Abstract
Background
The association between consumption of caffeinated and decaffeinated coffee and risk of mortality remains inconclusive.
Methods and Results
We examined the associations of consumption of total, caffeinated, and decaffeinated coffee with risk of subsequent total and cause-specific mortality among 74,890 women in the Nurses’ Health Study (NHS), 93,054 women in the NHS 2, and 40,557 men in the Health Professionals Follow-up Study. Coffee consumption was assessed at baseline using a semi-quantitative food frequency questionnaire. During 4,690,072 person-years of follow-up, 19,524 women and 12,432 men died. Consumption of total, caffeinated, and decaffeinated coffee were non-linearly associated with mortality. Compared to non-drinkers, coffee consumption one to five cups/d was associated with lower risk of mortality, while coffee consumption more than five cups/d was not associated with risk of mortality. However, when restricting to never smokers, compared to non-drinkers, the HRs of mortality were 0.94 (0.89 to 0.99) for ≤ 1 cup/d, 0.92 (0.87 to 0.97) for 1.1-3 cups/d, 0.85 (0.79 to 0.92) for 3.1-5 cups/d, and 0.88 (0.78 to 0.99) for > 5 cups/d (p for non-linearity = 0.32; p for trend < 0.001). Significant inverse associations were observed for caffeinated (p for trend < 0.001) and decaffeinated coffee (p for trend = 0.022). Significant inverse associations were observed between coffee consumption and deaths due to cardiovascular disease, neurological diseases, and suicide. No significant association between coffee consumption and total cancer mortality was found.
Conclusions
Higher consumption of total coffee, caffeinated coffee, and decaffeinated coffee was associated with lower risk of total mortality.
Keywords: caffeinated coffee, decaffeinated coffee, smoking, mortality
INTRODUCTION
Coffee is one of the most commonly consumed beverages worldwide. The associations between coffee consumption and risks of several disease outcomes have been investigated. Coffee consumption has been inversely associated with risks of type 2 diabetes 1, liver cancer 2, endometrial cancer 3, lethal prostate cancer 4, basal cell carcinoma of the skin 5, and neurological diseases 6, as well as with risk of cardiovascular disease (CVD) when consumed in moderation 7.
The association between coffee consumption and risk of total mortality has also been investigated. Recent studies showed an inverse association between moderate coffee consumption and risk of mortality, and an inverse or null association between heavy coffee consumption and risk of mortality 8-16. However, some earlier studies17-19 and a recent study20 found heavy coffee consumption to be associated with higher risk of mortality. Summarizing individual studies, meta-analyses have concluded that coffee consumption is not associated with higher risk of mortality; however, there was significant between-study heterogeneity in the effect estimates21-23. The associations between coffee consumption and cause-specific mortality, especially CVD and cancer mortality, have been sporadically investigated together with total mortality, 8, 10, 11, 23 with most studies finding an inverse association with CVD mortality, and no association with cancer mortality.
Based on the results of previous studies, four questions remain unanswered: first, does a non-linear relationship exist between coffee consumption and risk of mortality, i.e., is moderate coffee consumption associated with lower risk of mortality and heavy coffee drinking not associated with risk of mortality or even with an increased risk? Second, if a non-linear association exists, is it truly a biological effect of coffee or is it an artifact due to the confounding of smoking? Third, what are the associations of coffee consumption with risks of cause-specific mortality? Fourth, do caffeinated and decaffeinated coffee have similar associations with risk of mortality?
We therefore examined the association of coffee consumption with total and cause-specific mortality in three large, ongoing, independent cohort studies of men and women. This analysis updated our earlier publication on coffee consumption and total mortality in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) with 6,888 total deaths and extended to a younger cohort of nurses (Nurses’ Health Study II). These cohorts provide measures of caffeinated and decaffeinated coffee consumption, extensive data on known or suspected confounders, and up to 30 years of follow-up, during which more than 30,000 deaths have been recorded.
METHODS
Study Population
The NHS began in 1976, when 121,700 female registered nurses aged 30-55 y residing in 11 states were recruited to complete a baseline questionnaire about their lifestyle and medical history. The NHS II was established in 1989 and consisted of 116,671 younger female registered nurses, aged 25-42 y at baseline. These nurses responded to a baseline questionnaire similar to the NHS. The HPFS was initiated in 1986, and was composed of 51,529 male dentists, pharmacists, veterinarians, optometrists, osteopathic physicians, and podiatrists, aged 40-75 y at baseline. The male participants returned a baseline questionnaire about detailed medical history, lifestyle, and usual diet. In all three cohorts, questionnaires were collected at baseline and biennially thereafter, to update information on lifestyle factors and the occurrence of chronic diseases. All of the three cohorts consist of approximate 95% Caucasians.
For the current analysis, we excluded participants who reported CVD, or cancer at baseline (1984 for the NHS, 1991 for the NHS II, and 1986 for the HPFS). We further excluded participants with missing caffeinated or decaffeinated coffee consumption at baseline, those who left more than 70 food items blank or had daily energy intakes < 600 or > 3500 kcal for women and < 800 or > 4200 kcal for men. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Assessment of Coffee Consumption
In 1984, a 116-item food frequency questionnaire (FFQ) was administered to the NHS participants to obtain information on usual intake of food and beverages. Starting in 1986, an expanded 131-item FFQ was administered every 4 years to update diet. Using a similar FFQ, dietary data was collected every four years from the NHS II participants starting in 1991 and from the HPFS participants starting in 1986. In all FFQs, participants were asked how often (from “never or less than once per month” to “6 or more times per day”) on average they consumed a standard portion size of each food item during the previous year. The questionnaire items for coffee included “caffeinated coffee” and “decaffeinated coffee”. Consumption of total coffee was calculated as the sum of intakes of caffeinated and decaffeinated coffee. The validity and reproducibility of the FFQ has been described in detail elsewhere24-27. In brief, the validation study found a correlation coefficient of 0.78 between coffee intake assessed on the baseline FFQ and coffee intake assessed on four 1-week dietary records collected over a one year period26. As mean coffee consumption did not change in NHS 2 and decreased slightly in NHS and HPFS over time (Supplemental figure 1), we used baseline coffee consumption as primary exposure and further conducted several sensitivity analyses using updated dietary information.
Assessment of Covariates
In the biennial follow-up questionnaires, updated information was collected on age, weight, smoking status, physical activity, medication use, family history of diabetes, and self-reported diagnosis of diseases, including hypertension, hypercholesterolemia, CVD, and cancer. For NHS and NHS 2 participants, we also ascertained data on menopausal status and postmenopausal hormone use. We calculated the Alternate Healthy Eating Index (aHEI) as an overall measure of diet quality using FFQ data28.
Assessment of Deaths
Our primary end point was death from any cause. We performed systematic searches of the vital records of states and of the National Death Index. This search was supplemented by reports from family members and postal authorities. Using these methods, we were able to ascertain more than 98% of the deaths in each cohort 29. A physician who was blinded to data on coffee consumption and other risk factors reviewed death certificates and medical records to classify the cause of death according to the eighth and ninth revisions of the International Classification of Diseases. Deaths were grouped into nine major categories (Supplemental Table 1).
