Abstract
Background
Chronic rhinosinusitis (CRS) is a multifactorial disease of unknown etiology characterized by sinonasal inflammation, increased mucus production and defective mucociliary clearance. Pendrin, an epithelial anion transporter, is increased in asthma and chronic obstructive pulmonary disease. Pendrin increases mucus production and regulates mucociliary clearance.
Objectives
We sought to investigate the expression of pendrin and the mucus-related protein Muc5AC in sinonasal tissues of control and CRS patients, and to evaluate the regulation of pendrin expression in nasal epithelial cells (NECs) in vitro.
Methods
The expression and distribution of pendrin in sinonasal tissues was analyzed using real-time PCR, immunoblot analysis and immunohistochemistry. Differentiated NECs were used to study the regulation of pendrin expression.
Results
Increased pendrin was observed in NP tissue of CRS patients. Immunohistochemistry analysis revealed that pendrin was largely restricted to the epithelial layer. Pendrin expression significantly correlated with inflammatory cell markers, suggesting that the factors made by these cells may induce pendrin expression. Furthermore, both pendrin and periostin (a biomarker in asthma) correlated with IL-13, suggesting that pendrin may be induced by this cytokine in sinonasal tissues. The mucus component protein Muc5AC, correlated weakly with pendrin, indicating that pendrin might modulate mucus production in NPs. In cultured NECs, pendrin expression was induced by Th2 cytokines and was induced synergistically when Th2 cytokines were combined with IL-17A. Interestingly, human rhinovirus had a potentiating effect on IL-13 induced pendrin expression. Dexamethasone suppressed pendrin expression suggesting that the therapeutic benefit of dexamethasone in asthma and CRS may involve regulation of pendrin expression.
Conclusions
Th2-mediated pendrin expression is increased in nasal polyps of patients with CRS and may lead to increased inflammation, mucus production and a decreased mucociliary clearance.
Keywords: Pendrin, SLC26A4, Periostin, Muc5AC, Chronic rhinosinusitis, nasal polyp, mucus, mucociliary clearance, nasal epithelial cells
INTRODUCTION
Chronic rhinosinusitis (CRS) is a common, disease affecting 10% of the population in developed countries1. Patients with CRS have a poor quality of life comparable to sufferers of other chronic conditions such as congestive heart failure, chronic obstructive pulmonary disease (COPD), angina and back pain, yet have few therapeutic options2, 3. Most patients fail to respond to medical interventions and often undergo surgery in an attempt to alleviate symptoms, which results in a significant cost burden4.
CRS is characterized by persistent inflammation of the nasal and paranasal sinus mucosa that lasts longer than 12 weeks. Diagnosis requires endoscopic confirmation of inflammation, purulent discharge, or edema in the middle meatus or ethmoid region, presence of nasal polyposis, or radiographic evidence of paranasal sinus inflammation in the setting of rhinosinusitis symptoms (such as purulent discharge, nasal obstruction, facial pain/pressure/fullness, and hyposmia)5.
CRS can be further divided into two major subtypes: CRS without nasal polyposis (CRSsNP) and CRS with nasal polyposis (CRSwNP). Although the cytokines and cells driving CRSsNP are not clear, CRSwNP is often associated with an elevated Th2-cytokine profile and generally eosinophilic inflammation6. Recent studies have proposed the existence of multiple endotypes of the disease, highlighting the complex nature of CRS7. Although prior efforts have deepened our understanding of the inflammatory profiles and cells associated with CRS, the etiology and pathogenesis of CRS remain largely unclear8, 9. One potential contributing factor highlighted in previous studies is an imbalance in mucociliary clearance and mucus production10. Additional work has also demonstrated abnormalities in ion transport in the setting of CRS11, 12.
Pendrin/SLC26A4 is an apically-expressed ion exchanger originally identified as the causative mutation in Pendred syndrome, a condition characterized by prelingual hearing loss and iodide organification13. Pendrin is predominantly expressed in the inner ear, kidney, and thyroid gland, but is also present in airways13-16. A role for pendrin in regulating inflammation and mucus production in asthma, CRS and COPD has been previously proposed14-16. Pendrin has also been shown to regulate epithelial air-surface liquid levels and composition15, 17.
In this study, we determined the expression and localization of pendrin in sinonasal tissues, including uncinate and nasal polyp tissue, taken from patients with CRS and control subjects. We investigated the cellular and cytokine profiles associated with pendrin expression in sinonasal tissues. Finally, we assessed the regulation of pendrin expression in differentiated nasal epithelial cells (NECs) in response to inflammatory cytokines and glucocorticoid treatment in vitro.
METHODS
Patients and Tissue Samples
Patients were recruited from the Sinus and Allergy clinics at Northwestern Memorial Hospital using protocols approved by the Institutional Review Board of Northwestern University. All CRS patients (diagnosed using task force guidelines18, 19) and control subjects signed informed consent. Tissue samples (uncinates and nasal polyps (NPs)) and nasal scraping cells were collected from the patients who had failed conservative medical therapy (saline irrigations, decongestants, prolonged treatment with steroid and/or antibiotics) at the time of sinus surgery. Control uncinate tissues were collected from patients undergoing surgery for skull-based tumor excision. Control subjects did not have any history of inflammatory upper airway diseases. Patients with immunodeficiency, Churg-Strauss syndrome, or cystic fibrosis were excluded from this study. A detailed list of patient characteristics is presented in Table I.
Table I.
Subjects' characteristics
| Control | CRSsNP | CRSwNP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total no. of subjects | 37 (18M) | 47 (23M) | 84 (57M) | ||||||
| Age (y), median (range) | 48.5 (16-78) | 38 (20-71) | 45(23-74) | ||||||
| Y | N | U | Y | N | U | Y | N | U | |
| Atopy | 4 | 31 | 2 | 24 | 15 | 8 | 42 | 27 | 15 |
| Asthma | 0 | 36 | 1 | 11 | 34 | 2 | 35 | 47 | 2 |
| PCR | Uncinate | Uncinate | Uncinate | Polyp |
|---|---|---|---|---|
| No. of subjects | 18 (8M) | 23 (8M) | 23 (17M) | 20 (18M) |
| Age (y), median (range) | 46 (16-62) | 36 (20-64) | 43 (27-56) | 43 (26-74) |
| NEC PCR | ||||
| No. of subjects | 13 (6M) | 15 (8M) | 15 (10M) | 22 (17M) |
| Age (y), median (range) | 41(29-75) | 36 (21-64) | 52 (35-67) | 49 (27-72) |
| Immunoblot | ||||
| No. of subjects | 9 (5M) | 12 (7M) | 10 (6M) | 17 (6M) |
| Age (y), median (range) | 61 (20-78) | 46 (20-71) | 49 (32-71) | 45 (23-70) |
M:male, Y:yes, U: unknown, NEC: nasal epithelial cells
Cell Culture
NECs were collected during surgery, expanded and cultured under air-liquid interface (ALI) conditions20. A detailed protocol can be found in the online supplement section.
