Abstract
Aims
This study explored the factor structure of DSM III-R/IV symptoms for substance abuse and dependence across six illicit substance categories in a population-based sample of males.
Method
DSM III-R/IV drug abuse and dependence symptoms for cannabis, sedatives, stimulants, cocaine, opioids and hallucinogens from 4179 males born 1940-1970 from the population-based Virginia Adult Twin Study of Psychiatric and Substance Use Disorders were analyzed. Confirmatory factor analyses tested specific hypotheses regarding the latent structure of substance misuse for a comprehensive battery of 13 misuse symptoms measured across six illicit substance categories (78 items).
Results
Among the models fit, the latent structure of substance misuse was best represented by a combination of substance-specific factors and misuse symptom-specific factors. We found no support for a general liability factor to illicit substance misuse.
Conclusions
Results indicate that liability to misuse illicit substances is drug class specific, with little evidence for a general liability factor. Additionally, unique dimensions capturing propensity toward specific misuse symptoms (e.g., tolerance, withdrawal) across substances were identified. While this finding requires independent replication, the possibility of symptom-specific misuse factors, present in multiple substances, raises the prospect of genetic, neurobiological and behavioral predispositions toward distinct, narrowly defined features of drug abuse and dependence.
Keywords: Cannabis, cocaine, dependence, DSM, opioids, factor analysis
INTRODUCTION
Effective treatment of substance use disorder (SUD) depends on accurate models and measurement of the underlying psychopathological phenotypes. The importance of this issue has prompted extensive research examining the latent structure of substance misuse characteristics in dialogue with the models implicit in DSM diagnostic categories. Such research recently led to revising the long-standing DSM model of separate substance-specific SUD diagnoses for abuse and dependence, combining both criteria into single substance-specific SUDs in DSM-5 (1). This revision was supported by expert consensus that when considering substances individually, a single factor, known as liability, best explains the covariance between DSM abuse and dependence symptoms (2-11). However, less research has jointly considered misuse symptoms across multiple substance categories to examine the possibility of general poly-substance liability and/or more complex latent structures.
Among studies jointly considering misuse across multiple substance classes, results have been equivocal, primarily due to differing methodologies and substantive aims. Contrasting our current psychometric approach of modeling the latent structure of substance misuse phenotypes, previous studies have generally employed biometric approaches to decompose misuse phenotype variance into genetic and environmental components. Results from such research, including previous analyses of the present sample (12), have typically found a mix of substance-specific and general liability factors in both genetic and environmental risk (13-14). This conclusion has not received universal support, however, as other studies have found distinct, but correlated, genetic and environmental influences for cannabis versus other illicit substances (i.e., cocaine, sedatives, stimulants, hallucinogens or opioids) (15), as well as unique genetic liabilities toward illicit (cocaine and cannabis) versus licit (alcohol, nicotine and caffeine) substance dependence (16). Moreover, studies of illicit drug abuse/dependence using categorical, rather than dimensional, latent variable models have suggested distinct patterns of substance misuse (e.g., cannabis-only, prescription drugs), with truly general poly-substance misuse occurring rarely (17). Thus, while general liability to substance misuse has conceptual appeal and has received considerable empirical support, research on the topic has been inconclusive.
One major limitation of the studies described above is they rely on a count of dichotomous indicators to generate DSM diagnoses. For example, DSM V diagnosis of severe substance abuse requires at least 6 of 10 possible symptoms. This method may be sub-optimal because it assumes the symptoms function equivalently both within and between substances. Thus, all symptoms are implicitly assumed to be equally valid measures of SUD diagnosis, regardless of the substance in question. However, as shown by Gillespie et al. (18), identical symptoms measure different levels of liability across substances, suggesting that substance misuse symptoms do not function equivalently. Thus, condensing symptom data into binary diagnostic categories greatly decreases the amount of unique, relevant information compared to psychometric approaches directly modeling symptom-level data. The situation is only slightly improved in DSM-V, which sub-classifies SUDs into mild, moderate and severe. With symptom-level data it may be possible to identify novel latent dimensions of substance misuse, in addition to improved ability to adjudicate between substance-specific versus general misuse liability. Of particular interest, symptom-level data across a range of illicit substances allow the investigation of poly-substance liability to different misuse characteristics. For instance, there may exist a propensity to develop tolerance across multiple substances. The possibility of poly-substance liability was overlooked in previous analyses of the topic (e.g., Gillespie et al 2007) which only investigated liability structures within one specific substance at a time and not across substances. Identification of liability factors for specific misuse symptoms has the potential to yield novel targets for studies of, e.g., genetic association, neural substrates, prevention or treatment.
In this article, we build on previous research exploring SUD liability by jointly analyzing 13 individual DSM III-R/IV abuse and dependence symptoms across six inclusive illicit substance categories. This approach addresses limitations associated with examining substance categories independently, as well as those due to collapsing symptom data into binary diagnoses. Specifically, the study has two primary aims. The first is to determine whether there are substance-specific and/or misuse symptom-specific liability factors underlying DSM SUD symptom data. Second, we test whether the general SUD liability factor identified in previous research using diagnostic categories represents an accurate model of DSM illicit substance misuse symptoms when examining symptom-level data.
