Abstract
Rationale
The Sigma-1 receptor (Sig-1R) is a chaperone protein that has been implicated in drug abuse and addiction. Multiple studies have characterized the role the Sig-1R plays in psychostimulants addiction, but fewer studies have specifically investigated its role in alcohol addiction. We have previously shown that antagonism of the Sig-1R reduces excessive drinking and motivation to drink, whereas agonism induces binge-like drinking in rodents.
Objectives
The objectives of these studies were to investigate the impact of Sig-1R gene deletion in C57Bl/6J mice on ethanol drinking and other ethanol-related behaviors.
Methods
We used an extensive panel of behavioral tests to examine ethanol actions in male, adult mice lacking Oprs1, the gene encoding the Sig-1R. To compare ethanol drinking behavior, Sig-1 knockout (KO) and wild type (WT) mice were subject to a two-bottle choice, continuous access paradigm with different concentrations of ethanol (3%–20% v/v) vs. water. Consumption of sweet and bitter solutions was also assessed in Sig-1R KO and WT mice. Finally, motor stimulant sensitivity, taste aversion and ataxic effects of ethanol were assessed.
Results
Sig-1R KO mice displayed higher ethanol intake compared to WT mice; the two genotypes did not differ in their sweet or bitter taste perception. Sig-1R KO mice showed lower sensitivity to ethanol stimulant effects, but greater sensitivity to its taste aversive effects. Ethanol-induced sedation was unaltered in the mutants.
Conclusions
Our results suggest that the deletion of the Sig-1R increases ethanol consumption, likely by decreasing its rewarding effects, and therefore indicating that the Sig-1R is involved in modulation of the reinforcing effects of alcohol.
Keywords: Drinking, consumption, Oprs1, mutant, reinforcement, addiction
Introduction
Alcoholism constitutes one of the most serious global public health problems. The World Health Organization estimates that about 2 billion people worldwide consume alcoholic beverages [1], 76.3 million of which have alcohol use disorders. Hallmarks of alcohol addiction include a compulsion to seek and drink alcohol, a loss of control to limit intake, and the emergence of a negative emotional state reflecting a motivational withdrawal when access is prevented [2, 3]. Although significant progress has been made to understand the neurobiology of alcoholism, effective treatments remain elusive.
Originally, and mistakenly, categorized as members of the opiate receptor family or high-affinity phencyclidine binding sites [4, 5], sigma receptors have been proposed to play a role in the etiopathology of many psychiatric conditions. Today, two different isoforms are known, sigma-1 (Sig-1R) and sigma-2 (Sig-2R), which differ in binding profile and molecular weight [6, 7]; however, only Sig-1R has so far been cloned. Sig-1Rs are intracellular chaperones residing at the endoplasmic reticulum-mitochondrion interface [8–10] where they regulate calcium signaling. Sig-1Rs have been shown to translocate to other parts of the cell [10, 11] where they can bind to various ion channels, receptors and kinases, resulting in the modulation of multiple neurotransmitter systems such as glutamate, acetylcholine, and dopamine [12–17]. The existence of an endogenous ligand for Sig-1R is still under debate, although certain neurosteroids and the trace amine N,N-dimethyltryptamine have been proposed [18]. Sig-1Rs are predominantly expressed in the central nervous system, in particular in limbic regions and brainstem nuclei [19, 20].
Recent findings have suggested that compounds targeting Sig-1Rs may represent a new class of therapeutics aimed at treating alcohol use disorders. In vivo preclinical studies are starting to reveal that Sig-1R ligands can ameliorate the behavioral effects of many drugs of abuse including cocaine, methamphetamine, and alcohol [21–26]. Sig-1R antagonists have been shown in rodent models to reduce ethanol consumption, the motivation to work to obtain ethanol, and the alcohol deprivation effect selectively in animal models of excessive drinking [27–29]. Demonstrating selectivity of action, Sig-1R antagonists were shown not to affect the intake of sweet solutions [27, 28], suggesting that the Sig-1R is not involved in the motivation for natural rewards. Sig-1 antagonists have also been shown to attenuate ethanol-induced locomotion and ethanol-induced place and taste conditioning in mice [30]. On the other hand, Sig-1R agonists have been shown to induce alcohol binge-like drinking [31], suggesting bi-directionality of action. In addition, inbred mouse strains with greater ethanol preference display increased Sig-1R expression relative to more ethanol-averse mouse strains [32]. In humans, an association has been demonstrated between functional polymorphisms in the Sig-1R gene and alcoholism [33].
In light of the above-cited findings, the aim of the present study was to investigate the role of endogenous Sig-1Rs in the regulation of ethanol-related behaviors using a genetic approach. For this purpose, we used mutant mice lacking the Oprs1 gene, which encodes for the Sig-1R, to investigate ethanol drinking behavior, as well as sensitivity to ethanol-induced motor stimulation, aversion and ataxia, as compared to wild type mice.
Materials and Methods
Animals
Mice lacking the Oprs1 gene were generated as previously described in [34]. Mice, originally of a mixed background, were backcrossed onto a C57BL/6J strain for >10 generations to obtain a background pure null mutant mice (Oprs1−/−, Sig-1R KO). For control wild types (WT), age-matched C57BL6/J male mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). 9–13-week old mice were group-housed with food and water ad libitum, unless otherwise specified, in a humidity- and temperature-controlled AAALAC-approved vivarium on a 12 hr reverse light/dark cycle. All experiments were performed during the mice dark cycle. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Principles of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee (IACUC) of Boston University.
