Abstract
Mitochondria are undoubtedly changed in Parkinson’s disease (PD), and mitochondrial functions are disrupted in genetic and pharmacologic models of PD. However, many of these changes might not truly drive neurodegeneration. PD is defined by the particular susceptibility of nigrostriatal dopamine (DA) neurons, but little is understood about the mitochondria in these cells. Here, we critically review the evidence that mitochondrial stressors cause PD. We then consider how changes in the intrinsic function of mitochondria and in their mass, distribution, and dynamics might synergize with an increased need for mitochondria and produce PD, and the importance of understanding how mitochondria contribute to its pathogenesis.
1. Introduction
Mitochondria are heavily disrupted in Parkinson’s disease (PD), Alzheimer’s disease, Huntington’s disease, multiple sclerosis, and stroke. In these and other neurodegenerative and neurologic conditions, the enzymatic activity of respiratory chain enzymes is altered, mutations accumulate in mitochondrial DNA, and oxidative stress appears to be increased in affected brain areas at autopsy [1, 2]. Although mitochondrial morphology, behavior, and functions are disrupted in pharmacologic and genetic models of these diseases, the pathogenic role of mitochondria is unclear. Do changes in mitochondria initiate degeneration? If not, do they contribute to disease progression? Or are they simply epiphenomena that always accompany neuronal degeneration and death?
Among these conditions, perhaps only in those involving mutations in mitochondrial DNA can we be fully confident that primary changes in mitochondria actually cause disease. In most other diseases, changes in other cellular compartments have also been implicated in the pathophysiology. And even if mitochondrial changes cause these disorders, it is unclear which mitochondrial function or functions—such as loss of respiration, increased levels of reactive oxygen species (ROS), and impaired Ca2+ buffering—actually cause the degeneration.
In this review, we focus on mitochondria in PD, a neurodegenerative disease that is strongly linked to mitochondria. Indeed, the evidence for mitochondrial alterations in PD is as compelling as in any other neurodegenerative disorder. Support for a central pathogenic role of mitochondria is based on susceptibility to mitochondrial toxins, mitochondrial changes in autopsy tissue, genetic forms of PD caused by mutations in mitochondrial proteins, and model systems of PD. Here we evaluate the evidence that changes in mitochondria cause PD and consider how, specifically, mitochondria might be disrupted to cause PD.
2. Evidence That Mitochondrial Stressors Cause PD
2.1. Pharmacological evidence
DA neurons in the substantia nigra pars compacta (SN DA neurons) are uniquely vulnerable to specific mitochondrial stressors. The first direct evidence of this vulnerability was the discovery that the mitochondrial neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) preferentially kills SN DA neurons in humans [3]. MPTP proved to be toxic to DA neurons in primates and mice [4]. Its active metabolite, MPP+, concentrates in mitochondria as a result of their negative membrane potential [5]. MPP+ also weakly inhibits mitochondrial complex I [6], raising the possibility that this effect is responsible for the death of DA neurons. However, subsequent research revealed that MPP+ likely produces death, at least in part, through mechanisms that are independent of its effects on complex I [7–12].
MPP+ is a substrate for the DA transporter (DAT) and hence is preferentially imported into DA neurons [13, 14], likely resulting in higher cytosolic levels of MPP+ than in other types of neurons. It is unclear whether this is truly the case and, if it is, how much higher the cytosolic levels are. Cytosolic MPP+ is also sequestered in vesicles in DA neurons by the vesicular monoamine transporter 2 (VMAT2)[15, 16]. Therefore, the susceptibility to MPP+ highlights how preferential uptake might lead to the selective death of DA neurons, but does not provide clear evidence that DA neurons themselves are intrinsically vulnerable to complex I or other mitochondrial stresses.
More compelling evidence for the intrinsic vulnerability of SN DA neurons to complex I inhibition comes from studies with rotenone, a potent complex I inhibitor [17, 18]. Systemically delivered rotenone selectively kills these cells in rats [19, 20], and exposure to rotenone also appears to increase the risk of developing Parkinson’s disease (PD) [21]. Moreover, sensitivity to complex I inhibition appears to be somewhat specific, as DA neurons are not preferentially susceptible to all mitochondrial toxins. For instance, the complex II inhibitors 3-nitropropionic acid and malonate are toxic to medium spiny neurons in the striatum [22–24] not DA neurons. This specificity further supports a specific vulnerability of DA neurons to complex I inhibition. Nonetheless, the data are inconclusive. Rotenone has never been directly proven to cause PD in humans, and it also inhibits microtubules and has other nonspecific effects. In addition, SN DA neurons are also relatively resistant to toxicity from deletion of the complex I subunit NDUFS4, although the extent of complex I dysfunction achieved in DA neurons in vivo was unclear [25, 26].
2.2 Evidence from PD mutations
Autosomal-recessive PD
Genetic mutations associated with rare familial forms of PD provide near-definitive evidence that primary deficits in mitochondrial function cause PD. In particular, the finding that mutations in the serine/threonine-protein kinase PINK1 cause an autosomal-recessive form of PD [27, 28] is particularly compelling evidence that DA neurons are intrinsically susceptible to mitochondrial stressors. PINK1 is a mitochondria-targeted protein that regulates mitochondrial turnover (mitophagy) [29], complex I function [30], mitochondrial dynamics, [31] and mitochondrial transport [32]. Although it localizes to mitochondria and its effects on mitochondria are believed to be critical to its functions, PINK1 has functions outside of the mitochondria [33, 34], and a subset of PINK1 is cytosolic [35–37]. The relative significance of mitochondrial versus non-mitochondrial functions in PD pathogenesis is unknown.
Other recessive PD genes further support the unique vulnerability of SN DA neurons to mitochondrial stressors. In particular, the E3 ubiquitin ligase Parkin associates with mitochondria, especially damaged mitochondria, where it promotes the turnover of damaged mitochondria and decreases mitochondrial motility in axons through PINK1-dependent mechanisms [29, 32, 38]. Moreover, loss of PINK1 severely disrupts the morphology and function of mitochondria in Drosophila flight muscles, and these deficits are rescued by overexpression of Parkin [39, 40]. Thus, PINK1 and Parkin act in the same pathway to influence mitochondria.
Just as PINK1 inhibits complex I independently of Parkin [30], Parkin also influences mitochondria through pathways that are seemingly unrelated to PINK1. For instance, Parkin inhibits the transcriptional repressor Paris. When Parkin is absent, Paris accumulates and represses the expression of peroxisome proliferator–activated receptor-gamma coactivator 1-alpha (PGC-1α), a central regulator of mitochondrial biogenesis and functions [41]. In addition, the autosomal recessive PD protein DJ-1 decreases oxidative stress in mitochondria and also influences mitochondrial morphology and bioenergetics [42–45]. Interestingly, DJ-1 complements the mitochondrial effects of other PD proteins, including PINK1, Parkin, and α-synuclein [42, 46, 47]. Nonetheless, like PINK1, Parkin and DJ-1 have mitochondria-independent effects [48–52], and the relative contributions of mitochondrial versus non-mitochondrial pathways to degeneration in PD have not been established.
Autosomal-dominant PD
The evidence that mitochondrial dysfunction causes sporadic PD was strengthened by the discovery that typical adult-onset PD can be caused by mutations in the autosomal dominant gene CHCHD2 [53], which encodes a small protein localized to the mitochondrial intermembrane space. CHCHD2 binds to cytochrome c oxidase and maintains its level and activity [54, 55], and loss of CHCHD2 may compromise mitochondrial respiration. Like recessive PD genes, however, CHCHD2 also appears to have non-mitochondrial functions [54], and it is not known whether these functions also contribute to its role in PD pathogenesis.
Proteins causing other autosomal dominant forms of PD have been linked to mitochondria. In particular, a fraction of the central PD protein α-synuclein interacts with mitochondria, and α-synuclein promotes mitochondrial fragmentation and disrupts the function of mitochondrial complex I [47, 56–59]. The effects of α-synuclein may represent a toxic gain of function that is distinct from the proposed physiologic roles of α-synuclein in regulating synaptic vesicle release [60]. Increasing evidence, including evidence from human neurons, also shows that LRRK2 influences mitochondrial functions and movement [61–65]. However, both α-synuclein and LRRK2 are primarily cytosolic and have other biologic functions, and hence the role of their interactions with mitochondria in producing neurodegeneration in PD remains to be clarified.