Nasal protein extraction and immunoblot analysis
Proteins were isolated from tissues as previously described3. A detailed protocol is submitted in the online repository.
Real-time PCR
Tissues were lysed in Qiazol and RNA extraction and real-time PCR was performed as described previously3. A detailed protocol is submitted in the online repository.
Immunohistochemistry
The basic protocol for IHC has been described previously21. A detailed protocol for immunohistochemistry is submitted in the online repository.
Statistical Analysis
All multiple comparisons were carried out using an ANOVA test. Student's T-test was used to compare in vitro experimental data. All analyses were performed using software obtained from GraphPad Prism (La Jolla, CA). A p-value of less than 0.05 was considered statistically significant.
RESULTS
Increased expression of pendrin in nasal polyps of patients with chronic rhinosinusitis
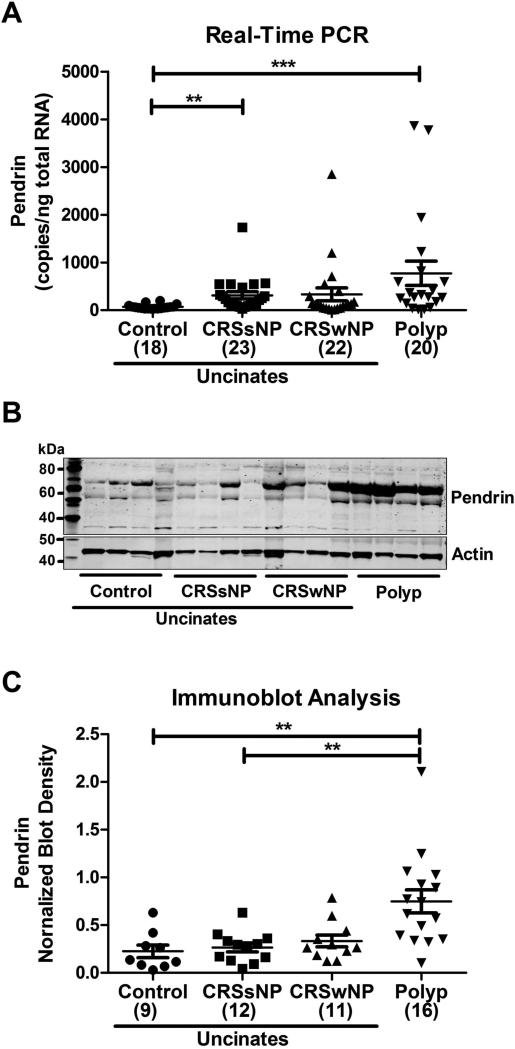
Th2 cytokine-mediated pendrin expression has been reported in asthma. Since nasal polyposis is often a Th2 cytokine dominated disease, we analyzed the expression of pendrin in 83 sinonasal tissues of control subjects (18) and CRS patients (CRSsNP UT (23), CRSwNP UT (22) and polyp (20). Pendrin expression was profoundly increased in nasal polyps of patients with CRSwNP (10 fold, 771.5±254.5 copies/ng total RNA) and uncinates of patients with CRSsNP (4 fold, 309.3±74.62 copies/ng total RNA) compared to control uncinates (71.68±11.31 copies/ng total RNA). Although there was a trend for increased pendrin expression in uncinates of patients with CRSwNP compared to control uncinates, it was not statistically significant (Fig. 1A). Next, we assessed whether mRNA data correlated to protein using immunoblot analysis in 48 sinonasal tissue samples (control UT (9), CRSsNP UT (12), CRSwNP UT (11) and polyp (16)). Surprisingly, we did not detect pendrin protein at the expected molecular weight (80 kDa: native and 100-120 kDa: glycosylated pendrin). Our pendrin specific antibody detected a cross-reacting band at a size of approximately 70 kDa. To confirm the specificity of the antibody, we performed peptide blocking experiments. Incubation of pendrin antibody with pendrin peptide prior to incubating the blot completely abrogated the 70 kDa band intensity indicating that the 70 kDa band detected by the antibody is indeed pendrin (Fig. E1). Next, we investigated whether there was any difference in the 70 kDa form of pendrin in our tissue samples. We found that the 70 kDa band density was significantly elevated (3 fold) in NPs of patients with CRS compared to control uncinates (Fig. 1, B and C). However, there was no significant difference between uncinates of control and CRS patients. These data suggest that there is a specific increase in the expression of pendrin in nasal polyps of patients with CRS compared with uncinate tissues from CRS patients or control subjects.
Fig. 1. Increased expression of pendrin in nasal polyps of patients with CRS.
Gene expression of pendrin by real-time PCR (A) in uncinates (UT) and nasal polyps. Pendrin expression was normalized based on median expression of the house keeping gene GUS and expressed as copies/ng of total RNA. (B) Representative immunoblot of pendrin and actin in sinonasal tissues. (C) Immunoblot analysis of pendrin in sinonasal tissues. Pendrin band density was normalized to actin band density and represented as blot density. *p<0.05, **P<0.01, ***p<0.001
Pendrin is expressed by the surface epithelial cells of sinonasal mucosa
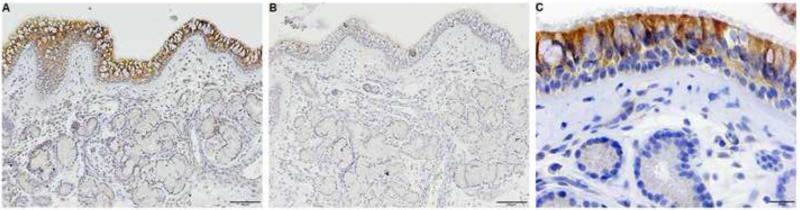
We used immunohistochemical (IHC) analysis to determine the distribution of pendrin in sinonasal tissues. Pendrin expression was detected in surface epithelial cells and in some cases, minor staining was also found in submucosal glands (Fig. 2, A and B). Closer examination of surface epithelium revealed that pendrin was expressed mostly by ciliated epithelial cells (Fig 2C). Since our IHC analysis indicated that pendrin was expressed by surface epithelial cells in sinonasal tissues, we performed real-time PCR on epithelial scrapings to specifically analyze whether epithelial cells contributed to increased pendrin expression seen in NP tissue. Pendrin mRNA trended towards an increase (3 fold) in NP epithelial cells from patients with CRSwNP compared to epithelial cells from controls uncinates (Fig. E2). Although not statistically significant, this trend suggests that the increased pendrin expression observed in nasal polyp tissue may partially be explained by an increase in epithelial pendrin expression.
