Abstract
Attention-deficit/hyperactivity disorder (ADHD) is comorbid with cocaine abuse. Whereas initiating ADHD medication in childhood does not alter later cocaine abuse risk, initiating medication during adolescence may increase risk. Preclinical work in the Spontaneously Hypertensive Rat (SHR) model of ADHD found that adolescent methylphenidate increased cocaine self-administration in adulthood, suggesting a need to identify alternatively efficacious medications for teens with ADHD. We examined effects of adolescent d-amphetamine treatment on strategy set shifting performance during adolescence and on cocaine self-administration and reinstatement of cocaine-seeking behavior (cue reactivity) during adulthood in male SHR, Wistar-Kyoto (inbred control), and Wistar (outbred control) rats. During the set shift phase, adolescent SHR needed more trials and had a longer latency to reach criterion, made more regressive errors and trial omissions, and exhibited slower and more variable lever press reaction times. d-Amphetamine improved performance only in SHR by increasing choice accuracy and decreasing errors and latency to criterion. In adulthood, SHR self-administered more cocaine, made more cocaine-seeking responses, and took longer to extinguish lever responding than control strains. Adolescent d-amphetamine did not alter cocaine self-administration in adult rats of any strain, but reduced cocaine seeking during the first of seven reinstatement test sessions in adult SHR. These findings highlight utility of SHR in modeling cognitive dysfunction and comorbid cocaine abuse in ADHD. Unlike methylphenidate, d-amphetamine improved several aspects of flexible learning in adolescent SHR and did not increase cocaine intake or cue reactivity in adult SHR. Thus, adolescent d-amphetamine was superior to methylphenidate in this ADHD model.
Keywords: Adolescence, Attention deficit/hyperactivity disorder, Cocaine cue reactivity, d-Amphetamine, Spontaneously Hypertensive Rat, Strategy set shifting task
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a widely prevalent neurodevelopmental disorder, affecting up to 11% of children and adolescents [1]. From 2003 to 2011, ADHD diagnoses rose 42%, with the largest increase observed in adolescents aged 14–17 [1,2]. Today, nearly one in five high school-age boys have an ADHD diagnosis [2]. Individuals with ADHD are at high risk for developing substance dependence. Comorbidity estimates of ADHD and substance use disorder (SUD) range from 9.5% up to 58%, depending on the sample [3,4]. While nicotine, alcohol, and cannabis are most frequently abused by individuals with ADHD [5], ADHD patients are more likely than those without ADHD to escalate use to other illicit drugs, including cocaine [6]. The lifetime prevalence of cocaine use disorder in individuals with ADHD is 23%, compared to 10.8% in those without ADHD [7]. Children with ADHD are twice as likely to abuse cocaine or become cocaine dependent in adulthood compared to children without an ADHD diagnosis [8].
Over two-thirds of individuals with ADHD are prescribed medication for their symptoms [1], the most common of which is the stimulant methylphenidate (Ritalin®) [9,10]. Methylphenidate acts by blocking dopamine and norepinephrine transporters (DAT and NET), prolonging catecholamine stimulation of post-synaptic receptors [11,12]. Questions have been raised regarding the relationship between methylphenidate treatment and development of SUD. Initiation of treatment in childhood neither increases nor decreases the risk of SUD [13–15]. Some evidence that ADHD medication initiation (methylphenidate in particular) during adolescence may have different long-term consequences for adult SUD than initiation in childhood is derived from research specifically analyzing age of treatment onset. The older the child is when ADHD medication is initiated, the greater the risk of developing SUD in adulthood [4,16–18]. Currently, there are no well-controlled studies on medication initiation after age 13 in individuals with ADHD. Evaluation of long-term effects of adolescent ADHD medication on SUD risk in human populations has been complicated by difficulty in controlling certain variables across patients, such as the presence of conduct disorder, the precise age at which treatment was initiated, and the prescription of one or more other medications, which may convey varying degrees of risk [4].
Preclinical models of ADHD, such as the SHR, can begin to address clinically important questions concerning ADHD in teens. SHR exhibit the core symptoms of ADHD, such as impulsivity, hyperactivity, inattention, increased novelty seeking, poor working memory and behavioral inflexibility [19–25]. Importantly, these deficits are unrelated to hypertension in SHR [24,26–28], and can be improved by ADHD medications [22,29–31]. The WKY, which is the progenitor strain of the SHR, does not exhibit hyperactivity, impulsivity, or hypertension, and is frequently used as an inbred control strain [26]. The WIS, a common ancestor to both SHR and WKY, is used as an outbred control strain [32]. Concurrent evaluation of these three strains is necessary to control for genetic homogeneity of the SHR, while representing the genetic heterogeneity of the general population.
Compared to WKY and WIS, SHR exhibit several aspects of elevated cocaine abuse risk, including faster acquisition of cocaine self-administration, heightened cocaine cue reactivity, and greater sensitivity and motivation to pursue cocaine reinforcement across a range of cocaine doses [30,33–35]. Initiation of methylphenidate treatment during adolescence increased nearly all of these aspects of cocaine abuse risk in SHR, but did not increase risk in WKY or WIS control strains [30,33,34]. These findings highlight the need to identify alternative medications for teens with ADHD that do not convey increased cocaine abuse risk, an issue that preclinical models are well suited to investigate [36,37]. Thus far, we have found that adolescent treatment with the non-stimulant atomoxetine (Strattera®), a selective NET inhibitor, does not increase cocaine abuse risk in SHR [34,35]. However, as a non-stimulant, atomoxetine alleviates different symptoms of ADHD than methylphenidate, and may not be as clinically efficacious in some individuals [38–40]. Therefore, it is important to determine whether other efficacious stimulant medications increase cocaine abuse risk in newly diagnosed teenagers with ADHD, who may be initiating treatment.
An alternative stimulant is d-amphetamine (Dexedrine®), which is more efficacious than both methylphenidate and atomoxetine in relieving most ADHD symptoms, and has a side-effect profile similar to that of methylphenidate [41,42]. d-Amphetamine was selected for the focus of the current investigation rather than l-amphetamine or the racemic mixture, as the d-isomer is more potent than the l-isomer in modulating dopamine transmission [43,44], and alleviates more ADHD-related symptoms [45]. Whereas both methylphenidate and d-amphetamine inhibit DAT and NET function, d-amphetamine also reverses transport [46], leading to greater increases in extracellular dopamine than methylphenidate at therapeutically relevant doses [47]. Because of this additional mechanism of action, we hypothesize that d-amphetamine may have long-term consequences on cocaine abuse risk that are different from methylphenidate.
In order to establish SHR as a viable model for adolescent d-amphetamine treatment, it is first necessary to determine whether d-amphetamine improves ADHD-related deficits in SHR, as it does clinically. In adult SHR, d-amphetamine reduces hyperactivity and impulsivity, and improves sustained attention and short- and long-term memory [48,49]. However, effects of d-amphetamine in adolescent SHR are not well known. The adolescent brain undergoes unique neurochemical changes, including altered expression of dopamine D1 and other monoamine receptors [50], suggesting that psychostimulants may differentially affect adolescents, relative to juveniles or adults [51]. The current study determined effects of d-amphetamine treatment in adolescent WKY, WIS and SHR on performance in a strategy set shifting task, analogous to the Wisconsin Card Sorting Task in humans [22,52]. If d-amphetamine improves ADHD-related cognitive deficits in adolescent SHR, it then becomes important to determine whether adolescent d-amphetamine treatment alters cocaine abuse risk in adulthood.
One facet of cocaine abuse risk is reactivity to cocaine cues. Environmental cues associated with cocaine use play primary roles in the development of compulsive drug seeking, craving, and relapse [53]. In cocaine users, drug-associated environmental cues trigger neural activity and dopamine release in the medial and dorsolateral prefrontal cortex (mPFC/DLPFC), orbitofrontal cortex (OFC) and striatum, which are regions also dysfunctional in ADHD [54–56.] Importantly, dysfunction in these regions is normalized by ADHD medication [54–56]. Therefore, the effect of adolescent d-amphetamine on cocaine cue reactivity was assessed in adult WKY, WIS, and SHR after treatment was discontinued, as is common in young adults with ADHD [57,58]. A second-order schedule of cocaine delivery and cue presentation was used to measure cocaine-seeking behavior when cocaine was (maintenance phase) and was not (reinstatement phase) available for self-administration [59]. Cocaine seeking reflects cue reactivity, a phenomenon associated with cocaine addiction that can result in heightened drug use [55,60].
2. Materials and methods
2.1 Subjects
Male WKY/Cr, WIS/Cr, and SHR/Cr rats (Charles River Laboratories, USA) arrived on postnatal day 25 (P25). Rats were individually housed in plastic cages with Aspen bedding in a temperature- (21–23°C) and light- (07:30 hours on; 19:30 hours off) controlled vivarium]. From P28-P55, constituting the rat adolescent period [61], food was restricted to achieve ~90% of a growth-adjusted free-feeding body weight to facilitate lever responding for food reinforcement during the strategy set-shifting task, and to mimic conditions of past comparator studies [22,30,33–35,62]. Animals had free access to water throughout experiments and to food after P55. All procedures were approved by the Institutional Animal Care and Use Committee at Boston University, and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Eighth Edition).
