Abstract
Chondroitin sulfate proteoglycans (CSPGs) play important roles in the developing and mature nervous system, where they guide axons, maintain stable connections, restrict synaptic plasticity, and prevent axon regeneration following CNS injury. The chondroitin sulfate glycosaminoglycan (CS GAG) chains that decorate CSPGs are essential for their functions. Through these sugar chains, CSPGs are able to bind and regulate the activity of a diverse range of proteins. CSPGs have been found both to promote and inhibit neuronal growth. They can promote neurite outgrowth by binding to various growth factors such as midkine (MK), pleiotrophin (PTN), brain-derived neurotrophic factor (BDNF) and other neurotrophin family members. CSPGs can also inhibit neuronal growth and limit plasticity by interacting with transmembrane receptors such as protein tyrosine phosphatase σ (PTPσ), leukocyte common antigen-related (LAR) receptor protein tyrosine phosphatase, and the Nogo receptors 1 and 3 (NgR1 and NgR3). These CS-protein interactions depend on specific sulfation patterns within the CS GAG chains, and accordingly, particular CS sulfation motifs are upregulated during development, in the mature nervous system, and in response to CNS injury. Thus, spatiotemporal regulation of CS GAG biosynthesis may provide an important mechanism to control the functions of CSPGs and to modulate intracellular signaling pathways. Here, we will discuss these sulfation-dependent processes and highlight how the CS sugars on CSPGs contribute to neuronal growth, axon guidance, and plasticity in the nervous system.
Keywords: Chondroitin sulfate (CS), Glycosaminoglycans, CSPG, Proteoglycan, CSPG receptor, Neuronal growth, Axon guidance, Axon regeneration, Neuronal injury, Plasticity
Introduction
Chondroitin sulfate proteoglycans (CSPGs) play critical roles in the developing central nervous system (CNS) and in response to adult CNS injury. During embryonic development, axons must elongate, navigate specific paths, and form synapses with their target neurons. To establish precise patterns of connectivity, a range of attractive or repulsive cues guide axons to their proper targets. Several families of extracellular receptors and their ligands are known to attract or repel growth cones, including netrins, ephrins, semaphorins, and slits (Bashaw and Klein, 2010; Dickson, 2002; Tessier-Lavigne and Goodman, 1996). In addition to these prototypical axon guidance molecules, increasing evidence suggests that the chondroitin sulfate (CS) sugars on CSPGs can serve as guidance cues for growth cones and contribute to the formation of neuronal boundaries in the developing CNS (Brittis et al., 1992; Carulli et al., 2005; Snow et al., 1990). CSPGs are also major components of perineuronal nets (PNNs), where they play crucial roles in the maturation of synapses and the closure of critical periods by limiting synaptic plasticity (Berardi et al., 2003; Dityatev et al., 2010; Kwok et al., 2011; Miyata and Kitagawa, 2015). In the adult CNS, CSPGs are dramatically upregulated in the glial scar around injury sites, where they restrict synaptic and anatomical plasticity, neuronal regeneration, and repair (Bartus et al., 2012; Galtrey and Fawcett, 2007; Silver and Miller, 2004; Yiu and He, 2006). Enzymatic digestion of the CS GAG chains on CSPGs can promote axon regeneration, sprouting, and functional recovery in in vivo models of CNS injury, underscoring again critical roles for CS sugars. In this review, we will highlight the various functions of the CS sugars on CSPGs and how they contribute to neuronal growth, axon guidance, and plasticity in the nervous system. We will also discuss strong evidence that specific sulfated motifs within CS chains can serve as ligands for extracellular receptors, thereby enabling CSPGs to activate key signaling pathways important for neuronal development and function.
Structure of chondroitin sulfate proteoglycans
CSPGs are composed of a core protein with one or more covalently attached CS GAG chains (Galtrey and Fawcett, 2007; Kjellén and Lindahl, 1991). They are major components of the extracellular matrix (ECM), where they provide structural support and modulate neuronal activity (Busch and Silver, 2007; Galtrey and Fawcett, 2007; Kwok et al., 2011). In addition, some CSPGs are inserted into the membrane via a single membrane-spanning domain or a glycosylphosphatidylinositol (GPI) anchor, or are localized to secretory granules. The most abundant CSPGs in the CNS are members of the lectican family, which is comprised of aggrecan, brevican, neurocan, and versican (Yamaguchi, 2000). Lecticans contain an N-terminal G1 domain, C-terminal G3 domain, and a central region decorated with varying numbers of CS chains (ranging from 1 to >100). Aggrecan, unlike the other lecticans, also contains a G2 domain following the N-terminal G1 domain (Yamaguchi, 2000). The phosphacan or receptor-type protein-tyrosine phosphatase zeta (PTPζ/RPTPβ) family, which consists of both transmembrane and soluble secreted forms, is expressed predominantly in the CNS and is found in neurons and astrocytes throughout development and adulthood (Hayashi et al., 2005; Maurel et al., 1994). The small leucine-rich proteoglycans (SLRPs) such as decorin and biglycan have N-terminal binding sites for 1 or 2 CS chains and leucine-rich repeats flanked by cysteine residues in their central domain (Hocking et al., 1998). Another prominent CSPG in the nervous system is NG2, a transmembrane proteoglycan with a CS chain attached to its large extracellular domain (Stallcup, 2002).
Structure of chondroitin sulfate sugars
Proteoglycan core proteins are decorated at certain serine residues with CS GAG chains via a tetrasaccharide linker. CS GAGs are linear polysaccharides comprised of a repeating disaccharide unit containing N-acetyl-D-galactosamine (GalNAc) and D-glucuronic acid (GlcA) (Gama and Hsieh-Wilson, 2005; Sugahara, 2003). Each chain contains up to 100 disaccharide units and undergoes extensive sulfation in the Golgi by chondroitin sulfotransferases (Kusche-Gullberg and Kjellen, 2003; Mikami and Kitagawa, 2013). The commonly occurring CS disaccharide units are characterized by the number and position of their sulfate modifications (Fig. 1A). For instance, the monosulfated CS-A and CS-C motifs contain sulfate groups at the 4-O and 6-O positions of the GalNAc residue, respectively, and are generated by the sulfotransferases chondroitin 4-O-sulfotransferase (C4ST) and chondroitin 6-O-sulfotransferase (C6ST), respectively (Fig. 1B). The disulfated CS-D unit is synthesized from the CS-C precursor via 2-O sulfation of the GlcA residue by uronyl 2-O-sulfotransferase (UST), while the CS-E unit is generated from a CS-A unit by 6-O sulfation of the GalNAc residue by the sulfotransferase N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST). Thus, a suite of sulfotransferase enzymes works in concert to produce a structurally complex, heterogeneously sulfated polysaccharide. This non-template driven process results in diverse patterns of sulfation that allow CS GAGs to interact with a wide range of proteins, including different growth factors, cytokines, and transmembrane receptors.
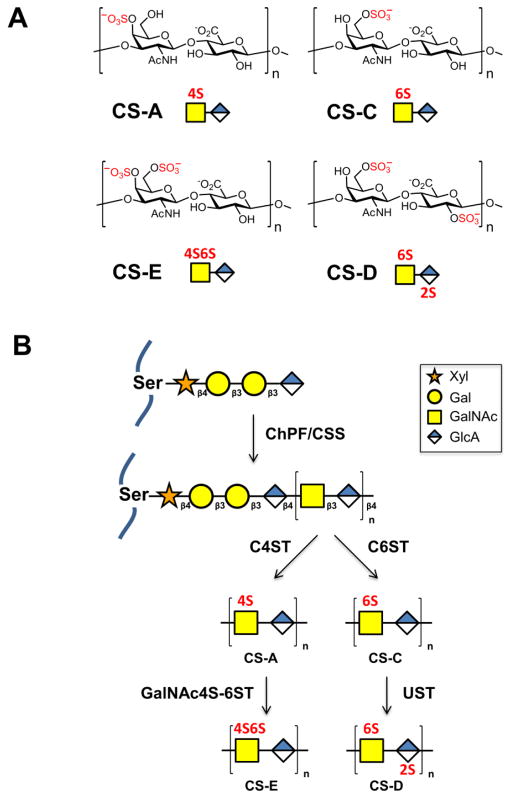
Figure 1.
