Abstract
Low-dose methotrexate (MTX) therapy is a well-recognized therapy for many inflammatory conditions such as rheumatoid arthritis (RA), psoriatic arthritis and psoriasis. More than 20 years ago the clinical efficacy of MTX was also established for steroid dependent Crohn’s disease (CD), but it was never broadly adapted as a treatment modality. More recently, MTX has become increasingly used in the pediatric CD population, both as a single agent as well as a concomitant therapy with anti-tumor necrosis factor-alpha (anti-TNF) treatment. This review outlines important pharmacological aspects for the therapeutic application of MTX and the current status of MTX as mono- or combination therapy in both pediatric and adult patients with IBD including new results of MTX monotherapy in steroid dependent ulcerative colitis (UC).
Keywords: methotrexate, IBD, Crohn’s disease, Ulcerative colitis, anti-TNF therapy
Introduction
The folate antagonist Methotrexate (MTX) was introduced around the late 1940’s as an anti-neoplastic therapy for the treatment of acute leukemia in children. It was later established as a treatment for other solid organ cancers.1 Antifolates such as MTX target thymidylate biosynthesis and the enzyme thymidylate synthase. In cancer chemotherapy, the rationale for the use of high dose MTX is that the rapidly proliferating malignant cells become starved of purine and pyrimidine precursors and therefore are unable to sufficiently maintain DNA and RNA synthesis, leading to decreased proliferation. Due to various toxicities, the high doses of MTX used for cancer chemotherapy were never meant to treat chronic inflammatory conditions. Low dose MTX therapy was tested and established as an anti-inflammatory regimen in psoriasis, psoriatric arthritis and rheumatoid arthritis (RA) in the late 1950’s and early 1960’s.2. In 1987 Kozarek was the first to report the results of intramuscular (im) MTX therapy in patients with refractory IBD (14 CD; 7 UC).3 Twenty-five mg MTX was given once weekly im for 12 weeks and then switched to an oral formulation, which was then tapered to a minimum dose of 7.5 mg in the event of therapeutic success. A significant number of patients, (11/14 patients with CD and 5/7 with UC,) demonstrated a response to MTX such that the prednisone doses could be tapered significantly. The study also described the effects of MTX on mucosal healing, which was observed in 5/11 CD responders (all of them diagnosed with Crohn’s colitis), but in none of the 5 patients with UC (table 1). Six years later, another small study of 18 steroid dependent or steroid refractory IBD patients (10 CD/8 UC) treated with oral low dose MTX (15 mg) suggested efficacy of this drug to lower the daily steroid dose.4
Table 1.
Studies reporting mucosal healing in CD or UC patients responding to MTX therapy.
| Study | Number of patients | IBD type | Evaluation after start of MTX | MTX dose | Mucosal healing (n) | % mucosal healing |
|---|---|---|---|---|---|---|
| Kozarek 19893 | 19 | CD (14) UC (5) |
3 months | 25 mg/week im | 5 (CD) 0 (UC) |
45% (CD) 0 % (UC) |
| Manosa 2010105 | 8 | 6–60 months | 25 mg/week sc or im for 16 weeks, then 15 mg/week | 5 (complete in 3, partial in 2) | 63 % | |
| Carbonnel 201554 | 60 | UC | 4 months | 25 mg/week sc | 21 | 35% |
The clinical use of MTX as induction and maintenance therapy in CD was more definitively established based on the results of the landmark placebo controlled trials of the North American Crohn’s Study Group (NACSG) Investigators published in 1995 and 2000 5, 6. In these studies, steroid dependent CD patients treated with 25 mg MTX im/week achieved steroid free remission rates in 39% compared to 19% on placebo and maintained remission with 15 mg MTX im/week in 65% compared to 39% on placebo.
Around the same time, 4 tertiary care centers in France confirmed the positive therapeutic effects of MTX in 39 therapy refractory CD patients and reported a probability of remission of 72% after 3 months and a probability of remission and steroid withdrawal of 42% after 12 months of therapy with 25 mg MTX im/week.7 In contrast, further exploration of the efficacy of MTX in UC stalled in the late 1990’s after an Israeli multi-center trial in patients with UC yielded a negative result (see below).8
Nevertheless, despite the excellent clinical data for MTX in CD, it has never been widely utilized as monotherapy in adults.9–11 This could be due to the inconvenience of parenteral application either subcutaneously (sc) or im resulting in a patient preference for orally available options such as azathioprine (AZA) or 6-mercaptopurine (6-MP). This may also be related to perceived side effects of MTX, particularly given the toxicities seen at higher doses in cancer chemotherapy. Also, European guidelines only recommend MTX as a second or third line therapy in CD after failure of thiopurine and/or anti-TNF therapy.12, 13 Treatment recommendations from the US do not explicitly advise a preference for AZA/6-MP over MTX, but do suggest the need for more dose ranging trials, maintenance studies and safety data for MTX 14–16. However, the therapeutic use of MTX is increasing pediatric patients. 17, 18 Due to the risk of hepatosplenic T-cell lymphoma associated with AZA/6-MP therapy, MTX is now often used instead of AZA/6-MP either as monotherapy or in combination with anti-TNF agents.
The purpose of this review is to 1) describe the clinical pharmacology of MTX and its implications on the mode of administration, 2) review the efficacy of MTX in CD and UC either as mono or combination therapy, and 3) highlight important aspects of adverse events of MTX therapy and their implications on the practical treatment approach.
Clinical pharmacology and bioavailability of oral and parenteral MTX
MTX has a relatively short serum half-life of 6 – 8 hours and more than 80% of the drug in its intact form is excreted in the urine by glomerular and tubular secretion.19, 20 Thus, drugs interfering with renal tubular secretion such as probenecid or the existence of low kidney function result in an increase in circulating MTX, which can lead to toxic side effects (table 2). Approximately 35% of MTX is bound to plasma proteins including albumin. Toxicity can be observed with concurrent low serum albumin concentrations or if MTX is displaced from albumin by drugs such as sulfonamides or tetracyclines. This drug is rapidly taken up from the plasma into a variety of cells and intracellular MTX is converted to MTX polyglutamates, which cross poorly through cellular membranes. The slow transmembrane migration leads to cellular entrapment, which is one of the mechanisms causing prolonged retention of the drug in tissues such as the liver and kidney.21 The MTX polyglutamates are thought to be responsible for most of the anti-inflammatory effects. However, studies in patients with RA and IBD have so far not found any meaningful correlation between the clinical efficacy of MTX and serum or plasma levels of either intact MTX or its metabolites, suggesting that there is little clinical value in monitoring these levels.22, 23
Table 2.