2.2 Drugs and treatments
d-Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline and injected intraperitoneally (i.p.) in a volume of 2 ml/kg to attain a therapeutically relevant dose of 0.5 mg/kg. The peak plasma concentration after i.p. administration of 0.5 mg/kg d-amphetamine is 60 ng/mL [63], which is consistent with the concentrations reported in patients with ADHD (64–84 ng/mL) after receiving 0.35–0.58 mg/kg/day oral d-amphetamine [64]. A dose of 0.5 mg/kg d-amphetamine is considered therapeutically relevant also because it is below the threshold for locomotor activation [65] and increases dopamine and norepinephrine signaling in PFC [66]. Injections of 0.9% sterile saline (2 ml/kg) were used for vehicle control. Treatments were administered once daily (Monday–Friday) during the rat adolescent period (P28-P55) to mimic the weekend medication holiday that is often elected by individuals with ADHD [67]. As the half-life of i.p. d-amphetamine is approximately 1 h in rats compared to 6–7 h in children with ADHD [68–70], treatments were administered 30 min prior to behavioral testing in the strategy set shifting task. This allowed peak monoamine responses to occur and ensured that plasma levels reached clinical significance during behavioral testing.
For intravenous (i.v.) self-administration, cocaine hydrochloride (NIDA, Bethesda, MD, USA) was dissolved in 0.9% sterile saline containing 3 IU/ml heparin. A 0.8 mg/ml solution of cocaine was infused at a rate of 1.8 ml/min. The infusion duration was adjusted for body weight (1.2 s/100 g) to attain a dose of 0.3 mg/kg for self-administration. This dose of cocaine produces maximal rates of responding under the second-order schedule of cocaine delivery used in the current study [71], and is also consistent with the dose employed in prior studies on cocaine self-administration in SHR [30,33–35].
2.3 Apparatus
Experimental chambers (Med Associates, St. Albans, VT, USA) were equipped with two response levers, a white stimulus light mounted above each lever, and a house light on the opposite wall. All chambers were fitted with a single channel fluid swivel and spring leash assembly, connected to a counterbalanced arm (Med Associates). Chambers were enclosed in a sound-attenuating cubicle that was equipped with a fan for ventilation and an 8-ohm speaker. Pellet dispensers for food delivery and motor-driven syringe pumps for cocaine delivery (Med Associates) were located within the cubicle. Experimental events were controlled from a PC-compatible computer programmed in Medstate Notation and connected to an interface (Med Associates).
2.4. Experiment 1: Strategy set shifting in adolescence
2.4.1 Habituation
An operant version of the strategy set shifting task was used [22,52]. On P28, WKY treated with vehicle (n=9) or d-amphetamine (n=9), WIS treated with vehicle (n=9) or d-amphetamine (n=9), and SHR treated with vehicle (n=10) or d-amphetamine (n=12), received 20 chocolate-flavored pellets in their home cages, to habituate rats to the novel food. On P31, all rats began training in experimental chambers. Rats were required to lever press under a fixed ratio 1 (FR1) schedule to earn a minimum of 25 chocolate pellet reinforcers within a 30-min period. Thereafter, rats began lever retraction training, requiring a response within 10-sec of lever insertion into the chamber to earn a chocolate pellet reinforcer. Once rats made fewer than 5 omissions for four consecutive 45-min daily sessions, lever position bias was established by allowing rats to freely press either lever for ~10-min. The lever on which the most responses were made was defined as preferred.
2.4.2. Initial set formation
On P42, rats were required to adopt a visual-based strategy to earn reinforcement. All set shift test sessions were 2-hr and conducted once daily, Monday–Friday. During the initial set formation, each trial began with a 20-sec timeout in a dark chamber. After 20 sec, one of the two stimulus lights was illuminated (randomly selected for each trial). The house light illuminated 3-sec later, and both response levers were inserted into the chamber. Rats were required to press the lever under the illuminated stimulus light, regardless of lever bias, to earn a chocolate pellet reinforcer. Levers were retracted after a response was made (whether correct or incorrect), or if 10-sec elapsed with no response (omission). After a correct response, the stimulus light remained illuminated for an additional 4 sec, and a chocolate pellet was delivered 15 sec later. The house light remained illuminated until 4 sec after pellet delivery. A 15-sec delay to reinforcement was imposed to increase cognitive demand and to better detect strain and treatment differences, as SHR are more sensitive to delayed reinforcers than WKY [22,72]. After an incorrect response or a trial omission, the stimulus and house lights were immediately extinguished and a new trial was initiated. Training on the initial set formation continued until criterion was reached, defined as making 8 consecutive correct responses, following Floresco et al [52] and Harvey et al [22].
2.4.3. Set shift
On the day following completion of the initial set formation, rats were required to shift responding to a spatial-based discrimination strategy. During this phase, animals were required to press the lever opposite the lever position bias to earn reinforcement, regardless of which stimulus light was illuminated. Trial contingencies were otherwise identical to the initial set formation phase. Training on the set shift phase continued until rats made 10 consecutive correct responses [22,52].
2.4.4. Reversal learning
On the day following completion of the set shift phase, reversal learning was assessed. During this final phase of the task, rats were required to press the lever on the same side as the lever position bias, regardless of which stimulus light was illuminated. Trial contingencies were otherwise identical to those of the initial set and set shift phases. Training on reversal learning continued until rats made 10 consecutive correct responses [22,52].
2.5. Experiment 2: Cocaine self-administration in adulthood
2.5.1. Surgery
d-Amphetamine treatment was discontinued after P55. On P67, rats were implanted with intravenous catheters connected to headmounts to enable cocaine self-administration. Catheters were made from silicon tubing (i.d. = 0.51, o.d. = 0.94 mm) and implanted into the right femoral vein. The unsecured end of the catheter was connected to a 22-gauge L-shaped pedestal mount (Plastics One, Roanoke, VA, USA), secured to the skull with four stainless-steel anchor screws and acrylic cement. When not in use, the pedestal was sealed with an obdurator and protected with a dust cap. Surgical anesthesia was achieved using i.p. ketamine (90 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (8 mg/kg; Akorn, Decatur, IL, USA). Buprenex (0.05 mg/kg, subcutaneous [s.c.]; Reckitt Benckiser Healthcare, UK) was used as a preemptive analgesic immediately preceding surgery and for up to 48 hrs post-surgery. The antibiotic Baytril (5 mg/kg, i.v.; Bayer HealthCare, Shawnee, Mission, KS, USA) was given for up to 5 days post-surgery, and the anti-inflammatory Meloxicam (0.3 mg/kg, s.c., Boehringer Ingelheim Vetmedica, St. Joseph, MO, USA) for 3 days post-surgery. Subcutaneous 0.9% sterile saline was administered as necessary to maintain hydration. Catheters were flushed Monday-Thursday with 0.2 ml of 0.9% sterile saline containing 30.0 IU/ml of the anticoagulant heparin and 67 mg/ml of the antibiotic Timentin (SmithKline Beecham Pharmaceuticals, Philadelphia, PA). To preserve catheter patency on weekends and holidays, catheters were filled with 0.08 ml of a locking solution consisting of glycerol (Sigma-Aldrich, St. Louis, MO, USA) and heparin (1000 mg/ml) in a 3:1 ratio. This solution was removed and replaced with 0.2 ml of a 3.0 IU/ml heparinized 0.9% saline solution prior to resumption of the next behavioral session. Catheter patency was assessed weekly by withdrawing blood and/or infusion of 0.1 ml (10 mg/ml) of methohexital sodium (JHP Pharmaceuticals, Rochester, MI, USA), and noting the rapid loss of muscle tone. In the event that patency was lost, catheters were repaired or replaced under surgical anesthesia and post-operative care, as above. Because a small number of rats (9 total) used for the set shifting procedures died prior to completing all cocaine self-administration procedures, group sizes for the cocaine maintenance, extinction and reinstatement tests (described below) were n=8 for vehicle- and d-amphetamine-treated WKY and WIS as well as for vehicle-treated SHR; the group size for d-amphetamine-treated SHR was n=9.
2.5.2. Maintenance testing
On P77, rats began self-administration training for 0.3 mg/kg cocaine under FR1 schedule contingencies. Self-administration sessions were initiated with illumination of the house light and onset of white noise (70 dB), which served as a background contextual cue. Cocaine infusions coincided with 20-sec presentation of the stimulus light, while the house light was extinguished. During this period, additional cocaine infusions could not be earned to reduce risk of accidental overdose. The house light was re-illuminated and the stimulus light extinguished after the 20-sec timeout period. Rats were gradually trained to a terminal fixed-interval (FI) based second-order schedule, designated FI 5-min [FR5:S]. Under this schedule, every fifth lever press (FR5) produced a 2-sec cue light, and the first FR5 response unit completed after the 5-min FI elapsed resulted in a cocaine infusion coinciding with 20-sec presentation of the stimulus light, after which the FI 5-min component was again in effect. Self-administration sessions were 2 hr in duration and conducted once daily, Monday–Friday, during the light phase at approximately the same time each day. Training under the second-order schedule continued until rats reached stable baseline levels of responding, defined as ≤ 15% variation in active lever responding and ≤ 33% of total responding on the inactive lever, for a minimum of 5 consecutive sessions.
