Abstract
Background
Gram-negative bacteria (GNB) are a leading cause of nosocomial infection and sepsis. Increasing multi-antibiotic resistance has left clinicians with fewer therapeutic options. Antibodies to GNB lipopolysaccharide (LPS, or endotoxin) have reduced morbidity and mortality as a result of infection and are not subject to the resistance mechanisms deployed by bacteria against antibiotics. In this phase 1 study, we administered a vaccine that elicits antibodies against a highly conserved portion of LPS with and without a CpG oligodeoxynucleotide (ODN) TLR9 agonist as adjuvant.
Methods
A vaccine composed of the detoxified LPS (dLPS) from E. coli O111:B4 (J5 mutant) non-covalently complexed to group B meningococcal outer membrane protein (OMP). Twenty healthy adult subjects received three doses at 0, 29 and 59 days of antigen (10 μg dLPS) with or without CPG 7909 (250 or 500 μg). Subjects were evaluated for local and systemic adverse effects and laboratory findings. Anti-J5 LPS IgG and IgM antibody levels were measured by electrochemiluminesence. Due to premature study termination, not all subjects received all three doses.
Results
All vaccine formulations were well-tolerated with no local or systemic events of greater than moderate severity. The vaccine alone group achieved a ≥4-fold “responder” response in IgG and IgM antibody in only one of 6 subjects. In contrast, the vaccine plus CPG 7909 groups appeared to have earlier and more sustained (to 180 days) responses, greater mean-fold increases, and a higher proportion of “responders” achieving ≥4-fold increases over baseline.
Conclusions
Although the study was halted before all enrolled subjects received all three doses, the J5dLPS/OMP vaccine, with or without CpG adjuvant, was safe and well-tolerated. The inclusion of CpG increased the number of subjects with a ≥4-fold antibody response, evident even after the second of three planned doses. A vaccine comprising J5dLPS/OMP antigen with CpG adjuvant merits further investigation.
Keywords: Lipopolysaccharide, Endotoxin, CpG ODN, adjuvant, Phase 1 study, sepsis
Introduction
Gram-negative bacteria (GNB) are a leading cause of nosocomial infection, exceeded in one recent survey only by C. difficile infections [1]. Despite decades of intensive research, the morbidity and mortality from Gram-negative bacteremia and sepsis is unacceptably high [2]. The situation is further exacerbated by the dramatic increase in multi-antibiotic resistant (MDR) bacteria accompanied by a steady decline in the antibiotic pipeline [3]. Despite governmental attempts to encourage the development of new antibiotics by pharmaceutical companies, the development of antibiotic resistance is inevitable, making the useful life of a new antibiotic uncertain [4,5]. Consequently, new approaches for the treatment of GNB are greatly needed.
Vaccines that elicit antibodies against bacterial pathogens have been successful in reducing the morbidity and mortality from infection, or in the case of H. influenzae, nearly eradicating lethal infections [6,7]. Antibodies against the lipopolysaccharide (LPS, or endotoxin) of GNB have been highly protective in experimental GNB infection, as well as in human infection [8-10]. While vaccines against nosocomial GNB pathogens have been developed and tested in human subjects, none have sought licensure in the United States, in part because of the effectiveness, until recently, of antibiotics [11-13].
Early investigation of LPS structure by several laboratories identified a highly conserved core region that joined the biologically active lipid A moiety to the highly variable carbohydrate region responsible for O antigen specificity. A whole killed vaccine was prepared from a mutant of E. coli O111:B4 (Rc chemotype, J5) that was unable to generate O antigens, thereby exposing conserved core LPS epitopes. Passive administration of post-immune antisera generated by the administration of this vaccine to healthy subjects demonstrated significant protection from shock and death in a large, multicenter randomized, control clinical trial [14]. We developed a new subunit formulation of the original J5 vaccine whereby purified J5 LPS was detoxified (J5dLPS) and non-covalently complexed with the outer membrane protein (OMP) of group B N. meningitides [15]. The resulting vaccine was highly immunogenic and protective in various preclinical models of sepsis caused by heterologous clinical isolates of GNB [15-20]. When tested in human subjects, this vaccine was well-tolerated with no systemic adverse effects and local reactions similar to those of licensed vaccines; however, this non-adjuvanted vaccine induced only a 2-4 fold increase in anti-J5 LPS antibodies over baseline levels [21].
Adjuvants are well established to increase antibody responses for kinetics, magnitude, breadth, and durability of antibody responses against co-administered antigens. Oligodeoxynucleotides (ODN) containing CpG motifs that activate immune cells via Toll-like receptor 9 (TLR9) have been shown to enhance antibody responses to a wide variety of antigens [22]. CPG 7909 is a 24-mer CpG ODN containing 3 CpG motifs that has been shown to significantly enhance antibody responses in several human clinical trials [23-27]. The subject of this report is a Phase 1 clinical study of J5dLPS vaccine administered alone or with CPG 7909 at two different doses.
Materials and Methods
Vaccine and Adjuvant
The J5dLPS/OMP vaccine was prepared at the Pilot Bioproduction Facility at the Walter Reed Army Institute of Research (WRAIR) in Silver Spring, MD under cGMP conditions as previously described [21]. E. coli O111:H4, J5 (Rc) mutant was originally obtained from Dr. Elizabeth Ziegler, San Diego, CA. The J5dLPS/OMP cGMP product (Lot 0376) was originally manufactured in 1996 and stored at -20° ±5° C.
CPG 7909 is a synthetic CpG ODN of sequence TCGTCGTTTTGTCGTTTTGTCGTT, is manufactured with a nuclease-resistant phosphorothioate backbone. CPG 7909, generously provided by Pfizer (PF-3512676), was stored at 2-8° C.
Study Protocol
This single-center study intended to recruit 28-34 healthy subjects aged 18-50 years. The subjects were randomized to one of four study groups: (1) vaccine alone (10 μg, based on LPS content), (2) vaccine + CPG 7909 (500 μg), (3) vaccine + CPG 7909 (250 μg), or (4) placebo (normal saline). The primary objective of the study was to establish the safety and tolerability of the combination of vaccine and CPG 7909 when given together. The secondary objective was to determine if the combination of vaccine with CPG 7909 was more immunogenic than the vaccine alone and if the antibody response occurred earlier. This study was approved by the IRB of the University of Maryland, Baltimore.
