Abstract
The breast cancer susceptibility gene protein, also known as γ-synuclein, is highly expressed in human breast cancer in a stage-specific manner, with highest expression in late stage cancer. In model systems, γ-synuclein binds phospholipase Cβ2 which is regulated by Gαq to generate intracellular Ca2+ signals. PLCβ2, which is also absent in normal tissue but highly expressed in breast cancer, is additionally regulated by Rac to promote migration pathways. We have found that γ-synuclein binds to the same region of PLCβ2 as Gαq. Using cells that mimic stage 4 breast cancer (MDA MB 231), we show that down-regulation of γ-synuclein reduces the protein level of PLCβ but increases the transcript level over 40 fold. γ-Synuclein down-regulation also promotes the interaction between Gαq and PLCβ resulting in a stronger Ca2+ response to Gαq agonists. The ability of γ-synuclein to interfere with Gαq-PLCβ interactions allows more PLCβ to colocalize with Rac impacting Rac-mediated pathways that may give rise to cancerous phenotypes.
Keywords: γ-synuclein, phospholipase Cβ2, calcium signaling, cell morphology, cell migration
1. INTRODUCTION
Synucleins are a family of small, intrinsically disordered proteins, consisting of three members: α, β and γ (for review see [1]). The synucleins share a conserved N-terminal domain but each member possesses a distinct C-terminal region. Synucleins are predominantly expressed in neuronal tissues, where they have been implicated in neurotransmitter homeostasis and release. However, their exact function remains unknown (for review see [1, 2])
γ-Synuclein is a 127 amino acid protein and possesses a shorter and slightly negative C-terminal domain that distinguishes it from other synucleins [3]. In contrast to the many studies involving α-synuclein which plays a key role in Parkinson’s disease, little research has been done on γ-synuclein. However, mouse studies of γ-synuclein have been carried out and these show that knocking-out expression leads to the improvement of working memory, suggesting that γ-synuclein has a role in cognitive function [4].
Aberrant overexpression of γ-synuclein has been observed in various pathological conditions especially in a variety of cancers including prostate, colorectal, pancreatic, ovarian and gall bladder [5, 6]. Surprisingly, γ-synuclein was first discovered in 1996 in breast cancers and was named the Breast Cancer Susceptibility Gene Product, but was later identified as being a member of the neuronal synuclein family [7]. In breast cancer, γ-synuclein is overexpressed in later-stage (Stage III and IV) cancer tissues, but not in healthy or early-stage (Stage I and II) cancer tissue [8]. Even though different types of breast cancers are classified by different markers [9], the presence of γ-synuclein been established as a biomarker for later stages of cancer and has been considered to be a prognosis of poor outcome [10].
Studies have shown that knockdown of γ-synuclein expression in prostate [5] and gall bladder [6] cancer cells greatly reduced the occurrence of cancerous phenotypes such as cell proliferation, migration, invasion and cell cycle arrest. Further studies have shown that downregulation of γ-synuclein expression in MCF7 cells (an early-stage breast cancer cell line) resulted in a drastic reduction in cell migration and proliferation [11], as well as the propensity to form tumors when xenografted into mice [12]. A study performed using the triple-negative breast cancer cell line, MDA MB 231 has revealed that knockdown of γ-synuclein results in an inhibition of cell migration and proliferation [13].
While the exact role of γ-synuclein in the signaling pathways that lead to cancer is currently not known, some studies have shown that γ-synuclein promotes cancerous phenotype by increasing ER-α (Estrogen Receptor) transcription [14], activating MAPK [15], enhancing AKT and ERK signaling [13], and binding and inhibiting BubR1, a mitotic checkpoint protein that prevents the formation of the anaphase promoting complex, thereby allowing the cells to rapidly undergo mitosis [16]. γ-Synuclein is known to regulate signaling pathways by changing its intracellular localization[17] and binding to transcription factors affecting gene expression[18].
Our lab has previously shown that both α- and γ-synuclein interact with the signaling enzyme phospholipase Cβ (PLCβ) to alter Ca2+ responses [19, 20]. There are 4 isoforms of PLCβ (PLCβ -4) which are all strongly activated by the Gαq family of heterotrimeric G proteins. Receptors that are coupled to Gαq include those that bind acetylcholine dopamine, angiotensin II, bradykinin as well as endothelin I [21]. Both PLCβ2 and β3 can be activated by Gβγ subunits which may be released in response to activation of other G protein families. PLCβ enzymes catalyze the hydrolysis of the signaling lipid phosphatidylinositol 4,5 bisphosphate to generate two second messengers (diacylglyercol and 1,4,5 inositol trisphosphate) which lead to the activation of protein kinase C and release of Ca2+ from intracellular stores, respectively (for review see [22, 23]). Even though they bind strongly to membranes, PLCβs are soluble and are found both on the plasma membrane and in the cytoplasm. It has been shown that membrane binding of PLCβ2 can be enhanced by Rac which better allows PLCβ to access its PI(4,5)P2 substrate and promote hydrolysis ([24] for review see [25]). It is notable that Rac1 which mediates cytoskeletal changes associated with migration and mobility leading to the idea that PLCβ may also be involved in these processes via its association with Rac. Rac1-RhoA signaling plays a role in cell motility, where Rac-mediated signaling is associated with forward movement and Rho-mediated signaling with the contractile movement. The movements associated with Rac or Rho mediated signaling have also been linked to protease-dependent mesenchymal and protease-independent amoeboid modes of invasion [26].
Using purified proteins, it was observed that both α- and γ-synuclein bind strongly to the C-terminal region of PLCβ2 [20]. This binding site of γ-synuclein is also the binding site of Gαq, resulting in the competitive inhibition of Gαq-mediated PLCβ2 activation. Conversely, the levels of γ-synuclein do not affect the binding of Gβγ or Rac to PLCβ2, since they bind to the N-terminal region which is distant from the Gαq binding site. [20]. It is also notable that the binding site of γ-synuclein on PLCβ2 overlaps the calpain cleavage site and therefore, the presence of γ-synuclein prevents PLCβ2 degradation [27].
Like γ-synuclein, PLCβ2 is abnormally overexpressed in late-stage breast cancer cells and tissues [28, 29]. Knockdown or overexpression of PLCβ2 affects cell migration only in late stage breast cancer cell lines, with no effect on cell proliferation or invasion [29], suggesting that PLCβ2 is part of a signaling pathway that influences transition into the late-stage cancer phenotypes.