Fig. 2. Pendrin is expressed by the surface epithelial cells of sinonasal mucosa.
(A and B) Representative immunohistochemical analysis of pendrin (A) and control IgG (B). Magnified image of A (C). Shown are representative pictures of at least 4 different donors)
Pendrin expression correlated with inflammatory cell markers expression in sinonasal tissues
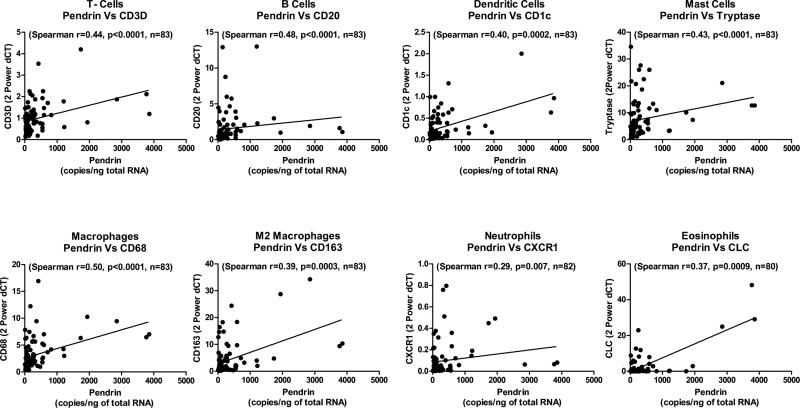
In an OVA-alum induced allergic mouse model of inflammation, pendrin knock out animals had less inflammation and inflammatory cell markers, suggesting that pendrin may regulate recruitment of inflammatory cells and therefore inflammation15. Therefore, we examined whether pendrin expression correlated with inflammatory cell markers in sinonasal tissues. Interestingly, we observed significant correlation between pendrin and CD3d (T cell), CD20 (B cell), CD1c (dendritic cell), tryptase (mast cell), CD68 (macrophage), CD163 (M2 macrophage), CLC (eosinophil) and CXCR1 (neutrophil) marker (Fig. 3). This suggests that pendrin may be involved in recruitment of these cells, or that product/s of these cells may be inducing pendrin expression in sinonasal tissues.
Fig. 3. Pendrin expression correlated with inflammatory cell markers expression in sinonasal tissues.
The relationship between pendrin and various inflammatory cell markers (CD3d (T cell), CD20 (B cell), CD1c (dendritic cell), tryptase (mast cell), Cd68 (macrophage), CD163 (M2 macrophage), CLC (eosinophil) and CXCR1 (neutrophil)) was assessed by Spearman Rank correlation.
Pendrin expression correlated with IL-13 in sinonasal tissues
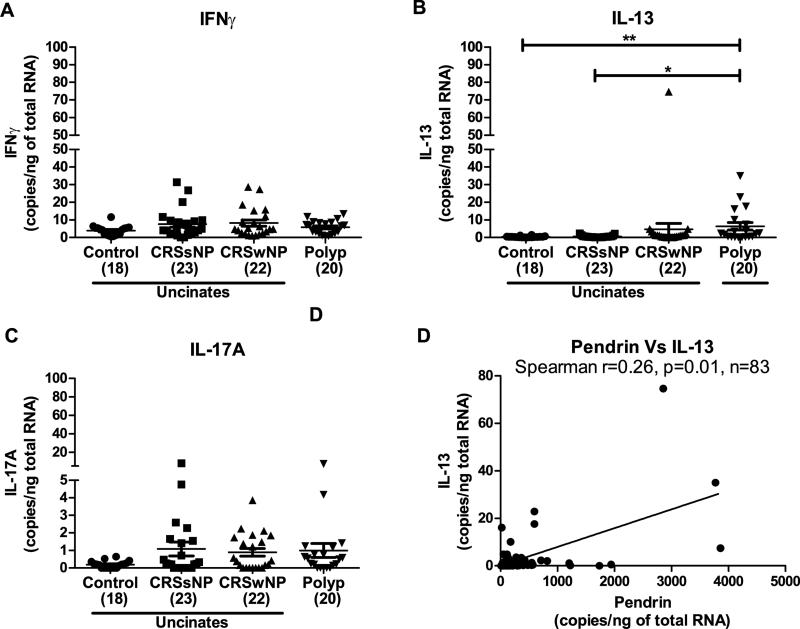
Since previous studies have reported pendrin expression to be induced by Th2 cytokines, we questioned whether the underlying factor/s responsible for the increased pendrin expression in NPs, may be the presence of elevated levels of Th2 cytokines, particularly IL-13. We confirmed an elevated expression of IL-13 mRNA in NPs of patients with CRS compared to control uncinates (Fig. 4B). mRNA levels of IFNγ and IL-17A were slightly increased, but not significantly, in NPs of patients with CRS compared to control uncinates (Fig. 4, A and C). Pendrin mRNA correlated weakly but significantly with mRNA levels of IL-13 in sinonasal tissues. (Fig. 4D). These observations suggest that pendrin expression in sinonasal tissues may be associated with the expression of IL-13. Of note, we also determined the expression of periostin, a Th2-induced protein used as a biomarker in asthma. As reported by others,22, 23 levels of periostin were significantly elevated in NPs of patients with CRS (Fig. E3A). Interestingly, mRNA of periostin also correlated strongly and significantly with IL-13 in sinonasal tissues (Fig. E3B). Taken together, our data suggests that IL-13 expression is associated with the expression of pendrin mRNA in sinonasal tissues.