Eligible subjects were to receive three immunizations in alternating deltoid muscle at Days 0, 29, and 59, based on the previous Phase 1 study performed with non-adjuvanted vaccine [21]. The subjects in the four study groups were to be immunized in three cohorts. Only the vaccinator, who did not monitor patient safety, was unblinded. For the first cohort, two subjects in each group received vaccine alone, vaccine + CPG 7909 (250 μg), or placebo. A second cohort received the vaccine alone (n=6), vaccine + CPG 7909 (250 μg, n=6), or vaccine + CPG 7909 (500 μg, n=2). The third cohort was to be immunized with the vaccine + CPG 7909 (500 μg, n=6) or placebo (n=2). After each immunization, subjects were examined for local and systemic reactions at 24 and 48 hr and reactogenicity and tolerability was recorded for 8 days. Blood samples for standard laboratory safety tests were obtained 7 days after each vaccination (i.e., days 7, 36, and 66) and for anti-core glycolipid antibody levels at days 14, 36, 66 as well as on days 120, 180, and 365. Baseline antibody levels were measured at day 0 before immunization. An immunology safety screen to monitor potential adverse effects of the adjuvant included ANA, anti-double-stranded DNA antibody, and TSH assays. EKGs were performed after each vaccination.
ELISA
A previously described ELISA assay for IgG and IgM antibody to J5 LPS was adapted to a proprietary platform that is a combination of electrochemiluminescence (ECL) detection and patterned arrays (Meso Scale Discovery [MSD]) according to the manufacturer's instructions. ECL detection uses secondary antibody labels that emit light when electrochemically stimulated that reduces background signals and improves sensitivity [28]. One microgram J5 LPS (List Biologics) in PBS was added to MSD MULTI-SPOT 96-well plates. Following addition of serum samples, incubation, and washing, SULFO-TAG® anti-human detection antibody was added to each well of the MSD plate and the wells were read in a SECTOR IMAGER 2400 reader. Data were analyzed using Microsoft Excel and MSD Discovery Workbench software (http://www.mesocale.com/CatalogSystemWeb/WebRoot/products/software.aspx).
Statistical analysis
IgG and IgM antibody levels to J5 LPS were analyzed by computing the geometric mean concentration (GMC), geometric mean-fold increase over baseline antibody levels (gMFI), and the proportion of responders (defined as >4-fold increase in antibody level over baseline) in each study group and at each time point. Confidence intervals for the geometric mean antibody levels were computed by transforming results to log scale, assuming normality assumptions were satisfied on this scale, and converting the computed interval back to the original scale. Exact 95% confidence intervals for the proportion of responders were also computed. An unplanned comparison was made between the proportion of responders in the vaccine alone and the combined vaccine + CPG 7909 groups. This analysis was performed with a Fisher Exact test and was consistent with the objective of determining if addition of CPG improved the level of antibody as well as the rapidity of response. Statistical analyses were performed using SAS software, version 9.3.
Results
Study Design and Subjects
Of the intended 28 subjects in this clinical trial, only 20 healthy adults aged 22-47 were enrolled. The study was prematurely terminated when inert particulate matter was detected in one vial of vaccine partway through the study. Of the 20 enrolled subjects, 12 (60%) were male, 12 (60%) were Black/African American, and the mean age was 33.9 years. All 20 subjects received 2 vaccine doses, but only 6 subjects received the third dose (2 in each of vaccine alone, vaccine + 250 μg CPG 7909, and placebo groups). As a result of the premature study halt, 14 enrolled subjects did not receive their third dose and cohort 3 was not enrolled. Eighteen of the 20 subjects (90%) completed the protocol. Two subjects (both in the vaccine + 250 μg CPG 7909 group) were lost to follow-up.
Safety Evaluation
All vaccine formulations were safe and well-tolerated, although all were more reactogenic than placebo treatment (Table 1).
Table 1. Maximum Severity of Systemic and Local Reactions by Study Group and Vaccination Number.
Subjects were examined for local and systemic reactions at 24 and 48 hr after each vaccination. Systemic and local reactogenicity events were collected for 8 days after each vaccination, or until resolution in subjects who experienced ongoing solicited events. Unsolicited adverse events were collected through 21 days after the subject's last immunization. All serious adverse events were collected throughout the study period. The proportion of subjects exhibiting the most severe local or systemic reaction that occurred after any vaccination and after each of the first two vaccinations is shown for each group. There were no adverse events of severe severity reported.
| Vaccine Alone | Vaccine + 250 μg CPG 7909 | Vaccine + 500 μg CPG 7909 | Placebo | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactogenicity | Vacc Number |
N | None n (%) |
Mild n (%) |
Mod n (%) |
N | None n (%) |
Mild n (%) |
Mod n (%) |
N | None n (%) |
Mild n (%) |
Mod n (%) |
N | None n (%) |
Mild n (%) |
Mod n (%) |
| Local | Any | 8 | 1 (13) | 4 (50) | 3 (38) | 8 | 1 (13) | 1 (13) | 6 (75) | 2 | 0 (0) | 1 (50) | 1 (50) | 2 | 1 (50) | 1 (50) | 0 (0) |
| 1 | 8 | 1 (13) | 5 (63) | 2 (25) | 8 | 2 (25) | 1 (13) | 5 (63) | 2 | 0 (0) | 1 (50) | 1 (50) | 2 | 2 (100) | 0 (0) | 0 (0) | |
| 2 | 8 | 3 (38) | 3 (38) | 2 (25) | 8 | 1 (13) | 4 (50) | 3 (38) | 2 | 1 (50) | 0 (0) | 1 (50) | 2 | 1 (50) | 1 (50) | 0 (0) | |
| 3 | 2 | 0 (0) | 2 (100) | 0 (0) | 2 | 2 (100) | 0 (0) | 0 (0) | 0 | NA | NA | NA | 2 | 2 (100) | 0 (0) | 0 (0) | |
| Systemic | Any | 8 | 1 (13) | 5 (63) | 2 (25) | 8 | 0 (0) | 1 (13) | 7 (88) | 2 | 0 (0) | 2 (100) | 0 (0) | 2 | 1 (50) | 0 (0) | 1 (50) |
| 1 | 8 | 1 (13) | 6 (75) | 1 (13) | 8 | 0 (0) | 1 (13) | 7 (88) | 2 | 0 (0) | 2 (100) | 0 (0) | 2 | 2 (100) | 0 (0) | 0 (0) | |
| 2 | 8 | 4 (50) | 3 (38) | 1 (13) | 8 | 0 (0) | 6 (75) | 2 (25) | 2 | 2 (100) | 0 (0) | 0 (0) | 2 | 1 (50) | 0 (0) | 1 (50) | |
| 3 | 2 | 0 (0) | 2 (100) | 0 (0) | 2 | 0 (0) | 1 (50) | 1 (50) | 0 | NA | NA | NA | 2 | 2 (100) | 0 (0) | 0 (0) | |
Systemic reactogenicity events were all mild to moderate in severity, with myalgia (12/20, 60%) and fatigue/malaise (12/20, 60%) being the most frequently observed. There was no evidence of increased reactogenicity with the inclusion of CPG 7909 compared to antigen alone (Table 1). Ten of 20 (50%) subjects had a moderate systemic reaction after any vaccination (Table 1), with the highest incidence after the first vaccination in the vaccine + CPG 7909 250 μg group (7/8, 88%) and a decreased incidence with subsequent vaccinations (Figure 1A).