2.3. Significance of PD genetics in understanding how mitochondria contribute to sporadic PD
In assessing the relevance of mitochondrial changes in genetic forms of PD to sporadic PD, we must consider the possibility that the pathophysiology of recessive PD differs from that of the sporadic and autosomal dominant forms of the disease [66]. If the disorders are truly distinct, establishing a causative role for mitochondria in recessive PD might not help us understand the role of mitochondria in the more common sporadic forms. However, the evidence that autosomal recessive PD is indeed distinct is limited. For example, although autosomal recessive PD usually begins at a younger age than sporadic PD, disease onset in the 50s or later has been reported [67, 68]. Furthermore, in a large proportion of early-onset cases, there is no clear familial inheritance, and no mutations were found in a panel of PD-associated genes (α-synuclein, Parkin, PINK1, DJ-1, LRRK2, with or without glucocerebrosidase) in ≈85–90% of patients with an age of onset of 30–50 years, and in ≈60–85% with onset at less than 30 years of age [69, 70]. Hence, younger patients are more likely to have genetic mutations, but the age of onset itself does not indicate a distinct pathophysiology.
Cognitive deficits appear to develop and progress more slowly in patients with mutant Parkin than in age-matched patients with sporadic PD [71]. However, this difference does not prove a distinct pathophysiology. Indeed, patients with mutant PINK1 and Parkin often develop cognitive deficits and other nonmotor changes that are indistinguishable from those in many patients with sporadic PD [28, 72, 73]. This substantial overlap of clinical features occurs because sporadic PD itself is clinically heterogeneous, and the motor changes, their responsiveness to levodopa [74], and the type and severity of nonmotor features, such as cognitive deficits, vary considerably [74, 75]. Moreover, patients with triplication of α-synuclein (a protein firmly linked to sporadic PD) develop disease at a younger age than those with duplication and can have a much more aggressive form of parkinsonism (that sometimes manifests as diffuse Lewy body disease) [76–78]. Thus, at least in this case, a greater dose of the same insult (rather than a distinct pathophysiology) leads to an earlier and more severe disease onset.
Finally, recessive PD has been proposed to have a distinct pathophysiology: patients with Parkin mutations usually do not have α-synuclein inclusions. However, some Parkin patients have Lewy bodies [79, 80], and the only PD patient with mutant PINK1 to come to autopsy also had typical Lewy bodies [81]; no autopsy data have been reported for DJ-1. Therefore, there is no evidence that most forms of recessive PD lack Lewy bodies. Conversely, despite having similar clinical presentations, some patients with autosomal dominant PD caused by mutations in LRRK2 also lack α-synuclein inclusions (in some cases having tau deposition instead), and others in the same family have typical Lewy bodies [82, 83]. Indeed, the clinical diagnosis of PD requires movement deficits, which result from the dysfunction or loss of SN DA neurons and can develop in the absence of Lewy bodies [82–84]. Moreover, Lewy bodies and Lewy neurites appear to develop in various regions, including the hippocampus, without causing significant neuronal death [85–88]. Lewy bodies can even accumulate at high levels in SN DA neurons in the absence of extrapyramidal symptoms (i.e., incidental Lewy bodies)[89].
Importantly, although PD is a heterogeneous disorder, SN DA neurons likely have a shared susceptibility that underlies its many different causes and forms. Mitochondria likely contribute to this shared pathogenesis, but the extent and nature of the contribution may differ markedly between different disease variants. Ultimately, autosomal recessive forms of PD may prove to have greater pathophysiologic overlap with subtypes of sporadic PD characterized by fewer cognitive deficits and greater responsiveness to levodopa than they do with other subtypes.
2.4. Evidence from sporadic PD
Do changes in mitochondria cause sporadic PD? The extensive mitochondrial changes in sporadic PD strongly suggest an important role for mitochondria in its pathogenesis, regardless of the indirect evidence from genetics and environmental toxins discussed above. These changes include decreased function of mitochondrial complex I in the SN [90, 91] and platelets [92, 93], decreased expression of PGC-1α and PGC-1α-regulated mitochondrial genes in DA neurons in PD[94, 95], as well as increased accumulation of mutations in the mitochondrial DNA of SN DA neurons with age and PD [96].
Nonetheless, it remains unclear which, if any, of these changes cause neurodegeneration. Definitive answers may require substantial technical advances, for instance new methods to longitudinally and simultaneously track mitochondrial functions and disease progression in susceptible neurons in patients, as well as new approaches to specifically correct the mitochondrial deficits and determine whether degeneration is blocked. Meanwhile, our understanding of whether and how changes in different mitochondrial functions cause sporadic PD depends heavily on insights from genetic and pharmacologic models.
Of particular importance to understanding mitochondria in sporadic PD is α-synuclein. Indeed, α-synuclein and mitochondria have important parallels. Changes in either α-synuclein or mitochondria can cause PD, and both α-synuclein and mitochondria are changed in sporadic PD. As reviewed in depth [59], increased α-synuclein influences mitochondria in several respects, including making them more fragmented [47, 56], inhibiting mitochondrial complex I function [57, 58], increasing transfer of Ca2+ from the endoplasmic reticulum (ER) to mitochondria [97], increasing mitophagy [98, 99], and modulating PGC-1α expression [100]. Endogenous α-synuclein also sensitizes mice to mitochondrial toxins [101, 102] and influences mitochondrial dynamics [47, 103], while endogenous PGC-1α protects against the toxicity of α-synuclein oligomers [104], suggesting that α-synuclein-mitochondria interactions also have physiologic roles. However, a central question about all physiologic and pathologic interactions between α-synuclein and mitochondria remains unanswered: Which, if any, of these changes actually contribute to neurodegeneration?
3. Pathophysiology of Mitochondrial Insufficiency in PD
Overwhelming evidence suggests that DA neurons are particularly vulnerable to specific mitochondrial stressors and that this susceptibility underlies certain rare genetic forms of PD. Furthermore, PD caused by mitochondrial insults can be clinically indistinguishable from sporadic PD, and mitochondrial insults in all likelihood contribute to the pathophysiology of at least some forms of sporadic PD. However the mechanisms governing this selective vulnerability are poorly understood. What is clear, however, it that the presence of DA alone cannot explain this susceptibility to mitochondrial insults. Indeed, DA neurons in the SN are more susceptible than DA neurons in the medial aspect of the immediately adjacent ventral tegmental area (VTA) [105, 106], and DA neurons in the hypothalamus are largely spared [107]. Furthermore, in sporadic PD, specific populations of nonDA neurons also die, including noradrenergic neurons in the locus coeruleus [108] and cholinergic neurons in the dorsal motor nucleus of the vagus [109] and in the nucleus basalis of Meynert [105, 110]. However, the extent to which neurons in these other susceptible areas degenerate and die varies considerably [78, 80]. How, then, do mitochondrial insults produce PD, and in what way are mitochondrial functions first disrupted in PD?
3.1. Insufficient mitochondrial function
Intrinsic mitochondrial function
The initial mitochondrial insult may involve a defect in one or more intrinsic mitochondrial functions—many of which have been implicated in PD pathogenesis, in particular changes in respiration, Ca2+ buffering, and the production of reactive oxygen species (ROS), as briefly discussed below. However, mitochondria are also important in apoptotic pathways and in lipid and neurotransmitter metabolism [111, 112], and changes in these functions may also contribute to degeneration in PD. Not surprisingly, many of the mitochondrial changes are closely related, and primary changes in one function may alter other functions. As a result, it is often difficult to discern which change occurs first and which changes drive degeneration. Thus, determining which mitochondrial function is affected first in PD and which mitochondrial functions are critical to degeneration is an extremely challenging (but very important) task.
Energy failure
As discussed earlier, a potential role for complex I dysfunction in PD pathogenesis has been proposed, largely because of the association of complex I toxins with PD and the decreased activity of complex I in the SN of PD patients. Disruptions in complex I or other parts of the respiratory chain may occur for several reasons. Mutations in mitochondrial DNA might accumulate in PD and with age [96, 113, 114]. Mitochondrial turnover and quality control might be impaired, as may occur with mutations in Parkin or PINK1[29, 38]. And mutant PINK1 [30] or environmental toxins, such as rotenone, might directly inhibit mitochondrial complex I.
Any disruption in complex I or other components of the respiratory chain may lead to “energy failure,” defined here as insufficient ATP to maintain normal cellular functions. Although energy failure has long been implicated in the pathophysiology PD and other neurodegenerative disorders, it is not known whether it truly occurs in dying neurons in PD [115]. We have shown that disruptions in complex I function or in mitochondrial fission can produce energy failure in hippocampal axons [116, 117]. It will be important to determine whether SN DA neurons are particularly susceptible to energy failure and, if so, why. If respiration is impaired in DA neurons in PD, it will also be important to know whether it is sufficient to impair function and produce degeneration.