Fig. 4. Pendrin expression correlated with IL-13 mRNA in sinonasal tissues.
Gene expression for IFNγ (A), IL-13 (B) and IL-17A (C) in sinonasal tissues was determined by real-time PCR. The relationship between pendrin and IL-13 was analyzed in mRNA samples by Spearman rank correlation (D). *p<0.05, **P<0.01, ***p<0.001
Pendrin is induced profoundly by Th2 cytokines in differentiated epithelial cells in vitro
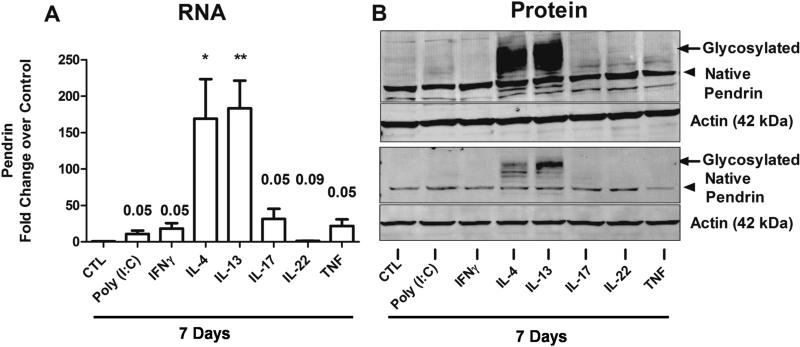
Next, we wanted to investigate whether there is a direct role of cytokines, especially Th2 cytokines, in the induction of pendrin in nasal epithelial cells (NECs). To this end, differentiated NECs (n=9) were stimulated with a Toll-like receptor 3 ligand (poly(I:C)) (10 μg/ml), Th1 (IFNγ, 10 ng/ml), Th2 (IL-4 or IL-13, 10 ng/ml), Th17 (IL-17A, 10 ng/ml) and Th22 (IL-22, 10 ng/ml) cytokines as well as a classic inflammatory cytokine, TNF (10 ng/ml), for 7 days to mimic chronic inflammation found in sinonasal tissues of patients with CRS. Pendrin mRNA and protein was analyzed by real-time PCR and immunoblotting, respectively. As expected, pendrin mRNA was increased dramatically by treatment with Th2 cytokines (IL-4 and IL-13) (Fig. 5A). We observed 169.2±54.5 fold and 183.4± 38 fold induction of pendrin mRNA by treatment with IL-4 and IL-13 respectively. Interestingly, pendrin mRNA was also modestly induced by treatment with IL-17A (31.4±13.8 fold). Moreover, treatment of airway epithelial cells with poly(I:C), IFNγ, and TNF induced pendrin mRNA, albeit to a lesser extent (10.9±4.5, 18.2±7.5, and 21.7±9.1 fold, respectively). IL-22, a cytokine involved in repair and epithelial proliferation, did not induce pendrin compared to control treated cells. The induction of pendrin in NECs by Th2 cytokines and IL-17A was dose-dependent (Fig. E4). Although induction of pendrin mRNA by Th2 cytokines is well characterized, there is no data on the regulation of pendrin protein by these cytokines. Therefore, we analyzed the expression of pendrin protein by immunoblot analysis in NECs from 8 donors. None of the cytokines or TLR stimuli tested affected the expression of native pendrin (80 kDa). Interestingly, Th2 cytokines (IL-4 and IL-13) were able to strongly induce an approximately 100 kDa glycosylated form of the pendrin protein (Fig. 5B). Although our mRNA data indicated that poly(I:C), IFNγ, IL-17A, and TNF induced pendrin mRNA in airway epithelial cells, surprisingly, we did not observe appreciable band intensity corresponding to the size of glycosylated pendrin. This observation was also true for bronchial epithelial cells (Data not shown), suggesting similar mechanisms of pendrin regulation between nasal and bronchial epithelial cells. Collectively, these results indicate that the glycosylated form, not the native pendrin, is induced profoundly by Th2 cytokines in differentiated airway epithelial cells.
Fig. 5. Pendrin is induced by Th2 cytokines and IL-17A in airway epithelial cells in vitro.
(A) Differentiated nasal epithelial cells (NECs) were untreated (CTL) or stimulated with Toll-like receptor 3 ligand (Poly (I:C)) or cytokines as indicated for 7 days. Pendrin gene expression was quantified using real-time PCR (n=9). Gene expression was normalized to actin and expressed as fold change over untreated control samples. Pendrin protein was verified using immunoblot analysis in stimulated ALI-cultured NECs. Arrow head and arrow indicates native (80 kDa) and glycosylated (100 kDa) pendrin respectively. Shown are representative blots for pendrin and actin from two donors out of 8 donors.
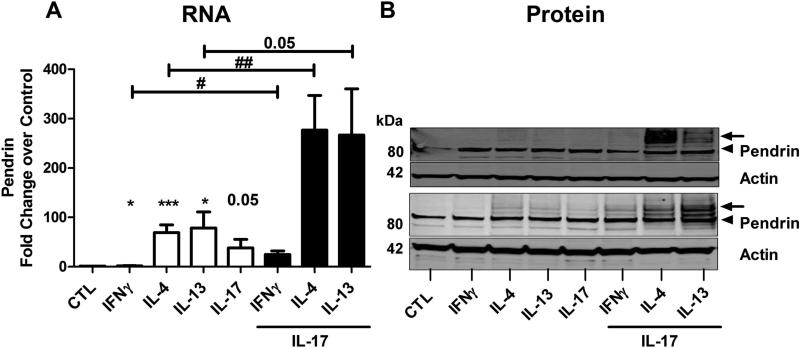
Synergistic induction of pendrin by Th2 cytokines and IL-17A in differentiated airway epithelial cells
As Th2 and Th17 cytokines are found to be expressed together and important in some forms of asthma and CRS, we wanted to investigate whether these two cytokines can work in concert to increase pendrin expression in NECs. Differentiated NECs were stimulated with media alone or with IFNγ, IL-4, IL-13 or IL-17A alone or in combination (IFNγ/IL-4/IL-13 and IL-17A) for 24 h and the mRNA was analyzed by real-time PCR. Treatment with individual cytokines induced pendrin expression (IFNγ- 1.9±0.4 fold, IL-4-69.2±15.4 fold, IL-13-78.5±32.6 fold, and IL-17A-38.0±17.3 fold, n=11-15). Surprisingly, combined treatment with either IL-4 or IL-13 and IL-17A profoundly induced pendrin mRNA expression (276.9±70.3 and 266.7±93.6 fold respectively), indicating a synergistic effect of these cytokines in inducing pendrin (Fig. 6A). Moreover, even very low concentrations of IL-17A (1 ng/ml) along with IL-13 were able to induce synergy, suggesting an amplification effect by IL-17A on Th2-induced pendrin (Data not shown). However, this synergy was observed to a lesser extent when IFNγ and IL-17A were used in combination, suggesting some specificity of the underlying Th2 and Th17 cytokine signaling mechanisms in the induction of pendrin. Synergistic induction by the combined treatment with Th2 cytokines and IL-17A was not observed with expression of periostin. Surprisingly, periostin, a Th2-induced gene that is elevated in asthmatics, was suppressed by the combined treatment (Fig. E5). Next, using immunoblot analysis, we confirmed the synergistic induction of pendrin at the protein level in NECs from 4 donors. In contrast with the extended treatment time that we used earlier (Fig.5, 7 days), a shorter treatment of airway epithelial cells with IL-4, IL-13 and IL-17A alone (for 48 h) induced very minimal levels of glycosylated form of pendrin protein (Fig. 6B). However, glycosylated pendrin levels were increased dramatically by the combined treatment of IL-17A with either IL-4 or IL-13, indicating that the synergistic induction of mRNA was faithfully recapitulated at the protein level. Taken together, these data indicate that a combination of Th2 cytokines and IL-17A induce pendrin in a synergistic manner in NECs.