Figure 1.
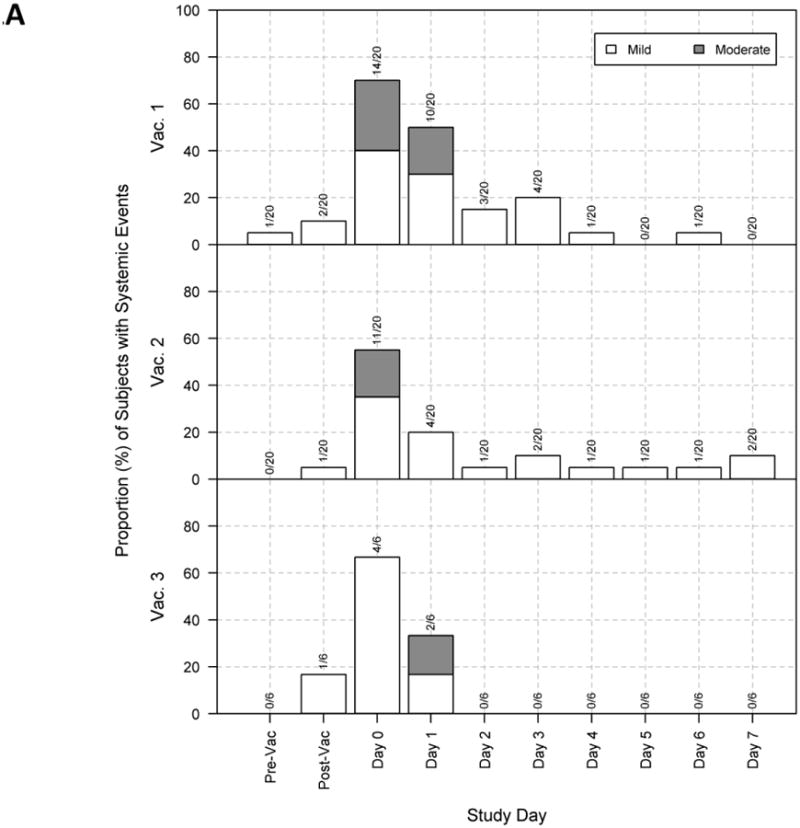
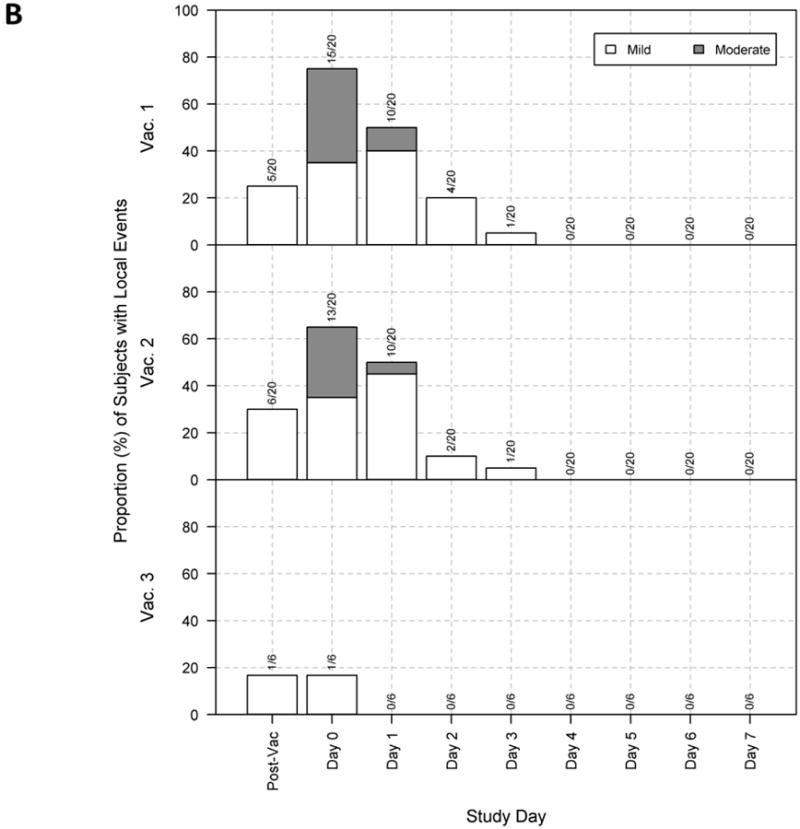
Maximum severity response per subject for all study groups by days post each of three vaccinations.
At 24 and 48 hr after each vaccination, subjects were examined for systemic (panel A) and local (panel B) reactions and subjects recorded reactogenicity and tolerability in diaries daily for 8 days. The proportion of subjects in each group that exhibited mild (light) or moderate (dark) systemic reactions are shown by day after each immunization. No subject experienced severe or life-threatening reactions. “Pre-Vac” and “Post-Vac” in Panel A refers to the evaluation in the clinic for systemic symptoms before and after each vaccination, while “Post-Vac” in panel B refers to the evaluation of local reactions in the clinic after immunization.
Local reactogenicity events were also all mild to moderate in severity. Moderate local reactions were observed in 10 of 20 subjects (50%) after any vaccination, and while there were no significant differences between groups, there was a trend for higher incidence with CPG 7909 compared to antigen alone, but not with the higher dose of CPG 7909 compared to the lower dose (Table 1). The local reactions for all groups were present by day 1 post-vaccination and usually resolved by day 3 post-vaccination; the number of local reactions were greatest after the first dose and declined with each subsequent immunization (Figure 1B and Table 1).
Although 19 of 20 (95%) subjects experienced 26 unsolicited adverse events, none were serious and only 5 were of moderate severity. Two the 26 events (8%) were vaccine-related and both were experienced by the same subject. No subject reported an adverse event of special interest while enrolled in this study.