(A) Common sulfation motifs of chondroitin sulfate, which consists of the repeating disaccharide N-acetyl-D-galactosamine-β(1,3)-D-glucuronic acid. n = 20–100. CS-A and CS-C are monosulfated at the 4-O and 6-O positions of GalNAc, respectively. CS-D is sulfated at the 2-O position of GlcA and 6-O position of GalNAc. CS-E is sulfated at the 4-O and 6-O positions of GalNAc. (B) Biosynthesis of chondroitin sulfate. A core tetrasaccharide (xylose (Xyl)-galactose (Gal)-galactose (Gal)-glucuronic acid (GlcA)) is appended to serine residues of the core proteoglycan. Chain extension is performed by chondroitin sulfate synthase (CSS) and chondroitin polymerizing factor (ChPF). The polysaccharide chains are then elaborated through sulfation by C4ST to generate CS-A or C6ST to generate CS-C, followed by GalNAc4S-6ST or UST to form CS-E or CS-D, respectively.
CSPG receptors
Many of the diverse functions of CSPGs arise from their ability to bind a large number of protein partners. CSPGs interact with various proteins in the ECM, including fibronectin, laminin, neural cell adhesion molecule (NCAM), and neural glial cell adhesion molecule (NgCAM) (Friedlander et al., 1994; Grumet et al., 1993; Wu et al., 2005b). Through these interactions, CSPGs can block laminin-mediated integrin activation, as well as cell adhesion molecules important for promoting neuronal migration and growth (Muir et al., 1989; Tan et al., 2011; Zuo et al., 1998). CSPGs are also known to interact with a variety of growth factors, such as midkine (MK), pleiotrophin (PTN), nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF), and in some cases, can help assemble growth factor-receptor complexes (Deepa et al., 2002; Gama et al., 2006; Maeda et al., 2003; Rogers et al., 2011; Zou et al., 2003). In this way, CSPGs can modulate growth factor signaling pathways by presenting soluble factors to their cell surface receptors and/or potentially sequestering them from their cell surface receptors. It is becoming increasing clear that CSPGs can also interact with and modulate the activity of many membrane-associated proteins, including the protein tyrosine phosphatases PTPσ and leukocyte common antigen-related (LAR) and the Nogo receptors NgR1 and NgR3 (Brown et al., 2012; Dickendesher et al., 2012; Fisher et al., 2011; Shen et al., 2009). For example, the interaction of CS and heparan sulfate (HS) GAGs with the thrombospondin repeats of semaphorin 5A (Sema5A) guides neurons in the developing diencephalon fiber tract, with each interaction resulting in different functional outcomes. While HS is required for Sema5A-mediated attraction, the interaction with CS converts Sema5A to a repulsive guidance cue, suggesting that neuronal responses to axon guidance cues can depend on their GAG binding status (Kantor et al., 2004). We will highlight additional examples below where the CS sugar chains mediate the interactions and activity of CSPGs toward their protein partners.
Regulation of the ‘sulfation code’
The sulfation profiles of CS are spatially and temporally regulated in a tissue-specific and cell type-specific manner. In the nervous system, particular CS sulfation motifs are upregulated during early and postnatal development (Fernaud-Espinosa et al., 1996; Ishii and Maeda, 2008a, b; Kitagawa et al., 1997; Maeda et al., 2003; Mitsunaga et al., 2006). For example, distinct immunohistochemical patterns are exhibited by monoclonal antibodies that recognize sulfated CS chains (antibodies CS-56, 2H6 and MO-225). Although these antibodies recognized a varied set of overlapping epitopes, each revealed distinct CS expression patterns in the brain (Sugahara and Mikami, 2007). For example the CS-56 and 2H6 epitopes were highly expressed in the postnatal day 7 (P7) mouse cortex and showed decreased expression in P12 and P20 cortical tissue (Maeda et al., 2003; Sugahara, 2003). In the cerebellum, CS-56 immunoreactivity was detected at P7 and P12 but was absent at P20. Interestingly, the MO-225 epitope was not observed in the cortex but showed strong expression in the cerebellum at P7, P12, and P22 (Maeda et al., 2003; Sugahara and Mikami, 2007).
Additional insights into the expression dynamics of CS motifs was obtained from high-performance liquid chromatography (HPLC) analysis of CS disaccharides following digestion of the GAG chains. The monosulfated CS-A motif was the most abundant motif in the embryonic mouse cortex and cerebellum, and its levels increased as development progressed (Ishii and Maeda, 2008a, b; Mitsunaga et al., 2006). Expression of the CS-C motif was highest during embryonic development and steadily decreased through development, but it remained the second most abundant CS motif (Ishii and Maeda, 2008a, b; Mitsunaga et al., 2006). Interestingly, the disulfated CS-E motif exhibited its highest expression in the embryonic mouse cortex, and its levels decreased into adulthood but remained higher in the cortex than in the cerebellum (Ishii and Maeda, 2008a, b; Mitsunaga et al., 2006). In contrast, expression of the disulfated CS-D motif was highest in the cerebellum and peaked around P10 (Ishii and Maeda, 2008a, b). Notably, deletion of these highly sulfated motifs by genetic knockdown of the sulfotransferases UST and GalNAc4S-6ST via in utero electroporation resulted in impaired migration of cortical neurons in vivo (Ishii and Maeda, 2008a). Electroporated neurons accumulated in the lower intermediate zone and the subventricular zone and did not migrate radially in the neocortex.
In addition to dynamic changes in CS sulfation, the expression of specific CS sulfotransferases is spatially and temporally regulated (Ishii and Maeda, 2008a, b; Kitagawa et al., 1997; Maeda, 2010; Purushothaman et al., 2007; Sugahara and Mikami, 2007). Widespread mRNA expression of C4ST and GalNAc4S-6ST was observed in the cortex, hippocampus, cerebellum, striatum, and the olfactory bulb during postnatal development and into adulthood, while UST was preferentially expressed in the developing cerebellum (Mitsunaga et al., 2006; Purushothaman et al., 2007). In the cerebellum, expression of GalNAc4S-6ST shifted during postnatal development from the external to the internal granular layer (Ishii and Maeda, 2008b; Purushothaman et al., 2007). This change in sulfotransferase mRNA expression profile matched the levels of CS-E expression observed by immunostaining and coincided with the migration and maturation of granular cells (Purushothaman et al., 2007).
Interestingly, studies suggest that proteoglycans possess well-defined GAG sequences. For example, early studies demonstrated that the CS chains of phosphacan purified from P20 mouse brains contained a higher abundance of the CS-D motif compared to those of phosphacan purified from P7 and P12 brains, and accordingly, they exhibited higher binding affinity for the growth factor PTN (Maeda et al., 2003). More recently, GAG fragments of similar size and charge were isolated from the heparan sulfate proteoglycan bikunin, and mass spectrometry sequencing revealed a single, defined sequence motif (Ly et al., 2011).
Together, multiple lines of evidence indicate that the sulfation patterns on CS chains are specific and highly controlled. Spatiotemporal regulation of CS GAG biosynthesis could provide an important mechanism to modulate the interactions and functions of CSPGs and activate intracellular signaling pathways. Moreover, the concerted expression of particular sulfation motifs on different proteoglycan core proteins could provide an elegant mechanism to coordinate the activities of various CSPGs. In this review, we will discuss current hypotheses regarding CSPGs and evidence supporting an important role for the sulfated CS chains in mediating the diverse activities of CSPGs toward neurons.
CSPGs as stimulatory cues for neuronal growth
Although CSPGs are traditionally considered inhibitory molecules, they are sometimes expressed in growth-permissive regions, such as the neocortical subplate where thalamocortical afferent axons grow (Bicknese et al., 1994; Sheppard et al., 1991). As such, CSPGs do not always constitute a barrier to axon initiation or outgrowth and may also participate in axon extension and pathfinding. In fact, numerous studies have established the ability of CSPGs to stimulate neurite outgrowth in vitro, and their growth-promoting activity depends on the CS sugar chains.