From the studies described above, we postulate that γ-synuclein might promote cancer phenotypes through its ability to increase levels of PLCβ2. This idea is supported by observations that in breast cancer cell line MDA MB 231, PLCβ2 and γ-synuclein co-localize with each other and PLCβ2 co-immunoprecipitates with γ-synuclein, suggesting a cellular interaction between the two proteins [20]. Here, we present evidence that γ-synuclein, by its ability to increase PLCβ2 levels, allows for enhanced Rac-mediated signals at the expense of Gαq signals thereby promoting cancerous phenotypes.
2. MATERIALS AND METHODS
2.1 Cell culture
MDA MB 231 cells were purchased from American Type Culture Collection (ATCC) and were cultured in Dulbecco's Minimum Essential Media (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 50 units/mL of penicillin and 50 μg/mL of streptomycin at 37°C and 5% CO2. To knockdown protein expression levels, cells were transfected using Dharmafect reagent with 40pg of γ-synuclein (Sigma), PLCβ2 (Sigma) or non-specific control (Ambien) siRNAs and were incubated for 96 hours before performing an experiment. For migration, invasion and viability assays, cells were transfected with 40pg each of γ-synuclein and PLCβ2 siRNAs or with 40pg each of γ-synuclein and control siRNAs, or with 80 pg of the control siRNA.
2.2 Quantitative Real Time Reverse Transcriptase PCR (qRT-PCR)
Total RNA were extracted using RNAeasy kit (Qiagen) according to the manufacturer's instructions. 2 μg of total RNA was used for cDNA synthesis with random hexamers using Reverse Transcriptase kit (Qiagen). Using TaqMan® Gene Expression Assays (ThermoFisher) for SNCG (γ-synuclein) , PLCΒ2 (PLCβ2) and ACTB (β Actin) as primers and using DNA Engine Opticon® 2 System (BioRad), real-time PCR was carried out with a denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and primer annealing at 60°C for 15 s and extension at 72°C for 30 s. Upon completion of the cycling steps, the reaction was stored at 4°C. Real-time PCR was carried out Reactions were run in triplicate in three independent experiments. The geometric mean of housekeeping gene ACTB was used as an internal control to normalize the variability in expression levels. Expression data were normalized to the geometric mean of housekeeping gene ACTB to control the variability in expression levels and were analyzed using the 2 −ΔΔCT method [30]
2.3 In vitro protein association studies
Binding between purified coumarin-PLCβ2 and purified C3PO in the presence and absence of purified γ-synuclein was carried out using the materials and procedure described in [20]. Briefly, purified proteins were labeled with the thiol-reactive probe7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin (CPM) at a probe:protein ratio of 4:1 on ice. The reaction was stopped after 60 minutes by adding 10 mM DTT and the protein was purified either by extensive dialysis. Protein solutions were diluted into 20 mM Hepes (pH 7.2), 160 mM NaCl, 1 mM DTT.
Spectral measurements were performed on an ISS spectrofluorometer (Champaign, IL) using 3 mm quartz cuvettes. Peptide and The emission spectrum of CPM-labeled protein was measured from 400 to 550 nm (λex = 380 nm). The background spectra of unlabeled protein or peptide were subtracted from each spectrum along the titration curve, which was also corrected for dilution.
2.4 Immunofluorescence
Cells were fixed using 3.7% formaldehyde and permeabilized with 0.2% nonyl phenoxypolyethoxylethanol (NP40) and incubated with 0.2% NP40 in phosphate buffered saline (PBS) for 5 min and then blocked in PBS containing 4% goat serum for 1 h. Cells were then incubated with the primary antibody (anti-PLCβ2, anti-Rac1 or anti-Gαq (Santa Cruz Biochemicals, Inc.) diluted to 1:1000 for 1.5 hours at 37°C, followed by incubation with Alexa-labeled secondary antibody for 0.5 hours at the same temperature. Cells were washed with Tris buffered saline (TBS) buffer after the incubations. Images of the cells were obtained using Olympus Fluoview FV1000 laser scanning confocal microscope, and were analyzed using Olympus (Fluoview) software and Image J (NIH).
2.5 Förster resonance energy transfer (FRET)
FRET measurements were performed using an Olympus Fluoview1000 instrument on MDA MB 231 cells co-expressing eCFP-tagged Rac1, eCFP-tagged Gαq and/or eYFP-tagged PLCβ2 proteins as described [31]. eCFP and eYFP were excited using 458 and 515 nm argon ion laser lines, respectively, and 480–495 and 535–565 nm bandpass filters to collect emission images, respectively. FRET efficiencies were calculated using the Olympus Fluoview software whose algorithm calculates FRET by sensitized emission after correcting for spectral bleed-through using images of control cells expressing only donor or acceptor proteins with the same intensity distributions as the sample.
2.6 Ca2+ Measurements
Intracellular Ca2+ levels in MDA MB 231 cells treated with γ-synuclein siRNA or sham control were harvested and incubated with 1 μM Fura 2-AM in Hanks Balanced Salt Solution (HBSS, Gibco) with 1% BSA. Cells (1 × 107) were incubated with 1 μM Fura 2-AM for 30 min, pelleted, washed twice with HBSS, and incubated for an additional 15 min for de-esterification of Fura 2-AM. Fluorescence measurements were taken as described in earlier publications [32].
2.7 Western blotting
MDA MB 231 cells were lysed with 500 ul of buffer containing 150 mM NaCl, 20 mM HEPES, 2 mM MgCl2, 5 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 10 ug/ml leupeptin and 10ug/ml aprotinin. The lysates were treated in sample buffer at 95°C for 3 minutes and were loaded onto a 12% PAGE for SDS-PAGE and were transferred into polyvinylidene difluoride membranes. After the transfer, the membranes were blotted with anti- γ-synuclein and anti-PLCβ2 antibodies. Protein levels were calculated using ImageJ and are normalized with the levels of housekeeping genes (β-actin).
2.8 Migration and Transwell matrigel invasion assays
For Transwell migration assays, MDA MB 231 cells were trypsinized and suspended in DMEM 96 hours after siRNA transfection. Equal numbers of cells (2.5 × 104 −6.25 × 104) were loaded on to each of transwell chambers (Corning) that were placed in a 24 well tissue culture plate (Falcon), containing DMEM supplemented with 10% FBS, 50 units/mL of penicillin and 50 μg/mL of streptomycin. The plate is then incubated at 37°C and 5% CO2 for 4 hours. The cells that migrated across the transwell membrane were then fixed with 3.7% Formaldehyde, stained with DAPI and imaged in situ.