2.5.3. Extinction training
Following establishment of maintenance baselines, rats underwent response extinction training. During this phase, the house light was illuminated throughout the session, but the stimulus light and white noise were not presented, and cocaine infusions could not be earned. Lever responding therefore had no consequences. Extinction sessions were 2 hr in duration, and conducted once daily, Monday–Friday. Extinction training continued for a minimum of 10 sessions and until criterion was reached, defined as active lever responding that was ≤ 10% of the maintenance baseline for 3 consecutive sessions. If rats did not reach criterion, a maximum of 21 extinction sessions was imposed.
2.5.4. Reinstatement testing
Following achievement of extinction criterion, rats underwent cue-induced reinstatement testing. During this phase, the discrete stimulus light and contextual white noise cues were presented under contingencies identical to self-administration training under the second-order schedule, but cocaine infusions could not be earned. Reinstatement testing sessions were 1 hr in duration and conducted once daily, Monday-Friday. All rats underwent 7 reinstatement sessions.
2.6. Data analysis
Each measure in the set shifting and cocaine self-administration studies was analyzed using two-factor (strain x treatment, or treatment x day) or three-factor (strain x treatment x phase, or strain x treatment x day) repeated-measures ANOVA, followed by post-hoc Tukey tests. The Tukey is a conservative test that controls for type-1 error when multiple comparisons are made [73]. In the strategy set shifting task, dependent measures included number of trials completed to reach criterion, latency to reach criterion (included the time for omitted trials), ratio of correct to incorrect lever responses (choice accuracy), and error subtypes. These measures of performance reflect the primary outcome measure, flexible learning, during the set shift and reversal learning phases of the task [52]. In addition to measures of flexible learning, the average lever press reaction time, variability in lever press reaction time, and number of omitted trials were recorded. Higher values in these measures are thought to represent attentional lapses during testing in children and teens with ADHD [74,75]. Several set shifting task measures failed the Shapiro-Wilk normality test and data were subsequently square root transformed prior to analysis. Square root-transformed measures included omissions, reaction time, reaction time variability and choice accuracy in the initial set formation phase; trials to criterion, latency to criterion, omissions, reaction time variability, choice accuracy, regressive and never-reinforced errors for the set shift phase; and trials to criterion, latency to criterion, omissions, reaction time variability, choice accuracy, and perseverative and regressive errors in the reversal learning phase. Data were normally distributed following square root transformation. Dependent measures for cocaine self-administration included number of cocaine infusions earned, active and inactive lever responses, and number of sessions required to reach extinction criterion.
Errors in the set shifting task were coded by subtype for the set shift and reversal learning phases [22,52]. During the set shift phase, perseverative and regressive errors were recorded when rats pressed a lever with the stimulus light illuminated above it on trials that required pressing of the opposite lever. Errors were perseverative when rats pressed the incorrect lever on six or more trials per block of eight trials. Once rats made five or fewer incorrect choices in a block of eight trials for the first time, the incorrect lever choices in subsequent blocks were then scored as regressive errors. Never-reinforced errors were recorded when a rat pressed the incorrect lever on trials when the correct lever had the stimulus light illuminated above it (i.e., a choice that was not reinforced during either the initial set or set shift phase). During the reversal learning phase, errors were examined in blocks of 16 trials and consisted of perseverative and regressive errors [22,76]. Once rats made ten or fewer incorrect choices in a block of 16 trials for the first time, the incorrect lever choices in subsequent blocks were then scored as regressive errors. Never-reinforced errors are not possible during the reversal-learning phase. Perseverative errors are an index of how well the previously acquired strategy is suppressed, regressive errors are an index of how well the new strategy is maintained, and never-reinforced errors are an index of how well the new strategy is acquired [52].
3. Results
3.1. Experiment 1: Strategy set shifting in adolescence
3.1.1. Initial set formation
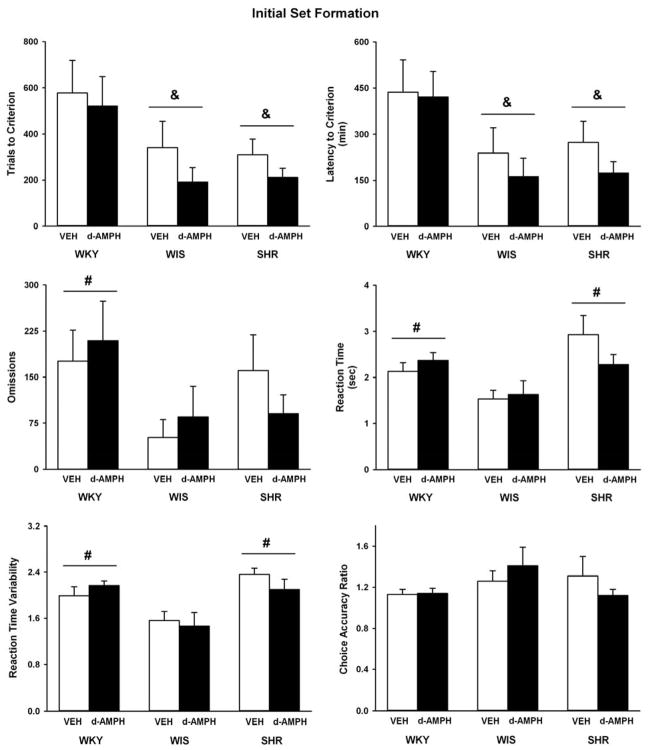
Figure 1 depicts data obtained during the initial set formation phase of the strategy set shifting task. Strains differed in trials to criterion (F(2, 52) = 6, p ≤ 0.005), latency to criterion (F(2, 52) = 5.8, p ≤ 0.005), trial omissions (F(2, 52) = 4.5, p ≤ 0.01), average reaction time (F(2, 52) = 9.2, p ≤ 0.001), and reaction time variability (F(2, 52) = 9.3, p ≤ 0.001). No strain differences were observed for choice accuracy. Adolescent SHR and the WIS control required fewer trials to reach criterion (p ≤ 0.008 and 0.01, respectively) and had shorter latencies to reach criterion (p ≤ 0.01) than the WKY control. Both SHR and the WKY control exhibited slower reaction time and greater reaction time variability than the WIS control (p ≤ 0.01), and the WKY control also made more trial omissions than the WIS control (p ≤ 0.01). d-Amphetamine treatment did not alter performance on any measure across strains during initial set formation.
Fig. 1.
Trials to criterion, latency to criterion, trial omissions, lever press reaction time, variability in reaction time, and choice accuracy during initial set formation in adolescent WKY, WIS, and SHR following vehicle or d-amphetamine treatment (Mean ± SEM). & p ≤ 0.05 compared to WKY overall. # p ≤ 0.05 compared to WIS overall.
3.1.2. Set shift
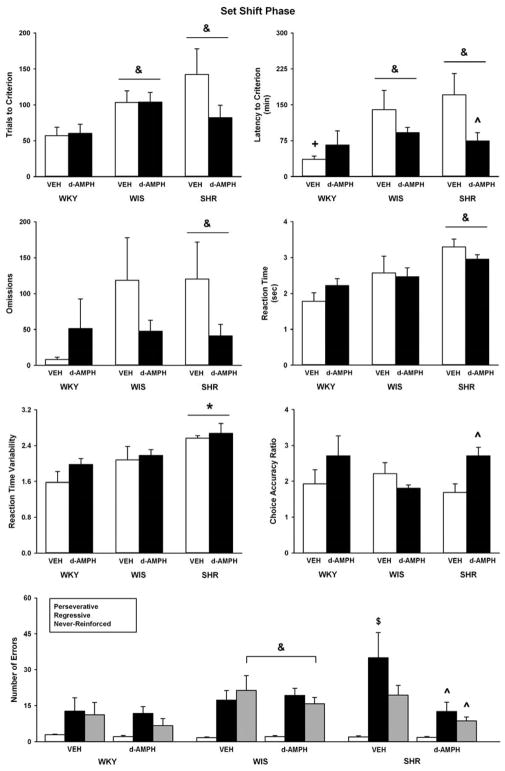
Figure 2 illustrates data from the set shift phase of the strategy set shifting task. Strains differed in trials to criterion (F(2, 52) = 4.8, p ≤ 0.01), latency to criterion (F(2, 52) = 6.4, p ≤ 0.003), trial omissions (F(2, 52) = 3.7, p ≤ 0.03), average reaction time (F(2, 52) = 10, p ≤ 0.001), and reaction time variability (F(2, 52) = 8.7, p ≤ 0.001). No strain differences were observed for choice accuracy. Adolescent SHR and the WIS control required more trials to reach criterion (ps ≤ 0.02) and had longer latencies to reach criterion (p ≤ 0.009 and 0.008, respectively) than the WKY control. Adolescent SHR also exhibited slower reaction time and greater reaction time variability compared to the WKY and WIS controls (p ≤ 0.05). Moreover, SHR made more omissions (p ≤ 0.03) than the WKY control.
Fig. 2.
Trials to criterion, latency to criterion, trial omissions, lever press reaction time, variability in reaction time, choice accuracy, and error subtypes during the set shift phase in adolescent WKY, WIS, and SHR following vehicle or d-amphetamine treatment (Mean ± SEM). * p ≤ 0.05 compared to both other strains. & p ≤ 0.05 compared to WKY overall. $ p ≤ 0.05 compared to vehicle-treated WKY. ^ p ≤ 0.05 compared to vehicle-treated SHR.