Factors associated with MTX toxicity.
|
In comparing modes of drug administration, oral versus parenteral applications in patients with RA, both response time and clinical efficacy of oral MTX is poorer, at least at a dose of ≥ 15 mg.24–31 Also, in patients with small bowel CD the bioavailability of oral MTX is highly variable and averages only 73% of that of sc administration. However, this is not solely due to small intestinal inflammation since a similar lower bioavailability of oral MTX has been reported for patients with RA.26, 32 Interestingly, an increased bioavailability in RA patients treated with 25–30 mg MTX orally can be achieved by splitting the total dose in two oral applications, taken 8 hours apart.33. As to the effectiveness of a specific parenteral form, (sc or im application), similar bioavailability and pharmacokinetics for both modes of application have been demonstrated.34 Hence, the preferred parenteral treatment method should be sc injections due to the easier and less painful mode of administration.
One small study with 11 children with IBD prospectively evaluated the bioavailability of oral and sc applied MTX and did not find a difference between both.35 However, no data comparing prospectively the clinical efficacy of oral and sc MTX are available for patients with IBD. Retrospective single or multi-center analyses of CD patients treated with MTX reported both a superiority of parenteral over oral MTX 36, 37 or no difference between both.38–42. Therefore a comparative effectiveness study of oral vs sc administered MTX in patients with IBD is undoubtedly needed, since adherence to therapy may be increased in case of equal efficacy of oral MTX compared to subcutaneous applications in maintenance therapy.
MTX monotherapy in CD
Two recently updated Cochrane reviews demonstrated the efficacy of MTX 25 mg administered im once weekly for induction of remission followed by MTX 15 mg, im, once weekly for maintenance of remission in adult CD.43, 44 (table 3) So far no formal dose-finding studies have been conducted in patients with IBD. In contrast to the Cochrane analyses, the most current “American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti–TNF-a biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease” concluded that MTX (and AZA/6-MP) is no more effective than placebo to induce remission in CD but is effective in maintaining remission.16
Table 3.
Cochrane analyses of azathioprine (AZA)/6-mercaptopurine and methotrexate for induction and maintenance of remission in Crohn’s disease.43, 44, 106, 107
A direct comparison of the clinical efficacy of MTX to AZA/6-MP in inducing and maintaining remission in CD is difficult, since only 2 small trials approached this question.45, 46 Both showed similar efficacy but the data quality and size of the trials were insufficient and thus these were not included in the Cochrane analyses. The available data for the analyses also reveal that in contrast to several trials with 6-MP or AZA with over 1761 patients the evidence for the clinical efficacy of MTX in CD is based on only 2 well designed trials including 217 patients.
In regard to the required dose of MTX to maintain remission, the only prospective study in patients with CD did not compare multiple doses. This study evaluated a dose of 15 mg MTX sc/week as a monotherapy. However, in a subgroup of 14 patients who relapsed on 15 mg MTX, retreatment with the 25 mg MTX im/week in combination with a steroid taper could re-induce lasting remission in more than half of the relapsed patients. This suggests that, for a subgroup of CD patients, a maintenance dose of > 15 mg/week may be required.
Subsequent to the above-described NACSG MTX landmark trials, several single or multi-center analyses of the long-term effectiveness of MTX in CD have been published. Hausmann et al. performed a retrospective analysis of 63 patients with CD treated with oral or parenteral MTX at a German tertiary care center with mean duration of treatment of 100 weeks (range 2–364 weeks).41 During induction, either MTX monotherapy or combination therapy with either infliximab (IFX) or steroids was used and clinical remission was achieved in 79% of the patients after 3 months. The cumulative probability to maintain remission on a sc or oral dose of 25 mg MTX/week was 90%, 71% and 63% after 1, 2 and 3 years of treatment, respectively. The same authors performed a meta-analysis placing their data in the context of 4 other long term follow-up MTX studies including a total of 267 CD patients.36, 41, 47–49 They calculated the cumulative probability of maintaining remission on MTX as 75% (95% CI: 64–86%), 53% (95% CI: 36–69%), and 43% (95% CI: 21–65%) after 1, 2 and 3 years of treatment, respectively. Seinen et al analyzed 174 CD patients, who received MTX monotherapy after AZA failure in 3 tertiary care centers in the Netherlands and reported sustained clinical benefit of 63%, 47% and 20% after 1,2 and 5 years of therapy, respectively.50 Similarly, a low sustained clinical benefit of MTX monotherapy has been reported by a single center study in Leeds, England, with a sustained clinical benefit of 29% (19/66 CD patients) after a median duration of 36 months of MTX therapy. 51 In comparison a large, English, single center study including 622 CD patients treated with AZA demonstrated remission rates of was 95%, 69%, and 55% for 1,3 and 5 years, respectively.52 Due to the heterogeneous patient populations it is difficult to compare the above-described studies spanning several years with each other or to AZA/6-MP. Nonetheless the overall effectiveness of MTX seems to decrease over 3–5 years of therapy. Multiple factors might influence the long term efficacy of MTX in CD including medication compliance, lower and ineffective maintenance doses of MTX or as yet undefined escape mechanisms of the immune system, which lead to re-occurrence of intestinal inflammation despite ongoing immunosuppressive therapy.
MTX monotherapy in UC
In contrast to CD, further exploration of MTX treatment in UC in prospective clinical studies was deterred by the negative result of an Israeli multi-center study investigating the clinical efficacy of 12.5 mg MTX given orally over 9 months to patients with steroid dependent UC.8 There were no significant differences among the groups with regard to the primary outcomes, monthly steroid use, clinical Mayo scores, or the sigmoidoscopy scores for inflammation. Interestingly, outside of clinical studies, IBD centers used MTX successfully for selected UC patients in clinical practice, as is reflected by numerous retrospectively analyses (reviewed in 53). The largest retrospective analysis was conducted using a drug prescription database of a UC cohort of 22,762 patients from the Veterans Affairs Health Care System between 2001–2011.40 Ninety-one UC patients were identified, who were either treated with oral MTX (75%; mean dose MTX/week 14 mg) or parenteral MTX (25%; mean dose MTX/week 25 mg). At the end of a 15 months follow-up period, 37% of the oral and 30% of the parenteral MTX group had stopped prednisone.
To definitively evaluate whether MTX has a significant clinical efficacy in UC if applied at the same dose (25 mg) and the same route (sc) as in the NACSG CD trials, 2 prospective clinical trials have been initiated. The first study was recently completed by the GETAID (Groupe d’Étude Thérapeutique des Affections Inflammatoires du Tube Digestif) in France and investigated the efficacy of MTX to induce steroid free remission.54 The second study, which is sponsored by the NIH (National Institute of Health) and performed by the CCFA - CRA (Crohn’s and Colitis Foundation of America - Clinical Research Alliance) is analyzing the efficacy of MTX in maintaining steroid free remission and is currently ongoing (clinicaltrials.gov; NCT01393405).