Role of CS sulfation patterns
The stimulatory effects of CSPGs on cultured neurons vary with the CS sulfation pattern and neuron type. CS chains enriched in highly sulfated motifs have been shown to promote neurite outgrowth of embryonic neurons in vitro (Bao et al., 2005; Clement et al., 1998; Clement et al., 1999; Faissner et al., 1994; Garwood et al., 1999; Hikino et al., 2003; Nadanaka et al., 1998; Nandini et al., 2004). For example, the phosphacan variant DSD-1-PG contains a specific sugar epitope, DSD-1, that stimulates the outgrowth of embryonic day 18 (E18) rat hippocampal neurons and E14 mesencephalic neurons (Faissner et al., 1994; Garwood et al., 1999). The growth-promoting effects of DSD-1-PG were blocked by removal of the sugar chains or by the monoclonal antibody (mAb) 473HD, which recognizes the DSD-1 sugar epitope (Clement et al., 1998; Faissner et al., 1994; Hikino et al., 2003). Polysaccharides enriched in the disulfated CS-D sulfation motif inhibited the interaction of mAb 473HD and DSD-1-PG, suggesting that CS-D may comprise part of the DSD-1 epitope (Clement et al., 1998; Clement et al., 1999; Nadanaka et al., 1998). Further characterization of mAb 473HD demonstrated that it recognized the tetrasaccharide sequence A-D or D-A (Ito et al., 2005). Both CS-D and DSD-1 interacted strongly with PTN (also known as HB-GAM) and promoted neurite outgrowth, at least in part, through those interactions (Bao et al., 2005; Maeda et al., 2003). PTN induced cortical cell migration in vitro through the proteoglycan receptor RPTPβ, and this activity was blocked by the addition of RPTPβ antibodies, the tyrosine phosphatase inhibitor sodium vanadate, or exogenous CS (Maeda and Noda, 1998).
Interestingly, while CS-D-enriched polysaccharides promoted the outgrowth of dendrite-like extensions in cultured embryonic hippocampal neurons, polysaccharides enriched in the disulfated CS-E motif promoted the outgrowth of a single, axon-like extension (Hikino et al., 2003). Moreover, the activity of CS-E was not blocked by mAb 473HD, suggesting that a structurally distinct epitope from DSD-1 is responsible for the growth-promoting effects (Clement et al., 1999; Nadanaka et al., 1998). Both CS-D- and CS-E-enriched polysaccharides bound preferentially to a variety of growth factors. MK and PTN appeared to have similar affinities for DSD-1, CS-D, and CS-E but did not interact strongly with CS-A- or CS-C-enriched polysaccharides (Deepa et al., 2002; Maeda et al., 2003; Ueoka et al., 2000). Similarly, other neurotrophic factors such as NGF, BDNF, neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) showed preferential binding to CS-E compared to CS-A or CS-C polysaccharides (Gama et al., 2006; Rogers et al., 2011).
In addition to CS, GAGs such as HS and dermatan sulfate (DS) can also bind to growth factors, including MK, PTN, FGF, and BDNF, and promote neurite outgrowth (Hacker et al., 2005; Nandini et al., 2005; Shipp and Hsieh-Wilson, 2007; Sugahara and Mikami 2007). Heparan sulfate proteoglycans (HSPGs) stimulated neurite outgrowth of cultured rat sympathetic neurons, spinal cord neurons, chick retinal neurons, and motor neurons in a GAG-dependent manner (Beller and Snow, 2014; Hantaz-Ambroise et al., 1987; Kim et al., 2003; Lander et al., 1982). Hybrid CS/DS chains, which are synthesized from CS through the action of chondroitin-glucuronate 5-epimerase to convert GlcA to L-iduronic acid (IdoA) (Sugahara et al., 2003), have been shown to promote the outgrowth of embryonic hippocampal neurons (Bao et al., 2004; Hikino et al., 2003; Li et al., 2007; Nandini et al., 2005). Moreover, DS chains enriched in the disulfated DS-D and DS-E motif, but not the disulfated DS-B motif (sulfated at the 2-O position of IdoA and 4-O position of GalNAc), stimulated neurite outgrowth, highlighting the importance of the sulfate group position for the activity of GAGs (Hikino et al., 2003; Li et al., 2007). Interestingly, the DS-D and DS-E motifs also promoted the formation of an increased number of neurites per cell compared to CS-D and CS-E, respectively (Hikino et al., 2003; Nandini et al., 2005). Thus, CS and DS structures with the same sulfation patterns can exhibit different activities towards developing neurons. In the future, comparative analyses of various GAG families and their distinct functions or mechanisms will be important for understanding the physiological roles of GAGs during development and in the mature nervous system.
Pure synthetic molecules for assessing structure-function relationships
The structural complexity of CS GAGs renders it difficult to purify well-characterized, homogeneous oligosaccharides and polysaccharides from natural sources. For instance, the CS-D polysaccharide used in the literature contains only ~20% of the CS-D sulfation motif, with the CS-C motif constituting the majority of the polysaccharide (Hikino et al., 2003). Thus, from a purity and selectivity standpoint, chemically-synthesized GAG structures with defined sulfation motifs are crucial for studying the structure–function relationships of CS GAGs (Gama and Hsieh-Wilson, 2005). To determine whether the growth-promoting activity of CS-E-enriched polysaccharides (which contain only ~60% CS-E) was due to the CS-E sulfation motif, defined tetrasaccharides were chemically synthesized (Gama et al., 2006; Tully et al., 2004). Interestingly, a tetrasaccharide structure was sufficient to promote neurite outgrowth, and only a tetrasaccharide containing the CS-E motif (E-E), not the CS-A (A-A) or CS-C motifs (C-C), strongly promoted the outgrowth of embryonic hippocampal, cortical, and dopaminergic neurons (Gama et al., 2006; Sotogaku et al., 2007; Tully et al., 2004). A different CS tetrasaccharide containing the same number of sulfate groups as CS-E had no appreciable effect on neuronal growth, indicating that the precise position of the sulfate groups was important (Gama et al., 2006). The CS-E tetrasaccharide bound preferentially to specific growth factors, including BDNF and MK, and the growth-promoting activity of the tetrasaccharide was abolished by the addition of function-blocking antibodies against these growth factors or their cell surface receptors (TrkB and RPTPβ/ζ, respectively) (Gama et al., 2006; Rogers et al., 2011). Together, these results demonstrate that the specific sequence of the sulfate groups along the sugar backbone, rather than electrostatics alone, modulates GAG–protein interactions and can direct important neuronal signaling events.
Mechanisms of growth promotion
Studies suggest that the CS chains on CSPGs promote neuronal growth in vitro by recruiting growth factors to the cell surface and facilitating interactions with their cell surface receptors (Fig. 2). CS-E polysaccharides can enhance the formation of neurotrophin-Trk complexes in a sulfation-dependent manner (Rogers et al., 2011). For instance, CS-E-enriched polysaccharides presented on a substratum increased NT-4/5-mediated TrkA activation in PC12 cells, while removal of endogenous CS chains by chondroitinase ABC (ChABC) reduced TrkA activation. A complex of CS-E with neurotrophins and their cognate Trk receptors was observed on CS GAG microarrays, and the colocalization of CS-E and TrkA increased in PC12 cells upon treatment with the neurotrophin NGF. On the other hand, the addition of exogenous CS-E polysaccharides into the culture medium inhibited NT-4/5-and NGF-mediated TrkA activation, consistent with the mechanism that CS-E polysaccharides in solution can sequester neurotrophins away from the cell surface and prevent them from activating TrkA receptors. In addition to neutrophin-Trk complexes, contactin-1 (CNTN-1) has been identified as a receptor for CS-E (Mikami et al., 2009). CNTN-1 regulates neurite outgrowth through the non-receptor-type tyrosine kinase Fyn. Phosphorylation and activation of Fyn was induced upon treatment with CS-E, but not CS-A or CS-C, polysaccharides. Antibodies against CNTN-1 inhibited CS-E-induced neurite outgrowth of embryonic hippocampal neurons. This stimulation was further inhibited when MK or BDNF antibodies were used in combination with CNTN-1 antibodies. Although it remains to be determined whether CSPGs modulate neurotrophin and CNTN-1 signaling in vivo, heparan sulfate proteoglycans are well established to utilize similar mechanisms for the regulation of numerous growth factors such as fibroblast growth factors, Sonic Hedgehog, and epidermal growth factor (Bernfield et al., 1999; Chan et al., 2009; Xu and Esko, 2014).
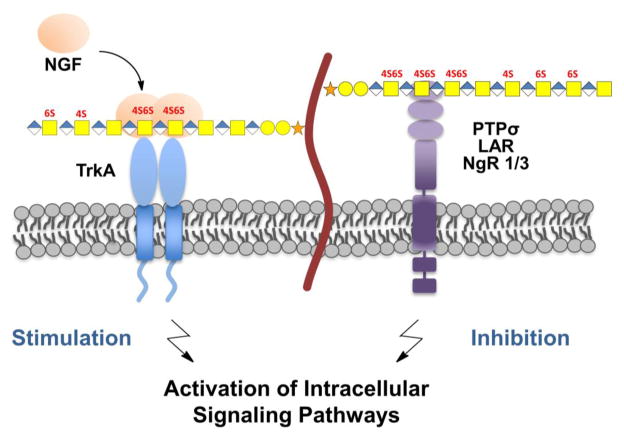
Figure 2.
