatric Depression Scale (GDS) | ||
| Control for baseline MCS only | 0.07 (−0.05, 0.20) | |
| N | 1,002 | |
| Control for baseline MCS, risk factors, and demographics | 0.14** (0.01, 0.26) | |
| N | 953 | |
Notes:
indicates p<0.10,
p<0.05,
p<0.01.
The Geriatric Depression Scale was reverse coded so that higher values indicated fewer depressive symptoms.
Regression models controlled for either: 1) only the baseline value of the outcome, or 2) risk factors and demographic covariates listed in Table 1, as well as the baseline value of the dependent variable (with the exception of the Geriatric Depression Scale regression, which controlled for baseline MCS score instead).
Associations of the at-risk drinking intervention with the outcomes controlling for risk factors, demographics and baseline health and HRQL
After adding controls for risk factors and demographics, the intervention remained unassociated with changes in 6- or 12-month PCS scores (Table 3). However, receiving the at-risk drinking intervention was associated with modest improvements in MCS scores. Specifically, assignment to treatment group was associated with a 0.58 point (95% CI: −0.06, 1.21) increase in 6-month MCS, although this association was only marginally significant (p<0.10).
Intervention effects on global HRQL and measures of geriatric depression were more robust. Compared to those receiving usual care, older adults in the treatment group reported a 0.01 point increase in SF-6D scores at both six and twelve months (6-month 95% CI: 0.01, 0.02; 12-month 95% CI: 0.01, 0.01). Older adults receiving the Project Share intervention also had a 0.14 point (95% CI: 0.01, 0.26) improvement in their 12-month GDS score, compared to those receiving usual care, suggesting they endorsed fewer depressive symptoms.
Importantly, our previous work has found the effectiveness of Project SHARE on reducing at-risk drinking among older adults varies by the intervention components received (Duru et al., 2015). Additional analyses of the association of the intervention components with health and HRQL (not shown) find evidence consistent with the earlier results for at-risk drinking; improvement in SF-6D scores associated with the intervention were primarily driven by whether patients engaged in an alcohol-related discussion with their physician at any time during the 12 month follow-up period rather than the health educator component of the intervention. However, changes in MCS and GDS associated with the Project SHARE intervention did not differ by receipt of a physician discussion. The physician component of the intervention may have been more important than the health educator component because it began at each subject’s baseline and continued for the full 12-month follow-up period. The health educators, as noted earlier, spoke to patients via telephone on three occasions between receiving the baseline Patient Report and the 6-month follow-up Patient Report.
DISCUSSION
Despite the previously shown effectiveness of the Project SHARE intervention in reducing at-risk drinking and health services use among older adults (Ettner et al., 2014), effects on health and HRQL were statistically but not necessarily clinically significant. These null findings may have arisen from at least two characteristics of our study. First, the patients participating in the study were in good health generally and thus there may have been little room for the intervention to improve some measures of health and HRQL outcomes. Additionally, it is likely a 12-month follow-up is too brief a duration for some of the health and HRQL outcomes of interest to be influenced by the at-risk drinking intervention.
The significant intervention effects found for the global measure of health-related quality of life were driven by receiving a physician discussion, suggesting that access to physicians who can provide alcohol counseling may be an important component of the intervention. This is consistent with other research suggesting that brief interventions in primary care are effective in reducing alcohol use, (Bertholet N, Daeppen J, Wietlisbach V, Fleming M, Burnand B., 2005), especially among older adults (Fleming, Manwell, Barry, Adams, & Stauffacher, 1999; Gordon et al., 2003). However, the size and type of the effects vary widely among these primary care interventions (Fleming et al., 1999; Moore et al., 2011) and may depend on factors like ethnicity, gender, education, baseline risk (Lin, Karno, Tang et al., 2010), and perception of physician advice (Lin, Karno, Barry et al., 2010).
We experienced several initial barriers/facilitators to implementation of the Project SHARE intervention. First, when asked to participate, the physicians expressed concerns about the time it would take to go over the personalized Patient Report with patients in the treatment group. However, after incorporating the patient report into the appointment, participating physicians found the report valuable and felt that it did not noticeably constrain their ability to discuss other medical concerns during a patient’s visit.
