Abstract
The initial accumulation of platelets after vessel injury is followed by thrombin-mediated generation of fibrin which is deposited around the plug. While numerous in vitro studies have shown that fibrin is highly adhesive for platelets, the surface of experimental thrombi in vivo contains very few platelets suggesting the existence of natural anti-adhesive mechanisms protecting stabilized thrombi from platelet accumulation and continuous thrombus propagation. We previously showed that adsorption of fibrinogen on pure fibrin clots results in the formation of a nonadhesive matrix, highlighting a possible role of this process in surface-mediated control of thrombus growth. However, the deposition of fibrinogen on the surface of blood clots has not been examined. In this study, we investigated the presence of intact fibrinogen on the surface of fibrin-rich thrombi generated from flowing blood and determined whether deposited fibrinogen is nonadhesive for platelets. Stabilized fibrin-rich thrombi were generated using a flow chamber and the time that platelets spend on the surface of thrombi was determined by video recording. The presence of fibrinogen and fibrin on the surface of thrombi was analyzed by confocal microscopy using specific antibodies. Examination of the spatial distribution of two proteins revealed the presence of intact fibrinogen on the surface of stabilized thrombi. By manipulating the surface of thrombi to display either fibrin or intact fibrinogen, we found that platelets adhere to fibrin- but not to fibrinogen-coated thrombi. These results indicate that the fibrinogen matrix assembled on the outer layer of stabilized in vitro thrombi protects them from platelet adhesion.
Keywords: Fibrin, Fibrinogen, Platelets, Thrombus, Hemostasis
1. Introduction
The formation of a hemostatic thrombus during blood vessel injury is a highly regulated process. It ensures that a blood clot is sufficiently strong to seal the breach and prevent the loss of blood, but is not overly robust to cause vessel occlusion. The response to vascular injury is initiated by adhesion of platelets to the exposed sub-endothelium followed by their aggregation (reviewed in [1,2]). Formation of the platelet plug is spatially coordinated with the activation of the blood coagulation system leading to thrombin generation and fibrin formation, the latter serving as a scaffold essential for the mechanical stability of the mixed fibrin/platelet thrombus. Depending on the type and severity of injury, a variable amount of fibrin has been detected within and around platelet thrombi in experimental animals [3–6]. Fibrin formation has also consistently been observed in ex vivo models of thrombosis [7–10].
Since uncontrolled blood coagulation is potentially dangerous, different anticoagulant mechanisms are activated to contain thrombus growth and localize it to the site of injury [11]. Even though the formation of fibrin ceases after some time, it is unclear why this fibrin remains nonthrombogenic. Fibrin supports strong integrin-mediated adhesion of both activated and resting platelets in vitro [12–16] and therefore, it would be expected to support accumulation of these cells on the surface of stabilized thrombi in vivo and thus promotion of continuous thrombus propagation. Nevertheless, many studies in experimental animals using traditional staining methods, isotopes, electron microscopy as well as advanced imaging techniques have not detected platelet accumulation on the surface of fibrin [17–20]. It has been reported that fibrin-rich thrombi produced in a model of repeated balloon injury in rabbit arteries do not propagate and only become occlusive after a significant reduction in blood flow [18,21]. Moreover, clinical findings indicate that non-occlusive fibrin-containing coronary thrombi are frequently detected during autopsies of noncardiac death and also present in a large number of subjects with evidence of silent plaque ruptures (reviewed in [22–25]). These observations suggest that non-occlusive thrombi are frequently formed and then followed by healing. While these various findings implicate the existence of processes that prevent the accumulation of platelets on the surface of fibrin formed around thrombi, the underlying mechanisms remain poorly understood.
In recent reports using purified proteins and isolated cells we showed that adsorption of fibrinogen on various surfaces, including fibrin clots, results in a dramatic loss of platelet and leukocyte adhesion [16,26]. The underlying mechanism of this process involves the adsorption of intact fibrinogen in a thin superficial layer of fibrin clots [27] and its self-assembly leading to the formation of a nanoscale (~10 nm) multilayer matrix [28,29]. The fibrinogen matrix is extensible, which makes it incapable of transducing strong mechanical forces via cellular integrins, resulting in weak intracellular signaling and infirm cell adhesion [16,28,29]. Consequently, platelet’s inability to adhere firmly and consolidate their grip on the extensible fibrinogen matrix leads to their detachment under flow. This interpretation is consistent with other studies that showed that fibrinogen deposited at high density reduces signaling in platelets [30]. Since thrombi in the circulation are continuously exposed to high (2–3 mg/mL) concentrations of fibrinogen, we hypothesize that the nonadhesive fibrinogen matrix assembles on the surface of fibrin developed around thrombi thereby preventing platelet adhesion and accumulation.
This study was undertaken to determine whether the surface of stabilized thrombi exposed to blood is covered with intact fibrinogen and whether deposited fibrinogen has anti-adhesive properties. Given the nanoscale nature of the fibrinogen multilayer, which would make the observation and manipulation of this structure in vivo challenging, we utilized a flow chamber to generate fibrin-rich thrombi that would mimic hemostatic clots formed under flow. Using specific monoclonal antibodies capable of discriminating between intact fibrinogen and fibrin, we analyzed the spatial distribution of fibrinogen and its association with fibrin. We also manipulated the surface of thrombi to display either intact fibrinogen or fibrin to demonstrate that fibrinogen prevents adhesion of platelets.
2. Materials and Methods
2.1 Reagents
Human fibrinogen and thrombin were obtained from Enzyme Research Laboratories (South Bend, IN). Monoclonal antibody (mAb) 59D8 which recognizes the N-terminal end of the β-chain of human fibrin [31,32] was obtained from Dr. H. Weiler, Blood Center of Wisconsin. MAb FPA directed against the fibrinopeptide A of human fibrinogen was a gift from Dr. J. Shainoff, the Cleveland Clinic. Polyclonal antibody against β3 integrin subunit was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The following secondary antibodies were obtained from Invitrogen (Life Technologies, Grand Island, NY): goat anti-mouse antibodies conjugated to Alexa 488, 633 or 647, and donkey anti-rabbit antibodies conjugated to Alexa 568. Alexa 488-labeled fibrinogen was from Invitrogen (Life Technologies, Grand Island, NY). MAb 7E3 was a gift from Dr. B. Coller. Thrombin inhibitors argatroban monohydrate and hirudin were from Sigma (St. Louis, MO) and D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) was from Haematologic Technologies Inc. (Essex Junction, VT). The chromogenic thrombin substrate S-2238 was purchased from Sigma.