Modulation of intracellular signaling pathways by chondroitin sulfate. (left) CS chains can localize soluble ligands such growth factors to the cell surface and facilitate interactions with their cognate receptors. For example, nerve growth factor (NGF) signaling and neurite outgrowth are enhanced when CS-E is presented on the cell surface. (right) Alternatively, CS chains can directly interact with transmembrane receptors such PTPσ, LAR, NgR1 and NgR3, and affect intracellular signaling. For example, CS interacts with lysine-rich IgG domains of PTPσ and inhibits neurite outgrowth.
As CS GAGs can modulate growth factor activation at the cell surface, the ability to engineer cells to express specific GAG structures would provide a novel means to control cell growth pathways. Recent studies have explored the ability to promote neuronal growth through the cell surface presentation of particular sulfated GAGs (Pulsipher et al., 2014). For example, liposomal-mediated delivery of CS-E, but not CS-A or CS-C, polysaccharides to cultured cortical neurons enhanced neurite outgrowth mediated by NGF (Pulsipher et al., 2014). The presentation of CS-E at neuronal cell membranes also stimulated NGF-mediated phosphorylation of Akt. The observed sulfation-dependent results are consistent with the high affinity of NGF for the disulfated CS-E motif (Rogers et al., 2011). In other studies, pluripotent embryonic stem cells engineered to display highly sulfated heparan sulfate underwent accelerated exit from self-renewal and differentiation into mature neuronal populations through increased activation of fibroblast growth factor/extracellular signal-regulated kinase (FGF/ERK)-mediated signaling pathways (Pulsipher et al., 2015). These studies lend strong support to the mechanism that specific sulfation motifs on GAG chains can recruit soluble factors to the cell surface and facilitate activation of their receptors (Fig. 2). Thus, controlled expression of GAG sulfation patterns at the cell surface may afford regulation of growth factor binding sites and tune intracellular signaling in a cell-type specific manner.
CSPGs as inhibitory cues for neuronal growth
CSPGs have been well documented to act as inhibitory cues in a variety of neuronal contexts. During development, CSPGs delineate boundaries that prevent extending axons from crossing (Brittis et al., 1992; Carulli et al., 2005; Snow et al., 1990). In the mature system, CSPGs have been shown to restrict synaptic plasticity and help stabilize existing connections (Bartus et al., 2012; Miyata and Kitagawa, 2015). Following CNS injury, CSPGs prevent axons from regenerating past the injury site to form functional connections (Bartus et al., 2012; Galtrey and Fawcett, 2007; Silver and Miller, 2004; Yiu and He, 2006). Although CSPGs were originally thought to function as non-specific, electrostatic or physical barriers to neuronal growth, it is becoming evident that the CS sugar chains on CSPGs can engage and modulate the activity of many inhibitory cell surface receptors. We will highlight recent studies that have demonstrated the importance of specific sulfation motifs in these systems.
Inhibition of neurite growth and axon elongation
While many studies have noted the stimulatory effects of CSPGs on embryonic hippocampal neurons, other studies have reported that CSPGs can inhibit the outgrowth of dorsal root ganglion (DRG), retinal ganglion cell (RGC) and cerebellar granule neurons (CGN) (Brown et al., 2012; Dou and Levine, 1994; Garwood et al., 1999; Koprivica et al., 2005; Monnier et al., 2003; Snow et al., 1990; Snow and Letourneau, 1992; Ughrin et al., 2003). The seemingly paradoxical activity of CSPGs appears to depend on the neuronal lineage, neuronal age, and expression of specific CSPG receptors. For example, certain CSPGs exhibit both stimulatory and inhibitory effects on neurons, depending on the neuron type. Whereas DSD-1-PG promoted the outgrowth of embryonic hippocampal neurons as described above, it potently inhibited the outgrowth of neonatal DRG explants (Garwood et al., 1999). In addition to their effects on neurite outgrowth, CSPGs also form an inhibitory barrier to elongating DRG, RGC, and CGN axons in vitro (Brown et al., 2012; Laabs et al., 2007; Snow et al., 1990). This activity depends on the CSPG concentration: neurons grown on a step gradient of immobilized CSPGs extended their axons at a reduced rate for each successively increasing CSPG concentration (Snow and Letourneau, 1992). Interestingly, RGC neurons extended axons further than DRG neurons on these CSPG step gradients, highlighting cell type-specific responses.
Many studies have established that CSPG-mediated inhibition occurs through activation of the small GTPase RhoA and its effector protein, Rho-associated, coiled-coil containing protein kinase ROCK (Borisoff et al., 2003; Brown et al., 2012; Monnier et al., 2003). Activation of the Rho/ROCK pathway leads to phosphorylation of LIM domain kinase 2 (LIMK2), myosin light chain (MLC), and other downstream proteins that induce cytoskeletal rearrangements such as neurite retraction and growth cone collapse (Maekawa et al., 1999; Wu et al., 2005a). CSPGs are also known to activate epidermal growth factor receptor (EGFR) pathways, and blocking the kinase activity of EGFR or mitogen-activated protein kinase (MAPK) reversed the inhibition by CSPGs (Brown et al., 2012; Kaneko et al., 2007; Koprivica et al., 2005).
The CS GAG chains on CSPGs are essential for the inhibitory activity of CSPGs. Enzymatic removal of the CS chains with ChABC rendered CSPGs significantly less inhibitory toward cultured DRG, RGC, and CGN neurons in neurite outgrowth and boundary assays (Dou and Levine, 1994; Laabs et al., 2007; Ughrin et al., 2003; Wang et al., 2008). Genetic disruption of CS biosynthesis by deletion of chondroitin polymerizing factor (ChPF) also reduced the inhibitory activity of CSPGs (Laabs et al., 2007). Specifically, CSPGs isolated from ChPF-deficient astrocytes failed to repel cultured CGN axons. Notably, decreasing the sulfation levels of CSPGs using the general sulfotransferase inhibitor chlorate reduced the ability of CSPGs to inhibit neurite outgrowth of DRG neurons (Smith-Thomas et al., 1995). Although the inhibitory activity of CSPGs resides primarily in their sugar chains, some core proteins also exhibit inhibitory properties (Beller and Snow, 2014; Inatani et al., 2001; Lemons et al., 2003; Schmalfeldt et al., 2000). For instance, the proteoglycan NG2 inhibited the outgrowth of neonatal CGN and embryonic DRG neurons even after ChABC treatment (Dou and Levine, 1994; Ughrin et al., 2003). Three independent domains within the extracellular portion of NG2 each exhibited comparable inhibitory activity as the full extracellular domain of NG2 (Ughrin et al., 2003). Notably, only the activity of the central domain containing CS attachment sites was affected by enzymatic digestion with ChABC. In some cases, the interaction of CSPGs with cell surface receptors does not require the GAG chains. For example, the binding of phosphacan to N-CAM and Ng-CAM was not significantly reduced by ChABC treatment, and phosphacan lacking GAG chains still bound to neurons and inhibited neurite growth comparable to intact phosphacan (Maeda and Noda, 1996; Milev el al 1994). Thus, the core protein, in addition to the GAG chains, can contribute to the inhibitory functions of certain CSPGs. Below, we will focus on the importance of the CS chains and specific sulfation motifs in mediating the inhibitory effects of CSPGs in the context of visual plasticity and CNS injury.