For latency to criterion, there was a trend towards a strain X treatment interaction (F(2, 52) = 2.7, p ≤ 0.07) and further testing revealed that vehicle-treated WKY took less time to reach criterion than vehicle-treated WIS and SHR (p ≤ 0.01 and 0.001, respectively). d-Amphetamine treatment reduced latency to criterion in adolescent SHR only (p ≤ 0.01). There also was a trend towards a strain X treatment interaction (F(2, 52) = 2.7, p ≤ 0.07) for choice accuracy, which for the adolescent SHR improved relative to vehicle following d-amphetamine treatment (p ≤ 0.01).
With respect to regressive errors, there was a significant strain X treatment interaction (F(2, 52) = 3.3, p ≤ 0.04). Vehicle-treated SHR made more regressive errors than vehicle-treated WKY (p ≤ 0.01). Compared to vehicle treatment, d-amphetamine reduced regressive errors in SHR only (p ≤ 0.006). For never-reinforced errors, both strain (F(2, 52) = 4.6, p ≤ 0.01) and treatment (F(1, 52) = 3.9, p ≤ 0.05) effects were observed. Overall, the WIS control made more never-reinforced errors than the WKY control (p ≤ 0.01). In addition, rats treated with d-amphetamine made fewer never-reinforced errors than vehicle-treated rats overall (p ≤ 0.05). Although the strain X treatment interaction was not significant, further testing revealed that SHR treated with d-amphetamine made fewer never-reinforced errors than vehicle-treated SHR (p ≤ 0.04). No strain or treatment differences were observed for perseverative errors during the set shift phase.
3.1.3. Reversal Learning
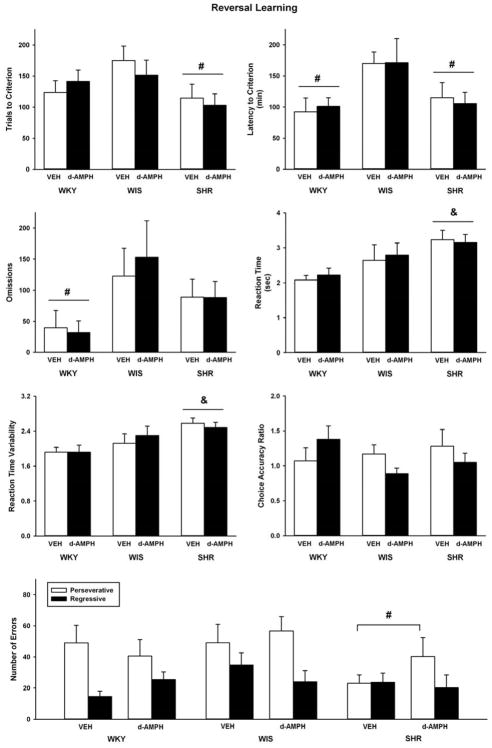
Figure 3 shows data obtained during the reversal learning phase of the strategy set shifting task. Strain differences were observed in trials to criterion (F(2, 52) = 3.8, p ≤ 0.03), latency to criterion (F(2, 52) = 5.1, p ≤ 0.01), trial omissions (F(2, 52) = 4.7, p ≤ 0.01), average reaction time (F(2, 52) = 6.7, p ≤ 0.003), and reaction time variability (F(2, 52) = 6.4, p ≤ 0.003). No strain differences were observed for choice accuracy. Overall, adolescent SHR required fewer trials to reach criterion than the WIS control (p ≤ 0.02), and both the WKY control and SHR had shorter latencies to reach criterion than the WIS control (p ≤ 0.01 and 0.03, respectively). Adolescent WKY also made fewer trial omissions than the WIS control (p ≤ 0.01), and SHR exhibited slower and more variable reaction times than the WKY control (p ≤ 0.002 and 0.003, respectively). For perseverative errors, there was a trend towards a main effect of strain (F(2, 52) = 2.9, p ≤ 0.06) and further testing revealed that SHR made fewer perseverative errors than the WIS control (p ≤ 0.05). No strain differences were observed for regressive errors. d-Amphetamine treatment did not alter performance on any measure across strains during the reversal learning phase.
Fig. 3.
Trials to criterion, latency to criterion, trial omissions, lever press reaction time, variability in reaction time, choice accuracy, and error subtypes during the reversal learning phase in adolescent WKY, WIS, and SHR following vehicle or d-amphetamine treatment (Mean ± SEM). & p ≤ compared to WKY overall. # p ≤ 0.05 compared to WIS overall.
3.2. Experiment 2: Cocaine self-administration in adulthood
3.2.1. Maintenance testing
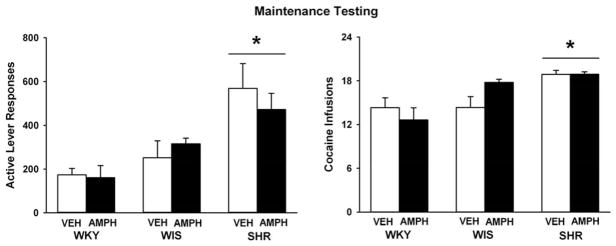
Figure 4 illustrates number of active lever responses and cocaine infusions during the maintenance-testing phase (last 5 sessions) of cocaine self-administration in adulthood. At baseline, strains differed in active lever responding (F(2, 43) = 13.6, p ≤ 0.001), with adult SHR making more active lever responses than the WKY and WIS control strains (p ≤ 0.001 and 0.005, respectively). Analysis of cocaine intake also revealed strain differences (F(2, 43) = 12.3, p ≤ 0.001), with adult SHR earning more cocaine infusions than the WKY and WIS control strains (p ≤ 0.001 and 0.03, respectively). Inactive lever responses did not differ by strain (average = 45 ± 11; ≤ 14% of active lever responses). Adolescent d-amphetamine treatment did not affect any measure across strains during maintenance testing in adult rats.
Fig. 4.
Active lever responses and cocaine infusions during cocaine self-administration maintenance testing under a second-order schedule (Mean ± SEM). Experiments were conducted in adult WKY, WIS, and SHR following vehicle or d-amphetamine treatment during adolescence. * p ≤ 0.05 compared to both other strains.
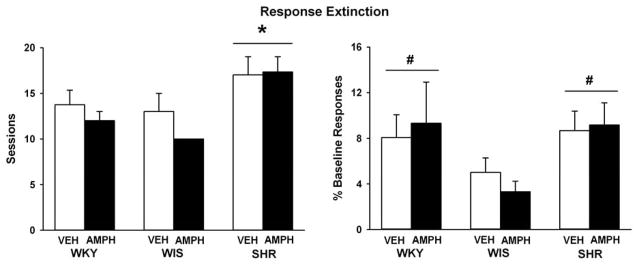
3.2.2. Extinction training
Figure 5 depicts data obtained during response extinction training following the cocaine self-administration maintenance phase in adulthood. Strains differed in the number of sessions to reach the extinction criterion (F(2, 43) = 8.1, p ≤ 0.001), with SHR requiring more sessions than the WKY or WIS controls (p ≤ 0.01 and 0.001, respectively). Strain differences also were observed for active lever responses made during the extinction baseline (F(2, 43) = 4, p ≤ 0.02), which was the average of the last three extinction sessions and expressed as a percentage of the maintenance testing baseline. SHR and the WKY control made relatively more active lever responses than the WIS control during the extinction baseline (p ≤ 0.05 and 0.04, respectively), though all strains extinguished to less than 10% of the maintenance baseline level of responding. Inactive lever responses during the extinction baseline also differed by strain (F(2, 43) = 3.7, p ≤ 0.03), with SHR making more inactive lever responses (31 ± 12) than the WIS control (6 ± 1; p ≤ 0.03), but not the WKY control (9 ± 2). Adolescent d-amphetamine treatment did not alter any measure across strains during extinction training in adult rats.
Fig. 5.
Number of sessions required to reach extinction criterion and active lever responses during the extinction baseline expressed as a percentage of the maintenance baseline (Mean ± SEM). Experiments were conducted in adult WKY, WIS, and SHR, following vehicle or d-amphetamine treatment during adolescence. * p ≤ 0.05 compared to both other strains. # p ≤ 0.05 compared to WIS overall.
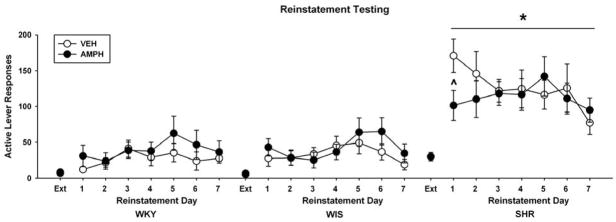
3.2.3. Reinstatement testing
Figure 6 illustrates active lever responses during cue-induced reinstatement testing following response extinction training in adulthood. Three-way ANOVAs of active lever responding revealed a main effect of phase (F(1, 43) = 116, p ≤ 0.001) and further testing indicated that in all strains, active lever responses were greater during the reinstatement phase than during the extinction phase (p ≤ 0.001). There was also a main effect of strain (F(2, 43) = 32.8, p ≤ 0.001) and reinstatement day (F(6, 252) = 2.5, p ≤ 0.02). Subsequently, two-way ANOVAs (strain X treatment) were conducted for each day of reinstatement. On day 1 of reinstatement testing, a strain X treatment interaction was observed (F(2, 43) = 5, p ≤ 0.01). Further testing revealed that SHR reinstated more active lever responses than the WKY and WIS controls overall (p ≤ 0.001), and that adult SHR treated with adolescent d-amphetamine reinstated fewer active lever responses than SHR treated with vehicle (p ≤ 0.003). However, this reduction in responding was not maintained across reinstatement sessions in amphetamine-treated SHR. For each subsequent day of reinstatement testing, strain differences were sustained (SHR > WKY = WIS; p ≤ 0.008). In WKY or WIS, adolescent d-amphetamine treatment did not alter active lever responses on day 1 or on any other reinstatement test day.