2.2 Parallel plate flow chamber
Glass coverslips (22×22 mm) were plasma cleaned and incubated with a solution of rat tail collagen type I (BD Biosciences, Bedford, MA) at 3.5 mg/mL. Glass coverslips were placed in a parallel plate flow chamber (flow path of 4 mm width, 16 mm length and 0.13 mm height), and the chamber was assembled for perfusion studies. Blood for perfusions was treated with 6 μM argatroban. This concentration of argatroban does not inhibit thrombin completely and allows for sufficient fibrin formation [33]. Blood was then drawn for 15 min through the chamber by a Harvard Apparatus pump producing estimated shear rates of 300, 1500 and 3000s−1. In selected experiments, 50 or 100 μM PPACK, 200 μM argatroban or 2.5 μM hirudin were perfused over the formed thrombi at 300–1500 s−1 for 15 min to inactivate thrombin generated on the surface of thrombi. In other selected experiments, a flow chamber containing thrombi generated at 3000 s−1 was mounted on the stage of a Leica inverted microsocope (DM IL, Buffalo Grove, IL), and platelets (1 ×107/mL) in Tyrode buffer (137 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 0.2 mM Na2HPO4, 12 mM NaHCO3, 5.5 mM glucose, 0.1% BSA, pH 7.2) were perfused at 1500 s−1. Images were obtained every second, and the contact time of platelets with selected thrombi was determined.
2.3 Preparation of thrombi covered with fibrinogen or fibrin
Three types of thrombi displaying on their surface either intact fibrinogen or fibrin were prepared as follows: (1) thrombi generated for 15 min at 3000 s−1 were perfused with Tyrode buffer containing 100 μM PPACK for 15 min at 1500 s−1 followed by 15 min resting in the perfusion solution (“fibrinogen” surface); (2) thrombi generated for 15 min at 3000 s−1 were perfused with Tyrode buffer supplemented with 0.1 U/mL thrombin for 15 min followed by 15 min resting in the same solution (“fibrin” surface); and (3) thrombi generated as in the second condition to obtain fibrin were incubated with 100 μM PPACK for 15 min to inhibit thrombin (“fibrin+PPACK” surface). Subsequently, calcein-labeled platelets (1×107) in Tyrode buffer, containing 5.5 mM glucose and 0.1% BSA, pH 7.2, were perfused over thrombi at a shear rate of 1500 s−1 for 15 min.
2.4 Fibrinogen and fibrin distribution in thrombi using confocal microscopy
Thrombi generated on the coverslips were washed with phosphate buffered saline (PBS) or Tyrode buffer and then fixed in 4 % paraformaldehyde for 15 min at 22 °C. Samples were washed with PBS and incubated with 0.3 % Triton X-100 for 2.5 min, then rinsed and incubated with 3% BSA for 60 min. Samples were washed and then incubated with solutions containing various combinations of anti-fibrin (20 μg/mL), anti-β3 (4 μg/mL) and anti-fibrinopeptide A (20 μg/mL) antibodies for 1.5 h at 37 °C. After washing, samples were incubated with appropriate secondary antibodies for 1.5 h at 37 °C, washed and mounted on glass slides, and viewed using a confocal microscope (Leica TSC SP5, Buffalo Grove, IL). When using different experimental conditions, consistent exposure and laser intensity settings were used. Care was taken to ensure that images were collected with the maximum dynamic range for 8-bit images (0–255 greyscales). Greyscale images were pseudocolored using Leica AS software and exported as maximum protection TIFF files. Where applicable, brightness and contrast were adjusted linearly and equally for the different channels using ImageJ to aid in visualization of printed images. In experiments where signal intensity was compared (i.e. signal vs. no signal), voltage corresponding to the photomultiplier tube gain remained the same between samples. Control experiments were performed with secondary antibodies only. Optical sections of images were produced using the z-stack function. Vertical cross-sections of the thrombus were generated using ImageJ software and analyzed for the distribution of fibrinogen and fibrin. The three-dimensional images were constructed using ImageJ.
2.5 Characterization of mAbs
To verify the specificity of anti-fibrinogen (termed FPA) and anti-fibrin (59D8) mAbs used in this study, the microtiter wells of an Immulon 4B plate were coated with different concentrations of fibrinogen overnight at 4 °C. Selected wells were incubated with 1 U/mL thrombin to convert fibrinogen into fibrin. In addition, some experiments were performed using fibrin-monomer prepared by dissolving preformed fibrin clots in 3 M urea and directly coating fibrin-monomer onto the wells. The wells were blocked with 1% BSA and incubated with 2 μg/mL of 59D8 or FPA mAbs for 1 h at 37 °C. Following this, the plates were incubated with goat anti-mouse secondary antibody conjugated to alkaline phosphatase for 1 h at 37 °C. The binding of mAbs was determined by adding the substrate p-nitrophenyl phosphate and measuring the absorbance at 405 nm.
2.6 Assessment of Thrombin Activity
To determine the thrombin activity associated with thrombi, the thrombi were generated in the flow chamber at 3000 s−1, and then Tyrode buffer alone or supplemented with 100 μM PPACK was perfused through the chamber at shear rate 300 s−1 for 15 min. The chamber was disassembled, washed with PBS, and the coverslip was placed in a 35 mm Petri dish containing 2 mL of 25 μM S-2238. The dishes were incubated at 37 °C and absorbance at 405 nm was determined at 10–30 min.