CSPGs and visual plasticity
During the critical period of development, the brain is most plastic, and experience-dependent activity shapes neuronal connections (Berardi et al., 2003; Hensch, 2005; Morishita and Hensch, 2008). Sensory input during the critical period is required for the formation of functional neural circuits. In the mouse visual cortex, monocular deprivation during the critical period leads to a shift in the responsiveness of neurons toward the non-deprived eye (Berardi et al., 2003; Morishita and Hensch, 2008). This shift in ocular dominance (OD) is not observed if monocular deprivation is performed after the close of the critical period, which is accompanied by a marked reduction in plasticity and the formation of perineuronal net (PNN) structures (Pizzorusso et al., 2002; Pizzorusso et al., 2006). PNNs, which consist of CSPGs, tenascin, link-proteins and hyaluronic acid, surround the cell body and extend along the dendrites of inhibitory neurons expressing the calcium-binding protein parvalbumin (PV). They serve to restrict synaptic plasticity and stabilize the network of existing neuronal connections (Berardi et al., 2003; Dityatev et al., 2010; Kwok et al., 2011; Miyata and Kitagawa, 2015). The CSPGs in PNNs, and in particular, their CS sugar chains, are essential to the structure and function of PNNs. Digestion of the CS sugars by ChABC in the visual cortex reactivated critical period plasticity following monocular deprivation in adult mice (Pizzorusso et al., 2002; Pizzorusso et al., 2006). ChABC treatment also increased dendritic spine dynamics and density in the visual cortex in vivo and in hippocampal organotypic slices (de Vivo et al., 2013; Orlando et al., 2012; Pizzorusso et al., 2006). These effects were prevented by pretreatment with β1-integrin blocking antibodies, suggesting that the increase in dendritic spine dynamics occurred through disruption of CSPG interactions with integrin and activation of β1-integrin signaling pathways (Orlando et al., 2012). Interestingly, CSPG interactions with hyaluronic acid (HA) chains are also required for the formation and stabilization of PNN structures. The N-terminal and C-terminal domains of lecticans allow these CSPGs to interact with HA and tenascins, two key components of PNNs (Grumet et al., 1994; Matsumoto et al., 2003). CSPG-HA interactions are stabilized by link protein, and genetic deletion of link protein or enzymatic digestion of HA or CS resulted in the loss of PNNs (Carulli et al., 2010; Galtrey and Fawcett, 2007; Koppe et al., 1997; Pizzorusso et al., 2002).
Specific sulfation motifs on CSPGs have been shown to regulate PNN formation and critical period plasticity (Miyata and Kitagawa, 2015). The sulfation patterns of CSPGs are tightly regulated during postnatal development in the mouse visual cortex. While 6-O sulfation of CS (CS-C) decreases, 4-O sulfation (CS-A) increases as the critical period comes to a close, resulting in an increase in the 4-O to 6-O sulfate (4S/6S) ratio (Miyata et al., 2012). Transgenic mice overexpressing C6ST-1 retain a low 4S/6S ratio and develop fewer PNNs around PV neurons (Miyata et al., 2012). Their PNNs are rich in CS-C and display a diffuse structure that is unable to tightly enwrap thalamocortical synaptic contacts. Importantly, the mice also exhibit persistent cortical plasticity into adulthood. When subjected to monocular deprivation, adult mice overexpressing C6ST-1 show ocular dominance plasticity similar to juvenile wild-type mice. Thus, the change from low to high 4S/6S sulfation ratio on CSPGs coincides with the close of the critical period when plasticity is restricted, and reducing this ratio can modulate PNN structure and enhance cortical plasticity.
CSPGs are believed to regulate plasticity in a sulfation-dependent manner by localizing plasticity-restricting factors to PNNs (Miyata and Kitagawa, 2015; Sugiyama et al., 2009). The transcription factor orthodenticle homeobox 2 (Otx2) is a key regulator of visual cortex plasticity (Spatazza et al., 2013; Sugiyama et al., 2008). The localization of Otx2 to PV neurons occurs through binding of Otx2 to sulfated CS GAG chains. In transgenic mice overexpressing C6ST-1, Otx2 was not incorporated in PV neurons surrounded by PNNs enriched in CS-C (Miyata et al., 2012). Otx2 fragments interact preferentially with highly sulfated CS-D- and CS-E-enriched polysaccharides (Beurdeley et al., 2012). The localization of Otx2 to PV neurons was disrupted by blocking Otx2-CS interactions through intracortical infusion of a CS-E hexasaccharide or an arginine-lysine rich N-terminal Otx2 fragment that interacts with CS-D and CS-E polysaccharides (Beurdeley et al., 2012; Despras et al., 2013). Infusion of this N-terminal Otx2 fragment, but not an alanine-containing mutant, also reopened critical period plasticity in adult mice (Beurdeley et al., 2012). Thus, the accumulation of Otx2 within PV neurons is sulfation pattern-dependent and appears to be mediated by highly sulfated CS sequences. Interestingly, the CS-E motif was also recently shown to interact with semaphorin 3A (Sema3A), a chemorepulsive guidance protein (Dick et al., 2013). Sema3A is enriched in PNNs, and ChABC treatment disrupted the localization of Sema3A to PNNs (Vo et al., 2013). Importantly, the abundance of Sema3A was reduced in PNNs during periods of enhanced synaptic remodeling in regions undergoing structural reorganization (Carulli et al., 2013). These examples suggest important roles for CS sugars in the regulation of synaptic plasticity and highlight the potential to modulate plasticity by altering the sulfation patterns of CSPGs and their sulfation-dependent interactions.
CSPGs and CNS injury
CSPGs are dramatically upregulated in the glial scar, which forms in response to CNS injury (Cregg et al., 2014; Silver and Miller, 2004; Yiu and He, 2006). Comprised of reactive astrocytes, microglia, and ECM molecules, the glial scar serves as a major barrier to regenerating axons (Cregg et al., 2014; Galtrey and Fawcett, 2007; Silver and Miller, 2004; Yiu and He, 2006). It also functions to restrict anatomical plasticity by inhibiting collateral sprouting and synaptic reorganization (Bartus et al., 2012; Galtrey and Fawcett, 2007; Silver and Miller, 2004). Within 24 h after injury, reactive astrocytes begin to synthesize and secrete CSPGs in high concentrations into the glial scar, where they persist for months (Jones et al., 2003; McKeon et al., 1999; Tang et al., 2003). One well-established strategy for overcoming the inhibition of the glial scar is localized delivery of the enzyme ChABC (Bradbury and Carter, 2011). ChABC digestion of the CS sugars on CSPGs induced axon regeneration following injury to the nigrostriatal, serotoninergic, or reticulospinal axon pathways (Fouad et al., 2005; García-Alías et al., 2011; Moon et al., 2001). ChABC treatment also resulted in improved regeneration and functional recovery in several models of spinal cord injury (Alilain et al., 2011; Barritt et al., 2006; Bartus et al., 2014; Bradbury et al., 2002; García-Alías et al., 2009). For example, mice treated with ChABC after a dorsal column crush injury exhibited growth of ascending sensory projections and descending corticospinal tract axons (Barritt et al., 2006). Enhanced functional recovery of locomotor and proprioceptive behaviors was also observed. In other studies, ChABC treatment increased conduction through intact fibers in the ventrolateral funiculus following unilateral hemisection of adult rat spinal cord (Hunanyan et al., 2010). The remarkable effects of ChABC have been proposed to occur both through regeneration of corticospinal tract axons, as well as enhanced plasticity and sprouting of spared axons.
Interestingly, enzymatic digestion of keratan sulfate (KS) GAGs using keratanase II (K-II) also led to axon regeneration and improved recovery of motor and sensory function following injury (Imagama et al. 2011; Ishikawa et al., 2015). KS, another component of the glial scar, is comprised of repeating disaccharide units of galactose (Gal) and N-acetyl-D-glucosamine (GlcNAc), and it can be sulfated at the 6-O position of Gal and GlcNAc residues (Funderburgh 2000; Kadomatsu and Sakamoto 2014). KS digestion by K-II led to comparable effects on axon regeneration and sprouting as CS digestion by ChABC (Imagama et al. 2011; Ishikawa et al., 2015). The effects of digestion with K-II and ChABC on axon regeneration were not additive, as treatment with both K-II and ChABC did not result in improved axon recovery compared to either treatment alone (Imagama et al. 2011; Ishikawa et al., 2015). It is unclear whether CS and KS inhibit axon regeneration through common receptors or if KS-specific receptors also exist.
In addition to enzymatic digestion of GAGs, genetic manipulation of CS sugar chains indicates a critical role for the sugars in inhibiting axon regeneration and plasticity after CNS injury. Mice lacking the enzyme chondroitin sulfate N-acetylgalactosaminyltransferase-1 (CSGalNAcT-1), which appends the first GalNAc residue to the core tetrasaccharide linker on CSPGs, synthesized less CS and exhibited reduced scar formation after a spinal cord compression injury (Takeuchi et al., 2013). The mice also displayed enhanced axon growth and a larger area encompassing serotonin-positive (5HT(+)) terminals beyond the lesion site. Notably, CSGalNAcT-1 knockout mice showed improved motor function and more complete, faster recovery in motor function assays compared to mice treated with ChABC. Likewise, genetic deletion of N-acetylglucosamine 6-O-sulfotransferase-1, an enzyme required for KS chain elongation, resulted in reduction of glial scar formation and promoted axonal regrowth of the corticospinal tract (Ito et al., 2010; Zhang et al., 2006).