Statistical Analysis
We calculated each individual’s person-time from the date of the return of the baseline questionnaire to the date of death or the end of follow-up (31 December 2012 for the NHS, 31 December 2012 for the NHS 2, and 31 December 2012 for the HPFS), whichever came first. We used Cox proportional hazards regression models to examine the association between coffee consumption (five categories) and risk of mortality. The regression models included calendar time in 2-y intervals as the time scale, and were stratified by age in years. In the multivariable analysis, we further adjusted for body mass index (BMI), physical activity, overall dietary pattern (aHEI), total energy intake, smoking status, sugar-sweetened beverage consumption and alcohol consumption, all of which were updated from follow-up questionnaires. We additionally adjusted for baseline hypertension, hypercholesterolemia, and diabetes status in both men and women, and menopausal status, and postmenopausal hormone use among women.
We also used restricted cubic splines with 3 knots to flexibly model the association between coffee consumption and risk of mortality. To test for a potential non-linear association between coffee consumption and risk of mortality, a likelihood ratio test (LRT) was used comparing the model with only the linear term of coffee consumption to the model with both the linear and the cubic spline terms, with P value < 0.05 denoting significant non-linearity. All analyses were performed separately in each cohort, and then pooled to obtain the overall hazard ratio using a fixed-effects model.
Stratified analyses were conducted according to BMI (≤ 25 kg/m2, > 25 kg/m2), age (≤ 70y, > 70y), aHEI (≤ median score, > median score), physical activity (≤ median, > median), smoking status (never smokers, ever smokers), sex (male, female), and individual cohort. We tested for potential effect modification by these stratification variables by including interaction terms between the exposure and potential effect modifier in the multivariate adjusted model, and conducting a likelihood ratio test (LRT) comparing the models with and without interaction terms.
The proportional hazard assumption of the Cox model was tested by adding interaction terms between exposure and the dichotomized indicator of time intervals to the multivariate adjusted model within each cohort, and conducting a likelihood ratio test comparing the models with and without interaction terms.
All statistical tests were 2-sided and performed using SAS version 9.2 for UNIX (SAS Institute Inc).
RESULTS
Coffee Consumption and Dietary and Lifestyle Factors
The percentages of never coffee drinkers were 12% in NHS, 30% in NHS 2, and 17% in HPFS. The percentages of those who drank more than 5 cups/d were 8% in NHS, 3% in NHS 2, and 5% in HPFS. There was a strong correlation between frequent coffee consumption and smoking status (Table 1). The proportions of never smokers among those who did not drink coffee were 63%, 80%, and 71% in NHS, NHS 2, and HPFS respectively, while the proportions of never smokers among those who drank more than 5 cups/d were 24%, 35%, and 25% in NHS, NHS 2, and HPFS. Those who drank coffee more frequently were also more likely to consume alcohol, and consumed less sugar-sweetened beverages and fruits, but more red meats.
Table 1.
Age-adjusted baseline characteristics of participants by frequency of total coffee consumption (including caffeinated and decaffeinated coffee) in NHS, NHS 2, and HPFS
| NHS (1984) | NHS 2 (1991) | HPFS (1986) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cups/d | 0 | ≤ 1 | 1.1 - 3 | 3.1-5 | > 5 | 0 | ≤ 1 | 1.1 - 3 | 3.1-5 | > 5 | 0 | ≤ 1 | 1.1 - 3 | 3.1-5 | > 5 |
| N | 9,233 | 14,740 | 30,420 | 14,760 | 5,737 | 27,888 | 22,837 | 29,239 | 10,049 | 3,041 | 6,863 | 11,402 | 14,264 | 5,861 | 2,167 |
| Age (year) | 48.4 | 50.5 | 50.7 | 50.5 | 50.0 | 35.2 | 35.5 | 37.6 | 38.0 | 38 | 50.9 | 54.0 | 53.7 | 52.9 | 52.0 |
| Caffeinated coffee (cups/d) | 0 | 0.4 | 1.7 | 3.2 | 4.7 | 0 | 0.4 | 1.9 | 3.4 | 5.15 | 0 | 0.4 | 1.6 | 3.0 | 4.3 |
| Decaffeinated coffee (cups/d) |
0 | 0.3 | 0.6 | 1.2 | 1.6 | 0 | 0.2 | 0.4 | 0.9 | 1.1 | 0 | 0.3 | 0. 7 | 1.3 | 2.0 |
| Physical activity (MET-h/wk) |
14.1 | 14.1 | 14.4 | 13.8 | 13.3 | 23.1 | 24.8 | 25.5 | 24.2 | 25.6 | 21.7 | 22.2 | 21.1 | 20.3 | 18.2 |
| aHEI # | 46.9 | 48.0 | 47.8 | 47.8 | 47.3 | 46.0 | 49.0 | 50.2 | 49.7 | 48.5 | 51.9 | 53.7 | 52.7 | 51.7 | 50.2 |
| Total energy intake (kcal/d) | 1720 | 1684 | 1748 | 1786 | 1815 | 1779 | 1768 | 1790 | 1836 | 1883 | 1934 | 1887 | 1973 | 2026 | 2086 |
| Sugar-sweetened beverages (serving/d) |
1.30 | 1.2 | 1.1 | 1.0 | 0.9 | 1.4 | 1.2 | 1.1 | 0.9 | 0.9 | 1.4 | 1.3 | 1.1 | 1.0 | 0.9 |
| Alcohol (grams/d) | 3.7 | 5.8 | 7.8 | 7.7 | 7.1 | 1.6 | 2.7 | 4.3 | 4.4 | 4.2 | 5.7 | 10.0 | 13.1 | 14.1 | 14.8 |
| Dairy (serving/d) | 1.9 | 1.9 | 2.0 | 2.07 | 2.1 | 2.1 | 2.2 | 2.4 | 2.4 | 2.6 | 2.2 | 2.1 | 2.2 | 2.4 | 2.5 |
| Fruits (serving/d) | 2.2 | 2.3 | 2.2 | 2.1 | 1.9 | 1.8 | 2.0 | 1.9 | 1.8 | 1.6 | 2.6 | 2.4 | 2.2 | 2.1 | 2.0 |
| Vegetables (serving/d) | 3.0 | 3.0 | 3.1 | 3.1 | 3.1 | 3.2 | 3.5 | 3.6 | 3.7 | 3.8 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Meats (serving/d) | 1.4 | 1.4 | 1.5 | 1.5 | 1.6 | 1.4 | 1.4 | 1.4 | 1.4 | 1.5 | 1.4 | 1.4 | 1.6 | 1.6 | 1. 8 |
| Fish (serving/d) | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| BMI (kg/m2) | 24.4 | 24.2 | 23.8 | 23.6 | 23.4 | 25.4 | 24.7 | 24.2 | 24.4 | 24.8 | 24.7 | 24.7 | 25.0 | 25.2 | 25. |
| Never smokers, % | 63 | 53 | 42 | 34 | 24 | 80 | 70 | 57 | 47 | 35 | 71 | 54 | 42 | 33 | 25 |
| Hypertension,% | 9 | 10 | 8 | 7 | 6 | 4 | 4 | 3 | 3 | 3 | 18 | 21 | 20 | 19 | 17 |
| Hypercholesterolemia, % | 4 | 4 | 4 | 3 | 3 | 10 | 10 | 9 | 9 | 11 | 10 | 11 | 11 | 10 | 11 |
| Postmenopausal women, % | 48 | 48 | 48 | 49 | 49 | 3 | 3 | 3 | 3 | 4 | NA | NA | NA | NA | NA |
| Current postmenopausal hormone use, (% among total women) |
15 | 14 | 14 | 13 | 12 | 3 | 3 | 3 | 3 | 3 | NA | NA | NA | NA | NA |
aHEI: alternative healthy eating index; BMI: body mass index.
aHEI ranges from 0 – 100, with a higher score indicating healthier diet.
