Abstract
Venous thromboembolism (VTE) is a preventable disease, yet it is one of the leading causes of death among patients with cancer. Improving risk stratification mechanisms will allow us to personalize thromboprophylaxis strategies. We sought to evaluate Collagen and Thrombin Activated Platelets (COAT-platelets) as well as protein C and factor VIII as biomarkers predictive of cancer-associated thrombosis in a prospective cohort of patients with cancer. Protein C was selected as a candidate based on bioinformatics prediction. Blood samples were collected before chemotherapy. All specimen processing was blinded to clinical data. Surveillance and adjudication of the main outcome of VTE was performed for up to 1 year. We used Cox proportional hazard regression to measure the association of biomarkers and incident events using SAS 9.2 for all statistical analysis. Death was modeled as a competing event. Among 241 patients followed for an average of 10.4 months, 15% died and 13% developed a VTE. COAT-platelets were not predictive of VTE. Low levels of pre-chemotherapy protein C (< 118 %) (HR 2.5; 95%CI 1.1–5.5) and high baseline factor VIII (> 261 % I) (HR 3.0; 95%CI 1.1–8.0) were predictive of VTE after adjusting for age, Khorana prediction risk, metastatic disease and D dimer. In addition, low protein C was predictive of overall mortality independent of age, metastatic disease and functional status (HR 2.8; 95%CI 1.3–6.0). Addition of these biomarkers to Cancer-VTE risk prediction models may add to risk stratification and patient selection to optimize thrombo-prophylaxis.
Keywords: Cancer, Venous Thromboembolism, Biomarkers, Bioinformatics
Introduction
One of the most important causes of death among patients with cancer is a preventable disease, venous thromboembolism (VTE) [1]. Thromboembolism is second only to cancer progression as a cause of death of outpatients with active malignancies. VTE is 5 to 6 times more likely among patients with cancer [2]. Moreover, cancer-associated thrombosis accounts for about 20% of the entire VTE burden. Patients with cancer who develop VTE have a 2-fold increase in mortality when compared to cancer patients without VTE; this remains true even after adjusting for stage of malignancy [3, 4]. In addition, cancer-associated VTE is also associated with increased therapy-associated bleeding and high healthcare cost burden [5–7]. Thromboprophylaxis is likely efficient in preventing cancer-associated thrombosis [8]. However, in order to better personalize preventive mechanisms and avoid unwarranted side effects, improved risk stratification tools are warranted.
There is a growing body of evidence on platelets as mediators of cancer-associated thrombosis and outcomes [9]. Alberio et al. [10] described a subset of platelets with a high level of factor V bound to their surface after stimulation with both thrombin and a collagen receptor agonist. These platelets are called COAT (Collagen And Thrombin) and they been extensively linked with arterial events in patients without cancer [11, 12]. COAT-platelets were recently determined to be predictive of arterial thrombosis [13].
To identify potential biomarkers for VTEs, we used a bioinformatics method called GAMMA [15], which uses an analysis of over 16,000 human microarray experiments to identify general correlations among genes. Paired with literature-mining software called IRIDESCENT [14], function, phenotype and disease relevance can be predicted for genes, regardless of whether or not the gene has appeared in any publications, via analysis of genes that are highly and consistently correlated with it in their own expression levels across a heterogeneous set of experiments. Protein C was selected from a list of potential biomarkers output by the GAMMA analysis as predicted to be associated with VTE risk. To date, GAMMA has been used successfully to validate phenotypes and disease relevance for several previously uncharacterized or poorly characterized genes [15–19]. While protein C is known to decrease after chemotherapy [20], there is paucity of prospective data measuring the value of this biomarker as a predictor of cancer-associated thrombosis. Protein C measurement may be confounded by factor VIII levels [21]. Levels of factor VIII are significantly higher among cancer patients with thrombosis than in those without thrombosis [22]. Moreover, in prospective trials, a high factor VIII level is predictive of cancer-associated thrombosis [23].
We aimed to prospectively evaluate COAT, protein C, factor VIII as predictors of cancer-associated thrombosis and to determine if these biomarkers add independent information beyond standard VTE risk factors.
POPULATION AND METHODS
We designed a phase 3 biomarker development PRoBE (Prospective sample collection, retrospective blinded evaluation) study [24–26]. We prospectively enrolled eligible patients ≥ 18 years old with solid tumors of any type or stage scheduled to receive chemotherapy. All patients were recruited at the University of Oklahoma Health Sciences Center. The enrolment was not consecutive. Patients were excluded if they were unable to sign informed consent, were receiving any anticoagulant therapy, pregnant, or hospitalized for trauma or major surgery within 2 weeks before the initial blood sample collection. We anticipated that the aforementioned clinical scenarios for exclusion would alter the baseline biomarker results adding to potential confounding. Patients with leukemia, lymphoma, multiple myeloma, other hematopoietic primary were not included. Patients with skin cancers were also excluded. The study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board.