Given the importance of the CS sugars, understanding the functions of different CS sulfation patterns in the glial scar and characterizing their mechanisms of action may provide new therapeutic strategies for promoting neuronal repair. Several studies indicate that particular CS sulfation patterns are upregulated in response to CNS injury. In addition to enhanced expression of CSPGs, the sulfotransferases C4ST, C6ST, and GalNAc4S-6ST are upregulated at the injury site following cortical lesions in mice and rats (Karumbaiah et al., 2011; Lin et al., 2011; Properzi et al., 2005). Consistent with these observations, disaccharide analysis of CS polysaccharides isolated from injured issue showed increased levels of the CS-A, CS-C, and CS-E motifs (Gilbert et al., 2005; Wang et al., 2008). For instance, a large increase in total CS and CS-A expression was observed one day after dorsal hemisection injury in mice (Wang et al., 2008). Others studies revealed an increase in CS-C and CS-E levels, but a decrease in CS-A levels, one week and one month after cortical lesion in rats (Properzi et al., 2005). Increased CS-E expression was also observed by immunohistochemistry 2 weeks after an optic nerve crush injury, as well as 24 h after cortical stab and dorsal spinal cord injuries in mice (Brown et al., 2012; Unpublished results). Comparison of these results are confounded by differences in the methods of CS analysis, the timing post injury, and the type of injury model. Nonetheless, these studies all observe an increase in the total amount of CS and in general, an increase in the CS-E sulfation motif. Systematic investigations into the CS patterns in different injury models and at various times points post injury are required for a better understanding of the temporal dynamics of CS sulfation following CNS injury.
Studies suggest that specific sulfation motifs within long CS polysaccharide chains enable CSPGs to interact with inhibitory receptors, possibly modulating the clustering and/or activation of those receptors (Fig. 2). Until recently, CSPGs were widely believed to inhibit axon growth through relatively non-specific mechanisms, such as steric blockage of the extracellular space, arrays of negatively charged sulfate, or steric hindrance of adhesive matrix molecules (Gilbert et al., 2005; Mckeon et al., 1995; Olson, 2002). However, recent studies suggest that CSPGs can interact directly with cell surface receptors expressed on injured axons and thereby activate growth-inhibitory signaling pathways (Sharma et al., 2012). Several important CSPG receptors have been identified, including the protein tyrosine phosphatases PTPσ and LAR and the Nogo receptors NgR1 and NgR3 (Brown et al., 2012; Coles et al., 2011; Fisher et al., 2011; Shen et al., 2009). DRG neurons from PTPσ-deficient mice crossed CSPG-rich boundaries and exhibited improved neurite outgrowth in response to CSPGs in vitro (Brown et al., 2012; Coles et al., 2011; Shen et al., 2009). Axons from PTPσ-deficient mice also extended further from the lesion site following a dorsal column crush injury. In a similar manner, modulating the activity of PTPσ using a peptide mimetic blocked CSPG-mediated inhibition and allowed DRG neurons to cross CSPG-rich barriers (Lang et al., 2015). Systemic treatment with this peptide following contusion spinal cord injury resulted in axon regeneration and recovery of locomotor function. It’s worth noting that PTPσ also binds to HS and this interaction promotes neurite outgrowth of cultured DRG neurons (Coles et al., 2011). Like Sema5A, PTPσ is another example where the neuronal response is dependent on its GAG binding status. Similarly, DRG and CGN neurons from LAR-deficient mice exhibited increased neurite outgrowth when grown on a CSPG substratum compared to neurons from wild-type mice (Fisher et al., 2011). As with PTPσ, blocking the activity of LAR using a peptide reversed the CSPG-mediated inhibition of neurite outgrowth in vitro and promoted axon growth and improved locomotor recovery in vivo following dorsal transection in mice. Likewise, genetic deletion of NgR1 and NgR3 resulted in improved neurite outgrowth of CGN neurons grown on a CSPG substratum and enhanced axon regeneration in mice following an optic nerve crush injury (Dickendesher et al., 2012). However, complete recovery was not observed in either PTPσ−/− or NgR1−/−; NgR2−/−; NgR3−/− triple knockout mice, and NgR1−/−; NgR3−/−; PTPσ−/− triple knockout mice displayed increased optic nerve regeneration compared to knockout of NgR1, NgR3, or PTPσ alone, suggesting that the inhibitory effects of CSPGs in the glial scar are mediated through multiple different receptors.
Importantly, the disulfated CS-E sulfation motif is critical for engaging these inhibitory CSPG receptors. PTPσ bound selectively to CS-E, but not to CS-A or CS-C, polysaccharides through its lysine-rich IgG domain (Brown et al., 2012). Moreover, the ability of CS-E polysaccharides to inhibit the outgrowth of DRG neurons was significantly attenuated in neurons from PTPσ-deficient mice (Brown et al., 2012). Interestingly, however, residual inhibition by CS-E (~22%) remained in PTPσ-deficient neurons, consistent with the idea that multiple different receptors interact with CSPGs to inhibit neuronal growth. NgR1 and NgR3 also interacted preferentially with CS chains containing the CS-E or CS-D motifs, but not those enriched in CS-A or CS-C, suggesting that these receptors also engage the CS-E structure on CSPGs (Dickendesher et al., 2012).
Additional evidence indicates that the CS-E sulfation motif is critical for the inhibitory activity of CSPGs. The CS-E motif has been shown to exert strong inhibitory effects on sensory neurons both in vitro and in vivo (Brown et al., 2012; Gilbert et al., 2005; Karumbaiah et al., 2011; Shimbo et al., 2013). Studies using CS-E-enriched polysaccharides and pure synthetic polymers containing CS-E showed that the CS-E motif was sufficient to inhibit CGN and DRG neurons in neurite outgrowth, growth cone collapse, and boundary assays (Brown et al., 2012; Gilbert et al., 2005; Shimbo et al., 2013). In contrast, CS-A- and CS-C-enriched polysaccharides and synthetic polymers had no appreciable inhibitory effects even when used at 10-fold higher concentrations or when combined with CS-E. Notably, CSPGs isolated from mice lacking GalNAc4S-6ST, the sulfotransferase that produces CS-E, showed significantly less inhibition of DRG neurite outgrowth (Brown et al., 2012). Similar effects were observed upon knockdown of C4ST or GalNAc4S-6ST in astrocytes: CSPGs isolated from these astrocytes lacked either CS-A and CS-E or CS-E alone, respectively, and were less inhibitory towards CGN and cortical neurons in neurite outgrowth and boundary assays (Karumbaiah et al., 2011; Wang et al., 2008). In contrast, reduced inhibition by CSPGs was not observed when C6ST-1, which produces CS-C, was knocked down (Wang et al., 2008). Importantly, blocking the CS-E, but not the CS-A, motif using a specific monoclonal antibody rescued the CSPG-mediated inhibition of DRG neurite outgrowth in vitro and promoted axon regeneration in vivo following an optic nerve crush injury (Brown et al., 2012). Interestingly, the CS-E-blocking antibody promoted regeneration in mice to a similar extent as complete digestion of CS chains using ChABC, suggesting that CS-E represents the major inhibitory determinant on CSPGs. The number of regenerating axons and the distance of regeneration was further enhanced by combining the CS-E antibody treatment with a small-molecule cyclic AMP analogue to activate intrinsic neuronal growth pathways. Given that CSPGs interact with several different receptors, blocking the CS-E motif may be an effective means to target multiple inhibitory receptors simultaneously.
Summary and conclusions
CSPGs are key components of the developing and mature nervous system, where they guide developing axons, restrict synaptic plasticity, and prevent axon regeneration following CNS injury. The functions of CSPGs are mediated largely by the CS sugar chains that decorate them and the ability of these sugars to engage various protein receptors in a sulfation-dependent manner. The different sulfation patterns on CS chains are tightly regulated, and the spatiotemporal expression of sulfation motifs offers a means to modulate a diverse range of neuronal processes in a cell type- and tissue-specific manner. Highly sulfated motifs, such as CS-E and CS-D, are important for modulating the functions of CSPGs and their interactions with stimulatory growth factors and inhibitory cell surface receptors. Further studies of the spatiotemporal expression of CS sulfation patterns and the identification of their interacting protein partners will provide new insights into many important neural processes. This understanding, coupled with the development of methods to disrupt CS sugars and specific sulfation motifs, such as blocking antibodies or pharmacological agents, should provide novel approaches for promoting neuronal growth, regeneration, and CNS plasticity.