For transwell invasion assay, 25ul of Matrigel (BD Biosciences) solution, diluted according to manufacturer’s instructions was added to transwell chambers, which were then allowed to dry overnight in a dry incubator. The chambers, now coated with Matrigel, are allowed to rehydrate by incubating with DMEM for 2 hours, after which the same protocol as transwell migration assay, but with an incubation time of 20 hours.
2.9 MTT assay
A total of 100,000 cells diluted in 0.5 ml growth media were plated on 12-well flat bottom plates and were then transfected as described earlier. At the indicated times, 120 ul of 5 mg/ml MTT solution in PBS were added to each well for 2.5 h. After removal of the medium, 900 ul of dissolving buffer (0.04M HCl diluted in Isopropanol) were added to each well to dissolve the formazan crystals. The contents of the wells were transferred into plastic cuvettes and absorbance at 570 nm and 650 nm was determined using a spectrophotometer.
2.10 Circularity
The shape of the cell was quantified by using the Circularity function of ImageJ (NIH) software, which was calculated using the formula below based on outline of a cell:
3. EXPERIMENTAL
3.1 Protein and mRNA levels of γ-synuclein and PLCβ2 in MDA MB 231 cells are correlated
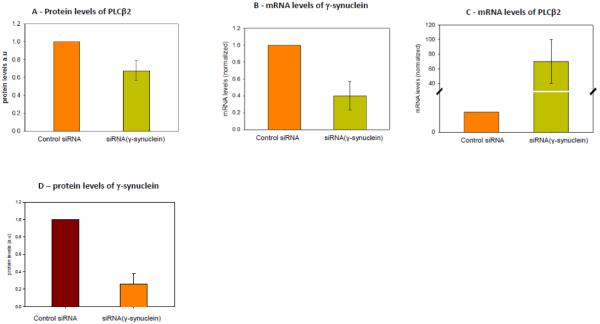
Our previous studies of α-synuclein showed that the binding site of α-synuclein on PLCβ2 overlaps with the calpain cleavage site[27]. Since calpain is activated by elevated Ca2+, then PLCβ2 degradation by calpain is thought to be involved as a feedback mechanism to help extinguish G protein-mediated Ca2+ signals. To determine whether this is the case for γ-synuclein, we down-regulated the protein with siRNA in MDA MB 231 cells and compared the protein levels of PLCβ2 with samples using control siRNA. Estimates from western blot analysis indicated that this treatment reduces γ-synuclein expression by 65%. In Fig. 1A, we show that reducing the level of γ-synuclein by ~65% also reduces the level of PLCβ2 by about 40%.
Figure 1. Correlation between PLCβ2 and γ-synuclein expression.
A- Compilation of band intensities from western blots (n=7) showing that the protein level of PLCβ2 in MDA MD 231 cells is reduced when γ-synuclein is knocked-down using siRNA relative to control siRNA. B- mRNA levels of γ-synuclein and (C) PLCβ2 showing that reducing the message level of γ-syncuclein results in a sharp increase in the mRNA level of PLCβ2 when compared to control. mRNA levels normalized to β-actin mRNA and to the control siRNA) (n=9). D- Reference plot showing the reduction of g-synuclein protein levels with siRNA treatment (n=12).
To determine whether the reduced production of PLCβ2 with γ-synuclein expression is on the transcriptional level, we measured the change in mRNA(PLCβ2) as γ-synuclein is down-regulated. Unexpectedly, we find that down-regulation of γ-synuclein increases the level of mRNA(PLCβ2) by a factor of 70 (Fig. 1B-C). This finding is consistent with γ-synuclein being involved in transcriptional regulation [15].
3.2 PLCβ2-Gαq interactions in MDA MB 231 cells are affected by γ-synuclein knockdown
In previous in-vitro studies using purified proteins, we found that the binding of γ-synuclein to PLCβ2 reduces enzymatic activity [20] . We also found that γ-synuclein binds to the same site as Gαq on PLCβ2 suggesting that γ-synuclein inhibits Gαq-mediated activation. Because MDA MB 231 cells over-express both γ-synuclein and PLCβ2, it is possible that Gαq-mediated activation of PLCβ2 is diminished due to competition by γ-synuclein.
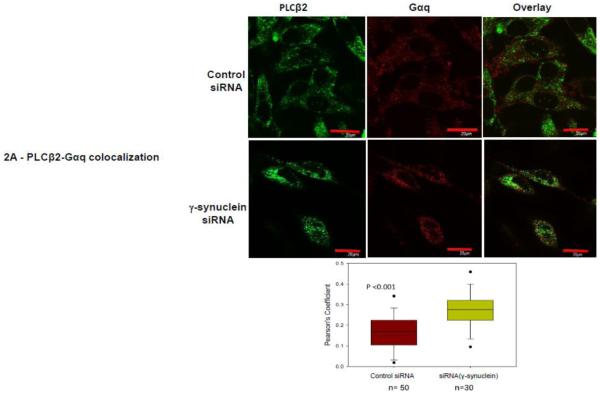
Based on our previous work, we postulate that lowering the levels of γ-synuclein would promote association between Gαq and PLCβ2. We first tested this idea by reducing the protein levels of γ-synuclein in MDA MB 231 cells using siRNA and monitoring the change in colocalization between PLCβ2 and Gαq by immunofluorescence. We find a significant increase in colocalization between PLCβ2 and Gαq when γ-synuclein levels are reduced (Fig. 2A) which is consistent with the idea that γ-synuclein competes with Gαq for PLCβ binding.
Figure 2. PLCβ2-Gαq interactions in MDA MB 231 cells are affected by γ-synuclein knock-down.