Drugs
The ethanol solution for injections (20% v/v, administered intraperitoneally -i.p.-) was prepared diluting 200-proof ethanol (Pharmco-Aaper Inc., Brookfield, CT) in isotonic saline. Ethanol solutions for drinking experiments (3%, 6%, 10% and 20% v/v) were prepared using 190-proof ethanol and tap water. Saccharin (0.02% and 0.07% w/v), quinine (0.03 and 0.1 mM) and sodium chloride (NaCl, 0.2 M) solutions were prepared dissolving saccharin sodium salt hydrate, quinine hemisulfate salt monohydrate (both from Sigma, St. Louis, MO), and sodium chloride (Fisher Scientific, Agawam, MA), respectively, in tap water.
The partial inverse agonist of the benzodiazepine (BDZ) receptor Ro 15-4513 (R&D Systems, Inc., Minneapolis, MN) was dissolved in DMSO (10% v/v) and then diluted with isotonic saline. Ro 15-4513 was injected i.p. in a volume of 10 ml/kg.
Voluntary drinking of ethanol
Ethanol naïve WT and Sig-1R KO mice were allowed to acclimate to single-housing in their home cage. After acclimation, the mice learned to drink water from two 50 ml conical tubes with rubber stoppers and metal double-ball sipper tubes, which produce negligible spillage. Mice body weights were recorded every 6 days.
A first set of WT and Sig-1R KO mice (body weight 26.9±0.4, mean±SEM) was exposed to escalating concentrations of an ethanol solution for 6 days each (3 %, 6 %, and 20% v/v), in their home cage in a continuous access (24 hr/day), two-bottle choice paradigm vs. water. The two tubes were weighed daily and offered right before the dark cycle onset. Bottle positions were alternately changed to avoid development of place preference (ethanol on the right side on days 1, 3 and 4); data from the first two days of access to each solution were excluded from data analysis in order to avoid bias due to the novelty of each tastant and the stress of cage changing.
A second set of WT and Sig-1R KO mice (body weight 30.1±0.7, mean±SEM) was exposed to a 10% v/v ethanol solution in their home cage in a continuous access (24 hr/day), two-bottle choice paradigm vs. water for 2 consecutive weeks.
Throughout the experiments, spillage estimates were calculated by weighing two bottles placed in empty cages, one filled with water and the other containing the appropriate solution. Spillage, however, was negligible. Solution intake was recorded by weighing the bottles before and after every access (precision 0.01 g). Solution intake was normalized to body weight; preference was calculated as the ratio percentage between the volume of tastant solution consumed and the total fluid intake.
Voluntary drinking of sweet and bitter solutions
Ethanol naïve WT and Sig-1R KO (body weight 28.9±0.3, mean±SEM) were tested for their preference for either sweet (saccharin) or bitter (quinine) solutions. The same two-bottle choice protocol used for ethanol was copied here, instead offering 6 days each of escalating concentrations of either saccharin (0.02 % and 0.07 %) or quinine (0.03 mM and 0.1 mM) solutions vs. water. Between tastants, mice were given a washout period of water for two weeks. Solution intake was recorded by weighing the bottles before and after every access (precision 0.01 g) and intake was normalized to body weight.
Locomotor activity test
Ethanol naïve WT and Sig-1R KO mice (body weight 31.8±1.3, mean±SEM) were tested for the ethanol-induced motor stimulation. The BDZ receptor partial inverse agonist Ro 15-4513 was administered right before ethanol injections in order to unmask its motor stimulating effect, previously reported to be unobservable in C57Bl/6 mice [35, 36]. A locomotor activity test was performed in Plexiglas chambers (27×48×20 cm) using an Opto-M3 activity system (Columbus Instruments, Columbus, OH), as reported before [37, 38]. The Opto-M3 system consists of a series of 16 sensor beams spaced 2.54 cm apart along the longest side of the cage; motor activity was recorded by a computer over a 20 min period, which began 2 min after mice were pre-treated with Ro 15-4513 (0, 3 mg/kg; i.p.), and 1 min after ethanol (0, 1.5 g/kg; i.p.). Doses and pre-treatment time were chosen based on previously published reports [38–40]; test days were separated by one or two treatment-free wash out days.
Conditioned taste aversion
The conditioned taste aversion (CTA) was performed as previously described with minor adaptations [41, 42]. WT and Sig-R1 KO (body weight 33.1±0.5, mean±SEM) were habituated to single-housing and water administration from a 50 ml conical tube with a rubber stopper and a metal double-ball sipper tube. Mice were then placed on a 2 hr restricted access to water for 8 consecutive days; water access began at the third hour of the dark cycle. Mice were matched for their water intake and body weight, and assigned to either a control group or an ethanol group. On day 9, 11 and 13 mice were given 1 hr access to a 0.2 M NaCl solution, instead of water, as a tasty conditioned stimulus. Immediately after NaCl access, mice were injected with ethanol (0, 3 g/kg, i.p.) using a between-subjects design. To prevent possible dehydration, mice were given access to water for an additional 30 min, 4 hr after each NaCl access. On day 10 and 12, mice were given access to water for 2 hr. Data from days 11 and 13 were used for data analysis.