The French METEOR (Comparison of Methotrexate vs Placebo in Corticosteroid-dependent Ulcerative Colitis) trial investigated the clinical efficacy of sc MTX 25 mg/week as an induction regimen over a total of 24 weeks. The primary endpoint of this study defined as “success” was a combined endpoint of a total Mayo score ≤ 2 with no item > 1, complete steroid withdrawal with a forced steroid tapering regimen and no further need for other immunosuppressants, anti TNF or colectomy at week 16. Of the 111 included patients (60 on MTX, 51 on placebo), 32% on MTX vs 20% on placebo achieved treatment success, which did not reach statistical significance (p<0.15). However, statistical significance was met for a secondary endpoint of clinical remission, which excludes the sigmoidoscopy subscore. Significantly more patients achieved clinical remission without need for steroids and immunosuppressant’s or anti-TNF drugs on MTX vs placebo (42% vs 24%; p≤0.04).
This discrepancy between the results of the full Mayo score, which includes sigmoidoscopy and the clinical Mayo score without sigmoidoscopy might be due to 2 scenarios. One possibility is that there was significant inter-observer variability leading to errors in discriminating an endoscopic score of 1, which in METEOR was defined as endoscopic remission, from an endoscopic score of 2 indicating active inflammation. This could have been avoided with central reading of the endoscopy pictures or videos, but this was not available for this investigator-initiated study.55 Alternatively, the data might indicate that MTX in patients with UC induces clinical remission in a significantly higher proportion of patients without leading to improvement of inflammation of the recto-sigmoid mucosa. Interestingly such a possibility was also initially suggested by the first study by Kozarek et al, who observed far less success in inducing endoscopic remission in his 7 UC patients compared to the 14 CD patients.3 However, the lack of significant differences in endoscopic results between the placebo and MTX group in the METEOR trial is even more astonishing since a significantly higher number of patients reported cessation of bleeding and improvement of diarrhea in the MTX group as compared to placebo. These clinical parameters have been associated with endoscopic healing, thus questioning the vailidity of the endoscopic scores.56, 57
Thus the results of METEOR are not as clear-cut as one would have hoped for. The US MERIT-UC trial (Methotrexate Response In Treatment of UC), a prospective, randomized, placebo controlled study to analyze the efficacy of MTX in maintaining steroid free remission over 54 weeks will hopefully be more definitive. This trial is still recruiting and initial results are expected in early 2017. MTX could represent a unique and affordable therapy for UC patients in need of immunosuppressive treatment.
MTX in combination with anti-TNF agents
Since the original report by Baert et al, showing the clinical impact of anti-IFX antibodies, which can be prevented with adding an immunomodulator therapy such as AZA/6-MP or MTX, combination therapy for IFX has been advocated.58 AZA/6-MP and MTX appear to be equally effective in suppressing antibody formation and preserving higher IFX trough levels.59 The Combination Of Maintenance Methotrexate-Infliximab Trial (COMMIT) is the most recent trial to demonstrate an effect of MTX on IFX trough and anti-IFX antibody levels (table 4).60 However, the study did not reveal a difference in clinical outcomes between combination MTX/IFX and IFX monotherapy, despite the effects of MTX on antibody formation to IFX and IFX trough levels. These results are in contrast to the results of the Study of Biologic and Immunomodulator Naive Patients in CD study (SONIC), which demonstrated an increased effectiveness of an AZA/IFX combination therapy compared to an IFX monotherapy in inducing and maintaining remission, which was corroborated by higher IFX trough levels and lower anti-IFX antibodies.61 The reasons for the differences between COMMIT and SONIC can only be speculated upon, but several factors, which might have influenced the differing outcomes seen in these trials have been postulated (table 5).62
Table 4.
Detectable infliximab (IFX), IFX trough levels and detectable anti-IFX antibodies in the COMMIT study (n=126 patients).60
| Detectable IFX | p-value | IFX trough- level (mg/ml) | p-value | Detectable IFX Antibody | p-value | |
|---|---|---|---|---|---|---|
| IFX+MTX | 20% | <0.08 | 6.4 | <0.08 | 4% | <0.01 |
| IFX | 14% | 3.8 | 20% |
Table 5.
|
MTX in pediatric IBD
Although there are no placebo-controlled, randomized trials of MTX for the treatment of pediatric CD, the use of MTX in this patient population is increasing and the body of published observational studies is growing. Mack et al. first reported on 14 patients with a mean age of 10.6 years who had active CD and were intolerant or unresponsive to 6-MP.63 Subcutaneous administration of MTX resulted in 64% of patients with clinical improvement by as early as 4 weeks. Steroid sparing was also demonstrated. Adverse events attributed to MTX were nausea and headache leading to withdrawal of therapy in two patients. No patients demonstrated bone marrow suppression, abnormal liver chemistries, or pulmonary complications. Another single center experience demonstrated a 12-month steroid-free remission rate of about 33% which is similar to that seen in reports of adult patients with CD.64 Good tolerance of the MTX therapy was reported.
Two multicenter retrospective studies published in 2006 and 2007 demonstrated a 40–45% 1-year steroid-free remission rate with MTX as a second-line immunomodulator in pediatric CD patients.65, 66 No difference in effect was seen regardless of whether the indication for the MTX was lack of thiopurine efficacy or intolerance. Again, overall good drug tolerance was demonstrated as was a steroid sparing effect and a positive effect on linear growth.65
More recently, a retrospective cohort study 172 children who received at least 3 months of MTX without thiopurine or biologicals and had ≥1 year of follow-up was reported.18 Twenty seven percent of those receiving MTX as a first line IMM achieved ≥12 months of sustained clinical remission without surgery, thiopurine, biologicals, or corticosteroids, as compared with 35% of those receiving MTX as a second line agent (p> 0.05). A recent European multi-center retrospective cohort of 113 children with CD in remission (median age 14 years) demonstrated a slightly higher effectiveness of MTX. In this study, 52% of patients remained in steroid- and biologics-free remission 12 months after starting MTX monotherapy.67
There are also emerging pediatric data regarding combination therapy with anti-TNF biologics and MTX. In a single center experience, pediatric CD patients treated with low-dose oral MTX (<10 mg/wk) demonstrated no benefit in terms of IFX effectiveness or durability as compared to children receiving IFX monotherapy.68 However, Grossi recently reported a retrospective, multicenter cohort study of 81 children receiving combination therapy with IFX and MTX (60 male, 21 female).69 Males receiving MTX for more than 6 months showed a significantly greater likelihood of remaining on IFX throughout the follow-up period compared to no MTX or other immunomodulator or ≤6 months MTX, with 5 year probabilities ranging from .41 ± .11 among patients who received no concomitant immunomodulators to .97±.03 in patients treated with MTX > 6 months (p<.001). Among females, the trend was in the same direction. Additionally, the durability of IFX was significantly greater with MTX as compared to thiopurines (probability to stay on IFX .97±.03 vs .58±.08 on placebo). In this study, the investigators were not able to identify an optimal dose or route of administration for concomitant MTX, as nearly all patients continued on IFX regardless of MTX dose/route. To compare the effectiveness of anti-TNF alone or in combination with oral MTX a large, multi-center pragmatic clinical trial has been recently funded by the Patient Centered Outcomes Research Institute (http://www.pcori.org/research-results/2015/anti-tnf-monotherapy-versus-combination-therapy-low-dose-methotrexate).
