Abstract
Limiting the environmental transmission of soil-transmitted helminths (STH), which infect 1.5 billion people worldwide, will require sensitive, reliable, and cost effective methods to detect and quantify STH in the environment. We review the state of the art of STH quantification in soil, biosolids, water, produce, and vegetation with respect to four major methodological issues: environmental sampling; recovery of STH from environmental matrices; quantification of recovered STH; and viability assessment of STH ova. We conclude that methods for sampling and recovering STH require substantial advances to provide reliable measurements for STH control. Recent innovations in the use of automated image identification and developments in molecular genetic assays offer considerable promise for improving quantification and viability assessment.
Environmental Detection of Soil-Transmitted Helminths for Public Health
Soil-transmitted helminths (STH), including hookworms (Necator americanus and Ancylostoma duodenale), roundworms (Ascaris spp.), and whipworms (Trichuris trichiura), impose a substantial burden of disease worldwide. An estimated 819 million people are infected with Ascaris lumbricoides, 465 million with T. trichiura, and 439 million with hookworms, globally [1]. In addition to a range of acute sequelae, including gastroenteritis, anemia, and intestinal obstruction, chronic helminth infection has been associated with poor physical and cognitive development, and is thought to reinforce poverty [2].
The World Health Organization has launched efforts to reduce the health and economic burden attributable to STH through programs that primarily focus on mass drug administration (MDA) of antihelminthic medicines to school-aged children [3]. These campaigns have succeeded in reducing global morbidity due to helminth infections. However MDA is unlikely to break cycles of STH transmission unless coupled with environmental measures to interrupt acquisition of new infections [4-6]. This is in part because STH ova are extremely resistant to environmental stressors, and may survive for years in soils [7]. This hardiness, combined with low infectious doses and high rates of excretion, contributes to the maintenance of STH transmission in the presence of MDA [8, 9]. Quantifying environmental contamination with helminths poses major technical challenges: methods are needed that are both sensitive enough to estimate low—but epidemiologically relevant—concentrations of STH, and cost-effective enough to be deployed in low resource settings where the impact of STH is highest. Here, a systematic review of peer-reviewed and grey literature is presented to assess the state of the art of methods to quantify STH in the environment. Where relevant, information regarding other helminthic parasites with similar biophysical and environmental characteristics (e.g. Toxocara spp., Taenia spp.) is included.
Literature concerning the four distinct methodological steps involved in quantifying STH in the environment is reviewed below: (i) environmental sampling; (ii) recovering and concentrating STH ova, larvae, or genetic material from the sample matrix; (iii) detecting and quantifying recovered STH or genetic material; and (iv) determining the viability of STH.
Spatial Sampling Regimes For STH
Sampling STH from environmental media requires consideration of their fundamental overdispersion in the environment, with localized clusters of high contamination existing within areas that otherwise exhibit very low STH concentration (Box 1). This follows from the aggregation of high worm burdens in particular individuals, whose feces become a localized source of contamination in the environment [10].
Box 1. Characteristics of the spatial and temporal distribution of STH in the environment.
STH ova exhibit the longest survival times in moist environmental conditions with little sunlight [33], and STH embryos develop most efficiently in aerobic environments [34]. Thus ova within sandy or loose soils, which retain water poorly, have been shown to be less resistant to desiccation and ultraviolet radiation [12, 35], while soils that are impermeable and anoxic have been observed to slow ova maturation [35]. Optimal conditions for hookworm larvae are similar, except that spacing between soil particles facilitates their migration through the soil column. Infective hookworm larvae have been observed more frequently in moist, shaded, sandy soils [36].
The vertical distribution of STH in the soil column is poorly understood. While exposure to viable STH ova that have passed below the surface layer is presumed unlikely, vertical transport by earthworms and disturbance of soil by rooting animals and human activity may return ova from deeper layers to the soil surface [37, 38]. Hookworm larvae actively move within the soil column, and are capable of migrating between the surface and depths of up to 20 cm to avoid dry conditions [22].
Among environmental media, STH tend to be sparsely concentrated in surface waters where ova settle rapidly out of the water column. In wastewater, however, STH may be present in high concentrations and relatively evenly dispersed due to mixing of numerous fecal sources [39]. Food crops grown close to, but above the ground are most frequently and heavily contaminated with STH [40]. Root crops are also prone to STH contamination [40], and the large surface area of leafy greens facilitates STH attachment and provides protection from drying and UV radiation, making these some of the most frequently contaminated crops [40, 41].
Seasonal fluctuations in temperature, moisture, and infection prevalence (due to MDA campaigns) are known to affect the seasonal distribution of STH in soils. Generally, more frequent soil contamination is found during wet seasons [19, 26-28, 42]. Some researchers have reported higher occurrence of STH, but lower STH viability, in soils during dry seasons, perhaps due to lack of rain to wash ova away [13, 33]. Temporal sampling regimes for STH often reflect these seasonal trends, with samples drawn during both wet and dry seasons to capture high and low contamination conditions [13, 19, 20, 27, 28].
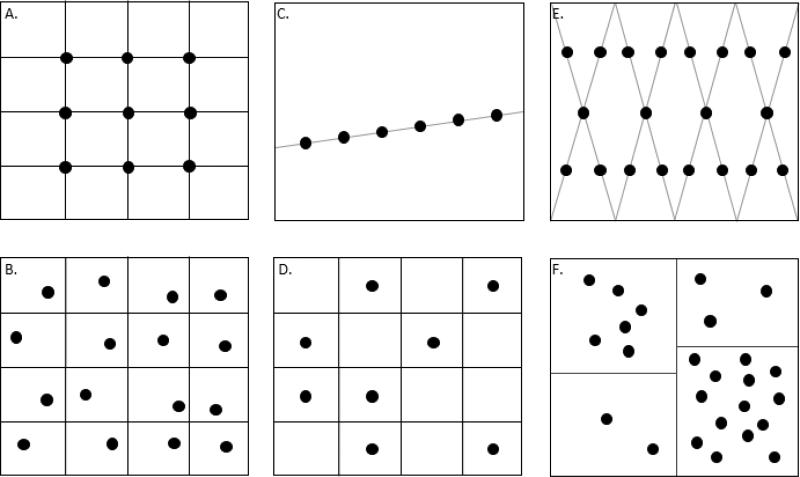
The spatial distribution of STH can be estimated using systematic aligned (Figure 1A – e.g., [11-13]) or unaligned (Figure 1B – e.g., [14, 15]) methods; or walking path transect (Figure 1C – e.g., [16-21]) sampling patterns. A grid-based form of random sampling has occasionally been pursued in which the surveyed area is divided into equal parts that are then randomly selected for sampling (Figure 1D; e.g., [22, 23]). Others have proposed a systematic sampling method combining aspects of grid-based and transect sampling (Figure 1E) [17, 18, 24]. Starting from the corner of a rectangular plot of land, an investigator walks diagonally, turning at the boundaries to create a W-shaped path, and taking samples at regular intervals.
Figure 1.
Spatial sampling regimes for soil transmitted helminths (STH) in soil and vegetation. (A) Systematic aligned sampling. (B) Systematic unaligned sampling. (C) Walking path transect sampling. (D) Random sampling of a grid. (E) W-route sampling. (F) Spatial stratified sampling with random samples designated according to expected variance within each stratum.
Purposive sampling, which relies on the investigator's judgment to determine appropriate locations from which to draw samples, has been used in some studies to sample from areas where STH are likely to survive, or where human exposures may occur, such as human and animal defecation sites [25, 26], shaded or moist areas [27-29], foot placements around latrine dropholes [30], or areas where children are observed to play [16, 31]. Alternatively, spatial stratified sampling is a method in which a survey site is subdivided into relatively homogeneous areas, from which a share of random or systematic samples is taken based on their contribution to the total area or spatial variance of the quantity of interest (Figure 1F). Spatial stratified sampling is efficient for sampling highly spatially heterogeneous quantities [32], but has yet to be applied to STH.