Loss of righting reflex and blood alcohol levels determination
The ethanol-induced loss of righting reflex (LORR) experiment was determined as previously described [43, 44]. Ethanol naïve WT and Sig-1R KO mice were administered ethanol (4 g/kg, i.p., as in [43]) and placed on a V-shaped surface. Latency to lose the righting reflex and sleep duration were recorded using a stop watch. The latency was defined as the time between the ethanol injection and the time when the mouse was unable to right itself from a supine position for at least 30 sec. Any mouse that did not lose the righting reflex within 5 min from the time of injection was excluded from the experiment. Mice remained undisturbed in a supine position until they could right themselves onto all four paws twice within a 30 sec period.
Blood from WT and Sig-1R KO mice (n=10/genotype) was collected from tails 12 min post-ethanol administration. Blood samples were centrifuged at 3,000 rpm for 20 min at 4 °C and plasma samples were then assayed to determine blood alcohol concentrations (BALs) using an oxygen-rate alcohol analyzer (Analox Instruments, Lunenburg, MA).
Statistical analysis
Data from the drinking behavior experiments were analyzed using a mixed design three-way ANOVA, with Genotype as a between-subjects factor, and Concentration and Day as within-subject factors. The incremental locomotor activity data were analyzed by a mixed design three-way ANOVA, with Genotype as a between-subjects factor, and Treatments and Time as within-subject factors. The CTA data were analyzed using a mixed design three-way ANCOVA with Genotype and Ethanol treatment as between-subjects factors, and Day as a within-subject factor. When a statistically significant overall effect and/or interactions were observed, pairwise post-hoc comparisons were performed using the Student’s t test to compare two groups, and Student Newman Keuls for all other comparisons.
The statistical software used were Systat 11.0 (SPSS, Chicago, IL), Instat 3.0 (GraphPad, San Diego, CA), and Statistica 7.0 (StatSoft. Inc., Tulsa, OK). The graphical software used was SigmaPlot 11.0 (Systat Sofware Inc., Chicago, IL).
Results
Sig-1R KO mice exhibit higher ethanol intake and preference
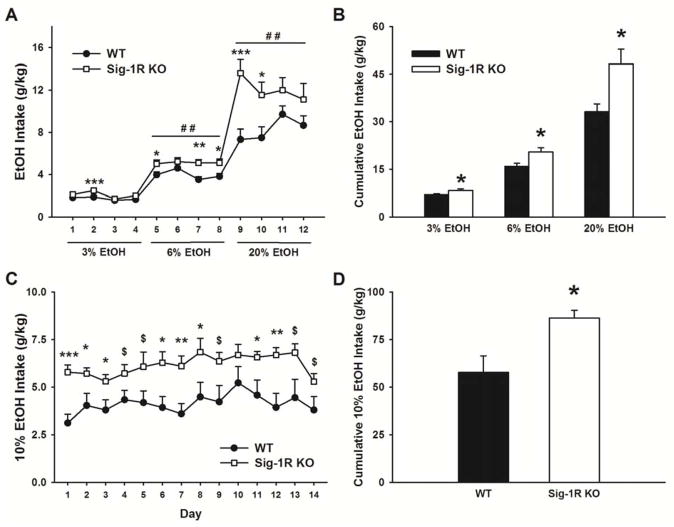
Mice lacking the Sig-1R showed significant differences in ethanol intake compared to WT mice when exposed to ascending concentrations of an alcohol solution (3 %, 6 %, and 20 % v/v) [Genotype: F(1,17)= 10.41, p<0.01; Genotype X Concentration: F(2,34)= 5.71, p<0.01; Genotype X Concentration X Day: F(6,102)= 3.26, p<0.01]. As shown in Fig. 1A and 1B, Sig-1R KO mice drank significantly more ethanol than WT mice at all 3 concentrations [3 % v/v: Genotype: F(1,17)= 5.68, p<0.05; 6 % v/v: Genotype: F(1,17)= 7.61, p<0.05; 20 % v/v: Genotype: F(1,17)= 7.81, p<0.05]. Fig. 1B shows that Sig-1R KO mice cumulatively drank 19.3% more of the 3% v/v solution, 28.4% more of the 6% v/v solution, and 45.4% more of the 20% v/v solution compared to WT mice.
Figure 1.
Ethanol drinking in WT and Sig-1R KO mice (n=9–10/genotype). (A, B) Mice were given access to escalating concentrations of ethanol (3%, 6% and 20% v/v) in a home cage, two bottle choice paradigm. (C, D) Mice were given access for 14 consecutive days to a 10% v/v ethanol solution in a two bottle choice paradigm. Data represent Mean ± SEM. $ p≤0.1, * p≤0.05, ** p≤0.01, *** p≤0.001 vs. WT mice (Student’s t test).
In addition, Sig-1R KO mice drank less water than WT mice [Genotype: F(1,17)= 2.33, n.s.; Genotype X Concentration X Day: F(6,102)= 2.59, p<0.05] (data not shown). As a consequence, the preference for ethanol was significantly higher in Sig-1R KO mice [Genotype: F(1,17)= 10.72, p<0.005; Genotype X Concentration X Day: F(6,102)= 3.44, p<0.005] (data not shown).