Baseline information was obtained via direct interview and confirmed by information from the electronic medical records. We collected demographic variables, clinical data, and cancer-specific variables including tumor type, presence of known metastatic disease, performance status and initial chemotherapy regimen. Type of cancer was grouped for analysis as by the Khorana score where patients with gastric or pancreatic cancers are considered [27].
Outcome measures
Outcome measures were defined a priori and blindly adjudicated via mutual agreement by 2 of the co-authors. The primary outcome measure was VTE, including symptomatic or incidentally found deep – vein thrombosis (DVT) of lower or upper limbs, pulmonary embolism (PE) and visceral venous thrombosis. Deep-vein thrombosis, including visceral thrombosis, was positive if imaged on CT scan, MRI, venogram or duplex ultrasound. PE was defined as positive in the presence of a high probability ventilation/perfusion scan, CT angiogram or a positive pulmonary angiogram. Objective documentation of VTE was searched every 3 months and the patients were followed for a maximum duration of 12 months. All patients were also followed and adjudicated for mortality. In addition, if there was no documentation of an event in the electronic medical records the patient or relative was contacted to confirm or exclude the outcomes of interest and obtain supporting documentation.
Blood sample collection and analysis
Blood samples were collected at the same time as the scheduled pre-chemotherapy laboratory evaluation including complete blood count. A complete methodology for the coated-platelet assay has been previously published [28, 29]. Briefly, 5 ml of blood was drawn into a plastic syringe containing 0.5 ml of acid citrate dextrose (ACD) and platelet rich plasma (PRP) was prepared. Coated-platelets were assayed with 1 μL of PRP in a 100 μL assay with the following reagents (final concentrations): 1.0 μL/mL biotin-fibrinogen, 0.4 mM gly-pro-arg-pro-amide, 500 ng/ml convulxin, 0.5 U/ml bovine thrombin, 2 mM CaCl2, 1 mM MgCl2, 150 mM NaCl and 10 mM HEPES, with pH 7.5. After 5 min at 37°C, 0.8 μg of phycoerythrin-streptavidin and 0.5 μg of FITC-abciximab were added. After an additional 5 min at 37°C, the reaction was stopped with 0.2 ml of 1.5% (w/v) formalin in 150 mM NaCl, 10 mM HEPES, pH 7.5. The percentage of abciximab-positive events (platelets) with bound biotin-fibrinogen was quantitated by flow cytometry. Results are reported as the percent of cells converted to coated-platelets. For Protein C (STA® – Staclot® Protein C kit) and Factor VIII (STA® – Deficient VIII kit) two 5 cc blood draws were collected in 0.109 M (i.e., 3.2 %) trisodium citrate anticoagulant (9:1 vol.). The samples were subsequently centrifuged for 15 minutes at 2000–2500g as per the standard suggestions of the assay manufacturers. The obtained plasma was separated into aliquots and centrally stored within 4 hours at −70 °C. Since D dimer (STA® – Liatest® D-Di kit) is known to be independently associated with cancer-associated thrombosis, baseline samples were also measured to control for potential confounding. The samples were centrally analyzed in batches according to the manufacturer (Diagnostica-Stago Inc.) instructions and reported as percent activity levels [21, 30]. In brief, equipment was calibrated prior to testing, the plasma was tested undiluted and Owren-Koller buffer, we used an automatic compatible analyzer. Quality control runs were done to ensure accuracy. All laboratory analysis was blinded to outcome and clinical data.
Sample size calculations
We estimated a required sample size of 200 patients, among whom we expected to observe 40 VTE events after 6 months of follow-up. This required sample size resulted in 80% power to detect a hazard ratio of 2.5 associated with a “high” biomarker level (above the median) assuming that at 6 months, 12% of subjects with a “low” biomarker level have developed a VTE and 27% with a “high” biomarker level will have developed a VTE. This calculation also assumed a 2-sided 0.05 alpha level. Sample size calculations were performed using PASS 2008 (Hintze, J. 2008. PASS 2008. NCSS, LLC. Kaysville, Utah). Due to low initial recruitment, the follow up period was extended to up to 1 year.