METHODS
Participants and measures
This study is based on data collected from Caucasian adult male twins in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD). Described in detail elsewhere (19), data came from a second wave of interviews between 1994 and 1998. Subjects were eligible for participation if they were successfully matched to birth records, a member of a multiple birth with at least one male, Caucasian, and born between 1940 and 1974 in Virginia, USA. Interviewers had a master's degree in a mental health-related field or a bachelor's degree in this area plus two years of clinical experience. Of 9,417 eligible individuals for the first wave (1993–1996), 6,814 (72.4%) completed the interview. The second interview was completed by 5,629 individuals (82.6%). Complete drug initiation data were available from 4,179 male subjects ranging in age from 20 to 58 years (μ = 36.9 yrs, σ2 = 9.1yrs). Unlike previous analyses (18), these data included an additional 1,602 males from opposite-sex and incomplete twin pairs. As recruitment focused on males, analyses of females were relatively underpowered in this sample. This fact, combined with previous research indicating gender differences in substance liability factor structure (20), led us to limit analyses to males only. Subjects were informed of the study’s goals and provided informed consent. The project was approved by the Virginia Commonwealth University institutional review board
The interview included assessments of lifetime drug use, abuse and dependence across six categories of substances using an adaptation of the Structured Clinical Interview (SCID) (21). Categories (examples) included: cannabis (marijuana and hashish); sedatives (quaalude, Seconal and Valium); stimulants (speed, ecstasy and Ritalin); cocaine (intranasal and crack); opioids (heroin and morphine); and hallucinogens (LSD and PCP). For substances that could be obtained legally, we defined non-medical use as, 1) without a doctor’s prescription, 2) in greater amounts or more often than prescribed, or 3) for any reason other than a doctor prescribed. For each substance, the abuse and dependence assessment comprised 13 symptoms listed in Table 1. Symptoms were structured this way to permit assignment of both DSM-III-R and DSM-IV diagnostic definitions. Initially, all 13 symptoms were measured on a three-point scale (definite/probable/no). Consistent with previous research in this sample (18), ‘probable’ responses were combined with ‘definite’ to minimize small cell optimization problems. The data were analyzed as dichotomous variables.
Table 1.
Percentage endorsement for abuse and dependence symptoms based on subjects who reported illicit drug use, experimentation or non-medical use of illicit substances. Symptoms that begin with an A are thought to measure abuse, while symptoms that begin with a D are thought to measure dependence.
| Cannabis | Sedatives | Stimulants | Cocaine | Opioids | Halluc. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Have you ever taken any of these drugs? |
n = 2313 | n = 539 | n = 843 | n = 767 | n = 292 | n = 636 | ||||||
| Symptoms | Freq. | % | Freq. | % | Freq. | % | Freq. | % | Freq. | % | Freq. | % |
|
| ||||||||||||
| A1-Hazardous use | 583 | 25 | 114 | 21 | 204 | 24 | 200 | 26 | 67 | 23 | 130 | 20 |
| A2-Consequences: Legal | 64 | 3 | 15 | 3 | 16 | 2 | 34 | 4 | 11 | 4 | 6 | 1 |
| A3-Consequences: Social | 221 | 10 | 41 | 8 | 48 | 6 | 114 | 15 | 28 | 10 | 35 | 6 |
| A4-Consequences: Physical and psychological |
189 | 8 | 33 | 6 | 147 | 17 | 138 | 18 | 28 | 10 | 63 | 10 |
| A5-Used often when doing something …important |
352 | 15 | 50 | 9 | 210 | 25 | 78 | 10 | 41 | 14 | 36 | 6 |
| A6-Stay away from school/ miss appointments |
135 | 6 | 36 | 7 | 30 | 4 | 81 | 11 | 21 | 7 | 37 | 6 |
|
| ||||||||||||
| D1-Used more or longer than thought/planned |
254 | 11 | 51 | 9 | 113 | 13 | 182 | 24 | 41 | 14 | 38 | 6 |
| D2-Loss of control: Unable to stop/desire to stop, tried to cut down or stop using |
428 | 19 | 87 | 16 | 208 | 25 | 210 | 27 | 53 | 18 | 89 | 14 |
| D3-Spend time taking/using it, recovering from it, or doing whatever |
220 | 10 | 36 | 7 | 63 | 7 | 123 | 16 | 37 | 13 | 42 | 7 |
| D4-Used instead of work/hobbies |
124 | 5 | 24 | 4 | 24 | 3 | 92 | 12 | 25 | 9 | 26 | 4 |
| D5-Need for larger amounts/doses (tolerance) |
305 | 13 | 40 | 7 | 98 | 12 | 141 | 18 | 32 | 11 | 36 | 6 |
| D6-Withdrawal symptoms: Feeling sick when cutting down/stopping |
56 | 2 | 26 | 5 | 67 | 8 | 102 | 13 | 27 | 9 | 13 | 02 |
| D7-Withdrawal symptoms: After not using … use to prevent sickness |
28 | 1 | 17 | 3 | 29 | 3 | 40 | 5 | 21 | 0.07 | 3 | 0.5 |
Statistical methods
We used Mplus V6.0 (22) to jointly model the diagnostic symptoms from the six substances. Specifically, the latent structure of SUD liability was modeled using confirmatory factor analysis. To handle twin clustering, we specified the “complex” analysis option, which implements a clustering corrected robust maximum likelihood estimator (23). This method uses a Huber/White/Sandwich clustered variance estimator to calculate standard errors (24-25), and has superior estimation properties for the analysis of dichotomous data with small cluster sizes (23), as observed here. Subjects not initiating use of a substance were considered missing for those symptoms.