Fig. 6.
Active lever responses during the extinction baseline (Ext) and each reinstatement test session (Mean ± SEM). Experiments were conducted in adult WKY, WIS, and SHR following vehicle or d-amphetamine treatment during adolescence. * p ≤ 0.05 compared to both other strains. ^ p ≤ 0.05 compared to vehicle-treated SHR.
For inactive lever responses, a three-way ANOVA revealed a day X strain X treatment interaction (F(12, 252) = 2.2, p ≤ 0.01). Further testing indicated that, overall, SHR made more inactive lever responses than the WKY and WIS controls (p ≤ 0.03). Moreover, on day 1 of reinstatement, adult SHR treated with adolescent d-amphetamine made fewer inactive lever responses than SHR treated with vehicle (p ≤ 0.006). In WKY and WIS, adolescent d-amphetamine treatment did not alter inactive lever responses on day 1 or on any other reinstatement test day.
4. Discussion
The performance of SHR, WKY and WIS strain rats were compared on a strategy set shifting task during adolescence and for cocaine self-administration and cue reinstatement during adulthood. Treatment with d-amphetamine or vehicle was provided during adolescence, from P28 through P55, with rats arriving on P25 and housed individually. Upon arrival on P25, animals were allowed three days to acclimate to the new housing conditions. A three-day acclimation period is standard protocol in animal studies assessing cocaine self-administration [77], neurochemistry [78], as well as in studies using adolescent rats [79]. One potential issue this design raises is the role of early life stress (recent shipping, individual housing, food restriction during adolescence) on the outcome measures. Previous studies have shown that rats housed individually in caging with wire mesh flooring and with no daily handing were more impulsive than group-housed rats living in an enriched environment with pulp bedding and novel objects for play [80]. Although rats in the current study were housed individually and food-restricted during adolescence, they were handled daily and were provided some enrichment in the form of Aspen bedding in the home cage and exposure to food reinforcement in operant chambers. With respect to cocaine self-administration, studies in outbred male rats have shown no reliable differences in individual- compared to pair- or group-housed peers [81,82]. In addition, although caloric restriction to 90% body weight can enhance initial locomotor responses to cocaine injections in adult outbred rats, this effect is not sustained after repeated exposure to cocaine [83]. Food restriction during adolescence in the current study likely did not impact cocaine responses during adulthood, as the cocaine self-administration training sessions began 22 days after a return to ad libitum feeding and lasted for several weeks before testing began. Thus, even if potential early life stress were not fully mitigated by our procedures and study design, all three strains were handled equivalently, yet strain and treatment differences were found in the set shifting and cocaine self-administration tasks. It remains possible that strain differences in stress vulnerability (SHR > WKY > WIS) [84,85] contributed to strain differences in set shifting and cocaine self-administration behavior. However, in most cases, WKY had the best performance during the latter phases of the set shifting task and had similar performance to WIS during cocaine self-administration testing, which is inconsistent with the rank order for stress vulnerability. Furthermore, early life stress has been shown to impact adolescent WKY and SHR differently, with WKY showing increased anxiogenic effects and SHR showing resilience to the anxiogenic effects of early life stress [86]. Thus, exposure to early life stress in the current study does not appear to systematically account for the strain and treatment differences in performance on the strategy set shifting task during adolescence and in cocaine self-administration and cue reinstatement tests during adulthood.
4.1. Strain differences in strategy set shifting in adolescence
During initial set formation, adolescent SHR needed fewer trials and shorter latencies to reach criterion relative to the WKY control, consistent with prior work in our laboratory [22]. Trial omissions also were fewer in SHR relative to the WKY control. However, SHR exhibited slower and more variable reaction times than the WIS control, suggesting that at this initial stage of learning the SHR display attentional lapses. These results are consistent with observations in individuals with ADHD who demonstrate high inter-individual reaction time variability, and in some studies, slower reaction time compared to controls without ADHD [87]. High reaction time variability is, moreover, a robust and highly consistent feature of ADHD, and may contribute to other symptoms such as poor sustained attention, behavioral inhibition, and working memory deficits [87]. Interestingly, these symptoms have been attributed to altered frontal cortex and DAT functioning [87,88], which also are observed in SHR [27,89–92].
The SHR may more readily respond than WKY for reinforcement under task conditions that do not require complex flexible learning, such as during acquisition of the initial attentional set [22,93]. However, performance in WKY also was consistently worse than performance in the WIS control during this initial stage of learning. Although WKY is frequently used as an inbred control strain for SHR [26], the WKY, particularly from Charles River Laboratories USA, has been used also as a model of anxiety, depression, and more recently, autism spectrum disorder [94,95]. Compared to outbred rat strains, WKY show reduced locomotor activity and social exploratory behavior in the open field test, elevated plus maze, and three-chamber social interaction test, as well as increased learned helplessness and immobility in forced swim and defensive burying paradigms [95–97]. Furthermore, in reinforcement-based tasks, WKY earn fewer sucrose pellets than WIS under FR 1 and progressive ratio schedules [98]. Behavioral abnormalities exhibited by WKY have been attributed to reduced cortical and hippocampal volume and plasticity, decreased D1 receptor and DAT binding in striatum, and decreased dopamine and dopamine metabolite levels in PFC, relative to outbred rat strains [99–102]. Importantly, the initial set formation of the strategy set shift task requires dopaminergic projections to frontal cortex, as 6-OHDA lesions in frontal cortex, but not caudate nucleus, impaired acquisition of an initial set [103]. Decreased dopamine levels in frontal cortex of WKY [100] may contribute, therefore, to slower learning of the initial strategy set in this strain. Although the WKY has been suggested to be the most appropriate comparator strain for SHR [22,26], current findings emphasize the importance of concurrent evaluation of WKY and an outbred control when examining set shifting performance in SHR.
In contrast to the initial set formation, the set shift phase of the task necessitates greater flexible learning, requiring rats to switch responding from a previously learned visual strategy to a spatial strategy. Consistent with previous studies [22], adolescent SHR showed the greatest deficits during the set shift phase, displaying poorer flexible learning and more attentional lapses than both control strains. On the Wisconsin Card Sorting Task, individuals with ADHD similarly exhibit poor flexible learning, making more perseverative responses and completing fewer categories than typically developing controls [75,104,105]. Poor flexible learning and other ADHD-related symptoms, such as hyperactivity, inattention, low motivation, and poor motor timing and response inhibition, may arise from abnormal neural activation and connectivity in frontostriatal circuitry both in humans and SHR [38,106,107].
During the set shift phase, WKY reached learning criterion faster than both WIS and SHR, and made fewer errors than WIS. It is possible that WKY exhibit superior flexible learning in strategy shifting compared to WIS or SHR. However, given that WKY were slower in acquiring the initial set, it is also possible that WKY experienced less proactive interference during the set shift than WIS or SHR. Proactive interference from a previously relevant stimulus dimension exacerbates cognitive inflexibility when individuals are required to attend to a new stimulus feature [108]. Previous work identified reduced proactive interference in WKY in an eyeblink conditioning paradigm, whereby pre-exposure to unconditioned or conditioned stimuli did not alter acquisition rate of the eyeblink response in WKY, but slowed acquisition in outbred Sprague-Dawley rats [109]. Though it is unclear to what degree proactive interference in classical conditioning generalizes to operant conditioning, weakened acquisition, memory storage, or retrieval of the initially reinforced visual strategy by WKY in the current study could plausibly lead to reduced proactive interference during subsequent testing phases, manifesting as fewer errors and an apparently faster ability to acquire new response strategies [110].
Reduced proactive interference may also help to explain some of the current observations during the reversal learning phase of the set shift task. During reversal learning, SHR continued to show slower and more variable reaction times, consistent with the ADHD phenotype. However, WKY and SHR reached the reversal learning criterion faster than WIS. Moreover, during this phase WKY made fewer trial omissions, and SHR fewer perseverative errors, than WIS rats. It is possible that, relative to SHR and WKY, WIS exhibit deficits in reversal learning, which is dependent on OFC functioning [76]. However, an alternative explanation is that WKY and SHR experienced less proactive interference than WIS during reversal learning. Deficient learning or memory of the previously acquired behavioral strategies by WKY and SHR would lead to the appearance of superior performance relative to WIS during the reversal learning phase. Conversely, normative learning and retrieval of the previously acquired behavioral strategy by WIS would lead to increased proactive interference, resulting in slower speed of reversal learning and a greater number of perseverative errors as observed in the current study.
