Abstract
Regulatory T (Treg) cells are critical players in the prevention of autoimmunity. Treg lineage commitment and functional stability are influenced by selected extracellular signals from the local environment, shaped by distinctive intracellular signaling network, and secured by their unique epigenetic profile. Recent advances in our understanding of the complex processes of Treg lineage differentiation, maintenance, and function has paved the way for developing strategies to manipulate these important cells for therapeutic benefit in many diseases. In this review, we will summarize recent advances in our understanding of Treg biology as well as Treg-targeted therapies in the context of autoimmune disease.
Introduction
Forkhead box P3 (Foxp3)-expressing regulatory T cells (Tregs) are a small subset of CD4+ T cells that are vital to immune homeostasis and prevention of autoimmunity in mice and man [1]. Expression of the transcription factor Foxp3 in these cells is essential for their development, maintenance, and function. Treg potency lies in their ability to deploy various immunosuppressive mechanisms depending on the immunological context as well as extending their influence through the process of infectious tolerance [2]. An emerging concept is that Tregs not only control immune responses, but also promote tissue homeostasis by suppressing inflammation and aiding in tissue repair [3]. Moreover, this system is exploited by tumor cells to evade immune surveillance [4]. Thus, changes in Treg number and function underlie many illnesses of the immune system and beyond.
Manipulating Tregs is a new therapeutic strategy for treating various diseases including autoimmunity, transplant rejection, and cancer [5,6]. Elucidating factors influencing Treg homeostasis and function has important implications in understanding disease pathogenesis and identifying therapeutic opportunities. This review will focus on recent advances in how Tregs integrate extracellular and intracellular signaling to control their survival and stability. We will discuss how these new insights can be utilized for the development of new approaches to promote and stabilize Tregs in autoimmunity and transplantation.
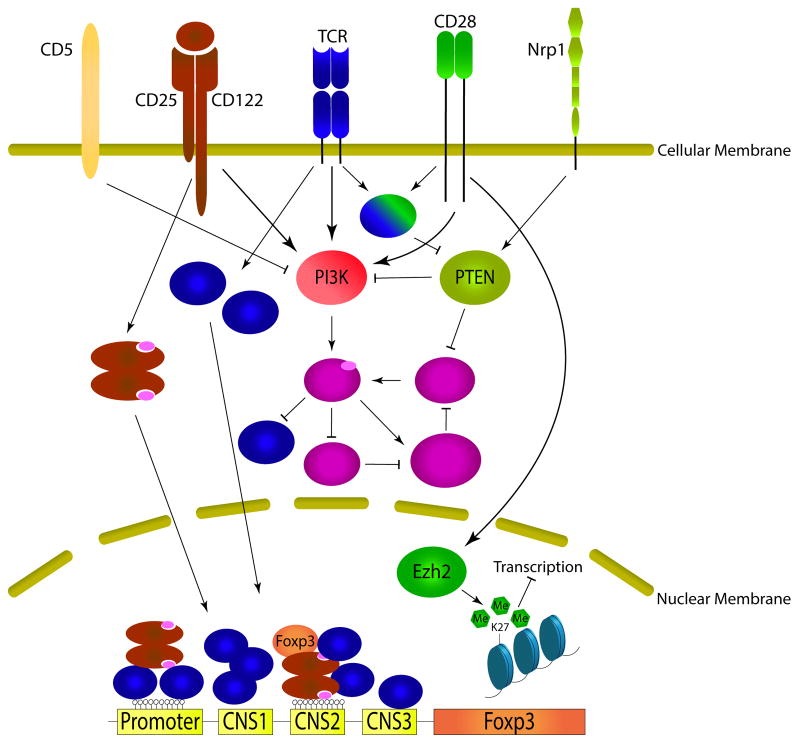
TCR, CD28, and IL-2: the essential triad for Treg lineage specification and maintenance
Thymic Treg (tTreg) development is initiated by T cell receptor (TCR) signaling followed by sequential activation of CD25 expression, IL-2 signaling, and then Foxp3 expression [7,8]. tTreg development can be enhanced through the constitutive activation of signal transducer and activator of transcription 5 (STAT5), which is downstream of the IL-2 receptor and directly binds cis elements in the Foxp3 promoter and enhancer to stabilize Foxp3 expression [9]. Indeed, the level of IL-2 in the circulation dictates the size of the thymic Treg compartment [10–12]. In addition to induction of CD25, TCR and CD28 signaling also contribute to establishing and stabilizing the Treg lineage commitment in the thymus by inducing epigenetic and differentiation events in Tregs [10,13–15]. Thus, antigen and IL-2 signaling transmitted via TCR, CD28, and CD25 are essential for Treg lineage specification in the thymus.