Confirmatory factor analyses (CFAs)
A series of CFA models were fit to the symptom data for all six substances. Using CFA we were able to test specific hypotheses about the structure of factors underlying liability to SUD. Specifically, we considered models specifying only substance-specific liability factors, only misuse symptom-specific liability factors, or a combination of both, as well as a model specifying a general liability factor underlying all illicit drug abuse and dependence symptoms. Each of these types of factors is explained in more detail below.
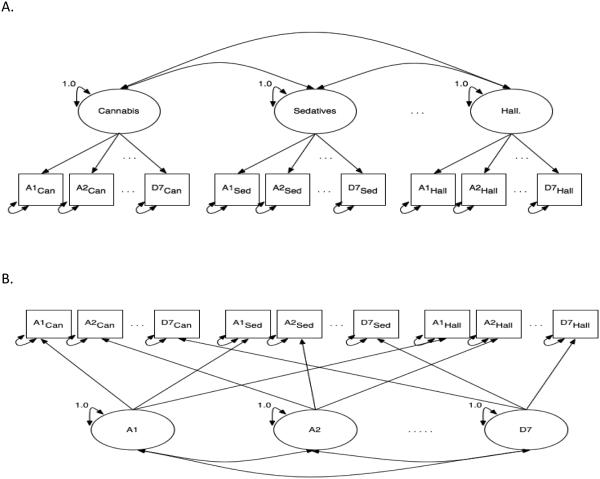
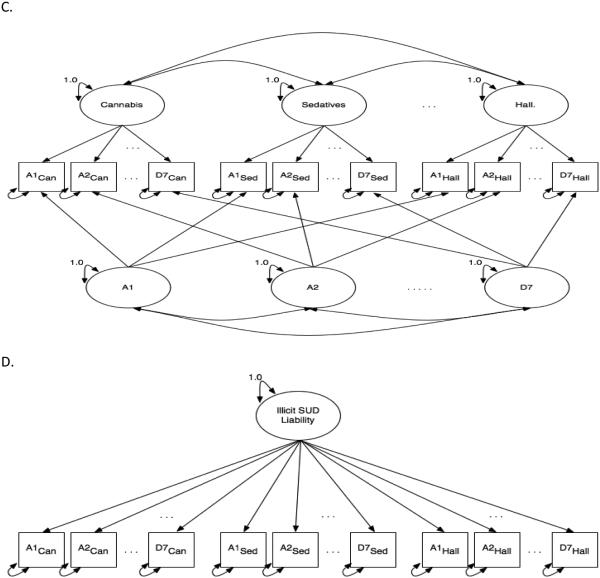
Model M1 tests whether substance-specific liability factors best explain the relationship between diagnostic symptoms. M1 specifies a unique liability for developing SUD for each specific substance. Thus, in M1 there are six latent factors, one for each substance (Figure 1A). Each substance factor is defined by the 13 DSM symptoms measured for that substance. Conversely, model M2 (Figure 1B) assumes no distinction between substances and instead models how well latent misuse symptom-specific factors explain the relationship between diagnostic symptoms. Thus, M2 examines if there are unique liabilities associated with specific misuse symptoms across the range of substances considered. For example, the factor pertaining to the symptom of hazardous use models a dimension capturing propensity toward engaging in dangerous behavior during drug use, irrespective of the specific substance.
Figure 1.
Path diagrams for confirmatory factor analytic models. Circles represent unobserved latent factors for cannabis (Can), sedatives (Sed), and hallucinogens (Hall). Not shown, but included in the modeling, are cocaine, opioids and stimulants. Boxes represent the 13 observed DSM abuse (A1-A6) and dependence (D1-D7) diagnostic symptoms for each substance. A. Path diagram for M1, which had only substance factors. B. Path diagram for M2, which had only misuse characteristic factors in the model. C. Path diagram for M3, which combines M1 and M2 together by having both substance and misuse characteristic factors in the same model. D. Path diagram for M4, which has one latent factor for overall illicit drug use defined by the 13 abuse and dependence symptoms for each substance (13 symptoms × 6 substances = 78 symptoms)
Model M3 combines M1 and M2 by modeling both substance and misuse symptom factors simultaneously. Under M3 (Figure 1C), there are 19 factors being modeled, six substance factors and 13 misuse symptom factors. This model specifies unique liabilities toward both substance-specific SUDs and misuse symptoms across substances. Model M4 (Figure 1D) tests whether there is a single overall liability for SUD across all substances and symptom types. Thus a single latent factor is measured by all 78 observed items (13 symptoms × 6 substances). Additional exploratory analyses were used to explore some minor modifications of these models, e.g., adding a second order poly-substance factor to model M3.
Choice of best fitting and most parsimonious model
All models were compared using omnibus fit indices. In addition to inspecting the difference in the chi-square statistics and traditional CFA model comparison indices, such as the Comparative Fit Index (CFI), Root Mean Square Error of Approximation (RMSEA) and the Akaike Information Criterion (AIC), we used the Bayesian Information Criterion (BIC) and sample-size adjusted BIC (saBIC) indices. For the CFI, values close to one are regarded as indicative of good model fit. For the RMSEA, values of 0.05 or less indicate good model fit, while values of 0.10 and greater are indicative of poor model fit. The lowest, most negative AIC, BIC and saBIC values are indicative of a superior balance of parsimony and explanatory power.
RESULTS
Table 1 displays the endorsement rates of the abuse and dependence symptoms by substance, as well as self-reported use of a substance. Cannabis had the highest self-reported use, while opioids had the lowest. The most frequently endorsed symptom across all drug categories was hazardous use. The two withdrawal symptoms had the lowest endorsement. When different substance users are compared, we see patterns consistent with the established illicit substance abuse literature (26). Cannabis and hallucinogen users reported experiencing fewer symptoms of withdrawal than did cocaine and opioid users. Stimulant users reported ‘using when doing something important’ more frequently than other substances, presumably because they are using the stimulant to enhance performance.