The suitability of this broad range of spatial sampling protocols depends on the investigator's objectives and on the expected distribution of STH within the site. Transect sampling is appropriate for investigating the variance of STH concentration along some other environmental gradient or with distance from a contamination source. Systematic sampling patterns are efficient approaches for arriving at an estimate of two-dimensional spatial distribution of STH, but given the spatial heterogeneity of STH in the environment, spatial stratified sampling may be more efficient, especially when investigators have reason to suspect greater variability in STH concentrations in certain zones within their study sites.
Few studies have investigated the relative performance of environmental sampling strategies for STH. Carabin et al. [43] found that a random sampling method and two purposive sampling methods—selecting areas where animals were thought to defecate and selecting areas where children were seen to play—underestimated Toxocara contamination at a daycare center relative to a comprehensive grid-based sampling of the entire area [43]. Thus comprehensive systematic sampling can provide more information to estimate STH contamination across a study site, and may also be significantly more reliable than purposive sampling [43]. Verschave et al. [44] compared the performance of W-route sampling with systematic unaligned sampling of sixteen 0.16 m2 plots for estimating local levels of pasture contamination with larval nematode parasites. They found no significant difference in the mean estimate of contamination between the two methods, but noted that the systematic unaligned sampling approach required less time to complete [44].
Recovering STH From Environmental Matrices
In order to quantify STH density in a sample, ova, larvae or their genetic material must be isolated from the environmental matrix and concentrated. Recovery of STH from soils, biosolids, and water samples typically involves five key processes: homogenization, chemical dissociation from the matrix, filtration, sedimentation, and flotation. As an initial step, sample homogenization can yield more reliable estimates of concentration because STHs are often unevenly distributed within environmental samples. Sample homogenization helps to lower variability between samples due to any disproportionate loss of STH associated with material discarded during sample processing.
STH ova tend to adhere to soils and other particles [45], and thus chemical dissociation from the particles in a matrix improves homogenization and prevents loss of ova as matrix particles are removed during subsequent processing steps [39]. Dissociation is usually achieved using ionic detergents such as 7X or Tween, which are thought to displace phosphate anions found on the outermost wall of ova from cationic sites on soil particles (http://www.ewisa.co.za/literature/files/155_107%20Hawksworth.pdf). Recovering STH from samples of plant matter requires a rinsing step (using Tris-buffered saline (TBS), Tween 20, Nacconol, or physiological saline, for instance), after which the rinsate is retained and processed using membrane filtration, sedimentation and/or flotation as necessary (see, e.g., [40]).
Filtration by sieving helps to remove larger particles that can interfere with STH recovery and detection [39]. When the contents retained on a sieve are to be discarded, there is a risk of loss of ova associated with the discarded material. Homogenization and dissociation of ova from the matrix prior to filtration helps to mitigate this risk. Some protocols use very fine mesh sieves or membrane filters to retain ova and larvae while passing flotation fluid and smaller particles through. Careful matching of mesh size to the target STH is called for in this instance, as meshes small enough to retain Ascaris ova, for example, may allow Trichuris ova to pass through if they are oriented along their long axis [46, 47].
Sedimentation is used to concentrate STH at the bottom of a sample suspension, resulting in a reduced sample volume that is suitable for examination or further processing. The sedimentation process may be accelerated with the assistance of a centrifuge, or allowed to proceed passively [39]. The passive settling velocities of STH depend on their size and density, as well as the properties of flocs they form with other particles, which may vary strongly between STH species. For instance, the settling of Ascaris ova has been found to be more rapid in wastewater compared with tap water (57.6 cm/h vs. 21.6 cm/h) [48], while for Trichuris ova slower settling rates were observed in wastewater than in tap water (32.4 cm/h, 54.0 cm/h) [48]. Since organic matter is available for floc formation in wastewater, this result suggests that the flocs formed by Trichuris settle more slowly than those formed by Ascaris [48].
Flotation refers to the use of high specific gravity (density relative to water, abbreviated as SG) solutions to separate STH from heavier particles. To accomplish this, flotation solutions must be denser than the STH of interest [39] (for densities of various helminth ova, see [49]). Advantages of flotation include increased sensitivity of microscopic and genetic quantification methods through removal of sediment and polymerase inhibitors [39, 46]. Like sedimentation, the partitioning of lighter and heavier particles via flotation may be accelerated through use of a centrifuge [39].
Some well-established methods simplify (and circumvent) homogenization, filtration and other steps. For example, STH ova may be recovered from low turbidity water samples using simple membrane filtration, followed by a chemical treatment to render the filter transparent for visual inspection [50]. As another example, hookworm larvae may be recovered using a technique known as the Baermann method: sample material is suspended inside a porous membrane submerged in water, and as larvae migrate out through pores in the membrane, they settle to the bottom of the water column and may be collected for examination [24].
Recovery rates of STH ova from soils and biosolids for various methods reported in the literature vary widely, ranging from less than 10% to over 100% (which indicates measurement error in enumerating seeded and/or recovered ova). The highest reported rates of recovery for each method, as well as the corresponding methods and parameters, are shown in Tables 1 and 2. Only one study has published recovery rates for the Baermann method for recovery of larvae, which were 80% on average, with a range of 58-99% [26].
Table 1.
Reported parameters yielding maximum rates of recovery of STH from soils.
| Organism | Soil type | Seeding level (EPGa) | Method type | Detergent | Flotation solution (SG) | Percent recovery | Estimated processing timeb | Refs. |
|---|---|---|---|---|---|---|---|---|
| Ascaris | Clay | 4 | Passive flotation | Bleach (20%) | NaNO3 (1.3) | 15.3% | 2 hours | [53] |
| Sandy | 20 | 24.32% | ||||||
| Loamy | 20 | 25.04% | ||||||
| Ascaris | NRc | 1452- 32393 |
Centrifugal flotation |
NaOH (0.5 N) |
Glucose monohydrate (1.27) |
82% | 2 days | [54] |
| Ascaris | NR | 10 | Centrifugal flotation |
Tween40 (0.5%) |
NaNO3 (1.35) | 47% | <1 hour | [55] |
| Tween80 (1%) |
NaNO3 (1.35) | 66.5% | <1 hour | |||||
| None | ZnSO4 (1.2) | 9.5% | 3 hours | |||||
| Trichuris | NR | 1452- 32393 |
Centrifugal flotation |
NaOH (0.5 N) |
Glucose monohydrate (1.27) |
80% | 2 days | [54] |
| Trichuris | NR | 1 | Centrifugal flotation, recovery by adherence to 4 coverslips |
None | MgSO4 (1.28) | 96.4% | 1-2 hours | [56] |
| Ancylostoma | NR | 10 | Centrifugal flotation |
Tween40 (0.5%) |
NaNO3 (1.35) | 39.33% | <1 hour | [55] |
| Tween80 (1%) |
NaNO3 (1.35) | 50% | <1 hour | |||||
| None | ZnSO4 (1.2) | 9% | 3 hours | |||||
| Toxocara | Sandy | 2 | Centrifugal flotation, recovery by adherence to 6 coverslips |
Tween80 (NR) |
NaNO3 (1.22) | 15% | 2-3 hours | [11] |
| 2 | Passive flotation, recovery by adherence to 4 coverslips |
Tween40 (NR) |
ZnSO4 (1.2) | 15% | ||||
| Toxocara | Sandy | 543 | Passive flotation | None | Saccharose (1.27) | 99.98% | <1 hour | [57] |
| Toxocara | Silty clay | 200 | Centrifugal flotation |
NaOH (0.1 N) |
Na2Cr2O7 (1.35) | 7.5% | <1 hour | [58] |
| Clay silt | 13.9% | |||||||
| Sandy | 38% | |||||||
| Sand | 62.5% | |||||||
| Toxocara | Sandy | 200 | Centrifugal flotation, recovery by adherence to coverslip |
Tween80 (0.2%) |
ZnSO4 (1.2) | 10.75% | 2 days | [59, 60] |
| NaOH (0.1 N) |
8.8% | |||||||
| Toxocara | NR | 1 | Centrifugal flotation, recovery by adherence to 4 coverslips |
None | MgSO4 (1.28) | 47.2% | 1-2 hours | [56] |
| Toxocara | NR | 10 | Centrifugal flotation (3 different methods: ([12, 60, 62]) |
Tween40 (0.5%) |
NaNO3 (1.35) | 46.83% | <1 hour | [55] |
| Tween80 (1%) |
NaNO3 (1.35) | 71% | <1 hour | |||||
| None | ZnSO4 (1.2) | 7.67% | 3 hours | |||||
| Toxocara | Clay | 210 | Centrifugal flotation X 3d, recovery by adherence to 4-5 coverslips |
NaOH (0.1 N) |
ZnSO4 (1.18) | 66.19% | 1-2 hours | [62] |
| Toxocara | Silt | 20 | Centrifugal flotation |
Tween80 (0.1%) |
NaCl (1.25) | 45.3% | 1-2 hours | [63] |
| Sand | NaCl (1.25) | 100.8% | ||||||
| Laterite | ZnSO4 (1.52) | 56.8% | ||||||
| Toxocara | NRd | 0.48 | Centrifugal flotation X 4 |
Tween80 (0.0025%) |
MgSO4 (1.275) | 82.5% | 1-2 hours | [64] |
| Toxocara | NR | NR | Centrifugal flotation, recovery by adherence to 2 coverslips |
Tween80 (1%) |
NaNO3 (1.35) | 69.8% | 1-2 hours | [12] |
| Echinococcus | NR | 100 | Centrifugal flotation, recovery by adherence to coverslip |
Tween80 (0.05%) |
Sucrose (1.27) | 10% | 2-3 hours | [65] |
| Echinococcus | Sand | NR | Centrifugal flotation, recovery by adherence to coverslip |
Tween80 (0.05%) |
ZnCl2 (1.4) | NR | 1-2 hours | [66] |
EPG - Eggs per gram.