In the periphery, mature Tregs continue to depend on TCR, CD28, and CD25 for their homeostasis and function, but their roles appear to be distinct from those in the thymus. Tregs proliferate more than conventional CD4+ T cells in steady state in a CD28 dependent fashion, suggesting that Tregs are constantly seeing antigens that drive their cell cycle progression [16,17]. Recently, analysis of Treg subsets in the periphery found that the CD62LloCD44hi effector Tregs (eTregs) were relatively more responsive to TCR stimulation and less IL-2 dependent than CD62Lhi CD44lo central Tregs (cTregs) [18]. Consistent with the idea that eTregs are TCR dependent, deletion of the TCR specifically in mature Tregs led to a selective loss of CD62LloCD44hi eTregs as soon as 9 days after excision of the TCR gene. This suggests that constant stimulation through the TCR is required to maintain this population [19,20]. These TCR-deficient Tregs proliferated less and expressed fewer eTreg molecules such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), IL-10, Ebi3, and, correspondingly, conventional T cells became activated to express cytokines. However, this immune activation profile is fairly mild, which is dramatically different from the catastrophic systemic autoimmunity observed after Treg depletion [21]. This is likely because the frequency of Tregs remained normal after TCR deletion and Tregs maintained their responsiveness to IL-2, high levels of Foxp3 expression, and Treg-specific epigenetic profile. Through these observations, it is suggested that the role of TCR signaling in mature Tregs is mainly to activate their proliferation and effector functions, but not for lineage maintenance.
Proliferating Tregs have a tendency to lose their Foxp3 expression and lineage stability in vitro and in vivo in lymphopenic hosts [22–24]. The conserved noncoding sequence 2 (CNS2) enhancer element, also known as Treg-specific demethylation region, is critical for safeguarding lineage stability of proliferating Tregs [25,26]. However, stimulation via TCR with limited IL-2 leads to a loss of Foxp3 expression in Tregs even in wild type cells with intact CNS2. CNS2 has binding sites for both the TCR-triggered transcription factor nuclear factor of activated T-cells (NFAT) and IL-2-induced transcription factor STAT5, providing a transcriptional basis for Treg stability by coordinating TCR and IL-2 signaling. Interestingly, forced expression of constitutively active STAT5 prevented the loss of Foxp3 in CNS2 deleted Tregs, demonstrating that STAT5 can stabilize Foxp3 expression independent of CNS2 [25]. This may be explained by the NFAT-mediated looping between CNS2 and the Foxp3 promoter, which also has binding sites for NFAT and STAT5 [26]. In conclusion, TCR-mediated signals are important for mature Treg function but pose a threat to their stability unless they are balanced by IL-2 signaling.
PI3K-Akt-mTOR: a critical signaling node for Treg development and homeostasis
Phosphatidylinositide 3 kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR) form an intracellular signaling hub common to the TCR, CD28, and IL-2 receptor. PI3K is directly activated when these receptors are engaged, leading to initial activation of Akt by the PH-domain containing protein PDK1 through phosphorylation of threonine 308. Akt is fully activated by additional phosphorylation on serine 473 by the mTOR complex 2 (mTORC2). Akt has many cellular targets, but the Forkhead box O (Foxo) transcription factors and mTORC1 are most relevant to Treg biology. Foxo family transcription factors are critical for Treg lineage specification [27–29] and are inhibited by Akt. mTORC1 coordinates anabolic activities in cells and inactivates mTORC2, thus limiting further Akt activation. In the thymus, Treg development is enhanced by mutating the p110d catalytic subunit of PI3K [30] and it is repressed by forced expression of a constitutively active Akt [31], demonstrating a negative role of the PI3K axis on tTreg development. However, deletion of mTOR (thus inactivating both mTORC1 and 2) or individual deletion of mTORC1 or 2 in T cells does not alter thymic development [32], suggesting that the negative effect of PI3K and Akt on tTreg development is mTOR independent and mainly due to their role in Foxo1 inactivation.
In the periphery, this axis controls peripheral Treg (pTreg) generation. Similar to the thymus, Foxp3 induction is favored after T cell activation in the presence of pharmacological inhibitors of PI3K [33]. However, distinct from tTregs, pTreg generation is significantly impacted by mTOR signaling. mTOR-deficient T cells exhibited mild proliferative defects, failed to express effector cytokines, and defaulted to Foxp3 induction after TCR activation. Inhibition of both mTORC1 and 2 was required for this effect [34]. Activation of PI3K is naturally antagonized by phosphatase and tensin homolog (PTEN). PTEN expression is progressively inhibited by stronger TCR stimulation, permitting efficient T cell activation and effector differentiation, an effect mediated by interleukin-2-inducible T-cell kinase (Itk) [35]. Thus, T cells with Itk deficiency fail to down regulate PTEN after activation and favor Foxp3 induction over Th17 differentiation. Similarly, loss of tuberous sclerosis 1 (TSC1), an inhibitor of mTORC1, results in excessive IL-17 production, defective pTreg induction, and severe chemical induced colitis [36]. Lastly, CD5 was found to block PI3K during pTreg induction, making pTregs refractory to destabilization [37]. Together, these data support the notion that PI3K and mTOR activity in mature T cells critically controls the bifurcation between effector verses pTreg cell fates.