Model fit comparisons
As seen in Table 2, both M1, which specified only substance factors, and M2, which specified only misuse symptom factors, provided poor fit to the data compared to M3, which specified both substance and misuse symptom factors. Regarding omnibus indices, CFI was higher for M3, while RMSEA and all three parsimony indices were lower for M3, consistently indicating superior fit for M3 compared to M1 and M2 (Table 2). Thus, both substance and misuse symptom factors made unique, significant contributions to model fit. Next, to test for the presence of an overall liability to illicit SUD, M4, which specified one overall liability factor, was compared to M3, the best fitting model with substance and misuse symptom factors. These models were non-nested, prohibiting formal chi-square comparisons; however, across all omnibus measures of model fit M3 markedly out-performed M4 (Table 2), indicating that a simple, general SUD liability CFA failed to accurately model these symptom-level data.
Table 2.
Confirmatory factor analysis model fit results. Model fit indices include: Comparative Fit index (CFI), Root Mean Square Error of Approximation (RMSEA), Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC) and sample size adjusted Bayesian Information Criterion (saBIC).
| Model | χ 2 | DF | p-value | CFI | RMSEA | AIC | BIC | saBIC |
|---|---|---|---|---|---|---|---|---|
| M1: Drug Factors Only | 4175 | 2910 | <0.001 | 0.78 | 0.017 | 4517 | 5431 | 4887 |
| M2: Misuse Characteristic | ||||||||
| Factors Only | 3647 | 2847 | <0.001 | 0.86 | 0.013 | 4115 | 5365 | 4622 |
| M3: Drug and Misuse | ||||||||
| Characteristic Factors | 2966 | 2754 | <0.001 | 0.96 | 0.007 | 3620 | 5367 | 4328 |
| M4: General Liability Factor | 4598 | 2925 | <0.001 | 0.71 | 0.019 | 4910 | 5744 | 5248 |
|
| ||||||||
| M1 vs. M3 | 1209 | 156 | <0.001 | |||||
| M2 vs. M3 | 681 | 93 | <0.001 | |||||
Characterizing the preferred model M3
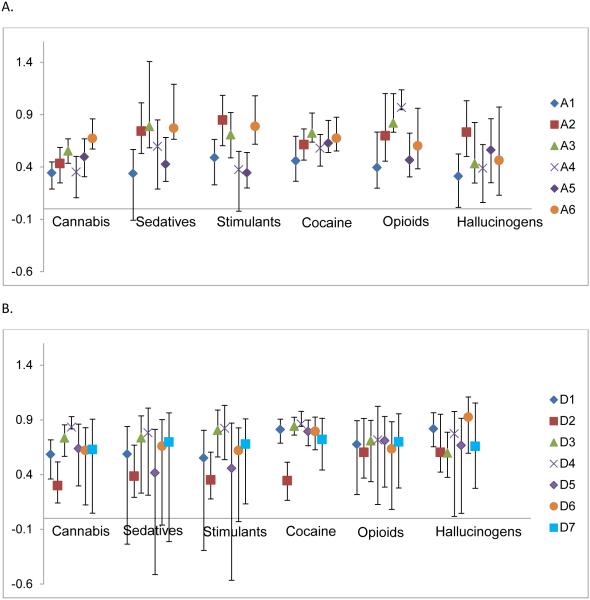
The factor loading estimates for the M3 substance factors are plotted with their 95% confidence intervals in Figure 2A, for the abuse symptoms, and in 2B for the dependence symptoms. Most of the symptoms loaded significantly on the substance factors with factors loadings ranging from 0.30-0.93 (See Supplementary Material Table S1). For each substance factor, D3 (Spend time using it or recovering from it) and D4 (Use instead of work or hobbies) had factor loadings greater than 0.70 suggesting that these two symptoms are particularly strong indicators of liability for these substances.
Figure 2.
Plot of substance factor loadings from Model 3 with 95% confidence intervals. A. Abuse symptom substance factor loadings. B) Dependence symptom substance factor loadings. Symptom meaning: A1= Hazardous use; A2 = Consequences: legal; A3 = Consequences: Social; A4 = Consequences: Physical and psychological; A5 = Used often when doing something …important; A6 = Stay away from school\miss appointments; D1 = Used more of longer than thought\planned; D2 = Loss of control: unable to stop\desire to stop; D3 = Spend time taking\using it, recovering from it; D4 = Used instead of work\hobbies; D5 = Need for larger amounts\doses (tolerance); D6 = Withdrawal symptoms: Feeling sick when cutting down\stopping; D7 = Withdrawal symptoms: After not using …use to prevent sickness.