Processing times estimated based on given times for incubation and centrifugation steps, and do not include time needed to examine the sample after recovery.
NR - not reported.
Multiple flotations performed (e.g. X 4 denotes four sequential flotations).
Table 2.
Reported parameters yielding maximum rates of recovery of Ascaris from biosolids and sludges.
| Organism | Type | Seeding level (EPGa) | Method type | Detergent | Flotation solution (SG) | Percent recovery | Estimated processing timeb | Reference |
|---|---|---|---|---|---|---|---|---|
| Ascaris | Acid-treated | NRc | Centrifugal flotation |
7X (NR) |
MgSO4 (1.2) |
80.5% | 3 days | [46] |
| Anaerobically composted |
75.5% | |||||||
| Soil-blended | 75.5% | |||||||
| Alkaline- treated |
58.5% | |||||||
| Ascaris | Urine-diverting toilet biosolids |
1200 | Centrifugal flotation, |
NH4HCO3 (NR) | ZnSO4 (1.4) |
77.28% | 2-3 hours, significant time to read slides due to debris |
(http://www.ewisa.co.za/literature/files/155_107%20hawksworth.pdf) |
| Centrifugal flotation with sequential sieving |
None | 57.14% | <1 hour | |||||
| Ascaris | Sewage Sludge | 125 (per gram total solids) |
Centrifugal flotation |
7X (1%) |
MgSO4 (1.2) |
82.00% | 5 days | [67] |
EPG – Eggs per gram.
Processing times estimated based on given times for incubation and centrifugation steps, and do not include time needed to examine the sample after recovery.
NR – not reported.
Few studies have been published describing the efficiency of recovery of STH from water samples or vegetation. De Souza et al. [51] were able to recover roughly 36% of Ascaris ova seeded into wastewater using flotation (ZnSO4 SG:1.18) and membrane filtration methods [51]. Maya et al. [50] were able to recover 80% and 83% of Ascaris ova seeded into well water and wastewater, respectively, and could detect as little as 1 ovum per liter using centrifugal flotation method (ZnSO4 SG:1.3) with 0.1% Tween 80 as detergent [50]. Using a flotation (ZnSO4 SG:1.2) and membrane filtration method without detergent, they were able to recover 86% and 63% of ova seeded into well and wastewater, respectively, and could also detect 1 ovum per liter [50]. Rude et al. [52] report an average recovery efficiency of 38.5% for a Nacconol-ether centrifugation method to recover Ascaris and Trichuris from large aggregate samples of vegetables [52].
Impact of method parameters on recovery from soils and biosolids
The efficiency of flotation is impacted by the properties of the flotation solution, characteristics of the soil matrix, and cross sectional area available for travel of STH in the flotation solution relative to the volume of matrix present [39, 46, 68]. To mitigate against poor recoveries, flotation steps may be repeated to recover any STH trapped between matrix particles during previous flotations. Sequential flotations have been reported to recover up to an additional 10-20% of seeded ova [64].
Soil texture and organic content have a substantial impact on recovery efficiencies. Recovery rates from clay soils are consistently lower and more variable than those from sandy soils [39, 45, 53, 58]. This may be because STH ova are among the lighter elements present in sandy soil, settling on the topmost layer of a suspension, while in suspensions of loam and clay they tend to sediment beneath a layer of lighter particles [69]. Similarly, the makeup of biosolids and sludge can affect recovery; heterogeneous biosolids (e.g., those mixed with soils) have been found to yield more variable recoveries [46]. Chemical treatment applied to biosolids has been found to impact the chemistry of dissociation and flotation processes, affecting recovery efficiency [46].
Properties of the flotation solution have been shown to influence recovery. Generally, higher SG solutions are thought to recover STH ova more efficiently (http://www.susana.org/en/resources/library/details/420). Several comparative studies have supported this assertion [53, 58, 63, 64], but others have reported either no improvement with flotation solutions of higher SG [59], or superior recovery with lower SG solutions [56, 62, 63]. Thus, it appears that in some cases, viscosity or chemical interactions with the outer surfaces of specific STH ova, the matrix, or other reagents may be more important determinants of recovery efficiency than SG.
Some authors have reported difficulties using specific flotation solutions. Concentrated NaNO3 has been observed to crystallize, interfering with examination of microscope slides [59], though the issue has been addressed by adding a drop of glycerol to prevent crystallization [53] and would not matter for methods that separate ova from the flotation solution with a fine-meshed filter. Concentrated sucrose solution was reported to yield low recoveries, presumably due to the interference of its high viscosity with the movement of ova and sediments during centrifugation and passive flotation [46, 63]. What is more, saturated saccharose has been reported to deform STH ova, potentially interfering with their quantification [70].
The choice of solution used to dissociate ova from soil particles has been found to affect recovery as well. NaOH is commonly used, although some authors have found this detergent to be ineffective [57, 62] or deleterious (e.g., through destruction of ova [59]) for recovering STH. The detergent Tween 40 has been reported to significantly improve recovery of Toxocara ova from soil and sand [61, 71], though Tween 20 has been reported as offering no improvement in at least one case [57]. The detergent 7X has been reported to be superior to other dissociation agents, including TritonX 100 and Tween 80 for recovering Ascaris ova from biosolids and soil [47], and benzethonium chloride and Tween 80 for recovering Ascaris ova in hand rinses [72].
Ova may be lost if they adhere to the walls of pipettes, tubes and beakers. To reduce this possibility, some protocols recommend treating labware with organosilane (Rain-X) to reduce adhesiveness. However, Jeandron et al. [71] found that organosilane treatment reduced yields from both glass and plastic materials, and recommended that non-coated pipettes and falcon tubes be used in protocols to recover helminth ova [72]. Adhesion can also be exploited for ova recovery, such as in the coverslip method commonly used for recovering Toxocara. In this method a test tube containing the sample is filled to the brim with flotation fluid in order to create a meniscus. A glass slide or coverslip is then placed so that ova traveling upwards via passive or centrifugal flotation may adhere to it and be counted (e.g., [61, 62, 71]). The relative sensitivity of this technique has not been compared with methods in which the entire final sample volume is examined for STH.