In committed Tregs, the PI3K-Akt-mTOR signaling axis continues to be repressed by high expression of PTEN. tTregs constitutively express high level of neuropilin [38,39], which directly binds PTEN and blocks Akt activation during immunological challenge [40]. Treg specific deletion of PTEN disrupted Treg homeostasis, function, and stability [41,42]. These PTEN-deficient Tregs lost both Foxp3 and CD25 expression but had a significant increase of mTORC2, but not mTORC1 activities. Additional deletion of mTORC2 in Tregs largely rescues the phenotype in mice with Treg-specific deletion of PTEN, demonstrating the normal function of PTEN in mature Tregs is to keep mTORC2 in check. In fact, intact mTORC1 function is required for Treg function because mice with selective deletion of mTORC1 in Tregs die of multi-organ autoimmune diseases similar to Foxp3-deficient mice [43]. Mechanistically, mTOR is found to control Treg function in part by regulating metabolic programming. T cells rely on mitochondrial oxidative phosphorylation at rest and switch to glycolysis after activation, a process essential for effector T cell differentiation [44]. In contrast, Tregs preferentially use oxidative metabolism even after activation. An emerging concept is that metabolic input can also dictate T cell fate decision [44]. PTEN-deficient Tregs show exaggerated glycolysis that is thought to contribute to Treg instability [41,42]. Additionally, functional defects in mTORC1-deficient Tregs are associated with disrupted lipid biosynthesis [43]. Thus, the impact of PI3K-Akt-mTOR axis on mature Treg function is far from black and white, while excessive activation of this pathway is clearly detrimental to Treg function as seen in PTEN-deficient Tregs, complete blockade of PI3K impairs Treg function as well [30,45].
Epigenome: a foundation for Treg stability
Treg lineage commitment and maintenance is ultimately secured by their epigenetic traits, which are governed by three complementary elements: histone modification, DNA methylation, and transcription factor binding [46,47]. Foxp3 binds to many histone-modifying proteins such as TIP60, Histone deacetylases (HDACs), p300, and Enhancer of zeste homolog 2 (Ezh2) to maintain epigenome stability. It is worth noting that Foxp3-mediated epigenetic changes lead to mostly gene repression, rather than activation, which is dependent on histone methyltransferase Ezh2 [48,49]. This genome wide repression is especially important for maintaining the Treg lineage under inflammatory conditions when activation of effector molecules normally expressed by conventional T cell need to be repressed in Tregs [50]. While Ezh2-deficient Tregs are phenotypically normal and have unaltered suppressive function in vitro, they lose Foxp3 expression after activation and are unable to control immune responses in vivo. Thus, antigen activation poses a threat to Treg stability and Tregs have intrinsic signaling and epigenetic mechanisms to safeguard their lineage stability.
Manipulating Tregs to treat autoimmune diseases
Elucidating the basic mechanisms underlying Treg biology is the key to manipulating these cells for therapeutic benefit. Changing the balance between effector cells and Tregs is a promising avenue to restore immune homeostasis and treat autoimmune diseases. Experimentally, all the critical elements in Treg biology described above have been targeted for the purpose of manipulating the balance between Tregs and effector cells and some of these approaches are being actively evaluated in the clinic.
Targeting TCR, CD28, and IL-2 triad
Although both Tregs and effector T cells express TCR and the associated CD3 complex, monoclonal antibodies to CD3 can tip the balance in favor of Tregs and induce long-lasting remission of type 1 diabetes in mouse models [51]. This change of Treg to effector T cell balance is due to higher resistance of Tregs to anti-CD3 induced cell death as well as increased induction of pTregs in the periphery [52,53]. Interestingly, delayed treatment with anti-CD3 reduced effector T cells and increased the proportion of Tregs in a mouse model of heart transplantation, resulting in long-term graft survival [54]. In humans, anti-CD3 antibodies induce the outgrowth of FOXP3+CD8+ Tregs in vitro and increase IL-10 in the serum in vivo [55]. These encouraging preclinical findings have led to clinical trials with promising results [56–60]. In type 1 diabetes, anti-CD3 treatment improves control of the disease and beta cell function during the first year after onset [56,57]. However, this therapy does not have efficacy for all patients [58] or in patients with long-standing disease [59].