Sig-1R KO mice exposed to a 10% v/v ethanol solution for 14 consecutive days also showed significant differences in ethanol intake, compared to WT mice [Genotype: F(1,16)= 7.46, p<0.05; Genotype X Day: F(13,208)= 0.84, n.s.]. As shown in Fig. 1C, Sig-1R KO mice drank more ethanol than WT mice during the whole 2 week period. As shown in Fig. 1D, Sig-1R KO mice cumulatively drank 49.3% more of the 10% v/v solution, compared to WT mice.
Water intake was not reliably affected in this paradigm [Genotype: F(1,16)= 1.64, n.s.; Genotype X Day: F(13,208)= 0.76, n.s.] and neither did the preference for ethanol (Genotype: F(1,16)= 2.64, p=0.12; Genotype X Day: F(13,208)= 1.16, n.s.] (data not shown).
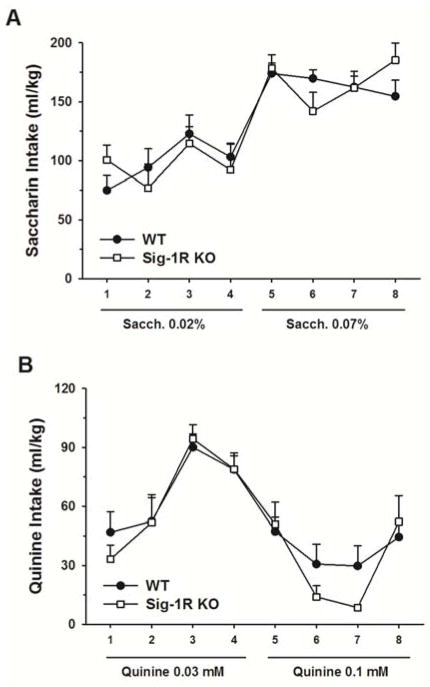
Sig-1R KO mice do not exhibit altered taste perception
As shown in Fig. 2, mice lacking the Sig-1R did not differ from WT mice for consumption of either sweet (saccharin, Fig. 2A) or bitter (quinine, Fig. 2B) solutions [Saccharin: Genotype: F(1,20)= 0.002, n.s.; Genotype X Concentration: F(1,20)= 0.12, n.s.; Genotype X Concentration X Day: F(3,60)= 1.43, n.s.] [Quinine: Genotype: F(1,21)= 0.29, n.s.; Genotype X Concentration: F(1,21)= 0.22, n.s;. Genotype X Concentration X Day: F(3,63)= 1.90, n.s.].
Figure 2.
Saccharin and quinine drinking in WT and Sig-1R KO mice (n=8–10/genotype). Mice were given access to escalating concentrations of either (A) a sweet saccharin solution (0.02% and 0.07% w/v) or (B) a bitter quinine solution (0.03 mM and 0.1 mM), in a home cage, two bottle choice paradigm. Data represent Mean ± SEM.
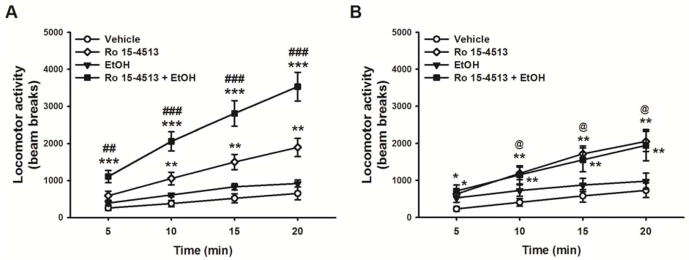
Sig-1R KO mice are less sensitive to ethanol-induced locomotor stimulation
A low dose of ethanol (1.5 g/kg, i.p.), administered right after the BDZ inverse agonist Ro 15-4513 (3 mg/kg, i.p.), had a differential effect on motor activity in WT and Sig-1R KO mice [Genotype X Ethanol: F(1,17)= 7.76; p<0.05; Genotype X Ro 15-4513: F(1,17)= 5.97, p<0.05; Genotype X Ethanol X Ro 15-4513: F(1,17)= 6.06, p<0.05]. Importantly, as shown in Fig. 3, pairwise comparisons revealed that locomotor activity of either genotype was not affected by ethanol treatment alone, whereas Ro 15-4513 by itself was able to stimulate it in both genotypes. Interestingly, while pretreatment with Ro 15-4513 was able to unmask the locomotor stimulant effect of ethanol in WT mice, interpreted as a significant difference between the Ro 15-4513+Ethanol group vs. the Ro 15-4513 group (Fig. 3A), no such potentiation was observed in Sig-1R KO mice (Fig. 3B).
Figure 3.
Locomotor stimulant effect of ethanol in (A) WT mice and (B) Sig-1R KO mice (9–10/genotype). Mice were treated with either ethanol (1.5 g/kg), or Ro 15-4513 (3 mg/kg), or co-administered both drugs, and locomotor activity in a familiar environment was evaluated. Data represent Mean ± SEM. * p≤0.05, ** p≤ 0.01, *** p≤0.001 vs. vehicle treated mice; ## p≤0.01, ### p≤0.001 vs. Ro 15-4513 treated mice, @ p≤0.05 vs. respective point in WT mice (Student Newman-Keuls test).