Some researchers, especially those conducting surveillance for Toxocara, pre-process samples by filtering them through coarse 4 mm2 sieves (e.g. [12, 64]), or fine 150 μm sieves (e.g. [21, 25, 27, 28]), prior to homogenization and other processing in order to remove twigs, rocks, and larger soil particles. The use of the finer sieve, in particular, prior to sample homogenization, seems likely to result in some loss of ova associated with retained materials. Nonetheless, one group has claimed that by drying a 200 gram soil sample, then sieving it down to 2 grams of powdery sand using the 150 μm sieve, up to a maximum of 40% efficient recovery may be achieved, and so this method may provide greater throughput for soil samples at the expense of some sensitivity [29]. Others have reported that mechanical blending to disrupt large particles provides better results than sieving [62].
Recovery methods for biosolids and sludge sometimes include a phase extraction step in which the sample is partitioned between an acidic aqueous phase, where ova sediment, and a lipophilic phase [70]. However, one group of researchers reported decreased recovery efficiency and lower Ascaris ova viability when using an acid-alcohol phase extraction compared with a method using a fine mesh sieve to retain ova after flotation [47]. Exposure of ova to an acid-alcohol extraction mixture for more than 30 minutes yielded reduced viability, and thus any acid-alcohol extraction procedure should be limited to short durations [47].
Storage conditions are important determinants of the recovery of viable STH ova and larvae. Ova of Ascaris and Trichuris are durable and can be stored at 4° C or room temperature for weeks or days, respectively, without loss of viability. However, hookworm ova are less resilient and have been shown to degrade within hours at room temperature if kept in a low moisture environment [73]. Hookworm larvae are even less hardy, and samples should be stored for no more than 7 days at 4° C to ensure a reliable assessment of viability [24]. It is important to note that differences in the physiochemical properties of ova across helminth species may necessitate adjustments to optimize other aspects of species-specific recovery methods, including flotation, dissociation, and sedimentation protocols.
Quantification Of STH Larvae And Ova
Light microscopy
After recovery from the sample matrix, STH have traditionally been quantified via light microscopy, a time-consuming and fatiguing process that requires expert knowledge of parasite morphology and is prone to human error, particularly when environmental processes or sampling and isolation methods distort parasite morphology. Furthermore, some species of STH, such as Ascaris lumbricoides and Ascaris suum, are nearly or completely indistinguishable by visual inspection. As an alternative to visual inspection, automated analysis of light microscopy images by computer could yield more rapid and reliable STH quantification. Such an approach has been described for detection of helminth ova isolated from cow feces [74]; identification of helminth ova isolated from human feces [75]; and quantification of different developmental stages of cultured Trichuris ova [76]. In concert with file sharing services, automated image identification has the potential to rapidly accelerate and standardize quantification of STH in environmental samples.
Nucleic acid-based methods
Genetic techniques for identifying and quantifying STH also have the potential to yield faster and more reliable sample analysis (see summary of genetic assay sensitivity for STH in Table 3). Genetic methods such as loop mediated isothermal amplification (LAMP) and polymerase chain reaction (PCR) have been used to detect STH in a wide range of contexts. Genetic methods can be species- or subspecies-specific, are highly sensitive (many assays target DNA sequences with multiple copies per cell, and can detect the equivalent of well under one mature ovum per gram of stool), and unlike microscopic or immunological assays, do not depend on parasite morphology remaining unaltered by environmental or laboratory processes [77]. Nonetheless, assays that rely on microscopic analysis are likely to be more practical for low-resource settings, where equipment for thermocycling and quantification of fluorescent reaction products may be unavailable. LAMP may be suitable for detection of STH in low-resource settings, since it can be performed with simple equipment such as a water bath (even a thermos of hot water [78]), and produces enough magnesium pyrophosphate precipitate that a positive reaction can be discerned with the naked eye [79]. Multiplex procedures to simultaneously detect multiple STH are available for PCR, but not yet for LAMP[80].
Table 3.
Reported sensitivities of genetic assays for STH detection and quantification.
| Organism | Type | Life stage | Target gene(s) | Sample matrix | Extraction kit | Detection method | Limit of detectiona | Refs |
|---|---|---|---|---|---|---|---|---|
|
Echinococcus multilocularis |
PCR | Ova | 12S rDNA | Soil | Sherlock AX Kit (A&A Biotechnology) |
Electrophoresis | 80% detection at 0.25 EPGb |
[66] |
|
Strongyloides stercoralis |
LAMP | Larva | 28S rRNA | Stool | Powersoil DNA Isolation
Kit (Mobio) |
Syto-82 fluorescent dye |
0.4 larvae / mL | [87] |
|
Taenia
solium Taenia asiatica Taenia saginata |
Multiplex PCR |
Ova | Valine tRNA NADH dehydrogenase |
Stool | NRc | 10 EPG | [89] | |
|
Taenia
solium Taenia asiatica Taenia saginata |
LAMP | Ova | COX1 CLP |
Stool | QIAamp DNA stool Minikit (QIAGEN) |
Electro-phoresis | 88.4% detection of single genome |
[78] |
| Taenia spp | Multiplex PCR |
Ova | COX1 | Stool | QIAamp DNA Stool
Minikit (QIAGEN) |
Electro-phoresis | 50 EPG | [90] |
|
Toxocara canis |
PCR | Ova (1 cell) |
ITS-2 rDNA |
Soil | NAd | Electro-phoresis | 33% detection at 2 EPG |
[82] |
|
Ancylostoma caninum |
0.4 EPG | |||||||
|
Toxocara canis Toxocara cati |
LAMP | Ova | ITS-2 rDNA |
Sand | NA | Electro-phoresis | 0.6 EPG | [79] |
|
Strongyloides stercoralis |
Multiplex quantitative PCR |
Larvae | 18S rRNA | Stool | QIAamp DNA Stool
Minikit (QIAGEN) |
Fluorescent Probes | 0.4 larvae per gram | [91] |
| Trichuris spp | Quantitative PCR |
Ova | ITS-1 rDNA |
Stool | NA2 | EvaGreen fluorescent dye |
1 EPG3 | [85] |
|
Necator/ Ancylostoma spp |
ITS-2 rDNA |
|||||||
| Toxocara spp | 23S rDNA | |||||||
|
Ascaris lumbricoides |
Quantitative PCR |
Ova | ITS-1 rDNA |
Sterile water |
UltraClean microbial DNA and RNA kits (Mobio) |
Fluorescent probes | 3,750 copies or ~ 4 cells |
[92] |
|
Toxocara canis Toxocara Cati |
Multiplex quantitative PCR |
Ova | ITS-2 rRNA |
Soil | PowerMax® soil
DNA isolation kit (Mobio) |
Fluorescent probes | 2 EPG | [88] |
|
Ancylostoma duodenale |
Multiplex quantitative PCR |
Ova | ITS-2 rDNA |
Stool | QIAamp DNA Stool Mini
kit (QIAGEN) |
Fluorescent probes | 1 copy per gram | [83, 93] |
|
Necator americanus |
Ova | ITS-2 rDNA |
100 copies per gram | |||||
|
Ascaris lumbricoides |
Ova | ITS-1 rDNA |
1 copy per gram | |||||
|
Strongyloides stercoralis |
Larvae | 18S rDNA | 1 copy per gram |
a Limits of detection are the lowest tested concentration at which 100% recovery was reported, unless otherwise stated.
EPG – eggs per gram.
NR – not reported.
NA – authors used their own in-house nucleic acid extraction techniques.