Acknowledgments
L.C. H-W. was supported by NIH grant R01 GM093627 and the Christopher & Dana Reeve Foundation; G.M.M. was supported by NIH training grant NRSAT32GM07616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Nishimura S, Mikami T, Yamada S, Itoh N, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of L-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J Biol Chem. 2004;279:9765–9776. doi: 10.1074/jbc.M310877200. [DOI] [PubMed] [Google Scholar]
- Bao X, Mikami T, Yamada S, Faissner A, Muramatsu T, Sugahara K. Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J Biol Chem. 2005;280:9180–9191. doi: 10.1074/jbc.M413423200. [DOI] [PubMed] [Google Scholar]
- Barritt A, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon S, Bradbury E. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235:5–17. doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller JA, Snow DM. Proteoglycans: road signs for neurite outgrowth. Neural Regen Res. 2014;9:343–355. doi: 10.4103/1673-5374.128235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknese AR, Sheppard AM, O’Leary DDM, Pearlman AL. Thalamocortidal Axons Extend Along a Chondroitin Sulfate Proteoglycan-enriched Pathway Coincident with the Neocortical Subplate and Distinct from the Efferent Path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Brown JM, Xia J, Zhuang B, Cho K, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA. 2012;109:4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F. Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci. 2013;57:10–22. doi: 10.1016/j.mcn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Chan JA, Balasubramanian S, Witt RM, Nazemi KJ, Choi Y, Pazyra-Murphy MF, Walsh CO, Thompson M, Segal RA. Proteoglycan interactions with Sonic Hedgehog specify mitogenic responses. Nat Neurosci. 2009;12:409–417. doi: 10.1038/nn.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nadanaka S, Masayama K, Mandl C, Sugahara K. The DSD-1 Carbohydrate Epitope Depends on Sulfation, Correlates with Chondroitin Sulfate D Motifs, and Is Sufficient to Promote Neurite Outgrowth. J Biol Chem. 1998;273:28444–28453. doi: 10.1074/jbc.273.43.28444. [DOI] [PubMed] [Google Scholar]
- Clement AM, Sugahara K, Faissner A. Chondroitin sulfate E promotes neurite outgrowth of rat embryonic day 18 hippocampal neurons. Neurosci Lett. 1999;269:125–128. doi: 10.1016/s0304-3940(99)00432-2. [DOI] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-Specific Clustering and Neuronal Extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, Spolidoro M, Pizzorusso T, Maffei L, Ratto GM. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013;4:1484. doi: 10.1038/ncomms2491. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Umehara Y, Higashiyama S, Itoh N, Sugahara K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J Biol Chem. 2002;277:43707–43716. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- Despras G, Bernard C, Perrot A, Cattiaux L, Prochiantz A, Lortat-Jacob H, Mallet JM. Toward libraries of biotinylated chondroitin sulfate analogues: from synthesis to in vivo studies. Chem Eur J. 2013;19:531–540. doi: 10.1002/chem.201202173. [DOI] [PubMed] [Google Scholar]
- Dick G, Tan CL, Alves JN, Ehlert EM, Miller GM, Hsieh-Wilson LC, Sugahara K, Oosterhof A, van Kuppevelt TH, Verhaagen J, Fawcett JW, Kwok JC. Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem. 2013;288:27384–27395. doi: 10.1074/jbc.M111.310029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular Mechanisms of Axon Guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Dou C, Levine JM. Inhibition of Neurite Growth by the NG2 Chondroitin Sulfate Proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Clement AM, Lochter A, Streit A, Mandl C, Schachner M. Isolation of a Neural Chondroitin Sulfate Proteoglycan with Neurite Outgrowth Promoting Properties. J Cell Biol. 1994;126:783–799. doi: 10.1083/jcb.126.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernaud-Espinosa I, Nieto-Sampedro M, Bovolenta P. Developmental distribution of glycosaminoglycans in embryonic rat brain: relationship to axonal tract formation. J Neurobiol. 1996;30:410–424. doi: 10.1002/(SICI)1097-4695(199607)30:3<410::AID-NEU9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gama CI, Hsieh-Wilson LC. Chemical approaches to deciphering the glycosaminoglycan code. Curr Opin Chem Biol. 2005;9:609–619. doi: 10.1016/j.cbpa.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, 3rd, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- García-Alías G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- García-Alías G, Petrosyan HA, Schnell L, Horner PJ, Bowers WJ, Mendell LM, Fawcett JW, Arvanian VL. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. J Neurosci. 2011;31:17788–17799. doi: 10.1523/JNEUROSCI.4308-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood J, Schnädelbach O, Clement A, Schütte K, Bach A, Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J Neurosci. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol. 1993;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Hantaz-Ambroise D, Vigny M, Koenig J. Heparan sulfate proteoglycan and laminin mediate two different types of neurite outgrowth. J Neurosci. 1987;7:2293–2304. [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Miyata S, Yamada M, Kamei K, Oohira A. Neuronal expression of the chondroitin sulfate proteoglycans receptor-type protein-tyrosine phosphatase β and phosphacan. Neuroscience. 2005;131:331–348. doi: 10.1016/j.neuroscience.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hikino M, Mikami T, Faissner A, Vilela-Silva AC, Pavao MS, Sugahara K. Oversulfated dermatan sulfate exhibits neurite outgrowth-promoting activity toward embryonic mouse hippocampal neurons: implications of dermatan sulfate in neuritogenesis in the brain. J Biol Chem. 2003;278:43744–43754. doi: 10.1074/jbc.M308169200. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biology. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Hunanyan AS, Garcia-Alias G, Alessi V, Levine JM, Fawcett JW, Mendell LM, Arvanian VL. Role of chondroitin sulfate proteoglycans in axonal conduction in Mammalian spinal cord. J Neurosci. 2010;30:7761–7769. doi: 10.1523/JNEUROSCI.4659-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z, Zhang H, Nishida Y, Asami N, Takeshita S, Sugiura N, Watanabe H, Yamashita T, Ishiguro N, Matsuama Y, Kadomatsu K. Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci. 2011;31:17091–17102. doi: 10.1523/JNEUROSCI.5120-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Honjo M, Otori Y, Oohira A, Kido N, Tano Y, Honda Y, Tanihara H. Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Invest Ophthalmol Vis Sci. 2001;42:1930–1938. [PubMed] [Google Scholar]
- Ishii M, Maeda N. Oversulfated chondroitin sulfate plays critical roles in the neuronal migration in the cerebral cortex. J Biol Chem. 2008a;283:32610–32620. doi: 10.1074/jbc.M806331200. [DOI] [PubMed] [Google Scholar]
- Ishii M, Maeda N. Spatiotemporal expression of chondroitin sulfate sulfotransferases in the postnatal developing mouse cerebellum. Glycobiology. 2008b;18:602–614. doi: 10.1093/glycob/cwn040. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Imagama S, Ohgomori T, Ishiguro N, Kadomatsu K. A combination of keratan sulfate digestion and rehabilitation promotes anatomical plasticity after rat spinal cord injury. Neurosci Lett. 2015;593:13–18. doi: 10.1016/j.neulet.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology. 2005;15:593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K, Ando K, Yamashita T, Ishiguro N, Kadomatsu K. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci. 2010;30:5937–5947. doi: 10.1523/JNEUROSCI.2570-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jung Kim M, Cotman SL, Halfter W, Cole GJ. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J Neurobiol. 2003;55:261–277. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- Kadomatsu K, Sakamoto K. Sulfated glycans in network rewiring and plasticity after neuronal injuries. Neurosci Res. 2014;78:50–54. doi: 10.1016/j.neures.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kubo T, Hata K, Yamaguchi A, Yamashita T. Repulsion of cerebellar granule neurons by chondroitin sulfate proteoglycans is mediated by MAPK pathway. Neurosci Lett. 2007;423:62–67. doi: 10.1016/j.neulet.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, Anand S, Thazhath R, Zhong Y, McKeon RJ, Bellamkonda RV. Targeted downregulation of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase significantly mitigates chondroitin sulfate proteoglycan-mediated inhibition. Glia. 2011;59:981–996. doi: 10.1002/glia.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Tsutsumi K, Tone Y, Sugahara K. Developmental Regulation of the Sulfation Profile of Chondroitin Sulfate Chains in the Chicken Embryo Brain. J Biol Chem. 1997;272:31377–31381. doi: 10.1074/jbc.272.50.31377. [DOI] [PubMed] [Google Scholar]