Targeting CD28 using CTLA4Ig is also effective in changing the Treg to effector T cell balance to prevent immune activation. Although Treg development and peripheral homeostasis depend on CD28, effector T cell differentiation is more sensitive to CTLA4Ig-mediated CD28 blockade; thus, a low dose of CTLA4Ig can block effector differentiation with minimal impact on Tregs [61]. This is also observed in kidney transplant patients treated with belatacept, a high affinity variant of humanized CTLA4Ig [62]. Currently, CTLA4Ig has been approved by the Food and Drug Administration for the treatment of rheumatoid arthritis and for the prevention of kidney transplant rejection [63,64]. Selectively targeting pathogenic effector cells may be particularly effective for restoring immune tolerance, especially when the pathology arises as a consequence of effector resistance to regulation [65]. In this regard, a CD2-targetting fusion protein, alefacept, has been recently shown to deplete effector T cells while preserving Tregs in type 1 diabetes patients [66]. It is worth mentioning that a form of agonist anti-CD28 was shown to selectively increase Tregs and prevent experimental allergic encephalitis, a model of multiple sclerosis (MS) [67]. When evaluated in a phase 1 clinical trial, TGN1412, the humanized agonist anti-CD28 induced pan T cell activation and severe cytokine storm in healthy volunteers [68]. Therefore, the potential impact on effector cells should be carefully considered when developing drugs that stimulate TCR and CD28. Alternatively, antagonistic antibodies may selectively preserve Tregs depending on the dosing [69].
Owning to their constitutive expression of the high affinity IL-2 receptor and distinct biochemical wiring, Tregs preferentially respond to low-dose IL-2 therapy. This therapy is effective in preventing and reversing type 1 diabetes in mouse models [70,71]. Low-dose IL-2 therapy has been effective in increasing Tregs in type 1 diabetes [72,73], GvHD [74], and alopecia areata [75]. In HCV-induced vasculitis, Tregs were induced by IL-2 therapy and 8 out of 10 patients showed clinical improvement [76]. Thus, IL-2 therapy is a promising avenue for increasing Tregs and improving clinical outcomes for patients with autoimmune disease.
However, since many cell types can respond to IL-2, one concern with IL-2 therapy is its Treg selectivity. For example, eosinophilia was observed in patients on IL-2 therapy, and in mouse models, it was found to be mediated by the CD25-expressing type 2 innate-lymphoid cells [77]. Increasing IL-2 dose in a mouse model of type 1 diabetes led to an increase of Natural Killer (NK) cell and cytotoxic CD8 T cells and exacerbation of diabetes [70]. Quantitative measurement of IL-2 sensitivity of various cell types in human blood showed that Tregs were most responsive followed by CD56hi NK cells and memory T cells [73,78]. Acute Treg depletion in mice [79–81] led to an increase in NK cells expressing cytotoxic effector molecules. Interestingly, this did not lead to an increase in NK killing of autologous cells, suggesting that NK activation does not contribute to the fatal autoimmunity after Treg depletion [80]. Similarly, anti-CD25 therapy led to a reduction of Tregs in patients with MS, which corresponded with increases of serum IL-2 and CD56hi NK cells, but dramatic disease protection [82,83]. In vitro analysis suggests that the CD56hi NK cells may substitute the function of Tregs and suppress immune responses by killing activated effector cells [84]. Thus, the rise of CD56hi NK cells after IL-2 therapy may actually be beneficial rather than problematic. Nonetheless, ongoing efforts are devoted to improving the safety of IL-2 therapy. One approach to more selectively target Tregs is to mutate the IL-2 molecule to make its binding to its receptor CD25 dependent, which has shown efficacy in a Lewis rat model of MS [85].
Targeting PI3K-Akt-mTOR axis
A myriad of inhibitors have been developed to target PI3K-Akt-mTOR pathways with the goal of inducing immunosuppression and as therapies for cancer. The most extensively studied inhibitor in the context of Tregs is rapamycin. Initially, rapamycin was thought to be a specific mTORC1 inhibitor but was later found to inhibit both mTORC1 and 2 when used at high concentrations or with prolonged exposure. As discussed above, ablation of both mTORC1 and 2 are required for the preferential induction of pTregs, and mature Treg function critically depends on mTORC1. In culture, Tregs are more resistant to rapamycin-mediated growth inhibition, thus, rapamycin has been a favored additive to Treg expansion cultures to increase their purity [86]. However, rapamycin does not expand Tregs and has clearly been shown to retard the growth of Tregs in vitro and in vivo [87,88]. In the clinic, rapamycin has been used in transplant recipients as an alternative immunosuppressive agent to the widely used calcineurin inhibitors (CNI). Converting patients from CNI to rapamycin or its analogs has been associated with a rise of Tregs in circulation. However, it is not clear if this effect is mainly a result of decreased use of CNI, which are clearly inhibitory to Tregs, or a direct effect of rapamycin. In type 1 diabetes, mouse studies found that rapamycin and IL-2 combination therapy prevented diabetes [89]. In patients, however, this treatment led to a transient worsening of beta cell function and increased NK cells and eosinophils despite the dramatic rise in Tregs [90]. The negative impact of this regimen in patients was attributed to a direct effect of rapamycin on beta cells. Thus, the effect of rapamycin can be seen on multiple immune and non-immune cells, and its utility in autoimmune diseases is yet to be determined. Additionally, findings from genetic ablation studies in mice suggest that the selective targeting of mTORC2 would be more effective for tipping the balance towards Tregs.