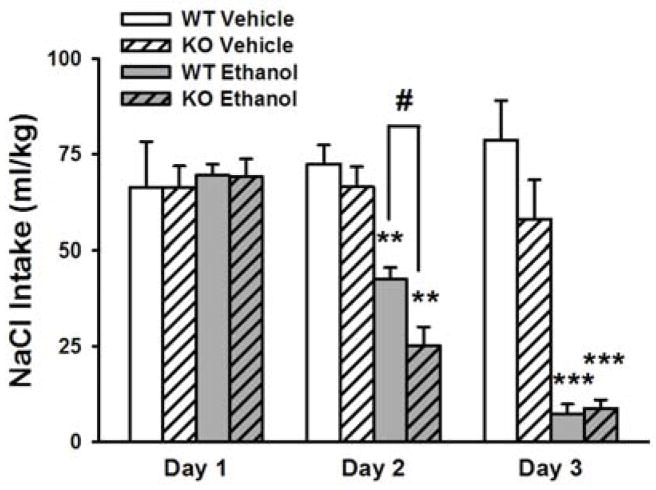
Sig-1R KO mice exhibit increased sensitivity to ethanol-induced conditioned taste aversion
WT and Sig-1R KO mice differed in the development of CTA induced by a high dose of ethanol differentially across days [Genotype: F(1,22)= 3.52, p=0.07; Genotype X Ethanol X Day: F(1,22)= 6.87, p<0.05]. Indeed, a significant difference between genotype in saline (NaCl) intake was observed on day 2 (i.e. after 1 pairing of the saline solution with ethanol), but not on day 3 of the CTA protocol [Day 2: Genotype: F(1,22)= 5.66, p<0.05]. As shown in Fig. 4, pairwise comparisons showed that ethanol-treated Sig-1R KO mice showed a greater reduction in NaCl solution intake after the first pairing with ethanol compared to WT mice (−63.7% vs. −38.8%, compared to intake of day 1, Sig-1R KO and WT respectively). The two genotypes equally reduced their saline intake after two pairings (−87.3% vs. −89.5%).
Figure 4.
Ethanol-induced conditioned taste aversion in WT and Sig-1R KO mice (n=11–15/genotype). A 0.2 M saline (NaCl) solution was offered to mice for 1 hr for 3 consecutive days following an injection of ethanol (0, 3 g/kg, i.p.) and intake was recorded. Data represent Mean ± SEM. ** p≤0.01, *** p≤0.001 vs. vehicle treated mice of the respective genotype; # p≤0.05 vs. ethanol-treated WT mice (Student Newman-Keuls).
Sig-1R KO mice show normal ethanol-induced loss of righting reflex
As shown in Supplementary Fig. 1, WT and Sig-1R KO mice did not differ in the latency to lose the righting reflex (LORR) following treatment with ethanol [t(18)= 0.97, n.s.] or the duration of ethanol-induced LORR (Suppl. Fig. 1B) [t(18)= 0.66, n.s.] (Suppl. Fig. 1A).
In addition, the blood alcohol levels following the administration of ethanol did not differ between genotypes. Blood alcohol levels 12 minutes after injection were 389±19.2 and 433±16.7 in WT and Sig-1R KO mice, respectively [Mean ± SEM, t(17)=1.73, n.s.] (data not shown).
Discussion
This series of studies demonstrates that the loss of the Sig-1R due to retroviral disruption of the Oprs1 gene causes: i) increased ethanol intake during both short and long periods of access, compared to WT mice; ii) no effect on consumption of either sweet (saccharin) or bitter (quinine) solutions; iii) reduced sensitivity to the locomotor stimulant effects of low doses of ethanol; iv) increased sensitivity to the taste aversive effect of high doses of ethanol; v) no effect on ethanol-induced sedation.
Sig-1R KO mice showed greater alcohol intake, compared to WT, when exposed to different ethanol concentrations; specifically, Sig-1R KO mice drank cumulatively 19.3%, 28.4% and 45.4% more alcohol than WT mice, when exposed to a 3%, 6% and 20% v/v alcohol solution, respectively. In addition, when exposed for 2 consecutive weeks to a 10% v/v solution, Sig-1R KO mice drank 49.3% more than WT. The higher intake was, therefore, more marked when mice were exposed to higher concentrations of ethanol. In contrast, neither water intake in the ethanol studies nor the intake or saccharin or quinine solutions was elevated in Sig-1R KO mice, ruling out that the deletion of the Sig-1R gene results in either altered taste perception or a general increase in intake of all fluids.
We have previously shown that the pharmacological blockade of the Sig-1R is able to reduce the ethanol drinking behavior in alcohol-preferring and in vapor-dependent rats [27–29], while pharmacological activation with a SigR agonist induces binge-like drinking in alcohol-preferring rats [31], suggesting that Sig-1R activation is involved in the reinforcing effects of alcohol. The lack of the Sig-1R is associated with increased ethanol intake showed in this paper may appear to be in contrast with the previous pharmacological studies. However, the species difference (mice vs. rats) may be responsible for the differential effects observed; indeed, to our knowledge, no studies have yet investigated the effect of Sig-1R ligands on alcohol intake in mice.