The laboratory evaluation monitored liver and renal function tests, complete blood count, urinalysis, and C-reactive protein. None of these laboratory values in any group were above grade 2 (moderate). Anti-nuclear antibody values were monitored for each of the subjects. No positive ANA values (or greater than 8 IU/mL) were observed in either CPG 7909 group, and 5 mild ANA value abnormalities occurred in two subjects in the vaccine alone group. One subject in the vaccine + 250 μg CPG 7909 group had a TSH level of 16.24 μIU/ml 127 days after the third vaccination that increased 56 days later; however there were no signs or symptoms of thyroid disorder and a further history revealed a family history (grandmother) of “thyroid problems”. No clinically significant EKG abnormalities were observed in any of the groups.
Antibody response
At the time of study halt, all subjects had received at least two vaccinations, and some of those in vaccine alone and vaccine + CPG 7909 250 μg had also received the third and final dose.
The IgG antibody responses in both vaccine + CPG 7909 groups were higher than those in the vaccine alone group, but the limited sample size in each group precludes formal statistical conclusions (Figure 2 and Table 2). While the vaccine alone group consistently displayed higher antibody levels than the placebo group, only 1 of 8 had a four-fold increase over baseline (i.e. “vaccine responder”) after the primary vaccination series (Table 2). In contrast, the vaccine + CPG 7909 250 μg group had a higher gMFI than did the vaccine alone group and a greater percentage of subjects in the CPG 7909 group had a >4-fold response. While only two subjects received vaccine + CPG 7909 500 μg, both subjects achieved a >4-fold response after only the second immunization and the gMFI was three times that of the 250 μg group (Table 2, Day 36, gMFI=2.75 vs. 9.03). Peak antibody levels were observed at Day 66 and elevated IgG levels persisted through day 180. When the number of responders after the second immunization were compared (Table 2, Day 36), the addition of CPG (either dose) produced a higher proportion of responders, but the difference did not achieve statistical significance (1/8 vaccine alone vs. 5/10 vaccine + CPG 7909, p=0. 0758, one sided Fisher's Exact Test).
Figure 2.
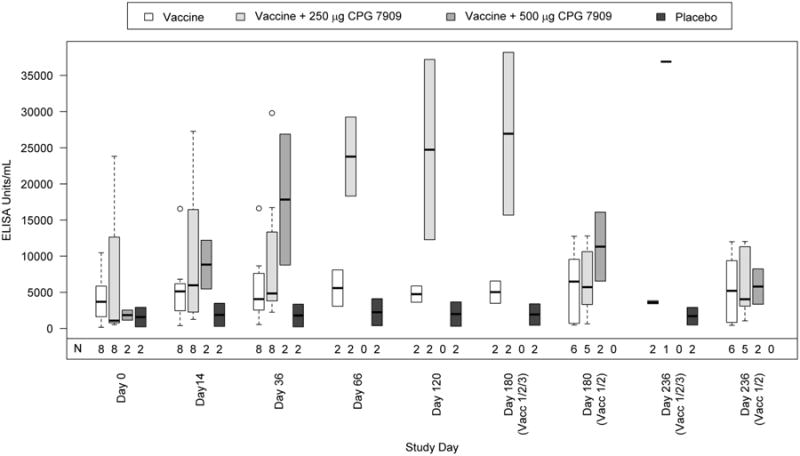
Summary of IgG response to J5 antigen by visit and study group.
Boxplots of ECL ELISA IgG antibody responses to J5 LPS are shown by study group and visit. The number of subjects evaluated at each study day is indicated (N) and the numbers of subjects receiving all 3 or only 2 vaccine doses are also shown. The dark horizontal bar within each boxplot represents the median antibody response with the 25-75th percentiles indicated by the lower and upper borders of the vertical box. The dotted lines extending from the box (“whiskers”) represent responses of up to 1.5 times the height of the box. Open circles at days 14 and 36 are outliers (i.e. beyond the whiskers).
Table 2. Summary statistics of ELISA IgG antibody response to J5dLPS/OMP vaccine + CPG.
Healthy subjects were immunized with 10 ug (based on LPS content) of vaccine alone, vaccine + 250 μg CPG. 7909, vaccine + 500 μg CPG 7909or placebo (PBS) on Days 0, 29 and 59. Blood sampling was obtained on days 0, 14, 36, 66, 120, 180 and 365 for antibody determinations by ECL ELISA. The IgG antibody levels to J5 LPS were analyzed by computing the geometric mean concentration (GMC), geometric mean-fold increase over baseline (Day 0) antibody levels (gMFI) and the proportion of responders in each study group and at each time point as described in Materials and Methods. The number (N) of subjects in each group that were seen for followup after Dose 1 of the vaccine (Day 14), Dose 2 (Day 36) and Dose 3 (Day 66) are indicated. Long term followup (Days 180 and 236) regardless of the number of immunizations received are also shown. Since there was little change in antibody levels on Days 120 and Day 365, these values are not shown in this table (but shown in Figure 2). At each day of followup the geometric mean concentration (GMC) and 95% confidence interval is shown. The proportion of subjects with >four-fold increase in GMC over the baseline GMC (Day 0) (i.e. “responders”) was calculated and also expressed as geometric mean fold increases (gMFI) with 95% confidence interval.