3. Results
3.1 Thrombus formation and fibrin deposition on the surface of thrombi at different shear rates
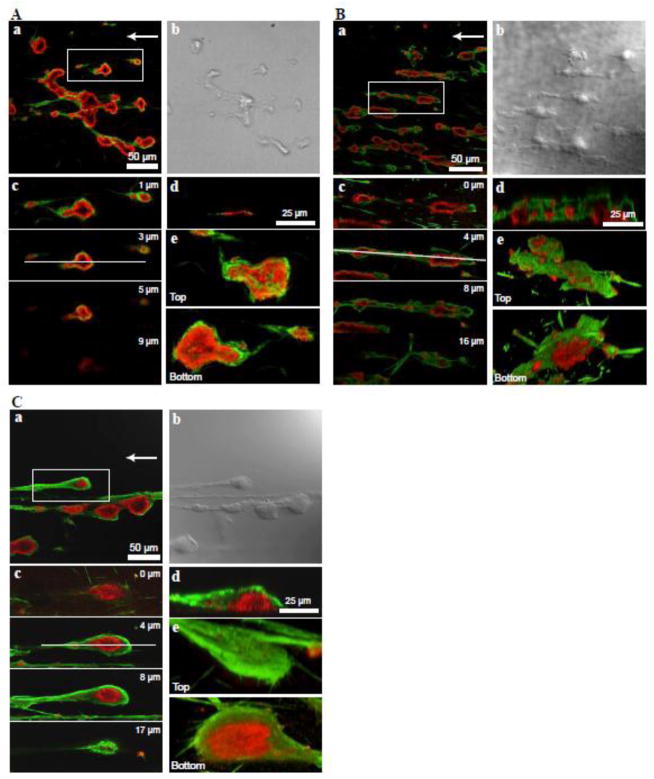
To examine whether fibrinogen accumulates on the surface of fibrin covering the surface of stabilized thrombi, we initially tested the conditions that produce the largest amount of fibrin. Blood was treated with 6 μM argatroban to allow partial anticoagulation and perfused over collagen-coated coverslips at 300, 1500 and 3000 s−1 shear rates. These shear rates mimic blood flow conditions found in veins, arteries and small or stenosed arteries, respectively. After 15 min, thrombi were fixed and incubated with antibodies against fibrin and the platelet β3 integrin subunit. The mAb 59D8 was selected based on its ability to selectively interact with fibrin, but not fibrinogen (Fig. S1A). Small platelet thrombi formed at 300 s−1 had an irregular shape with little fibrin deposited mainly at their periphery (Fig. 1A). The thrombi formed at high shear rates (1500 s−1 and 3000 s−1) were elongated with a distinct thrombus head and tail and contained a core consisting of aggregated platelets (Fig. 1B and Fig. 1C). The morphometric analyses indicated that a significant portion of thrombi (~80%) generated at 3000 s−1 had a width to length ratio in a range of 2–4 (Fig. S2). Fibrin was deposited not only around thrombi but also many thrombi were often connected by fibrin strands. The vertical cross-sections, z-stacks, and the three-dimensional reconstruction showed the presence of fibrin deposited on the surface of thrombi generated at 1500 and 3000 s−1 (Fig. 1B and 1C). However, variability in the coverage by fibrin was noted as the uppermost surface of platelet thrombi was not always fully shielded by fibrin. Representative fields containing thrombi and a summary of experiments performed at 1500 s−1 and 3000 s−1 showing variability of the surface appearance are presented in Figure S3. The inconsistency in thrombus surface coverage by fibrin may reflect an inter-individual variability in platelet responses to collagen, heterogeneity in collagen coating or other reasons. Despite these variations, thrombi generated at 1500 s−1 and 3000 s−1 showed extensive deposition of fibrin. Since thrombi formed at 3000 s−1 had more fibrin than those at 1500 s−1, the shear rate of 3000 s−1 was selected to generate thrombi for subsequent analyses.
Figure 1. Analysis of fibrin distribution in thrombi formed at a shear rate of 300 s−1, 1500 s−1 and 3000 s−1.
Whole blood was treated with 6 μM argatroban and perfused through the flow chamber containing a collagen-coated coverslip for 15 min at a shear rate of (A) 300 s−1 (B) 1500 s−1 and (C) 3000 s−1. The generated thrombi were stained for fibrin (green) using mAb 59D8 and for platelet β3 integrin (red) using polyclonal anti-β3 antibody. a, a representative confocal image of several thrombi. b, a DIC image of the field shown in a. c, series of cross-sections (z-stacks) of one of the thrombi shown in a (box) taken at 1, 2,5, and 9 μm (A), 0, 4, 8, and 16 μm (B), or 0, 4, 8,and 17 μm (C) from the bottom of the chamber. d, vertical cross-section of the thrombus shown in c taken at the position shown by a horizontal white line. e, three dimensional reconstructions of the thrombus shown in a–c (top and bottom views).
3.2 Adhesion of platelets to the surface of preformed thrombi
As shown in Figure 1, there was no platelet-associated immunostaining on the surface of thrombi above the fibrin layer. Moreover, platelets were not detected on the fibrin fibers connecting thrombi (Fig. 1). To further confirm that platelets do not adhere to the surface of thrombi, we performed video recording of flowing platelets. Thrombi were generated by perfusing whole blood at 3000 s−1 followed by washing with platelet-poor plasma to remove blood cells. Platelets in Tyrode buffer were then perfused over the formed thrombi. Live video microscopic images were captured with a frame interval of one second. A representative snap shot of the thrombus with platelets in contact with its surface is shown in Figure 2A.
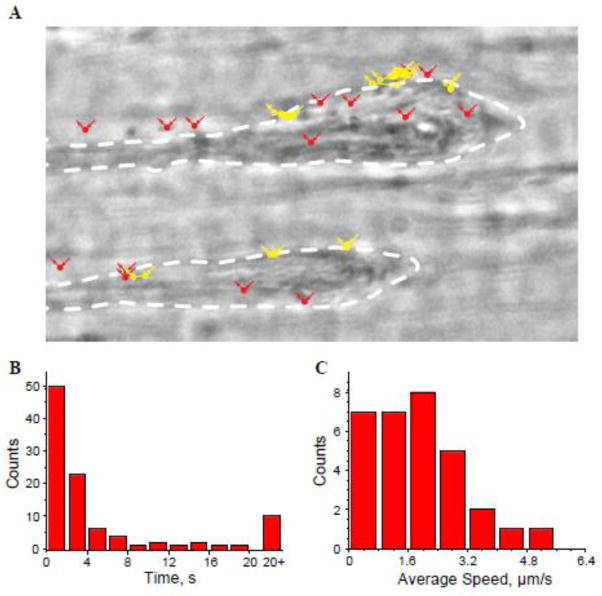
Figure 2. Tracking platelet contacts with the surface of thrombi.
Thrombi generated at 3000 s−1 for 15 min were washed with platelet poor plasma (PPP) for additional 15 min, and then platelets in Tyrode buffer were perfused over the preformed thrombi for 15 min. A, Live video microscopic images of platelets in contact with the thrombus surface were captured with a frame interval of 1 sec. The platelets that stayed on the surface less than 2 s are marked in solid red. The platelets that interacted with the surface of thrombi for 2–30 s are marked in yellow. All platelet positions are marked with circles and displacements are shown as lines. The contours of two thrombi are outlined. B, a distribution of times each platelet spends on the surface of thrombus. The data shown are from 100 platelets. C, a distribution of the average rolling speed of each platelet while on the surface of thrombus expressed as μm/s. n=3 experiments.
The distribution of the average interaction times between platelets and the surface of the thrombus is shown in Figure 2B. The majority of platelets (>70%) do not contact the surface of thrombi longer than 3–4 s, and approximately 10% remain on the surface for more than 20 s (20–100 s). While interacting with the surface of thrombi, these latter platelets remained in motion rolling over the surface with a short stop before complete detachment. Since no statistically significant pattern regarding this motion within the interaction time was found, we limited ourselves to the average displacement speed, which was calculated as the distance that a platelet travels on the surface between consecutive frames (Fig. 2C). The analyses showed that the majority of rolling platelets moved with a speed of 2–3 μm/sec. These results indicate that platelets only transiently adhere to the surface of a thrombus, suggesting that it is largely nonreactive for platelets.