Adverse reactions to MTX therapy
When comparing the prospective placebo controlled trials of MTX in CD, more patients in the induction trial withdrew related to adverse events than in the maintenance trial (17% vs 3%), which might be due to different dosing (25 mg vs 15 mg) or the fact that adverse effects leading to the stop of MTX appear early in the treatment course; however, overall adverse event rates were quite low. Interestingly, single center analyses show a higher absolute rate of adverse events leading to discontinuation of MTX with approximately 20–33% of patients discontinuing therapy due to adverse events.41, 50, 51, 70 This seems to be comparable to AZA/6-MP reported adverse events leading to discontinuation of therapy.71–73
The most frequently observed adverse event of MTX therapy is nausea (in up to 25% of treated patients), which often can be well-controlled and/or avoided with concomitant ondansetron tablets given 1–2 hours before and 12–24 hours after the MTX application. Other significant adverse events of MTX can include hepatotoxicity, bone marrow suppression, hypersensitivity pneumonitis, gastrointestinal toxicity and infectious complications as a consequence of immunosuppression.74, 75 In contrast to treatments with anti-TNF agents and AZA/6-MP, until now now no increased incidence of cancer or lymphoma has been reported for MTX; albeit overall rates of use are lower than that of the other therapies.76 In clinical practice, MTX therapy is often recommended for IBD patients with a history of malignant disease who are in need of immunosuppressive treatment to control their disease. However, the current data landscape for such an assumption is too small to draw this conclusion.77
MTX Hepatotoxicity
MTX may promote a rise of liver enzymes, which is observed in 3–33% of IBD patients treated with MTX and necessitates discontinuing therapy in around 6–8%.6, 50, 78 Recommendations for stopping therapy are based on degree of absolute change in liver enzymes and duration of abnormalities on serial measurements. Underlying liver diseases such as non-alcoholic fatty liver disease (NAFLD), diabetes or excess alcohol consumption are important cofactors for MTX induced liver toxicity. However, MTX associated advanced liver fibrosis is rarely seen in patients with IBD or RA, but is more often found in patients treated with MTX for psoriasis.79 In the past, guidelines recommended a liver biopsy after a cumulative dose of 1,500 mg MTX. These recommendations were based on expert opinion and have been revised for IBD and RA patients.14, 80 Current recommendations only include an initial liver biopsy for patients with psoriasis after reaching a cumulative threshold at 1,000–1,500 mg MTX in patients with baseline risk factors (e.g. NAFLD) or 3,500 – 4,000 mg in those without risk factors.81, 82 No scheduled liver biopsies are recommended in IBD patients as several studies have found no association between the cumulative dose of MTX and the development of liver fibrosis or cirrhosis (table 6). Altogether the risk of end stage liver cirrhosis seems to be very low, as reflected by the small total number (117) of cases of MTX induced end stage liver disease (0.07%) out of the 158,904 adults who have been listed for, and/or received, liver transplantation in the USA in the past 24 years.83, 84
Table 6.
Cumulative MTX dose and histologic evaluated liver toxicity.
| Study | Number of patients | Mean cumulative dose MTX (mg) | Range cumulative dose MTX (mg) | Histological evaluation110 | |
|---|---|---|---|---|---|
| Early changes (Roenigk I, II) | Advanced changes Roenigk III, IV) | ||||
| Te 2000111 | 20 | 2,633 | 1,500 – 5,140 | 19 | 1 |
| Leman 200048 | 11 | 1,225* | 220 – 3,400* | 9 | 2 |
| Fraser 2002112 | 3 | >1,500 | not reported | 3 | 0 |
| Fournier 201078 | 17 | 2,653 | 1,400 – 6,025 | 16 | 1 |
| Laharie 2006 and 2010102, 104 | 7 | 3,152 | 2,170 – 5,705 | 5 | 2 |
Mean MTX dose and MTX dose range of 41 patients, of whom 11 underwent liver biopsy
MTX induced bone marrow suppression and pneumonitis
Bone marrow suppression (pancytopenia) in the context of low-dose MTX therapy occurs in approximately 1% of patients treated for RA.85 Severe pancytopenia has been associated with low folate levels in the absence of folate supplementation, low serum albumin concentrations (<2.6 g/dl) and renal impairment. Depressed levels of serum albumin and decreased kidney function lead to an increase of circulating MTX and MTX metabolites thus leading to increased toxicity.86
Pulmonary toxicity is a rare side effect of MTX therapy, which clinically is characterized by the new onset of dyspnea, a dry cough, and fever and usually presents radiologically as an acute interstitial pneumonitis.87, 88 Meta-analyses in patients with RA suggest a decreasing incidence of pulmonary toxicity; most likely due to improved selection criteria of patients for MTX therapy.89 This is also supported by the fact that no increased incidence of pulmonary complications is reported in more strictly controlled randomized trials in IBD, psoriatic arthritis and psoriasis.89, 90 Most cases of MTX-induced lung injury are reversible following discontinuation of therapy.
MTX therapy: Contraindications and adverse events
Due to the potential teratogenicity, MTX is contraindicated in pregnancy 91. Currently it is recommended that females and males stop MTX therapy 3 months before planned pregnancy and that MTX should not be used during pregnancy or breast feeding.80, 92 The contraindication for females with planned pregnancy is supported by data showing that women exposed to MTX at dosages typically used in RA therapy after conception have increased risk of spontaneous abortion or of delivering babies with major birth defects. Such risks are not demonstrated if MTX therapy is stopped 12 weeks prior to conception.93 The recommendations that females on MTX should not to breast feed due to adverse or immunosuppressive effects of MTX in breast milk are based solely on expert opinion.80, 92, 94, 95 For males planning a pregnancy with their partner, more recent data point to no increase in major birth defects, spontaneous abortion, gestational age at delivery or birth weight due to continuing MTX therapy.96
Relative contraindications for MTX therapy are pre-existing liver disease (e.g. Hepatitis B or C) and conditions associated with NAFLD such as diabetes and obesity with a body mass index > 30. Additionally, impaired kidney function, known excessive alcohol consumption, immunosuppressed state (other than caused by concomitant medications), clinically significant leukopenia or thrombopenia, active infections, and an unreliable or noncompliant patient (due to required lab monitoring) are contraindications for MTX treatment.14, 80, 81
MTX at doses of 10 mg once weekly appears safe to be continued peri-operatively based on data collected in prospective studies involving mainly orthopedic surgeries in patients with RA.80, 97 However, no such data exists for the perioperative risk of abdominal or wound infections for patients with CD, who are normally treated with of 15–25 mg MTX weekly.