Oxidative damage from excessive mitochondrial ROS
Respiration is closely tied to other mitochondrial functions, including ROS production. A small proportion of electrons escapes from the respiratory chain and reduce molecular oxygen to form superoxide, and ROS are also produced by certain enzymes in the mitochondrial matrix [118]. The extent of this electron leak can increase when the respiratory chain is blocked at key sites, including complex I [119, 120]. The mechanisms of changes in ROS production are often poorly understood [118]. Furthermore, just as the respiratory rate can influence ROS production, the converse is also true, as primary changes in ROS levels can compromise respiration [121]. Therefore, although the effects of mitochondria on ROS and respiration can be dissociated under certain circumstances[122], it is often challenging to identify the initiating factor when both are altered.
In PD, there is considerable evidence for weakened antioxidant defenses (e.g., less glutathione [123]) and increased oxidative damage [124–127], and the potential contribution of ROS to the pathophysiology of PD has been reviewed extensively [128, 129]. There are three key points to emphasize here.
First, DA neurons may produce and be exposed to more ROS, in part because the degradation of DA produces ROS [130, 131]. However, DA neurons likely have intrinsic defenses against ROS. For instance, they express VMAT2, which maintains low levels of cytosolic DA by sequestering it in vesicles [15, 132], although, SN DA neurons may have less capacity to do this than VTA DA neurons[133]. DA neurons also express tetrahydrobiopterin which, in addition to functioning as a cofactor for tyrosine hydroxylase in catecholamine synthesis, also functions a free radical scavenger [134, 135], and cultured embryonic DA neurons appear to maintain lower levels of superoxide than other mesencephalic neurons [9]. On the other hand, compelling evidence indicates that SN DA neurons in slices and in postnatal cultures have a higher level of oxidation than adjacent VTA DA neurons [43, 136], and its unclear whether these seemingly discrepant findings reflect differences in the culture paradigms or in the specificity of the probes studied (e.g., hydroethidine to measure superoxide[134] versus mito-roGFP to assess oxidation state [43]). Therefore, it is unclear whether DA neurons are exposed to higher ROS levels than non-DA neurons, especially in vivo, where differences in neural activity and environment (e.g., increased iron levels in the midbrain [137]) are also likely to influence ROS levels.
Second, although mitochondria are thought to be the major source of ROS in most cell types, their contribution to total ROS levels in DA neurons, versus other sources of ROS (e.g., NADPH oxidase [138] and the metabolism of DA itself [130, 131]) are not known [118, 139]. However, as the oxidation state of mitochondria in DA neurons depends on DJ-1 and mitochondrial Ca2+ uptake [43, 45], mitochondria do at least appear to contribute significantly to mitochondrial ROS levels. Whether changes in mitochondrial ROS levels initiate or contribute to degeneration in PD is still unclear.
Third, ROS changes may be particularly critical in DA axons. However, very little is known about ROS levels in DA axons in PD or in cellular or animal models of PD. Therefore, it is unclear whether mitochondria-derived ROS contribute significantly to neurodegeneration in PD.
Mitochondrial Ca2+ buffering and ER–mitochondria contacts
Mitochondrial Ca2+ buffering is another intrinsic mitochondrial function that may be disrupted in PD. Cytosolic Ca2+ crosses the outer mitochondrial membrane freely and is transferred across the inner mitochondrial membrane into the mitochondrial matrix by the mitochondrial calcium uniporter [140, 141]. Its capacity to import Ca2+ depends primarily on the electrochemical gradient defined by the mitochondrial membrane potential and the Ca2+ gradient between the cytosol and the mitochondrial matrix[140, 142–144]. Indeed, mitochondrial Ca2+ buffering and respiration are closely tied, and respiration is regulated by both cytosolic and mitochondrial Ca2+ levels—a potentially important mechanism for cells, particularly neurons, to upregulate energy production to meet energy needs [145, 146]. Although not fully understood, this complex process includes both the upregulated transfer of metabolites into the mitochondrial matrix by cytosolic Ca2+-sensitive transporters [147] and direct activation by mitochondrial Ca2+ of enzymes involved in energy metabolism and oxidative phosphorylation, including α-ketoglutarate, isocitrate, and pyruvate dehydrogenases [148, 149].
Besides stimulating respiration, increased cytosolic Ca2+ is also predicted to increase energy consumption by increasing the energy needed to restore normal Ca2+ levels [150]. Nonetheless, increased Ca2+ has an overall positive effect on respiration and ATP levels in cortical neurons [146, 151]. It is not known whether this beneficial effect on the energy level also applies to axons, which are more susceptible to energy depletion from neural activity [116, 117], or to SN DA and other neurons, which may have both a greater Ca2+ burden and fewer mechanisms to buffer Ca2+ [152]. Furthermore, at excessively high levels, mitochondrial Ca2+ depolarizes mitochondria and inhibits respiration, increases mitochondrial ROS production, and triggers apoptotic pathways [153–156]. Excessive mitochondrial toxicity may contribute, for instance, to PINK1-based PD, as PINK1-deficient neurons have increased mitochondrial Ca2+ from decreased mitochondrial Ca2+ efflux by the Na+/Ca2+ exchanger [157].
Of particular interest is Ca2+ transfer from the ER (the main calcium storage organelle) to the mitochondria at the areas of apposition between the two organelles known as mitochondria-associated membranes (MAMs) [158, 159]. Basal transfer of Ca2+ at MAMs appears to be required for normal bioenergetics [159, 160], and several PD proteins, including α-synuclein [97, 161], DJ1 [162] and Parkin [163], disrupt MAM function or ER-Ca2+ transfer—and may have important and convergent roles in neurodegeneration. MAMs also participate in functions other than Ca2+ transfer, including lipid transfer, cell signaling, autophagy, and apoptosis[159]. It is not known whether these processes contribute to neurodegeneration in PD.
Mitochondrial mass (biogenesis, turnover)
The primary mitochondrial deficit in certain forms of PD might not be impaired mitochondrial function. Rather, it might be a decrease in the number of functional mitochondria as a result of decreased synthesis or decreased turnover of dysfunctional mitochondria. Indeed, the decreased level of PGC-1α-responsive genes in DA neurons in sporadic PD [94], the association of PGC-1α pathway activation with a decreased incidence of PD[164], and the finding that Parkin provides critical support for PGC-1α activity[41] suggest that mitochondrial biogenesis is impaired, perhaps leading to a decrease in mitochondrial mass. Consistent with this possibility, deletion of Parkin produces a decrease in mitochondrial content specifically in human DA neurons and not in non-DA neurons[165]. Conversely, impaired mitophagy due to defects in Parkin or PINK1 [29, 38] might increase mitochondrial mass, but with a greater proportion of dysfunctional mitochondria (i.e., intrinsic dysfunction as described above). Thus, it is surprising that mitochondrial mass is normal in brain lysates from PINK1- and Parkin-knockout mice [166–168], although it will be important to learn whether mitochondrial mass changes specifically in DA neurons in vivo.
SN DA neurons have lower mitochondrial mass at the cell body and dendrites than VTA DA and other non-DA midbrain neurons [169]. It will be important to determine whether this is also the case in humans and whether SN DA neurons in patients with genetic or sporadic PD have any changes in mitochondrial mass, especially in axons, which degenerate before the cell body. Moreover, mitochondrial turnover is complicated, and although the entire organelle can be degraded at once, multiple processes also regulate the turnover of individual compartments. Indeed, proteins in the matrix and inner and outer membranes appear to be degraded at different rates, as can specific proteins in the same compartment [170–172]. Disruption of Parkin differentially affects the turnover of mitochondrial compartments [171]. Therefore, the biosynthesis or turnover of mitochondrial components may be deficient in PD, even if the total mass of mitochondria proves to be unchanged.
Mitochondrial distribution in axons
Yet another possibility is that the function and total mass of mitochondria are normal, but their subcellular distribution is disrupted and may lead to regional energy failure or regional deficits in other key mitochondrial functions. As discussed above, SN DA neurons have unusually large axonal arbors and, hence, could be more susceptible to a disruption in axonal transport or localization. Indeed, PINK1 directly modulates mitochondrial movement in axons through effects on Miro in a Parkin-dependent manner[32]. Other PD proteins, including LRRK2 and α-synuclein, also affect mitochondrial transport [173, 174]. Interestingly, deletion of PINK1 does not affect the morphology of synaptic mitochondria [175]. However, it is unclear whether the mass or distribution of axonal mitochondria changes in PD or in models of PD, or whether any such changes in distribution would produce areas of insufficient energy [116, 176] or deficiency in other mitochondrial functions. To answer these questions, we might need more sensitive analyses of different models systems of PD (including those in which there is clear neurodegeneration), as well as a systematic examination of mitochondria in DA synapses in patients with PD. There is also very little information on whether or how mitochondrial distribution in dendrites is altered in PD.