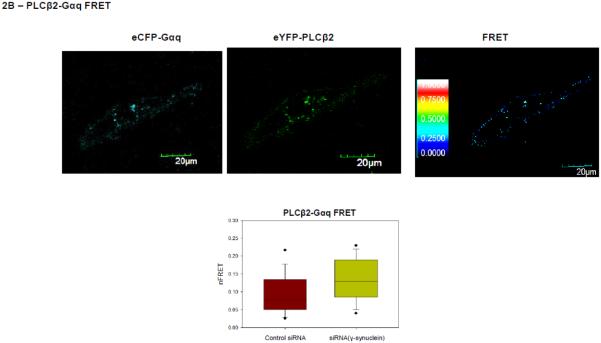
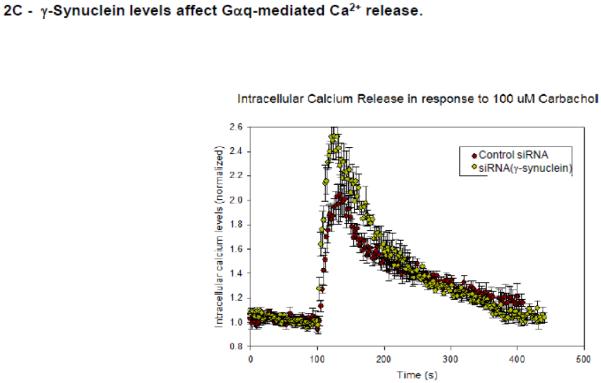
A- Co-immunofluorescence studies showing the colocalization of PLCβ2 (green,) and Gαq (red) in MDA MB 231 cells treated with control (n=50) or γ-synuclein (n=30) siRNA where the compiled colocalization values are shown in the box plot. Pearson’s coefficient between the two groups is statistically significant (p<0.001, Student’s t-test. Positive control= 0.65 +/− 0.12, negative control= 0.03 +/− 0.08). B- Sample raw FRET image of a MDA MB 231cell expressing eCFP- Gαq and eYFP- PLCβ2 with corresponding images at CFP and YFP wavelengths where the normalized FRET efficiencies for cells treated with γ-synuclein (n=30) and control (n=50) are given in the box plot were the values are found to be significantly different (p<0.001, Rank Sum Test. (Positive control = 0.44+/− 0.06, Negative control = 0.03 +/− 0.02). C- Functional results showing that reduction of γ-synuclein causes a more robust Ca2+ response. Ca2+ release was measured in MDA MB 231 cell suspensions upon the addition of 100 μM carbachol using Fura-2, where cells were treated with control or γ-synuclein siRNA, n = 6. The mean ± SEM is shown.
To confirm that loss of γ-synuclein promotes physical association between PLCβ and Gαq, we measured the FRET between eCGP-Gαq and eYFP-PLCβ2 expressed in MDA MB 231 cells. We find a reduction in the amount of γ-synuclein causes a small but significant increase in FRET (p<0.001) (Fig. 2B). The values of FRET, when compared to the value obtained for the positive control (eCFP-X-eYFP) and negative controls (free eCFP and free eYFP, or eCFP-TRAX and YFP-membrane marker), show an increase in association from 19.1±0.2% to 32.2±0.6% when γ-synuclein is reduced approximately in half.
If reducing γ-synuclein promotes PLCβ2-Gαq association, then it is likely Ca2+ signals generated through Gαq will be enhanced. We tested this idea by measuring the release of Ca2+ with carbachol addition to suspensions of MDA MB 231 cells loaded with the fluorescent Ca2+ indicator Fura-2. We find that cells in which γ-synuclein has been down-regulated by 65% using siRNA show a significant increase in Ca2+ release despite the correlative reduction in PLCβ levels (Fig. 2C) suggesting that high levels of γ-synuclein expression may quench Ca2+ signals mediated through Gαq.
3.3 γ-Synuclein inhibits the association between PLCβ2 and C3PO
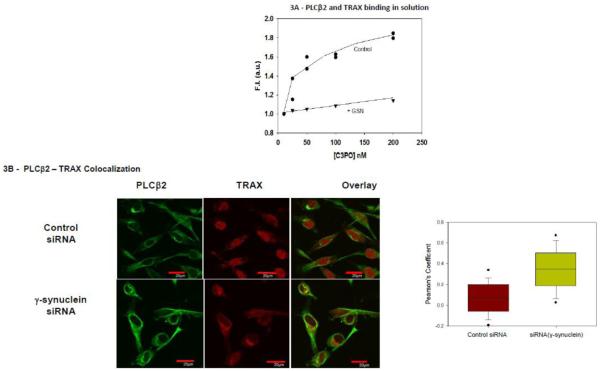
In a yeast two hybrid study, we found that the C-terminal region of PLCβ1 associates to the nuclease Translin-Associated Factor X (TRAX) [33]. TRAX binds to the oligonucleotide binding protein translin to form the octamer C3PO [34]. C3PO has been shown to play an integral role in RNA-induced silencing by removing the passenger strand of the double-stranded silencing siRNA [35]. The association between PLCβ and C3PO reduces the ability of C3PO to process specific genes [36]. Because the binding site for Gαq and C3PO on PLCβ2 overlap, we determined whether γ-synuclein could interfere with PLCβ2-C3PO association as well. To answer this question, we labeled purified PLCβ2 with a fluorescent probe and monitored the change in fluorescence as C3PO binds in the absence and presence of γ-synuclein (Fig. 3A). This observation was corroborated by enhanced colocalization between TRAX and PLCβ2 upon knockdown of γ-synuclein in MDA MB 231 cells (Fig. 3B). This result suggests that γ-synuclein may indirectly affect miR population by interfering with PLCβ-C3PO association.
Figure 3. PLCβ2-TRAX interactions in MDA MB231 cells are affected by γ-synuclein knockdown.
A- Normalized change in fluorescence intensity of 2 nM CPM- PLCβ2 in solution as purified TRAX is added in the presence or absence of 100nM γ-synuclein. B- Co-immunofluorescence studies showing the colocalization of PLCβ2 (green) and TRAX (red) in MDA MB 231 cells treated with control (n=41) or γ-synuclein (n=75) siRNA. Pearson’s coefficient between the two groups is statistically significant (p<0.001, Student’s t-test. Positive control= 0.65 +/− 0.12, negative control= 0.03 +/− 0.08).
3.4 The presence of γ-synuclein affects PLCβ2- Rac interactions and Rac-mediated events
Phosphoinositides are required by RhoGEFS to regulate Rac (e.g [37]). Rac has been shown to bind to the N-terminal region of PLCβ2 which is structurally distant than the region where γ-synuclein and Gαq bind [38]. Rac promotes the activation of PLCβ2 by recruiting and stabilizing its association to the plasma membrane (see [39]). Because the PLCβ2 /γ-synuclein complex does not bind to Gαq, the presence of γ-synuclein would make more PLCβ available to impact Rac-mediated pathways, such as cell invasion and cell morphology [40-43]. Alternately, down-regulating γ-synuclein would make less PLCβ2 available to Rac due to reduction of the cellular amount of enzyme and by promoting Gαq association.