4.2. Effects of d-amphetamine treatment on strategy set shifting in adolescence
d-Amphetamine improved several measures of flexible learning in adolescent SHR during the set shift phase. Specifically, SHR treated with d-amphetamine reached the set shift learning criterion faster, were more accurate in response choices, and made fewer regressive and never-reinforced errors than vehicle-treated SHR. In children with ADHD, treatment with d-amphetamine, as well as other ADHD medications, was found to normalize abnormal striatal glutamate levels that may contribute to ADHD-related symptoms in both humans and SHR [56,91,107,111–113]. However, future work is needed in the SHR model to determine whether d-amphetamine alters glutamate signaling in striatum or substantia nigra. Although d-amphetamine did not improve reaction time measures or reduce the number of omitted trials in SHR during any phase of the set shift task, rarely are all ADHD symptoms alleviated by drug therapy [114]. It is possible that a higher d-amphetamine dose would improve these additional ADHD-related deficits in SHR [49], but higher doses may also produce plasma levels, monoamine responses, and behavioral consequences that are not clinically relevant [68]. In adult outbred rats, prior adolescent (P27-P45) or adult (P95-P103) treatment with 3 mg/kg d-amphetamine (i.p.) every other day produced sensitization to a d-amphetamine challenge, and increased errors during reversal learning of a compound stimulus discrimination [115]. Because reversal learning is dependent on OFC [76], these findings suggest that higher doses of d-amphetamine may impair OFC functioning long-term [115]. Moreover, the same 3 mg/kg adolescent d-amphetamine treatment regimen reduced choice latency during attentional set shifting, and increased perseverative responding during strategy set shifting and differential reinforcement of low rates of responding (DRL) tasks, suggesting that higher doses of d-amphetamine heighten the saliency of reward-related cues [115,116]. Interestingly, adolescent exposure to 3 mg/kg d-amphetamine also reduced trials to criterion during set shifting and reversal learning in adulthood, compared to vehicle-treated rats as well as rats receiving d-amphetamine in adulthood [115]. These findings further confirm that psychostimulants differentially affect adolescents relative to adults [51], and also emphasize the importance of task selection when considering the long-term effects of d-amphetamine and its potential therapeutic use relative to other ADHD medications.
In contrast to d-amphetamine, a past study shows that adolescent treatment with the stimulant methylphenidate improved flexible learning in adolescent SHR only during the initial set formation phase [22]. Conversely, the non-stimulant atomoxetine improved flexible learning and reduced the number of omitted trials in the SHR during the set shift phase [22], more similar to the current observations with d-amphetamine. The ability of d-amphetamine and atomoxetine to improve flexible learning in adolescent SHR may relate in part to direct effects not only on glutamatergic signaling, but also on DAT function in PFC. Chronic treatment with atomoxetine during adolescence decreased DAT function in OFC and striatum of SHR [35]. In contrast, chronic treatment with methylphenidate during adolescence increased DAT function in mPFC of SHR [62], potentially exacerbating pre-existing elevations of DAT [27,92]. We hypothesize that chronic treatment with d-amphetamine during adolescence produces long-term decreases in DAT function and expression, similar to that observed after atomoxetine treatment. Although the effect of d-amphetamine on PFC DAT function in SHR is unknown, recently, acute amphetamine infusions were found to reduce DAT function and expression in nucleus accumbens of adult outbred rats [117]. Importantly, activation of post-synaptic D1 and D2 receptors in mPFC facilitates flexible learning [118], as infusion of D1 and D2 antagonists in mPFC impairs set shifting performance in outbred rats [119,120]. Therefore, by decreasing DAT function and elevating dopaminergic tone, adolescent d-amphetamine may produce optimal activation at D1 and D2 receptors in mPFC of SHR, thereby improving flexible learning during the set shift. However, additional studies are needed to test our hypothesis in mPFC of the SHR model, using a chronic dosing regimen of d-amphetamine during adolescence that is clinically relevant to ADHD.
4.3. Strain differences in cocaine cue reactivity in adulthood
The current study replicated previous findings suggesting that SHR are a viable model for comorbid ADHD and cocaine abuse risk. During maintenance testing under a second-order schedule, SHR earned more cocaine infusions and made more cocaine-seeking responses than WKY and WIS control, consistent with our prior work [34]. SHR also required more sessions than WKY or WIS to reduce lever responding during extinction training. This is consistent with prior findings that SHR, like individuals with ADHD, exhibit slower extinction rates than controls, possibly as a result of low sensitivity to changes in reinforcement contingencies and abnormal nigrostriatal dopamine functioning [121–123]. Lastly, SHR exhibited greater reinstatement of cocaine-seeking responses than WKY and WIS upon re-exposure to cocaine-paired cues, in the absence of cocaine reinforcement. These results suggest that SHR are more reactive to cocaine-paired cues than WKY or WIS. Although studies on cocaine cue reactivity in ADHD are lacking, cigarette smokers with ADHD report more frequent encounters with smoking-associated cues and perceive a stronger relationship between cues and smoking motivation [124], suggesting heightened drug cue reactivity relative to smokers without ADHD. However, further studies evaluating physiological measures such as skin conductance, heart rate, or hemodynamic neural responses to drug cues in individuals with ADHD are necessary to confirm this hypothesis.
4.4. Effects of adolescent d-amphetamine treatment on cocaine cue reactivity in adulthood
Adolescent d-amphetamine did not alter cocaine intake, cocaine seeking, or response extinction rates in adult rats of any strain, with the exception that adult SHR treated with d-amphetamine during adolescence made fewer cocaine-seeking responses on the first day of reinstatement testing compared to vehicle-treated SHR. Together, these findings suggest that adolescent d-amphetamine treatment does not further increase cocaine abuse risk in adult SHR. The effects of adolescent atomoxetine treatment were similar to d-amphetamine; however, cocaine-seeking responses were reduced across the entire 7-day reinstatement test phase in adult SHR after adolescent atomoxetine [34]. In contrast, adolescent methylphenidate treatment speeded acquisition of cocaine self-administration, increased cocaine intake, and augmented the efficacy and motivating influence of cocaine reinforcement in adult SHR under FR and progressive-ratio schedules [30,33]. Under a second-order schedule, adolescent methylphenidate treatment also further increased cocaine intake during maintenance testing in adult SHR, but it did not further enhance cocaine-seeking behavior during reinstatement testing [34]. The differential effects of adolescent d-amphetamine, atomoxetine, and methylphenidate on cocaine-seeking behavior in adulthood might relate in part to differential long-term effects on DAT function within PFC, as reviewed above. It is probable that additional neurochemical adaptations contribute to the long-term effects of ADHD medications on cocaine abuse risk, such as changes at post-synaptic receptors or norepinephrine and serotonin transporters, to which d-amphetamine also potently binds and reverses transport [43,125]. However, future investigations are needed to investigate these changes in SHR.
5. Conclusions
Questions regarding long-term effects of adolescent ADHD medication on later substance use disorders, and particularly cocaine abuse risk, have been historically difficult to examine in a systematic manner in individuals with ADHD. The results of the current investigation, together with previous work, indicate that the SHR has important heuristic value in modeling aspects of cognitive dysfunction, cocaine abuse risk, and effects of d-amphetamine treatment for adolescents with ADHD. The failure of a clinically relevant dose of d-amphetamine to alter set shifting behavior in adolescence or cocaine cue reactivity in adulthood of WKY and WIS control strains emphasizes the importance of employing an appropriate animal model wherever feasible when investigating the effects of ADHD medications.
Although cocaine cue reactivity, as examined in the current study, is an important aspect of cocaine abuse risk, the most robust effects of adolescent methylphenidate treatment were observed during the acquisition of cocaine self-administration and on the efficacy and motivating influence of cocaine reinforcement in adulthood [30,33]. Future studies are needed to assess whether adolescent d-amphetamine treatment alters other important aspects of cocaine abuse risk in adult SHR by examining full cocaine dose-response functions using fixed and progressive ratio schedules of reinforcement. Nonetheless, the current results suggest that d-amphetamine may be an alternative stimulant medication to methylphenidate for newly diagnosed teenagers with ADHD. While concern has been raised regarding the abuse liability of d-amphetamine as a prescription medication in itself, there is little actual abuse of d-amphetamine by ADHD patients [43]. The pharmacokinetics of orally administered d-amphetamine make the drug less reinforcing than other stimulants, such as cocaine or methamphetamine, and clinically relevant doses of d-amphetamine are unlikely to produce the euphoria associated with compulsive drug use [43]. Moreover, the pro-drug, lisdexamfetamine (Vyvanse®), has recently been developed to further deter abuse, by requiring enzymatic hydrolysis within red blood cells in order to be converted into psychoactive d-amphetamine [43,126].
Illicit drug use costs the United States over $193 billion each year [127]. Nearly two-thirds of treatment-seeking drug users report sampling drugs before age 20 [128,129]. Thus, with approximately 1,600 teens and adults initiating cocaine use each day [130], it is critically important to reduce the risk of cocaine abuse, particularly in vulnerable populations such as those with ADHD. Application of our preclinical findings to a clinical setting could help minimize the risk of SUD in ~700,000 individuals, which is the estimated number of first-time ADHD diagnoses in adolescents aged 12–17 [131].
Highlights.
Adolescent SHR exhibited set shift deficits compared to WKY and WIS controls
Low dose d-amphetamine improved set shift performance only in adolescent SHR
During adulthood, cocaine seeking and intake were greater in SHR than controls
Adolescent d-amphetamine did not increase cocaine seeking or intake in adult SHR
d-Amphetamine may be an alternate to methylphenidate for teens diagnosed with ADHD
Acknowledgments
This study was supported by DA011716, the Clara Mayo Memorial Fellowship, and the Undergraduate Research Opportunities Program at Boston University. We thank Britahny Baskin, Sae-Mi Jeon, and Laura Tabbaa for research assistance.