| Study Group Vaccination 1, 2 & 3 | Vaccine Alone | Vaccine + 250 μg CPG 7909 | Vaccine + 500 μg CPG7909 | Placebo |
|---|---|---|---|---|
| Day 0: Enrollment, Dose 1 | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 2395.0 (742.5 - 7725) | 2412.0 (657.7 - 8845) | 1700.5 (10.4 - 2.8E+05) | 802.7 (0.0 - 1.1E+10) |
| Day 14: Clinic Visit | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 3508.7 (1285.4 - 9578) | 5925.2 (2334.4 - 1.5E+04) | 8167.5 (49.0 - 1.4E+06) | 1001.9 (0.0 - 7.4E+09) |
| Four-fold Increase - %(95% CI) | 0 (0 - 37) | 25 (3 - 65) | 50 (1 - 99) | 0 (0 - 84) |
| gMFI (95% CI) | 1.47 (1.10 - 1.95) | 2.46 (1.07 - 5.62) | 4.80 (0.00 - >1000) | 1.25 (0.67 - 2.33) |
| Day 36: Clinic Visit | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 3885.4 (1643.5 - 9185) | 6639.9 (3212.6 - 1.4E+04) | 15362.6 (12.4 - 1.9E+07) | 852.1 (0.0 - 3.2E+10) |
| Four-fold Increase - %(95% CI) | 13 (0 - 53) | 38 (9 - 76) | 100 (16 - 100) | 0 (0 - 84) |
| gMFI (95% CI) | 1.62 (0.94 - 2.79) | 2.75 (1.08 - 7.00) | 9.03 (1.19 - 68.54) | 1.06 (0.39 - 2.91) |
| Day 66: Clinic Visit (All subjects received vaccination 1, 2, and 3) | ||||
| N | 2 | 2 | 0 | 2 |
| GMC (95% CI) | 4988.9 (10.4 - 2.4E+06) | 23144.1 (1181.6 - 4.5E+05) | ---- | 1252.7 (0.0 - 4.6E+09) |
| Four-fold Increase - %(95% CI) | 0 (0 - 84) | 50 (1 - 99) | ---- | 0 (0 - 84) |
| gMFI (95% CI) | 0.91 (0.08 - 10.89) | 4.87 (0.00 - >1000) | ---- | 1.56 (0.42 - 5.86) |
| Day 180: Clinic Visit (Subjects receiving vaccination 1 and 2 only) | ||||
| N | 6 | 5 | 2 | 0 |
| GMC (95% CI) | 3363.8 (755.2 - 1.5E+04) | 4397.2 (986.3 - 2.0E+04) | 10264.0 (33.5 - 3.1E+06) | ---- |
| Four-fold Increase - %(95% CI) | 0 (0 - 46) | 40 (5 - 85) | 100 (16 - 100) | ---- |
| gMFI (95% CI) | 1.85 (1.09 - 3.15) | 3.56 (0.59 - 21.37) | 6.04 (3.21 - 11.36) | ---- |
| Day 236: Final Visit (Subjects receiving vaccination 1 and 2 only) | ||||
| N | 6 | 5 | 2 | 0 |
| GMC (95% CI) | 3189.4 (759.9 - 1.3E+04) | 4473.3 (1274.6 - 1.6E+04) | 5258.3 (17.1 - 1.6E+06) | ---- |
| Four-fold Increase - %(95% CI) | 0 (0 - 46) | 40 (5 - 85) | 0 (0 - 84) | ---- |
| gMFI (95% CI) | 1.76 (1.25 - 2.47) | 3.62 (0.84 - 15.69) | 3.09 (1.64 - 5.85) | ---- |
After the first vaccination there was a robust IgM response in the two CPG groups, with >4-fold increase in both 500 μg CPG 7909 group subjects and nearly a three-fold average increase in the 250 μg CPG 7909 group (Figure 3, Table 3, Day 14). Following the second vaccination, the Day 36 IgM level increased further in the 500 μg CPG 7909 group (gMFI 11.54) and in the 250 μg CPG 7909 group (gMFI 4.82), while the vaccine alone group had a gMFI of 2.43. The gMFI in the vaccine alone group was essentially unchanged through the third vaccination. The difference between IgM responders after the second vaccination in the two combined CPG 7909 vs. vaccine alone groups (Table 2, Day 36) did not achieve statistical significance (2/8 [25%] vs 6/10 [60%], p=0.0940, one-sided Fisher Exact Test). The nonsignificant result can be partly attributed to the low sample size in each group.
Figure 3.
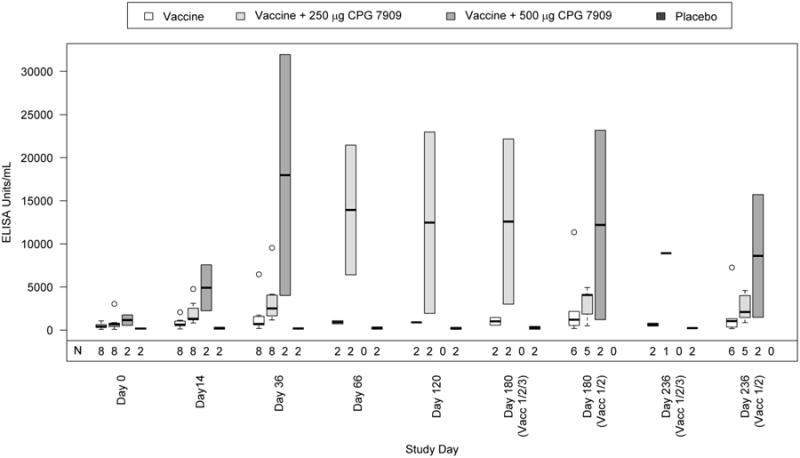
Summary of IgM response to J5 antigen by visit and study group.
Boxplots of ECL ELISA IgM antibody responses to J5 LPS are shown by study group and visit. The number of subjects evaluated at each study day is indicated (N) and the number of subjects receiving all 3 or only 2 vaccine doses are also shown. The dark horizontal bar within each boxplot represents the median antibody response with the 25-75th percentiles indicated by the lower and upper borders of the vertical box. The dotted lines extending from the box (“whiskers”) represent responses of up to 1.5 times the height of the box. Open circles are outliers (beyond the whiskers).
Table 3. Summary statistics of ELISA IgM antibody response to J5dLPS/OMP vaccine + CPG.
The IgM antibody levels determined by ECL ELISA following vaccination are shown in a format similar to Table 1. The antibody values for visits Days 120 and 365 were similar to those shown for Days 180 and 236 and therefore not shown.