Second, the research staff at the collaborating clinic also had some initial concerns about the training time and effort that would be required in order to use the online patient recruitment and tracking system. After becoming more familiar with the system and its advantages, however, they concurred that the online system was more efficient and training with the new system proceeded relatively quickly. The online tracking system facilitated implementation of the intervention and research evaluation by making recruitment, retention and tracking of the intervention and survey data collection activities easier and more reliable than would otherwise have been possible, for example if Excel spreadsheets and Outlook calendars had been used for patient tracking. The online system also enabled the project director to monitor the research staff and health educators long-distance, saving project resources.
Finally, our initial recruitment plan targeted patients with an upcoming physician visit within the next month to enroll; furthermore, we only had enough resources to recruit a subsample of the total eligible patient population. An increase in the resources for data collection enabled us to change the recruitment strategy so that we were able to attempt data collection on the entire eligible population, randomly selecting a subsample each month. The advantage of the new recruitment strategy was that it allowed us to avoid sample selection bias (e.g., recruiting only individuals who were frequent/high utilizers).
When interpreting our findings, several limitations to our study are worth noting. First, our estimates may not generalize beyond our sample. Compared to the U.S. Census population over 60, our sample was more likely to be white, married, well-educated, and higher-income (U.S. Census Bureau, 2006). However, increased access to primary care resulting from recent coverage expansions among lower-socioeconomic populations may result in larger provider-based alcohol intervention effects if individuals with previously poor access to providers benefit more from additional care than patients who already had good healthcare. While we do not have any information about the reasons for withdrawal, we suspect from anecdotal reports that participants dropped out because they did not want to talk about their alcohol use. We empirically examined the correlates of dropping out between baseline and 12 months by estimating a regression of the predictors of dropout, including treatment assignment and all of the covariates listed in Table 1 of the manuscript. We find that the only indicator that is significantly correlated with dropout is assignment to the intervention group.
Further, selection bias based on unobservable differences in the intervention and control groups that may be correlated with participation in the intervention and in health and HRQL is a potential threat to the validity of our estimates. To assess this bias, we conducted a “worst-case” analysis similar to Ettner et al (2014), by “imputing” a conservative value for 12-month HRQL outcomes to individuals who drop out of the sample in order to include them in the analysis. For each individual who dropped out of the sample, we took the baseline value of that individual’s HRQL and adjusted it by the average percent change between baseline and follow-up HRQL among control group participants. This adjusted value was then assigned as the follow-up HRQL value. (If t-tests showed that changes over time among the control group were non-significant, we instead assigned the baseline value without any adjustments.) Estimates from these analyses were quantitatively similar to the original estimates, suggesting that potential bias due to unobservables correlated with intervention participation and health and HRQL outcomes was not a major threat to the consistency of our estimates.
Additionally, our study period may have been too short to allow improvements in health and HRQL to develop. The changes in at-risk drinking resulting from the Project SHARE intervention may require a longer time horizon to translate into improved health and HRQL. Studies of HRQL improvements after alcohol dependence treatment show gains after long (e.g. 12 months) and short time horizons, but these studies also used an alcohol-dependent population (Donovan et al., 2005; Kraemer et al., 2002). Few studies appear to follow seniors past 12 months; this is an important area for future research.
In summary, our results suggest that interventions to reduce at-risk drinking among older adults that include a provider component are modestly effective at improving health and HRQL. Given the limited evidence on how best to integrate behavioral health treatment with primary care to improve the physical and mental health of older adults, our findings offer an important contribution. One “take-home message” is that it may take longer than the typical timeline of most intervention studies to see changes in behaviors (in our case, at-risk drinking) translate into changes in health and health-related quality of life; in turn, this suggests that intervention studies may miss important effects if the evaluation does not include intermediate outcome measures. With the expansion of coverage for behavioral health conditions resulting from recent U.S. health reforms such as the Affordable Care Act and the Mental Health Parity and Addiction Equity Act, future inquiry assessing the effectiveness of provider-based behavioral health interventions is needed.
HIGHLIGHTS.
At-risk drinking affects 10% of elderly in U.S.