Mitochondrial dynamics
Changes in mitochondrial dynamics—the balance between the fusion and fission of mitochondria—are yet another potential initial insult in PD. Such changes could affect respiration [117, 177], mitochondrial mass and turnover [178, 179], mitochondrial transport, or other intrinsic mitochondrial functions [180]. Indeed, nigrostriatal DA neurons are more susceptible to loss of mitochondrial fission than other midbrain DA neurons [179] and seem to have a similar (albeit less severe) preferential susceptibility to loss of the mitochondrial fusion protein Mfn2 [180]. Although a specific mitochondrial fusion or fission protein has not been implicated in classic PD, recent data suggest that mutations in Opa1 manifest as parkinsonism in the absence of clinically significant optic atrophy [181]. Furthermore, Parkin and PINK1 have both been proposed to act as mitochondrial fission proteins in Drosophila, likely by degrading Mfn [182]. In addition, increased α-synuclein causes the fragmentation of mitochondria independent of Drp1, likely by interacting directly with the mitochondrial membrane [47, 56]. In a PD mouse model, inhibition of DRP1 reduces neurotoxicity and synaptic transmission deficits [175]. This finding raises an important question: Is manipulating mitochondrial dynamics an effective therapeutic strategy[175]?
Secondary mitochondrial dysfunction due to non-mitochondrial causes
Mitochondrial insults likely initiate several some forms of genetic and sporadic PD. In other forms, the primary insult is probably non-mitochondrial. For instance, primary changes in the extent or pattern of synaptic transmission [183], ER-to-Golgi transport [184], and lysosomal function [185] may also initiate certain forms of PD. Are mitochondria disrupted in these non-mitochondrial cases? If so, do these disruptions occur early enough to contribute to the initiation or progression of degeneration, or are they simply epiphenomena to the primary processes of degeneration? These questions cannot be answered until the pathophysiologic subtypes of PD have been better defined.
3.2. Increased need for mitochondria
Insufficient mitochondrial function might result from impaired, insufficient, or misdistributed mitochondria. However, it might also result from excessive demand for mitochondrial functions. Here we consider this possibility from the perspective of energy levels, but the same principle might also apply to other mitochondrial functions.
Intrinsic pacemaking and Ca2+ buffering
There are several reasons—none of them proved—why SN DA neurons may theoretically have greater metabolic needs than other neuron types. First, their intrinsic pacemaking function may entail greater overall neural activity than is required by other types of neurons. Supporting synaptic transmission produces the greatest energy demand in the brain [186], and increased neural activity predisposes axons to energy failure when mitochondria are compromised [116, 117]. SN DA neurons may also be particularly susceptible because they are enriched in L-type Ca2+ channels (Cav1.3) [43, 187], rather than Na+ channels used by most other pacemaking neurons, including adjacent DA neurons in the VTA, which are relatively resistant in PD [150, 187]. Indeed, pacemaking supported by Ca2+ influx may be especially demanding energetically because Ca2+ must be removed from the cell against a steep concentration gradient [150]. SN DA neurons in PD also have more K-ATP channels, which may facilitate burst firing and further increase Ca2+ levels [188, 189], and the activity of SN DA neurons further increases in pharmacologic and certain genetic models of PD [190–195].
Vulnerable SN DA neurons also have low levels of the Ca2+-binding protein calbindin [108, 152] and a low overall capacity for intrinsic Ca2+ buffering [196]. Other vulnerable neuronal populations in PD besides SN DA neurons are also spontaneously active and tend to have lower Ca2+ buffering capacity[150]. Nonetheless, it has not been established that Ca2+ pacemaking and decreased buffering place SN DA neurons under greater metabolic stress. Indeed, very little is known about the energy status of DA neurons, and both cytosolic and mitochondrial Ca2+ can also stimulate respiration. Therefore, it is not known whether Ca2+ influx also stimulates respiration in SN DA neurons and, if so, whether the extent of increase compensates for the increased energy demands that the influx may impose. A better understanding of the balance between energy production and consumption in DA neuron subtypes—including the relative contributions of different Ca2+ channels, intrinsic pacemaking, and Ca2+-binding mechanisms—will help clarify the intrinsic vulnerability of this neuronal population.
Large axonal arbors and axonal mitochondria
To understand how changes in mitochondria contribute to PD, we must also focus on axons. SN DA neurons have remarkably extensive axonal arbors, which almost certainly contain the majority of mitochondria. A single rat SN DA neuron may form synapses with some 75,000 striatal target neurons, and make up to 245,000 synapses [197, 198]. In contrast, adjacent VTA DA neurons that project to the nucleus accumbens and other areas in the ventral striatum may form synapses with fewer than 30,000 target neurons, and the axons of other key neuronal types in the basal ganglia may give rise to fewer than 5000 synapses. Remarkably, in humans, SN DA neurons are even larger: they are estimated to form up to 2.4 million synapses and average 4.5 meters in length [198]. Since support for neural activity is the greatest consumer of energy in the brain [186, 199], it seems likely that neurons with larger axonal arbors and more synaptic connections will have proportionally greater energy requirements.
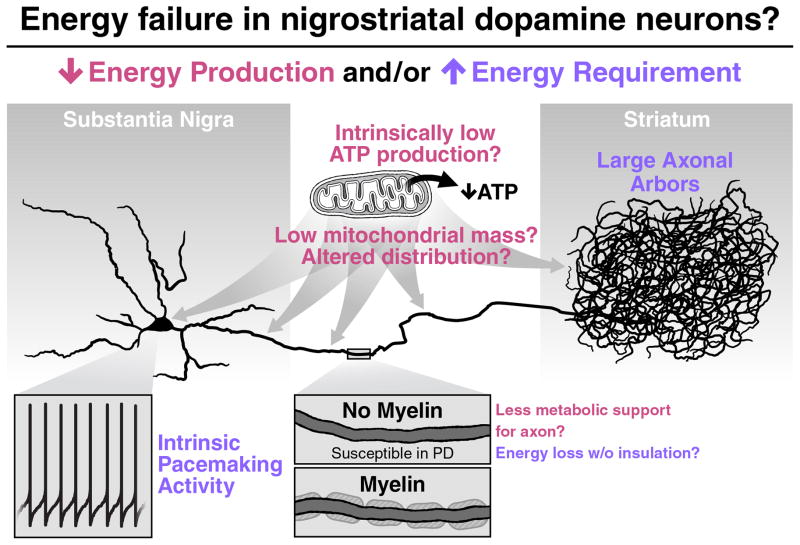
SN DA neurons and susceptible non-DA neurons are unmyelinated or only lightly myelinated [200, 201]. Thus, they may be more susceptible to energy stressors because of increased energy demands—myelin decreases the energy required for synaptic transmission [202], but it is not known whether the energy savings exceeds the costs of myelin synthesis and maintenance [199]—and a decreased supply of energy precursors such as lactate to underlying axons [110, 203] (Fig. 1). Axons are also where key disease proteins such as α-synuclein and tau accumulate and, importantly, where neurodegeneration begins in PD, Alzheimer’s disease, Huntington’s disease, and other neurodegenerative diseases and in most mitochondrial models of neurodegeneration [19, 180, 204–208].
Fig. 1.
SN DA neurons may be susceptible to energy failure if they cannot produce enough energy to meet normal demand or have increased energy requirements. The former might result if their mitochondria have less capacity to make ATP or if they have fewer or misdistributed mitochondria, especially in their axons, which degenerate first in PD. SN DA axons also lack myelin, which may mean they have a decreased abundance of energy substrates to support energy production. Increased energy requirements might result if DA neurons require significantly more energy to support intrinsic pacemaking and their massive axonal arbors, which project to the striatum. If SN DA neurons are indeed more energetically challenged, they could be predisposed to the toxicity of additional bioenergetic and other mitochondrial insults, which may underlie their unique susceptibility to mitochondrial stressors in PD.
