Abstract
There is clear evidence that patients with type 2 diabetes mellitus (T2D) have increased fracture risk, despite having high bone mineral density (BMD) and body mass index (BMI). Thus, poor bone quality has been implicated as a mechanism contributing to diabetic skeletal fragility. Poor bone quality in T2D may result from the accumulation of advanced glycation end-products (AGEs), which are post-translational modifications of collagen resulting from a spontaneous reaction between extracellular sugars and amino acid residues on collagen fibers. This review discusses what is known and what is not known regarding AGE accumulation and diabetic skeletal fragility, examining evidence from in vitro experiments to simulate a diabetic state, ex vivo studies in normal and diabetic human bone, and diabetic animal models. Key findings in the literature are that AGEs increase with age, affect bone cell behavior, and are altered with changes in bone turnover. Further, they affect bone mechanical properties and microdamage accumulation, and can be inhibited in vitro by various inhibitors and breakers (e.g. aminoguanidine, N-Phenacylthiazolium Bromide, vitamin B6). While a few studies report higher AGEs in diabetic animal models, there is little evidence of AGE accumulation in bone from diabetic patients. There are several limitations and inconsistencies in the literature that should be noted and studied in greater depth including understanding the discrepancies between glycation levels across reported studies, clarifying differences in AGEs in cortical versus cancellous bone, and improving the very limited data available regarding glycation content in diabetic animal and human bone, and its corresponding effect on bone material properties in T2D.
Keywords: diabetes, bone strength, bone mechanics, bone quality, advanced glycation end-products
1. Introduction
Men and women with type 2 diabetes mellitus (T2D) have 20% to 3-fold increased fracture risk, depending on the skeletal site and severity of disease [1-5]. A systematic review of 16 observational studies including over 800,000 participants and 135,000 incident fractures found that T2D was associated with a 2-3 fold increased risk of hip fracture [5]. Whereas T2D is associated with a modest increase in overall fracture risk, the huge and growing number of persons with T2D renders this as a compelling clinical issue. Notably, among those aged 65 years and older, a group already at increased risk of fracture, prevalence of T2D exceeds 25% and is predicted to increase by 4.5-fold by 2050 [6].
The increased risk of fracture in T2D patients is paradoxical, given they tend to have normal to high bone mineral density (BMD) [7] and high body mass index (BMI), two factors which are generally associated with reduced fracture risk. Thus, several mechanisms have been proposed to contribute to the increased fracture risk seen in individuals with T2D, including an increased propensity to fall, deficits in bone microarchitecture, and poor bone quality. Notably, the increased fracture risk persists even after adjustment for a higher incidence of falls [8], implicating altered bone microarchitecture and/or poor quality as key factors. Interestingly, reports to date indicate relatively preserved trabecular bone, but increased cortical porosity in those with T2D (see review in this issue by Farr and Khosla for more detail).
Accumulation of advanced glycation end-products (AGEs) underlies the pathogenesis of many diabetic complications, and thus, we focus here on their possible role in diabetic skeletal fragility. It is now generally reported that AGEs accumulate in bone, stiffen the collagen matrix, and alter biomechanical properties of the bone matrix (see for example, a recent review by Saito and Marumo [9]). Numerous literature reviews have been conducted on the role of nonenzymatic glycation on diabetic skeletal fragility [10-16]. The primary conclusions in these reviews are that AGEs affect various factors including bone mineralization, material properties, microstructure, and microdamage accumulation, and that these factors may ultimately contribute to diabetic skeletal fragility. However, these conclusions should be evaluated in light of limited and some contradicting data in the literature. Our goal was to indicate the areas in which data is lacking and where data is inconsistent so that future work can complete these gaps in knowledge.
We conducted a literature search for English language articles in the PubMed database using the following keywords in various combinations: “diabetes,” “bone,” “aging,” “fracture,” “fracture risk,” “skeletal fragility,” “collagen,” “advanced glycation end-products,” “nonenzymatic glycation,” “in vitro,” “ex vivo,” “strength,” “mechanics,” “mechanical properties,” “cancellous,” “trabecular,” “cortical,” “microdamage,” “turnover,” “remodeling,” “breaker,” “inhibitor,” “review”. Approximately 100 relevant articles were reviewed to discuss the experimental evidence for a relationship between AGEs in bone and bone's biomechanical properties as investigated through in vitro experiments to simulate a diabetic state, and comparing these findings to ex vivo studies conducted in normal and diabetic human bone as well as in diabetic animal models.
2. Posttranslational modifications of collagen: enzymatic and non-enzymatic crosslinks
The main organic constituent of bone is type I collagen, comprised of two non-helical domains and a triple helical region. This structural protein is composed of three polypeptide chains with a very specific sequence of amino acids that allows the chains to wind into a triple helical structure (e.g. glycine-X-hydroxyproline with X representing an amino acid such as lysine). Amino acids that lie on the surface of the helix are involved in collagen crosslink formation [17].