| Study Group Vaccination 1, 2 & 3 | Vaccine Alone | Vaccine + 250 μg CPG 7909 | Vaccine + 500 μg CPG7909 | Placebo |
|---|---|---|---|---|
| Day 0: Enrollment, Dose 1 | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 378.4 (195.1 - 734) | 562.8 (240.8 - 1315) | 981.0 (0.6 - 1.6E+06) | 151.2 (0.3 - 7.9E+04) |
| Day 14: Clinic Visit | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 642.7 (329.9 - 1252) | 1651.6 (1011.6 - 2696) | 4115.9 (1.8 - 9.5E+06) | 171.9 (0.0 - 6.7E+05) |
| Four-fold Increase - %(95% CI) | 0 (0 - 37) | 13 (0 - 53) | 100 (16 - 100) | 0 (0 - 84) |
| gMFI (95% CI) | 1.70 (1.34 - 2.15) | 2.93 (1.20 - 7.16) | 4.20 (3.05 - 5.76) | 1.14 (0.15 - 8.41) |
| Day 36: Clinic Visit | ||||
| N | 8 | 8 | 2 | 2 |
| GMC (95% CI) | 918.8 (385.8 - 2188) | 2710.6 (1526.0 - 4815) | 11316.4 (0.0 - 6.0E+09) | 152.1 (0.2 - 1.3E+05) |
| Four-fold Increase - %(95% CI) | 25 (3 - 65) | 50 (16 - 84) | 100 (16 - 100) | 0 (0 - 84) |
| gMFI (95% CI) | 2.43 (1.41 - 4.18) | 4.82 (1.91 - 12.13) | 11.54 (0.04 - >1000) | 1.01 (0.62 - 1.62) |
| Day 66: Clinic Visit (All subjects received vaccination 1, 2, and 3) | ||||
| N | 2 | 2 | 0 | 2 |
| GMC (95% CI) | 886.4 (58.3 - 1.3E+04) | 11728.7 (5.4 - 2.5E+07) | ---- | 174.2 (0.0 - 1.5E+06) |
| Four-fold Increase - %(95% CI) | 0 (0 - 84) | 100 (16 - 100) | ---- | 0 (0 - 84) |
| gMFI (95% CI) | 2.71 (0.03 - 259.75) | 7.88 (1.91 - 32.47) | ---- | 1.15 (0.07 - 18.67) |
| Day 180: Clinic Visit (Subjects receiving vaccination1 and 2 only) | ||||
| N | 6 | 5 | 2 | 0 |
| GMC (95% CI) | 1224.4 (284.8 - 5263) | 2379.3 (725.4 - 7804) | 5281.9 (0.0 - 7.7E+11) | ---- |
| Four-fold Increase - %(95% CI) | 33 (4 - 78) | 60 (15 - 95) | 50 (1 - 99) | ---- |
| gMFI (95% CI) | 3.08 (1.45 - 6.54) | 6.81 (0.97 - 47.65) | 5.38 (0.00 - >1000) | ---- |
| Day 236: Final Visit (Subjects receiving vaccination 1 and 2 only) | ||||
| N | 6 | 5 | 2 | 0 |
| GMC (95% CI) | 913.5 (236.1 - 3533) | 2172.2 (913.5 - 5165) | 4824.1 (0.0 - 1.6E+10) | ---- |
| Four-fold Increase - %(95% CI) | 17 (0 - 64) | 60 (15 - 95) | 50 (1 - 99) | ---- |
| gMFI (95% CI) | 2.30 (1.20 - 4.41) | 6.21 (1.36 - 28.40) | 4.92 (0.00 - >1000) | ---- |
As expected, the anti-J5 LPS IgG or IgM antibody levels did not change significantly over time for subjects in the placebo group (Tables 2 and 3).
Discussion
In recent years there has been a dramatic increase in the frequency and spread of MDR GNB accompanied by an increased mortality, length of hospital stay, and increased costs [29-31]. There also has been a collapse of the antibiotic research-and-development pipeline, with no new class of antimicrobials approved for treatment of GNB in over 40 years [3]. Since bacteria possess or can quickly develop resistance mechanisms to antibiotics [4, 5] it is likely that the useful lifespan of any new antimicrobial will be relatively short-lived. There have also been supply shortages of licensed antimicrobial agents [32]. Thus, there is an urgent need for alternative therapeutic approaches to the treatment of serious GNB infections. Indeed, the prevalence of MDR GNB is such a high priority security concern that it prompted a national strategy to develop new therapeutic measures and vaccines [33].
After decades of intensive work on immune modulators that target the host response, not one product is currently approved for adjunctive therapy for severe GNB infections. In contrast, the strategy of targeting bacteria with antibodies has been effective, likely because such antibodies are not subject to or drive the same mechanisms by which bacteria evade antimicrobials. They also avoid the risk of immune compromise of immune modulators that target the host response. Further, there may be synergistic interaction between immunoglobulins and antimicrobials [34].
While antibodies directed at the highly conserved lipid A portion of the LPS molecule buried within the bacterial outer membrane have not been protective, antibodies against the immunodominant LPS O-polysaccharide have afforded protection against lethal infection in multiple pre-clinical studies and have also been effective in human clinical studies [8-11]. GNB mutants that lacked O polysaccharide, thereby exposing a highly conserved glycolipid core, could induce antibodies that were highly protective against a broad range of clinically relevant GNB [35-37]. The anti-core endotoxin antibodies elicited by a heat-killed J5 E. coli vaccine provided significant protection when passively administered to patients with sepsis and shock caused by a broad range of GNB, and prevented the onset of GNB shock and death when given prophylactically to surgical patients admitted to intensive care units [14, 38]. Subsequent studies with anti-core glycolipid antibodies had mixed results, perhaps due to the failure to insure adequate levels of circulating antibody [39]. Multiple studies had found a positive correlation between the presence of anti-core LPS antibodies and better clinical outcomes [40-43]. In any event, the unacceptable reactogenicity of the heat-killed bacterial vaccine limited its further development.
Based on the robust protection reported by Ziegler et al [35], we prepared a subunit vaccine from the J5 mutant bacteria provided by the Braude laboratory. This J5dLPS/OMP vaccine was highly effective in multiple animal models of lethal GNB infection including neutropenic rat, cecal ligation/puncture, and murine pneumonia models when given actively or when the vaccine-induced antibodies were given passively [15-20]. While this vaccine was highly immunogenic in preclinical models of sepsis, in the absence of adjuvant it failed to induce a robust antibody response in a Phase 1 study [21].
CPG 7909, a CpG ODN TLR9 adjuvant, has been shown to enhance and accelerate the immune response in human subjects to a number of different antigens, and to be well-tolerated [23, 24]. Since the combination of J5dLPS/OMP vaccine and CPG 7909 was highly immunogenic and protective in preclinical studies [17], and did not cause dysbiosis in mice [44], we studied its safety, tolerability, and immunogenicity in the present study. We hypothesized that addition of CPG 7909 would enhance and accelerate the human response to the J5dLPS/OMP vaccine while being safe and well-tolerated. Since the study was prematurely terminated, there were insufficient data to formally evaluate the hypothesis for enhanced immunogenicity, but the data trended in this direction. As well, the limited data suggest that the vaccine formulations containing 250 or 500 μg CPG 7909 were safe and well tolerated. These findings will require a larger number of subjects for confirmation.