3.3 Intrinsic vulnerability and Aging
Aging is the strongest risk factor for PD [209]. Hence it is important to consider whether aging interacts with mitochondria to produce degeneration and, if so, how. One likely possibility is a progressive decline in key intrinsic mitochondrial functions with age [210], such that the level of mitochondrial function in DA neurons drops below the threshold for normal function and survival. In addition, dysfunctional mitochondria may in some cases produce damaging byproducts that accumulate over time. For instance, oxidative damage from mitochondrial ROS is thought to accumulate with age [211], and may make neurons more susceptible to the toxicity of disease processes. The increase in mitochondrial DNA mutations with age may also predispose neurons to degeneration by impairing respiration and compromising other mitochondrial functions [212, 213]. Chronic energy deficiency may well have other long-term adverse effects on a multitude of cellular functions, including protein synthesis [214], neurogenesis, and synaptic integrity [215, 216]. However, very little is known about these relationships.
The energy requirements of the brain may also change with age, although this remains controversial. A number of studies have found that total brain glucose consumption decreases with age, which could reflect decreased energy requirements, decreased substrate availability at the brain or neuron level, or a decreased capacity to produce energy [217]. It is not known if energy requirements of individual neurons change with age. Interestingly, during postnatal development in mice, SN DA neurons increasingly rely on L-type Cav1.3 Ca2+ channels to support autonomous pacemaking, which may place mature DA neurons under greater oxidative and energetic stress [187]. It is not known whether similar changes occur in older adults and influence energy requirements.
Summary of mitochondrial pathophysiology in PD
Mitochondria may be chronically stressed by multiple mechanisms in PD. The stressors can be broadly classified into those resulting from insufficient mitochondrial function and those resulting from excessive demand. Insufficient mitochondrial function might reflect a defect in an intrinsic function of the mitochondria or a change in their number, distribution, or dynamics. Finally, mitochondrial functions are often intertwined—making it particularly challenging to determine their relative contributions to degeneration.
4. Significance/Rationale for Understanding Disease Pathophysiology
Why do we need to understand how mitochondria cause and contribute to PD? Certainly, understanding how changes in mitochondria cause PD would provide important insights into mitochondrial metabolism and selective neuronal vulnerability. But would it also lead to new disease-modifying therapies? Can mitochondria be targeted therapeutically for neurologic diseases? These questions are important, as therapies to boost mitochondrial functions, including creatine [218] and coenzyme Q10 [219], have failed to slow PD progression in large clinical trials.
These failures likely resulted largely from an inadequate understanding of how, exactly, mitochondria contribute to the disease and a consequent lack of basic science evidence that the therapies would be beneficial. Indeed, although creatine failed for PD, it is an effective disease-modifying therapy for guanidinoacetate methyltransferase deficiency, where there is a defect in creatine biosynthesis [220, 221]. Similarly, CoQ10 is an effective disease-modifying therapy for primary CoQ10 deficiency [222, 223]. Another successful energy-based therapy for neurologic disease, a ketogenic diet for glucose transporter type 1 deficiency, appears to help by providing an alternate fuel source when glucose import into the brain is impaired [224, 225].
In all of these examples, a specific therapy was used to target a specific metabolic defect. However, as mitochondrial therapies for PD have not been based on a solid foundation of mechanistic understanding, it isn’t surprising that they failed. Nevertheless, our understanding of the mechanistic basis for mitochondrial therapies has advanced, a new line of therapeutic approaches has emerged. For instance, PINK1-mediated PD is likely caused by a loss of kinase activity [226], and efforts to boost kinase activity [227] might be expected to help at least the small subset of patients with PD caused by PINK1 mutations. Strategies to boost Parkin function carry a similar rationale [228], and it will be important to discover whether these approaches are beneficial in treating sporadic forms of PD. Another promising approach targets an intrinsic susceptibility of SN DA neurons by blocking L-type Ca2+ channels, an intervention that protects against mitochondrial inhibitors and oxidative stress in PD model systems [43, 187, 229]. Important caveats for this and other approaches targeting sporadic PD, however, are the heterogeneity of sporadic PD and the risk that a therapy that helps only a subset of sporadic forms of PD may not be recognized. Nonetheless, these and other approaches give reasons for optimism about the future of mitochondria-based therapies for PD. They also highlight the need for concerted research efforts to deepen our understanding of how, exactly, mitochondria contribute to the various genetic and sporadic forms of the disease.
Acknowledgments
This work was supported by Betty Brown’s Family and the Joan and David Traitel Family Trust, NIH (1RO1NS091902, KN) and a Larry L. Hillblom Foundation Fellowship (DH). We thank Stephen Ordway and Gary Howard for editorial assistance and Giovanni Maki for graphical design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lezi E, Swerdlow RH. Mitochondria in neurodegeneration. Adv Exp Med Biol. 2012;942:269–86. doi: 10.1007/978-94-007-2869-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol. 2008;34:577–89. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 4.Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–74. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoppel CL, Grinblatt D, Kwok HC, Arora PK, Singh MP, Sayre LM. Inhibition of mitochondrial respiration by analogs of 4-phenylpyridine and 1-methyl-4-phenylpyridinium cation (MPP+), the neurotoxic metabolite of MPTP. Biochem Biophys Res Commun. 1987;148:684–93. doi: 10.1016/0006-291x(87)90931-4. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay RR, Salach JI, Dadgar J, Singer TP. Inhibition of mitochondrial NADH dehydrogenase by pyridine derivatives and its possible relation to experimental and idiopathic parkinsonism. Biochem Biophys Res Commun. 1986;135:269–75. doi: 10.1016/0006-291x(86)90972-1. [DOI] [PubMed] [Google Scholar]
- 7.Miyako K, Kai Y, Irie T, Takeshige K, Kang D. The content of intracellular mitochondrial DNA is decreased by 1-methyl-4-phenylpyridinium ion (MPP+) J Biol Chem. 1997;272:9605–8. doi: 10.1074/jbc.272.15.9605. [DOI] [PubMed] [Google Scholar]
- 8.Soldner F, Weller M, Haid S, Beinroth S, Miller SW, Wullner U, Davis RE, Dichgans J, Klockgether T, Schulz JB. MPP+ inhibits proliferation of PC12 cells by a p21(WAF1/Cip1)-dependent pathway and induces cell death in cells lacking p21(WAF1/Cip1) Exp Cell Res. 1999;250:75–85. doi: 10.1006/excr.1999.4504. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ. The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol. 2000;58:271–278. doi: 10.1124/mol.58.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Xu Z, Fang H, Duhart HM, Patterson TA, Ali SF. Gene expression profiling of MPP+-treated MN9D cells: a mechanism of toxicity study. Neurotoxicology. 2007;28:979–87. doi: 10.1016/j.neuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–41. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno Y, Saitoh T, Sone N. Inhibition of mitochondrial alpha-ketoglutarate dehydrogenase by 1-methyl-4-phenylpyridinium ion. Biochem Biophys Res Commun. 1987;143:971–6. doi: 10.1016/0006-291x(87)90346-9. [DOI] [PubMed] [Google Scholar]
- 13.Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-induced neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pifl C, Giros B, Caron MG. Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J Neurosci. 1993;13:4246–4253. doi: 10.1523/JNEUROSCI.13-10-04246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992;89:9074–8. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tormo JR, Gallardo T, Peris E, Bermejo A, Cabedo N, Estornell E, Zafra-Polo MC, Cortes D. Inhibitory effects on mitochondrial complex I of semisynthetic mono-tetrahydrofuran acetogenin derivatives. Bioorg Med Chem Lett. 2003;13:4101–5. doi: 10.1016/j.bmcl.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Giordano S, Lee J, Darley-Usmar VM, Zhang J. Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS One. 2012;7:e44610. doi: 10.1371/journal.pone.0044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 20.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009;34:279–90. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119:866–72. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massieu L, Del Rio P, Montiel T. Neurotoxicity of glutamate uptake inhibition in vivo: correlation with succinate dehydrogenase activity and prevention by energy substrates. Neuroscience. 2001;106:669–77. doi: 10.1016/s0306-4522(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 23.Brouillet E, Jacquard C, Bizat N, Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J Neurochem. 2005;95:1521–40. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- 24.Greene JG, Greenamyre JT. Characterization of the excitotoxic potential of the reversible succinate dehydrogenase inhibitor malonate. J Neurochem. 1995;64:430–6. doi: 10.1046/j.1471-4159.1995.64010430.x. [DOI] [PubMed] [Google Scholar]
- 25.Choi WS, Palmiter RD, Xia Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson’s disease model. J Cell Biol. 2011;192:873–82. doi: 10.1083/jcb.201009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterky FH, Hoffman AF, Milenkovic D, Bao B, Paganelli A, Edgar D, Wibom R, Lupica CR, Olson L, Larsson NG. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21:1078–89. doi: 10.1093/hmg/ddr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardi L, Petrucci S, Guidubaldi A, Ialongo T, Serra L, Ferraris A, Spano B, Bozzali M, Valente EM, Bentivoglio AR. Phenotypic variability of PINK1 expression: 12 Years’ clinical follow-up of two Italian families. Mov Disord. 2014;29:1561–6. doi: 10.1002/mds.25994. [DOI] [PubMed] [Google Scholar]