3.3 Fibrinogen distribution on the surface of thrombus
To examine the presence of intact fibrinogen on the surface of the fibrin coat, the generated thrombi were washed with Tyrode buffer, to remove blood cells, and then incubated with fibrinogen-specific mAb FPA, which selectively binds intact fibrinogen but not fibrin (Fig. S1B). As shown in Fig. 3A, the presence of intact fibrinogen on the surface of thrombi was observed. However, fibrinogen was not detected as a uniform coat in association with fibrin but rather appeared in a punctate manner. One reason for this uneven distribution is the presence of fibrin-associated thrombin, which continues to convert fibrinogen into fibrin while the coverslips were being processed for immunostaining. As expected, we detected the presence of thrombin activity associated with thrombi using a chromogenic thrombin substrate S-2238 (Fig. S4).
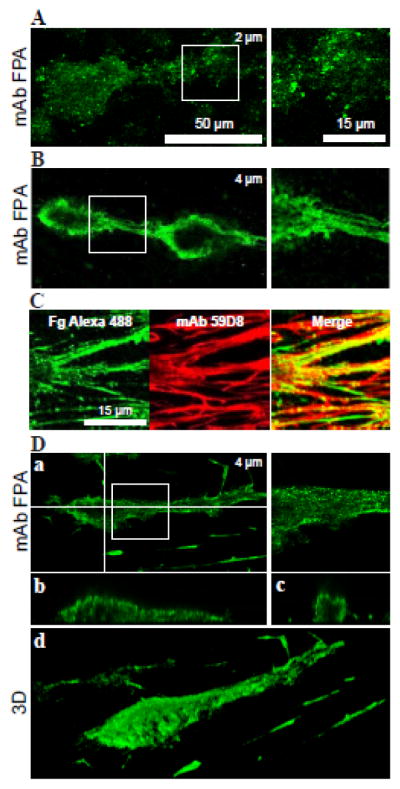
Figure 3. Deposition of fibrinogen on the surface of thrombi.
Whole blood containing 6 μM argatroban was perfused over collagen-coated surfaces for 15 min at 3000 s−1 and the generated thrombi were washed with Tyrode buffer for 15 min to remove blood cells. A, thrombi were incubated with anti-fibrinogen mAb FPA followed by Alexa 488-secondary antibody (green). The right panel shows an enlarged area (box) from the left panel. The image shown is taken at 2 μm from the bottom of the chamber. A representative image from 3 experiments is shown. B, generated thrombi were washed to remove blood cells followed by 15 min perfusion with PBS+50 μM PPACK at 1500 s−1. Thrombi were incubated with mAb FPA (green). The right panel shows an enlarged area (box) from the left panel. The image shown is taken at 4 μm from the bottom of the chamber. A representative image from 3 experiments is shown. C, thrombi washed with PPACK as in B were subsequently perfused with PRP containing 50 μg/mL Alexa 488-labeled fibrinogen (green) for 15 min at 300 s−1, after which the thrombi were incubated with anti-fibrin mAb 59D8. Left panel, a representative thrombus image shows accumulation of Alexa 488-fibrinogen (green); middle panel, the same area showing staining for fibrin (mAb 59D8) followed by incubation with Alexa 633-secondary antibody (red); and right panel, merging of green and red labels to highlight colocalization of fibrinogen and fibrin. n=14 experiments. D, generated thrombi were treated with PPACK and then perfused with a solution of 2.7 mg/mL unlabeled fibrinogen containing 100 μM PPACK for 15 min. After washing, thrombi were stained with mAb FPA for intact fibrinogen (green). The image shown is taken at 4 μm from the bottom of the chamber (a). Horizontal (b) and vertical (c) cross-sections of the thrombus were taken at the positions shown by white lines. (d), three-dimensional reconstruction of the thrombus. A representative image from 3 experiments is shown.
To investigate the possibility that fibrin-bound thrombin decreased the amount of intact fibrinogen, the generated thrombi were washed with Tyrode buffer supplemented with 50–100 μM PPACK. PPACK-treated thrombi were incubated with mAb FPA and found to exhibit a stronger and more uniform staining for fibrinogen (Fig. 3B). To further explore the capacity of fibrinogen to adsorb on fibrin, PPACK-treated thrombi were perfused with platelet-rich plasma containing Alexa 488-labeled fibrinogen. As shown in Figure 3C, thrombi accumulated Alexa 488-labeled fibrinogen, which predominantly colocalized with fibrin. Finally, nonlabeled fibrinogen (2.7 mg/mL) was perfused over PPACK-treated thrombi and found to adsorb on the surface of thrombi as disclosed by staining with mAb FPA (Fig. 3D, a–d). Exogenously added unlabeled (Fig. 3D) and Alexa 488-labeled fibrinogen (Fig. 3C) decorated the thrombus head and tail and also coated fibrin fibers connecting thrombi. Since, in each case, fibrinogen was added after thrombi formation, the deposition of fibrinogen evidently occurs on the surface of thrombus-associated fibrin.
Even though colocalization of Alexa 488-labeled fibrinogen with fibrin in the experiments described above (Fig. 3C) is an indicator of the newly deposited material, the Alexa 488 label on its own cannot discriminate between intact fibrinogen and fibrin since part of the labeled fibrinogen could be converted into fibrin. Therefore, we sought to verify that Alexa 488-associated protein was intact fibrinogen. The generated thrombi (Fig. 4A) were washed and then allowed to rest for an additional 15 min in the flow chamber to allow conversion of surface-associated fibrinogen into fibrin. Subsequently, Alexa 488-labeled fibrinogen (green) in Tyrode buffer + PPACK (100μM) was perfused over the thrombi, which were then stained for intact fibrinogen with mAb FPA (red). As shown in Figure 4B, a significant amount of fibrinogen was adsorbed on the surface of thrombi and the majority of this protein was intact (Fig. 4C). The intact state of fibrinogen was further confirmed by the colocalization of the two labels (Fig. 4D). Inspection of their distribution also showed that there were small areas where only the green label (Alexa-488 labeled fibrinogen) was observed (Fig. 4D–E, boxed areas), indicating the presence of residual thrombin activity resulting in the conversion of Alexa 488-labeled fibrinogen into fibrin. The addition of other thrombin inhibitors including hirudin (2.5 μM) or argatroban (200 μM) during perfusion with Alexa 488-labeled fibrinogen produced similar results. Together, these data indicate that intact fibrinogen is deposited on the surface of preformed thrombi and its quantity depends on the presence of thrombin activity.
Figure 4. Detection of intact fibrinogen on the surface of thrombi.
Thrombi were generated by perfusing whole blood containing 6 μM argatroban over collagen-coated surfaces for 15 min at high shear rate of 3000 s−1. Thrombi were allowed to rest in Tyrode buffer for an additional 15 min to convert surfaceassociated fibrinogen into fibrin. Subsequently, Alexa 488-labeled fibrinogen in Tyrode buffer containing 100 μM PPACK was perfused at 1500 s−1 for 15 min. A, a representative DIC image of thrombi and connecting fibrin fibers. B, thrombi were perfused with 20 μg/mL fibrinogen containing 1:100 Alexa 488-labeled fibrinogen (green). C, thrombi were subsequently stained with mAb FPA (red). D, fluorescent images of fibrinogen and fibrin were merged to highlight colocalization of the two labels. E, enlarged images of the boxed areas shown in D. n=3 experiments.