Coffee Consumption and All-cause Mortality
During 28 years of follow-up (1,894,292 person-years) among women in the NHS, we documented 17,468 deaths; during 21 years of follow up (1,882,464 person-years) among women in the NHS 2, we documented 2,056 deaths; during 26 years of follow-up (913,316 person-years) among men in the HPFS, we documented 12,432 deaths. In total, 31,956 deaths were recorded during 4,690,072 person-years of follow-up across all three cohorts.
Age-adjusted analysis showed that the highest categories of consumption of total and caffeinated coffee were associated with a higher risk of all-cause mortality across the three cohorts. The association between consumption of total, caffeinated, and decaffeinated coffee and all-cause mortality attenuated significantly after further adjusting for smoking. Multivariate-adjusted analysis showed a non-linear association between consumption of total, caffeinated, and decaffeinated coffee and all-cause mortality (P values for non-linearity using LRT < 0.001; P values for non-linear trend < 0.001) (Table 2). Relative to no consumption of coffee, the pooled hazard ratio for death was 0.95 (95% CI: 0.91 to 0.99) for ≤ 1cup of total coffee per day, 0.91 (95% CI: 0.88 to 0.95) for 1.1 - 3 cups per day, 0.93 (95% CI: 0.89 to 0.97) for 3.1 - 5 cups per day, and 1.02 (95% CI: 0.96 to 1.07) for > 5 cups per day. Similar results were found when caffeinated and decaffeinated coffee was examined separately. Examining the three cohorts individually, the non-linear associations between consumption of total coffee, caffeinated coffee, and decaffeinated coffee and risk of all-cause mortality were most pronounced in NHS (Table 2, Supplemental figures 2 - 4).
Table 2.
HRs (95% CI) for the association between consumption of total coffee, caffeinated coffee, and decaffeinated coffee and risk of mortality
| Total coffee | ||||||||
| Intake (cups/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | 3.1-5 cups/d | >5 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
| Cases/Person- time |
4,166/ 958,267 |
7,826/ 1,086,683 |
12,198/ 1,681,922 |
5,456/ 709,646 |
2,310/ 253,554 |
|||
| NHS | ||||||||
| Age-adjusted | 1.00 | 0.98 (0.93, 1.04) |
0.91 (0.87, 0.96) |
0.96 (0.91, 1.02) |
1.24 (1.16, 1.33) |
1.02 (1.01, 1.03) |
< 0.001 | < 0.001 |
| Age and smoking-adjusted |
1.00 | 0.94 (0.89, 0.99) |
0.82 (0.78, 0.86) |
0.81 (0.76, 0.86) |
0.92 (0.86, 0.98) |
0.98 (0.97, 0.99) |
< 0.001 | < 0.001 |
| Multivariate- adjusted model |
1.00 | 0.94 (0.88, 0.99) |
0.90 (0.86, 0.95) |
0.93 (0.88, 0.99) |
1.02 (0.95, 1.09) |
1.00 (0.99, 1.01) |
< 0.001 | 0.50 |
| NHS 2 | ||||||||
| Age-adjusted | 1.00 | 0.89 (0.79, 1.00) |
0.83 (0.74, 0.92) |
0.93 (0.80, 1.07) |
1.37 (1.12, 1.67) |
0.98 (0.95, 1.01) |
0.003 | 0.23 |
| Age and smoking-adjusted |
1.00 | 0.86 (0.76, 0.97) |
0.74 (0.66, 0.83) |
0.74 (0.64, 0.86) |
0.92 (0.75, 1.13) |
0.93 (0.90, 0.96) |
0.015 | < 0.001 |
| Multivariate- adjusted model |
1.00 | 0.91 (0.81, 1.03) |
0.84 (0.75, 0.95) |
0.86 (0.74, 1.01) |
1.02 (0.83, 1.26) |
0.96 (0.93, 1.00) |
0.17 | 0.03 |
| HPFS | ||||||||
| Age-adjusted | 1.00 | 1.06 (1.00, 1.12) |
1.05 (0.99, 1.11) |
1.09 (1.02, 1.16) |
1.30 (1.19, 1.42) |
1.02 (1.00, 1.03) |
0.98 | 0.007 |
| Age and smoking-adjusted |
1.00 | 1.00 (0.94, 1.06) |
0.95 (0.90, 1.01) |
0.94 (0.88, 1.01) |
1.05 (0.96, 1.15) |
0.98 (0.94, 1.02) |
0.24 | 0.36 |
| Multivariate- adjusted model |
1.00 | 1.00 (0.94, 1.06) |
0.97 (0.90, 1.01) |
0.95 (0.88, 1.02) |
1.02 (0.93, 1.12) |
0.99 (0.97, 1.00) |
0.13 | 0.04 |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.95 (0.91, 0.99) |
0.91 (0.88, 0.95) |
0.93 (0.89, 0.97) |
1.02 (0.96, 1.07) |
0.98 (0.97, 0.99) |
< 0.001 | < 0.001 |
|
Caffeinated
coffee |
||||||||
| Intake (cups/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | 3.1-5 cups/d | >5 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
| Cases/Person- time |
8,615/ 1,454,869 |
10,105/ 1,404,192 |
8,495/ 1,255,722 |
3,304/ 419,049 |
1,437/ 156,238 |
|||
| NHS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.96 (0.92, 1.00) |
0.92 (0.89, 0.96) |
1.01 (0.96, 1.07) |
1.07 (0.99, 1.14) |
1.00 (0.99, 1.01) |
< 0.001 | 0.78 |
| NHS 2 | ||||||||
| Multivariate- adjusted model |
1.00 | 0.97 (0.86, 1.09) |
0.91 (0.80, 1.02) |
0.99 (0.83, 1.17) |
1.11 (0.88, 1.40) |
0.99 (0.96, 1.02) |
0.38 | 0.47 |
| HPFS | ||||||||
| Multivariate- adjusted model |
1.00 | 1.00 (0.96, 1.05) |
0.94 (0.90, 0.99) |
1.00 (0.93, 1.07) |
1.11 (1.00, 1.24) |
0.99 (0.98, 1.00) |
0.046 | 0.11 |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.97 (0.94, 1.00) |
0.93 (0.90, 0.96) |
1.00 (0.96, 1.05) |
1.08 (1.02, 1.14) |
0.98 (0.97, 0.99) |
0.015 | < 0.001 |
|
Decaffeinated
coffee |
||||||||
| Intake (mg/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | >3 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
|
| Cases/Person- time |
16,393/ 2,607,891 |
10,637/ 1,516,930 |
3,777/ 445,908 |
1,149/ 119,341 |
||||
| NHS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.94 (0.90, 0.97) |
0.92 (0.87, 0.96) |
0.96 (0.89, 1.04) |
0.96 (0.95 0.98) |
0.008 | < 0.001 | |
| NHS 2 | ||||||||
| Multivariate- adjusted model |
1.00 | 0.83 (0.74, 0.92) |
0.86 (0.70, 1.04) |
0.93 (0.64, 1.35) |
0.92 (0.86, 1.00) |
0.009 | 0.035 | |
| HPFS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.91 (0.88, 0.95) |
0.92 (0.87, 0.98) |
0.91 (0.83, 1.01) |
0.97 (0.95, 0.99) |
0.006 | 0.014 | |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.92 (0.89, 0.94) |
0.91 (0.88, 0.94) |
0.94 (0.88, 1.00) |
0.96 (0.94, 0.97) |
< 0.001 | < 0.001 |
A likelihood ratio test was performed.