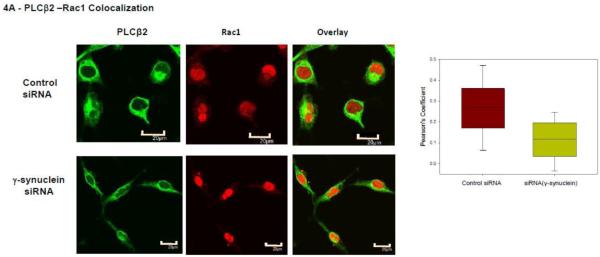
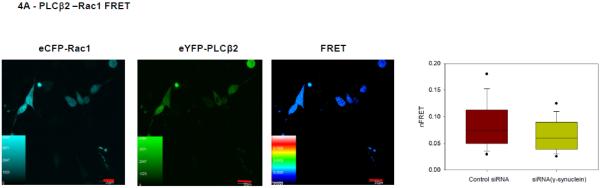
We first measured changes in the colocalization between PLCβ2 and Rac in control and γ-synuclein knockdown MDA MB 231 cells and found a significant reduction with reduced γ-synuclein expression (Fig. 4A). We note that western blotting shows that the protein level of Rac is not affected by γ-synuclein knock-down. We supported the co-immunofluorescence studies by FRET in which cells were transfected with eYFP-PLCβ2 and eCFP-Rac1. We find that the level of FRET slightly decreases with γ-synuclein knock-down. We note that this small change in FRET may reflect the over-expression of the proteins which mask larger changes at endogenous levels (Fig. 4B).
Figure 4. PLCβ2 and Rac1 interactions in MDA MB 231 cells are affected by γ-synuclein knock-down.
A- Co-immunofluroescence studies showing the colocalization of PLCβ2 (green) and Rac1 (red) in MDA MB 231 cells treated with control (n=29) or γ-synuclein (n=30) siRNA. As shown in the accompanying box plots, Pearson’s coefficient between the two groups is statistically significant (p<0.001, Student’s t-test. Positive control= 0.65 +/− 0.12, negative control= 0.03 +/− 0.08). B- Sample FRET images of an MDA MB 231 cell expressing eCFP- Rac1 and eYFP- PLCβ2 where the accompanying box plot shows changes in the normalized FRET efficiencies of cells treated with γ-synuclein (n=83) or control (n=80) siRNA (p<0.001, Rank Sum Test., Positive control = 0.44+/− 0.06, Negative control = 0.03 +/− 0.02).
3.5 γ-Synuclein / PLCβ2 interactions may be linked to cancerous phenotypes
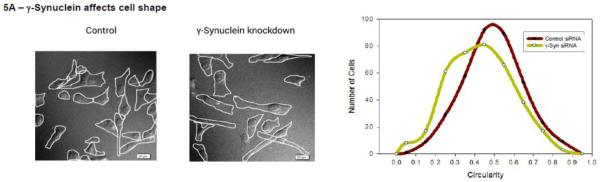
MDA MB 231 cells are known to be able to follow both amoeboid and mesenchymal pathways of invasion. The elongated morphology is often associated with a mesenchymal pathway, whereas circular morphology is often associated with an amoeboid pathway. We determined the ability of γ-synuclein to affect the morphology of MDA MB 231 cells by comparing cells treated with siRNA(γ-synuclein) or control siRNA. We find that knocking down γ-synuclein expression results in cells with a more elongated and less circular morphology (Fig. 5A). Because γ-synuclein increases the protein level of PLCβ2, then more of its N-terminus is available for Rac1 binding resulting in a decrease in inhibition of RhoA. RhoA signaling is associated with a more circular amoeboid morphology [44], while Rac is associated with more elongated mesenchymal morphology (for review see [25]).
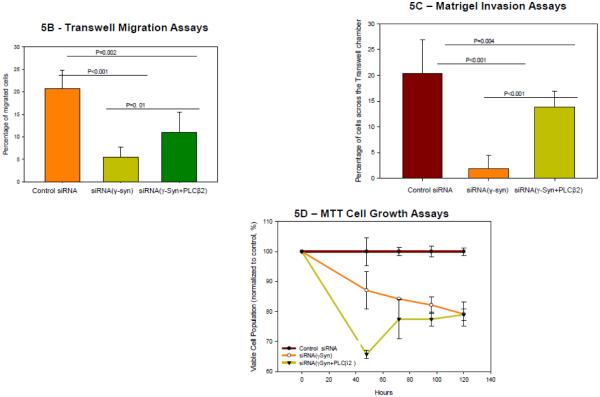
Figure 5. γ-Synuclein and PLCβ2 affect MDA MD 231 cell phenotypes.
A- Sample DIC images of MDA MB 231 cells mock treated or treated with γ-synuclein siRNA and compiled results showing a significant difference in the circularity of cells between the two groups (both n=360, p<0.001, Student’s t-test). B- Transwell migration assay showing the average number of cells treated with control siRNA, γ-synuclein and control siRNA, or γ-synuclein and PLCβ2 siRNA, that migrated per field, determined after fixing the transwell membrane and staining with DAPI after 4 hours of incubation (n=5, ANOVA). C- Transwell Matrigel invasion assay showing the average number of cells treated with control siRNA or γ-synuclein siRNA, that migrated per field, which was determined after fixing the transwell membrane and staining with DAPI after 4 hours of incubation (n=5, p<0.001 Student’s t-test). D- MTT assays were used to evaluate cell growth after treatment with control siRNA, γ-synuclein and control siRNA, or γ-synuclein and PLCβ2 siRNA. Data were expressed as a percentage of the absorbance of control cells at 570nm. The mean ± SEM is shown (n=6).
Migration pathways can be mediated by Rac [45]. Previous studies [13, 29] found that the levels of both γ-synuclein and PLCβ2 independently affect the migration of MDA MB 231 cells. Based on this work, we performed Transwell migration assay using cells treated with two siRNAs (γ-synuclein and control siRNAs, γ-synuclein and PLCβ2 siRNAs, PLCβ2 and control siRNAs, or double the amount of control siRNA). We observe that knocking down the levels of both PLCβ2 and γ-synuclein results in decreased cell migration, corroborating previous results [11]. However, treatment with both PLCβ2 and γ-synuclein siRNA revealed that knockdown of PLCβ2 attenuates the decrease in cell migration caused by the knockdown of γ-synuclein in breast cancer cells, without completely eliminating the effect. This result suggests that γ-synuclein/ PLCβ2/Rac1 complexes is one of the interactions that utilized by γ-synuclein to induce cell migration (Fig. 5B). Treatment with γ-synuclein siRNA on MDA MB 231 cells decreases cell invasion as quantified by Transwell matrigel assay which was also attenuated by simultaneous treatment with PLCβ2 siRNA (Fig. 5C).