3.4 Platelet adhesion to the surface of thrombi displaying fibrinogen or fibrin
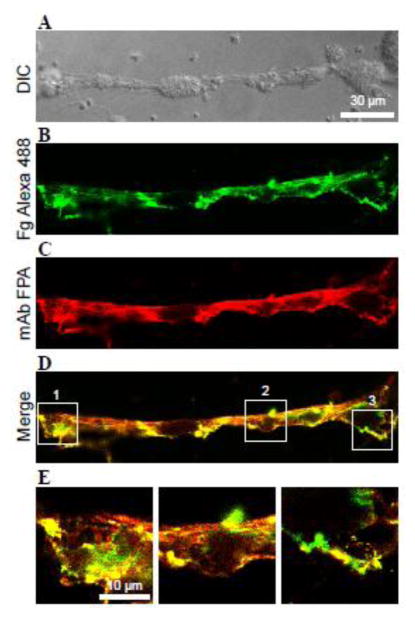
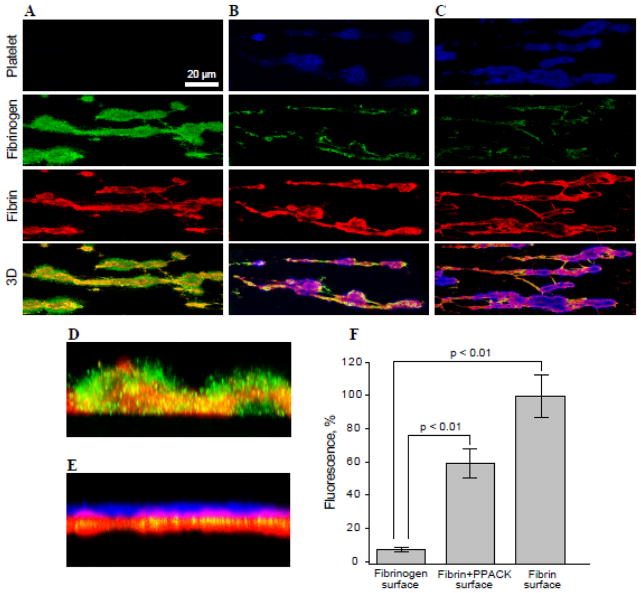
Having established conditions for the generation of stabilized thrombi covered with intact fibrinogen, we sought to verify that the fibrinogen coat protects thrombi from platelet adhesion. Three types of surfaces were prepared: “Fibrinogen”, “Fibrin” and “Fibrin+PPACK” (described in Materials and methods). Subsequently, calcein-labeled platelets in Tyrode buffer were perfused over thrombi. Thrombi were then stained for fibrin (red) and fibrinogen (green) with the respective mAbs. As shown in Figure 5A (upper panel), when thrombin was inhibited by PPACK to halt the conversion of fibrinogen deposited on the surface of fibrin, no platelets were detected on the surface of thrombi. The presence of fibrinogen on the surface of fibrin was confirmed by staining of thrombi for fibrinogen and fibrin (Fig. 5A and 5D). In contrast, pronounced accumulation of platelets (blue) was seen on the surface of thrombi and connecting fibrin fibers when fibrin-bound fibrinogen was allowed to convert into fibrin (Fig. 5C, upper panel, and 5E). After blocking fibrin-bound thrombin with PPACK, a considerable quantity of platelets (~60%) adhered to fibrin, suggesting that although fibrin-bound thrombin enhanced adhesion perhaps by activating platelet receptors, nonstimulated platelets were still able to adhere (Fig. 5B). Platelet adhesion to both “Fibrin” surfaces was largely mediated by αIIbβ3 as mAb 7E3 at 10 μg/ml inhibited adhesion by ~90%. Quantification of platelet adhesion showed that ~12- and 20-fold more platelets adhered to “Fibrin+PPACK” and “Fibrin” surfaces, respectively, than to the “Fibrinogen” surface (Fig. 5F). Since the presence of red blood cells (RBCs) may affect platelet adhesion, we perfused platelets over preformed thrombi in the presence of RBCs (25–50%). No increase in platelet adhesion to thrombi coated with fibrinogen was found. Furthermore, pre-incubation of platelets with ADP (10 μM) before their perfusion did not induce their adhesion to the surface of fibrinogen-coated thrombi, although single platelets and platelet aggregates were observed adherent to the collagen substrate (Fig. S5). It should be noted that our system models a step of thrombogenesis when fibrin-rich thrombi have been stabilized and the generation of platelet agonists ceased. Therefore, ADP was used only as a means to convert αIIbβ3 into its active form. Together, these results indicate that the deposition of fibrinogen on the surface of fibrin associated with thrombi prevents adhesion of either resting or activated platelets.
Figure 5. Deposition of platelets on the surface of thrombi expressing intact fibrinogen or fibrin.
Whole blood containing 6 μM argatroban was perfused over collagen-coated surfaces for 15 min at 3000 s−1. Generated thrombi were then incubated with PPACK (A), thrombin+PPACK (B) or thrombin only (C) to prepare the “Fibrinogen” (n=7 experiments), “Fibrin+PPACK” (n=5) or “Fibrin” (n=5) surfaces, respectively, as described in Materials and Methods. Calcein-labeled platelets (blue) in Tyrode buffer were perfused at 1500 s−1 for 15 min (A–C, upper panels). Thrombi were incubated with mAb 59D8 for fibrin (red) or mAb FPA for fibrinogen (green). The fourth row from the top in each column shows merging of fluorescent images for fibrin and fibrinogen in A (orange) and that for fibrin and platelets in B and C (purple). D and E, vertical cross-sections of representative thrombi generated under conditions shown in A and C, respectively. F, platelet adhesion was quantified using ImageJ software and expressed as a percentage of maximal fluorescence observed in C (“fibrin” surface). Fluorescence of platelets was measured from 254×254 μm random fields of 40 representative images. Fluorescence of fibrin in each field was determined and used to normalize the number of adherent platelets. Statistical analysis was performed using one-way ANOVA followed by Tukey’s test to determine significance (p< 0.01).