Certain compounds present in environmental matrices may inhibit LAMP and PCR reactions, including clays, humic and fulvic acids, polysaccharides, salts, heavy metals, and various organic molecules [39], though the BstDNA polymerase used in LAMP tends to be more resistant to inhibitors than Taq polymerases used in PCR [80]. Removal of inhibitors can be accomplished in part by using centrifugal flotation to recover STH ova [81], though even after flotation, adding anti-inhibition agents has been found to increase the sensitivity of genetic assays for STH [82]. To determine whether inhibitors are affecting reactions in a genetic assay, internal control targets can be used (e.g., [83]).
While most researchers who use genetic methods to detect sth in environmental matrices perform nucleic acid extraction after recovering ova by flotation [66, 84-86], some have successfully performed extraction directly on the environmental matrix [87, 88]. Some protocols call for disrupting the outer shell of certain helminth ova (primarily trichuris and baylisascaris procyonis) in order to efficiently extract genetic material; this can be accomplished through freeze-thaw cycles [85, 86], heating/boiling [85], shaking with glass beads [84, 85], or digestion with proteinase k [84, 86].
Determination Of STH Viability
Traditionally, STH ova viability has been assessed microscopically using an embryonation assay: after incubation under favorable conditions for 10-21 days, ova containing visibly developed larvae are recorded as viable, and undeveloped ova are assumed to be non-viable. The minimum time required for embryonation has been reported as 13 days for Ascaris and 16 days for Trichuris when incubated at 30 °C under constant aeration [94]. Hookworm ova hatch in soil within 1-2 days of excretion, and larvae survive in the environment for a few weeks at most (http://www.cdc.gov/dpdx/hookworm/index.html). Therefore, there is a risk that quantification of viable Ascaris and Trichuris ova via an embryonation assay may not generate accurate estimates of hookworm contamination in a sample – by the time Ascaris or Trichuris ova have matured, hookworm ova will have hatched and the larvae may have died and decomposed.
Determination of STH viability by microscopy is laborious and prone to human error, and as for STH detection and quantification generally, developments in automated image analysis —particularly when used with dyes that stain only live or dead cells—have the potential to improve the efficiency and reliability of microscopy-based viability assessment. Dabrowska et al. [95] were able to differentiate Ascaris, Toxocara, and Trichuris ova with high efficiency using SYTO 9 to stain live cells and propidium iodide to stain dead cells. The sensitivity of the assay decreased slightly when applied to sewage sludge samples, most likely due to interference of bile salts with the uptake of fluorescent dyes [95].
Genetic assays also show promise for establishing the viability of STH ova. In one such approach, ITS-1 DNA (internal transcribed spacer region of ribosomal DNA, present in multiple copies per genome) is amplified by quantitative PCR in order to track the development and viability of Ascaris ova [92, 96]. Raynal et al. [96] determined that ITS-1 DNA signals in embryonated ova decreased to negligible levels 10 days after inactivation, but persisted in viable ova [96]. Pecson et al. [92] proposed that persistent DNA in inactivated ova could be removed by treatment with proteinases and nucleases prior to PCR, and demonstrated that RTPCR targeting ITS-1 mRNA detected only viable ova, though the RNA assay was not sensitive nor consistent enough to be quantitative [92]. Other possible means of quantifying viability using genetic assays include using vital stains such as ethidium mono azide as inhibitors that only affect dead cells [97], and using quantitative RT-PCR to measure heat shock protein mRNA after exposure to elevated temperatures [39], but neither approach has been published for STH.
Concluding Remarks
Methods for reliably, rapidly, and cost-effectively assessing environmental contamination with STH will be crucial for understanding and disrupting STH transmission. Dominant methodologies are time and labor intensive, unreliable, and unstandardized in their approach. Key unresolved issues remain regarding optimal environmental sampling methods for STH, and optimal parameters for recovery, quantification, and viability assessment protocols (see Outstanding Questions Box). Future research examining sampling error under various spatial sampling strategies for STH and the effect of systematic variations in method parameters on recovery efficiency, will be critical to establishing standardized, efficient and reliable STH detection methods. To begin to address the many gaps in our understanding of the optimal strategies for recovering STH ova from environmental samples, some have called for universal use of internal process controls during analysis of environmental samples [98]. Stained control ova can be introduced to environmental samples, providing a standardized means of estimating efficiency of STH recovery for each published assay. Such a strategy could help to accelerate a consensus on optimal techniques.
Outstanding questions.
Despite the long history of methods for the environmental detection of STHs in soils and other media, key methodological issues remain:
What spatial sampling strategies are most efficient for estimating the environmental distribution of STHs in soil, surface water, and vegetation?
Few studies have taken a systematic approach to environmental sampling for STHs, and thus optimal sampling strategies that reduce measurement error and uncertainty for specific STHs in particular environmental media remain poorly understood.
What aspects of recovery protocols are most important for maximizing STH recovery for specific species and environmental media?
Limited comparative studies have been conducted examining the efficiency of recovery methods conducted with controlled differences in procedural parameters. Parameters whose effects need clarification include the choice of flotation solution, its specific gravity or exact chemical nature, the choice of dissociation agent, lengths of sedimentation, and preprocessing and lipid extraction steps. There is a particular absence of data on the efficiency of methods to recover STHs from plant matter.
Trends.
- The state of the art and key developments in environmental methods for sampling, recovery and concentration, quantification and viability assessment of soil transmitted helminths (STHs) are reviewed.
- Optimal protocols for sampling and recovery of STHs from environmental samples have not been developed, and systematic investigation is needed.
- Recent advances in genetic assays and automated image analysis for quantification and viability assessment offer improved sensitivity, reliability, and sample throughput.
Acknowledgments
M.C.F., A.E.K., and P.A.C. were funded by a grant from the Taskforce for Global Health's NTD Support Center. D.G.A. was supported by Children without Worms. J.V.R. and P.A.C. acknowledge additional support from the National Institute of Allergy and Infectious Diseases (grant K01AI091864), the National Science Foundation Water Sustainability and Climate Program (grant 1360330), and the National Institutes of Health/National Science Foundation Ecology of Infectious Disease program funded by the Fogarty International Center (grant R01TW010286).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nokes C, Bundy DA. Does helminth infection affect mental processing and educational achievement? Parasitology today (Personal ed.) 1994;10:14–18. doi: 10.1016/0169-4758(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 3.Vercruysse J, Levecke B, Prichard R. Human soil-transmitted helminths: implications of mass drug administration. Current Opinion in Infectious Diseases. 2012;25:703–708. doi: 10.1097/QCO.0b013e328358993a. [DOI] [PubMed] [Google Scholar]