Crosslinking, a prominent post-translational modification of collagen, occurs by two distinct processes: 1) enzymatic crosslinking, mediated by lysyl hydroxylase and lysyl oxidase; and 2) non-enzymatic crosslinking, mediated by glycation and/or oxidation. The enzymatic and non-enzymatic crosslinks differentially affect collagen stability and mechanical properties.
Enzymatic crosslinking requires an enzyme (e.g. lysyl oxidase) to create intra- or inter-fibrillar crosslinks [18]. During the process, bivalent crosslinks transform into trivalent and stable crosslinks. Two commonly assessed enzymatic crosslinks, deoxypyridinoline and pyridinoline, represent collagen maturity and are bone resorption markers [19]. These crosslinks increase collagen fibril stiffness and contribute to increased tissue strength [20, 21]. Enzymatic crosslinks are typically characterized by high performance liquid chromatography (HPLC) [22], mass spectrometry [23], and Fourier transform infrared spectroscopy [24].
AGEs are produced by non-enzymatic glycation (NEG), which is an irreversible and spontaneous biochemical reaction that occurs between free-floating sugars and exposed amino acid residues on proteins [21, 25]. This process occurs in various proteins including hemoglobin, albumin, osteocalcin, and collagen, among others [26, 27]. NEG incorporates a biochemical reaction between the ε-amino group of lysine or hydroxylysine and an aldehyde group of a sugar such as glucose. This reaction forms glucosyl-lysine, a product that then experiences additional reactions to form a Schiff base adduct or an Amadori product. These intermediate products endure further biochemical reactions to eventually create post-translational modifications of collagen (AGEs) that accumulate in numerous tissues including tendons, skin, cartilage, and bone [28]. AGEs include both crosslinking modifications that form within or across collagen fibers (e.g. pentosidine, vesperlysines, crossline) and non-crosslinking modifications (e.g. carboxymethyllysine, carboxyethyllysine, pyrraline) [21].
3. Assessment of AGEs in bone
The two methods available for quantifying AGEs in bone are based on measuring AGE fluorescence, and thus require a specimen of bone. Pentosidine is the single AGE that has been isolated and measured in bone specimens, and is quantified by HPLC [28, 29]. Current HPLC methods use lyophilized and acid-hydrolyzed bone samples in which pentosidine is separated from enzymatic crosslinks via a solid phase extraction column and is then subsequently quantified with a fluorescence detector [28]. Pentosidine amounts are normalized to the amount of collagen present in the sample, which is estimated from hydroxyproline content. Thus pentosidine content is typically expressed in units of mmol / mol collagen. However, pentosidine composes <1% of total fluorescent AGEs in bone [30], and is only weakly correlated to the amount of total fluorescent AGEs in human cortical and cancellous bone [31]. Thus, it is important to also measure total fluorescent AGEs rather than pentosidine alone.
The second technique quantifies the bulk fluorescence of AGEs from papain-digested or acid-hydrolyzed bone samples relative to a quinine sulfate standard [32, 33]. The amount of quinine-based fluorescence is normalized to the amount of collagen present in the sample, which is estimated from hydroxyproline content [34], and thus total fluorescent AGE content is usually expressed in units of ng quinine / mg collagen. The fluorometric assay utilizes wavelengths (370/440 nm excitation/emission) that encompass the excitation and emission spectra of several crosslinking and non-crosslinking AGEs such as pentosidine (335/385 nm excitation/emission), crossline (379/400 nm excitation/emission), vesperlysines A and B (366/442 nm excitation/emission), vesperlysine C (345/405 nm excitation/emission), carboxymethyllysine (345/455 nm excitation/emission), and carboxyethyllysine (345/455 nm excitation/emission) [29, 35-38]. However, the contributions of each of these crosslinks to the total fluorescence cannot be determined from this assay and are currently unknown. Furthermore, although the fluorescence spectra for enzymatic crosslinks, pyridinoline and deoxypyridinoline, are known (297/395 nm excitation/emission), to our knowledge no work has been conducted to clarify the extent to which these crosslinks may be captured using the fluorometric assay.
4. Differences in AGEs in human cortical versus cancellous bone
Two studies have compared differences in AGE levels between cancellous and cortical bone and showed differing results. Odetti et al. reported greater pentosidine content in cortical compared to cancellous bone, possibly due to higher turnover rates in cancellous bone that results in increased removal of AGEs [39]. In contrast, Karim et al. reported that there were more total fluorescent AGEs and pentosidine in cancellous than in cortical bone, and this was attributed to the greater surface-to-volume ratio in cancellous bone that allows for increased access of sugars to form AGEs [31]. Further studies are needed to determine whether there are differences in glycation content between cortical and cancellous bone.