4. Discussion
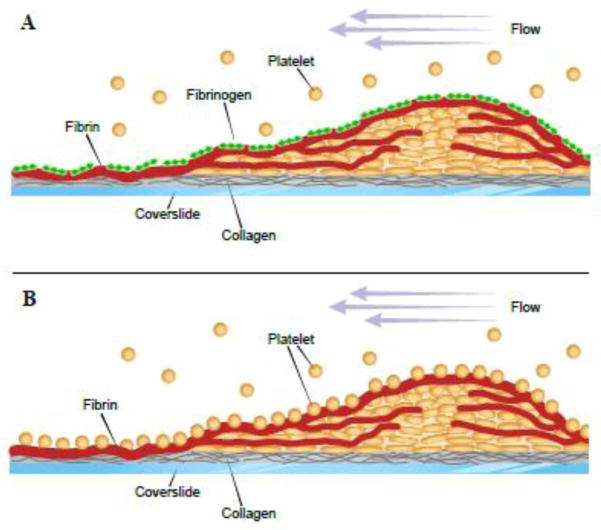
In previous studies we identified a process in which fibrinogen adsorbed on various surfaces, including fibrin, dramatically reduces adhesion of platelets and leukocytes, and determined the molecular basis for this phenomenon [16,26,28,29]. However, these studies have been performed using pure fibrin clots formed under static conditions and with isolated blood cells. As for thrombi formed in flowing blood, they have a distinctive composition and architecture. Moreover, fibrin deposited around the platelet plug incorporates other blood proteins that may modify its fibrinogen binding capacity. Therefore, it was mandatory to show the presence of intact fibrinogen on the surface of fibrin deposited on the surface and around fibrin-rich thrombi and investigate its anti-adhesive properties. The major finding of this study is that thrombi generated from blood flowing over collagen-containing surfaces are coated with intact fibrinogen deposited on the boundary layer of thrombus-associated fibrin and on fibrin fibers around thrombi. This fibrinogen shell renders thrombi nonadhesive for platelets thrombi (schematically depicted in Fig. 6). These results provide direct experimental evidence for the presence of intact fibrinogen on the surface of in vitro thrombi and suggest that the fibrin shell, in addition to serving as a mechanical scaffold, functions as a platform for the self-assembly of the nonadhesive fibrinogen matrix.
Figure 6. A model of the proposed fibrinogen-dependent antiadhesive mechanism operating on the surface of stabilized thrombi.
A, when fibrin on the surface of a thrombus is covered with the fibrinogen matrix (green diamonds), platelets do not adhere. B, in contrast, platelets accumulate on the surface of a thrombus covered with fibrin (red lines). Blood flow is shown from right to left (purple arrows).
Since fibrin is essential for stabilization of fibrin/platelet thrombi, numerous studies using in vivo models of thrombosis have examined its deposition [3,5,6,17,18,34,35]. It was also shown that while platelet accumulation peaked within 30 min to one hour of arterial injury and then ceased [36–38], fibrin formation continued for several hours [6]. Remarkably, no further platelet accumulation onto the injury site has been observed when labeled platelets were injected several hours following injury [6]. Likewise, in vitro models, in which platelet accumulation and fibrin formation were induced either on sections of an excised vessel wall or on substrates that mimic the sub-endothelial matrices, showed fibrin deposition that initially increased and then gradually stabilized [7–9,39,40]. Two recent studies in living mice have shown that as the thrombus grows, its surface, consisting primarily of platelets, changes to that composed of fibrin [19,20]. However, fibrin overlaying platelet thrombi is not a universal finding among intravital studies in mice. The reason why some intravital investigations were able to detect a fibrin coat on the luminal thrombus surface [19,20] whereas other studies detected fibrin only within and around thrombi [41,42] and in the extravascular space underlying the thrombus [41,43] is unclear. Differences in the methods used to induce thrombi, the times to monitor the course of thrombogenesis, the nature of vascular beds, and specificity of mAbs employed to detect fibrin may account for these discrepancies.
The in vitro thrombi produced in our study show a strong resemblance, both in the overall morphology and the presence of a fibrin coat, to those produced in vivo [19,20] and thus may represent a suitable model of fibrin-rich thrombi. The characteristic architecture of platelet-fibrin thrombi with a central platelet core and fibrin fibers aligned in the direction of flow [44,45] suggests that this structure may have specific functions. Consequently, only those in vitro thrombi that are generated from flowing blood and under conditions that allow for both the development of platelet thrombi and blood coagulation may be an appropriate model for studies of thrombus growth and dissolution. In contrast, a homogeneous structure of clots generated from platelet-rich plasma or pure fibrin under static conditions may be less informative. Nevertheless, while the in vitro perfusion chamber thrombosis model used in this as well as in many previous studies allows for the generation of thrombi with some morphological similarities to those formed in vivo, this reductive system clearly has many limitations. Thus, further studies of thrombi generated on excised vessel walls or in animal models of thrombosis may help to define the role of fibrin as a substrate for the deposition of the anti-adhesive fibrinogen layer.
Based on the fact that the fibrin cap is a feature of stabilized thrombi, it has been proposed that fibrin limits thrombus growth by physically separating procoagulant platelets from coagulation zymogens in blood [19]. Although it is possible that fibrin may preclude the contact of circulating coagulation factors with the procoagulant platelet surface, it is also known to be a highly adhesive substrate for both resting and activated platelets mediating their attachment via integrin αIIbβ3 [12–16]. Therefore, if fibrin on the surface of stabilized thrombi is not shielded, it would remain thrombogenic, continually attracting platelets. Indeed, we show that when thrombi were manipulated to display fibrin (Fig. 5B, C, E and F) platelets readily accumulated on such surfaces. In contrast, no platelets were detected on the surface of thrombi that contained intact fibrinogen (Fig. 5A, D). Moreover, when platelets were tracked by time-lapsed video microscopy, they contacted the surface of thrombi only for a short period of time (Fig. 2), presumably because of the nonadhesive fibrinogen coat assembled on the surface of fibrin. This is in keeping with in vivo studies that did not detect platelets above the fibrin cap [19,20] and in agreement with a finding that thrombi generated in Par 4−/− mice, which showed a robust fibrin accumulation, had no platelets adherent to the topmost surface of the fibrin clot [46]. It is also consistent with previous reports showing that no platelets have been observed on the surface of fibrin produced in ex vivo models of thrombosis [7,10,47].
Discussion of the mechanisms that are responsible for the cessation of thrombus growth has traditionally focused on those that halt the accrual of platelets to the platelet plug [48]. However, it is the deposition of fibrin around the initial platelet plug and in its vicinity, as documented in numerous studies, that appears to be the end stage of thrombus formation. Therefore, the anti-adhesive mechanisms that prevent the accumulation of blood cells on this highly adhesive and, thus, dangerous substrate should also play an important role in the control of thrombus propagation. The long-held view that fibrin serves as a mechanical scaffold stabilizing the thrombus structure is supported by numerous in vitro and in vivo studies. Our results indicate that fibrin may perform yet another important function by serving as a platform for the assembly of the nonadhesive fibrinogen layer. It is also possible that the fibrinogen matrix deposited atop of the fibrin cap may separate fibrin-bound thrombin, which remains enzymatically active [40,49,50], from the flowing blood. Although thrombin would obviously convert first arriving fibrinogen molecules into fibrin, this newly formed fibrin will not contain thrombin. Therefore, subsequent fibrinogen molecules that will interact with this thrombin-free fibrin will remain intact and initiate the formation of the fibrinogen multilayer through the interaction between the αC domains of the neighboring molecules [29].