Footnotes
Authors’ contribution
CJ was responsible for data collection, data analysis, and writing of the report. KK and LD were involved in the study concept and provided important intellectual content during the writing of the manuscript. DT was involved in data collection. All authors critically reviewed, edited, and approved the content and final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adol Psychiatry. 2014;53:34–46.e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarz A, Cohen S. ADHD seen in 11% of US children as diagnoses rise. [Accessed 23 June 2015];NY Times. 2013 Available at http://www.nytimes.com/2013/04/01/health/more-diagnoses-of-hyperactivity-causing-concern.html.
- 3.Sizoo B, van den Brink W, Koeter M, Gorissen van Eenige M, van Wijngaarden-Cremers P, et al. Treatment seeking adults with autism or ADHD and co-morbid Substance Use Disorder: prevalence, risk factors and functional disability. Drug Alcohol Depend. 2010;107:44–50. doi: 10.1016/j.drugalcdep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Steinhausen H-C, Bisgaard C. Substance use disorders in association with attention-deficit/hyperactivity disorder, co-morbid mental disorders, and medication in a nationwide sample. European Neuropsychopharm. 2014;24:232–241. doi: 10.1016/j.euroneuro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Wilens TE, Petty C, Antshel K, Spencer T, Biederman J. Substance use among ADHD adults: implications of late onset and subthreshold diagnoses. Am J Addict. 2007;16:24–34. doi: 10.1080/10550490601082767. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Monuteaux MC, Mick E, Wilens TE, Fontanella JA, Poetzl KM, et al. Is cigarette smoking a gateway to alcohol and illicit drug use disorders? A study of youths with and without attention deficit hyperactivity disorder. Biol Psych. 2006;59:258–264. doi: 10.1016/j.biopsych.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Lambert N. The contribution of childhood ADHD, conduct problems, and stimulant treatment to adolescent and adult tobacco and psychoactive substance abuse. Ethical Human Psychol Psychiatry. 2005;7:197–221. [Google Scholar]
- 8.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psych Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in Medication Treatment for ADHD. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 10.Raman SR, Marshall SW, Gaynes BN, Haynes K, Naftel AJ, Stürmer T. An observational study of pharmacological treatment in primary care of children with ADHD in the United Kingdom. Psychiatr Serv. 2015;66:617–624. doi: 10.1176/appi.ps.201300148. [DOI] [PubMed] [Google Scholar]
- 11.Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding Y-S. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sciences. 1996;58:231–239. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilens T. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67:32–37. [PubMed] [Google Scholar]
- 13.Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes. JAMA. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina BSG, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adol Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry. 2008;165:553–555. doi: 10.1176/appi.ajp.2008.08020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood – a naturalistic long-term follow-up study. Addict Behav. 2014;39:325–328. doi: 10.1016/j.addbeh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 18.Mannuzza S, Klein RG, Truong NL, Moulton JL, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Bayless DW, Perez MC, Daniel JM. Comparison of the validity of the use of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder in males and females. Behav Brain Res. 2015;286:85–92. doi: 10.1016/j.bbr.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 21.dela Peña I, Gonzales EL, de la Peña JB, Kim B-N, Han DH, Shin CY, Cheong JH. Individual differences in novelty-seeking behavior in spontaneously hypertensive rats: enhanced sensitivity to the reinforcing effect of methylphenidate in the high novelty-preferring subpopulation. [Accessed 23 June 2015];J Neurosci Methods. doi: 10.1016/j.jneumeth.2014.08.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: methylphenidate vs atomoxetine treatments. Behav Brain Res. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill JC, Herbst K, Sanabria F. Characterizing operant hyperactivity in the spontaneously hypertensive rat. Behav Bran Func. 2012;8:5. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- 25.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 26.Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Func. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe Y, Fujita M, Ito Y, Okada T, Kusuoka H, Nishimura T. Brain dopamine transporter in spontaneously hypertensive rats. J Nuclear Med. 1997;38:470–474. [PubMed] [Google Scholar]
- 28.Wyss JM, Kadish I, van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertension. 2003;25:455–474. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 29.Cao A-H, Yu L, Wang Y-W, Wang J-M, Yang L-J, Lei G-F. Effects of methylphenidate on attentional set-shifting in a genetic model of attention-deficit/hyperactivity disorder. Behav Brain Func. 2012;8:10–19. doi: 10.1186/1744-9081-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharm. 2011;36:837–847. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon SJ, Kim CJ, Lee YJ, Hong M, Han J, Bahn GH. Effect of atomoxetine on hyperactivity in an animal model of attention-deficit/hyperactivity disorder (ADHD) PLoS ONE. 2014;9:e108918. doi: 10.1371/journal.pone.0108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dela Peña I, Lee JC, Lee HL, Woo TS, Lee HC, Sohn AR, Cheong JH. Differential behavioral responses of the spontaneously hypertensive rat to methylphenidate and methamphetamine: lack of a rewarding effect of repeated methylphenidate treatment. Neurosci Letters. 2012;514:189–193. doi: 10.1016/j.neulet.2012.02.090. [DOI] [PubMed] [Google Scholar]
- 33.Baskin BM, Dwoskin LP, Kantak KM. Methylphenidate treatment beyond adolescence maintains increased cocaine self-administration in the spontaneously hypertensive rat model of attention deficit/hyperactivity disorder. Pharm Biochem Behav. 2015;131:51–56. doi: 10.1016/j.pbb.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan CJ, Harvey RC, Baskin BB, Dwoskin LP, Kantak KM. Cocaine-seeking behavior in a genetic model of attention-deficit/hyperactivity disorder following adolescent methylphenidate or atomoxetine treatments. Drug Alcohol Depend. 2014;140:25–32. doi: 10.1016/j.drugalcdep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somkuwar SS*, Jordan CJ*, Kantak KM, Dwoskin LP. Adolescent atomoxetine treatment in a rodent model of ADHD: effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharm. 2013;38:2588–2597. doi: 10.1038/npp.2013.163. *co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huskinson SL, Naylor JE, Rowlett JK, Freeman KB. Predicting abuse potential of stimulants and other dopaminergic drugs: overview and recommendations. Neuropharm. 2014;87:66–80. doi: 10.1016/j.neuropharm.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharm Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. Med Gen Med. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 40.Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: an updated systematic review of the literature. Clin Psych Rev. 2014;34:218–232. doi: 10.1016/j.cpr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Arnold LE. Methylphenidate vs amphetamine: Comparative review. J Atten Disord. 2000;3:200–211. [Google Scholar]
- 42.Faraone SV, Buitelaar J. Comparing the efficacy of medications for ADHD in children and adolescents using meta-analysis. Eur Child Adols Psych. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- 43.Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present - a pharmacological and clinical perspective. J Psychopharm. 2013;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrick KS, Morowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Human Psychopharm. 1997;12:527–546. [Google Scholar]
- 45.Arnold LE, Kirilcuk V, Corson SA, Corson EOL. Levoamphetamine and dextroamphetamine: differential effect on aggression and hyperkinesis in children and dogs. Am J Psychiatry. 1973;130:165–170. doi: 10.1176/ajp.130.2.165. [DOI] [PubMed] [Google Scholar]
- 46.Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobio. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharm Exp Therapeutics. 2001;296:876–883. [PubMed] [Google Scholar]
- 48.Meneses A, Ponce-Lopez T, Tellez R, Gonzalez R, Castillo C, Gasbarri A. Effects of d-amphetamine on short- and long-term memory in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Behav Brain Res. 2011;216:472–476. doi: 10.1016/j.bbr.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Sagvolden T, Xu T. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Func. 2008;4:3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanis JJ, Andersen SL. Reducing substance use during adolescence: a translational framework for prevention. Psychopharm (Berl) 2014;231:1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psych. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J. Effect of psychostimulants on brain structure and function in ADHD. J Clin Psychiatry. 2013;74:902–917. doi: 10.4088/JCP.12r08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarthy S, Asherson P, Coghill D, Hollis C, Murray M, Potts L, Sayal K, de Soysa R, Taylor E, Williams T, Wong ICK. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Brit J Psychiatry. 2009;194:273–277. doi: 10.1192/bjp.bp.107.045245. [DOI] [PubMed] [Google Scholar]
- 58.Zetterqvist J, Asherson P, Halldner L, Långström N, Larsson H. Stimulant and non-stimulant attention deficit/hyperactivity disorder drug use: total population study of trends and discontinuation patterns 2006–2009. Acta Psychiatrica Scandinavica. 2012;128:70–77. doi: 10.1111/acps.12004. [DOI] [PubMed] [Google Scholar]
- 59.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharm. 2005;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 61.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 62.Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol. 2013;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West CHK, Bonsall RW, Emery MS, Weiss JM. Rats selectively bred for high and low swim-test activity show differential responses to dopaminergic drugs. Psychopharm. 1999;146:241–251. doi: 10.1007/s002130051113. [DOI] [PubMed] [Google Scholar]
- 64.Brown GL, Hunt RD, Ebert MH, Bunney WE, Jr, Kopin IJ. Plasma levels of d-amphetamine in hyperactive children. Serial behavior and motor responses. Psychopharm (Berl) 1979;62:133–40. doi: 10.1007/BF00427126. [DOI] [PubMed] [Google Scholar]
- 65.Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behaviour in adulthood. Int J Neuropsychopharm. 2011:1319–1330. doi: 10.1017/S1461145711001544. [DOI] [PubMed] [Google Scholar]
- 66.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharm. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharm. 2004;14:195–205. doi: 10.1089/1044546041649066. [DOI] [PubMed] [Google Scholar]