Targeting epigenetic regulation
Although epigenetic programming is important for safeguarding Treg lineage identity, it is also dynamically regulated, providing an opportunity for pharmacological manipulations. Histone acetylation contributes epigenetic regulation and the process is balanced by the histone acetyltransferases (HATs) and HDACs. HDAC inhibitors have been extensively explored as anti-inflammatory and immunosuppressive agents. Particularly, inhibition of certain HDACs has been shown to selectively enhance Tregs, although these effects are likely more complicated than just histone acetylation because HDACs have many other cellular targets [91]. Ezh2-mediated repression is essential for Treg stability during antigenic challenge, suggesting that preserving and enhancing Ezh2 function would have an impact for promoting tolerance in the face of autoimmune diseases and inflammation. Much of the pharmacological development targeting Ezh2 focuses on inhibiting the enzyme in cancer cells with the added benefit of destabilizing Tregs. The activity of Ezh2 is naturally opposed by the histone demethylase Jmjd3 and UTX. Ablation of Jmjd3 in T cells inhibits Th1 and Th17 differentiation and preserves Tregs under Th1 polarizing conditions [92,93]. Thus targeting Jmjd3/UTX pathway may be effective for promoting Treg stability.
Achieving antigen-specific tolerance
Research in animal models shows that antigen-specific Tregs are more effective for controlling organ-specific autoimmune diseases and transplantation rejection when compared to polyclonal Tregs [94–96]. A long-term global increase in Tregs may impair effective immune surveillance against infections and malignancies; therefore, antigen-specific therapies are more effective and safer for organ-specific autoimmune diseases. Self-antigens coupled to killed splenocytes or erythrocytes via chemical crosslinking can inactivate self-reactive effector cells and induce expansion of antigen-specific Tregs in mouse models of MS, type 1 diabetes, and transplant rejection [97–99]. These pioneering studies are just beginning to be translated into the clinic [100]. Various newer experimental approaches have been explored to increase antigen-specific Tregs. For example, apoptotic cells pulsed with peptide have been described to have therapeutic effect in both experimental allergic encephalitis and type 1 diabetes by producing TGFβ and inducing antigen-specific pTregs in vivo [101]. Additionally, CD45 ligation on Tregs resulted in increased antigen-specific Treg-DC interactions and selective expansion of antigen-specific Tregs [102]. Synthetic nanoparticles represent an exciting new therapeutic platform to achieve antigen-specific manipulation of the immune system [103].
Treg cell therapy
Infusion of Tregs is a direct approach to selectively increase Tregs. Several phase 1 clinical trials of Treg cell therapy for the prevention of GvHD [104–106] and one trial in type 1 diabetes have been reported [107]. Currently, more than a dozen Treg cell therapy trials are registered on clinicaltrials.gov website, mostly in GvHD and solid organ transplantations using polyclonal Tregs. While it is feasible to produce large numbers of alloantigen-reactive Tregs by selective stimulation using allogeneic antigen presenting cells [108], large-scale manufacturing of tissue antigen-specific Tregs for autoimmunity is far more challenging because of their low precursor frequency and the tendency of Tregs to destabilize with repeated in vitro stimulation in an attempt to expand them [22,87]. New technology using chimeric antigen receptor (CAR) engineered T cells is promising for cancer immunotherapy [109], and may have applications for engineering antigen-specific Tregs to combat autoimmune disease. Indeed, engineered Tregs do have utility in mouse models of autoimmunity [110–113]. In addition to therapeutic development, CAR-engineering of Tregs also offers an opportunity for investigating fundamental biology of Tregs by defining the optimal CAR design to preserve Treg stability and function.
Conclusion and future prospects
In the past several years, we have gained deeper mechanistic understanding of the molecular control of Treg development, maintenance, and function thanks to genetic tools in mouse models. These discoveries are instrumental for the development of better targeted therapies for alternating the balance between Tregs and effector cells in various disease settings. It is clear that no specific molecule or pathway is uniquely utilized by Tregs and the distinction between Tregs and effector T cells may be quantitative. Tregs may preferentially use a combination of pathways; therefore, combination therapies may be able to more specifically target Tregs with lower and less toxic doses of drugs. Additionally, drugs that are not Treg-specific may be used for ex vivo manipulation of purified Tregs to increase their number while preserving their stability for therapeutic use. In the future, advanced tools for faster and more specific genetic manipulation of human cells [114] will allow us to more directly investigate the critical molecular pathways of human Tregs, such as engineering better CAR Tregs for achieving antigen specific tolerance.
Figure 1.
Coordinated signaling from extracellular inputs and their downstream targets in Treg cells.
Highlights.