5. Age-related changes in AGEs in human bone
AGE contents reported in the literature are highly variable (Table 1). For instance, two studies report extremely low concentrations of cortical bone pentosidine: ~0.44-1.39 mmol / mol collagen (19-89 years) [40] and ~56.7×10−8-62.0×10−8 mmol / mol collagen (34-92 years) [39], while another reports much higher values ranging from ~0.1-60 mmol / mol collagen (18-97 years) [31]. One study reported a very low concentration of cancellous bone pentosidine ranging from ~4.5×10−8-16.3×10−8 mmol / mol collagen (34-92 years) [39], while two others report higher values ranging from ~0.1-70 mmol / mol collagen (18-97 years) [31, 33]. Only two of these studies reported total fluorescent AGE content ranging from ~0.1-330 ng quinine / mg collagen in cortical bone and ~0.1-2000 ng quinine / mg collagen in cancellous bone (18-97 years) [31, 33], and these values are on the same order of magnitude as several other reports in bovine, murine, and human bone [32, 41, 42]. The reasons for vastly different ranges in glycation content among the various studies are not completely clear.
Table 1.
Pentosidine and/or total fluorescent advanced glycation end-product (AGEs) quantities measured in various models. Quantities are reported as average ± standard deviation or range of values unless otherwise noted. Values estimated from figures are preceded by (~).
| Model/bone | Genders included (n) | In vitro induced? | Age | Group description | Cortical Pentosidine (mmol / mol collagen) | Cortical AGEs (ng quinine / mg collagen) | Cancellous pentosidine (mmol / mol collagen) | Cancellous AGEs (ng quinine / mg collagen) | References |
|---|---|---|---|---|---|---|---|---|---|
|
In vitro glycation levels
| |||||||||
| Human/femur | Female (n=8) |
Yes | 42—97 yr | Vehicle | -- | -- | -- | 169.9 ± 120.7 | [41] |
| Glycated | -- | -- | -- | 322.4 ± 256.5 | |||||
| Bovine/femur | ? (n=22) |
Yes | 18 mo | Vehicle | -- | 75.9 ± 14.8 | -- | -- | [32] |
| Glycated | -- | 1274 ± 141.3 |
-- | -- | |||||
|
In vivo glycation levels in human bone | |||||||||
| Human/femur | Female (n=32 cortical, n=50 cancellous) |
No | 7—86 yr | Hip fracture, low bone density | 24.1 ± 16.7 | -- | 19.1 ± 12.5 | -- | [65, 66] |
| No fracture, low bone density | 4.1 ± 2.5 | -- | 4.7 ± 1.8 | -- | |||||
| Hip fracture, high bone density | 13.3 ± 8.0 | -- | 15.8 ± 10.3 | -- | |||||
| No fracture, high bone density | 7.8 ± 3.5 | -- | 7.7 ± 2.4 | -- | |||||
| Human/femur | Both (n=30) |
No | 19—89 yr | Young | 0.44 ± 0.21 | -- | -- | -- | [40] |
| Middle aged | 0.90 ± 0.23 | -- | -- | -- | |||||
| Elderly | 1.39 ± 0.29 | -- | -- | -- | |||||
| Human/femur,tibia | Both (n=104) |
No | 34—92 yr | -- | 59.42×10−8 ± 2.63×10−9 |
-- | 10.41×10−8 ± 5.9×10−10 |
-- | [39] |
| Human/femur,tibia | Both (n=170) |
No | 18—97 yr | -- | ~0.1—60 | ~0.1—330 | ~0.5—70 | ~0.1—2000 | [31] |
| Human/tibia | Both (n=17) |
No | 51—90 yr | Middle aged | 0.3 ± 0.1 | -- | -- | -- | [53] |
| Elderly | 0.5 ± 0.1 | -- | -- | -- | |||||
| Human/tibia | Both (n=42) |
No | 18—97 yr | -- | -- | -- | ~0.1—50 | ~0.1—1500 | [33] |
| Human/tibia | Male (n=20) |
No | 45—80 yr | Non-diabetic | -- | -- | ~7 ± 2* | -- | [52] |
| Diabetic | -- | -- | ~9 ± 3* | -- | |||||
| Human/vertebrae | Both (n=49) |
No | 54—95 yr | -- | -- | -- | 20.4 ± 9.4 | -- | [55] |
| Human/vertebrae | Both (n=19) |
No | 26—93 yr | -- | 75 ± 126 | -- | 30 ± 42 | -- | [28] |
| Human/vertebrae (individual trabeculae) | Both (n=32) |
No | 54—94 yr | -- | -- | -- | 2.25 (median) |
-- | [54] |
|
In vivo glycation levels in animal bone | |||||||||
| Monkey/vertebrae | Female (n=76) |
No | 12 ± 1.5 yr | Sham | -- | -- | 1.1 ± 0.4 | -- | [50] |
| OVX | -- | -- | 1.8 ± 1.1 | -- | |||||
| Low-PTH treatment | -- | -- | 1.0 ± 0.5 | -- | |||||
| High-PTH treatment | -- | -- | 0.8 ± 0.3 | -- | |||||
| Mouse/femur | Male (n=36) |