Patients randomly assigned to patient-provider education intervention or usual care
Tested effect of the intervention on health & health-related quality of life
Improvements were significant but not necessarily clinically meaningful
Effects were most prominent for patients who received physician discussions
Provider counseling may be a critical component of primary care interventions
Acknowledgments
This project was funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant 1RO1AA013990 (Principal investigator: Susan L. Ettner). Alison A. Moore’s time was additionally supported by NIAAA Grants R01 AA15957 and K24 AA15957 (Principal investigator: Alison A. Moore). O. Kenrik Duru’s time was supported by National Institute on Aging Grants 5P30AG021684-12 and 5K08AG033630-05. The authors thank the following: the leadership, research staff, and information technology staff of Sansum Clinic, in particular Mr. Paul Jaconette, Ms. Chris McNamara, Ms. Linda Chapman, and Mr. Tom Colbert; the University of California at Los Angeles research staff; the Project SHARE (Senior Health and Alcohol Risk Education) health educators; and the members of our research advisory board. Finally, we are indebted to the Sansum Clinic patients and physicians who participated in Project SHARE, without whom the study could not have been conducted. This study is registered as a clinical trial with ClinicalTrials.gov Identifier: NCT00107640.
Appendix 1. Participant Flow through Project SHARE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakhshi S, While AE. Older people and alcohol use. British Journal of Community Nursing. 2014;19(8):370–374. doi: 10.12968/bjcn.2014.19.8.370. [DOI] [PubMed] [Google Scholar]
- Barnes AJ, Moore AA, Xu H, Ang A, Tallen L, Mirkin M, Ettner SL. Prevalence and correlates of at-risk drinking among older adults: The project SHARE study. Journal of General Internal Medicine. 2010;25(8):840–846. doi: 10.1007/s11606-010-1341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AJ, Robert N, Bradley CJ. Job attributes, job satisfaction and the return to health after breast cancer diagnosis and treatment. Psycho-Oncology. 2014;23(2):158–164. doi: 10.1002/pon.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet N, Daeppen J, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: Systematic review and meta-analysis. Archives of Internal Medicine. 2005;165(9):986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- Breslow RA, Faden VB, Smothers B. Alcohol consumption by elderly americans. J Stud Alcohol. 2003;64(6):884–92. doi: 10.15288/jsa.2003.64.884. [DOI] [PubMed] [Google Scholar]
- Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women’s use of alcohol and their Health-Related quality of life. Journal of the American Geriatrics Society. 2006;54(9):1341–1347. doi: 10.1111/j.1532-5415.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Health related quality of life (HRQoL) 2011 Retrieved from http://www.cdc.gov/hrqol/concept.htm.
- Chen C, Storr CL. Alcohol use and health-related quality of life among youth in taiwan. Journal of Adolescent Health. 2006;39(5):752.e9–752.e16. doi: 10.1016/j.jadohealth.2006.04.019. http://dx.doi.org/10.1016/j.jadohealth.2006.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca d’Alessandro E, Bonacci S, Giraldi G. Aging populations: The health and quality of life of the elderly. La Clinica Terapeutica. 2011;162(1):e13–8. [PubMed] [Google Scholar]
- Donovan D, Mattson ME, Cisler RA, Longabaugh R, Zweben A. Quality of life as an outcome measure in alcoholism treatment research. Journal of Studies on Alcohol. Supplement. 2005:15–39. doi: 10.15288/jsas.2005.s15.119. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- Duru OK, Xu H, Moore AA, Mirkin M, Ang A, Tallen L, Ettner SL. Examining the impact of separate components of a multicomponent intervention designed to reduce at-risk drinking among older adults: The project SHARE study. Alcoholism, Clinical and Experimental Research. 2015 doi: 10.1111/acer.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettner SL, Xu H, Duru OK, Ang A, Tseng CH, Tallen L, Moore AA. The effect of an educational intervention on alcohol consumption, at-risk drinking, and health care utilization in older adults: The project SHARE study. Journal of Studies on Alcohol and Drugs. 