Statistical analysis
Descriptive statistics and plots were created to summarize the distribution of baseline biomarker levels, clinical parameters, cancer diagnosis information, medications and therapy, and comorbidities. Continuous measures, including the proposed biomarker levels, were categorized into equally spaced intervals to explore the assumption that the log hazard of VTE was a linear function of the biomarker level [24–26, 31, 32]. The associations between the proposed biomarker levels and the standard risk factors for VTE (type of cancer, metastatic disease, Khorana risk score [27], BMI, age, gender, type of chemotherapy agent, antiplatelet agent, D dimer) were investigated using a log-rank test, comparing the time-to-VTE distributions between independent groups defined by the independent factors. Kaplan-Meier curves were then created to summarize the distributions of the time to VTE [33]. The cut off value for D dimer was 1.44 μg/mL as previously validated [34]. Time-to-event distributions were compared among groups using a log-rank test. All time to event outcomes were estimated accounting for competing risk of mortality [35] and censored for last point of contact. The association between each biomarker measure and the hazard of VTE was examined using Cox proportional hazards regression modelling with the biomarker measure. All-cause mortality was studied as a secondary outcome. The biomarker categories were collapsed to better satisfy modelling assumptions when indicated by residual plots or data sparseness. For variables with no events, a Fisher’s exact test was used to compare the proportion of events between independent groups. Multivariate approaches were limited given sample size. For all statistical analyses we used SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A p value of <0.05 was considered as statistically significant. JS and AT were responsible for the statistical analysis.
RESULTS
We recruited 252 eligible outpatients with solid organ tumors; three samples were not pre-chemotherapy, one patient had a thrombosis between recruitment and sample acquisition, and seven samples hemolysis. As per protocol, none of the samples were collected within 2 weeks of cancer related surgery. Thus 241 patients were followed for an average of 10.4 mo (SD 3.2) and analyzed for the primary outcome. The patients were 60 years-old on average and predominantly white females (Table 1). Most of the patients had a gynecological (33%), breast (21%) or pancreas (12%) tumor and 32% of the cohort had metastatic disease at recruitment. Based on the Khorana prediction score, the likelihood of VTE was intermediate to high in 77% of our cohort. There were no patients lost to follow up, 37 patients died (15%) and 31 (13%) developed a VTE. The thrombotic events were 16 (52%) in upper extremity (including superior vena cava extension n=2), 8 (26%) pulmonary embolisms, 5 (16%) in lower extremity and 2 (6%) organ related. Protein C data were unavailable on 7 (3%) patients, and 14 (6%) patients did not have factor VIII values, and COAT-platelet percentage was not measured on 42 (17%) patients. The median (Inter quartile range IQR) values for the biomarkers were: protein C 137.5 % (118–164); factor VIII 197% (151–261) and COAT-platelets 37.8% (30.8–46.2).
Table 1.
Baseline Cohort Characteristics (N=241)
| Variable | Count or Median | % or IQR |
|---|---|---|
| *Age (years) (Median, IQR) | 60 | 53–68 |
| Female | 173 | 72 |
| White Race | 196 | 82 |
| BMI (Median, IQR) Kg/m2 | 28.0 | 24.2–35.0 |
| Hypertension | 137 | 57 |
| Diabetes Mellitus | 38 | 16 |
| History of TIA/Stroke | 23 | 10 |
| Statin | 50 | 21 |
| ACEi ARB | 83 | 34 |
| Aspirin | 42 | 17 |
| Gemcitabine | 28 | 12 |
| Bevacizumab | 16 | 7 |
| Platinum | 163 | 68 |
| Cancer Type by Thrombosis Risk | ||
| - Very High risk | 33 | 14 |
| - High risk | 99 | 41 |
| - Other | 109 | 45 |
| ECOG ≥ 2 | 20 | 8 |
| Metastatic | 78 | 32 |
| Khorana score | ||
| - High | 32 | 13 |
| - Intermediate | 154 | 64 |
| - Low | 55 | 23 |
Distribution of baseline cohort variables. Quantitative data presented as median and interquartile range (IQR 25th percentile–75th percentile). ACEi = Angiotensin converting enzyme inhibitor. ARB = Angiotensin receptor blocker. BMI = body mass index. TIA = transient ischemic attack. ECOG = Eastern Cooperative Oncology Group Performance Score
In the univariate analysis, VTE was more likely among (Hazard Ratio [HR], 95% Confidence Interval [95%CI]) those who received bevacizumab at baseline (HR 3.4; 95%CI 1.3–9) and those patients with a high risk Khorana score (HR 2.6; 95%CI 1.1–6.2). Females were less likely to develop cancer-associated VTE (HR 0.4; 95%CI 0.2–0.9). COAT-platelets percentage at baseline did not predict cancer-associated thrombosis in our cohort (Table 2). There were 26 patients who had surgery within 1 month prior to recruitment and only 2 of them developed an adjudicated event. One event was a right atrial thrombus detected 49 days after the blood draw and the other one was a PE diagnosed 35 days after sample collection. The levels of protein C (128.1 + 27 vs. 142.7 + 37; p=0.12) and factor VIII (214.2 ± 63 vs. 211.7 ± 91; p=0.48) were not different among those who had surgery within 1 month of recruitment versus those who did not. There was no interaction for factor VIII (p=0.99) or for protein C (p=0.30).
Table 2.