No | 8 wk | Non-diabetic SWR mouse | -- | ~225 ± 50 | -- | -- | [42] |
| Diabetic Tallyho mouse | -- | ~275 ± 75 | -- | -- | |||||
| 17 wk | Non-diabetic SWR mouse | -- | ~213 ± 50 | -- | -- | ||||
| Diabetic Tallyho mouse | -- | ~225 ± 50 | -- | -- | |||||
| Rat/femur | Male (n=30) |
No | ? | Non-diabetic Sprague-Dawley rat | -- | 14.8 ± 0.7× | -- | -- | [67] |
| Type 1 diabetic Sprague-Dawley rat | -- | 27.3 ± 4.8× | -- | -- | |||||
| Rat/femur | Male (n=41) |
No | 11—12 wk |
Non-diabetic Sprague-Dawley rat | 44 ± 12 | -- | -- | -- | [68] |
| Type 1 diabetic Sprague-Dawley rat | 65 ± 38 | -- | -- | -- | |||||
| Rat/femur | Male (n=70) |
No | 0—18 mo | Non-diabetic Wistar rat | ~0.01—0.35 | -- | -- | -- | [64] |
| Diabetic WBN/Kob rat | ~0.01—0.8 | -- | -- | -- | |||||
Study does not explicitly state whether pentosidine content is measured from cortical or cancellous bone, only that it is measured in a specimen from the tibial plateau.
Units are expressed in fluorescence units / mg collagen
Despite the vastly different values reported, all of these studies indicate that there is an increase in AGEs with age within a very wide age range (~20-100 years) [31, 33, 39, 40]. However, only 2 of these studies investigated how the age-related changes in AGEs vary between cancellous and cortical bone. Odetti et al. reported that pentosidine increased exponentially with age from ~56.7×10−8 to 62.0×10−8 mmol / mol collagen in human cortical bone from femurs and tibias, but this trend was not evident in cancellous bone [39]. The authors suggested that the lack of age-related changes in cancellous bone may be attributed to the more variable levels of pentosidine in cancellous than in cortical bone, which may make it difficult to observe relationships. In comparison, Karim et al. reported that total fluorescent AGEs but not pentosidine increased with age in cortical bone, from ~0.1-330 ng quinine / mg collagen, whereas AGEs did not increase with age in cancellous bone [31].
6. Influence of AGEs on bone cells
Studies indicate that AGEs may alter the behavior of bone cells, which in turn may influence bone biomechanical properties. Specifically, in vitro studies indicate that AGEs decrease osteoblast activity by impairing adhesion of osteoblasts to the collagen matrix [43], inhibiting osteoblast proliferation and differentiation [44], and decreasing mRNA expression of key osteoblastic products [45]. Additionally, in vitro studies have shown that AGEs can affect osteoclast behavior, although there are contradictory findings. Specifically, one study showed that resorption was significantly inhibited in bone treated with pentosidine in vitro [46], while another found that there were more and larger resorption pits in bone areas containing high in vivo AGE levels [47]. Further, only a single study has examined the effect of glycation on osteocyte behavior. This study showed that treatment of an osteocytic cell line (MLO-Y4-A2) with laboratory-synthesized AGEs increased SOST and decreased RANKL expression [48]. Further work is needed to characterize the effect of naturally occurring AGEs versus chemically-induced AGEs on bone cell behavior and the implications for bone biomechanical properties.
7. Effect of bone turnover on AGE accumulation
Alterations in bone turnover may influence accumulation of AGEs. In adult beagles after one year treatment with bisphosphonates, cortical bone AGE content was unchanged using doses equivalent to those used in postmenopausal women, but was increased following treatment at doses 5-fold higher than the standard clinical dose [49]. In comparison, increased bone turnover due to once-weekly parathyroid hormone treatment for 18 months in ovariectomized monkeys led to a decrease in pentosidine content [50]. However, to our knowledge, no studies have been conducted investigating the effect of bone turnover modulation via anabolic or anti-resorptive treatment on AGEs in humans.