Fig. 6. Synergistic induction of pendrin by Th2 cytokines and IL-17A in airway epithelial cells.
Real-time PCR analysis (A) and immunoblot analysis (B) of pendrin expression in differentiated nasal epithelial cells stimulated with cytokines alone (10 ng/ml) or in combination (10 ng each) as indicated. Shown in A is data from 11-15 experiments. Arrow head and arrow indicates native (80 kDa) and glycosylated (100 kDa) pendrin respectively. Representative blots for pendrin and actin from two experiments of 4 experiments are shown in (B). #; *p<0.05, ##; **p<0.01 by paired t-test * comparing control to treated.
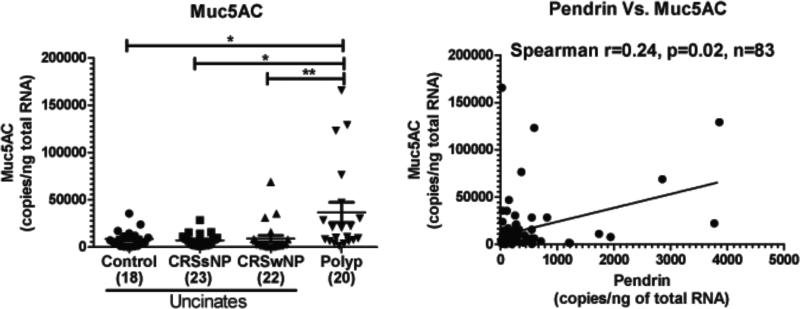
Fig. 7. Expression of Muc5AC is increased in nasal polyps of patients with CRS and pendrin expression correlated with Muc5AC mRNA in sinonasal tissues.
Muc5AC mRNA levels were measured in sinonasal tissues using real-time PCR. The relationship between pendrin and Muc5AC was analyzed in mRNA samples by real-time PCR. Correlations were assessed by Spearman rank correlation. *p<0.05, **p<0.01.
Muc5AC is increased in nasal polyps of patients with CRS and pendrin expression correlated with Muc5AC expression in sinonasal tissues
Since pendrin expression has been linked to increased mucus production, we wanted to test whether the expression of the major mucus protein component, Muc5AC, was elevated in NPs of patients with CRSwNP and whether the expression of pendrin and Muc5AC were associated in sinonasal tissues. As reported by others24, 25, we confirmed the increased expression of Muc5AC in NPs of patients with CRSwNP compared to uncinates from control or CRS patients (Fig. 7A). Muc5AC mRNA was increased by four fold in NPs compared to control uncinates (36654±10790 vs 8553±2238, p<0.05, n=83). This indicates that NPs have increased expression of Muc5AC that may contribute to the increased mucus production in polyps of patients with CRSwNP. We correlated the levels of gene expression of pendrin with Muc5AC mRNA in sinonasal tissues using a Spearman rank correlation. There was a weak but statistically significant correlation between pendrin and Muc5AC in sinonasal tissues (r=0.24, p=0.02, n=84) (Fig. 7B). One interpretation of these data is that the expression of Muc5AC and pendrin in sinonasal tissues are associated and that an increase in pendrin may promote increased mucus production in patients with CRS.
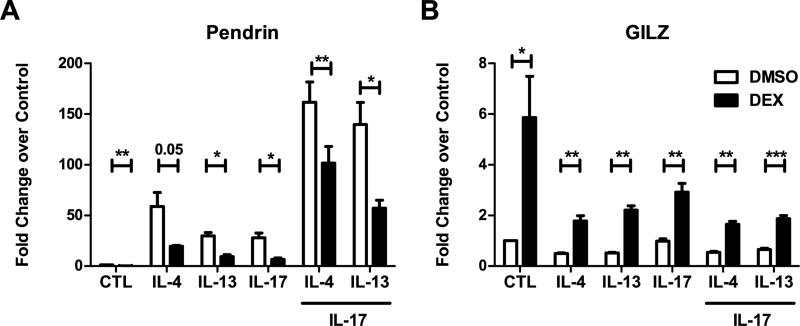
Glucocorticoids suppressed pendrin expression in airway epithelial cells
Glucocorticoids are leading drugs for treatment of diseases of airway inflammation in which mucus expression is prominent. We therefore investigated whether dexamethasone treatment can suppress pendrin expression in NECs. Differentiated NECs (n=5) were pretreated with DMSO or dexamethasone for 1 h prior to treatment with cytokines. Cells were then stimulated with media, IL-4, IL-13 or IL-17A alone or in combination for 24 h. RNA was extracted and subjected to real-time PCR. As expected, stimulation of NECs with IL-4, IL-13 and IL-17A induced pendrin expression. Interestingly, pretreatment with dexamethasone suppressed basal as well as cytokine induced pendrin expression by 50% in NECs (Fig. 8A). As a positive control for steroid treatment, we analyzed the expression of glucocorticoid induced leucine zipper (GILZ), a gene that is induced by dexamethasone. As expected, dexamethasone induced significant quantities of GILZ in airway epithelial cells (Fig. 8B).