To understand whether these migration effects could be due to changes in cell viability (see [13]), we determined whether simultaneous knockdown of PLCβ2 attenuated reduced viability due to γ-synuclein. We find that this is not the case (Fig. 5D) supporting the idea that PLCβ2 / Rac pathways are promoted by γ-synuclein.
4. DISCUSSION
In this study, we have shown that changes in the interaction between PLCβ2 and γ-synuclein may promote cancerous phenotypes though several mechanisms. Breast cancer is typified by changes in cell morphology and migration, which are brought about by changes in the levels of specific genes and their expressed proteins. Changes in gene expression were first examined by Ji and coworkers [7] who identified a gene that is absent in normal tissue but highly overexpressed in breast cancer tissue. Surprisingly, this gene was later identified to be the neurological protein, γ-synuclein. γ-Synuclein has since been found in other types of cancer where it may be involved in transcriptional regulation of cellular proteins. This regulation was seen here by the impact of γ-synuclein on the message level of PLCβ2.
Most likely γ-synuclein levels significantly affect the mRNA levels of other regulatory proteins but in ways that are difficult to predict. For example, in breast cancer cells, activator protein AP-1, a complex composed of c-Jun homodimers or c-Jun/c-Fos heterodimers, was found to promote the expression of γ-synuclein [46]. Conversely γ-synuclein was found to bind to and mediate AP-1 activity in neuronal and retinoblastoma cells [18, 47], and to activate c-JunN-terminal kinase 1 (JNK1) in breast cancer [15]. Meanwhile PLCs, including PLCβ2 and transcription factors c-Jun and c-Fos reciprocally regulate their respective expression levels in cardiomyoctes [48].
Previous studies have shown that like γ-synuclein, PLCβ2 is absent in normal breast tissue but is found at high levels in breast cancer [28]. It is noteworthy that changes in PLCβ expression appear in many types of cancer [49] showing the complexity of lipid signaling pathways [50]. Here, we show the positive correlation between protein levels of γ-synuclein and PLCβ2. A similar correlation was previously found in analogous studies of α-synuclein and PLCβ1 where we have shown that α-synuclein protects PLCβ2 from degradation by the calcium-activated protein enzyme, calpain [27]. While we do not know the extent of regulation of PLCβ2 levels by calpain under normal cellular conditions, it is probable that γ-synuclein physically protects PLCβs from other forms of degradation as well. It is possible that the interactions between γ-synuclein, PLCβ2 and transcription factors might cause a feedback effect in response to the higher PLCβ2 degradation in γ-synuclein knockdown MDA MB 231 cells compared to control, causing an increase in PLCβ2 mRNA in response to γ-synuclein knockdown.
Cancer cells are less responsive to external regulation. Our studies showing that reduction of γ-synuclein by 40% significantly enhances Ca2+ signals mediated through Gαq stimulation is consistent with a loss in regulation by external sources in cancerous states when γ-synuclein is over-produced. Additionally, we find that down regulation of γ-synuclein increases both the co-localization of PLCβ and Gαq, and their physical association as seen by FRET, which also contributes to a stronger Ca2+ response.
PLCβ enzymes are distinguished from other PLCs by their long ~400aa C-terminal tails which contain the Gαq binding site (see [23] ). We have previously found that PLCβs can bind to the promoter of RNA interference and this binding impacts siRNA down-regulation. Because the binding region between PLCβ and C3PO overlaps with Gαq , it is not surprising that γ-synuclein interferes with PLCβ2 – C3PO association. Although much more work would be needed to determine whether specific targets or pathways are altered by γ-synuclein through this mechanism, existing literature and our results suggest that the association between γ-synuclein and PLCβ may influence transcriptional and post-transcriptional gene regulation.
Rac1 is involved with processes related to cytoskeletal rearrangements and has been shown to recruit PLCβ2 from the cytosol to the plasma membrane promoting PLCβ activation [24, 51]. Similarly, PLCb2 has been shown to promote migration pathways in breast cancer cells. It is interesting that PLCβ2 is the only PLC isoform that correlates with breast cancer and is specifically activated by Rac1. Because Rac binds to the N-terminal domain of PLCβ2, it should be unaffected by γ-synuclein levels. However, our studies show that when γ-synuclein is down-regulated, association between PLCβ and Rac is reduced. We interpret this reduction as being due to increased interaction between PLCβ and Gαq due to the loss of γ-synuclein and due to a decrease in the protein levels of PLCβ. It is known that Rac1 inhibits Rho activity, and that the balance between Rac and Rho signaling influences cell migration and invasion [26, 41, 52]. Therefore it is possible that γ-synuclein promotes Rho signaling by sequestering Rac by binding to PLCβ2. This theory is partly corroborated by Pan and co-workers’ findings that γ-synuclein was found to activate ERK pathway by enhancing Rho GTPase activation which may play a major role in cell migration [14]. The overall functional effects of the regulation of Rac-Rho signaling by γ-synuclein and PLCβ, can be seen by the changes in the Rac-mediated processes of cell migration and morphology.
5. CONCLUSIONS
In conclusion, we have shown that γ-synuclein can have multiple effects on cell function through its association with PLCβ, such as reduced calcium responses due to G protein activation, changes in modulation of RNA interference, and changes in Rac-mediated cytoskeletal arrangement that modulate cell shape and migration. While it is certain that γ-synuclein works through alternate mechanism to promote cancerous phenotypes, these studies show that PLC-γ-synuclein interactions may play an important role.
HIGHLIGHTS.
γ-Synuclein and PLCβ2 are linked to cancer and γ-synuclein increases PLCβ2 levels.
γ-Synuclein competes with Gαq for PLCβ2 binding impacting Gαq-mediated Ca2+ responses.
Reducing γ-synuclein promotes PLCβ2-C3PO association which may affect RNA interference mediated by RNA-induced silencing complex (RISC).
γ-Synuclein impacts PLCβ2- Rac interactions changing cell morphology and migration.