- 4.Anderson R, Truscott J, Hollingsworth TD. The coverage and frequency of mass drug administration required to eliminate persistent transmission of soil-transmitted helminths. Philosophical Transactions of the Royal Society B-Biological Sciences. 2014:369. doi: 10.1098/rstb.2013.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truscott J, Hollingsworth T, Brooker S, Anderson R. Can chemotherapy alone eliminate the transmission of soil transmitted helminths? Parasites & Vectors. 2014;7:266. doi: 10.1186/1756-3305-7-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remais JV, Eisenberg JNS. Balance between clinical and environmental responses to infectious diseases. The Lancet. 379:1457–1459. doi: 10.1016/S0140-6736(11)61227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordin A, Nyberg K, Vinnerås B. Inactivation of Ascaris Eggs in Source-Separated Urine and Feces by Ammonia at Ambient Temperatures. Applied and Environmental Microbiology. 2009;75:662–667. doi: 10.1128/AEM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinniah B. Daily egg production of Ascaris lumbricoides: the distribution of eggs in the faeces and the variability of egg counts. Parasitology. 1982;84:167–175. doi: 10.1017/s0031182000051763. [DOI] [PubMed] [Google Scholar]
- 9.Shuval HI, Adin A, Fal B, Rawitz E, Yekutiel P. Integrated Resource Recovery: Wastewater Irrigation in Developing Countries - Health Effects and Technical Solutions. World Bank. 1986 [Google Scholar]
- 10.Guyatt HL, Bundy DAP. Are wormy people parasite prone or just unlucky? Parasitology Today. 1990;6:282–283. doi: 10.1016/0169-4758(90)90252-y. [DOI] [PubMed] [Google Scholar]
- 11.Oge S, Oge H. Prevalence of Toxocara spp. eggs in the soil of public parks in Ankara, Turkey. DTW. Dtsch. Tierarztl. Wochenschr. 2000;107:72–75. [PubMed] [Google Scholar]
- 12.O'Lorcain P. Prevalence of Toxocara canis ova in public playgrounds in the Dublin area of Ireland. J. Helminthol. 1994;68:237–241. doi: 10.1017/s0022149x00014401. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz de Ybáñez MR, Garijo MM, Alonso FD. Prevalence and viability of eggs of Toxocara spp. and Toxascaris leonina in public parks in eastern Spain. J. Helminthol. 2001;75:169–173. [PubMed] [Google Scholar]
- 14.Alonso JM, Stein M, Chamorro MC, Bojanich MV. Contamination of soils with eggs of Toxocara in a subtropical city in Argentina. J. Helminthol. 2001;75:165–168. [PubMed] [Google Scholar]
- 15.Rocha S, Pinto RM, Floriano AP, Teixeira LH, Bassili B, Martinez A, Caseiro MM. Environmental analyses of the parasitic profile found in the sandy soil from the Santos municipality beaches, SP, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:277–281. doi: 10.1590/s0036-46652011000500007. [DOI] [PubMed] [Google Scholar]
- 16.Jarosz W, Mizgajska-Wiktor H, Kirwan P, Konarski J, Rychlicki W, Wawrzyniak G. Developmental age, physical fitness and Toxocara seroprevalence amongst lower-secondary students living in rural areas contaminated with Toxocara eggs. Parasitology. 2010;137:53–63. doi: 10.1017/S0031182009990874. [DOI] [PubMed] [Google Scholar]
- 17.Mejer H, Roepstorff A. Oesophagostomum dentatum and Trichuris suis infections in pigs born and raised on contaminated paddocks. Parasitology. 2006;133:295–304. doi: 10.1017/S0031182006000382. [DOI] [PubMed] [Google Scholar]
- 18.Mejer H, Roepstorff A. Ascaris suum infections in pigs born and raised on contaminated paddocks. Parasitology. 2006;133:305–312. doi: 10.1017/S0031182006000394. [DOI] [PubMed] [Google Scholar]
- 19.Paquet-Durand I, Hernández J, Dolz G, Zuñiga JJR, Schnieder T, Epe C. Prevalence of Toxocara spp., Toxascaris leonina and ancylostomidae in public parks and beaches in different climate zones of Costa Rica. Acta Tropica. 2007;104:30–37. doi: 10.1016/j.actatropica.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Tiyo R, Guedes TA, Falavigna DLM, Falavigna-Guilherme AL. Seasonal contamination of public squares and lawns by parasites with zoonotic potential in southern Brazil. J. Helminthol. 2008;82:1–6. doi: 10.1017/S0022149X07870829. [DOI] [PubMed] [Google Scholar]
- 21.Zibaei M, Abdollahpour F, Birjandi M, Firoozeh F. Soil contamination with Toxocara spp. eggs in the public parks from three areas of Khorram Abad, Iran. Nepal Med Coll J. 2010;12:63–65. [PubMed] [Google Scholar]
- 22.Udonsi JK, Nwosu AB, Anya AO. Necator americanus: population structure, distribution, and fluctuations in population densities of infective larvae in contaminated farmlands. Z Parasitenkd. 1980;63:251–259. doi: 10.1007/BF00931987. [DOI] [PubMed] [Google Scholar]
- 23.Wong MS, Bundy DA, Golden MH. The rate of ingestion of Ascaris lumbricoides and Trichuris trichiura eggs in soil and its relationship to infection in two children's homes in Jamaica. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:89–91. doi: 10.1016/0035-9203(91)90172-u. [DOI] [PubMed] [Google Scholar]
- 24.Roepstorff A, Nansen P. Epidemiology, diagnosis and control of helminth parasites of swine. Food and Agriculture Organization of the United Nations; 1998. [Google Scholar]
- 25.Fajutag AJ, Paller VG. Toxocara egg soil contamination and its seroprevalence among public school children in Los Banos, Laguna, Philippines. Southeast Asian J. Trop. Med. Public Health. 2013;44:551–560. [PubMed] [Google Scholar]
- 26.Hominick WM, Dean CG, Schad GA. Population biology of hookworms in west Bengal: analysis of numbers of infective larvae recovered from damp pads applied to the soil surface at defaecation sites. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:978–986. doi: 10.1016/0035-9203(87)90371-3. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi S, Paller VG, Uga S. Soil contamination by parasite eggs in rural village in the Philippines. Tropical biomedicine. 2013;30:495–503. [PubMed] [Google Scholar]
- 28.Shrestha A, Rai SK, Basnyat SR, Rai CK, Shakya B. Soil transmitted helminthiasis in Kathmandu, Nepal. Nepal Med Coll J. 2007;9:166–169. [PubMed] [Google Scholar]
- 29.Uga S, Nagnaen W, Chongsuvivatwong V. Contamination of soil with parasite eggs and oocysts in southern Thailand. Southeast Asian J. Trop. Med. Public Health 28 Suppl. 1997;3:14–17. [PubMed] [Google Scholar]
- 30.Baker SM, Ensink JHJ. Helminth transmission in simple pit latrines. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012;106:709–710. doi: 10.1016/j.trstmh.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Sinniah B, Sabaridah I, Soe MM, Sabitha P, Awang IPR, Ong GP, Hassan AKR. Determining the prevalence of intestinal parasites in three Orang Asli (Aborigines) communities in Perak, Malaysia. Tropical biomedicine. 2012;29:200–206. [PubMed] [Google Scholar]
- 32.Wang J-F, Stein A, Gao B-B, Ge Y. A review of spatial sampling. Spatial Statistics. 2012;2:1–14. [Google Scholar]
- 33.Maikai BV, Umoh JU, Ajanusi OJ, Ajogi I. Public health implications of soil contaminated with helminth eggs in the metropolis of Kaduna, Nigeria. J. Helminthol. 2008;82:113–118. doi: 10.1017/S0022149X07874220. [DOI] [PubMed] [Google Scholar]
- 34.Brown HW. A Quantitative Study of the Influence of Oxygen and Temperature on the Embryonic Development of the Eggs of the Pig Ascarid (Ascaris suum Goeze). The Journal of Parasitology. 1928;14:141–160. [Google Scholar]
- 35.Brown HW. Studies on the Rate of Development and Viability of the Eggs of Ascaris lumbricoides and Trichuris trichiura under Field Conditions. The Journal of Parasitology. 1927;14:1–15. [Google Scholar]