In conclusion, in this in vitro model, the fibrin-rich thrombi formed from blood flowing over the collagen surfaces were coated with intact fibrinogen which strongly reduced platelet adhesion. While there is little doubt that fibrinogen, upon its contact with fibrin, undergoes self-assembly, the exact structure of the fibrinogen matrix remains to be determined. The nanoscale nature of the fibrinogen multilayer presents a formidable challenge for studies of this structure deposited on the surface of fibrin. At present, even state-of-the art microscopy methods such as high-resolution AFM-based imaging are not amenable to reveal the molecular structure of the fibrinogen matrix deposited on the soft fibrin polymers. Therefore, the conclusions and models generated in our previous studies of fibrinogen deposition on hard surfaces such as mica or glass [28,29] should provide guidance for future analyses. Likewise, our present studies and previous low resolution demonstration that fibrinogen accumulates in a thin superficial layer of fibrin clots [27] may guide future analyses of spatial distribution of fibrinogen and other proteins on the flow surface of thrombi in vivo.
Supplementary Material
Highlights.
Intact fibrinogen deposited on the surface of pure fibrin clots dramatically reduces adhesion of platelets.
It is unknown whether the surface of fibrin-rich platelet thrombi is covered with fibrinogen.
This in vitro study shows that fibrin on the surface of stabilized thrombi is covered with intact fibrinogen.
The fibrinogen layer is nonadhesive for platelets, halting platelet accumulation.
This finding may potentially explain a long-standing puzzle as to why hemostatic thrombi formed in the circulation do not propagate.
Acknowledgments
Financial support: This work was supported by the NIH grant HL 107539
This work was supported by the NIH grant HL 107539.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 2.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen L, Rowsell HC, Hovig T, Mustard JF. Resolution and organization of platelet-rich mural thrombi in carotid arteries of swine. Am J Pathol. 1967;51:681–719. [PMC free article] [PubMed] [Google Scholar]
- 4.Sixma JJ, Wester J. The hemostatic plug. Semin Hematol. 1977;14:265–299. [PubMed] [Google Scholar]
- 5.Hatton MW, Ross B, Southward SM, DeReske M, Richardson M. Pretreatment of rabbits with either hirudin, Ancrod, or Warfarin significantly reduces the emmediate uptake of fibrinogen and platelets by the deendothelialized aorta wall after balloon-cathether injury in vivo. Arterioscler Thromb Vasc Biol. 1998;18:816–824. doi: 10.1161/01.atv.18.5.816. [DOI] [PubMed] [Google Scholar]
- 6.van Ryn J, Lorenz M, Merk H, Buchanan MR, Eisert WG. Accumulation of radiolabeled platelets and fibrin on the carotid artery of rabbits after angioplasty: effects of heparin and dipyridamole. Thromb Haemost. 2003;90:1179–1186. doi: 10.1160/TH03-05-0305. [DOI] [PubMed] [Google Scholar]
- 7.Weiss HJ, Turitto VT, Baumgartner HR. Role of shear rate and platelets in promoting fibrin formation on rabbit subendothelium. J Clin Invest. 1986;78:1072–1082. doi: 10.1172/JCI112663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gast A, Tschopp TB, Baumgartner HR. Thrombin plays a key role in late platelet thrombus growth and/or stability. Effect of a specific thrombin inhibitor on thrombogenesis induced by aortic subendothelium exposed to flowing rabbit blood. Arterioscler Thromb. 1994;14:1466–1474. doi: 10.1161/01.atv.14.9.1466. [DOI] [PubMed] [Google Scholar]
- 9.Mailhac A, Badimon JJ, Fallon JT, Fernández-Ortiz A, Meyer B, Chesebro JH, Fuster V, Badimon L. Effect of an eccentric severe stenosis on fibrin(ogen) deposition on severely damaged vessel wall in arterial thrombosis. Relative contribution of fibrin(ogen) and platelets. Circulation. 1994;90:988–996. doi: 10.1161/01.cir.90.2.988. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhofer D, Tschopp TB, Steiner B, Baumgartner HR. Role of collagen-adherent platelets in mediating fibrin formation in flowing whole blood. Blood. 1995;86:3815–3822. [PubMed] [Google Scholar]
- 11.Dahlback B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. Journal of Internal Medicine. 2005;257:209–223. doi: 10.1111/j.1365-2796.2004.01444.x. [DOI] [PubMed] [Google Scholar]
- 12.Hantgan RR, Hindriks G, Taylor RG, Sixma JJ, de Groot PG. Glycoprotein Ib, von Willebrand factor, and glycoprotein IIb:IIIa are all involved in platelet adhesion to fibrin in flowing whole blood. Blood. 1990;76:345–353. [PubMed] [Google Scholar]
- 13.Jen CJ, Lin JS. Direct observation of platelet adhesion to fibrinogen- and fibrin-coated surfaces. Am J Physiol. 1991;261(5 Pt 2):H1457–1463. doi: 10.1152/ajpheart.1991.261.5.H1457. [DOI] [PubMed] [Google Scholar]
- 14.Hantgan RR, Endenburg SC, Cavero I, Marguerie G, Uzan A, Sixma JJ, de Groot PG. Inhibition of platelet adhesion to fibrin(ogen) in flowing whole blood by arg-gly-asp and fibrinogen gamma-chain carboxy terminal peptides. Thromb Haemost. 1992;68:694–700. [PubMed] [Google Scholar]
- 15.Endenburg SC, Hantgan RR, Sixma JJ, de Groot PG, Zwaginga JJ. Platelet adhesion to fibrin(ogen) Blood Coagul Fibrinolysis. 1993;4:139–142. [PubMed] [Google Scholar]
- 16.Podolnikova NP, Yermolenko IS, Fuhrmann A, Lishko VK, Magonov S, Bowen B, Enderlein J, Podolnikov A, Ros R, Ugarova TP. Control of integrin αIIbβ3 outside-in signaling and platelet adhesion by sensing the physical properties of fibrin(ogen) substrates. Biochemistry. 2010;49:68–77. doi: 10.1021/bi9016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groves HM, Kinlough-Rathbone RL, Richardson M, Jorgensen L, Moore S. Thrombin generation and fibrin formation following injury to rabbit neointima. Lab Invest. 1982;46:605–612. [PubMed] [Google Scholar]
- 18.Kinlough-Rathbone RL, Packman MA, Mustard JF. Vessel injury, platelet adherence, and platelet survival. Arteriosclerosis. 1983;3:529–546. doi: 10.1161/01.atv.3.6.529. [DOI] [PubMed] [Google Scholar]