Venous Thromboembolism Hazard Ratios. Univariate analysis
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Age (>68 years) | 0.9 | 0.4–2.0 | 0.74 |
| Female | 0.4 | 0.2–0.9 | 0.03 |
| White Race | 1.2 | 0.4–3.5 | 0.7 |
| BMI > 30 Kg/m2 | 0.9 | 0.4–2.3 | 0.92 |
| Hypertension | 0.6 | 0.3–1.4 | 0.26 |
| Diabetes Mellitus | 0.6 | 0.2–2.2 | 0.5 |
| History of TIA/Stroke | 0.6 | 0.08–4.5 | 0.62 |
| Statin | 1 | 0.4–2.6 | 0.95 |
| Aspirin | 0.5 | 0.1–1.9 | 0.28 |
| ACEi ARB | 0.5 | 0.2–1.3 | 0.13 |
| Gemcitabine | 1.7 | 0.6–4.9 | 0.33 |
| Bevacizumab | 3.4 | 1.3–9.0 | 0.02 |
| Platinum | 1.3 | 0.5–3.2 | 0.60 |
| Cancer Type by Thrombosis Risk | |||
| - Very High risk | 2.7 | 0.9–7.7 | 0.07 |
| - High risk | 1.3 | 0.5–3.3 | 0.52 |
| - Other | REF | ||
| - Very high vs not | 2.2 | 0.9–5.7 | 0.07 |
| ECOG ≥ 2 | 2.1 | 0.6–6.9 | 0.24 |
| Metastatic | 1.9 | 0.9–4.2 | 0.10 |
| Khorana score | |||
| - High | 2.2 | 0.7–6.8 | 0.19 |
| - Intermediate | 0.8 | 0.3–2.2 | 0.65 |
| - Low | 1 | REF | |
| - High vs not | 2.6 | 1.1–6.2 | 0.034 |
Age cut-off for categorized analysis was 75th percentile. ACEi = Angiotensin converting enzyme inhibitor. ARB = Angiotensin receptor blocker. BMI = body mass index. TIA = transient ischemic attack. ECOG = Eastern Cooperative Oncology Group Performance Score
Patients with protein C levels less than or equal to 118% (lower quartile) were almost 3 times as likely to develop a thrombotic event (HR 2.9; 95%CI 1.3–6.2). Patients with cancer and a baseline factor VIII level over 261 % were also 3 times as likely to develop VTE (HR 3.16; 95%CI 1.4–7) (Figures 1 and 2). Both low protein C (HR 2.3; 95%CI 1.0–5.4) and high factor VIII (HR 3.6; 95%CI 1.6–8) remained associated with new thrombotic events after adjusting for gender. Similarly, patients with protein C less than or equal to 118 % (HR 2.8; 95%CI 1.3–6.2) and those with baseline factor VIII levels over 261 % (HR 2.8; 95%CI 1.3–6.3) were more likely to develop VTE after controlling for administration of bevacizumab, an agent associated with thrombosis. Multivariate evaluation controlling for cancer-specific risk factors is presented on Table 3. In a model adjusting for age, Khorana score, metastatic disease D dimer and both protein C (HR 2.5; 95%CI 1.1–5.5) and factor VIII (HR 3.0; 95%CI 1.1–8.0) remained significantly associated with new cancer associate thrombosis.
Figure 1. Protein C and Cancer-associated Venous thromboembolism.
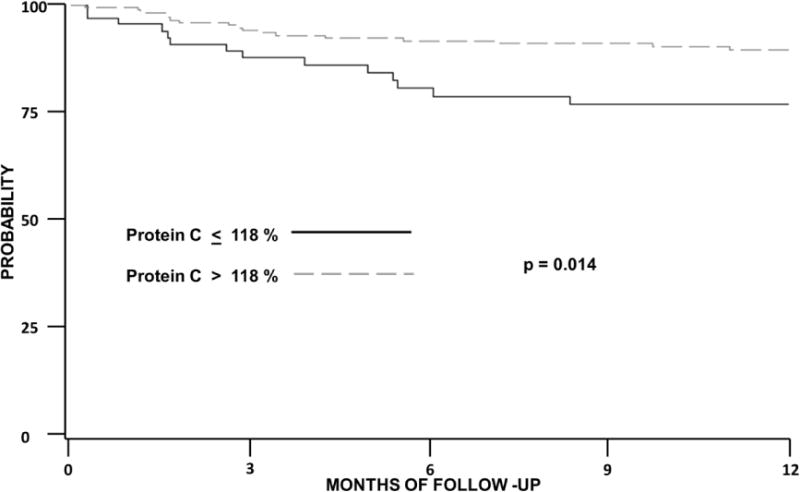
Kaplan Meier curve of the probability of thrombosis-free survival among patients with cancer. Log rank used for statistical testing.
Figure 2. Factor VIII and Cancer-associated Venous Thromboembolism.