2014;75(3):447–457. doi: 10.15288/jsad.2014.75.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Morton SC, Beck JC, Hays RD, Spritzer K, Oishi S, Moore AA. The alcohol-related problems survey: Identifying hazardous and harmful drinking in older primary care patients. J Am Geriatr Soc. 2002;50(10):1717–22. doi: 10.1046/j.1532-5415.2002.50467.x. jgs50467. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Manwell LB, Barry KL, Adams W, Stauffacher EA. Brief physician advice for alcohol problems in older adults: A randomized community-based trial. The Journal of Family Practice. 1999;48(5):378–384. [PubMed] [Google Scholar]
- Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, Kind P. US norms for six generic health-related quality-of-life indexes from the national health measurement study. Medical Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Conigliaro J, Maisto SA, McNeil M, Kraemer KL, Kelley ME. Comparison of consumption effects of brief interventions for hazardous drinking elderly. Subst use Misuse. 2003;38(8):1017–1035. doi: 10.1081/JA-120017649. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Huguet N, Feeny D, McFarland BH, Caetano R, Bernier J, Ross N. Alcohol use patterns and trajectories of health-related quality of life in middle-aged and older adults: A 14-year population-based study. Journal of Studies on Alcohol and Drugs. 2012;73(4):581–590. doi: 10.15288/jsad.2012.73.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner JE, Zubritsky C, Cody M, Coakley E, Chen H, Ware JH, Levkoff S. Alcohol consumption among older adults in primary care. J Gen Intern Med. 2007;22(1):92–7. doi: 10.1007/s11606-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer K, Maisto S, Conigliaro J, McNeil M, Gordon A, Kelley M. Decreased alcohol consumption in outpatient drinkers is associated with improved quality of life and fewer alcohol-related consequences. Journal of General Internal Medicine. 2002;17(5):382–386. doi: 10.1007/s11606-002-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Karno MP, Barry KL, Blow FC, Davis JW, Tang L, Moore AA. Determinants of early reductions in drinking in older at-risk drinkers participating in the intervention arm of a trial to reduce at-risk drinking in primary care. Journal of the American Geriatrics Society. 2010;58(2):227–233. doi: 10.1111/j.1532-5415.2009.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Karno MP, Tang L, Barry KL, Blow FC, Davis JW, Moore AA. Do health educator telephone calls reduce at-risk drinking among older adults in primary care? Journal of General Internal Medicine. 2010;25(4):334–339. doi: 10.1007/s11606-009-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Erwin CW, Cleveland WP, Logue PE, Gentry WD. Effects of alcohol on psychomotor performance of men and women. Journal of Studies on Alcohol. 1978;39(5):745–758. doi: 10.15288/jsa.1978.39.745. [DOI] [PubMed] [Google Scholar]
- Makai P, Brouwer WB, Koopmanschap MA, Stolk EA, Nieboer AP. Quality of life instruments for economic evaluations in health and social care for older people: A systematic review. Social Science & Medicine (1982) 2014;102:83–93. doi: 10.1016/j.socscimed.2013.11.050. [DOI] [PubMed] [Google Scholar]
- Martinez P, Lien L, Landheim A, Kowal P, Clausen T. Quality of life and social engagement of alcohol abstainers and users among older adults in south africa. BMC Public Health. 2014;14(1):316. doi: 10.1186/1471-2458-14-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick EL, Hodgkin D, Garnick DW, Horgan CM, Panas L, Ryan M, Blow FC. Unhealthy drinking patterns and receipt of preventive medical services by older adults. Journal of General Internal Medicine. 2008;23(11):1741–1748. doi: 10.1007/s11606-008-0753-3. 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Beck JC, Babor TF, Hays RD, Reuben DB. Beyond alcoholism: Identifying older, at-risk drinkers in primary care. J Stud Alcohol. 2002;63(3):316–24. doi: 10.15288/jsa.2002.63.316. [DOI] [PubMed] [Google Scholar]