- 29.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–7. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin W, Wadlington NL, Chen L, Zhuang X, Brorson JR, Kang UJ. Loss of PINK1 attenuates HIF-1alpha induction by preventing 4E-BP1-dependent switch in protein translation under hypoxia. J Neurosci. 2014;34:3079–89. doi: 10.1523/JNEUROSCI.2286-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haque ME, Thomas KJ, D’Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–21. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–8. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weihofen A, Ostaszewski B, Minami Y, Selkoe DJ. Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet. 2008;17:602–16. doi: 10.1093/hmg/ddm334. [DOI] [PubMed] [Google Scholar]
- 37.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106:464–74. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–6. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 40.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–61. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 41.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A. 2010;107:9747–52. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X. Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem. 2012;121:830–9. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–12. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–46. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 47.Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. Embo J. 2010;29:3571–89. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–42. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Q, Ren Y, Feng J. Direct binding with histone deacetylase 6 mediates the reversible recruitment of parkin to the centrosome. J Neurosci. 2008;28:12993–3002. doi: 10.1523/JNEUROSCI.2860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.da Costa CA, Sunyach C, Giaime E, West A, Corti O, Brice A, Safe S, Abou-Sleiman PM, Wood NW, Takahashi H, Goldberg MS, Shen J, Checler F. Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol. 2009;11:1370–5. doi: 10.1038/ncb1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Coelln R, Dawson VL, Dawson TM. Parkin-associated Parkinson’s disease. Cell Tissue Res. 2004;318:175–84. doi: 10.1007/s00441-004-0924-4. [DOI] [PubMed] [Google Scholar]
- 52.Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SM. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid Med Cell Longev. 2013;2013:683920. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S, Li Y, Ogaki K, Ando M, Yoshino H, Tomiyama H, Nishioka K, Hasegawa K, Saiki H, Satake W, Mogushi K, Sasaki R, Kokubo Y, Kuzuhara S, Toda T, Mizuno Y, Uchiyama Y, Ohno K, Hattori N. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14:274–82. doi: 10.1016/S1474-4422(14)70266-2. [DOI] [PubMed] [Google Scholar]
- 54.Aras S, Bai M, Lee I, Springett R, Huttemann M, Grossman LI. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion. 2015;20:43–51. doi: 10.1016/j.mito.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Baughman JM, Nilsson R, Gohil VM, Arlow DH, Gauhar Z, Mootha VK. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet. 2009;5:e1000590. doi: 10.1371/journal.pgen.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–26. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loeb V, Yakunin E, Saada A, Sharon R. The transgenic over expression of alpha-synuclein and not its related pathology, associates with complex I inhibition. J Biol Chem. 2010;285:7334–43. doi: 10.1074/jbc.M109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K. alpha-Synuclein and mitochondria: partners in crime? Neurotherapeutics. 2013;10:391–9. doi: 10.1007/s13311-013-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–66. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–8. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L, Xie Z, Osborn T, Hargus G, Deleidi M, Lawson T, Bogetofte H, Perez-Torres E, Clark L, Moskowitz C, Mazzulli J, Chen L, Volpicelli-Daley L, Romero N, Jiang H, Uitti RJ, Huang Z, Opala G, Scarffe LA, Dawson VL, Klein C, Feng J, Ross OA, Trojanowski JQ, Lee VM, Marder K, Surmeier DJ, Wszolek ZK, Przedborski S, Krainc D, Dawson TM, Isacson O. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med. 2012;4:141ra90. doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherra SJ, 3rd, Steer E, Gusdon AM, Kiselyov K, Chu CT. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol. 2013;182:474–84. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–44. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanders LH, Laganiere J, Cooper O, Mak SK, Vu BJ, Huang YA, Paschon DE, Vangipuram M, Sundararajan R, Urnov FD, Langston JW, Gregory PD, Zhang HS, Greenamyre JT, Isacson O, Schule B. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol Dis. 2014;62:381–6. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahlskog JE. Parkin and PINK1 parkinsonism may represent nigral mitochondrial cytopathies distinct from Lewy body Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:721–7. doi: 10.1016/j.parkreldis.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishioka K, Kefi M, Jasinska-Myga B, Wider C, Vilarino-Guell C, Ross OA, Heckman MG, Middleton LT, Ishihara-Paul L, Gibson RA, Amouri R, Ben Yahmed S, Ben Sassi S, Zouari M, El Euch G, Farrer MJ, Hentati F. A comparative study of LRRK2, PINK1 and genetically undefined familial Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:391–5. doi: 10.1136/jnnp.2009.185231. [DOI] [PubMed] [Google Scholar]
- 68.Klein C, Pramstaller PP, Kis B, Page CC, Kann M, Leung J, Woodward H, Castellan CC, Scherer M, Vieregge P, Breakefield XO, Kramer PL, Ozelius LJ. Parkin deletions in a family with adult-onset, tremor-dominant parkinsonism: expanding the phenotype. Ann Neurol. 2000;48:65–71. [PubMed] [Google Scholar]
- 69.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Ross BM, Verbitsky M, Kisselev S, Louis ED, Comella C, Colcher A, Jennings D, Nance MA, Bressman SB, Scott WK, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Rezak M, Novak K, Friedman JH, Pfeiffer R, Marsh L, Hiner B, Siderowf A, Ottman R, Marder K, Clark LN. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67:1116–22. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macedo MG, Verbaan D, Fang Y, van Rooden SM, Visser M, Anar B, Uras A, Groen JL, Rizzu P, van Hilten JJ, Heutink P. Genotypic and phenotypic characteristics of Dutch patients with early onset Parkinson’s disease. Mov Disord. 2009;24:196–203. doi: 10.1002/mds.22287. [DOI] [PubMed] [Google Scholar]
- 71.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, Ruiz D, Louis ED, Comella CL, Nance MA, Bressman SB, Scott WK, Tanner CM, Mickel SF, Waters CH, Fahn S, Cote LJ, Frucht SJ, Ford B, Rezak M, Novak KE, Friedman JH, Pfeiffer RF, Marsh L, Hiner B, Payami H, Molho E, Factor SA, Nutt JG, Serrano C, Arroyo M, Ottman R, Pauciulo MW, Nichols WC, Clark LN, Marder KS. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol. 2014;71:62–7. doi: 10.1001/jamaneurol.2013.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohmann E, Thobois S, Lesage S, Broussolle E, du Montcel ST, Ribeiro MJ, Remy P, Pelissolo A, Dubois B, Mallet L, Pollak P, Agid Y, Brice A. A multidisciplinary study of patients with early-onset PD with and without parkin mutations. Neurology. 2009;72:110–6. doi: 10.1212/01.wnl.0000327098.86861.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caccappolo E, Alcalay RN, Mejia-Santana H, Tang MX, Rakitin B, Rosado L, Louis ED, Comella CL, Colcher A, Jennings D, Nance MA, Bressman S, Scott WK, Tanner CM, Mickel SF, Andrews HF, Waters C, Fahn S, Cote LJ, Frucht S, Ford B, Rezak M, Novak K, Friedman JH, Pfeiffer RF, Marsh L, Hiner B, Siderowf AD, Ross BM, Verbitsky M, Kisselev S, Ottman R, Clark LN, Marder KS. Neuropsychological Profile of Parkin Mutation Carriers with and without Parkinson Disease: The CORE-PD Study. J Int Neuropsychol Soc. 2011;17:91–100. doi: 10.1017/S1355617710001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marras C, Lang A. Parkinson’s disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry. 2013;84:409–15. doi: 10.1136/jnnp-2012-303455. [DOI] [PubMed] [Google Scholar]
- 75.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71:499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- 76.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 77.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–71. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 78.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–9. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 79.Farrer M, Chan P, Chen R, Tan L, Lincoln S, Hernandez D, Forno L, Gwinn-Hardy K, Petrucelli L, Hussey J, Singleton A, Tanner C, Hardy J, Langston JW. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 80.Pramstaller PP, Schlossmacher MG, Jacques TS, Scaravilli F, Eskelson C, Pepivani I, Hedrich K, Adel S, Gonzales-McNeal M, Hilker R, Kramer PL, Klein C. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58:411–22. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 81.Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J, Pastor MA, Marrero C, Isla C, Herrera-Henriquez J, Pastor P. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133:1128–42. doi: 10.1093/brain/awq051. [DOI] [PubMed] [Google Scholar]
- 82.Wszolek ZK, Pfeiffer RF, Tsuboi Y, Uitti RJ, McComb RD, Stoessl AJ, Strongosky AJ, Zimprich A, Muller-Myhsok B, Farrer MJ, Gasser T, Calne DB, Dickson DW. Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology. 2004;62:1619–22. doi: 10.1212/01.wnl.0000125015.06989.db. [DOI] [PubMed] [Google Scholar]
- 83.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Mori H, Kondo T, Yokochi M, Matsumine H, Nakagawa-Hattori Y, Miyake T, Suda K, Mizuno Y. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51:890–2. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- 85.Joelving FC, Billeskov R, Christensen JR, West M, Pakkenberg B. Hippocampal neuron and glial cell numbers in Parkinson’s disease--a stereological study. Hippocampus. 2006;16:826–33. doi: 10.1002/hipo.20212. [DOI] [PubMed] [Google Scholar]