Anti-endotoxin vaccines against O polysaccharide or core glycolipid may be used to generate hyperimmune immunoglobulins for intravenous treatment of acute infections, as was done in the Ziegler et al study [35]. However, passive antibody therapies are hampered by the need to administer these therapies rapidly after the onset of infection in order to succeed. Furthermore, even with successful treatment, patients who survive sepsis suffer from long-term cognitive defects, disabilities, and increased mortality from events unrelated to the septic episode [45-48]. Thus, it is not sufficient to just treat sepsis, but it is highly desirable to develop a vaccine to prevent infection through active immunization. Of particular interest, the vaccine/adjuvant combination elicited an IgM antibody response at 14 days after the first immunization, the first time-point assayed, and persisted for 180 days. Previous studies found that acute trauma victims and ICU patients developed antibody responses to experimental GNB vaccines within as few as 7-14 days [49, 50]. Response and efficacy might be further improved by the addition of an adjuvant, such as a CpG ODN. Based on our limited results in this prematurely terminated study, the J5dLPS/OMP vaccine in conjunction with CPG 7909 might be of value to patients at risk of developing GNB infections later in their hospitalization, and merits further investigation.
Highlights.
A vaccine elicits antibodies against a highly conserved core region of GNB endotoxin.
This detoxified endotoxin vaccine with and without two different doses of CPG 7909 adjuvant was given to healthy subjects.
The adverse effects of those given vaccine + CPG 7909, vaccine alone or placebo were similar.
Addition of CPG 7909 appeared to increase and accelerate the IgG and IgM antibody responses to the core glycolipid.
This vaccine merits further investigation as an adjunct to therapy against GNB infections
Acknowledgments
This clinical trial was supported by federal funds from the NIAID/NIH, Department of HHS, under Vaccine Training Evaluation Unit Contract No. HHSN2722008000057C to the University of Maryland, Baltimore, Center for Vaccine Development. The authors acknowledge the strong and valuable support of the entire team at the Division of Microbiology and Infectious Diseases (DMID) at NIAID.
Abbreviations
- TSH
Thyroid Stimulating Hormone
- ECL
electrochemiluminescence
- GNB
Gram-negative bacteria
- MDR
multidrug resistant
- gMFI
geometric mean fold increase
- OMP
outer membrane protein
- dLPS
detoxified LPS
- CpG
Cytosine and guanosine triphosphate oligodeoxynucleotides joined by phosphorothioate
Footnotes
Conflict of Interest. A patent was issued to AC and colleagues for this vaccine.
Contributors: AC designed and conducted the study and prepared the manuscript; NG was the study nurse who enrolled subjects, administered the vaccine, followed up each of the subjects and maintained study documents. MB was the unblinded vaccinator who did not evaluate patient safety. LZ performed the antibody assays. RM and KB collated the data, prepared the figures and tables, and performed statistical analyses; CD reviewed all of the EKGs for safety.
ClinicalTrials.gov Identifier: NCT01164514
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alan S. Cross, Email: across@medicine.umaryland.edu.
Nancy Greenberg, Email: Ngreenbe@medicine.umaryland.edu.
Melissa Billington, Email: mbilling@medicine.umaryland.edu.
Lei Zhang, Email: lzhang@medicine.umaryland.edu.
Christopher DeFilippi, Email: cdefilip@medicine.umaryland.edu.
Ryan C. May, Email: rmay@emmes.com.
Kanwaldeep K. Bajwa, Email: kbajwa@emmes.com.
References
- 1.Magill SS, Edwards JR, Fridkin SK. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Survey of health care-associated infections. N Engl J Med. 2014 Jun 26;370(26):2542–3. doi: 10.1056/NEJMc1405194. [DOI] [PubMed] [Google Scholar]
- 2.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. National Hospital Discharge Survey Data Brief. 62. CDC; 2011. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals; pp. 1–8. [PubMed] [Google Scholar]
- 3.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:4. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, Johnston MD, et al. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS ONE. 2012p;7:e34953. doi: 10.1371/journal.pone.0034953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Costa VM, King CE, Kalan L, Morar M, Sung WWL, Schwarz C, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–61. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 6.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–62. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 7.Robbins JB, Schneerson R, Anderson P, Smith DH. The 1996 Albert Lasker Medical research Awards. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenza type b. Impact on public health and implications for other polysaccharide-based vaccines. JAMA. 1996;276:1181–5. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 8.Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, et al. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect Immun. 2011;79:4240–9. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins JB, Kubler-Kielb J, Vinogradov E, Mocca C, Pozsgay V, Shiloach J, et al. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc Natl Acad Sci U S A. 2009 May 12;106(19):7974–78. doi: 10.1073/pnas.0900891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryz SJ, Jr, Furer E, Cross AS, Wegmann A, Germanier R, Sadoff JC. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–6. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryz SJ, Jr, Cross AS, Sadoff JC, Furer E. Synthesis and characterization of Escherichia coli O18 O-polysaccharide conjugate vaccines. Infect Immun. 1990 Feb;58(2):373–77. doi: 10.1128/iai.58.2.373-377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryz SJ, Mortimer P, Cross AS, Furer E, Germanier R. Safety and immunogenicity of polyvalent Klebsiella capsular polysaccharide vaccine in humans. Vaccine. 1986;4:15–20. doi: 10.1016/0264-410x(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 13.Edelman R, Taylor KN, Wasserman SS, McClain JB, Cross AS, Sadoff JC, et al. Phase I trial of a 23-valent Klebsiella capsular polysaccharide vaccine and an eight valent Pseudomonas O-polysaccharide conjugate vaccine administered simultaneously. Vaccine. 1994;12:1288–94. doi: 10.1016/s0264-410x(94)80054-4. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler EJ, McCutchan JA, Fierer J, Glauser MP, Sadoff JC, Douglas H, et al. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–30. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharjee A, Opal SM, Taylor R, Naso R, Semenuk M, Zollinger WD, et al. A non-covalent complex vaccine prepared with detoxified Escherichia coli J5 (Rc chemotype) lipopolysaccharide and Neisseria meningitidis group B outer membrane protein produces protective antibodies against Gram-negative bacteremia. J Infect Dis. 1996;173:1157–63. doi: 10.1093/infdis/173.5.1157. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee AK, Opal SM, Palardy JE, Drabick JJ, Collins H, Taylor R, et al. Affinity-purified Escherichia coli J5 lipopolysaccharide specific IgG protects neutropenic rats against Gram-negative bacterial sepsis. J Inf Dis. 1994;170:622–29. doi: 10.1093/infdis/170.3.622. [DOI] [PubMed] [Google Scholar]