- 68.Heijtz RD, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? European J Neurosci. 2003;18:3394–99. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 69.Rowley HL, Kulkarni R, Gosden J, Brammer R, Hackett D, Heal DJ. Lisdexamfetamine and immediate release d-amfetamine - differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity. Neuropsychopharm. 2012;63:1064–1074. doi: 10.1016/j.neuropharm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Cesari N, Fontana S, Montanari D, Braggio S. Development and validation of a high-throughput method for the quantitative analysis of D-amphetamine in rat blood using liquid chromatography/MS3 on a hybrid triple quadrupole-linear ion trap mass spectrometer and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:21–28. doi: 10.1016/j.jchromb.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johansen EB, Sagvolden T, Kvande G. Effects of delayed reinforcers on the behavior of an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2005;162:47–61. doi: 10.1016/j.bbr.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 73.Winer BJ, Brown BD, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill; 1991. [Google Scholar]
- 74.Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hinshaw SP, Swanson JM, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–40. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 75.Seidman LJ, Biederman J, Faraone SV, Weber W, Ouelette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psych. 1997;65:150–160. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- 76.Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobio Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 78.Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- 79.Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, et al. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirkpatrick K, Marshall AT, Smith AP, Koci J, Park Y. Individual differences in impulsive and risky choice: Effects of environmental rearing conditions. Behav Brain Res. 2014;269:115–127. doi: 10.1016/j.bbr.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharm, Biochem, Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- 82.Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stamp JA, Mashoodh R, van Kampen JM, Roberton HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and ΔFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 84.Roman O, Seres J, Pometlova M, Jurcovicova J. Neuroendocrine or behavioral effects of acute or chronic emotional stress in Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Endocr Regul. 2004;38:151–155. [PubMed] [Google Scholar]
- 85.Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sterley TL, Howells FM, Russell VA. Effects of early life trauma are dependent on genetic predisposition: A rat study. Behav Brain Func. 2011;7:11. doi: 10.1186/1744-9081-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psych Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 88.MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50:848–857. doi: 10.1016/j.neuint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1in SHR and WKY rat strains. Behav Brain Func. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller EM, Pomerleau F, Huettl P, Gerhardt GA, Glaser PEA. Aberrant glutamate signaling in the prefrontal cortex and striatum of the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Psychopharm (Berl) 2014;231:3019–3029. doi: 10.1007/s00213-014-3479-4. [DOI] [PubMed] [Google Scholar]
- 92.Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neurosci. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 93.Johansen EB, Aase H, Meyer A, Sagvolden T. Attention-deficit/hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behav Brain Res. 2002;130:37–45. doi: 10.1016/s0166-4328(01)00434-x. [DOI] [PubMed] [Google Scholar]
- 94.Paré W, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physio Behav. 1997;62:643–648. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- 95.Zhang-James Y, Yang L, Middleton FA, Yang L, Patak J, Faraone SV. Autism-related behavioral phenotypes in an inbred rat substrain. Behav Brain Res. 2014;269:103–114. doi: 10.1016/j.bbr.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paré W. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physio Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 97.Pardon M-C, Gould GG, Garcia A, Phillips L, Cook MC, Miller A, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neurosci. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 98.De La Garza R., II Wistar Kyoto rats exhibit reduced sucrose pellet reinforcement behavior and intravenous nicotine self-administration. Pharm Biochem Behav. 2005;85:330–337. doi: 10.1016/j.pbb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 99.Cominski TP, Jiao X, Catuzzi JE, Stewart AL, Pang KC. The role of the hippocampus in avoidance learning and anxiety vulnerability. Front Behav Neurosci. 2014;8:273. doi: 10.3389/fnbeh.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De La Garza R, II, Mahoney JJ., III A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 101.Jiao X, Pare WP, Tejani-Butt SM. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharm Biol Psych. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 102.Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar–Kyoto (WKY) and Wistar rats. Life Sci. 2008;83:74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–26. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 104.Kado Y, Sanada S, Yanagihara M, Ogino T, Ohno S, Watanabe K, Nakano K, Morooka T, Oka M, Ohtsuka Y. Executive function in children with pervasive developmental disorder and attention-deficit/hyperactivity disorder assessed by the Keio version of the Wisconsin card sorting test. Brain Dev. 2012;34:354–359. doi: 10.1016/j.braindev.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Reeve WV, Schandler SL. Frontal lobe functioning in adolescents with attention deficit hyperactivity disorder. Adolescence. 2001;36:749–765. [PubMed] [Google Scholar]
- 106.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 107.Warton FL, Howells FM, Russell VA. Increased glutamate-stimulated release of dopamine in substantia nigra of a rat model for attention-deficit/hyperactivity disorder—lack of effect of methylphenidate. Metab Brain Dis. 2009;24:599–613. doi: 10.1007/s11011-009-9166-1. [DOI] [PubMed] [Google Scholar]
- 108.Dick AS. Sources of cognitive inflexibility in set-shifting tasks: insights into developmental theories from adult data. J Cog Dev. 2012;13:82–110. doi: 10.1080/15248372.2011.573516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ricart TM, De Niear MA, Jiao X, Pang KC, Beck KD, Servatius RJ. Deficient proactive interference of eyeblink conditioning in Wistar-Kyoto rats. Behav Brain Res. 2015;216:59–65. doi: 10.1016/j.bbr.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kantak KM, Barlow N, Tassin DH, Brisotti MF, Jordan CJ. Performance on a strategy set shifting task in rats following adult or adolescent cocaine exposure. Psychopharm (Berl) 2014;231:4489–4501. doi: 10.1007/s00213-014-3598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Courvoisie H, Hooper SR, Fine C, Kwock L, Castillo M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsych Clin Neurosci. 2004;16:63–69. doi: 10.1176/jnp.16.1.63. [DOI] [PubMed] [Google Scholar]
- 112.MacMaster FP, Carrey N, Sparkes S, Kusumakar V. Proton spectroscopy in medication- free pediatric attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;53:184–187. doi: 10.1016/s0006-3223(02)01401-4. [DOI] [PubMed] [Google Scholar]
- 113.Carrey N, MacMaster FP, Fogel J, Sparkes S, Waschbusch D, Sullivan S, Schmidt M. Metabolite changes resulting from treatment in children with ADHD: a 1H-MRS study. Clin Neuropharm. 2003;26:218–221. doi: 10.1097/00002826-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 114.Wilens TE, Hammerness P, Utzinger L, Schillinger M, Georgiopoulous A, Doyle RL, Martelon MK, Brodziak K. An open study of adjunct OROS-methylphenidate in children and adolescents who are atomoxetine partial responders: I effectiveness. J Child Adol Psychopharm. 2009;19:485–492. doi: 10.1089/cap.2008.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–125. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hankosky ER, Gulley JM. Performance on an impulse control task is altered in adult rats exposed to amphetamine. Dev Psychobiol. 2013;55:733–744. doi: 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. A single amphetamine infusion reverses deficits in dopamine nerve-terminal function caused by a history of cocaine self-administration. Neuropsychopharm. 2015;40:1826–1836. doi: 10.1038/npp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Floresco SB. Dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:1–12. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharm. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 120.Ragozzinno ME. The effects of dopamine D1 receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brackney RJ, Cheung THC, Herbst K, Hill JC, Sanabria F. Extinction learning deficit in a rodent model of attention-deficit hyperactivity disorder. Behav Brain Func. 2012;8:59–68. doi: 10.1186/1744-9081-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kollins SH, Lane SD, Shapiro SK. Experimental analysis of childhood psychopathology: a laboratory matching analysis of the behavior of children diagnosed with attention-deficit hyperactivity disorder (ADHD) Psychol Record. 1997;47:25–44. [Google Scholar]
- 123.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 124.Mitchell JT, McIntyre EM, McClernon FJ, Kollins SH. Smoking motivation in adults with attention-deficit/hyperactivity disorder using the Wisconsin inventory of smoking dependence motives. Nicotine Tobacco Res. 2014;16:120–125. doi: 10.1093/ntr/ntt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Easton N, Steward C, Marshall F, Fone K, Marsden C. Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharm. 2007;52:405–414. doi: 10.1016/j.neuropharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 126.Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;24:317–327. doi: 10.2147/ndt.s9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.National Institute of Drug Abuse (NIDA) [Accessed 24 July 2015];Trends & statistics: Costs of substance abuse. Available at http://www.drugabuse.gov/related-topics/trends-statistics#costs.
- 128.Substance Abuse and Mental Health Services Administration (SAMHSA) National and state estimates of the drug abuse treatment gap: 2000 National Household Survey on Drug Abuse. Office of Applied Studies; Rockville, MD: 2002. NSDUH Series H-14, DHHS Publication No. SMA 02-3640. [Google Scholar]
- 129.Stanis JJ, Andersen SL. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology (Berl) 2014;231:1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 131.National Survey of Children’s Health Child and Adolescent Health Measurement Initiative. [Accessed 23 June 2015];Data Resource Center on Child and Adolescent Health Website. 2011 Available at http://www.nschdata.org/browse/survey/results?q=2390&r=1&g=451.