6. ACKNOWLEDGEMENTS
The authors would like to thank Jessica Desamero for her assistance with the construction of eCFP-Rac1 construct, Dr. Yuanjian Guo for providing the MDA MB 231 cell line, and Dr. Urszula Golebiewska (Queensborough Community College) for her advice. This work was supported by funding from the Carol Baldwin Foundation and NIH GM116178.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- [1].George JM. The synucleins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3002. REVIEWS3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Surguchov A. Synucleins: are they two-edged swords? J Neurosci Res. 2013;91:161–166. doi: 10.1002/jnr.23149. [DOI] [PubMed] [Google Scholar]
- [3].Ducas VC, Rhoades E. Quantifying interactions of beta-synuclein and gamma-synuclein with model membranes. Journal of molecular biology. 2012;423:528–539. doi: 10.1016/j.jmb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kokhan VS, Van'kin GI, Bachurin SO, Shamakina IY. Differential involvement of the gamma-synuclein in cognitive abilities on the model of knockout mice. BMC Neurosci. 2013;14:53. doi: 10.1186/1471-2202-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen J, Jiao L, Xu C, Yu Y, Zhang Z, Chang Z, Deng Z, Sun Y. Neural protein gamma-synuclein interacting with androgen receptor promotes human prostate cancer progression. BMC Cancer. 2012;12:593. doi: 10.1186/1471-2407-12-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han S, She F, Wang D, Yao X, Jiang L, Chen Y. SNCG gene silencing in gallbladder cancer cells inhibits key tumorigenic activities. Front Biosci. 2012;17:1589–1598. doi: 10.2741/4005. [DOI] [PubMed] [Google Scholar]
- [7].Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997;57:759–764. [PubMed] [Google Scholar]
- [8].Wu K, Quan Z, Weng Z, Li F, Zhang Y, Yao X, Chen Y, Budman D, Goldberg ID, Shi YE. Expression of neuronal protein synuclein gamma gene as a novel marker for breast cancer prognosis. Breast Cancer Res Treat. 2007;101:259–267. doi: 10.1007/s10549-006-9296-7. [DOI] [PubMed] [Google Scholar]
- [9].McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Libra M, Nicoletti F, D'Assoro AB, Cocco L, Martelli AM, Steelman LS. Targeting breast cancer initiating cells: Advances in breast cancer research and therapy. Advances in Biological Regulation. 2014;56:81–107. doi: 10.1016/j.jbior.2014.05.003. [DOI] [PubMed] [Google Scholar]
- [10].Wu K, Huang S, Zhu M, Lu Y, Chen J, Wang Y, Lin Q, Shen W, Zhang S, Zhu J, Shi YE, Weng Z. Expression of synuclein gamma indicates poor prognosis of triple-negative breast cancer. Med Oncol. 2013;30:612. doi: 10.1007/s12032-013-0612-x. [DOI] [PubMed] [Google Scholar]
- [11].Liang B, Wang XJ, Shen PH, Li XY, Cheng HW, Shan Q, Guo KY, Cao YW, Fan QX, Zheng RF, Li B, Zhang W, Li YW, Yang K. Synuclein-gamma suppression mediated by RNA interference inhibits the clonogenicity and invasiveness of MCF-7 cells. Oncol Lett. 2013;5:1347–1352. doi: 10.3892/ol.2013.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shen PH, Fan QX, Li YW, Zhang W, He XK, Wang Z, Zhang YH. SNCG shRNA suppressed breast cancer cell xenograft formation and growth in nude mice. Chin Med J (Engl) 2011;124:1524–1528. [PubMed] [Google Scholar]
- [13].He J, Xie N, Yang J, Guan H, Chen W, Wu H, Yuan Z, Wang K, Li G, Sun J, Yu L. siRNA-Mediated Suppression of Synuclein gamma Inhibits MDA-MB-231 Cell Migration and Proliferation by Downregulating the Phosphorylation of AKT and ERK. J Breast Cancer. 2014;17:200–206. doi: 10.4048/jbc.2014.17.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jiang Y, Liu YE, Lu A, Gupta A, Goldberg ID, Liu J, Shi YE. Stimulation of estrogen receptor signaling by gamma synuclein. Cancer Res. 2003;63:3899–3903. [PubMed] [Google Scholar]
- [15].Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. The Journal of biological chemistry. 2002;277:35050–35060. doi: 10.1074/jbc.M201650200. [DOI] [PubMed] [Google Scholar]
- [16].Panneerselvam M, Muthu K, Jayaraman M, Sridharan U, Jenardhanan P, Ramadas K. Molecular dynamic simulations of the tubulin-human gamma synuclein complex: structural insight into the regulatory mechanism involved in inducing resistance against Taxol. Mol Biosyst. 2013;9:1470–1488. doi: 10.1039/c3mb25427e. [DOI] [PubMed] [Google Scholar]
- [17].Surgucheva I, McMahon B, Surguchov A. gamma-synuclein has a dynamic intracellular localization. Cell motility and the cytoskeleton. 2006;63:447–458. doi: 10.1002/cm.20135. [DOI] [PubMed] [Google Scholar]
- [18].Surgucheva I, Surguchov A. Gamma-synuclein: cell-type-specific promoter activity and binding to transcription factors. Journal of molecular neuroscience : MN. 2008;35:267–271. doi: 10.1007/s12031-008-9074-6. [DOI] [PubMed] [Google Scholar]
- [19].Narayanan V, Guo Y, Scarlata S. Fluorescence studies suggest a role for alpha-synuclein in the phosphatidylinositol lipid signaling pathway. Biochemistry. 2005;44:462–470. doi: 10.1021/bi0487140. [DOI] [PubMed] [Google Scholar]
- [20].Golebiewska U, Guo Y, Khalikaprasad N, Zurawsky C, Yerramilli VS, Scarlata S. gamma-Synuclein interacts with phospholipase Cbeta2 to modulate G protein activation. PloS one. 2012;7:e41067. doi: 10.1371/journal.pone.0041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annual Review of Pharmacology & Toxicology. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- [22].Suh P, Park J, Manzoli L, Cocco L, Peak J, Katan M, Fukami K, Kataoka T, Yun S, Ryu S. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB reports. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- [23].Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiological reviews. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- [24].Illenberger D, Walliser C, Strobel J, Gutman O, Niv H, Gaidzik V, Kloog Y, Gierschik P, Henis Y. Rac2 regulation of phospholipase Cb2 activity and mode of membrane interactions in intact cells. J.Biol.Chem. 2003;278:8645–8652. doi: 10.1074/jbc.M211971200. [DOI] [PubMed] [Google Scholar]