8. Effect of AGEs on bone's biomechanical behavior
8.1. A review of basic bone biomechanics
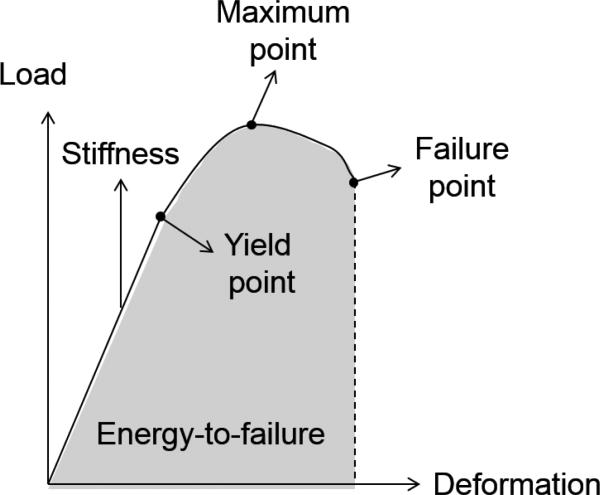
Key biomechanical properties describing bone's monotonic behavior are based on the relationship between applied loads and the resulting deformation in bone. Two important measures are stress and strain where stress is defined as force per unit area (intensity of the applied force) and strain is defined as the change in length divided by the initial length (normalized deformation). Many important mechanical variables are calculated from the load-deformation or stress-strain curves acquired from a mechanical test (Figure 1). In performing a test of a whole bone, structural properties are assessed as follows: load and deformation are linearly related until the yield point is reached, and the slope of the curve in the pre-yield region represents bone stiffness. Loading beyond the yield point results in permanent deformation upon removal of the load. In the post-yield region the ultimate or failure load for bone is reached, and the energy needed to result in failure is calculated as the area under the load-displacement curve. Materials that are brittle undergo little deformation prior to failure, while materials that are ductile undergo significant deformation prior to failure. In comparison, the intrinsic properties of bone material are derived from a stress-strain curve that is generated by conducting a mechanical test on a specimen that has a standardized shape. The slope of the stress-strain curve up until the yield point is known as the elastic modulus. After this point, the bone undergoes irrecoverable plastic strain. The maximum stress (or strength) occurs when the bone nears the ultimate or failure point, and finally the ultimate stress and strain are the points at which the bone breaks. The total area under the load-displacement curve represents the toughness of the specimen, or its ability to absorb energy until failure.
Figure 1.
Schematic diagram of a load-deformation curve acquired from a mechanical test.
8.2. Influence of AGEs on bone biomechanics
The pre-yield mechanical behavior of bone tissue is largely influenced by its mineral composition while post-yield mechanical properties are influenced by the organic matrix [51]. Because NEG directly modifies the organic matrix, it is likely that primarily the post-yield properties of bone will be affected by NEG, although there is evidence that NEG can affect pre-yield mechanical behavior as well.
A few in vitro studies have incubated bones in a sugar solution to simulate a diabetic state. The incubation process incorporates a sugar in the presence of protease inhibitors in a buffer solution, at physiological temperature and pH [41]. Very few in vitro studies have been conducted, and they suggest the mechanical responses to in vitro glycation may be different in cancellous and cortical bone. Specifically, in vitro glycation of bovine cortical bone specimens for 38 days resulted in approximately 17-fold greater AGE content in glycated versus vehicle bone (vehicle: 75.9 ± 14.8 ng quinine / mg collagen, glycated: 1274 ± 141.3 ng quinine / mg collagen). In vitro glycation resulted in stiffer collagen fibers (i.e. equilibrium modulus assessed in demineralized bone), greater yield stress and strain, less post-yield displacement, but similar post-yield strain energy compared to vehicle-treated specimens [32]. The AGE levels in the vehicle group were on the same order of magnitude as reported in a study conducted in human bone, but the in vitro induced levels were much greater than that observed in human bone [31], and thus the physiologic relevance of these results are unclear.
In comparison, in vitro glycation of human cancellous bone specimens for 7 days resulted in approximately 2-fold greater AGE content in glycated versus vehicle bone (vehicle: 169.9 ± 120.7 ng quinine / mg collagen, glycated: 322.4 ± 256.6 ng quinine / mg collagen). This study suggests that a 7-day incubation of human cancellous bone specimens in a 0.6 M ribose solution produces AGE levels equivalent to that measured after approximately 2-3 decades of natural aging. Specifically, the authors report that the in vitro induced AGE content in bone specimens from a 42 year old donor had similar AGE levels as in vehicle-incubated bone specimens from a 74 year old donor. Glycated trabecular bone specimens had similar yield stress and strain, but lower post-yield strain energy than vehicle-treated specimens [41]. These two investigations were conducted in different models (i.e. bovine versus human), making it difficult to compare results. Furthermore, the clinical relevance of the in vitro induced AGE levels is difficult to deduce as these values cannot be compared to glycation content in diabetic human bone, as the only study that measured glycation in diabetic bone assessed pentosidine content but not total fluorescent AGEs [52].
Ex vivo studies using normal human bone report a negative relationship between AGE levels and post-yield bone mechanical properties. For example, total fluorescent AGEs and pentosidine content in human cancellous bone specimens from the tibial plateau (age range 18-97 years) is negatively associated with ultimate strain (r = −0.38 and r = −0.44, respectively) [33], while pentosidine content in human cortical bone specimens from the femoral midshaft (age range 19-89 years) and from the tibial midshaft (age range 51-90 years) are negatively associated with ultimate stress and fracture toughness [40, 53]. Another investigation similarly reports that pentosidine content is weakly correlated (r = -−.3) with ultimate strain of individual trabeculae extracted from human vertebrae (age range 54-94 years) [54]. However, one ex vivo study using human vertebral bone (age range 54-94 years) shows contradictory results, reporting that glycation content does not influence bone biomechanical properties as suggested by previously described studies (i.e. no relationship between pentosidine and compressive biomechanical properties) [55].