Monitoring of MTX therapy
Before and after starting MTX or increasing the dose it is recommended to check for indicators off MTX toxicity including liver chemistries, creatinine and CBC (table 7, figure 1 and 2). In the case of normal liver function tests and no risk factors for cirrhosis (NASH, alcohol), planned liver biopsies after cumulative thresholds of MTX are not required for IBD patients (see above). In the case of persistently abnormal liver-associated chemistries or a low serum albumin, guidelines of the American Gastroenterology Association and the American College of Gastroenterology recommend either a discontinuation of MTX therapy or a liver biopsy.14, 15 These recommendations are based on the suggestions of the American College of Rheumatology made in 1994, which advised a liver biopsy if the aspartate-aminotransferase (AST) value is above the upper limit of laboratory normal in 5 of 9 determinations within a given 12-month interval or in 6 of 12 measurements if tests are performed monthly or a decrease in serum albumin below the normal range.98 These recommendations are suboptimal for patients with IBD on MTX therapy. Serum albumin levels can fall below normal levels in CD or UC patients due to active intestinal inflammation and liver fibrosis may develop despite normal liver function tests. European guidelines also do not recommend a surveillance liver biopsy, but state “if the AST doubles then it is sensible to withhold methotrexate until it returns to normal before a rechallenge”.12, 13
Table 7.
Recommended tests before starting MTX therapy53
| Assess for clinical risk factors for MTX liver toxicity | Laboratory work up | Radiology | Consideration of following tests: |
|---|---|---|---|
|
| |||
| Obesity1 Diabetes mellitus1 Alcohol intake |
AST, ALT Albumin2 CBC Creatinine3 |
Chest X-ray to rule out interstitial lung disease 4 | Serology testing for: Hepatitis B, C HIV Pregnancy Test Lipid profile1 Blood fasting glucose1 |
Associated with hepatotoxicity in the context of non-alcoholic fatty liver disease (NAFLD) and the risk of preexisting liver fibrosis.
Low albumin is associated with thrombocytopenia, liver and pulmonary toxicity.
Creatinine clearance <79 ml/min reduces methotrexate clearance and is associated with more severe toxicity.
Obtained within the previous year before start of methotrexate therapy.
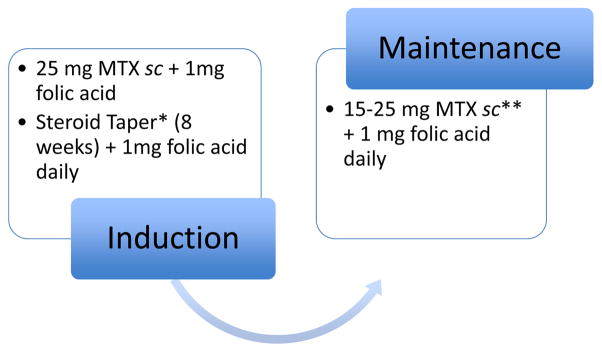
Figure 1.
Induction and maintenance of MTX therapy. Folic acid decreases the systemic hematologic and hepato-gastrointestinal side effects of MTX and can be given as 1 mg daily or 5 mg once weekly.80
*Due to the delayed onset of the therapeutic efficacy of MTX of around 6–8 weeks, one should consider an additional induction therapy such as steroids in case of MTX monotherapy. ** A decrease of the MTX dose from 25 to 15 mg sc or a switch to oral MTX can be considered. However, both may be associated with a higher risk for relapse due to either substandard dosing or decreased bioavailability.
In case of nausea: Ondansentron 4–8 mg before and on the day after injection.
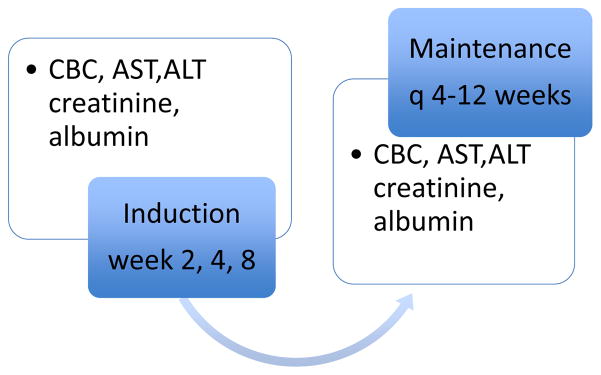
Figure 2.
Monitoring of MTX therapy * currently not recommended by guidelines but may be a reasonable approach based on current literature.42, 102, 104
Liver biopsy: Not generally recommended except for persistent elevation of Liver function tests. * Consider Liver elastography as a monitoring tool especially for patients with risk factors such as obesity, NAFLD and alcohol consumption.
When starting MTX therapy a prescription of at least 5mg folic acid or folinic acid either as a single weekly dose of given daily (1 mg folic acid) is strongly recommended (figure 1).80 Folic acid or folinic acid reduces the gastrointestinal (including the incidence of mouth sores) and liver toxicity of methotrexate and increases the ability of the patient to continue on MTX therapy.99 The therapeutic efficacy of MTX is not affected by this supplementation and it does not matter if the folic acid is given on the same day of MTX or delayed by 24 hours (in case of weekly administration of folic acid).100, 101
In regard to the development of fibrosis or cirrhosis aside of checking liver function tests, all current IBD guidelines do not advise on other monitoring strategies or on specific monitoring of patients with risk factors for liver fibrosis such as diabetes, NAFLD or obesity. However, liver fibrosis or cirrhosis may develop in patients while being on MTX therapy with normal liver function tests due to predisposing risk factors.42, 102, 103 Transient elastography (TE; Fibroscan) is a non-invasive method to measure liver stiffness.103 In a Spanish study, screening TE of 46 IBD patients treated with MTX detected significant fibrosis according Metavir criteria in 6 patients (13%).42 Laharie et al analyzed 518 patients with various inflammatory diseases, who were all treated with MTX with a combination of TE and laboratory derived FibroTest scores and found that 8% (43 patients) had results suggesting severe liver fibrosis.102 Liver biopsies were only performed in 13 of these patients and showed advanced fribrosis in 6/13 (46%). Predictive factors for a TE value of > 7.9kPa indicating severe fibrosis in a multivariate analysis were a BMI > 28 kg/m2 and alcohol consumption > 14 drinks/week. So far no cost effectiveness analyses have been performed for TE, but apart from regularly checking liver function tests it seems reasonable to screen patients with known risk factors for liver fibrosis with TE and perform liver biopsies in the event of pathologic TE results.