Examining each substance factor individually revealed unique features. The cannabis factor was moderately defined by the two withdrawal symptoms (D6, D7) and A6 (staying away from school/missed appointments). Hazardous use (A1) and having physical or psychological consequences (A4) were not good indicators of cannabis liability, having lower loadings than other symptoms. Liability for sedatives was highly defined by staying away from school and missing appointments (A6) and having social consequences (A3), but like cannabis, was not defined by hazardous use (A1). The stimulant factor had large loadings for legal consequences (A2) and staying away from school/missing appointments (A6). However, stimulant liability was not indexed highly by using when doing something important (A5), physical or psychological consequences (A4) or inability to stop using or quit (D2). Liability to cocaine SUD is highly defined by the tolerance (D5) and withdrawal symptoms (D6 and D7) as well as using longer than thought or planned (D1). It was not measured well by being unable to stop or cut-down on use (D2). Opioid liability was highly indexed by social (A3) and physical and psychological consequences (A4) and was moderately indexed by the tolerance and withdrawal symptoms (D5-D7). Hallucinogen liability was highly defined by using more or longer than intended (D1) and feeling sick when cutting down/stopping (D6), but was not defined well by hazardous use (A1) and physical or psychological consequences (A4). These findings must be interpreted in the light of the misuse symptom factor loadings.
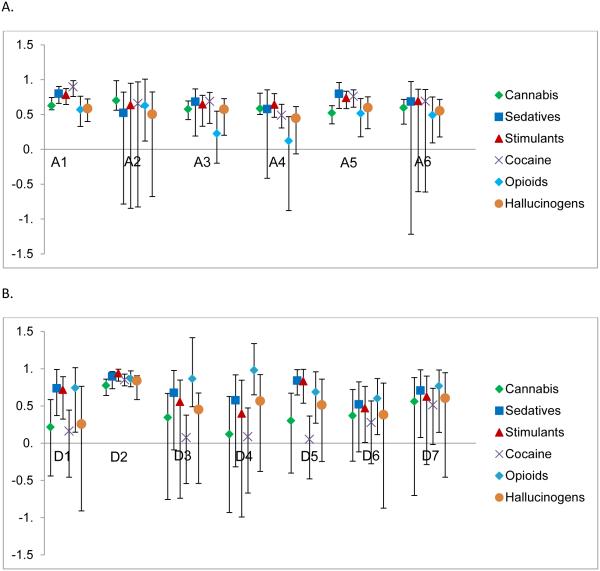
The factor loading estimates and 95% confidence intervals for the misuse symptom factors are plotted in Figure 3A and 3B (See Supplementary Material Table S2 for the misuse factor loadings and standard errors). Their interpretation is that substances with higher loadings index the liability to the specific misuse symptom factor better than those substances with lower loadings. Cannabis tended to have lower loadings in comparison to the other substances on most of the misuse symptom factors and did not load significantly on any of the dependence misuse symptom factors with the exception of D2 (Loss of control). Several of the dependence misuse symptom factors (D1, D3, D4, D6 and D7) had opioids as the highest loading substance while several other misuse symptom factors had sedatives as the highest loading substance (A5, D1 and D5). Examining the factors individually yields additional interesting findings. Cocaine involvement was the strongest indicator of liability toward hazardous use (A1). Cannabis indexed the liability for legal consequences (A2) better than other substances. Use instead of work or hobbies (D4) had only one significant loading, from opioids. For the tolerance factor (D5), sedatives and stimulants had the highest loadings. The two withdrawal factors (D6 and D7) both had opioids as the best index of the underlying liability and cocaine as the worst.
Figure 3.
Plot of misuse characteristic factor loadings from Model 3 with 95% confidence intervals. A. Abuse misuse characteristic factor loadings. B) Dependence misuse characteristic factor loadings. Symptom meaning: A1= Hazardous use; A2 = Consequences: legal; A3 = Consequences: Social; A4 = Consequences: Physical and psychological; A5 = Used often when doing something …important; A6 = Stay away from school\miss appointments; D1 = Used more of longer than thought\planned; D2 = Loss of control: unable to stop\desire to stop; D3 = Spend time taking\using it, recovering from it; D4 = Used instead of work\hobbies; D5 = Need for larger amounts\doses (tolerance); D6 = Withdrawal symptoms: Feeling sick when cutting down\stopping; D7 = Withdrawal symptoms: After not using …use to prevent sickness.
DISCUSSION
Overall, results indicate that a model including both substance-specific and misuse symptom-specific factors provides superior fit for symptom-level data across illicit substances relative to the other models considered and in omnibus fit. Furthermore, the relevance of individual questionnaire items varied considerably across substance- and symptom-specific factors. Notably, misuse characteristics pertaining to using instead of work or hobbies (D4) and spending time using or recovering from the substances (D3) emerged as the best indices of symptom-specific SUD liability.
Perhaps the most controversial finding was the lack of support for a general poly-substance liability factor. Several potential explanations for this result exist. First, the general liability model tested here was specified as a single factor measured by all items (Figure 1D). While intuitive and parsimonious, this model is only one of a multitude of potential general liability specifications; so it is possible that other, more complex, poly-substance liability models that were not tested may have provided superior fit. Although comprehensive comparison of the full universe of possible latent structures is impractical and contrary to the aims of CFA, we did explore alternative models of general liability, including adding a second-order factor loading on all substance factors to M3. However, no tested model out-performed M3. The failure of these alternative models is clarified by considering the non-significant correlations observed between substance factors in M3 (See Supplementary Material Table S3), indicating little shared variance. Specifically, out of the 15 covariances modeled between substance factors in M3, only 3 were significant (p <0.05)—opioids-cocaine (p=0.037), hallucinogens-stimulants (p=0.018) and hallucinogens-sedatives (p=0.006). The cannabis factor showed the lowest intercorrelation among substance factors, supporting previous research indicating greater uniqueness for cannabis liability compared to other illicit drugs (15). It is also important to note that substance-factor correlations were attenuated by modeling misuse symptom factors (i.e., comparing M1 v. M3), suggesting that a portion of the variance modeled as general liability in former studies may be more accurately modeled as symptom-specific poly-substance liabilities. However, given the indeterminate nature of latent variable modeling, we cannot claim to have definitively debunked general liability, only to have found no evidence among the models considered. In addition to the possibility of a superior, untested model of general liability, it is also possible that the population may be qualitatively heterogeneous. Thus, future research should test for subpopulations of individuals with distinct liability structures (e.g., factor mixture models) (17).