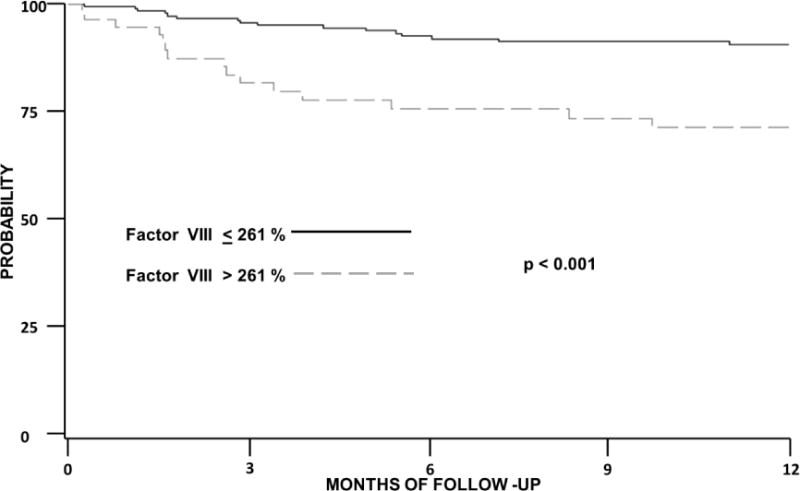
Kaplan Meier curve of the probability of thrombosis free survival among patients with cancer. Log rank used for statistical testing. Factor VIII is presented as % of activity.
Table 3.
Hazard Ratio of venous thromboembolism
| Low Protein C % activity | High Factor VIII % activity | |||
|---|---|---|---|---|
| Model | HR | 95% CI | HR | 95% CI |
| Model 1 | 2.8 | 1.3–6.2 | 3.6 | 1.4–8.7 |
| Model 2 | 2.6 | 1.2–5.9 | 2.9 | 1.3–6.6 |
| Model 3 | 2.8 | 1.2–6.1 | 2.9 | 1.2–6.8 |
| Model 4 | 2.5 | 1.1–5.5 | 3.0 | 1.1–8.0 |
Model 1= Adjusted for elevated D Dimer (> 1.44 ug/mL), Model 2= Adjusted for Metastatic disease, Model 3= Adjusted for Khorana score, Model 4 Adjusted for Khorana score, D Dimer, metastatic disease, Protein C, Factor VIII.
Among 209 patients with a low to intermediate Khorana risk score, there were 54 (26%) patients with low protein C and 169 (81%) with high factor VIII levels. Within this subgroup, patients with low protein C (HR 3.3; 95%CI 1.3–8.2) and patients with high factor VIII (HR 3.4; 95%CI 1.3–8.7) were 3 times as likely to develop a VTE.
We analyzed the impact of protein C and factor VIII in all-cause mortality. Patients with low levels of protein C were more likely to die (HR 3.4; 95%CI 1.6–6.9). Our findings remained statistically significant after controlling for age, ECOG status and metastatic disease at inclusion (HR 2.8; 95%CI 1.3–6.0). Although high factor VIII levels were associated with mortality in the univariate analysis (HR 2.5; 95%CI 1.2–5.2), the effect was not statistically significant when controlled for confounders.
DISCUSSION
In our prospectively collected cohort of 241 patients with solid tumors, low protein C levels were predictive of cancer-associated thrombosis, as predicted by our bioinformatics analysis. These results were independent of factor VIII levels, which were also independently predictive of thrombotic events. After adjusting for cancer-specific risk factors of thrombosis, patients whose levels of protein C 118% or lower, and the patients with pre-chemotherapy levels of factor VIII levels above 261 % were over two times more likely to develop cancer-associated thrombosis. These results were replicated on a stratum of patients with low to intermediate Khorana risk score. The association of low protein C with cancer thrombosis was independent of factor VIII and D dimer levels. Levels of COAT-platelets were not associated with cancer-associated VTE in our cohort.
Bioinformatics have been successfully used in the discovery of prognostic and diagnostic biomarkers of patients with cancer [36, 37]. There is however paucity of data validating the use of bioinformatics in the prediction of cancer-associated thrombosis. To our knowledge, this is the first publication providing evidence of pretreatment protein C levels as an independent predictor of cancer-associated thrombosis and mortality. The most widely validated construct to predict cancer-associated thrombosis is the Khorana score which uses a combination of clinical and laboratory characteristics (Cancer type, white blood cells, BMI, hemoglobin/erythropoiesis stimulating agent, platelet count) to predict VTE [27]. The score has gained acceptance to stratify the likelihood of cancer-associated thrombosis [38]. Pre chemotherapy levels of both protein C and factor VIII provided additional prediction value in addition to the risk prediction score. Pending external validation of our results, these biomarkers may be used to further improve VTE risk prediction among patients with intermediate Khorana scores.