Conclusion
MTX is an inexpensive and well-characterized immunosuppressive drug, which has demonstrated proven efficacy for more than 20 years in patients with CD. MTX is currently being increasingly used in combination with anti-TNF agents to prevent immunogenicity. Apart from the recent data from the French METEOR study, more data are necessary to definitively assess the clinical efficacy of MTX in patients with UC. These data will be available around the end of 2017 once the current US study MERIT-UC is fully recruited. Currently sc application, at least in monotherapy, is advisable for all patients treated with MTX, since the bioavailability of parenteral MTX at doses > 15 mg seems to be superior. Optimal dosing and route of administration of MTX when used in combination therapy are not known. Adverse events associated with MTX occur in comparable rates to those seen with alternate therapies, such as 6MP/azathioprine. Special consideration should be given to the use of MTX in young women of childbearing age, due to fetal birth defects associated with MTX use. To prevent drug toxicity and avert unnecessary adverse events of MTX therapy, one should be well aware of the screening and surveillance assessments of MTX therapy.
Acknowledgments
This work is supported by National Institutes of Health grants NIH P30 DK3498 and 1U01-DK092239-01
References
- 1.Farber S. Chemotherapy in the treatment of leukemia and Wilms’ tumor. JAMA. 1966;198:826–36. [PubMed] [Google Scholar]
- 2.Benedek TG. Methotrexate: from its introduction to non-oncologic therapeutics to anti-TNF-alpha. Clin Exp Rheumatol. 2010;28:S3–8. [PubMed] [Google Scholar]
- 3.Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–6. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 4.Baron TH, Truss CD, Elson CO. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci. 1993;38:1851–6. doi: 10.1007/BF01296109. [DOI] [PubMed] [Google Scholar]
- 5.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627–32. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292–7. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 7.Lemann M, Chamiot-Prieur C, Mesnard B, et al. Methotrexate for the treatment of refractory Crohn’s disease. Aliment Pharmacol Ther. 1996;10:309–14. doi: 10.1111/j.0953-0673.1996.00309.x. [DOI] [PubMed] [Google Scholar]
- 8.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–21. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 9.Saibeni S, Bollani S, Losco A, et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123–7. doi: 10.1016/j.dld.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Herfarth HH, Long MD, Isaacs KL. Methotrexate: underused and ignored? Dig Dis. 2012;30 (Suppl 3):112–8. doi: 10.1159/000342735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benchimol EI, Cook SF, Erichsen R, et al. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:878–89. doi: 10.1016/j.crohns.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein GR, Abreu MT, Cohen R, et al. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940–87. doi: 10.1053/j.gastro.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein GR, Hanauer SB, Sandborn WJ, et al. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 16.Dassopoulos T, Sultan S, Falck-Ytter YT, et al. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1464–78. e1–5. doi: 10.1053/j.gastro.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–52. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 18.Sunseri W, Hyams JS, Lerer T, et al. Retrospective cohort study of methotrexate use in the treatment of pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:1341–5. doi: 10.1097/MIB.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 19.Shen DD, Azarnoff DL. Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet. 1978;3:1–13. doi: 10.2165/00003088-197803010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165–71. doi: 10.1002/jps.2600780219. [DOI] [PubMed] [Google Scholar]
- 21.Stamp LK, Barclay M. Therapeutic drug monitoring in rheumatic diseases: utile or futile? Rheumatology (Oxford) 2014;53:988–97. doi: 10.1093/rheumatology/ket355. [DOI] [PubMed] [Google Scholar]
- 22.Lafforgue P, Monjanel-Mouterde S, Durand A, et al. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1995;22:844–9. [PubMed] [Google Scholar]
- 23.Egan LJ, Sandborn WJ, Tremaine WJ, et al. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1597–604. doi: 10.1046/j.1365-2036.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86–90. doi: 10.1093/rheumatology/36.1.86. [DOI] [PubMed] [Google Scholar]
- 25.Jundt JW, Browne BA, Fiocco GP, et al. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol. 1993;20:1845–9. [PubMed] [Google Scholar]
- 26.Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645–8. [PubMed] [Google Scholar]
- 27.Braun J, Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum. 2008;58:73–81. doi: 10.1002/art.23144. [DOI] [PubMed] [Google Scholar]
- 28.Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32:563–71. [PubMed] [Google Scholar]
- 29.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses >/=15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549–51. doi: 10.1136/annrheumdis-2014-205228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis. 2009;68:1094–9. doi: 10.1136/ard.2008.092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206504. [DOI] [PubMed] [Google Scholar]
- 32.Kurnik D, Loebstein R, Fishbein E, et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:57–63. doi: 10.1046/j.1365-2036.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoekstra M, Haagsma C, Neef C, et al. Splitting high-dose oral methotrexate improves bioavailability: a pharmacokinetic study in patients with rheumatoid arthritis. J Rheumatol. 2006;33:481–5. [PubMed] [Google Scholar]
- 34.Brooks PJ, Spruill WJ, Parish RC, et al. Pharmacokinetics of methotrexate administered by intramuscular and subcutaneous injections in patients with rheumatoid arthritis. Arthritis Rheum. 1990;33:91–4. doi: 10.1002/art.1780330112. [DOI] [PubMed] [Google Scholar]
- 35.Stephens MC, Baldassano RN, York A, et al. The bioavailability of oral methotrexate in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2005;40:445–9. doi: 10.1097/01.mpg.0000157588.27125.50. [DOI] [PubMed] [Google Scholar]
- 36.Chong RY, Hanauer SB, Cohen RD. Efficacy of parenteral methotrexate in refractory Crohn’s disease. Aliment Pharmacol Ther. 2001;15:35–44. doi: 10.1046/j.1365-2036.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 37.Turner D, Doveh E, Cohen A, et al. Efficacy of oral methotrexate in paediatric Crohn’s disease: a multicentre propensity score study. Gut. 2014 doi: 10.1136/gutjnl-2014-307964. [DOI] [PubMed] [Google Scholar]
- 38.Nathan DM, Iser JH, Gibson PR. A single center experience of methotrexate in the treatment of Crohn’s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol. 2008;23:954–8. doi: 10.1111/j.1440-1746.2007.05006.x. [DOI] [PubMed] [Google Scholar]