Role of TCR, CD28, and IL2 in Treg identity and function
Impact of PI3K-Akt-mTOR signaling on Treg development, homeostasis, and function
Importance of epigenome in safeguarding Treg lineage stability after activation
Rationalize design for Treg targeted therapies for autoimmune diseases
Acknowledgments
This work was supported by grants from the National Institutes of Health R01 DK08231 (Q.T.), JDRF grants # 17-2013-549, # 2-SRA-2014-150 and 17-2011-661 and R01 AI046643 (J.B.). The authors would like to thank members of the Tang and Bluestone labs for their helpful discussions and Susanna Cheng for editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Current Opinion in Immunology. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 5.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA. Regulatory T-Cell Therapy in Transplantation: Moving to the Clinic. Cold Spring Harbor Perspectives in Medicine. 2013:3. doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lio C-WJ, Hsieh C-S. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3+ regulatory T cells. Immunological Reviews. 2014;259:11–22. doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015 doi: 10.1038/ni.3171. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi Z, Lin WW, Stunz LL, Bishop GA. The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol. 2014;15:866–874. doi: 10.1038/ni.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 Costimulation Is Essential for the Homeostasis of the CD4+CD25+ Immunoregulatory T Cells that Control Autoimmune Diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franckaert D, Dooley J, Roos E, Floess S, Huehn J, Luche H, Fehling HJ, Liston A, Linterman MA, Schlenner SM. Promiscuous Foxp3-cre activity reveals a differential requirement for CD28 in Foxp3(+) and Foxp3(−) T cells. Immunol Cell Biol. 2015;93:417–423. doi: 10.1038/icb.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 17.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. This study finds two distinct Treg subsets in the periphery that are either IL-2 responsive and localized primarily to lymphoid organs or TCR/co-stimulation reliant and localize primarily to tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. This study ablates the TCR in mature Tregs, finding a critical role for the TCR in Treg effector function but not lineage maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. This study ablates the TCR in mature Tregs, finding that Treg homeostasis, Treg-specific gene expression, and suppressive function are linked to TCR triggering, while Foxp3 expression and methylation patterns are not. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the Regulatory T Cell Lineage in Vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. A Foxp3 intronic element, CNS2, is critical for maintenance of Foxp3 expression after proinflammatory cytokine signaling or when IL-2 is limiting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158:734–748. doi: 10.1016/j.cell.2014.07.030. A Foxp3 intronic element, CNS2, senses TCR/NFAT activation to maintain Foxp3 expression in a proinflammatory cytokine environment, demonstrating a critical role for epigenetically marked cis-elements in the protection of cell identity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada Y, Harada Y, Elly C, Ying G, Paik J-H, DePinho RA, Liu Y-C. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. The Journal of Experimental Medicine. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LSK, Vanhaesebroeck B, et al. Cutting Edge: The Phosphoinositide 3-Kinase p110δ Is Critical for the Function of CD4+CD25+Foxp3+ Regulatory T Cells. The Journal of Immunology. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 31.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, Kirby MR, Belkaid Y, Schwartzberg PL. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med. 2014;211:529–543. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin HS, Park Y, Elly C, Liu YC. Itch expression by Treg cells controls Th2 inflammatory responses. J Clin Invest. 2013;123:4923–4934. doi: 10.1172/JCI69355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity. 2015;42:471–483. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgoffe GM, Woo S-R, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. PTEN-deficient Tregs fail to regulate Th1 and B cell responses. These Tregs become unstable, losing CD25 and Foxp3 expression, demonstrating a requirement for control of PI3K signaling by PTEN in Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. This study linked the phosphatase PTEN to Treg stability and metabolism through its role in blocking mTORC2 activation, which is important for Treg effector function in Th1 and Tfh responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. Tregs deficient in mTORC1 fail to mediate their suppressive function at least in part due to a block in lipid biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 45.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110delta regulates expression of CD38 on regulatory T cells. PLoS One. 2011;6:e17359. doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huehn J, Beyer M. Epigenetic and transcriptional control of Foxp3+ regulatory T cells. Semin Immunol. 2015;27:10–18. doi: 10.1016/j.smim.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Morikawa H, Sakaguchi S. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol Rev. 2014;259:192–205. doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 48.Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. This study demonstrates that a CD28 responsive chromatin modifier Ezh2 is required for Treg lineage stability and supports the Foxp3 gene-expression program after activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morikawa H, Ohkura N, Vandenbon A, Itoh M, Nagao-Sato S, Kawaji H, Lassmann T, Carninci P, Hayashizaki Y, Forrest ARR, et al. Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proceedings of the National Academy of Sciences. 