We found that Sig-1R KO mice were insensitive to the locomotor stimulant effects of alcohol (1.5 g/kg) compared to WT mice. These results are in line with previous studies showing that pharmacological blockade of the Sig-1R in Swiss mice is able to attenuate ethanol-induced hyperlocomotion [30]. The stimulant effects of alcohol has been suggested to represent a measure of its motivational, euphoric and rewarding properties [45, 46] and to be directly related to its addictive properties [47]. Even though such theories are subject of debate [48], we may hypothesize that the reduced sensitivity of Sig-1R KO mice to the stimulant effects of alcohol may reflect a reduced sensitivity to its motivational effects. Consistent with this interpretation is the observation that Sig-1R KO mice show increased alcohol intake compared to WT; therefore, if the reinforcing effects of alcohol are reduced in Sig-1R KO mice, they would need to consume more alcohol to achieve euphoria. Even though such compensatory drinking behavior is more commonly observed in operant conditions rather than free drinking, it is important to consider the possibility that the deletion of the Sig-1R may alter the rewarding nature of ethanol. Future studies, for instance involving alcohol-induced place preference, will be needed to confirm a putative reduction in the sensitivity to its rewarding effects.
Lack of alcohol-induced activation has been observed in the C57BL/6J strain, which is particularly insensitive to the locomotor stimulant effects of low doses ethanol (despite showing high levels of voluntary drinking), while being equally or more sensitive to the sedative effects of higher doses [36, 40, 49, 50]. One way to overcome this issue experimentally has been the pretreatment of mice with the benzodiazepine (BDZ) partial inverse agonist Ro 15-4513 [51], which is able to unmask the stimulant effects of ethanol by virtue of its ability to antagonize the depressant properties of ethanol [38], likely by interacting with γ2 containing GABA-1 receptors [52]. Thus, while 1.5 g/kg ethanol administered alone does not influence activity in this mouse strain, the co-administration of Ro 15-4513 and ethanol significantly increases locomotor activity. Importantly, this potentiation did indeed occur in WT mice, but not in Sig-1R KO mice, suggesting a reduced, or a complete lack of, sensitivity to an ethanol stimulant effect. As the lack of ethanol stimulation in some strains has been suggested to be related to a greater sensitivity to the depressant properties of ethanol [53], an alternative interpretation of our data could be that Sig-1R KO mice, rather than being insensitive to the stimulant effects of ethanol, could instead be more sensitive to its depressant effects; therefore the dose of BDZ partial inverse agonist used here would be effective in WT mice to unmask ethanol stimulant properties, but insufficient in antagonizing the depressogenic effects of alcohol in Sig-1R KO mice. While this hypothesis cannot be completely ruled out based on the present experiments, the observation that ethanol induced a comparable sedative effect in WT and Sig-1R KO mice, as assessed by ethanol-induced loss of righting reflex (see below), would advise against this alternative hypothesis. Therefore, the observation that Sig-1R KO mice did not show an overall change in motor activity rules out the possible influence of the Sig-1R in all of the experiments presented here.
Results from the conditioned taste aversion (CTA) experiment showed that Sig-1R KO mice developed a greater taste aversion to alcohol after only one pairing, compared to WT mice, suggesting a greater sensitivity to its aversive effect. This result is in contrast with previous findings observing a disruption of CTA acquisition induced by a pharmacological blockade of Sig-1R [30]. However, three major differences between the reported methods and our experiments may be responsible for the different results obtained, i.e. different doses of ethanol used (3 vs. 1 g/kg), different strains of mice (C57BL6/J vs. Swiss), and different housing conditions (single vs. grouped).
Ethanol-induced CTA has been proposed to negatively correlate with drinking, suggesting that in some cases, ethanol’s aversive actions may limit oral consumption [54, 55]. This hypothesis, however, does not seem to fit in this context based on the results obtained, as one would expect Sig-1R KO mice, showing higher levels of drinking, to show a reduced – rather than increased – response to ethanol-induced taste aversion. On the other hand, aversion induced by self-administered drugs, such as ethanol, has been proposed to arise from the novelty of the subjective intoxication rather than from toxicity, and therefore the aversive stimulus properties would be positively correlated with the positive reinforcing properties [56, 57].
It should also be noted that the dose of ethanol used in CTA experiments is usually much higher than the dose of ethanol mice consume orally and voluntarily. Therefore, it can be speculated that Sig-1R KO mice may be more sensitive to the aversive effects of high doses of alcohol, but less sensitive to the positive reinforcing effects of low doses. Again, it cannot be ruled out that possible developmental compensatory changes in expression of other genes may occur in Sig-1R KO mice and instead be responsible for the results obtained. The alternative hypothesis that increased CTA response in Sig-1R KO mice is due to improved learning is highly unlikely. Indeed, Sig-1R KO mice were previously shown not to have learning and memory alterations [58], and pharmacological studies have shown that Sig-1R agonists, and not antagonists, ameliorate cognitive impairments and memory [59, 60]. Importantly, the two genotypes showed no difference in alcohol-induced sedation or in the blood alcohol levels resulting from ethanol administration.