- 86.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–2. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 87.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64:485–91. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 89.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 90.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–7. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 91.Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AHV. Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol. 1994;36:876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- 92.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 93.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26:719–23. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 94.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elstner M, Morris CM, Heim K, Bender A, Mehta D, Jaros E, Klopstock T, Meitinger T, Turnbull DM, Prokisch H. Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol. 2011;122:75–86. doi: 10.1007/s00401-011-0828-9. [DOI] [PubMed] [Google Scholar]
- 96.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–7. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 97.Cali T, Ottolini D, Negro A, Brini M. alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem. 2012;287:17914–29. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, Zharkovsky A, Kaasik A. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286:10814–24. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–9. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Melov S, Andersen JK. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free Radic Biol Med. 2012;53:993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–9. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–8. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 103.Norris KL, Hao R, Chen LF, Lai CH, Kapur M, Shaughnessy PJ, Chou D, Yan J, Taylor JP, Engelender S, West AE, Lim KL, Yao TP. Convergence of Parkin, PINK1, and alpha-synuclein on stress-induced mitochondrial morphological remodeling. J Biol Chem. 2015;290:13862–74. doi: 10.1074/jbc.M114.634063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eschbach J, von Einem B, Muller K, Bayer H, Scheffold A, Morrison BE, Rudolph KL, Thal DR, Witting A, Weydt P, Otto M, Fauler M, Liss B, McLean PJ, Spada AR, Ludolph AC, Weishaupt JH, Danzer KM. Mutual exacerbation of peroxisome proliferator-activated receptor gamma coactivator 1alpha deregulation and alpha-synuclein oligomerization. Ann Neurol. 2015;77:15–32. doi: 10.1002/ana.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014;137:2493–508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- 106.McRitchie DA, Cartwright HR, Halliday GM. Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Exp Neurol. 1997;144:202–13. doi: 10.1006/exnr.1997.6418. [DOI] [PubMed] [Google Scholar]
- 107.Matzuk MM, Saper CB. Preservation of hypothalamic dopaminergic neurons in Parkinson’s disease. Ann Neurol. 1985;18:552–5. doi: 10.1002/ana.410180507. [DOI] [PubMed] [Google Scholar]
- 108.German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 109.Greene JG. Causes and consequences of degeneration of the dorsal motor nucleus of the vagus nerve in Parkinson’s disease. Antioxid Redox Signal. 2014;21:649–67. doi: 10.1089/ars.2014.5859. [DOI] [PubMed] [Google Scholar]
- 110.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord. 2013;28:715–24. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 111.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waagepetersen HS, Sonnewald U, Schousboe A. Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: functional implications. Neuroscientist. 2003;9:398–403. doi: 10.1177/1073858403254006. [DOI] [PubMed] [Google Scholar]
- 113.Gu M, Cooper JM, Taanman JW, Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol. 1998;44:177–86. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 114.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–71. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 115.Pathak D, Berthet A, Nakamura K. Energy failure: does it contribute to neurodegeneration? Ann Neurol. 2013;74:506–16. doi: 10.1002/ana.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, Kim H, Brand MD, Edwards RH, Nakamura K. The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem. 2015;290:22325–36. doi: 10.1074/jbc.M115.656405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shields LY, Kim H, Zhu L, Haddad D, Berthet A, Pathak D, Lam M, Ponnusamy R, Diaz-Ramirez LG, Gill TM, Sesaki H, Mucke L, Nakamura K. Dynamin-related protein 1 is required for normal mitochondrial bioenergetic and synaptic function in CA1 hippocampal neurons. Cell Death Dis. 2015;6:e1725. doi: 10.1038/cddis.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–72. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A. 2005;102:19126–31. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramsay RR, Singer TP. Relation of superoxide generation and lipid peroxidation to the inhibition of NADH-Q oxidoreductase by rotenone, piericidin A, and MPP+ Biochem Biophys Res Commun. 1992;189:47–52. doi: 10.1016/0006-291x(92)91523-s. [DOI] [PubMed] [Google Scholar]
- 121.Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orr AL, Ashok D, Sarantos MR, Shi T, Hughes RE, Brand MD. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic Biol Med. 2013;65:1047–59. doi: 10.1016/j.freeradbiomed.2013.08.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 124.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 125.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalized increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 127.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the Parkinsonian brain: an apparent selective increase in 8-hudroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 128.Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2014;16:217–30. doi: 10.1007/s12017-014-8294-x. [DOI] [PubMed] [Google Scholar]
- 129.Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montine TJ, Picklo MJ, Amarnath V, Whetsell WO, Jr, Graham DG. Neurotoxicity of endogenous cysteinylcatechols. Exp Neurol. 1997;148:26–33. doi: 10.1006/exnr.1997.6662. [DOI] [PubMed] [Google Scholar]
- 131.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem. 1999;73:1127–37. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 132.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–48. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakamura K, Bindokas VP, Kowlessur D, Elas M, Milstien S, Marks JD, Halpern HJ, Kang UJ. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J Biol Chem. 2001;276:34402–7. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- 135.Nakamura K, Wright DA, Wiatr T, Kowlessur D, Milstien S, Lei XG, Kang UJ. Preferential resistance of dopaminergic neurons to the toxicity of glutathione depletion is independent of cellular glutathione peroxidase and Is mediated by tetrahydrobiopterin. J Neurochem. 2000;74:2305–2315. doi: 10.1046/j.1471-4159.2000.0742305.x. [DOI] [PubMed] [Google Scholar]
- 136.Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT, Surmeier DJ. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci. 2013;33:10154–64. doi: 10.1523/JNEUROSCI.5311-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirsch EC. Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson’s disease. Ann Neurology. 1992;32:S88–S93. doi: 10.1002/ana.410320715. [DOI] [PubMed] [Google Scholar]
- 138.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–63. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kovac S, Domijan AM, Walker MC, Abramov AY. Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 2014;5:e1442. doi: 10.1038/cddis.2014.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 141.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–5. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–12. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 143.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A. 1961;47:1744–50. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978;176:463–74. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moreno-Sanchez R. Regulation of oxidative phosphorylation in mitochondria by external free Ca2+ concentrations. J Biol Chem. 1985;260:4028–34. [PubMed] [Google Scholar]
- 146.Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol. 2015;593:3447–62. doi: 10.1113/JP270254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–73. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–35. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 149.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–16. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem. 2013;288:10736–41. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, Satrustegui J. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci. 2013;33:13957–71. 13971a. doi: 10.1523/JNEUROSCI.0929-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: co-localization with calbindin-D28K and calretinin. Neuroscience. 1996;75:523–33. doi: 10.1016/0306-4522(96)00228-x. [DOI] [PubMed] [Google Scholar]
- 153.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–9. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 154.Arduino DM, Esteves AR, Cardoso SM, Oliveira CR. Endoplasmic reticulum and mitochondria interplay mediates apoptotic cell death: relevance to Parkinson’s disease. Neurochem Int. 2009;55:341–8. doi: 10.1016/j.neuint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 155.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial Ca2+ and apoptosis. Cell Calcium. 2012;52:36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–6. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 159.Lopez-Crisosto C, Bravo-Sagua R, Rodriguez-Pena M, Mera C, Castro PF, Quest AF, Rothermel BA, Cifuentes M, Lavandero S. ER-to-mitochondria miscommunication and metabolic diseases. Biochim Biophys Acta. 2015;1852:2096–2105. doi: 10.1016/j.bbadis.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 160.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–59. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ottolini D, Cali T, Negro A, Brini M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet. 2013;22:2152–68. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- 163.Cali T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta. 2013;1832:495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 164.Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, Douglas I. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: A retrospective cohort study. PLoS Med. 2015;12:e1001854. doi: 10.1371/journal.pmed.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shaltouki A, Sivapatham R, Pei Y, Gerencser AA, Momcilovic O, Rao MS, Zeng X. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports. 2015;4:847–59. doi: 10.1016/j.stemcr.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA. 2008;105:11364–9. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, Garcia-Arencibia M, Fernandez-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rub U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ. Endogenous Parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron. 2015;87:371–81. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol. 2007;203:370–80. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 170.Beattie DS. The turnover of the protein components of the inner and outer membrane fractions of rat liver mitochondria. Biochem Biophys Res Commun. 1969;35:721–7. doi: 10.1016/0006-291x(69)90465-3. [DOI] [PubMed] [Google Scholar]