- 39.Wahed M, Louis-Auguste JR, Baxter LM, et al. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Aliment Pharmacol Ther. 2009;30:614–20. doi: 10.1111/j.1365-2036.2009.04073.x. [DOI] [PubMed] [Google Scholar]
- 40.Khan N, Abbas AM, Moehlen M, et al. Methotrexate in ulcerative colitis: a nationwide retrospective cohort from the Veterans Affairs Health Care System. Inflamm Bowel Dis. 2013;19:1379–83. doi: 10.1097/MIB.0b013e31828133e8. [DOI] [PubMed] [Google Scholar]
- 41.Hausmann J, Zabel K, Herrmann E, et al. Methotrexate for maintenance of remission in chronic active Crohn’s disease: long-term single-center experience and meta-analysis of observational studies. Inflamm Bowel Dis. 2010;16:1195–202. doi: 10.1002/ibd.21166. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Lama Y, Taxonera C, Lopez-Sanroman A, et al. Methotrexate in inflammatory bowel disease: a multicenter retrospective study focused on long-term efficacy and safety. The Madrid experience. Eur J Gastroenterol Hepatol. 2012;24:1086–91. doi: 10.1097/MEG.0b013e3283556db5. [DOI] [PubMed] [Google Scholar]
- 43.McDonald JW, Wang Y, Tsoulis DJ, et al. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD003459. doi: 10.1002/14651858.CD003459.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel V, Wang Y, MacDonald JK, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD006884. doi: 10.1002/14651858.CD006884.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mate-Jimenez J, Hermida C, Cantero-Perona J, et al. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–33. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 46.Ardizzone S, Bollani S, Manzionna G, et al. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619–27. doi: 10.1016/s1590-8658(03)00372-4. [DOI] [PubMed] [Google Scholar]
- 47.Domenech E, Manosa M, Navarro M, et al. Long-term methotrexate for Crohn’s disease: safety and efficacy in clinical practice. J Clin Gastroenterol. 2008;42:395–9. doi: 10.1097/MCG.0b013e31802e6875. [DOI] [PubMed] [Google Scholar]
- 48.Lemann M, Zenjari T, Bouhnik Y, et al. Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000;95:1730–4. doi: 10.1111/j.1572-0241.2000.02190.x. [DOI] [PubMed] [Google Scholar]
- 49.Charpignon C, Beau P. Methotrexate as single therapy in Crohn’s disease: is its long-term efficacy limited? Gastroenterol Clin Biol. 2008;32:153–7. doi: 10.1016/j.gcb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Seinen ML, Ponsioen CY, de Boer NK, et al. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2013;11:667–72. doi: 10.1016/j.cgh.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 51.Suares NC, Hamlin PJ, Greer DP, et al. Efficacy and tolerability of methotrexate therapy for refractory Crohn’s disease: a large single-centre experience. Aliment Pharmacol Ther. 2012;35:284–91. doi: 10.1111/j.1365-2036.2011.04925.x. [DOI] [PubMed] [Google Scholar]
- 52.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–9. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herfarth HH, Osterman MT, Isaacs KL, et al. Efficacy of methotrexate in ulcerative colitis: failure or promise. Inflamm Bowel Dis. 2010;16:1421–30. doi: 10.1002/ibd.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carbonnel F, Colombel J-F, Filippi J, et al. 745 Methotrexate for Corticosteroid-Dependent Ulcerative Colitis: Results of a Placebo Randomized Controlled Trial. Gastroenterology. 2015;148:S-140. [Google Scholar]
- 55.Feagan BG, Sandborn WJ, D’Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149–157. e2. doi: 10.1053/j.gastro.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 56.Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782–8. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–6. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 59.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–31. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681–688. e1. doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 62.Narula N, Peyrin-Biroulet L, Colombel JF. Combination therapy with methotrexate in inflammatory bowel disease: time to COMMIT? Gastroenterology. 2014;146:608–11. doi: 10.1053/j.gastro.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Mack DR, Young R, Kaufman SS, et al. Methotrexate in patients with Crohn’s disease after 6-mercaptopurine. J Pediatr. 1998;132:830–5. doi: 10.1016/s0022-3476(98)70313-0. [DOI] [PubMed] [Google Scholar]
- 64.Boyle B, Mackner L, Ross C, et al. A single-center experience with methotrexate after thiopurine therapy in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2010;51:714–7. doi: 10.1097/MPG.0b013e3181dd861a. [DOI] [PubMed] [Google Scholar]
- 65.Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn’s disease. Am J Gastroenterol. 2007;102:2804–12. doi: 10.1111/j.1572-0241.2007.01474.x. quiz 2803, 2813. [DOI] [PubMed] [Google Scholar]
- 66.Uhlen S, Belbouab R, Narebski K, et al. Efficacy of methotrexate in pediatric Crohn’s disease: a French multicenter study. Inflamm Bowel Dis. 2006;12:1053–7. doi: 10.1097/01.mib.0000235103.47280.bb. [DOI] [PubMed] [Google Scholar]
- 67.Haisma SM, Lijftogt T, Kindermann A, et al. Methotrexate for maintaining remission in paediatric Crohn’s patients with prior failure or intolerance to thiopurines: a multicenter cohort study. J Crohns Colitis. 2015;9:305–11. doi: 10.1093/ecco-jcc/jjv031. [DOI] [PubMed] [Google Scholar]
- 68.Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-year, single tertiary care center experience on the durability of infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:606–13. doi: 10.1097/MIB.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossi V, Lerer T, Griffiths A, et al. Concomitant Use of Immunomodulators Affects the Durability of Infliximab Therapy in Children With Crohn’s Disease. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Din S, Dahele A, Fennel J, et al. Use of methotrexate in refractory Crohn’s disease: the Edinburgh experience. Inflamm Bowel Dis. 2008;14:756–62. doi: 10.1002/ibd.20405. [DOI] [PubMed] [Google Scholar]
- 71.Peyrin-Biroulet L, Deltenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2009;104:2089–96. doi: 10.1038/ajg.2009.301. [DOI] [PubMed] [Google Scholar]
- 72.Reinisch W, Angelberger S, Petritsch W, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut. 2010;59:752–9. doi: 10.1136/gut.2009.194159. [DOI] [PubMed] [Google Scholar]
- 73.Herfarth H, Tjaden C, Lukas M, et al. Adverse events in clinical trials with azathioprine and mesalamine for prevention of postoperative recurrence of Crohn’s disease. Gut. 2006;55:1525–6. [PMC free article] [PubMed] [Google Scholar]
- 74.Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol. 1987;14:1164–71. [PubMed] [Google Scholar]
- 75.Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429–48. doi: 10.2165/00002018-200023050-00006. [DOI] [PubMed] [Google Scholar]