- [25].Harden TK, Hicks SN, Sondek J. Phospholipase C isozymes as effectors of Ras superfamily GTPases. Journal of lipid research. 2009;50(Suppl):S243–248. doi: 10.1194/jlr.R800045-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell communication and signaling : CCS. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guo Y, Rosati B, Scarlata S. alpha-Synuclein increases the cellular level of phospholipase Cbeta1. Cell Signal. 2012;24:1109–1114. doi: 10.1016/j.cellsig.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bertagnolo V, Benedusi M, Querzoli P, Pedriali M, Magri E, Brugnoli F, Capitani S. PLC-beta2 is highly expressed in breast cancer and is associated with a poor outcome: a study on tissue microarrays. International journal of oncology. 2006;28:863–872. [PubMed] [Google Scholar]
- [29].Bertagnolo V, Benedusi M, Brugnoli F, Lanuti P, Marchisio M, Querzoli P, Capitani S. Phospholipase C-beta 2 promotes mitosis and migration of human breast cancer-derived cells. Carcinogenesis. 2007;28:1638–1645. doi: 10.1093/carcin/bgm078. [DOI] [PubMed] [Google Scholar]
- [30].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [31].Philip F, Scarlata S. Real-time measurements of protein affinities on membrane surfaces by fluorescence spectroscopy. Science's STKE : signal transduction knowledge environment. 2006;2006:pl5. doi: 10.1126/stke.3502006pl5. [DOI] [PubMed] [Google Scholar]
- [32].Calizo RC, Scarlata S. A role for G-proteins in directing G-protein-coupled receptor-caveolae localization. Biochemistry. 2012;51:9513–9523. doi: 10.1021/bi301107p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aisiku OR, Runnels LW, Scarlata S. Identification of a novel binding partner of phospholipase cbeta1: translin-associated factor X. PloS one. 2010;5:e15001. doi: 10.1371/journal.pone.0015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tian Y, Simanshu DK, Ascano M, Diaz-Avalos R, Park AY, Juranek SA, Rice WJ, Yin Q, Robinson CV, Tuschl T, Patel DJ. Multimeric assembly and biochemical characterization of the Trax–translin endonuclease complex. Nat Struct Mol Biol. 2011;18:658–664. doi: 10.1038/nsmb.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Y, Ye X, Jiang F, Liang C, Chen D, Peng J, Kinch LN, Grishin NV, Liu Q. C3PO, an Endoribonuclease That Promotes RNAi by Facilitating RISC Activation. Science. 2009;325:750–753. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Philip F, Guo Y, Aisiku O, Scarlata S. Phospholipase Cbeta1 is linked to RNA interference of specific genes through translin-associated factor X. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:4903–4913. doi: 10.1096/fj.12-213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Seasholtz TM, Radeff-Huang J, Sagi SA, Matteo R, Weems JM, Cohen AS, Feramisco JR, Brown JH. Rho-mediated cytoskeletal rearrangement in response to LPA is functionally antagonized by Rac1 and PIP2. J Neurochem. 2004;91:501–512. doi: 10.1111/j.1471-4159.2004.02749.x. [DOI] [PubMed] [Google Scholar]
- [38].Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-beta2. Nat.Struc.& Mol.Biol. 2006;13:1135–1139. doi: 10.1038/nsmb1175. [DOI] [PubMed] [Google Scholar]
- [39].Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-beta2 by the Rho GTPases Cdc42Hs and Rac1. Embo J. 1998;17:6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sommers CL, Thompson EW, Torri JA, Kemler R, Gelmann EP, Byers SW. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ. 1991;2:365–372. [PubMed] [Google Scholar]
- [41].Pozarowski P, Grabarek J, Darzynkiewicz Z. Flow cytometry of apoptosis. Curr Protoc Cytom. 2003 doi: 10.1002/0471142956.cy0719s25. Chapter 7. Unit 7 19. [DOI] [PubMed] [Google Scholar]
- [42].Chao C, Ives K, Hellmich HL, Townsend CM, Jr., Hellmich MR. Gastrin-releasing peptide receptor in breast cancer mediates cellular migration and interleukin-8 expression. J Surg Res. 2009;156:26–31. doi: 10.1016/j.jss.2009.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marshall J. Transwell((R)) invasion assays. Methods Mol Biol. 2011;769:97–110. doi: 10.1007/978-1-61779-207-6_8. [DOI] [PubMed] [Google Scholar]
- [44].Tkach V, Bock E, Berezin V. The role of RhoA in the regulation of cell morphology and motility. Cell motility and the cytoskeleton. 2005;61:21–33. doi: 10.1002/cm.20062. [DOI] [PubMed] [Google Scholar]
- [45].Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental Biology. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- [46].Lu A, Zhang F, Gupta A, Liu J. Blockade of AP1 transactivation abrogates the abnormal expression of breast cancer-specific gene 1 in breast cancer cells. The Journal of biological chemistry. 2002;277:31364–31372. doi: 10.1074/jbc.M201060200. [DOI] [PubMed] [Google Scholar]
- [47].Surgucheva IG, Sivak JM, Fini ME, Palazzo RE, Surguchov AP. Effect of gamma-synuclein overexpression on matrix metalloproteinases in retinoblastoma Y79 cells. Archives of biochemistry and biophysics. 2003;410:167–176. doi: 10.1016/s0003-9861(02)00664-1. [DOI] [PubMed] [Google Scholar]
- [48].Singal T, Dhalla NS, Tappia PS. Reciprocal regulation of transcription factors and PLC isozyme gene expression in adult cardiomyocytes. J Cell Mol Med. 2010;14:1824–1835. doi: 10.1111/j.1582-4934.2009.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Advances in Biological Regulation. 2015;57:10–16. doi: 10.1016/j.jbior.2014.10.004. [DOI] [PubMed] [Google Scholar]
- [50].Blind RD. Disentangling biological signaling networks by dynamic coupling of signaling lipids to modifying enzymes. Advances in Biological Regulation. 2014;54:25–38. doi: 10.1016/j.jbior.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Illenberger D, Stephan I, Gierschik P, Schwald F. Stimulation of phospholipase C-beta 2 by Rho GTPases. Methods Enzymol. 2000;325:167–177. doi: 10.1016/s0076-6879(00)25441-4. [DOI] [PubMed] [Google Scholar]
- [52].Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]