Fig. 8. Glucocorticoids suppressed pendrin expression in airway epithelial cells.
Differentiated nasal epithelial cells were pretreated with 100 mM dexamethasone or DMSO for 1 h prior to cytokine treatment for 24 h. RNA was analyzed by real-time PCR for pendrin (A) and glucocorticoid induced leucine zipper (GILZ), a positive control, induced by glucocorticoid treatment (B). Shown are data from 5 donors.*p<0.05, **p<0.01 by paired t-test.
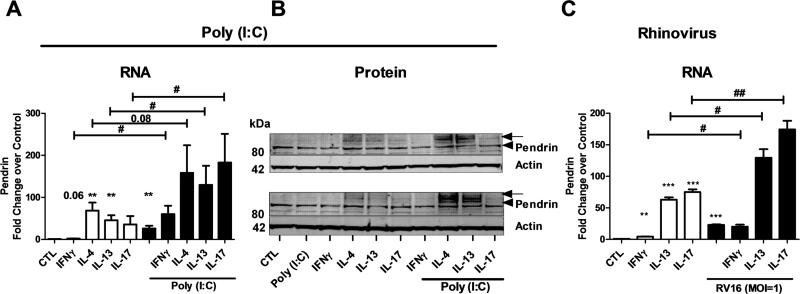
Potentiation effect of IL-13/IL-17A and poly(I:C)/ human Rhinovirus infection on pendrin expression in differentiated airway epithelial cells
As our experiments suggest that pendrin is induced by poly(I:C) in NECs, we next investigated whether poly(I:C) or rhinovirus infection has a potentiating effect on cytokine-induced pendrin expression. NECs were either untreated or treated with poly(I:C) or cytokines alone or in combination for 24 h (RNA, n=8-12) and 48 h (protein (n=2) and rhinovirus infection (n=4)). RNA was analyzed for pendrin by real-time PCR. As observed previously, treatment with poly(I:C) and other cytokines alone minimally induced pendrin mRNA. This induction was further enhanced by the combined treatment of poly(I:C) and Th1/Th2/Th17 cytokines (Fig. 9A). Moreover, treatment with IL-4, IL-13 or IL-17A along with poly(I:C) induced high levels of pendrin mRNA. We confirmed pendrin protein expression using immunoblot analysis. As observed earlier, treatment with poly(I:C) or Th1/Th2/Th17 cytokines alone did not induce the glycosylated form of pendrin. Glycosylated pendrin was only induced by the combined treatment with poly(I:C) and Th2 cytokines. Although combined treatment with IL-17A and poly(I:C) induced pendrin mRNA, we did not observe induction of the glycosylated form of pendrin (Fig. 9B). This suggests that among Th-cell cytokines, Th2 cytokines are the only ones that strongly induce glycosylated pendrin. We next used a rhinovirus (RV16) infection to test the relevance of the poly(I:C) data to infection. RV16 infection and treatment of NECs with individual cytokines (IL-13 and IL-17A) induced pendrin mRNA, which was further enhanced by the combined influence of IL-13/IL-17A with rhinovirus infection (Fig. 9C). This suggests that pendrin is induced by Th2/Th17 cytokines and the induction is further enhanced by rhinovirus infection, implying a role for pendrin either during cold virus infections, asthma exacerbations or both.
Fig. 9. Potentiation effect of IL-13/IL-17A and Poly(I:C)/ Human Rhinovirus infection on pendrin expression in airway epithelial cells.
Differentiated nasal epithelial cells were stimulated with cytokines and/ or Poly(I:C) (A and B) or cytokines and/or human rhinovirus infection (C). Cells were harvested at 24 h (A) or 48 h (B and C) after stimulation. RNA (A and C) was analyzed using real-time PCR. Pendrin mRNA was normalized to actin and expressed as fold change over control. Data from 8-12 experiments (A); 2 experiments (B) and 4 experiments (C) are shown. B (2) and C (4) experiments. In B, the arrow head and the arrow indicate native (80 kDa) and glycosylated (100 kDa) pendrin respectively. #; *p<0.05, ##; **p<0.01by paired t-test, * comparing control to treated.
DISCUSSION
Chronic rhinosinusitis is a complex multifactorial disease of the upper airways of which very little is known about the etiology and pathogenesis. Several theories have been proposed to explain the pathogenesis of CRS, but there is no direct evidence linking a single phenomenon/process to the etiology of this disease 26, 27. Among several possible factors contributing to the pathogenesis of CRS, dysregulated mucociliary clearance28, 29 and increased mucus production10 are important events that may increase susceptibility to recurrent sinus infections. In this regard, we became interested in pendrin, a Th2 cytokine induced gene that has been shown to be involved in these processes14, 15. Studies by Nakao et al. showed that enforced expression of pendrin in airway epithelial cells causes increased mucus production14. Moreover, pendrin knockout animals have decreased inflammatory cell recruitment and decreased inflammation in an OVA-alum induced asthma model15. Pendrin also functions as an anion transporter, exchanging bicarbonate, thiocyanate and iodide for chloride in different tissues. Pendrin, CFTR, and sodium channels regulate chloride secretion in airway epithelial cells, thereby regulating air surface liquid levels. In this regard, epithelial cells from pendrin knock out animals have increased air-surface liquid, possibly due to dysregulation of ion transport activity in absence of pendrin. Since CRSwNP is associated with heavy secretions of mucus and Th2 inflammation6, we hypothesized that Th2 cytokines would increase pendrin expression and function, thereby causing a defect in mucociliary clearance through the previously described functions of pendrin.
We found that pendrin was dramatically increased in nasal polyp tissues of CRSwNP patients. It was notable that the size of the immuno-reactive band was smaller than would be expected from the native protein. This finding could be due to several factors, including: the presence of alternative splice variants in sinonasal tissues, abnormal migration of pendrin during electrophoresis, or cleavage of pendrin either in vivo or during the protein extraction procedure. More investigation into the specific cause of the size variance we observed is warranted.