While congenital protein C deficiency is well known as a risk factor of venous thromboembolism [39], the levels and age of our patients were not suggestive of the inclusion of subjects with congenital disease. In addition, some chemotherapies agents may lower protein C levels, and potentially exacerbate the thrombotic likelihood [20, 40, 41]. We did not measure post-chemotherapy levels of protein C, thus this change is speculative in our cohort. Bevacizumab [42] is strongly associated with increased VTE likelihood, yet the predictive effect of protein C levels persisted after adjusting for its administration. Thus we do not think that the prediction of VTE was confounded by specific chemotherapy administration. High factor VIII activity increases the risk of primary and recurrent VTE [23, 43]. Moreover, in a cohort of 840 patients with cancer, including hematological malignancies, with2 years of follow up, high factor VIII levels were associated with new VTE events [23]. Factor VIII activity above 232% was chosen as the cut off value and was associated with a hazard ratio of VTE of 2.8 (95% CI 1.7–4.6). Low protein C, but not high factor VIII, was associated with higher mortality even after controlling for potential confounders. Besides controlling thrombosis, protein C has additional mechanisms of action that modulate inflammatory response and endothelial apoptosis [39]. These mechanisms are plausibly associated with the predictive value on mortality for population of patients with cancer. Moreover, there are emerging data linking the protein C pathway and malignancy progression via cancer cell extravasation and immune mediated cancer cell elimination [44–46]. The survival effect of protein C levels is not likely to be due only to fatal VTE.
There is a growing body of evidence on the importance of platelets in incident venous thromboembolism among patients with cancer. In the Vienna CATS group, a cohort of 665 prospectively followed patients with cancer, a platelet level above 443,000 (95th percentile) was associated with a 25% incidence rate of venous thromboembolism [47]. The Khorana Score uses a platelet level cut off value of 350,000 as an indicator of high likelihood of thrombosis [27]. High mean platelet volume has been recently associated with decreased risk of VTE [48]. Platelets however, are heterogeneous and some subtypes hold a stronger thrombotic capacity. Indeed, in our study higher levels of COAT-platelets were not predictive of cancer-associated thrombosis. Although platelets are relatively uniformly distributed in the hemostatic plugs due to vessel injury, super-activated platelets are preferentially located near the collagen exposure sites [49]. The mechanism of activation of COAT-platelets is probably not prevalent in patients with cancer. Our findings contrast with the value of this platelet subset in the prediction of stroke in a population of 329 patients with carotid disease [13]. Our findings highlight the specificity of platelet subsets in different thrombotic events.
The main strength of our study is that we specifically designed it for evaluation of novel biomarkers. All laboratory analyses were blinded to the clinic and outcome data and no patients were lost to follow up. Our study however is relatively small and we are not able to present a validation cohort of our findings. In addition, our report is based on single center demographics and our institution has a higher prevalence of gynecological malignancies, which limits external validity. Of note, we did not systematically screen for VTE at baseline. Although we reached our sample size, all multivariate analysis should be considered exploratory since we only observed 31 patients who developed VTE events. Our VTE incidence of 13% for a cohort of cancer patients was higher than the 1–8% reported in epidemiological series [50]. This is result is plausible given its representation of a patient population at a single tertiary center and it is not a standardized population of patients with cancer. While we externally validate the use of factor VIII as a predictor of cancer-associated thrombosis, we believe that protein C levels need external validation before being able to apply this biomarker to alternative cancer thrombosis risk prediction models.
In summary, as predicted by a bioinformatics model, pre-chemotherapy protein C was predictive of cancer-associated thrombosis. In addition, we validate high factor VIII levels as an independent predictor of VTE in patients with solid organ cancer. COAT-platelets were not predictive of VTE in our cohort. Finally, low protein C levels were also a predictor of all-cause mortality among patients with cancer. Our findings will add to the risk stratification of cancer-associated thrombosis, which needs to be considered in future preventive trials.
Highlights.
We have validated a bioinformatics based selection process for biomarker discovery in cancer-associated thrombosis.
Patients with low levels of pre-chemotherapy protein C had higher likelihood of cancer-associated thrombosis and all-cause mortality.