- Moore AA, Blow FC, Hoffing M, Welgreen S, Davis JW, Lin JC, Barry KL. Primary care-based intervention to reduce at-risk drinking in older adults: A randomized controlled trial. Addiction (Abingdon, England) 2011;106(1):111–120. doi: 10.1111/j.1360-0443.2010.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Giuli L, Gould R, Hu P, Zhou K, Reuben D, Karlamangla A. Alcohol use, comorbidity, and mortality. Journal of the American Geriatrics Society. 2006;54(5):757–762. doi: 10.1111/j.1532-5415.2006.00728.x. JGS728. [DOI] [PubMed] [Google Scholar]
- Moore AA, Hays RD, Reuben DB, Beck JC. Using a criterion standard to validate the alcohol-related problems survey (ARPS): A screening measure to identify harmful and hazardous drinking in older persons. Aging (Milano) 2000;12(3):221–7. doi: 10.1007/BF03339839. [DOI] [PubMed] [Google Scholar]
- Moore AA, Whiteman EJ, Ward KT. Risks of combined alcohol/medication use in older adults. The American Journal of Geriatric Pharmacotherapy. 2007;5(1):64–74. doi: 10.1016/j.amjopharm.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Drinking levels defined. 2015 Retrieved from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Oishi SM, Morton SC, Moore AA, Beck JC, Hays RH, Spritzer KL, Fink A. Using data to enhance the expert panel process. International Journal of Technology Assessment in Health Care. 2001;17:125–136. doi: 10.1017/s0266462301104113. [DOI] [PubMed] [Google Scholar]
- Okoro CA, Brewer RD, Naimi TS, Moriarty DG, Giles WH, Mokdad AH. Binge drinking and health-related quality of life: Do popular perceptions match reality? American Journal of Preventive Medicine. 2004;26(3):230–233. doi: 10.1016/j.amepre.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Saarni SI, Joutsenniemi K, Koskinen S, Suvisaari J, Pirkola S, Sintonen H, Lönnqvist J. Alcohol consumption, abstaining, health utility, and quality of life--a general population survey in finland. Alcohol and Alcoholism (Oxford, Oxfordshire) 2008;43(3):376. doi: 10.1093/alcalc/agn003. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Bhatia MS. Quality of life as an outcome measure in the treatment of alcohol dependence. Industrial Psychiatry Journal. 2013;22(1):41. doi: 10.4103/0972-6748.123617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 10. 10.1. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Strandberg TE, Strandberg AY, Salomaa VV, Pitkala K, Tilvis RS, Miettinen TA. Alcoholic beverage preference, 29-year mortality, and quality of life in men in old age. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2007;62(2):213–218. doi: 10.1093/gerona/62.2.213. 62/2/213. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Current population survey, annual social and economic supplement, 2006. 2006 Retrieved from http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- Ugochukwu C, Bagot KS, Delaloye S, Pi S, Vien L, Garvey T, Ishak WW. The importance of quality of life in patients with alcohol abuse and dependence. Harvard Review of Psychiatry. 2013;21(1):1–17. doi: 10.1097/HRP.0b013e31827fd8aa. [DOI] [PubMed] [Google Scholar]
- Valencia-Martín JL, Galán I, Guallar-Castillón P, Rodríguez-Artalejo F. Alcohol drinking patterns and health-related quality of life reported in the spanish adult population. Preventive Medicine. 2013;57(5):703–707. doi: 10.1016/j.ypmed.2013.09.007. http://dx.doi.org/10.1016/j.ypmed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Vestal RE, McGuire EA, Tobin JD, Andres R, Norris AH, Mezey E. Aging and ethanol metabolism. Clinical Pharmacology and Therapeutics. 1977;21(3):343–354. doi: 10.1002/cpt1977213343. 0009-9236(77)90129-1. [DOI] [PubMed] [Google Scholar]
- Volk RJ, Cantor SB, Steinbauer JR, Cass AR. Alcohol use disorders, consumption patterns, and health-related quality of life of primary care patients. Alcoholism: Clinical and Experimental Research. 1997;21(5):899–905. doi: 10.1111/j.1530-0277.1997.tb03855.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12 health survey (with a supplement documenting version 1) QualityMetric Incorporated; 2002. [Google Scholar]
- Ware John E, Kosinski M, Keller SD. A 12- item short- form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wen XJ, Kanny D, Thompson WW, Okoro CA, Town M, Balluz LS. Binge drinking intensity and health-related quality of life among US adult binge drinkers. Preventing Chronic Disease. 2012;9:E86–E86. doi: 10.5888/pcd9.110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. http://dx.doi.org/10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]