- 19.Kamocka MM, Mu J, Chen N, Zollman A, Sturonas-Brown B, Dunn K, Xu Z, Chen DZ, Alber MS, Rosen ED. Two-photon intravital imaging of thrombus development. Journal of Biomedical Optics. 2010;15:0160201-016020-7. doi: 10.1117/1.3322676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita A, Furukoji E, Marutsuka K, Hatakeyama K, Yamamoto H, Tamura S, Ikeda Y, Sumiyoshi A, Asada Y. Increased vascular wall thrombogenicity conbined with reduced blood flow promotes occlusive thrombus formation in rabbit remoral artery. Arterioscler Thromb Vasc Biol. 2004;24:2420–2424. doi: 10.1161/01.ATV.0000147767.61336.de. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. Journal of Internal Medicine. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikanth S, Ambrose JA. Pathophysiology of coronary thrombus formation and adverse consequences of thrombus during PCI. Current Cardiology Reviews. 2012;8:168–176. doi: 10.2174/157340312803217247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronaty syndromes: the pathologists’ view. European Heart Journal. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 26.Lishko VK, Burke T, Ugarova TP. Anti-adhesive effect of fibrinogen: A safeguard for thrombus stability. Blood. 2007;109:1541–1549. doi: 10.1182/blood-2006-05-022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lishko VK, Yermolenko IS, Owaynat H, Ugarova TP. Fibrinogen counteracts the antiadhesive effect of fibrin-bound plasminogen by preventing its activation by adherent U937 monocytic cells. Journal of Thrombosis and Haemostasis. 2012;10:1081–1090. doi: 10.1111/j.1538-7836.2012.04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yermolenko IS, Fuhrmann A, Magonov SN, Lishko VK, Oshkaderov SP, Ros R, Ugarova TP. Origin of nonadhesive properties of fibrinogen matrices probed by force spectroscopy. Langmuir. 2010;26:17269–17277. doi: 10.1021/la101791r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yermolenko IS, Gorkun OV, Fuhrmann A, Podolnikova NP, Lishko VK, Oshkaderov SP, Lord ST, Ros R, Ugarova TP. The assembly of nonadhesive fibrinogen matrices depends on the αC regions of the fibrinogen molecule. J Biol Chem. 2012;287:41979–41990. doi: 10.1074/jbc.M112.410696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jirouskova M, Jaisawal JK, Coller BS. Ligand density dramatically affects integrin αIIbβ3-mediated platelet signaling and spreading. Blood. 2007;109:5260–5269. doi: 10.1182/blood-2006-10-054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui KY, Haber E, Matsueda GR. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983;222:1129–1132. doi: 10.1126/science.6648524. [DOI] [PubMed] [Google Scholar]
- 32.Runge MS, Bode C, Matsueda GR, Haber E. Antibody-enhanced thrombolysis: targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci USA. 1987;84:7659–7662. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno T, Sugimoto M, Matsui H, Hamada M, Shida Y, Yoshioka A. Visual evaluation of blood coagulation during mural thrombogenesis under high shear blood flow. Thromb Res. 2008;121:855–864. doi: 10.1016/j.thromres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Hattori A, Watanabe T, Izumi T. Scanning electron microscope study on hemostatic reaction. Mural thrombus after the removal of endothelium, with special references to platelet behavior, site of fibrin formation and microhemolysis. Arch Histol Jap. 1978;41:205–227. doi: 10.1679/aohc1950.41.205. [DOI] [PubMed] [Google Scholar]
- 35.Hatton MW, Ross B, Southward SM, Timleck-DeReske M, Richardson M. Platelet and fibrinogen turnover at the exposed subendothelium measured over 1 year after a balloon catheter de-endothelializing injury to the rabbit aorta: Thrombotic eruption at the late re-endothelializing stage. Atherosclerosis. 2002;165:57–67. doi: 10.1016/s0021-9150(02)00195-8. [DOI] [PubMed] [Google Scholar]
- 36.Steele PM, Chesebro JH, Stanson AW, Holmes DR, Dewanjee MK, Badimon L, Fuster V. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985;57:105–112. doi: 10.1161/01.res.57.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Groves HM, Kinlough-Rathbone RL, Richardson M, Moore S, Mustard JF. Platelet interaction with damaged rabbit aorta. Lab Invest. 1979;40:194–1979. [PubMed] [Google Scholar]
- 38.Kelly AB, Marzec UM, Krupski W, Bass A, Hanson SR, Harker LA. Hirudin interruption of heparin-resistant arterial thrombus formation in baboons. Blood. 1991;77:1006–1012. [PubMed] [Google Scholar]
- 39.Barstad RM, Kierulf P, Sakariassen KS. Collagen-induced thrombus formation at the apex of eccentric stenosis - a time course study with non-anticoagulated blood. Thromb Haemost. 1996;75:685–692. [PubMed] [Google Scholar]
- 40.Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, Bock PE, Watson SP, McCarty OJ, Heemskerk JW. Spatial distribution of Factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PloS ONE. 2010;5:10415. doi: 10.1371/journal.pone.0010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falati S, Cross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nature Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Mogami H, Murakami Y, Nakamura T, Kanayama N, Konno H, Urano T. Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflugers Arch -Eur J Physiol. 2008;456:1239–1251. doi: 10.1007/s00424-008-0466-9. [DOI] [PubMed] [Google Scholar]
- 43.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signalling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuijper PH, Gallardo Torres HI, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood. 1997;89:166–175. [PubMed] [Google Scholar]
- 45.Gersh KC, Edmundsonm KE, Weisel JW. Flow rate and fibrin fiber alignment. Journal of Thrombosis and Haemostasis. 2014;8:2826–2828. doi: 10.1111/j.1538-7836.2010.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandendries ER, Hamiltom JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation is a mouse model of thrombosis. Proc Natl Acad Sci USA. 2007;104:288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakariassen KS, Joss R, Muggli R, Kuhn H, Tschopp TB, Sage H, Baumgartner HR. Collagen type III induced ex vivo thrombogenesis in humans. Role of platelets and leukocytes in deposition of fibrin. Arteriosclerosis. 1990;10:276–284. doi: 10.1161/01.atv.10.2.276. [DOI] [PubMed] [Google Scholar]
- 48.Brass LF, Wannemacher KM, Ma P, Stalker TJ. Regulating thrombus growth and stability to achieve an optimal response to injury. J Thromb Haemost. 2011;9(Suppl 1):66–75. doi: 10.1111/j.1538-7836.2011.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meh DA, Siebenlist KR, Mosesson MW. Identification and characterization of the thrombin binidng sites on fibrin. J Biol Chem. 1996;271:23121–23125. doi: 10.1074/jbc.271.38.23121. [DOI] [PubMed] [Google Scholar]
- 50.Fredenburgh JC, Stafford AR, Leslie BA, Weitz JI. Bivalent binding to gamma A/gamma’-fibrin engages both exosites of thrombin and protects it from inhibition by the antithrombin-heparin complex. J Biol Chem. 2008;283:2470–2477. doi: 10.1074/jbc.M707710200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.