When interpreting results obtained with mutant mice, it is noteworthy to remember the question of whether compensatory changes in expression of other genes occur as a result of the deletion of a target gene [61]. It is indeed possible that developmental compensatory changes in expression of other genes may happen in Sig-1R KO mice, and in particular, an up-regulation of Sig-2Rs, which may explain some of the observed effects. While a role for the Sig-2R in the effects of drugs of abuse has been proposed [62, 63], progress in this field has been hampered by the lack of potent and selective ligands. Future studies employing inducible deletions will be needed to overcome the developmental issue of constitutive knockouts and the resulting intrinsic limitations of this model.
In summary, our data strongly suggest that Sig-1Rs modulate several actions of ethanol, including drinking, locomotor stimulation and conditioned taste aversion, and strengthen the notion that drugs acting on the Sig-1R system may have potential for treating alcohol-use disorders.
Supplementary Material
Highlights.
Sig-1 receptor knockout mice (Sig-1R KO) display elevated alcohol drinking.
Consumption of sweet or bitter solutions is unaltered in Sig-1R KO.
Sig-1R KO show lower sensitivity to the ethanol locomotor stimulant effects.
Sigma-1R KO are more sensitive to ethanol taste aversive and hypothermic effects.
Ethanol-induced sedation does not differ in Sigma-1R KO and WT.
Acknowledgments
Source of support: Grant numbers MH093650, MH091945, AA016731, DA030425 and DA023680 from the National Institute of Mental Health (NIMH), the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA), by the Peter McManus Trust (V.S.), by the Peter Paul Career Development Professorship (P.C.) and by the Boston University Undergraduate Research Opportunity Program.
We thank Aditi N. Narayan and Aditya Khedkar for technical assistance. This publication was made possible by grant numbers MH093650, MH091945, AA016731, DA030425 and DA023680 from the National Institute of Mental Health (NIMH), the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA), by the Peter McManus Charitable Trust (V.S.), by the Peter Paul Career Development Professorship (P.C.) and by the Boston University Undergraduate Research Opportunity Program.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global Status Report on Alcohol. World Health Organization DoMHaSA; Geneva: 2004. [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 4.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–32. [PubMed] [Google Scholar]
- 5.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–6. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 6.Bowen WD, DeCosta B, Hellewell SB, Thurkauf A, Walker JM, Rice KC. Characterization of [3H] (+)-pentazocine, a highly selective sigma ligand. Progress in clinical and biological research. 1990;328:117–20. [PubMed] [Google Scholar]
- 7.Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain research. 1990;527:244–53. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 8.Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. JNeurosci. 1986;6:1757–70. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker JM, Bowen WD, Walker FO, Matsumoto RR, De CB, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 10.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–33. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 12.Skuza G, Kolasiewicz W. Repeated treatment with SA4503, a selective sigma1 receptor agonist, up-regulates alpha-adrenergic system. a behavioral study. Pol J Pharmacol. 2001;53:547–50. [PubMed] [Google Scholar]
- 13.Ault DT, Radeff JM, Werling LL. Modulation of [3H]Dopamine release from rat nucleus accumbens by neuropeptide Y may involve a sigma1-like receptor. J Pharmacol Exp Ther. 1998;284:553–60. [PubMed] [Google Scholar]
- 14.Ault DT, Werling LL. Phencyclidine and dizocilpine modulate dopamine release from rat nucleus accumbens via sigma receptors. Eur J Pharmacol. 1999;386:145–53. doi: 10.1016/s0014-2999(99)00769-4. [DOI] [PubMed] [Google Scholar]
- 15.Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–80. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- 16.Gronier B, Debonnel G. Involvement of sigma receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. Eur J Pharmacol. 1999;368:183–96. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 17.Weatherspoon JK, Gonzalez-Alvear GM, Frank AR, Werling LL. Regulation of [3H] dopamine release from mesolimbic and mesocortical areas of guinea pig brain by sigma receptors. Schizophr Res. 1996;21:51–62. doi: 10.1016/0920-9964(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 18.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacology & therapeutics. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–77. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- 20.Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–70. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–45. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1967–73. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S, Miwa T, Horikomi K. Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett. 2000;289:21–4. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- 24.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 25.Nonatsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 26.Hiranita T, Tanda G, Kopajtic TA, Newman AH, Katz JL. Reinforcing effects of sigma-receptor agonists in cocaine-experienced and naïve rats; Neuroscience Meeting Planner.: Program No 496.1; Chicago, IL. Society for Neuroscience; 2009. [Google Scholar]
- 27.Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1482–93. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabino V, Cottone P, Zhao Y, Steardo L, Koob GF, Zorrilla EP. Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology. 2009;205:327–35. doi: 10.1007/s00213-009-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blasio A, Valenza M, Iyer MR, Rice KC, Steardo L, Hayashi T, et al. Sigma-1 receptor mediates acquisition of alcohol drinking and seeking behavior in alcohol-preferring rats. Behavioural brain research. 2015;287:315–22. doi: 10.1016/j.bbr.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacology, biochemistry, and behavior. 2003;74:869–76. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 31.Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:1207–18. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phan VL, Urani A, Romieu P, Maurice T. Strain differences in sigma(1) receptor-mediated behaviours are related to neurosteroid levels. Eur J Neurosci. 2002;15:1523–34. doi: 10.1046/j.1460-9568.2002.01989.x. [DOI] [PubMed] [Google Scholar]