Multivariate-adjusted model: further adjusted for baseline disease status (hypertension, hypercholesterolemia, diabetes), BMI (< 20.9, 21-22.9, 23-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2), physical activity ( < 3, 3-8.9, 9-17.9, 18-26.9, ≥ 27 MET-h/wk), overall dietary pattern (aHEI score, in quintiles), total energy intake (quintiles), smoking status (never, former (1 - 4 cigarettes/d), former (5 - 14 cigarettes/d), former (15 - 24 cigarettes/d), former (25 - 34 cigarettes/d), former (35 - 44 cigarettes/d), former (≥ 45 cigarettes/d), former (unknown cigarettes/d), current (1 - 4 cigarettes/d), current (5 - 14 cigarettes/d), current (15 - 24 cigarettes/d), current (25 - 34 cigarettes/d), current (35 - 44 cigarettes/d), current (≥ 45 cigarettes/d), current (unknown cigarettes/d)), sugar-sweetened beverage consumption (quintiles) and alcohol consumption (0, 0-5, 5-10, 10-15, ≥ 15 g/d). We additionally adjusted for menopausal status (yes vs. no), and postmenopausal hormone use (yes vs. no) for women. Caffeinated and decaffeinated coffee adjusted for each other.
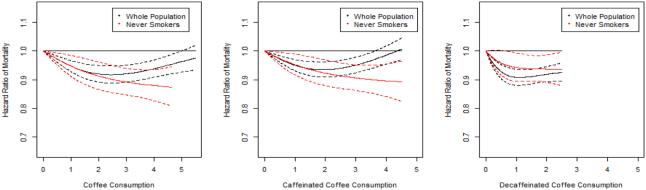
As smoking is a strong confounder of the coffee-mortality relationship, we repeated the analysis among never smokers only. In this analysis, 10,505 deaths were documented during 2,451,970 person-years of follow-up after pooling data from the three cohorts. Overall, the association of total coffee, caffeinated coffee, and decaffeinated coffee consumption with risk of all-cause mortality changed from a non-linear association in the overall population to a linear inverse association when restricting to never smokers (total coffee: P for non-linearity = 0.32, P for linear trend < 0.001; Caffeinated coffee: P for non-linearity = 0.40, P for linear trend < 0.001; Decaffeinated coffee: P for non-linearity = 0.18, P for linear trend = 0.02) (Table 3, Figure 1).
Table 3.
HRs (95% CI) for the association between consumption of total coffee, caffeinated coffee, and decaffeinated coffee and risk of mortality among never smokers
| Total coffee | ||||||||
| Intake (cups/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | 3.1-5 cups/d | >5 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
| Cases/Person-time | 2,190/ 704,010 |
3,032/ 638,687 |
3,759/ 779,966 |
1,211/ 262,069 |
313/ 67,238 |
|||
| NHS | ||||||||
| Age-adjusted model | 1.00 | 0.92 (0.85, 0.99) |
0.80 (0.74, 0.86) |
0.73 (0.66, 0.80) |
0.76 (0.65, 0.88) |
0.93 (0.91, 0.95) |
0.09 | < 0.001 |
| Multivariate- adjusted model |
1.00 | 0.92 (0.85, 0.99) |
0.88 (0.81, 0.95) |
0.84 (0.76, 0.92) |
0.82 (0.71, 0.96) |
0.96 (0.95, 0.98) |
0.09 | < 0.001 |
| NHS 2 | ||||||||
| Age-adjusted model | 1.00 | 0.84 (0.71, 0.98) |
0.84 (0.72, 0.98) |
0.76 (0.60, 0.97) |
0.85 (0.55, 1.32) |
0.95 (0.91, 1.00) |
0.20 | 0.07 |
| Multivariate- adjusted model |
1.00 | 0.89 (0.76, 1.05) |
0.98 (0.84, 1.15) |
0.88 (0.69, 1.13) |
0.91 (0.59, 1.42) |
1.00 (0.95, 1.05) |
0.77 | 0.96 |
| HPFS | ||||||||
| Age-adjusted model |
1.00 | 0.99 (0.90, 1.08) |
0.96 (0.88, 1.05) |
0.83 (0.73, 0.95) |
1.06 (0.84, 1.33) |
0.96 (0.94, 0.99) |
0.20 | 0.005 |
| Multivariate- adjusted model |
1.00 | 0.98 (0.89, 1.08) |
0.97 (0.88, 1.06) |
0.84 (0.73, 0.97) |
0.98 (0.78, 1.24) |
0.96 (0.94, 0.99) |
0.38 | 0.009 |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.94 (0.89, 0.99) |
0.92 (0.87, 0.97) |
0.85 (0.79, 0.92) |
0.88 (0.78, 0.99) |
0.97 (0.95, 0.98) |
0.32 | < 0.001 |
| Caffeinated coffee | ||||||||
| Intake (cups/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | 3.1-5 cups/d | >5 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
| Cases/Person-time | 3,702/ 962,732 |
3,637/ 771,531 |
2,409/ 542,629 |
595/ 138,375 |
162/ 36,700 |
|||
| NHS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.95 (0.89, 1.01) |
0.89 (0.83, 0.96) |
0.84 (0.75, 0.94) |
0.82 (0.67, 1.00) |
0.96 (0.94, 0.98) |
0.34 | < 0.001 |
| NHS 2 | ||||||||
| Multivariate- adjusted model |
1.00 | 0.99 (0.85, 1.15) |
1.07 (0.90, 1.27) |
1.05 (0.78, 1.42) |
1.04 (0.60, 1.81) |
1.02 (0.98, 1.08) |
0.95 | 0.42 |
| HPFS | ||||||||
| Multivariate- adjusted model |
1.00 | 1.03 (0.95, 1.11) |
0.92 (0.84, 1.02) |
0.95 (0.80, 1.14) |
1.06 (0.78, 1.44) |
0.97 (0.95, 1.00) |
0.76 | 0.08 |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.98 (0.93, 1.03) |
0.92 (0.87, 0.97) |
0.89 (0.81, 0.97) |
0.90 (0.77, 1.06) |
0.97 (0.95, 0.99) |
0.40 | < 0.001 |
|
Decaffeinated
coffee |
||||||||
| Intake (mg/d) | 0 | ≤ 1 cup/d | 1.1-3 cups/d | >3 cups/d | Per cup increase |
P for non- linearity* |
P for linear trend |
|
| Cases/Person-time | 5,436/ 1,423,563 |
3,775/ 799,017 |
1,084/ 191,219 |
210/ 38,171 |
||||
| NHS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.97 (0.91, 1.03) |
0.92 (0.85, 1.00) |
0.97 (0.82, 1.14) |
0.97 (0.93, 1.00) |
0.56 | 0.03 | |
| NHS 2 | ||||||||
| Multivariate- adjusted model |
1.00 | 0.82 (0.70, 0.95) |
0.87 (0.65, 1.16) |
0.70 (0.33, 1.47) |
0.93 (0.83, 1.04) |
0.06 | 0.21 | |
| HPFS | ||||||||
| Multivariate- adjusted model |
1.00 | 0.96 (0.88, 1.03) |
0.94 (0.83, 1.06) |
0.70 (0.52, 0.95) |
0.98 (0.93, 1.02) |
0.61 | 0.33 | |
| Pooled | ||||||||
| Multivariate- adjusted model |
1.00 | 0.95 (0.91, 0.99) |
0.93 (0.86, 0.99) |
0.89 (0.77, 1.02) |
0.97 (0.94, 0.99) |
0.18 | 0.02 |
A likelihood ratio test was performed.