Others and we have found using IHC analysis that pendrin is localized mainly at the sinonasal epithelial cells. 14, 16. Consistently, pendrin mRNA in nasal polyp scraping cells was increased but not significant compared to control UT scraping cells, indicating that the increase in pendrin expression seen in nasal polyp tissue may not be solely contributed by surface epithelial cells. An important goal of future work is identification of the pendrin expressing cells in NP of CRSwNP patients.
To study the factors that are responsible for increased pendrin expression in NP tissue, we evaluated various T-helper cell cytokines such as IFNγ, IL-13 and IL-17A. Levels of mRNA of IL-17A were not significantly elevated in CRS, as we have shown previously21. We have also not found elevations in IFNγ, and other interferons, in CRS (W. Stevens et al, in preparation). Pendrin did not correlate with IFNγ and showed a weak correlation with IL-17A (Data not shown). Pendrin mRNA expression also correlated weakly with IL-13, as would be expected by the ability of IL-13 to induce its expression in vitro (Fig. 4). In cultured NECs, both IL-13 and IL-17A induced pendrin expression, although only Th2 cytokines induced the glycosylated form. We speculate that glycosylation may lead to increased membrane targeting, a necessary step in pendrin's function; if this is the case, then IL-13 may be the main cytokine among the ones tested that induces functionally active pendrin. Although it is well established that Th2 cytokines induce pendrin through a STAT6 dependent mechanism30, the induction of pendrin by IL-17A demonstrated in our study is a novel observation. As IL-17A plays a critical role in lung innate host defense, further studies are needed to elucidate the mechanism by which IL-17A induces pendrin and the role of IL-17A-induced pendrin in epithelial innate host defense.
Furthermore, combined treatment of Th2 cytokine and IL-17A synergistically induced pendrin but not periostin in NECs. As IL-13 and/or IL-17A contribute to pathogenesis of some forms of asthma31, 32 and some groups implicate both in CRS33-36, we speculate a role of pendrin in these diseases. Although other studies have shown the presence of IL-17A in nasal polyps of patients with CRS correlating positively with symptom severity and radiological score33, 35, our previous study failed to detect the presence of IL-17A in sinonasal tissues21: we therefore re-analyzed the presence of IL-17A protein using a more sensitive luminex assay. We detected measurable IL-17A in only 25% of the samples, suggesting that the synergy between Th2 cytokines and IL-17A is not likely to explain the increased pendrin expression in all nasal polyps. However, we cannot rule out the role of IL-17 in synergistic expression of pendrin in nasal polyps, as very minute quantities (below the detection limit of ELISA), may be sufficient to induce synergistic expression in vivo. Additionally, the timing of when the polyps were obtained, may also play an important role in the detection of IL-17A in sinonasal tissues. Interestingly, supporting a role for IL-17A, recent studies have shown that IL-17A is also induced by diesel exhaust particles which may be potentially important in Th2 diseases such as asthma and CRS32. Of note, we found that IL-1β can also co-operate additively with IL-13 to induce pendrin in epithelial cells (Fig. E6). Interestingly, IL-1β mRNA correlates moderately but significantly with pendrin mRNA (Data not shown). Since IL-1β is a pleiotropic cytokine involved in infection and inflammation, we speculate that IL-1β-induced pendrin might play a role in inflammatory and infectious diseases of the upper and lower airways. We have some evidence that inflammasome activation is occurring in nasal polyps (S. Seshadri, unpublished observations), so it is possible that IL-1β is contributing to the CRS phenotype.
As pendrin expression has been shown to be increased in asthma, and because rhinovirus infections are the leading cause of asthma exacerbations leading to emergency room visits, we analyzed pendrin expression upon treatment of NECs with human rhinovirus infection in the presence of IL-13 or IL-17A. Interestingly, both IL-13 and IL-17A along with rhinovirus infection led to the induction of pendrin in an additive manner, suggesting that pendrin expression may be increased during rhinovirus infection and contribute to the profound mucus secretion observed with the common cold. In asthmatic patients, pendrin thus might play a role in rhinovirus induced asthma exacerbations.
Lastly, we show that the glucocorticoid significantly suppressed pendrin mRNA at rest and after stimulation with IL-4, IL-13 or IL-17A alone; dexamethasone also suppressed the synergistic induction by these cytokines. This suggests that the therapeutic efficacy of glucocorticoids in asthma and CRS may be due in part by suppressing pendrin expression. Although we did not test dexamethasone against the combination of rhinovirus and cytokines, if it suppresses synergistic activation of pendrin in that situation, it might be relevant to the well-known ability of glucocorticoids to suppress asthma exacerbations.
When our study was in progress, Ishida et al. reported increased expression of pendrin in nasal polyps of CRS and aspirin exacerbated respiratory disease patients16. We confirmed and extended their findings to show the inflammatory cells and cytokines associated with pendrin expression in sinonasal tissues. Interestingly, the increase in pendrin in nasal polyps seems to be a common phenomenon observed both in Japanese and American polyps, although the pathology of nasal polyps may differ18, 37. We speculate that increased levels of pendrin found in both Japanese and American polyps may be an independent phenomenon depending on the cytokine milieu present in these tissues. Also, our data identifies previously unknown regulators of pendrin expression in epithelial cells such as induction of pendrin by IL-17A, synergistic induction by the combination of Th2 and Th17 cytokines and the potentiating effect of HRV infection in Th2 induced pendrin.
There are few studies that have attempted to elucidate the function of pendrin in airway epithelial cells. Based on these, we can presume that pendrin plays a role in innate immunity, inflammation, mucus production and ion transport. Pendrin levels correlated with inflammatory cell markers expression levels suggesting either that pendrin may be induced by products secreted from these cells or may regulate recruitment of these cells and therefore inflammation through other mechanisms. Since overexpression of pendrin in epithelial cells increases mucus production and mucus production is a hallmark feature of patients with CRS, we examined the association of pendrin with Muc5AC. Our data suggests that pendrin and Muc5AC expression are weakly associated in sinonasal tissues and that increased pendrin might contribute to elevated mucus production. This weak association may be due to small sample size, variability of human samples, variability introduced due to sample isolation and processing and importantly, suggests alternate modes of regulation and function. Since pendrin is also involved in regulating ion transport in airway epithelial cells, we speculate that elevated pendrin in nasal polyps may lead to dysregulated ion transport activity (increased Cl− absorption) as proposed by others11, 12.