The findings of this study suggest several directions for future research. Since SUDs are heritable, with about 9-45% of phenotypic variation being explained by additive genetic effects (27-29), it is reasonable to investigate genetic variance in liability to symptom-specific factors. Beseler et al. (30) found that the 11 symptoms of the DSM-III-R for alcohol, cannabis, cocaine, opioids, sedatives and stimulants were heritable. Previous studies have found genomic regions associated with DSM abuse and dependence symptoms (31-32). Instead of using SUD diagnosis in genetic studies, as is common practice, we suggest using factor scores from the substance and symptom dimensions found here to better identify genetic factors underlying each component of liability and behaviors associated with addiction. The loss of control symptom may be particularly useful in this respect as all substances loaded highly on this factor, suggests that loss of control is a central poly-substance liability characteristic.
Liability structure implications by substance
The results of our liability structure analysis reveal that each substance and symptom indexes the liability of SUD differently. We review these results by substance.
Cannabis
The effects of cannabis use are among the least disruptive. Users were less likely to endorse using instead of work/hobbies, and were least likely to experience physical and psychological consequences. Along with hallucinogen users, they were also less prone to encounter legal consequences. Kosten et al. (33) have shown that cannabis use is associated with fewer dependence symptoms than the use of other drugs. The misuse symptom factors in our analyses confirm this result. Cannabis symptoms tended to have lower loadings on most of the misuse symptom factors and did not load significantly on any of the dependence symptom factors with the exception of loss of control. Notably, the cannabis-specific substance factor was moderately defined by the two withdrawal symptoms. While it is has been previously shown that withdrawal symptoms are commonly reported among cannabis users (7), it has been suggested that there is no evidence to support a clear cannabis withdrawal syndrome (34). In recent years, however, there has been increasing evidence in both the human and animal literature of physical and psychological effects associated with cannabis withdrawal (26). Our findings do not suggest that there is a definitive cannabis withdrawal syndrome, but instead suggest that those individuals who do experience withdrawal effects from cannabis have a greater risk of developing cannabis SUD.
Sedatives
Compared to other substances, sedative users were somewhat less likely to report misuse symptoms in this study. Liability for sedative SUD was highly defined by staying away from school/missing appointments and having social consequences. Notably, sedatives were one of the highest loading substances on several of the misuse symptom factors, including using when doing something important. Thus, while misuse is relatively uncommon among sedative users, when it does occur it is a superior indicator of liability for various poly-substance misuse symptoms.
Stimulants
Stimulants were comparatively frequently associated with several symptoms including physical and psychological consequences, using while doing something important and difficulty stopping or reducing. However, the most frequently endorsed stimulant symptoms were relatively poor indicators of the stimulant factor. Thus, characteristics like using when doing something important and physical and psychological consequences were more typical features of illicit stimulant use, while symptoms including social consequences and using instead of work/hobbies occurred rarely but were more indicative of psychopathology. This may reflect the fact that some stimulants (e.g., Ritalin, Adderall) may be used illicitly during work or school for cognitive enhancement in the absence of broader psychiatric dysfunction (35).
Cocaine
Cocaine users were comparatively most likely to report misuse, particularly dependence, symptoms. Dependence symptoms, including tolerance and withdrawal, also accurately indexed liability to cocaine SUD. Cocaine users are known to develop tolerance quickly, though there is differential tolerance to cocaine based on the route of administration(26). Oral administration is less likely to produce tolerance effects, presumably because of the lower absorption rate, than intravenous or smoking, which produce rapid acute tolerance(36). Cocaine withdrawal is associated with few physical symptoms, but is associated with psychological symptoms including dysphoria, depression and craving(26) due, in part, to remodeling of the mesolimbic dopaminergic pathway(37). However, cocaine misuse was generally a poor indicator of dependence symptom factors. This seems counterintuitive since cocaine is one of the more addictive substances under consideration here. Perhaps because it is so highly addictive, the endorsement rate is of these symptoms is very high, which increases the error of the factor loadings. Substances with lower rates of endorsement of dependence symptoms may serve as superior indices.
Opioids
Withdrawal symptom endorsement was comparatively frequent among opioid users, particularly for using to prevent sickness. Similarly, liability for opioid SUD was indexed by the dependence symptoms and physical and psychological consequences. Opioids also had the highest factor loadings on the two withdrawal symptom factors. These results are consistent with previous findings of both high mean levels of, and significant inter-individual variation in, opioid withdrawal severity(38). Thus, results highlight the centrality of physical dependence to opioid SUD and suggest that sensitivity to opioid withdrawal is an excellent indicator of poly-substance withdrawal liability.
Hallucinogens
Hallucinogen users generally had lower symptom endorsement rates than the other substances considered. Hallucinogen liability was highly defined by using more or longer than intended and feeling sick when cutting down/stopping. The strong loadings of these items seems counterintuitive because hallucinogens are typically not associated with physical dependence (39), but they may relate to nausea which is a common side-effect of many hallucinogenic compounds (e.g. mushrooms(40) ). This anomaly may reflect study participants interpreting these items to mean that hallucinogenic experiences lasted longer than desired, perhaps related to the commonly occurring panic symptom colloquially known as a "bad trip"(41). Similar to cannabis, hallucinogens did not load significantly on the dependence symptom factors, with the exception of loss of control. This result is not unexpected given that hallucinogens are typically used in small doses and in recreational settings (42).