8.3. Effect of AGE content on microdamage accumulation
A few studies have reported that AGEs influence microdamage accumulation. For instance, in dogs treated with the bisphosphonate incadronate for 3 years, pentosidine content was positively associated with microdamage accumulation in ribs (e.g. crack density r2 = 40%, crack length r2 = 22%) [56]. In vitro glycation of human cancellous bone leads to increased microcracks following compressive loading [57], and higher levels of AGEs are reported in regions with increased amounts of crack-like microdamage [33]. Although increased NEG appears to be associated with increased microdamage content, further work is needed to elucidate the relevance of this microdamage accumulation to overall skeletal fragility.
8.4. AGE inhibitor and reversal of mechanical property degradation
Work is now being done to investigate therapeutic interventions to inhibit AGE formation or to cleave existing AGEs, as several AGE inhibitors and breakers have been described, including aminoguanidine, N-Phenacylthiazolium Bromide (PTB), and pyridoxamine (vitamin B6) [58]. However, very few studies have been conducted using these agents in bone tissue. One study showed that whereas aminoguanidine treatment reduced the pentosidine content induced by in vitro glycation of bovine cortical bone, it did not influence hardness assessed by microindentation or mechanical properties assessed by 3-point bending [59]. In contrast, a recent study showed that human cancellous bone specimens treated with PTB in vitro resulted in decreased AGE content and a corresponding increase in ductility (e.g., post-yield strain) [60]. It has also been shown that vitamin B6 inhibits AGEs [61, 62]. Specifically, there is decreased pentosidine content in diabetic rats treated with vitamin B6 with a corresponding increase in elastic modulus and toughness [62]. Further work needs to be done to determine whether AGE inhibitors and breakers can alter bone mechanical properties in animal models and/or in human bone.
9. AGEs and diabetic skeletal fragility
Studies conducted in diabetic animal models play an important role in the goal of identifying the underlying mechanisms of diabetic skeletal fragility. However, diabetic rodent models have some limitations such as having low bone mass and/or the onset of diabetes occurring before reaching skeletal maturity, which is in contrast to what occurs in adult humans [63]. With these limitations in mind, Saito et al. reported that diabetic WBN/Kob rats have a sharp increase in bone pentosidine content at the onset of diabetes, associated with a lower femoral bending stiffness (pre-yield mechanical property), energy absorption, and maximum load compared to controls, despite having no difference in BMD [64]. In Tallyho mice, a model of early onset T2D, total fluorescent AGEs were increased in femoral cortical bone, while femoral maximum load was higher and post-yield deformation lower (suggesting increased brittleness) than non-diabetic controls [42]. Additionally, Tallyho mice had increased indentation distance as measured by cyclic reference point indentation, suggesting that the diabetic bone matrix is more susceptible to cracks propagating into the bone after multiple loading cycles.
Detailed characterization of AGEs in bone from patients with diabetes is currently lacking. To our knowledge, only one study has reported AGE levels in bone patients with T2D, finding ~30% higher pentosidine levels in cortico-cancellous bone specimens from men with T2D versus non-diabetics (9 ± 3 vs 7 ± 2 mmol / mol collagen).
10. Comparison of AGE levels in bone specimens exposed to in vitro glycation, diabetic animal models, and bone tissues from T2D patients
It is important to compare AGE levels measured in animal studies and in vitro glycation studies to quantities in diabetic human bone. As previously mentioned, total fluorescent AGEs and pentosidine content reported in the literature (Table 1) are highly variable. These differences are likely the result of several factors including: animal model, bone type, location of specimen within the bone, gender, and age range, but further work needs to be done to explain these differences.
In vitro induced glycation in human cancellous bone (7-day incubation) shows AGE levels similar to that measured after 2-3 decades of natural aging [41], but in vitro induced AGE levels in bovine cortical bone (38-day incubation) results in AGE values beyond that observed in human bone [31, 32]. These values are higher than that observed in human bone even after only ~7 days of the total 38-day incubation. Further, the in vitro induced quantities are difficult to compare to that in diabetic human bone because the single study that assessed glycation in diabetic human bone measured only pentosidine, but not total fluorescent AGEs [52]. Given the weak relationship between pentosidine and total fluorescent AGEs [31], it is difficult to extrapolate and compare the extent of glycation across these studies.