Patients with high factor VIII at baseline had higher likelihood of cancer-associated thrombosis
Acknowledgments
Partial funding provided by National Institutes of Health, National Institute of General Medical Sciences [grant 1 U54GM104938; JAS, JW] and research grant by Stago Diagnostica (AT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Addendum, Authors participation:
Alfonso Tafur. Design, Data analysis, Data interpretation, Manuscript preparation
Mohamad Cherry. Data interpretation, Manuscript preparation
Jonathan Wren: Design, Manuscript preparation
Aaron Mansfield. Data interpretation, Manuscript preparation
Philip Comp. Data interpretation, Manuscript preparation
Suman Rathbun Design, Manuscript preparation
Julie Stoner. Design, Data analysis, Data interpretation, Manuscript preparation
References
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of thrombosis and haemostasis: JTH. 2007;5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of internal medicine. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 4.Kourelis TV, Wysokinska EM, Wang Y, Yang P, Mansfield AS, Tafur AJ. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer. 2014;86:358–62. doi: 10.1016/j.lungcan.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tafur AJ, Wysokinski WE, McBane RD, Wolny E, Sutkowska E, Litin SC, Daniels PR, Slusser JP, Hodge DO, Heit JA. Cancer effect on periprocedural thromboembolism and bleeding in anticoagulated patients. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2012;23:1998–2005. doi: 10.1093/annonc/mds058. [DOI] [PubMed] [Google Scholar]
- 6.Kakkar A, Kessler C, Goldhaber S, Kovacs M, Spyropoulos A, Ortel T, Pabinger I, Huisman M, Bergqvist D, Monreal M, Turpie G, Francis CW. Dalteparin sodium for the long-term management of venous thromboembolism in cancer patients. In: ISTH, editor. the Daltecan study. 2013. p. AS 12.3. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Dalal MR, Lin J, Connolly GC. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res. 5:101–8. doi: 10.2147/CEOR.S39964ceor-5-101. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan M, John S, Casanegra AI, Rathbun S, Mansfield A, Stoner JA, Tafur AJ. Primary venous thromboembolism prophylaxis in patients with solid tumors: a meta-analysis. Journal of thrombosis and thrombolysis. 2014;38:241–9. doi: 10.1007/s11239-013-1014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riedl J, Pabinger I, Ay C. Platelets in cancer and thrombosis. Hamostaseologie. 34:54–62. doi: 10.5482/HAMO-13-100054. 13-10-0054 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–702. [PubMed] [Google Scholar]
- 11.Kirkpatrick AC, Stoner JA, Dale GL, Prodan CI. Elevated coated-platelets in symptomatic large-artery stenosis patients are associated with early stroke recurrence. Platelets. 25:93–6. doi: 10.3109/09537104.2013.775570. [DOI] [PubMed] [Google Scholar]
- 12.Prodan CI, Dale GL. Coated-platelets in ischemic stroke – potential insight into the etiology of stroke subtypes. International journal of stroke: official journal of the International Stroke Society. 2008;3:249–50. doi: 10.1111/j.1747-4949.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick AC, Tafur AJ, Vincent AS, Dale GL, Prodan CI. Coated-platelets improve prediction of stroke and transient ischemic attack in asymptomatic internal carotid artery stenosis. Stroke; a journal of cerebral circulation. 2014;45:2995–3001. doi: 10.1161/STROKEAHA.114.006492. [DOI] [PubMed] [Google Scholar]
- 14.Wren JD, Bekeredjian R, Stewart JA, Shohet RV, Garner HR. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics. 2004;20:389–98. doi: 10.1093/bioinformatics/btg421. [DOI] [PubMed] [Google Scholar]
- 15.Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, Gorbsky GJ. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Current biology: CB. 2009;19:1467–72. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupu C, Zhu H, Popescu NI, Wren JD, Lupu F. Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood. 2011;118:4463–71. doi: 10.1182/blood-2011-05-355370. blood-2011-05-355370 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemmensen SN, Bohr CT, Rorvig S, Glenthoj A, Mora-Jensen H, Cramer EP, Jacobsen LC, Larsen MT, Cowland JB, Tanassi JT, Heegaard NH, Wren JD, Silahtaroglu AN, Borregaard N. Olfactomedin 4 defines a subset of human neutrophils. Journal of leukocyte biology. 2012;91:495–500. doi: 10.1189/jlb.0811417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towner RA, Jensen RL, Colman H, Vaillant B, Smith N, Casteel R, Saunders D, Gillespie DL, Silasi-Mansat R, Lupu F, Giles CB, Wren JD. ELTD1, a potential new biomarker for gliomas. Neurosurgery. 2013;72:77–90. doi: 10.1227/NEU.0b013e318276b29d. discussion 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhapudi SK, Tan C, Marino JH, Taylor AA, Pack CC, Gaikwad J, Van De Wiele CJ, Wren JD, Teague TK. IL-18 Acts in Synergy with IL-7 To Promote Ex Vivo Expansion of T Lymphoid Progenitor Cells. J Immunol. 2015;194:3820–8. doi: 10.4049/jimmunol.1301542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers JS, 2nd, Murgo AJ, Fontana JA, Raich PC. Chemotherapy for breast cancer decreases plasma protein C and protein S. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1988;6:276–81. doi: 10.1200/JCO.1988.6.2.276. [DOI] [PubMed] [Google Scholar]
- 21.Baron JM, Johnson SM, Ledford-Kraemer MR, Hayward CP, Meijer P, Van Cott EM. Protein C assay performance: an analysis of North American specialized coagulation laboratory association proficiency testing results. American journal of clinical pathology. 2012;137:909–15. doi: 10.1309/AJCP8MWU4QSTCLPU. [DOI] [PubMed] [Google Scholar]