Multivariate-adjusted model: further adjusted for baseline disease status (hypertension, hypercholesterolemia, diabetes), BMI (< 20.9, 21-22.9, 23-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2), physical activity (< 3, 3-8.9, 9-17.9, 18-26.9, ≥ 27 MET-h/wk), overall dietary pattern (AHEI score, in quintiles), total energy intake (quintiles), sugar-sweetened beverages consumption (quintiles) and alcohol consumption (0, 0-5, 5-10, 10-15, ≥ 15 g/d). We additionally adjusted for menopausal status (yes vs. no), and postmenopausal hormone use (yes vs. no) for women. Caffeinated and decaffeinated coffee adjusted for each other.
Figure 1.
The association between coffee consumption and risk of mortality in the overall population and among never smokers pooled across the three cohorts. 1a. Total coffee consumption and risk of mortality 1b. Caffeinated coffee consumption and risk of mortality 1c. Decaffeinated coffee consumption and risk of mortality. Multivariate-adjusted models adjusted for age, baseline disease status (hypertension, hypercholesterolemia, diabetes), BMI (< 20.9, 21-22.9, 23-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2), physical activity (< 3, 3-8.9, 9-17.9, 18-26.9, ≥ 27 MET-h/wk), smoking status (never, former (1 - 4 cigarettes/d), former (5 - 14 cigarettes/d), former (15 - 24 cigarettes/d), former (25 - 34 cigarettes/d), former (35 - 44 cigarettes/d), former (≥ 45 cigarettes/d), former (unknown cigarettes/d), current (1 - 4 cigarettes/d), current (5 - 14 cigarettes/d), current (15 - 24 cigarettes/d), current (25 - 34 cigarettes/d), current (35 - 44 cigarettes/d), current (≥ 45 cigarettes/d), current (unknown cigarettes/d)), overall dietary pattern (AHEI score, in quintiles), total energy intake (quintiles), sugar-sweetened beverages consumption (quintiles) and alcohol consumption (0, 0-5, 5-10, 10-15, ≥ 15 g/d). We additionally adjusted for menopausal status (yes vs. no), and postmenopausal hormone use (yes vs. no) for women. Caffeinated and decaffeinated coffee adjusted for each other.
Coffee Consumption and Cause-specific Mortality
The association between coffee consumption and leading causes of mortality was further investigated (Supplemental table 2). In the whole population, coffee consumption was inversely associated with risk of mortality due to CVD, non-linearly associated with risk mortality due to type 2 diabetes, and positively associated with risks of mortality due to lung cancer and respiratory diseases (Supplemental table 3). However, when restricting to never smokers, coffee consumption was no longer associated with risk of mortality due to lung cancer and respiratory disease, but was inversely associated only with risks of mortality due to CVD, neurological disease, and suicide. No associations of coffee consumption with risks of mortality due to colorectal cancer and breast cancer were found (Table 4).
Table 4.
Multivariate HRs (95% CI) for the association between consumption of total coffee and risk of cause-specific mortality among never smokers
| 0 | ≤ 1 cup/d | 1.1-3 cups/d | 3.1-5 cups/d | >5 cups/d | P for nonlinearity* |
|
|---|---|---|---|---|---|---|
|
CVD mortality
(2587 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.95 (0.85, 1.07) |
0.94 (0.84, 1.05) |
0.81 (0.70, 0.95) |
0.91 (0.71, 1.17) |
0.77 |
|
CHD mortality
(1815 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.90 (0.78, 1.03) |
0.90 (0.79, 1.03) |
0.81 (0.68, 0.98) |
0.89 (0.66, 1.20) |
0.90 |
|
Stroke mortality
(656 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 1.04 (0.82, 1.31) |
1.01 (0.80, 1.27) |
0.76 (0.56, 1.04) |
0.93 (0.56, 1.54) |
0.81 |
|
Cancer mortality
(3664 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.99 (0.90, 1.09) |
0.99 (0.90, 1.08) |
0.88 (0.77, 0.99) |
0.84 (0.68, 1.03) |
0.34 |
|
Colorectal cancer mortality
(380 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.88 (0.65, 1.19) |
0.92 (0.69, 1.23) |
0.92 (0.63, 1.33) |
0.94 (0.51, 1.75) |
0.78 |
|
Lung cancer mortality
(217 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 1.11 (0.73, 1.69) |
1.14 (0.76, 1.71) |
1.15 (0.70, 1.90) |
0.89 (0.37, 2.12) |
0.32 |
|
Pancreatic cancer mortality
(321 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 1.47 (1.04, 2.06) |
1.10 (0.78, 1.55) |
1.06 (0.69, 1.62) |
0.41 (0.15, 1.14) |
0.056 |
|
Breast cancer mortality
(567 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.96 (0.76, 1.22) |
0.91 (0.72, 1.14) |
0.79 (0.58, 1.07) |
0.62 (0.36, 1.09) |
0.84 |
|
Premenopausal breast cancer
mortality (77 cases) |
||||||
| Multivariate-adjusted model | NA | |||||
|
Postmenopausal breast cancer
mortality (490 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.94 (0.72, 1.21) |
0.83 (0.64, 1.07) |
0.79 (0.57, 1.08) |
0.67 (0.38, 1.18) |
0.57 |
|
Ovary cancer mortality
(250 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.73 (0.50, 1.05) |
0.85 (0.61, 1.19) |
0.51 (0.31, 0.83) |
0.63 (0.30, 1.33) |
0.71 |
|
Endometrial cancer mortality
(115 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.99 (0.55, 1.80) |
1.24 (0.72, 2.15) |
0.90 (0.43, 1.85) |
2.17 (0.94, 5.05) |
0.26 |
|
Prostate cancer mortality
(210 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.75 (0.51, 1.10) |
0.87 (0.59, 1.28) |
0.74 (0.42, 1.29) |
0.83 (0.30, 2.35) |
0.42 |
|
Respiratory disease mortality
(385 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.91 (0.67, 1.23) |
0.88 (0.66, 1.19) |
0.94 (0.65, 1.36) |
0.62 (0.30, 1.31) |
0.31 |
|
Neurological disease mortality
(243 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.91 (0.62, 1.32) |
0.80 (0.55, 1.15) |
0.63 (0.39, 1.01) |
0.79 (0.38, 1.62) |
0.83 |
|
Diabetes mortality
(128 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.96 (0.59, 1.56) |
0.67 (0.40, 1.11) |
0.76 (0.38, 1.49) |
0.76 (0.26, 2.20) |
0.19 |
|
Injury mortality
(233 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 1.01 (0.70, 1.46) |
0.90 (0.62, 1.31) |
0.71 (0.42, 1.22) |
1.28 (0.62, 2.65) |
0.28 |
|
Suicide mortality
(134 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 1.36 (0.88, 2.10) |
0.73 (0.45, 1.21) |
0.64 (0.30, 1.35) |
0.80 (0.24, 2.65) |
0.58 |
|
Other disease mortality
(3108 cases) |
||||||
| Multivariate-adjusted model | 1.00 | 0.86 (0.77, 0.95) |
0.81 (0.73, 0.90) |
0.81 (0.71, 0.93) |
0.94 (0.76, 1.16) |
0.011 |
A likelihood ratio test was performed.