Of the two studies reported here on glycation content in T2D animal models, one assessed total fluorescent AGEs [42]. The other assessed pentosidine in cortical bone, which reported ~23-fold lower pentosidine content than that measured in diabetic human bone [52, 64]. Furthermore, in the study on diabetic human bone specimens, findings are difficult to interpret as it is unclear whether the specimens were cortical or cancellous bone or a mixture of both. [52].
11. Summary and Recommendations for Future Research
Overall there are several important points that are well-known regarding non-enzymatic glycation in bone: 1) AGEs increase with age, 2) glycation is associated with altered osteoblast activity, 3) glycation content is not affected by treatment with a clinically relevant dose of bisphosphonate, but increases with high bisphosphonate doses and decreases with parathyroid hormone treatment as shown in animal models, 4) in vitro glycation alters bone biomechanical properties and influences the type and extent of microdamage formed, 5) AGE-inhibitors tested in vitro can decrease AGE accumulation and restore biomechanical properties as shown in animal models, and 6) bone from diabetic rodents has higher AGE content than non-diabetic controls with corresponding changes in mechanical properties.
However, our review indicates there are still many gaps in knowledge and inconsistencies in reported results that require further investigation. Specifically, the following points are still unknown and/or need to be clarified: 1) the reasons why there are drastic differences in glycation levels across the various published studies, 2) whether glycation content differs between cancellous and cortical bone as the two studies that have compared bone types show opposite findings, 3) how glycation affects osteoclast and osteocyte behavior, 4) how bone cell behavior is affected by chemically-induced versus naturally-produced AGEs, 5) whether AGE levels change in patients treated with osteoporosis drugs (as this has only been assessed in animal models), 6) whether AGEs are increased in patients with T2D and how AGE levels are influenced by T2D duration and/or severity, and 7) how glycation levels induced in vitro or in diabetic animal models relate to levels in human diabetic bone.
In summary, despite a few reports of altered cortical bone microarchitecture in T2D, there are still several open questions regarding the possible mechanisms underlying skeletal fragility in T2D. While altered bone quality has been suggested as a possible contributor to diabetic skeletal fragility, additional research is needed. In particular, further studies of bone tissue from patients with T2D and assessment of the biomechanical consequences of accumulation of physiologically-relevant levels of AGEs are warranted. Our current limited understanding of mechanisms underlying diabetic skeletal fragility impedes development of guidelines to assess fracture risk and to determine optimal treatments to prevent fractures in this population.
Highlights.
Diabetics have increased fracture risk despite having high BMD, implicating poor bone quality as one mechanism of diabetic skeletal fragility.
Poor bone quality in diabetics may result from the accumulation of advanced glycation end-products (AGEs).
It is generally reported that AGEs accumulate in bone, stiffen the collagen matrix, and alter bone's biomechanical properties.
The effect of AGEs on bone mechanics should be assessed in light of limited and some contradicting data in literature.
Acknowledgements
This work was supported by a pilot and feasibility grant from the Boston Area Diabetes and Education Center (NIH P30 DK057521).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–10. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone. 2015 doi: 10.1016/j.bone.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Laguna D, Tebe C, Javaid MK, Nogues X, Arden NK, Cooper C, Diez-Perez A, Prieto-Alhambra D. Incident type 2 diabetes and hip fracture risk: a population-based matched cohort study. Osteoporos Int. 2015;26:827–33. doi: 10.1007/s00198-014-2986-9. [DOI] [PubMed] [Google Scholar]
- 5.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 6.Kirkman SM, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–56. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TT, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57:2057–65. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito M, Marumo K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif Tissue Int. 2015 doi: 10.1007/s00223-015-9985-5. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis International. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 11.McCabe LR, Zhang J, Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2011;21:187–206. doi: 10.1615/critreveukargeneexpr.v21.i2.70. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus. Endocrine J. 2011;58:613–24. doi: 10.1507/endocrj.ej11-0063. [DOI] [PubMed] [Google Scholar]
- 13.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–7. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 14.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012;19:128–35. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes. 2013;4:101–13. doi: 10.4239/wjd.v4.i4.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dede AD, Tournis S, Dontas I, Trovas G. Type 2 diabetes mellitus and fracture risk. Metabolism. 2014;63:1480–90. doi: 10.1016/j.metabol.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Vashishth D. Advanced Glycation End-products and Bone Fractures. IBMS BoneKEy. 2009;6:268–278. doi: 10.1138/20090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnero P. The contribution of collagen crosslinks to bone strength. BoneKEy Rep. 2012;1:182. doi: 10.1038/bonekey.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep. 2012;10:141–50. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Depalle B, Qin Z, Shefelbine SJ, Buehler MJ. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater. 2014 doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 22.Sroga GE, Vashishth D. UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. Journal of Chromatography B. 2011;879:379–85. doi: 10.1016/j.jchromb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyre DR, Weis MA, Wu JJ. Advances in collagen cross-link analysis. Methods. 2008;45:65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011;469:2170–8. doi: 10.1007/s11999-010-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 26.Neelofar K, Ahmad J. Amadori albumin in diabetic nephropathy. Indian J Endocrinol Metab. 2015;19:39–46. doi: 10.4103/2230-8210.146863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundberg CM, Anderson M, Dickson I, Gallop PM. “Glycated” Ostecalcin in Human and Bovine Bone. Journal of Biological Chemistry. 1986;261:14557–14561. [PubMed] [Google Scholar]