- 171.Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci USA. 2013;110:6400–5. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–56. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun. 2014;5:5245. doi: 10.1038/ncomms6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li L, Nadanaciva S, Berger Z, Shen W, Paumier K, Schwartz J, Mou K, Loos P, Milici AJ, Dunlop J, Hirst WD. Human A53T alpha-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons. PLoS One. 2013;8:e85815. doi: 10.1371/journal.pone.0085815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ, Tieu K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun. 2014;5:5244. doi: 10.1038/ncomms6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–9. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–9. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 178.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–80. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Berthet A, Margolis EB, Zhang J, Hsieh I, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, Nakamura K. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci. 2014;34:14304–17. doi: 10.1523/JNEUROSCI.0930-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pham AH, Meng S, Chu QN, Chan DC. Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum Mol Genet. 2012;21:4817–26. doi: 10.1093/hmg/dds311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Carelli V, Musumeci O, Caporali L, Zanna C, La Morgia C, Del Dotto V, Porcelli AM, Rugolo M, Valentino ML, Iommarini L, Maresca A, Barboni P, Carbonelli M, Trombetta C, Valente EM, Patergnani S, Giorgi C, Pinton P, Rizzo G, Tonon C, Lodi R, Avoni P, Liguori R, Baruzzi A, Toscano A, Zeviani M. Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann Neurol. 2015;78:21–38. doi: 10.1002/ana.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA. 2010;107:5018–23. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–72. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- 184.Cho HJ, Yu J, Xie C, Rudrabhatla P, Chen X, Wu J, Parisiadou L, Liu G, Sun L, Ma B, Ding J, Liu Z, Cai H. Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER-Golgi export. EMBO J. 2014;33:2314–31. doi: 10.15252/embj.201487807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 186.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 187.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 188.Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–51. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- 189.Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, Zaghloul KA, Schneider G, Liss B, Roeper J. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15:1272–80. doi: 10.1038/nn.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch Neurol. 1984;41:856–61. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
- 191.Stachowiak MK, Keller RW, Jr, Stricker EM, Zigmond MJ. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 1987;7:1648–54. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- 193.Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990;533:203–12. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- 194.Bishop MW, Chakraborty S, Matthews GA, Dougalis A, Wood NW, Festenstein R, Ungless MA. Hyperexcitable substantia nigra dopamine neurons in PINK1- and HtrA2/Omi-deficient mice. J Neurophysiol. 2010;104:3009–20. doi: 10.1152/jn.00466.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, Fakler B, Roeper J. Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci. 2014;34:13586–99. doi: 10.1523/JNEUROSCI.5069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Foehring RC, Zhang XF, Lee JC, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol. 2009;102:2326–33. doi: 10.1152/jn.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–53. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bolam JP, Pissadaki EK. Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord. 2012;27:1478–83. doi: 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–71. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25:19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 201.Orimo S, Uchihara T, Kanazawa T, Itoh Y, Wakabayashi K, Kakita A, Takahashi H. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson’s disease. Neuropathol Appl Neurobiol. 2011;37:791–802. doi: 10.1111/j.1365-2990.2011.01194.x. [DOI] [PubMed] [Google Scholar]
- 202.Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40:319–84. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- 203.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 205.Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci. 2001;21:8473–81. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–25. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135:2058–73. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, Nakamura K. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci. 2014;34:14304–17. doi: 10.1523/JNEUROSCI.0930-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12:359–66. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jang YC, Van Remmen H. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–60. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 212.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–7. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–91. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 214.Pontes MH, Sevostyanova A, Groisman EA. When too much ATP Is bad for protein synthesis. J Mol Biol. 2015;427:2586–94. doi: 10.1016/j.jmb.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xavier JM, Rodrigues CM, Sola S. Mitochondria: major regulators of neural development. Neuroscientist. 2015 doi: 10.1177/1073858415585472. [DOI] [PubMed] [Google Scholar]
- 216.Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia Mh, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition (Burbank, Los Angeles County, Calif) 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators. Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB, Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Perez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313:584–93. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, Juncos JL, Nutt JG, Voss TS, Ravina B, Shults CM, Helles K, Snively V, Lew MF, Griebner B, Watts A, Gao S, Pourcher E, Bond L, Kompoliti K, Agarwal P, Sia C, Jog M, Cole L, Sultana M, Kurlan R, Richard I, Deeley C, Waters CH, Figueroa A, Arkun A, Brodsky M, Ondo WG, Hunter CB, Jimenez-Shahed J, Palao A, Miyasaki JM, So J, Tetrud J, Reys L, Smith K, Singer C, Blenke A, Russell DS, Cotto C, Friedman JH, Lannon M, Zhang L, Drasby E, Kumar R, Subramanian T, Ford DS, Grimes DA, Cote D, Conway J, Siderowf AD, Evatt ML, Sommerfeld B, Lieberman AN, Okun MS, Rodriguez RL, Merritt S, Swartz CL, Martin WR, King P, Stover N, Guthrie S, Watts RL, Ahmed A, Fernandez HH, Winters A, Mari Z, Dawson TM, Dunlop B, Feigin AS, Shannon B, Nirenberg MJ, Ogg M, Ellias SA, Thomas CA, Frei K, Bodis-Wollner I, Glazman S, Mayer T, Hauser RA, Pahwa R, Langhammer A, Ranawaya R, Derwent L, Sethi KD, Farrow B, Prakash R, Litvan I, Robinson A, Sahay A, Gartner M, Hinson VK, Markind S, Pelikan M, Perlmutter JS, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71:543–52. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 220.Mercimek-Mahmutoglu S, Sinclair G, van Dooren SJ, Kanhai W, Ashcraft P, Michel OJ, Nelson J, Betsalel OT, Sweetman L, Jakobs C, Salomons GS. Guanidinoacetate methyltransferase deficiency: first steps to newborn screening for a treatable neurometabolic disease. Mol Genet Metab. 2012;107:433–7. doi: 10.1016/j.ymgme.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 221.Braissant O. GAMT deficiency: 20 years of a treatable inborn error of metabolism. Mol Genet Metab. 2014;111:1–3. doi: 10.1016/j.ymgme.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 222.Rotig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, Lebideau M, Dallner G, Munnich A, Ernster L, Rustin P. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–5. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 223.Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, Lopez LC, Hirano M, Quinzii CM, Sadowski MI, Hardy J, Singleton A, Clayton PT, Rahman S. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–66. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ramm-Pettersen A, Stabell KE, Nakken KO, Selmer KK. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014;39:111–5. doi: 10.1016/j.yebeh.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 225.Klepper J. GLUT1 deficiency syndrome and ketogenic diet therapies: missing rare but treatable diseases? Dev Med Child Neurol. 2015 doi: 10.1111/dmcn.12807. [DOI] [PubMed] [Google Scholar]
- 226.Song S, Jang S, Park J, Bang S, Choi S, Kwon KY, Zhuang X, Kim E, Chung J. Characterization of PINK1 (PTEN-induced putative kinase 1) mutations associated with Parkinson disease in mammalian cells and Drosophila. J Biol Chem. 2013;288:5660–72. doi: 10.1074/jbc.M112.430801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell. 2013;154:737–47. doi: 10.1016/j.cell.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, Snyder SH. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Parkinson Study G. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD) Mov Disord. 2013;28:1823–31. doi: 10.1002/mds.25639. [DOI] [PubMed] [Google Scholar]