The model adjusted for age, baseline disease status (hypertension, hypercholesterolemia, diabetes), BMI (< 20.9, 21-22.9, 23-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2), physical activity (< 3, 3-8.9, 9-17.9, 18-26.9, ≥ 27 MET-h/wk), overall dietary pattern (AHEI score, in quintiles), total energy intake (quintiles), sugar-sweetened beverages consumption (quintiles) and alcohol consumption (0, 0-5, 5-10, 10-15, ≥ 15 g/d). We additionally adjusted for menopausal status (yes vs. no), and postmenopausal hormone use (yes vs. no) for women. Caffeinated and decaffeinated coffee adjusted for each other.
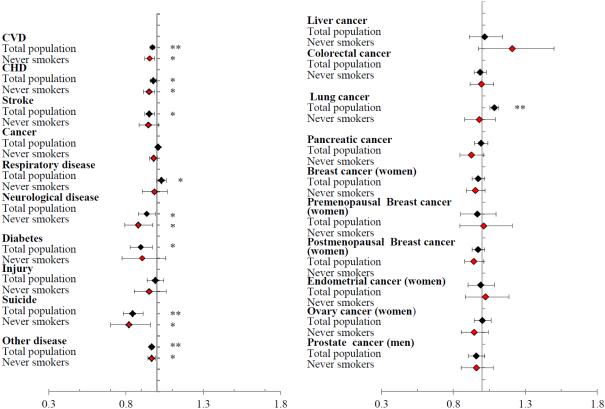
In the total population, a 1-cup per day increment in coffee consumption was positively associated with mortality due to lung cancer (P < 0.0001) and respiratory disease (P < 0.05), and inversely associated with mortality due to CHD, stroke, neurological disease, and type 2 diabetes (P < 0.05). However, after restricting to never smokers, the positive association disappeared for lung cancer and respiratory disease and significant inverse associations remained for mortality due to CHD, neurological disease, and suicide (P < 0.05) (Figure 2).
Figure 2.
The association of a 1-cup per day increment in coffee consumption with risk of cause-specific mortality pooled across the three cohorts. The black squares stand for the overall population. The red squares stand for never smokers. * P value < 0.05. ** P value < 0.001. Multivariate-adjusted models adjusted for age, baseline disease status (hypertension, hypercholesterolemia, diabetes), BMI (< 20.9, 21-22.9, 23-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2), physical activity (< 3, 3-8.9, 9-17.9, 18-26.9, ≥ 27 MET-h/wk), smoking status (never, former (1 - 4 cigarettes/d), former (5 - 14 cigarettes/d), former (15 - 24 cigarettes/d), former (25 - 34 cigarettes/d), former (35 - 44 cigarettes/d), former (≥ 45 cigarettes/d), former (unknown cigarettes/d), current (1 - 4 cigarettes/d), current (5 - 14 cigarettes/d), current (15 - 24 cigarettes/d), current (25 - 34 cigarettes/d), current (35 - 44 cigarettes/d), current (≥ 45 cigarettes/d), current (unknown cigarettes/d)), overall dietary pattern (AHEI score, in quintiles), total energy intake (quintiles), sugar-sweetened beverages consumption (quintiles) and alcohol consumption (0, 0-5, 5-10, 10-15, ≥ 15 g/d). We additionally adjusted for menopausal status (yes vs. no), and postmenopausal hormone use (yes vs. no) for women. Caffeinated and decaffeinated coffee adjusted for each other.
Stratified Analysis
Significant interactions were found between coffee consumption and risk of mortality by age (P for interaction = 0.003) and smoking (P for interaction = 0.015) (Supplemental table 4). The association appeared to be stronger among those aged < 70 years than older individuals and was stronger among never smokers than smokers. There were no significant differences in the associations between coffee consumption and risk of total mortality when stratified by aHEI score, BMI, physical activity, sex, and cohort.
The proportional hazard assumption was violated in NHS (but not in NHS 2 or HPFS), with a stronger association between coffee consumption and mortality in earlier time intervals. However, non-linear associations between coffee consumption and risk of mortality were found in both subgroups stratified by time interval in NHS (Supplemental table 5). We further assessed the proportional hazard assumption among never smokers in NHS, and the proportional hazard assumption was no longer violated (P for interaction = 0.60).
Sensitivity Analysis
We further evaluated the association between coffee consumption and mortality using cumulatively updated coffee consumption and stopping updating of coffee consumption when intermediate diseases developed (hypertension, hypercholesterolemia, type 2 diabetes, cancer, and CVD); using time-varying coffee consumption with 4-year lag; using time-varying coffee consumption adjusting for hypercholesterolemia as a time-varying covariate; and using baseline coffee consumption excluding the hypertensive or hypercholesterolemia cases at baseline. The associations between consumption of total coffee, caffeinated coffee, and decaffeinated coffee and risk of mortality did not change substantially in these analyses (Supplemental table 6-7, Supplemental figure 5 - 9).
To further evaluate whether the change of the association from non-linear in the whole population to inverse linear among never smokers was due to the differences in the composition of total mortality between the overall population and never smokers, Cox models with inverse probability weighting were applied in the never smokers assessing the association between coffee consumption and risk of mortality and the results did not change substantially (Supplemental table 8).
DISCUSSION
In this analysis of three large ongoing cohort studies, we observed a non-linear association between coffee consumption and risk of mortality in the overall population, with moderate coffee consumption being associated with lower mortality risk, and high coffee consumption not being associated with mortality risk. Given that this association became linear and inverse after restricting to never smokers, it is likely that the non-linear association observed in the total population was due to the residual confounding by smoking. This was further strengthened by the observation that the positive association between coffee consumption and death due to lung cancer and respiratory diseases in the overall population, for both of which smoking is an important risk factor, disappeared when restricting to never smokers. The inverse association between coffee consumption and risk of mortality did not change substantially when using a weighted Cox model among never smokers, excluding the possibility that the different associations in overall population and never smokers were due to the different composition of total mortality. For both caffeinated and decaffeinated coffee consumption, the non-linear associations in the total population and the inverse associations among the never smokers decreased the possibility that the non-linear association was due to the biological effect of caffeine.