In conclusion, this study demonstrates elevated expression of pendrin in nasal polyps of patients with CRS that is associated with inflammatory cytokines. Increased pendrin expression may contribute to the chronic inflammatory response by increasing mucus production and decreasing mucociliary clearance leading to frequent bacterial infection and colonization. Further understanding of the molecular mechanisms involved in the regulation of pendrin expression and function will help in the design of novel therapeutics for treating patients with CRS and or asthma.
Supplementary Material
Clinical implications.
Increased pendrin expression may lead to increased mucus production and a defective mucociliary clearance in Th2 mediated diseases such as asthma and nasal polyposis.
Capsule summary.
Elevated levels of pendrin in nasal polyps of patients with CRSwNP suggest a hypothetical role of pendrin in nasal polyp formation. Increased pendrin may lead to decreased mucociliary clearance and increased susceptibility to infection and thereby promote chronic inflammation.
ACKNOWLEDGEMENTS
This research was supported in part by NIH Grants R37HL068546, R01HL078860 and U19AI106683 and the Ernest S. Bazley foundation to RPS and by National Natural Science Foundation of China (NSFC) grant 81020108018 to ZL.
Abbreviations
- CRS
Chronic rhinosinusitis
- CRSwNP
CRS with nasal polyps
- CRSsNP
CRS without nasal polyps
- UT
Uncinate tissue
- NEC
nasal epithelial cells
- CLC
Charcot-Leyden crystal
- CXCR1
chemokine (C-X-C) receptor-1
- ASL
air-surface liquid
- ALI
air-liquid interface
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinol Suppl. 20122012:3. preceding table of contents, 1-298. [PubMed] [Google Scholar]
- 2.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–9. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67:920–8. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118:S17–61. doi: 10.1016/j.jaci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. 41, e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fokkens WJ, van Drunen C, Georgalas C, Ebbens F. Role of fungi in pathogenesis of chronic rhinosinusitis: the hypothesis rejected. Curr Opin Otolaryngol Head Neck Surg. 2012;20:19–23. doi: 10.1097/MOO.0b013e32834e9084. [DOI] [PubMed] [Google Scholar]
- 9.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:631–43. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein JM, Yankaskas JR. Increased ion transport in cultured nasal polyp epithelial cells. Arch Otolaryngol Head Neck Surg. 1994;120:993–6. doi: 10.1001/archotol.1994.01880330071013. [DOI] [PubMed] [Google Scholar]
- 12.Dejima K, Randell SH, Stutts MJ, Senior BA, Boucher RC. Potential role of abnormal ion transport in the pathogenesis of chronic sinusitis. Arch Otolaryngol Head Neck Surg. 2006;132:1352–62. doi: 10.1001/archotol.132.12.1352. [DOI] [PubMed] [Google Scholar]
- 13.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997;17:411–22. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 14.Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol. 2008;180:6262–9. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- 15.Nakagami Y, Favoreto S, Jr., Zhen G, Park SW, Nguyenvu LT, Kuperman DA, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181:2203–10. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012;61:589–95. doi: 10.2332/allergolint.11-OA-0370. [DOI] [PubMed] [Google Scholar]
- 17.Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, et al. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem. 2011;286:41069–82. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearlman AN, Conley DB. Review of current guidelines related to the diagnosis and treatment of rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2008;16:226–30. doi: 10.1097/MOO.0b013e3282fdcc9a. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Souza N, Avila PC, Widdicombe JH. Polarized cultures of human airway epithelium from nasal scrapings and bronchial brushings. In Vitro Cell Dev Biol Anim. 2003;39:266–9. doi: 10.1290/1543-706X(2003)039<0266:PCOHAE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403 e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Hubin G, Endam LM, Al-Mot S, Filali-Mouhim A, Desrosiers M. Expression of the extracellular matrix gene periostin is increased in chronic rhinosinusitis and decreases following successful endoscopic sinus surgery. Int Forum Allergy Rhinol. 2012;2:471–6. doi: 10.1002/alr.21056. [DOI] [PubMed] [Google Scholar]
- 23.Stankovic KM, Goldsztein H, Reh DD, Platt MP, Metson R. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope. 2008;118:881–9. doi: 10.1097/MLG.0b013e31816b4b6f. [DOI] [PubMed] [Google Scholar]
- 24.Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007;21:359–66. doi: 10.2500/ajr.2007.21.3037. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH, Lee HM. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130:747–52. doi: 10.1001/archotol.130.6.747. [DOI] [PubMed] [Google Scholar]
- 26.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:640–53 e4. doi: 10.1016/j.jaci.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passali D, Ferri R, Becchini G, Passali GC, Bellussi L. Alterations of nasal mucociliary transport in patients with hypertrophy of the inferior turbinates, deviations of the nasal septum and chronic sinusitis. Eur Arch Otorhinolaryngol. 1999;256:335–7. doi: 10.1007/s004050050158. [DOI] [PubMed] [Google Scholar]
- 29.Majima Y, Sakakura Y, Matsubara T, Murai S, Miyoshi Y. Mucociliary clearance in chronic sinusitis: related human nasal clearance and in vitro bullfrog palate clearance. Biorheology. 1983;20:251–62. doi: 10.3233/bir-1983-20215. [DOI] [PubMed] [Google Scholar]
- 30.Nofziger C, Vezzoli V, Dossena S, Schonherr T, Studnicka J, Nofziger J, et al. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2011;90:399–405. doi: 10.1038/clpt.2011.128. [DOI] [PubMed] [Google Scholar]
- 31.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–204 e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu XD, Bao YY, Zhou SH, Yao HT, Mao JY, Ji XX, et al. Interleukin-17A expression in patients with chronic rhinosinusitis and its relationship with clinical features. J Int Med Res. 2013;41:777–84. doi: 10.1177/0300060513478089. [DOI] [PubMed] [Google Scholar]
- 34.Jiang XD, Li GY, Li L, Dong Z, Zhu DD. The characterization of IL-17A expression in patients with chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2011;25:e171–5. doi: 10.2500/ajra.2011.25.3645. [DOI] [PubMed] [Google Scholar]
- 35.Makihara S, Okano M, Fujiwara T, Kariya S, Noda Y, Higaki T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol. 2010;126:397–400, e1-11. doi: 10.1016/j.jaci.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010;151:8–16. doi: 10.1159/000232566. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011;49:392–6. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.