- 36.Mabaso MLH, Appleton CC, Hughes JC, Gouws E. Hookworm (Necator americanus) transmission in inland areas of sandy soils in KwaZulu-Natal, South Africa. Tropical Medicine & International Health. 2004;9:471–476. doi: 10.1111/j.1365-3156.2004.01216.x. [DOI] [PubMed] [Google Scholar]
- 37.Storey GW, Phillips RA. The survival of parasite eggs throughout the soil profile. Parasitology. 1985;91(Pt 3):585–590. doi: 10.1017/s003118200006282x. [DOI] [PubMed] [Google Scholar]
- 38.Mizgajska H. Eggs of Toxocara spp. in the environment and their public health implications. J. Helminthol. 2001;75:147–151. [PubMed] [Google Scholar]
- 39.Smith HV. Detection of parasites in the environment. Parasitology. 1998;117(Suppl):S113–141. [PubMed] [Google Scholar]
- 40.Uga S, Hoa NTV, Noda S, Moji K, Cong L, Aoki Y, Fujimaki Y. Parasite egg contamination of vegetables from a suburban market in Hanoi, Vietnam. Nepal Med Coll J. 2009;11:75–78. [PubMed] [Google Scholar]
- 41.Abougrain AK, Nahaisi MH, Madi NS, Saied MM, Ghenghesh KS. Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 2010;21:760–762. [Google Scholar]
- 42.Bariweni PA, Ekweozor IK, Ogbonna DN. Assessment of nonzoonotic soil-transmitted helminth levels in soils in Yenagoa Metropolis, Niger Delta. Journal of environmental health. 2014;76:108–112. [PubMed] [Google Scholar]
- 43.Carabin H, Gyorkos TW, Kokoskin E, Payment P, Joseph L, Soto J. Comparison of methods of sampling for Toxocara species and fecal coliforms in an outdoor day care environment. Can J Infect Dis. 1998;9:149–156. doi: 10.1155/1998/613048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verschave SH, Levecke B, Duchateau L, Vercruysse J, Charlier J. Measuring larval nematode contamination on cattle pastures: Comparing two herbage sampling methods. Veterinary Parasitology. 2015;210:159–166. doi: 10.1016/j.vetpar.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 45.Landa-Cansigno O, Durán-Álvarez JC, Jiménez-Cisneros B. Retention of Escherichia coli, Giardia lamblia cysts and Ascaris lumbricoides eggs in agricultural soils irrigated by untreated wastewater. Journal of Environmental Management. 2013;128:22–29. doi: 10.1016/j.jenvman.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 46.Bowman DD, Little MD, Reimers RS. Precision and accuracy of an assay for detecting Ascaris eggs in various biosolid matrices. Water Research. 2003;37:2063–2072. doi: 10.1016/S0043-1354(02)00597-3. [DOI] [PubMed] [Google Scholar]
- 47.Nelson KL, Darby JL. Inactivation of Viable Ascaris Eggs by Reagents during Enumeration. Applied and Environmental Microbiology. 2001;67:5453–5459. doi: 10.1128/AEM.67.12.5453-5459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta ME, Thamsborg SM, Andersen TJ, Olsen A, Dalsgaard A. Sedimentation of helminth eggs in water. Water Research. 2011;45:4651–4660. doi: 10.1016/j.watres.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Stott R. Fate and behaviour of parasites in wastewater treatment systems. In: Mara D, Horan N, editors. Handbook of Water and Wastewater Microbiology. Academic Press; 2003. pp. 491–521. [Google Scholar]
- 50.Maya C, Jimenez B, Schwartzbrod J. Comparison of techniques for the detection of helminth ova in drinking water and wastewater. Water Environ. Res. 2006;78:118–124. doi: 10.2175/106143005x89571. [DOI] [PubMed] [Google Scholar]
- 51.de Souza GSMB, Rodrigues LA, de Oliveira WJ, Chernicharo CAL, Guimarães MP, Massara CL, Grossi PA. Disinfection of domestic effluents by gamma radiation: effects on the inactivation of Ascaris lumbricoides eggs. Water Research. 2011;45:5523–5528. doi: 10.1016/j.watres.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Rude RA, Jackson GJ, Bier JW, Sawyer TK, Risty NG. Survey of fresh vegetables for nematodes, amoebae, and Salmonella. J Assoc Off Anal Chem. 1984;67:613–615. [PubMed] [Google Scholar]
- 53.Ajala MO, Asaolu SO. Efficiency of the salt flotation technique in the recovery of Ascaris lumbricoides eggs from the soil. J. Helminthol. 1995;69:1–5. doi: 10.1017/s0022149x00013754. [DOI] [PubMed] [Google Scholar]
- 54.Larsen MN, Roepstorff A. Seasonal variation in development and survival of Ascaris suum and Trichuris suis eggs on pastures. Parasitology. 1999;119(Pt 2):209–220. doi: 10.1017/s0031182099004503. [DOI] [PubMed] [Google Scholar]
- 55.Charitha VG, Rayulu VC, Kondaiah PM, Ch S. Comparative evaluation of flotation techniques for the detection of soil borne parasites. Journal of parasitic diseases : official organ of the Indian Society for Parasitology. 2013;37:260–263. doi: 10.1007/s12639-012-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenner L, Gounel JM, Chauve CM. A standardized method for detecting parasite eggs and oocysts in soils. Revue Méd. Vét. 2002;153:729–734. [Google Scholar]
- 57.Ruiz De Ybáñez MR, Garijo M, Goyena M, Alonso FD. Improved methods for recovering eggs of Toxocara canis from soil. J. Helminthol. 2000;74:349–353. doi: 10.1017/s0022149x00000512. [DOI] [PubMed] [Google Scholar]
- 58.Nunes CM, Sinhorini IL, Ogassawara S. Influence of soil texture in the recovery of Toxocara canis eggs by a flotation method. Vet. Parasitol. 1994;53:269–274. doi: 10.1016/0304-4017(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 59.Santarém VA, Magoti LP, Sichieri TD. Influence of variables on centrifuge-flotation technique for recovery of Toxocara canis eggs from soil. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:163–167. doi: 10.1590/s0036-46652009000300007. [DOI] [PubMed] [Google Scholar]
- 60.Rosa Xavier IG, Ramos BC, Santarém VA. Recovery threshold of Toxocara canis eggs from soil. Vet. Parasitol. 2010;167:77–80. doi: 10.1016/j.vetpar.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 61.Kazacos KR. Improved method for recovering ascarid and other helminth eggs from soil associated with epizootics and during survey studies. Am. J. Vet. Res. 1983;44:896–900. [PubMed] [Google Scholar]
- 62.Dada BJ, Lindquist WD. Studies on flotation techniques for the recovery of helminth eggs from soil and the prevalence of eggs of Toxocara spp in some Kansas public places. J. Am. Vet. Med. Assoc. 1979;174:1208–1210. [PubMed] [Google Scholar]
- 63.Loh AG, Israf DA. Tests on the centrifugal flotation technique and its use in estimating the prevalence of Toxocara in soil samples from urban and suburban areas of Malaysia. J. Helminthol. 1998;72:39–42. doi: 10.1017/s0022149x0000095x. [DOI] [PubMed] [Google Scholar]
- 64.Quinn R, Smith HV, Bruce RG, Girdwood RW. Studies on the incidence of Toxocara and Toxascaris spp. ova in the environment. 1. A comparison of flotation procedures for recovering Toxocara spp. ova from soil. J Hyg (Lond) 1980;84:83–89. doi: 10.1017/s0022172400026553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuo K, Kamiya H. Modified sugar centrifugal flotation technique for recovering Echinococcus multilocularis eggs from soil. The Journal of Parasitology. 2005;91:208–209. doi: 10.1645/GE-3388RN. [DOI] [PubMed] [Google Scholar]
- 66.Szostakowska B, Lass A, Kostyra K, Pietkiewicz H, Myjak P. First finding of Echinococcus multilocularis DNA in soil: preliminary survey in Varmia-Masuria Province, northeast Poland. Vet. Parasitol. 2014;203:73–79. doi: 10.1016/j.vetpar.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 67.Bastos VK, Cutolo SA, Doria M.d.C.O., Razzolini MTP. Detection and quantification of viable Ascaris sp. and other helminth eggs in sewage sludge. Int J Environ Health Res. 2013;23:352–362. doi: 10.1080/09603123.2012.733939. [DOI] [PubMed] [Google Scholar]
- 68.Moodley P, Archer A, Hawksworth D. Standard Methods for the Recovery and Enumeration of Helminth Ova in Wastewater, Sludge, Compost, and Urine Diversion Waste in South Africa. Water Research Commission; 2008. [Google Scholar]