Our results for the associations between coffee consumption and cause-specific mortality are consistent with the associations between coffee consumption and cause-specific diseases from previous studies. Numerous prospective cohort studies have shown coffee consumption to be associated with lower risk of type 2 diabetes 1. There are several plausible biological mechanisms that could explain this observation. The chlorogenic acid, lignans, quinides, trigonelline, and magnesium in coffee reduce insulin resistance and systematic inflammation 30, 31,32-34,35. Chlorogenic acid may have this putative effect by reducing glucose absorption in the intestine by competitively inhibiting glucose-6-phosphate translocase and reducing sodium-dependent glucose transport in the brush border membrane vesicles 36; by reducing oxidative stress as a result of its antioxidant properties; and by reducing liver glucose output 37. In our study, an inverse association between coffee drinking and risk of mortality due to CVD was observed. Given that diabetes and CVD share common disease pathways, the mechanism of inverse association between coffee consumption and risk of CVD mortality might be similar to that for diabetes mortality. Studies have also shown coffee consumption to be associated with a lower risk of Parkinson’s diseases (PD) 6, 38, 39, which is consistent with our finding of an inverse association between coffee consumption and risk of neurological mortality. In a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin model of PD, caffeine was shown to attenuate MPTP - induced striatal dopamine loss, striatal dopamine transporter binding sites loss, and dopaminergic neurons loss, which might be mediated through A2A adenosine receptors 40. Three published cohort studies have shown an inverse association between coffee consumption and risk of suicide 17, 41, 42, however, one study showed a J-shaped association where heavy coffee consumption was associated with a higher risk of suicide 43. Our study had shown an inverse association of both caffeinated and decaffeinated coffee consumption with risk of suicide in both the whole population and never smokers, indicating that coffee consumption might have antidepressant effects. Studies have shown an inverse association between coffee consumption and risk of liver diseases or risk of mortality due to liver diseases2, 44-47, however, no association of coffee consumption with risk of mortality due to liver diseases was found in our study, which might be due to the limited power given the small number of cases. Previous cohort studies showed no association between coffee consumption and risk of colorectal cancer 48, 49, which was consistent with our results.
Our results showed similar associations of caffeinated and decaffeinated coffee consumption with risk of total and cause-specific mortality in both the overall population and never smokers, further showing that other components in coffee besides caffeine might play a beneficial role mediating the association between long-term coffee consumption and risk of mortality. However, short-term metabolic studies have shown that caffeine could acutely increase blood pressure by antagonizing the adenosine A1 and A2A receptor 50-52, and could also acutely adversely affect arterial stiffness and endothelium dependent vasodilation 53, 54. Case crossover studies showed that coffee consumption transiently increased the risk of nonfatal myocardial infarction, ischemic stroke onset, and sudden cardiac death 55-57. One cohort study assessed the association of coffee consumption with total mortality in subsequent 2 years among CVD participants, and no association was found 14. However, it is still difficult to differentiate acute effects from long-term effects of habitual coffee consumption.
Our analysis has several strengths. The large sample size, long follow-up time, and a large number of deaths provided sufficient power to detect a non-linear association in the overall population and to perform further analyses among never smokers. The large number of deaths also allowed us to conduct analyses on cause-specific mortality. In addition, we had detailed measures of both caffeinated and decaffeinated coffee consumption as well as other dietary and lifestyle factors.
Several potential limitations also need to be considered. First, given the observational nature of the study design, we could not directly establish a cause-effect relationship between coffee and mortality. Second, assessment of coffee intake was based on FFQs and thus measurement errors are inevitable. However, our validation studies have demonstrated high validity (Pearson correlation = 0.74) of the coffee intake by the FFQs as compared to multiple week diet records, and high reproducibility (Pearson correlation = 0.80) by comparing two consecutive FFQs 26. Moreover, coffee intake was also one of the food items showing the highest validity and reproducibility by the FFQs in Europe 58 and Asia 59, 60, indicating that coffee was a beverage less prone to misreporting. In addition, the use of the repeated measures of diet not only represented long-term habitual intake, but also reduced the influences of measurement errors. Finally, since our cohort participants comprise medical and health professionals and the vast majority of them are white, the results may not be generalizable to other populations.
In conclusion, regular consumption of coffee was inversely associated with risk of total mortality and mortality due to CVD, and neurological disease. Similar associations of caffeinated and decaffeinated coffee consumption with risk of total and cause-specific mortality were found. Results from this and previous studies indicate that coffee consumption can be incorporated into a healthy lifestyle.
Supplementary Material
Acknowledgements
We would like to thank the participants and staff of the NHS, NHS2, and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding Sources: The study was supported by research grants UM1 CA186107, UM1 CA176726, UM1 CA167552, P01 CA87969, P01 CA055075, R01 HL034594, HL088521, HL35464 and HL60712 from the National Institutes of Health.
RVD received research funding from Nestec S.A., Vevey, Switzerland.
Footnotes
Disclosures: None of the authors had any financial or personal conflict of interest to disclose.
References
- 1.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, Tamakoshi A, Group JS. Coffee and risk of death from hepatocellular carcinoma in a large cohort study in japan. Br J Cancer. 2005;93:607–610. doi: 10.1038/sj.bjc.6602737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Je Y, Giovannucci E. Coffee consumption and risk of endometrial cancer: Findings from a large up-to-date meta-analysis. Int J Cancer. 2012;131:1700–1710. doi: 10.1002/ijc.27408. [DOI] [PubMed] [Google Scholar]
- 4.Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, Giovannucci E, Mucci LA. Coffee consumption and prostate cancer risk and progression in the health professionals follow-up study. J Natl Cancer Inst. 2011;103:876–884. doi: 10.1093/jnci/djr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song F, Qureshi AA, Han J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012;72:3282–3289. doi: 10.1158/0008-5472.CAN-11-3511. [DOI] [PubMed] [Google Scholar]
- 6.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 7.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama K, Kuriyama S, Akhter M, Kakizaki M, Nakaya N, Ohmori-Matsuda K, Shimazu T, Nagai M, Sugawara Y, Hozawa A, Fukao A, Tsuji I. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in japanese women. J Nutr. 2010;140:1007–1013. doi: 10.3945/jn.109.109314. [DOI] [PubMed] [Google Scholar]
- 9.Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr. 2013;143:1299–1308. doi: 10.3945/jn.112.173807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–914. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Lopez-Garcia E, Li TY, Hu FB, van Dam RM. Coffee consumption and risk of cardiovascular diseases and all-cause mortality among men with type 2 diabetes. Diabetes Care. 2009;32:1043–1045. doi: 10.2337/dc08-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang WL, Lopez-Garcia E, Li TY, Hu FB, van Dam RM. Coffee consumption and risk of cardiovascular events and all-cause mortality among women with type 2 diabetes. Diabetologia. 2009;52:810–817. doi: 10.1007/s00125-009-1311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Mukamal KJ, Hu FB, van Dam RM. Coffee consumption and mortality in women with cardiovascular disease. Am J Clin Nutr. 2011;94:218–224. doi: 10.3945/ajcn.110.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, Tavani A. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol. 2013;28:527–539. doi: 10.1007/s10654-013-9834-7. [DOI] [PubMed] [Google Scholar]