2014;111:5289–5294. doi: 10.1073/pnas.1312717110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proceedings of the National Academy of Sciences. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 Therapy Promotes Tolerance by Selectively Depleting Pathogenic Cells while Preserving Regulatory T Cells. The Journal of Immunology. 2011;187:2015–2022. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belghith M, Bluestone JA, Barriot S, Megret J, Bach J-F, Chatenoud L. TGF-[beta]-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 54.Goto R, You S, Zaitsu M, Chatenoud L, Wood KJ. Delayed Anti-CD3 Therapy Results in Depletion of Alloreactive T Cells and the Dominance of Foxp3+CD4+ Graft Infiltrating Cells. American Journal of Transplantation. 2013;13:1655–1664. doi: 10.1111/ajt.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. The Journal of Clinical Investigation. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, et al. Anti-CD3 Monoclonal Antibody in New-Onset Type 1 Diabetes Mellitus. New England Journal of Medicine. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 57.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. Insulin Needs after CD3-Antibody Therapy in New-Onset Type 1 Diabetes. New England Journal of Medicine. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 58.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, et al. Teplizumab (Anti-CD3 mAb) Treatment Preserves C-Peptide Responses in Patients With New-Onset Type 1 Diabetes in a Randomized Controlled Trial: Metabolic and Immunologic Features at Baseline Identify a Subgroup of Responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebastchi J, Deng S, Lebastchi AH, Beshar I, Gitelman S, Willi S, Gottlieb P, Akirav EM, Bluestone JA, Herold KC. Immune Therapy and β-Cell Death in Type 1 Diabetes. Diabetes. 2013;62:1676–1680. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vudattu NK, Herold KC. Treatment of new onset type 1 diabetes with teplizumab: successes and pitfalls in development. Expert Opinion on Biological Therapy. 2014;14:377–385. doi: 10.1517/14712598.2014.881797. [DOI] [PubMed] [Google Scholar]
- 61.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. European Journal of Immunology. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 62.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The Effect of Costimulatory and Interleukin 2 Receptor Blockade on Regulatory T Cells in Renal Transplantation. American Journal of Transplantation. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: Bridging the Basic Immunology with Clinical Application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Wojciechowski D, Vincenti F. Belatacept in kidney transplantation. Current Opinion in Organ Transplantation. 2012;17:640–647. doi: 10.1097/MOT.0b013e32835a4c0d. [DOI] [PubMed] [Google Scholar]
- 65.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The Effector T Cells of Diabetic Subjects Are Resistant to Regulation via CD4+FOXP3+ Regulatory T Cells. The Journal of Immunology. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:0–0. doi: 10.1172/JCI81722. A CD2 targeting therapy, alefacept, that depletes effector memory and central memory T cells and was able to preserve C-peptide secretion, reduce insulin use, and reduce hypoglycemic events in new onset type 1 diabetic patients. This therapy had favorable immunological profiles 1 year after cessation of therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beyersdorf N, Gaupp S, Balbach K, Schmidt J, Toyka KV, Lin C-H, Hanke T, Hünig T, Kerkau T, Gold R. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. The Journal of Experimental Medicine. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. New England Journal of Medicine. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 69.Haanstra KG, Dijkman K, Bashir N, Bauer J, Mary C, Poirier N, Baker P, Scobie L, ’t Hart BA, Vanhove B. Selective Blockade of CD28-Mediated T Cell Costimulation Protects Rhesus Monkeys against Acute Fatal Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2015;194:1454–1466. doi: 10.4049/jimmunol.1402563. [DOI] [PubMed] [Google Scholar]
- 70.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central Role of Defective Interleukin-2 Production in the Triggering of Islet Autoimmune Destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. The Journal of Experimental Medicine. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 73**.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR. Selective IL-2 Responsiveness of Regulatory T Cells Through Multiple Intrinsic Mechanisms Supports the Use of Low-Dose IL-2 Therapy in Type 1 Diabetes. Diabetes. 2015;64:2172–2183. doi: 10.2337/db14-1322. The higher sensitivity of Tregs to IL-2 is due to their distinct intracellular signaling pattern downstream of IL-2 receptor complex in additional to their constitutive expression of CD25. [DOI] [PubMed] [Google Scholar]
- 74.Matsuoka K-i, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, et al. Low-Dose Interleukin-2 Therapy Restores Regulatory T Cell Homeostasis in Patients with Chronic Graft-Versus-Host Disease. Science Translational Medicine. 2013;5:179ra143. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castela E, Le Duff F, Butori C, et al. EFfects of low-dose recombinant interleukin 2 to promote t-regulatory cells in alopecia areata. JAMA Dermatology. 2014;150:748–751. doi: 10.1001/jamadermatol.2014.504. [DOI] [PubMed] [Google Scholar]
- 76.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-Cell Responses to Low-Dose Interleukin-2 in HCV-Induced Vasculitis. New England Journal of Medicine. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 77.Van Gool F, Molofsky AB, Morar MM, Rosenzwajg M, Liang H-E, Klatzmann D, Locksley RM, Bluestone JA. Interleukin-5–producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Q. Therapeutic Window of Interleukin-2 for Autoimmune Diseases. Diabetes. 2015;64:1912–1913. doi: 10.2337/db15-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, Huse M, Sun JC, Rudensky AY. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210:1153–1165. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue E-W, Stefoski D, Robinson R, Riester K, Rana J, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. The Lancet. 2013;381:2167–2175. doi: 10.1016/S0140-6736(12)62190-4. [DOI] [PubMed] [Google Scholar]