- Kjellén L, Lindahl U. Proteoglycans: Structures and Interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Koppe G, Bruckner G, Hartig W, Delpech B, Bigl V. Characterization of proteoglycan-containing perineuronal nets by enzymatic treatments of rat brain sections. Histochem J. 1997;29:11–20. doi: 10.1023/a:1026408716522. [DOI] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Kjellen L. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 2003;13:605–611. doi: 10.1016/j.sbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982;94:574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Sandy JD, Anderson DK, Howland DR. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp Neurol. 2003;184:981–990. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- Li F, Shetty AK, Sugahara K. Neuritogenic Activity of Chondroitin/Dermatan Sulfate Hybrid Chains of Embryonic Pig Brain and Their Mimicry from Shark Liver involvment of the pleiotrophin and hepatocyte growth factor signaling pathways. J Biol Chem. 2007;282:2956–2966. doi: 10.1074/jbc.M609296200. [DOI] [PubMed] [Google Scholar]
- Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok J. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One. 2011;6:e21499. doi: 10.1371/journal.pone.0021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Leach FE, 3rd, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7:827–833. doi: 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Sys Agents Med Chem. 2010;10:22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- Maeda N, He J, Yajima Y, Mikami T, Sugahara K, Yabe T. Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule. J Biol Chem. 2003;278:35805–35811. doi: 10.1074/jbc.M305530200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Dev. 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase ζ/RPTPβ and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278:41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Mikami T, Yasunaga D, Kitagawa H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J Biol Chem. 2009;284:4494–4499. doi: 10.1074/jbc.M809227200. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga C, Mikami T, Mizumoto S, Fukuda J, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains in the development of cerebellum. Spatiotemporal regulation of the expression of critical disulfated disaccharides by specific sulfotransferases. J Biol Chem. 2006;281:18942–18952. doi: 10.1074/jbc.M510870200. [DOI] [PubMed] [Google Scholar]
- Miyata S, Kitagawa H. Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulphate. J Biochem. 2015;157:13–22. doi: 10.1093/jb/mvu067. [DOI] [PubMed] [Google Scholar]
- Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–422. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Muir D, Engvall E, Varon S, Manthorpe M. Schwannoma cell-derived inhibitor of the neurite-promoting activity of laminin. J Cell Biol. 1989;109:2353–2362. doi: 10.1083/jcb.109.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Clement A, Masayama K, Faissner A, Sugahara K. Characteristic hexasaccharide sequences in octasaccharides derived from shark cartilage chondroitin sulfate D with a neurite outgrowth promoting activity. J Biol Chem. 1998;273:3296–3307. doi: 10.1074/jbc.273.6.3296. [DOI] [PubMed] [Google Scholar]
- Nandini CD, Mikami T, Ohta M, Itoh N, Akiyama-Nambu F, Sugahara K. Structural and functional characterization of oversulfated chondroitin sulfate/dermatan sulfate hybrid chains from the notochord of hagfish. Neuritogenic and binding activities for growth factors and neurotrophic factors. J Biol Chem. 2004;279:50799–50809. doi: 10.1074/jbc.M404746200. [DOI] [PubMed] [Google Scholar]
- Nandini CD, Itoh N, Sugahara K. Novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chains with a unique heterogenous sulfation pattern from shark skin, which exhibit neuritogenic activity and binding activities for growth factors and neurotrophic factors. J Biol Chem. 2005;280:4058–4069. doi: 10.1074/jbc.M412074200. [DOI] [PubMed] [Google Scholar]
- Olson L. Medicine: clearing a path for nerve growth. Nature. 2002;416:589–590. doi: 10.1038/416589a. [DOI] [PubMed] [Google Scholar]
- Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32:18009–18017. 18017a. doi: 10.1523/JNEUROSCI.2406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, ten Dam GB, Furukawa Y, Mikami T, Sugahara K, Toida T, Geller HM, Fawcett JW. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–390. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. Directing neuronal signaling through cell-surface glycan engineering. J Am Chem Soc. 2014;136:6794–6797. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Hsieh-Wilson LC. Long-Lived Engineering of Glycans to Direct Stem Cell Fate. Angew Chem Int Ed Engl. 2015;54:1466–1470. doi: 10.1002/anie.201409258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Fukuda J, Mizumoto S, ten Dam GB, van Kuppevelt TH, Kitagawa H, Mikami T, Sugahara K. Functions of chondroitin sulfate/dermatan sulfate chains in brain development. Critical roles of E and iE disaccharide units recognized by a single chain antibody GD3G7. J Biol Chem. 2007;282:19442–19452. doi: 10.1074/jbc.M700630200. [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Clark PM, Tully SE, Abrol R, Garcia KC, Goddard WA, Hsieh-Wilson LC. Elucidating glycosaminoglycan–protein–protein interactions using carbohydrate microarray and computational approaches. Proc Natl Acad Sci USA. 2011;108:9747–9752. doi: 10.1073/pnas.1102962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonalgrowth. J Cell Sci. 2000;113:807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Hamilton SK, Pearlman AL. Changes in the distribution of extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J Neurosci. 1991;11:3928–3942. doi: 10.1523/JNEUROSCI.11-12-03928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo M, Ando S, Sugiura N, Kimata K, Ichijo H. Moderate repulsive effects of E-unit-containing chondroitin sulfate (CSE) on behavior of retinal growth cones. Brain Res. 2013;1491:34–43. doi: 10.1016/j.brainres.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Shipp EL, Hsieh-Wilson LC. Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem Biol. 2007;14:195–208. doi: 10.1016/j.chembiol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci. 1995;108:1307–1315. doi: 10.1242/jcs.108.3.1307. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated Proteoglycans in Astroglial Barriers Inhibit Neurite Outgrowth in Vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Snow DM, Letourneau PC. Neurite Outgrowth on a Step Gradient of Chondroitin Sulfate Proteoglycan (CS-PG) J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- Sotogaku N, Tully SE, Gama CI, Higashi H, Tanaka M, Hsieh-Wilson LC, Nishi A. Activation of phospholipase C pathways by a synthetic chondroitin sulfate-E tetrasaccharide promotes neurite outgrowth of dopaminergic neurons. J Neurochem. 2007;103:749–760. doi: 10.1111/j.1471-4159.2007.04849.x. [DOI] [PubMed] [Google Scholar]
- Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, Prochiantz A. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 2013;3:1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocyto. 2002;31:423. doi: 10.1023/a:1025731428581. pl=-435. [DOI] [PubMed] [Google Scholar]
- Sugahara K. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Prochiantz A, Hensch TK. From brain formation to plasticity: insights on Otx2 homeoprotein. Dev Growth Differ. 2009;51:369–377. doi: 10.1111/j.1440-169X.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun. 2013;4:2740. doi: 10.1038/ncomms3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tully SE, Mabon R, Gama CI, Tsai SM, Liu X, Hsieh-Wilson LC. A Chondroitin Sulfate Small Molecule that Stimulates Neuronal Growth. J Am Chem Soc. 2004;126:7736–7737. doi: 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- Ueoka C, Kaneda N, Okazaki I, Nadanaka S, Muramatsu T, Sugahara K. Neuronal Cell Adhesion, Mediated by the Heparin-binding Neuroregulatory Factor Midkine, Is Specifically Inhibited by Chondroitin Sulfate E: Structural and Functional Implications of the Over-sulfated Chondroitin Sulfate. J Biol Chem. 2000;275:37407–37413. doi: 10.1074/jbc.M002538200. [DOI] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo T, Carulli D, Ehlert EM, Kwok JC, Dick G, Mecollari V, Moloney EB, Neufeld G, de Winter F, Fawcett JW, Verhaagen J. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci. 2013;56:186–200. doi: 10.1016/j.mcn.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005a;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, LA PIERRE DP, Jin W, Albert JY, Burton BY. The interaction of versican with its binding partners. Cell Res. 2005b;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annual review of biochemistry. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K. N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology. 2006;16:702–710. doi: 10.1093/glycob/cwj115. [DOI] [PubMed] [Google Scholar]
- Zou P, Zou K, Muramatsu H, Ichihara-Tanaka K, Habuchi O, Ohtake S, Ikematsu S, Sakuma S, Muramatsu T. Glycosaminoglycan structures required for strong binding to midkine, a heparin-binding growth factor. Glycobiology. 2003;13:35–42. doi: 10.1093/glycob/cwg001. [DOI] [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]