- 33.Miyatake R, Furukawa A, Matsushita S, Higuchi S, Suwaki H. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol Psychiatry. 2004;55:85–90. doi: 10.1016/j.biopsych.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behavioural brain research. 2009;198:472–6. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcoholism, clinical and experimental research. 2011;35:1351–60. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randall CL, Carpenter JA, Lester D, Friedman HJ. Ethanol-induced mouse strain differences in locomotor activity. Pharmacology, biochemistry, and behavior. 1975;3:533–5. doi: 10.1016/0091-3057(75)90069-6. [DOI] [PubMed] [Google Scholar]
- 37.Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:2593–604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker HC, Hale RL. Ethanol-induced locomotor stimulation in C57BL/6 mice following RO15-4513 administration. Psychopharmacology. 1989;99:333–6. doi: 10.1007/BF00445553. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer GJ, Michael RP. Interactions between RO 15-4513 and ethanol on brain self-stimulation and locomotor activity in rats. Pharmacology, biochemistry, and behavior. 1989;34:785–90. doi: 10.1016/0091-3057(89)90275-x. [DOI] [PubMed] [Google Scholar]
- 40.Crabbe JC, Jr, Johnson NA, Gray DK, Kosobud A, Young ER. Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. Journal of comparative and physiological psychology. 1982;96:440–51. doi: 10.1037/h0077898. [DOI] [PubMed] [Google Scholar]
- 41.Houchi H, Warnault V, Barbier E, Dubois C, Pierrefiche O, Ledent C, et al. Involvement of A2A receptors in anxiolytic, locomotor and motivational properties of ethanol in mice. Genes, brain, and behavior. 2008;7:887–98. doi: 10.1111/j.1601-183x.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 42.Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcoholism, clinical and experimental research. 2011;35:1842–51. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behavior genetics. 2006;36:536–52. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- 44.Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcoholism, clinical and experimental research. 2009;33:464–76. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thrasher MJ, Freeman PA, Risinger FO. Clozapine’s effects on ethanol’s motivational properties. Alcoholism, clinical and experimental research. 1999;23:1377–85. [PubMed] [Google Scholar]
- 46.Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. International review of neurobiology. 1996;39:243–82. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- 47.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological review. 1987;94:469–92. [PubMed] [Google Scholar]
- 48.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 49.Frye GD, Breese GR. An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacology. 1981;75:372–9. doi: 10.1007/BF00435856. [DOI] [PubMed] [Google Scholar]
- 50.Tabakoff B, Kiianmaa K. Does tolerance develop to the activating, as well as the depressant, effects of ethanol? Pharmacology, biochemistry, and behavior. 1982;17:1073–6. doi: 10.1016/0091-3057(82)90496-8. [DOI] [PubMed] [Google Scholar]
- 51.Miczek KA, Weerts EM. Seizures in drug-treated animals. Science. 1987;235:1127–8. [PubMed] [Google Scholar]
- 52.Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, Luddens H, et al. Ro 15-4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma2-type GABA(A) Receptors. Frontiers in neuroscience. 2011;5:3. doi: 10.3389/fnins.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masur J, dos Santos HM. Response variability of ethanol-induced locomotor activation in mice. Psychopharmacology. 1988;96:547–50. doi: 10.1007/BF02180038. [DOI] [PubMed] [Google Scholar]
- 54.Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behavioral neuroscience. 2002;116:138–48. [PubMed] [Google Scholar]
- 56.Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neuroscience and biobehavioral reviews. 1987;11:107–30. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- 57.Davis CM, Riley AL. Conditioned taste aversion learning: implications for animal models of drug abuse. Annals of the New York Academy of Sciences. 2010;1187:247–75. doi: 10.1111/j.1749-6632.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- 58.Chevallier N, Keller E, Maurice T. Behavioural phenotyping of knockout mice for the sigma-1 (sigma(1)) chaperone protein revealed gender-related anxiety, depressive-like and memory alterations. Journal of psychopharmacology. 2011;25:960–75. doi: 10.1177/0269881111400648. [DOI] [PubMed] [Google Scholar]
- 59.Moriguchi S, Yamamoto Y, Ikuno T, Fukunaga K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. Journal of neurochemistry. 2011;117:879–91. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- 60.Senda T, Matsuno K, Okamoto K, Kobayashi T, Nakata K, Mita S. Ameliorating effect of SA4503, a novel sigma 1 receptor agonist, on memory impairments induced by cholinergic dysfunction in rats. Eur J Pharmacol. 1996;315:1–10. doi: 10.1016/s0014-2999(96)00572-9. [DOI] [PubMed] [Google Scholar]
- 61.Ponomarev I, Maiya R, Harnett MT, Schafer GL, Ryabinin AE, Blednov YA, et al. Transcriptional signatures of cellular plasticity in mice lacking the alpha1 subunit of GABAA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:5673–83. doi: 10.1523/JNEUROSCI.0860-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Effects of UMB24 and (+/−)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacology, biochemistry, and behavior. 2007;86:86–91. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garces-Ramirez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, et al. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biological psychiatry. 2011;69:208–17. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.