- 76.Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut. 2012;61:476–83. doi: 10.1136/gutjnl-2011-301133. [DOI] [PubMed] [Google Scholar]
- 77.Beaugerie L, Carrat F, Colombel JF, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2014;63:1416–23. doi: 10.1136/gutjnl-2013-305763. [DOI] [PubMed] [Google Scholar]
- 78.Fournier MR, Klein J, Minuk GY, et al. Changes in liver biochemistry during methotrexate use for inflammatory bowel disease. Am J Gastroenterol. 2010;105:1620–6. doi: 10.1038/ajg.2010.21. [DOI] [PubMed] [Google Scholar]
- 79.Maybury CM, Jabbar-Lopez ZK, Wong T, et al. Methotrexate and liver fibrosis in people with psoriasis: a systematic review of observational studies. Br J Dermatol. 2014;171:17–29. doi: 10.1111/bjd.12941. [DOI] [PubMed] [Google Scholar]
- 80.Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086–93. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalb RE, Strober B, Weinstein G, et al. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60:824–37. doi: 10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 82.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–85. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 83.Dawwas MF, Aithal GP. End-stage methotrexate-related liver disease is rare and associated with features of the metabolic syndrome. Aliment Pharmacol Ther. 2014;40:938–48. doi: 10.1111/apt.12912. [DOI] [PubMed] [Google Scholar]
- 84.Conway R, Low C, Coughlan RJ, et al. Risk of liver injury among methotrexate users: A meta-analysis of randomised controlled trials. Semin Arthritis Rheum. 2015 doi: 10.1016/j.semarthrit.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68:1100–4. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morgacheva O, Furst DE. Use of MTX in the elderly and in patients with compromised renal function. Clin Exp Rheumatol. 2010;28:S85–94. [PubMed] [Google Scholar]
- 87.Rondon F, Mendez O, Spinel N, et al. Methotrexate-induced pulmonary toxicity in psoriatic arthritis (PsA): case presentation and literature review. Clin Rheumatol. 2011;30:1379–84. doi: 10.1007/s10067-011-1765-7. [DOI] [PubMed] [Google Scholar]
- 88.Margagnoni G, Papi V, Aratari A, et al. Methotrexate-induced pneumonitis in a patient with Crohn’s disease. J Crohns Colitis. 2010;4:211–4. doi: 10.1016/j.crohns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Conway R, Low C, Coughlan RJ, et al. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. BMJ. 2015;350:h1269. doi: 10.1136/bmj.h1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conway R, Low C, Coughlan RJ, et al. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:803–12. doi: 10.1002/art.38322. [DOI] [PubMed] [Google Scholar]
- 91.Lewden B, Vial T, Elefant E, et al. Low dose methotrexate in the first trimester of pregnancy: results of a French collaborative study. J Rheumatol. 2004;31:2360–5. [PubMed] [Google Scholar]
- 92.Feagins LA, Kane SV. Sexual and reproductive issues for men with inflammatory bowel disease. Am J Gastroenterol. 2009;104:768–73. doi: 10.1038/ajg.2008.90. [DOI] [PubMed] [Google Scholar]
- 93.Weber-Schoendorfer C, Chambers C, Wacker E, et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014;66:1101–10. doi: 10.1002/art.38368. [DOI] [PubMed] [Google Scholar]
- 94.Mahadevan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006;55:1198–206. doi: 10.1136/gut.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding) Clin Exp Rheumatol. 2009;27:678–84. [PubMed] [Google Scholar]
- 96.Weber-Schoendorfer C, Hoeltzenbein M, Wacker E, et al. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: an observational cohort study. Rheumatology (Oxford) 2014;53:757–63. doi: 10.1093/rheumatology/ket390. [DOI] [PubMed] [Google Scholar]
- 97.Loza E, Martinez-Lopez JA, Carmona L. A systematic review on the optimum management of the use of methotrexate in rheumatoid arthritis patients in the perioperative period to minimize perioperative morbidity and maintain disease control. Clin Exp Rheumatol. 2009;27:856–62. [PubMed] [Google Scholar]
- 98.Kremer JM, Alarcon GS, Lightfoot RW, Jr, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994;37:316–28. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- 99.Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951. doi: 10.1002/14651858.CD000951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morgan SL, Baggott JE, Vaughn WH, et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 1990;33:9–18. doi: 10.1002/art.1780330102. [DOI] [PubMed] [Google Scholar]
- 101.de van EAE, Laan RF, Rood MJ, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001;44:1515–24. doi: 10.1002/1529-0131(200107)44:7<1515::AID-ART273>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 102.Laharie D, Seneschal J, Schaeverbeke T, et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J Hepatol. 2010;53:1035–40. doi: 10.1016/j.jhep.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 103.Kaffenberger BH, Kaffenberger JA, Wong H, et al. Magnetic resonance elastography and transient elastography as non-invasive analyses for liver fibrosis: can they obviate the need for liver biopsy in psoriasis patients treated with methotrexate? Int J Dermatol. 2015;54:752–6. doi: 10.1111/ijd.12923. [DOI] [PubMed] [Google Scholar]
- 104.Laharie D, Zerbib F, Adhoute X, et al. Diagnosis of liver fibrosis by transient elastography (FibroScan) and non-invasive methods in Crohn’s disease patients treated with methotrexate. Aliment Pharmacol Ther. 2006;23:1621–8. doi: 10.1111/j.1365-2036.2006.02929.x. [DOI] [PubMed] [Google Scholar]
- 105.Manosa M, Naves JE, Leal C, et al. Does methotrexate induce mucosal healing in Crohn’s disease? Inflamm Bowel Dis. 2010;16:377–8. doi: 10.1002/ibd.21015. [DOI] [PubMed] [Google Scholar]
- 106.Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;4:CD000545. doi: 10.1002/14651858.CD000545.pub4. [DOI] [PubMed] [Google Scholar]
- 107.Prefontaine E, Sutherland LR, Macdonald JK, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009:CD000067. doi: 10.1002/14651858.CD000067.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Arora S, Katkov W, Cooley J, et al. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology. 1999;46:1724–9. [PubMed] [Google Scholar]
- 109.Oren R, Moshkowitz M, Odes S, et al. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol. 1997;92:2203–9. [PubMed] [Google Scholar]
- 110.Roenigk HH, Jr, Auerbach R, Maibach H, et al. Methotrexate in psoriasis: consensus conference. J Am Acad Dermatol. 1998;38:478–85. doi: 10.1016/s0190-9622(98)70508-0. [DOI] [PubMed] [Google Scholar]
- 111.Te HS, Schiano TD, Kuan SF, et al. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150–6. doi: 10.1111/j.1572-0241.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 112.Fraser AG, Morton D, McGovern D, et al. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:693–7. doi: 10.1046/j.1365-2036.2002.01227.x. [DOI] [PubMed] [Google Scholar]