- 28.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–9. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- 30.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–60. [PubMed] [Google Scholar]
- 31.Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24:2441–7. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 33.Karim L, Vashishth D. Heterogeneous Glycation of Cancellous Bone and its Association with Bone Quality and Fragility. PLoS ONE. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. Journal of Experimental Medicine. 1958;107:247–263. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–9. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Nakazawa Y, Ienaga K. Acid-stable fluorescent advanced glycation end products: vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem Biophys Res Commun. 1997;232:227–30. doi: 10.1006/bbrc.1997.6262. [DOI] [PubMed] [Google Scholar]
- 37.Obayashi H, Nakano K, Shigeta H, Yamaguchi M, Yoshimori K, Fukui M, Fujii M, Kitagawa Y, Nakamura N, Nakamura K, Nakazawa Y, Ienaga K, Ohta M, Nishimura M, Fukui I, Kondo M. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun. 1996;226:37–41. doi: 10.1006/bbrc.1996.1308. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19338–45. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 39.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Annals of the New York Academy of Sciences. 2005;1043:710–717. doi: 10.1196/annals.1333.082. DO - 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 41.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devlin MJ, Van Vliet M, Motyl K, Karim L, Brooks DJ, Louis L, Conlon C, Rosen CJ, Bouxsein ML. Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology. 2014;155:3806–16. doi: 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol. 2004;36:840–8. doi: 10.1016/j.biocel.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–58. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 45.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008;1126:166–72. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 46.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 47.Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49:174–83. doi: 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461:193–9. doi: 10.1016/j.bbrc.2015.02.091. [DOI] [PubMed] [Google Scholar]
- 49.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporosis International. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, Kuroda T. Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1-34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int. 2011;22:2373–83. doi: 10.1007/s00198-010-1454-4. [DOI] [PubMed] [Google Scholar]
- 51.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am. 1975;57:956–961. [PubMed] [Google Scholar]
- 52.Oren TW, Botolin S, Williams A, Bucknell A, King KB. Arthroplasty in veterans: Analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. The Journal of Rehabilitation Research and Development. 2011;48:1195. doi: 10.1682/jrrd.2010.09.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007;25:646–55. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez CJ, van Der Ham F, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and nonenzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–832. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Follet H, Viguet-Carrin S, Burt-Pichat B, Depalle B, Bala Y, Gineyts E, Munoz F, Arlot M, Boivin G, Chapurlat RD, Delmas PD, Bouxsein ML. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011;29:481–8. doi: 10.1002/jor.21275. [DOI] [PubMed] [Google Scholar]
- 56.Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporosis International. 2008;19:1343–54. doi: 10.1007/s00198-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 57.Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010;46:148–154. doi: 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549–59. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viguet-Carrin S, Farlay D, Bala Y, Munoz F, Bouxsein ML, Delmas PD. An in vitro model to test the contribution of advanced glycation end products to bone biomechanical properties. Bone. 2008;42:139–49. doi: 10.1016/j.bone.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 60.Bradke BS, Vashishth D. N-phenacylthiazolium bromide reduces bone fragility induced by nonenzymatic glycation. PLoS One. 2014;9:e103199. doi: 10.1371/journal.pone.0103199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culbertson SM, Vassilenko EI, Morrison LD, Ingold KU. Paradoxical impact of antioxidants on post-Amadori glycoxidation: Counterintuitive increase in the yields of pentosidine and Nepsilon-carboxymethyllysine using a novel multifunctional pyridoxamine derivative. J Biol Chem. 2003;278:38384–94. doi: 10.1074/jbc.M305099200. [DOI] [PubMed] [Google Scholar]
- 62.Saito M, Shoshi S, Tanaka T, Fujii K. Effects of vitamin B6 and vitamin K2 on bone mechanical properties and collagen cross-links in spontaneously diabetic WBN/Kob rats. J Bone Miner Res. 2005;20:S190–S313. [Google Scholar]
- 63.Fajardo RJ, Karim L, Calley VI, Bouxsein ML. A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res. 2014;29:1025–40. doi: 10.1002/jbmr.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporosis International. 2006;17:1514–1523. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 65.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcified Tissue International. 2006;79:160–168. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 66.Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986–95. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 67.Tomasek JJ, Meyers SW, Basinger JB, Green DT, Shew RL. Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci. 1994;55:855–61. doi: 10.1016/0024-3205(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 68.Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, Wang X. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res. 2009;24:1618–27. doi: 10.1359/JBMR.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]