- 16.Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: A dose-response meta-analysis. Am J Epidemiol. 2014;180:763–775. doi: 10.1093/aje/kwu194. [DOI] [PubMed] [Google Scholar]
- 17.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3:375–381. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- 18.LeGrady D, Dyer AR, Shekelle RB, Stamler J, Liu K, Paul O, Lepper M, Shryock AM. Coffee consumption and mortality in the chicago western electric company study. Am J Epidemiol. 1987;126:803–812. doi: 10.1093/oxfordjournals.aje.a114717. [DOI] [PubMed] [Google Scholar]
- 19.Lindsted KD, Kuzma JW, Anderson JL. Coffee consumption and cause-specific mortality. Association with age at death and compression of mortality. J Clin Epidemiol. 1992;45:733–742. doi: 10.1016/0895-4356(92)90051-n. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Sui X, Lavie CJ, Hebert JR, Earnest CP, Zhang J, Blair SN. Association of coffee consumption with all-cause and cardiovascular disease mortality. Mayo Clin Proc. 2013;88:1066–1074. doi: 10.1016/j.mayocp.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Je Y, Giovannucci E. Coffee consumption and total mortality: A meta-analysis of twenty prospective cohort studies. Br J Nutr. 2014;111:1162–1173. doi: 10.1017/S0007114513003814. [DOI] [PubMed] [Google Scholar]
- 22.O'Keefe JH, Bhatti SK, Patil HR, DiNicolantonio JJ, Lucan SC, Lavie CJ. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J Am Coll Cardiol. 2013;62:1043–1051. doi: 10.1016/j.jacc.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: A dose-response meta-analysis. Am J Epidemiol. 2014;180:763–75. doi: 10.1093/aje/kwu194. doi: 10.1093/aje/kwu194. Epub 2014 Aug 24. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 26.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: The alternate healthy eating index. Public Health Nutr. 2006;9:152–157. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 29.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 30.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 31.Williams CJ, Fargnoli JL, Hwang JJ, Van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care. 2008;31:504–507. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dam RM. Coffee and type 2 diabetes: From beans to beta-cells. Nutr Metab Cardiovasc Dis. 2006;16:69–77. doi: 10.1016/j.numecd.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Van Dijk AE, Olthof MR, Meeuse JC, Seebus E, Heine RJ, Van Dam RM. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care. 2009;32:1023–1025. doi: 10.2337/dc09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr. 2006;84:682–693. doi: 10.1093/ajcn/84.4.682. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27:134–140. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 36.Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, Schubert G, Below P, Herling AW. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339:315–322. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 37.Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Strom EC, Jacobs DR, Jr., Ose L, Blomhoff R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr. 2004;134:562–567. doi: 10.1093/jn/134.3.562. [DOI] [PubMed] [Google Scholar]
- 38.Saaksjarvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Mannisto S. Prospective study of coffee consumption and risk of parkinson's disease. Eur J Clin Nutr. 2008;62:908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 39.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 40.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr., Schwarzschild MA. Neuroprotection by caffeine and a(2a) adenosine receptor inactivation in a model of parkinson's disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas M, O'Reilly EJ, Pan A, Mirzaei F, Willett WC, Okereke OI, Ascherio A. Coffee, caffeine, and risk of completed suicide: Results from three prospective cohorts of american adults. World J Biol Psychiatry. 2014;15:377–386. doi: 10.3109/15622975.2013.795243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawachi I, Willett WC, Colditz GA, Stampfer MJ, Speizer FE. A prospective study of coffee drinking and suicide in women. Arch Intern Med. 1996;156:521–525. [PubMed] [Google Scholar]
- 43.Tanskanen A, Tuomilehto J, Viinamaki H, Vartiainen E, Lehtonen J, Puska P. Heavy coffee drinking and the risk of suicide. Eur J Epidemiol. 2000;16:789–791. doi: 10.1023/a:1007614714579. [DOI] [PubMed] [Google Scholar]
- 44.Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 45.Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: A meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 46.Lai GY, Weinstein SJ, Albanes D, Taylor PR, McGlynn KA, Virtamo J, Sinha R, Freedman ND. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. Br J Cancer. 2013;109:1344–1351. doi: 10.1038/bjc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tverdal A, Skurtveit S. Coffee intake and mortality from liver cirrhosis. Ann Epidemiol. 2003;13:419–423. doi: 10.1016/s1047-2797(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 48.Tavani A, La Vecchia C. Coffee, decaffeinated coffee, tea and cancer of the colon and rectum: A review of epidemiological studies, 1990-2003. Cancer Causes Control. 2004;15:743–757. doi: 10.1023/B:CACO.0000043415.28319.c1. [DOI] [PubMed] [Google Scholar]
- 49.Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. Int J Cancer. 2009;124:1662–1668. doi: 10.1002/ijc.24124. [DOI] [PubMed] [Google Scholar]
- 50.Nurminen ML, Niittynen L, Korpela R, Vapaatalo H. Coffee, caffeine and blood pressure: A critical review. Eur J Clin Nutr. 1999;53:831–839. doi: 10.1038/sj.ejcn.1600899. [DOI] [PubMed] [Google Scholar]
- 51.Mesas AE, Leon-Munoz LM, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: A systematic review and meta-analysis. Am J Clin Nutr. 2011;94:1113–1126. doi: 10.3945/ajcn.111.016667. [DOI] [PubMed] [Google Scholar]
- 52.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 53.Karatzis E, Papaioannou TG, Aznaouridis K, Karatzi K, Stamatelopoulos K, Zampelas A, Papamichael C, Lekakis J, Mavrikakis M. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: Should we consider monitoring central blood pressure? Int J Cardiol. 2005;98:425–430. doi: 10.1016/j.ijcard.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: The role of caffeine. Clin Sci (Lond) 2005;109:55–60. doi: 10.1042/CS20040358. [DOI] [PubMed] [Google Scholar]
- 55.Baylin A, Hernandez-Diaz S, Kabagambe EK, Siles X, Campos H. Transient exposure to coffee as a trigger of a first nonfatal myocardial infarction. Epidemiology. 2006;17:506–511. doi: 10.1097/01.ede.0000229444.55718.96. [DOI] [PubMed] [Google Scholar]
- 56.Selb Semerl J, Selb K. Coffee and alcohol consumption as triggering factors for sudden cardiac death: Case-crossover study. Croat Med J. 2004;45:775–780. [PubMed] [Google Scholar]
- 57.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: The stroke onset study. Neurology. 2010;75:1583–1588. doi: 10.1212/WNL.0b013e3181fb443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Date C, Fukui M, Yamamoto A, Wakai K, Ozeki A, Motohashi Y, Adachi C, Okamoto N, Kurosawa M, Tokudome Y, Kurisu Y, Watanabe Y, Ozasa K, Nakagawa S, Tokui N, Yoshimura T, Tamakoshi A. Reproducibility and validity of a self-administered food frequency questionnaire used in the jacc study. J Epidemiol. 2005;15(Suppl 1):S9–23. doi: 10.2188/jea.15.S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the tehran lipid and glucose study. J Epidemiol. 2010;20:150–158. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferraroni M, Tavani A, Decarli A, Franceschi S, Parpinel M, Negri E, La Vecchia C. Reproducibility and validity of coffee and tea consumption in italy. Eur J Clin Nutr. 2004;58:674–680. doi: 10.1038/sj.ejcn.1601864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.