- 17.Opal SM, Palardy JE, Chen WH, Parejo NA, Bhattacharjee AK, Cross AS. Active immunization with a detoxified endotoxin vaccine protects against lethal polymicrobial sepsis: its use with CpG adjuvant and potential mechanisms. J Infect Dis. 2005;192:2074–80. doi: 10.1086/498167. [DOI] [PubMed] [Google Scholar]
- 18.Chen WH, Kang TJ, Bhattacharjee AK, Cross AS. Intranasal administration of a detoxified endotoxin vaccine protects against heterologous Gram-negative bacillary pneumonia. Innate Immunity. 2008;14:269–78. doi: 10.1177/1753425908095959. [DOI] [PubMed] [Google Scholar]
- 19.Neely AN, Bhattacharjee AK, Babcock GF, Holder IA, Cross AS. Differential effects of two different routes of immunization on protection against gram-negative sepsis by a detoxified E.coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine in a burned mouse model. J Burn Care Rehabil. 2002;23:333–40. doi: 10.1097/00004630-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cross AS, Opal SM, Warren HS, Palardy JE, Glaser K, Parejo NA, Bhattacharjee AK. Active immunization with a detoxified E.coli J5 lipopolysaccharide-group B meningococcal outer membrane protein complex vaccine protects animals from experimental sepsis. J Infect Dis. 2001;183:1079–86. doi: 10.1086/319297. [DOI] [PubMed] [Google Scholar]
- 21.Cross AS, Opal SM, Palardy JE, Drabick JJ, Warren HS, Huber C, et al. Phase I study of detoxified Escherichia coli J5 lipopolysaccharide/group B meningococcal outer membrane protein complex vaccine to human subjects. Vaccine. 2003;21:4576–87. doi: 10.1016/s0264-410x(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM, Davis HL. CpG ODN as a TH1 immune enhancer for prophylactic and therapeutic vaccines. In: Hackett CJ, Harn DA Jr, editors. Vaccine Adjuvants: Immunological and Clinical Principles. Vol. 6. 2005. pp. 87–110. [Google Scholar]
- 23.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46:1310–4. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
- 24.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 25.Søgaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a TLR9 agonist-adjuvant: A randomized trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 26.Ellis RD, Wu Y, Martin LB, Shaffer D, Miura K, Aebig J, et al. Phase 1 study in malaria naïve adults of BSAM2/Alhydrogel®+CPG 7909, a blood stage vaccine against P. falciparum malaria. PLoS One. 2012;7(10):e46094. doi: 10.1371/journal.pone.0046094. Epub 2012 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, et al. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine. 2013 Jun 26;31(30):3051–8. doi: 10.1016/j.vaccine.2013.04.063. Epub 2013 May 10 Vaccine 31 (2013): 3051-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter MM. Electroluminescence (ECL) Chem Rev. 2004;104:3003–36. doi: 10.1021/cr020373d. [DOI] [PubMed] [Google Scholar]
- 29.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–7. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 30.Neidel MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S, et al. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis. 2012;55:807–15. doi: 10.1093/cid/cis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century-a clinical superchallenge. N Engl J Med. 2009:439–42. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 32.Quadri F, Mazer-Amirshahi M, Fox ER, Hawley KL, Pines JM, Zocchi MS, et al. Antibacterial drug shortages from 2001-2013: Implications for clinical practice. Clin Infect Dis. 2015 Apr 22;pii:civ201. doi: 10.1093/cid/civ201. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Executive Order—National Strategy for Combating Antibiotic-Resistant Bacteria. Issued by the Office of the Press Secretary, The White House; Sep 18, 2014. [Google Scholar]
- 34.Manning MC, Galliardi LA, Fisher MW. The therapeutic activity of gamma globulin; chloramphenicol combinations in mouse infections due to Salmonella, pneumococci, Escherichia coli, or Pasteurella. Antibiot Annu. 1957-1958;5:566–71. [PubMed] [Google Scholar]
- 35.Ziegler EJ, Douglas H, Sherman JE, Davis CE, Braude AI. Treatment of E. coli and Klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-Gal epimerase-deficient mutant. J Immunol. 1973;111:433–38. [PubMed] [Google Scholar]
- 36.DeMaria A, Jr, Johns MA, Berberich H, McCabe WR. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988 Aug;158(2):301–11. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- 37.Chedid L, Parant M, Parant F, Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968;100:292–301. [PubMed] [Google Scholar]
- 38.Baumgartner JD, Glauser MP, McCutchan JA, Ziegler EJ, van Melle G, Klauber MR, et al. Prevention of Gram negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985;2(8446):59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- 39.Cross AS, Opal SM, Bhattacharjee AK, Peduzzi P, Donta S, Furer E, et al. Immunotherapy of sepsis: Flawed concept or faulty implementation? Vaccine. 1999;17:S13–S21. doi: 10.1016/s0264-410x(99)00230-3. [DOI] [PubMed] [Google Scholar]
- 40.Pollack M, Huang AI, Prescott RK, Young LS, Hunter KW, Cruess DF, et al. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983;72:1874–81. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nys M, Damas P, Joassin L, Lamy M. Sequential anti-core glycolipid immunoglobulin antibody activities in patients with and without septic shock and their relation to outcome. Ann Surg. 1993;217:300–6. doi: 10.1097/00000658-199303000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schedel I, Dreikhausen U, Nentwig B, Hockenschneider M, Rauthmann D, Balikcioglu S, Coldeway R, Deicher H. Treatment of Gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med. 1991;9:1104–13. doi: 10.1097/00003246-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Fomsgaard A, Baek L, Fomsgaard JS, Engquist A. Preliminary study on treatment of septic shock patients with antilipopolysaccharide IgG from blood donors. Scand J Infect Dis. 1989;21:697–708. doi: 10.3109/00365548909021700. [DOI] [PubMed] [Google Scholar]
- 44.Alaish SM, Mongodin EF, Zhang L, Murphy E, Cross A. Intestinal dysbiosis following cholestasis is reduced by active immunization with a detoxified endotoxin vaccine. J Bacter Mycol. 2015;2:1–5. [Google Scholar]
- 45.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–4. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 47.Kahn J, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospitalization after critical illness. JAMA. 2010;303:2253–59. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–63. [PubMed] [Google Scholar]
- 49.Campbell WN, Hendrix E, Cryz S, Jr, Cross AS. Immunogenicity of a 24-valent Klebsiella Capsular polysaccharide vaccine and an 8-valent Pseudomonas O-polysaccharide conjugate vaccine administered to acute trauma victims. Clin Infect Dis. 1931;23:179–81. doi: 10.1093/clinids/23.1.179. [DOI] [PubMed] [Google Scholar]
- 50.Intercell News release, November 9, 2010.