- 83.Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, O’Neill G, Neyer L, Sheridan J, Wang C, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. The Lancet Neurology. 2010;9:381–390. doi: 10.1016/S1474-4422(10)70033-8. [DOI] [PubMed] [Google Scholar]
- 84.Jiang W, Chai NR, Maric D, Bielekova B. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol. 2011;187:781–790. doi: 10.4049/jimmunol.1100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weishaupt A, Paulsen D, Werner S, Wolf N, Köllner G, Rübsamen-Schaeff H, Hünig T, Kerkau T, Beyersdorf N. The T cell-selective IL-2 mutant AIC284 mediates protection in a rat model of Multiple Sclerosis. Journal of Neuroimmunology. 2015;282:63–72. doi: 10.1016/j.jneuroim.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Battaglia M, Stabilini A, Roncarolo M-G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 87.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, et al. Massive ex Vivo Expansion of Human Natural Regulatory T Cells (Tregs) with Minimal Loss of in Vivo Functional Activity. Science Translational Medicine. 2011;3:83ra41–83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Camirand G, Lin Y, Froicu M, Deng S, Shlomchik WD, Lakkis FG, Rothstein DM. Regulatory T Cells Require Mammalian Target of Rapamycin Signaling To Maintain Both Homeostasis and Alloantigen-Driven Proliferation in Lymphocyte-Replete Mice. The Journal of Immunology. 2011;186:2809–2818. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rabinovitch A, Suarez-Pinzon WL, Shapiro AMJ, Rajotte RV, Power R. Combination Therapy With Sirolimus and Interleukin-2 Prevents Spontaneous and Recurrent Autoimmune Diabetes in NOD Mice. Diabetes. 2002;51:638–645. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 90.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, et al. Rapamycin/IL-2 Combination Therapy in Patients With Type 1 Diabetes Augments Tregs yet Transiently Impairs β-Cell Function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hancock WW, Akimova T, Beier UH, Liu Y, Wang L. HDAC inhibitor therapy in autoimmunity and transplantation. Annals of the Rheumatic Diseases. 2012;71:i46–i54. doi: 10.1136/annrheumdis-2011-200593. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Zou J, Wang M, Ding X, Chepelev I, Zhou X, Zhao W, Wei G, Cui J, Zhao K, et al. Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation. Nat Commun. 2014:5. doi: 10.1038/ncomms6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z, Cao W, Xu L, Chen X, Zhan Y, Yang Q, Liu S, Chen P, Jiang Y, Sun X, et al. The histone H3 lysine-27 demethylase Jmjd3 plays a critical role in specific regulation of Th17 cell differentiation. Journal of Molecular Cell Biology. 2015 doi: 10.1093/jmcb/mjv022. [DOI] [PubMed] [Google Scholar]
- 94.Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. doi: 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunological Reviews. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 98.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 99.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proceedings of the National Academy of Sciences. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lutterotti A, Yousef S, Sputtek A, Stürner KH, Stellmann J-P, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, et al. Antigen-Specific Tolerance by Autologous Myelin Peptide–Coupled Cells: A Phase 1 Trial in Multiple Sclerosis. Science Translational Medicine. 2013;5:188ra175–188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, Zanvit P, Jin W, Konkel JE, Chen W. In Vivo–Generated Antigen-Specific Regulatory T Cells Treat Autoimmunity Without Compromising Antibacterial Immune Response. Science Translational Medicine. 2014;6:241ra278. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 102.Camirand G, Wang Y, Lu Y, Wan YY, Lin Y, Deng S, Guz G, Perkins DL, Finn PW, Farber DL, et al. CD45 ligation expands Tregs by promoting interactions with DCs. J Clin Invest. 2014;124:4603–4613. doi: 10.1172/JCI74087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews. 2015 doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 105.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2010;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 107.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, et al. Administration of CD4+CD25highCD127- Regulatory T Cells Preserves beta-Cell Function in Type 1 Diabetes in Children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, Trotta E, Szot GL, Liu W, Lares A, et al. Clinical Grade Manufacturing of Human Alloantigen-Reactive Regulatory T Cells for Use in Transplantation. American Journal of Transplantation. 2013;13:3010–3020. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Themeli M, Riviere I, Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell. 2015;16:357–366. doi: 10.1016/j.stem.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, Ehrenstein MR, Stauss HJ. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 112.Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–2024. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 113.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, Harris RA, Magnusson PU, Brittebo E, Loskog AS. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114**.Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proceedings of the National Academy of Sciences. 2015;112:10437–10442. doi: 10.1073/pnas.1512503112. This study was able to successfully use Cas9 ribonucleoproteins (RNPs) to edit the genome of primary human T cells. This establishes Cas9 RNP as a technology for future use in manipulating these cells for many therapeutic applications. [DOI] [PMC free article] [PubMed] [Google Scholar]