Limitations
Our findings must be interpreted in the context of a few limitations. First, as these data were restricted to white males, it is unclear whether these results will generalize to females or other races and ethnicities. Future research in other samples should test whether the same liability structure is seen in females and other races and ethnicities. Second, these analyses did not include cohort effects. However, preliminary analyses with age at interview and age of first drug use as covariates suggested no significant changes. Third, endorsement rates for several items were low. This may result in unreliable parameter estimates, but this uncertainty should be accurately quantified by robust standard error estimates. As a sensitivity analysis, the models were fit to the symptoms from only the three substances with highest reported use from Table 1—cannabis, stimulants, and cocaine. Results were similar to the full six substance analyses. Finally, the current study did not address the possibility of subpopulation-specific liability structures, as fitting factor mixture models to these data led to optimization problems due to model complexity and sparse data. Future research to address this line of inquiry might consider the use of clinical addiction case-control samples because they are likely to provide denser item endorsement to support the mixture models needed to investigate subpopulation-specific liability structures.
CONCLUSION
Results from the CFAs support a model of illicit substance misuse liability including substance specific factors, as well as factors capturing poly-substance symptom-specific liability. Inspection of the substance factors reveals that individual symptoms do not index the liability for misuse to the same degree across the six major illicit drug classes considered. These results also showed variation in the relevance of substances as indices of misuse symptom factors. We identified several symptoms as reliable, poly-substance features of SUD and recommend that they be investigated in genetic and neurobiological studies of substance misuse. Finally, in these symptom-level data, there is no support for a general liability to illicit substance misuse after modeling symptom-specific factors. Further research should consider the possibility of that SUD liability structure may vary across subpopulation classes. A potentially high impact application of these results would be to use the factor scores from the substance and symptom dimensions to better identify measured genetic variants in samples with genome-wide association study data.
Supplementary Material
Highlights.
The liability to misuse illicit substance is drug class specific.
There is no evidence to support a general liability for illicit substance misuse.
We identified dimensions capturing propensity toward specific misuse symptoms.
Acknowledgments
Role of Funding Sources
This work was supported by National Institute on Alcohol Abuse and Alcoholism grant K01AA021266, National Institute of Mental Health grant K01MH093731, and National Institute on Drug Abuse grants DA026119, DA018673 and R00DA023549. The ascertainment of twins by the Mid-Atlantic Twin Registry (MATR) was supported by CTSA Grant Number UL1TR00058 from the National Center for Advancing Translational Sciences. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Clark and Neale designed the study. Drs. Clark and Gillespie conducted the statistical analysis. Drs. Adkins and Neale provided guidance for the statistical analysis. Dr. Kendler aided in the substantive interpretation of the statistical analyses. Dr. Clark wrote the first draft of the manuscript and all authors contributed to and approved of the final manuscript.
Conflict of Interest
The authors declare they have no conflicts of interest.
References
- 1.Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. American Journal of Psychiatry. 2013 Aug;170(8):834–51. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie AJ, Teesson M. Continuous, categorical and mixture models of DSM-IV alcohol and cannabis use disorders in the Australian community. Addiction. 2010 Jul;105(7):1246–53. doi: 10.1111/j.1360-0443.2010.02951.x. [DOI] [PubMed] [Google Scholar]
- 3.Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings M, Young SE, et al. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. J Am Acad Child Adolesc Psychiatry. 2008 Feb;47(2):165–73. doi: 10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynskey MT, Agrawal A. Psychometric properties of DSM assessments of illicit drug abuse and dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychol Med. 2007 Sep;37(9):1345–55. doi: 10.1017/S0033291707000396. [DOI] [PubMed] [Google Scholar]
- 5.Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, et al. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. J Abnorm Psychol. 2004 Feb;113(1):72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- 6.Teesson M, Lynskey M, Manor B, Baillie A. The structure of cannabis dependence in the community. Drug Alcohol Depend. 2002 Dec 1;68(3):255–62. doi: 10.1016/s0376-8716(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 7.Swift W, Hall W, Teesson M. Characteristics of DSM-IV and ICD-10 cannabis dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Drug Alcohol Depend. 2001 Jul 1;63(2):147–53. doi: 10.1016/s0376-8716(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 8.Nelson CB, Rehm J, Ustun TB, Grant B, Chatterji S. Factor structures for DSM-IV substance disorder criteria endorsed by alcohol, cannabis, cocaine and opiate users: results from the WHO reliability and validity study. Addiction. 1999 Jun;94(6):843–55. doi: 10.1046/j.1360-0443.1999.9468438.x. [DOI] [PubMed] [Google Scholar]