- 22.Dogan M, Demirkazik A, Konuk N, Yalcin B, Buyukcelik A, Utkan G, Tek I, Akbulut H, Sencan O, Icli F. The effect of venous thromboembolism on survival of cancer patients and its relationship with serum levels of factor VIII and vascular endothelial growth factor: a prospective matched-paired study. Int J Biol Markers. 2006;21:206–10. [PubMed] [Google Scholar]
- 23.Vormittag R, Simanek R, Ay C, Dunkler D, Quehenberger P, Marosi C, Zielinski C, Pabinger I. High factor VIII levels independently predict venous thromboembolism in cancer patients: the cancer and thrombosis study. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:2176–81. doi: 10.1161/ATVBAHA.109.190827. [DOI] [PubMed] [Google Scholar]
- 24.Pepe MS, Feng Z. Improving biomarker identification with better designs and reporting. Clinical chemistry. 2011;57:1093–5. doi: 10.1373/clinchem.2011.164657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC medicine. 2012;10:51. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost. 2005;3:2185–92. doi: 10.1111/j.1538-7836.2005.01274.x. JTH1274 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Prodan CI, Szasz R, Vincent AS, Ross ED, Dale GL. Coated-platelets retain amyloid precursor protein on their surface. Platelets. 2006;17:56–60. doi: 10.1080/09537100500181913. [DOI] [PubMed] [Google Scholar]
- 30.Guglielmone HA, Vides MA. A novel functional assay of protein C in human plasma and its comparison with amidolytic and anticoagulant assays. Thrombosis and haemostasis. 1992;67:46–9. [PubMed] [Google Scholar]
- 31.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. Journal of the National Cancer Institute. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 32.Grund B, Sabin C. Analysis of biomarker data: logs, odds ratios, and receiver operating characteristic curves. Current opinion in HIV and AIDS. 2010;5:473–9. doi: 10.1097/COH.0b013e32833ed742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O [pii]. [DOI] [PubMed] [Google Scholar]
- 34.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 35.Ay C, Posch F, Kaider A, Zielinski C, Pabinger I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. Journal of thrombosis and haemostasis: JTH. 2015;13:390–7. doi: 10.1111/jth.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remo A, Simeone I, Pancione M, Parcesepe P, Finetti P, Cerulo L, Bensmail H, Birnbaum D, Van Laere SJ, Colantuoni V, Bonetti F, Bertucci F, Manfrin E, Ceccarelli M. Systems biology analysis reveals NFAT5 as a novel biomarker and master regulator of inflammatory breast cancer. Journal of translational medicine. 2015;13:138. doi: 10.1186/s12967-015-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towner RA, Wren JD. Prioritizing uncharacterized genes in the search for glioma biomarkers. CNS oncology. 2014;3:93–5. doi: 10.2217/cns.14.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:654–6. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clouse LH, Comp PC. The regulation of hemostasis: the protein C system. The New England journal of medicine. 1986;314:1298–304. doi: 10.1056/NEJM198605153142006. [DOI] [PubMed] [Google Scholar]
- 40.Pectasides D, Tsavdaridis D, Aggouridaki C, Tsavdaridou V, Visvikis A, Tsatalas K, Fountzilas G. Effects on blood coagulation of adjuvant CNF (cyclophosphamide, novantrone, 5-fluorouracil) chemotherapy in stage II breast cancer patients. Anticancer research. 1999;19:3521–6. [PubMed] [Google Scholar]
- 41.von Tempelhoff GF, Dietrich M, Hommel G, Heilmann L. Blood coagulation during adjuvant epirubicin/cyclophosphamide chemotherapy in patients with primary operable breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1996;14:2560–8. doi: 10.1200/JCO.1996.14.9.2560. [DOI] [PubMed] [Google Scholar]
- 42.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. Jama. 2008;300:2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 43.Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–5. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 44.van Sluis GL, Bruggemann LW, Esmon CT, Kamphuisen PW, Richel DJ, Buller HR, van Noorden CJ, Spek CA. Endogenous activated protein C is essential for immune-mediated cancer cell elimination from the circulation. Cancer letters. 2011;306:106–10. doi: 10.1016/j.canlet.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, Van Noorden CJ, Spek CA. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–73. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spek CA, Arruda VR. The protein C pathway in cancer metastasis. Thrombosis research. 2012;129(Suppl 1):S80–4. doi: 10.1016/S0049-3848(12)70022-1. [DOI] [PubMed] [Google Scholar]
- 47.Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J Thromb Haemost. 2010;8:114–20. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 48.Riedl J, Kaider A, Reitter EM, Marosi C, Jager U, Schwarzinger I, Zielinski C, Pabinger I, Ay C. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS) Thromb Haemost. 111 doi: 10.1160/TH13-07-0603. 13-07-0603 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Mazepa M, Hoffman M, Monroe D. Superactivated platelets: thrombus regulators, thrombin generators, and potential clinical targets. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1747–52. doi: 10.1161/ATVBAHA.113.301790. [DOI] [PubMed] [Google Scholar]
- 50.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]