- 69.Beaver PC. Observations on the epidemiology of ascariasis in a region of high hookworm endemicity. J Parasitol. 1952;38:445–453. [PubMed] [Google Scholar]
- 70.Ayres RM, Mara D. A Laboratory Manual of Parasitological and Bacteriological Techniques. World Health Organization; 1996. Analysis of Wastewater for Use in Agriculture. [Google Scholar]
- 71.Oge H, Oge S. Quantitative comparison of various methods for detecting eggs of Toxocara canis in samples of sand. Vet. Parasitol. 2000;92:75–79. doi: 10.1016/s0304-4017(00)00276-4. [DOI] [PubMed] [Google Scholar]
- 72.Jeandron A, Ensink JHJ, Thamsborg SM, Dalsgaard A, Sengupta ME. A Quantitative Assessment Method for Ascaris Eggs on Hands. PLoS ONE. 2014;9:e96731. doi: 10.1371/journal.pone.0096731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krauth SJ, Coulibaly JT, Knopp S, Traoré M, N'Goran EK, Utzinger J. An In-Depth Analysis of a Piece of Shit: Distribution of Schistosoma mansoni and Hookworm Eggs in Human Stool. PLoS Negl Trop Dis. 2012;6:e1969. doi: 10.1371/journal.pntd.0001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mes TH, Ploeger HW, Terlou M, Kooyman FN, Van Der Ploeg MP, Eysker M. A novel method for the isolation of gastro-intestinal nematode eggs that allows automated analysis of digital images of egg preparations and high throughput screening. Parasitology. 2001;123:309–314. doi: 10.1017/s0031182001008496. [DOI] [PubMed] [Google Scholar]
- 75.Yang YS, Park DK, Kim HC, Choi MH, Chai JY. Automatic identification of human helminth eggs on microscopic fecal specimens using digital image processing and an artificial neural network. IEEE Trans. Biomed. Eng. 2001;48:718–730. doi: 10.1109/10.923789. [DOI] [PubMed] [Google Scholar]
- 76.Bruun JM, Carstensen JM, Vejzagic N, Christensen S, Roepstorff A, Kapel CMO. OvaSpec - A vision-based instrument for assessing concentration and developmental stage of Trichuris suis parasite egg suspensions. Comput. Biol. Med. 2014;53:94–104. doi: 10.1016/j.compbiomed.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Khouja L, Cama V, Xiao L. Parasitic contamination in wastewater and sludge samples in Tunisia using three different detection techniques. Parasitol Res. 2010;107:109–116. doi: 10.1007/s00436-010-1844-8. [DOI] [PubMed] [Google Scholar]
- 78.Nkouawa A, Sako Y, Li T, Chen X, Wandra T, Swastika IK, Ito A. Evaluation of a Loop-Mediated Isothermal Amplification Method Using Fecal Specimens for Differential Detection of Taenia Species from Humans. J. Clin. Microbiol. 2010;48:3350–3352. doi: 10.1128/JCM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macuhova K, Kumagai T, Akao N, Ohta N. Loop-Mediated Isothermal Amplification Assay for Detection and Discrimination of Toxocara canis and Toxocara cati Eggs Directly From Sand Samples. Journal of Parasitology. 2010;96:1224–1227. doi: 10.1645/GE-2394.1. [DOI] [PubMed] [Google Scholar]
- 80.Raoul F, Li T, Sako Y, Chen X, Long C, Yanagida T, Ito A. Advances in diagnosis and spatial analysis of cysticercosis and taeniasis. Parasitology. 2013;140:1578–1588. doi: 10.1017/S0031182013001303. [DOI] [PubMed] [Google Scholar]
- 81.Harmon AF, Williams ZB, Zarlenga DS, Hildreth MB. Real-time PCR for quantifying Haemonchus contortus eggs and potential limiting factors. Parasitol Res. 2007;101:71–76. doi: 10.1007/s00436-006-0428-0. [DOI] [PubMed] [Google Scholar]
- 82.Krämer F, Vollrath T, Schnieder T, Epe C. Improved detection of endoparasite DNA in soil sample PCR by the use of anti-inhibitory substances. Vet. Parasitol. 2002;108:217–226. doi: 10.1016/s0304-4017(02)00199-1. [DOI] [PubMed] [Google Scholar]
- 83.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Noordin R. A Pentaplex Real-Time Polymerase Chain Reaction Assay for Detection of Four Species of Soil-Transmitted Helminths. The American journal of tropical medicine and hygiene. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dangoudoubiyam S, Vemulapalli R, Kazacos KR. PCR assays for detection of Baylisascaris procyonis eggs and larvae. The Journal of Parasitology. 2009;95:571–577. doi: 10.1645/GE-1905.1. [DOI] [PubMed] [Google Scholar]
- 85.Demeler J, Ramünke S, Wolken S, Ianiello D, Rinaldi L, Gahutu JB, Krücken J. Discrimination of Gastrointestinal Nematode Eggs from Crude Fecal Egg Preparations by Inhibitor-Resistant Conventional and Real-Time PCR. PLoS ONE. 2013;8:e61285. doi: 10.1371/journal.pone.0061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khademvatan S, Abdizadeh R, Tavalla M. Molecular characterization of Toxocara spp. from soil of public areas in Ahvaz southwestern Iran. Acta Tropica. 2014;135:50–54. doi: 10.1016/j.actatropica.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 87.Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, Lee R. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. The American journal of tropical medicine and hygiene. 2014;90:306–311. doi: 10.4269/ajtmh.13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durant JF, Irenge LM, Fogt-Wyrwas R, Dumont C, Doucet JP, Mignon B, Gala JL. Duplex quantitative real-time PCR assay for the detection and discrimination of the eggs of Toxocara canis and Toxocara cati (Nematoda, Ascaridoidea) in soil and fecal samples. Parasit Vectors. 2012;5:288. doi: 10.1186/1756-3305-5-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeon H-K, Eom KS. Molecular Approaches to Taenia asiatica. Korean J Parasitol. 2013;51:1–8. doi: 10.3347/kjp.2013.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Ito A. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–553. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janwan P, Intapan P, Thanchomnang T, Lulitanond V, Anamnart W, Maleewong W. Rapid detection of Opisthorchis viverrini and Strongyloides stercoralis in human fecal samples using a duplex real-time PCR and melting curve analysis. Parasitol Res. 2011;109:1593–1601. doi: 10.1007/s00436-011-2419-z. [DOI] [PubMed] [Google Scholar]
- 92.Pecson BM, Barrios JA, Johnson DR, Nelson KL. A Real-Time PCR Method for Quantifying Viable Ascaris Eggs Using the First Internally Transcribed Spacer Region of Ribosomal DNA. Applied and Environmental Microbiology. 2006;72:7864–7872. doi: 10.1128/AEM.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basuni M, Mohamed Z, Ahmad M, Zakaria NZ, Noordin R. Detection of selected intestinal helminths and protozoa at Hospital Universiti Sains Malaysia using multiplex real-time PCR. Tropical biomedicine. 2012;29:434–442. [PubMed] [Google Scholar]
- 94.Gaspard P, Wiart J, Schwartzbrod J. A Method for Assessing the Viability of Nematode Eggs in Sludge. Environmental Technology. 1996;17:415–420. [Google Scholar]
- 95.Dąbrowska J, Zdybel J, Karamon J, Kochanowski M, Stojecki K, Cencek T, Kłapeć T. Assessment of viability of the nematode eggs (Ascaris, Toxocara, Trichuris) in sewage sludge with the use of LIVE/DEAD Bacterial Viability Kit. Ann Agric Environ Med. 2014;21:35–41. [PubMed] [Google Scholar]
- 96.Raynal M, Villegas EN, Nelson KL. Enumeration of viable and non-viable larvated Ascaris eggs with quantitative PCR. J Water Health. 2012;10:594–604. doi: 10.2166/wh.2012.101. [DOI] [PubMed] [Google Scholar]
- 97.Rudi K, Moen B, Drømtorp SM, Holck AL. Use of Ethidium Monoazide and PCR in Combination for Quantification of Viable and Dead Cells in Complex Samples. Applied and Environmental Microbiology. 2005;71:1018–1024. doi: 10.1128/AEM.71.2.1018-1024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malicki J, Montusiewicz A, Bieganowski A. Improvement of counting helminth eggs with internal standard. Water Res. 2001;35:2333–2335. doi: 10.1016/s0043-1354(00)00517-0. [DOI] [PubMed] [Google Scholar]