- 9.Feingold A, Rounsaville B. Construct validity of the abuse-dependence distinction as measured by DSM-IV criteria for different psychoactive substances. Drug Alcohol Depend. 1995 Aug;39(2):99–109. doi: 10.1016/0376-8716(95)01142-l. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern J, Langenbucher J, Labouvie EW. The generalizability of the dependence syndrome across substances: an examination of some properties of the proposed DSM-IV dependence criteria. Addiction. 1994 Sep;89(9):1105–13. doi: 10.1111/j.1360-0443.1994.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 11.Bryant KJ, Rounsaville BJ, Babor TF. Coherence of the dependence syndrome in cocaine users. Br J Addict. 1991 Oct;86(10):1299–310. doi: 10.1111/j.1360-0443.1991.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 12.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003 Apr;160(4):687–95. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 13.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998 Nov;55(11):967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 14.Vanyukov MM. Substance-Specific Symptoms and General Liability to Addiction. American Journal of Psychiatry. 2012 Oct;169(10):1016–8. doi: 10.1176/appi.ajp.2012.12070923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behav Genet. 2004 May;34(3):217–28. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007 Nov;64(11):1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal A, Lynskey MT, Madden PAF, Bucholz KK, Heath AC. A latent class analysis of illicit drug abuse/dependence: results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction. 2007 Jan;102(1):94–104. doi: 10.1111/j.1360-0443.2006.01630.x. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie NA, Neale MC, Prescott CA, Aggen SH, Kendler KS. Factor and Item-Response analysis of DSM-IV criteria for abuse and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opiods. Addiction. 2007;102:920–30. doi: 10.1111/j.1360-0443.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- 19.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the causes of psychiatric and substance use disorders. First The Guilford Press; New York: 2006. [Google Scholar]
- 20.Palmer RHC, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, et al. Genetic etiology of the common liability to drug dependence: Evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug and Alcohol Dependence. 2012 Jun;123:S24–S32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-III-R-Patient Version (SCID-P,4/1/87) New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- 22.Muthén LK, Muthén BO. MPlus User's Guide. Sixth Muthen & Muthen; Los Angeles: 1998-2010. [Google Scholar]
- 23.Muthén BO, Satorra A. Complex sample data in structural equation modeling. Sociological Methodology. 1995;25:267–316. [Google Scholar]
- 24.Huber PJ, editor. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability.1967. [Google Scholar]
- 25.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroscedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 26.Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; London: 2006. [Google Scholar]
- 27.Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, Alcohol and Drug use in Eight- to sixteen- year old twins: The Virginia twin study of adolescent behavioral development. Journal of Studies on Alcohol and Drugs. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- 28.McGue MK, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard twin study of substance abuse: What we have learned. Harvard review of psychiatry. 2001;9:267–79. [PubMed] [Google Scholar]
- 30.Beseler C, Jacobson KC, Kremen WS, Lyons MJ, Glatt SJ, Faraone SV, et al. Is there heterogeneity among syndromes of substance use disorder for illicit drugs? Addictive Behaviors. 2006;31(6):929–47. doi: 10.1016/j.addbeh.2006.03.037. [doi: DOI: 10.1016/j.addbeh.2006.03.037] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehlers CL, Gizer IR, Vieten C, Wilhelmsen KC. Linkage analyses of cannabis dependence, craving, and withdrawal in the San Francisco family study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2010;153B(3):802–11. doi: 10.1002/ajmg.b.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo PH, Neale MC, Riley BP, Webb BT, Sullivan PF, Vittum J, et al. Identification of susceptibility loci for alcohol-related traits in the Irish Affected Sib Pair Study of Alcohol Dependence. Alcohol Clin Exp Res. 2006 Nov;30(11):1807–16. doi: 10.1111/j.1530-0277.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 33.Kosten TR, Rounsaville BJ, Babor TF, Spitzer RL, Williams JB. Substance-use disorders in DSM-III-R. Evidence for the dependence syndrome across different psychoactive substances. Br J Psychiatry. 1987 Dec;151:834–43. doi: 10.1192/bjp.151.6.834. [DOI] [PubMed] [Google Scholar]
- 34.Smith NT. A review of the published literature into cannabis withdrawal symptoms in human users. Addiction. 2002 Jun;97(6):621–32. doi: 10.1046/j.1360-0443.2002.00026.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith ME, Farah MJ. Are Prescription Stimulants "Smart Pills"? The Epidemiology and Cognitive Neuroscience of Prescription Stimulant Use by Normal Healthy Individuals. Psychological Bulletin. 2011 Sep;137(5):717–41. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE. Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacol Biochem Behav. 1989 May;33(1):167–73. doi: 10.1016/0091-3057(89)90446-2. [DOI] [PubMed] [Google Scholar]
- 37.Gao WY, Lee TH, King GR, Ellinwood EH. Alterations in baseline activity and quinpirole sensitivity in putative dopamine neurons in the substantia nigra and ventral tegmental area after withdrawal from cocaine pretreatment. Neuropsychopharmacology. 1998 Mar;18(3):222–32. doi: 10.1016/S0893-133X(97)00132-2. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Bi J, Chan G, Oslin D, Farrer L, Gelernter J, et al. Improved methods to identify stable, highly heritable subtypes of opioid use and related behaviors. Addictive Behaviors. 2012 Oct;37(10):1138–44. doi: 10.1016/j.addbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gable RS. Toward a Comparitive Overview of dependence potential and acute toxicity of psychoactive substances used nonmedically. American Journal of Drug and Alcohol Abuse. 1993;19(3):263–81. doi: 10.3109/00952999309001618. [DOI] [PubMed] [Google Scholar]
- 40.Barbee G, Berry-Caban C, Barry J, Borys D, Ward J, Salyer S. Analysis of mushroom exposures in Texas requiring hospitalization, 2005-2006. J Med Toxicol. 2009 Jun;5(2):59–62. doi: 10.1007/BF03161087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. Journal of Psychopharmacology. 2008 Aug;22(6):603–20. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein M, Kramer F. Rave drugs: pharmacological considerations. AANA J. 2004 Feb;72(1):61–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.