Abstract
Voice and swallowing deficits can occur with aging. Tongue exercise paired with a swallow may be used to treat swallowing disorders, but may also benefit vocal function due to cross-system activation effects. It is unknown how exercise-based neuroplasticity contributes to behavior and maintenance following treatment.
Eighty rats were used to examine behavioral parameters and changes in neurotrophins after tongue exercise paired with a swallow. Tongue forces and ultrasonic vocalizations were recorded before and after training/detraining in young and old rats. Tissue was analyzed for neurotrophin content.
Results showed tongue exercise paired with a swallow was associated with increased tongue forces at all ages. Gains diminished after detraining in old rats. Age-related changes in vocalizations, neurotrophin 4 (NT4), and brain derived neurotrophic factor (BDNF) were found. Minimal cross-system activation effects were observed. Neuroplastic benefits were demonstrated with exercise in old rats through behavioral improvements and up-regulation of BDNF in the hypoglossal nucleus. Tongue exercise paired with a swallow should be developed, studied, and optimized in human clinical research to treat swallowing and voice disorders in elderly people.
Keywords: voice, swallowing, tongue exercise, neurotrophins, exercisedependent neuroplasticity, aging
1.1 Introduction
Clinicians use exercise-based therapies to treat a variety of age-related cognitive and sensorimotor impairments (Butefisch, 2006,Ding, et al., 2011,Dishman, et al., 2006,Intlekofer and Cotman, 2013,Vaynman and Gomez-Pinilla, 2005), including impairments in voice and swallowing (Behrman, et al., 2008,Burkhead, et al., 2007,Carnaby and Harenberg, 2013,Fox, et al., 2006,Green and Bavelier, 2008,Robbins, et al., 2007,Roy, et al., 2003,Stemple, et al., 1994). However, treatment efficacy for swallowing problems (dysphagia) and voice problems (dysphonia) has not been optimized because the underlying functional changes associated with exercise in the cranial sensorimotor system are unknown. As a result, clinicians have limited evidence available when choosing an exercise-based treatment for the specific needs of their patients. Thus, clinicians are required to try a plethora of “possible” exercises to see if one of them will lead to improvement, resulting in inefficient use of therapy time and a lack of evidenced-based therapy guidelines (Carnaby and Harenberg, 2013,Ludlow, et al., 2008,Robbins, et al., 2008). Without a clear understanding of changes to underlying neural substrates with exercise and age, clinicians are at a disadvantage because they lack physiological and mechanistic evidence to support the decision to use specific therapy parameters or to determine when and if exercise follow-up should occur (Carnaby and Harenberg, 2013,Cramer, et al., 2011). Thus, research must systematically examine therapy parameters and the resulting changes to neural substrates to provide evidence needed to guide clinical decision-making, maximize therapeutic outcomes, and encourage neuroplasticity.
Neurotrophic factors, or neurotrophins, represent molecular candidates for neural substrates responsible for neuroplastic change. Neurotrophins are the molecular mediators of synaptic plasticity within the motor system (Zhan, et al., 2003), contribute to maintaining and restoring synaptic function in neurons of both the central and peripheral nervous systems (Barde, 1990,Hennigan, et al., 2007), and have been proposed as mechanisms of exercise-based change in the limb sensorimotor system (Gomez-Pinilla, et al., 2002,Gomez-Pinilla, et al., 2011). However, little information exists regarding the potential for neuroplastic change in the cranial sensorimotor system and the role of neurotrophins in this context, making development of mechanistically-based neuroplastic therapy approaches for voice and swallowing challenging.
Research in our laboratory has demonstrated that aging results in degenerative changes to the nucleus ambiguus (NA), hypoglossal nucleus (HN), and muscles involved in voice and swallowing (Basken, et al., 2012,Hodges, et al., 2004,Ota, et al., 2005,Schaser, et al., 2011,Schwarz, et al., 2009,Suzuki, et al., 2002) and that 8 weeks of tongue exercise leads to increased tongue forces across the lifespan in a rat model (Behan, et al., 2012,Connor, et al., 2009,Schaser, et al., 2012). In addition, our research suggests that brain derived neurotrophic factor (BDNF) and its receptor TrkB are a putative mechanism underlying age and exercise related change in tongue function, because the HN showed decreased TrkB with age and increased BDNF with exercise in adult rats (Schaser, et al., 2012). However, questions exist regarding the enduring benefits and generalizability (cross-activation potential) of tongue exercise paired with a swallow. Furthermore, it is unknown how neurotrophic factors mediate adaptations in sensorimotor function with age and exercise, and how other neurotrophic factors, such as neurotrophin 4 (NT4) (Barbacid, 1995) exert effects through the TrkB receptor in the cranial sensorimotor system.
To examine exercise specificity and cross-activation in our study, tongue exercise paired with a swallow was used as the exercise-based therapy intervention. This exercise is relevant, because exercises that use cross-activation of shared inputs during training to induce treatment efficiency are being explored in the clinical world for dysphagia and dysphonia (Easterling, 2008,El Sharkawi, et al., 2002). For the exercise task trained in this study, muscles of both the tongue and larynx were activated during the exercise to allow for a safe and accurate swallow (Fig. 1), because the vocal folds are known to close to protect the airway during the swallow (Fink, 1974,Logemann, et al., 1992,McCulloch, et al., 1996,Perlman, et al., 1999,Pressman, 1941,Stuart, 1891,Van Daele, et al., 2005). In addition, muscles involved in both vocalization and swallowing receive input from similar neural control elements and are both involved in respiration (McFarland and Paydarfar, 2011,McFarland and Tremblay, 2006,Nishio and Niimi, 2004). Therefore, the task used in this study had the potential to affect both lingual and laryngeal structures and their sensorimotor outputs. As such, it is theoretically possible and reasonable that our exercise, which targets a specific component, the tongue, within a larger sensorimotor framework (swallowing) may provide cross-system benefits to other components not specifically targeted but activated, such as the larynx (McFarland and Tremblay, 2006). However, based on the principles of specificity and saliency proposed in a seminal paper outlining the 10 principles of neuroplasticity (Kleim and Jones, 2008), it was hypothesized that changes in physiological and underlying neural substrates would be greater in measures involving the tongue (genioglossus (GG) muscle, tongue forces, and HN) after tongue exercise than for those involving the larynx (thyroarytenoid (TA) muscle, vocalization acoustics and NA), because this exercise was specific to tongue activation, and resulted in a greater proportion of tongue presses, with laryngeal activation occurring passively during the swallow.
Fig. 1.
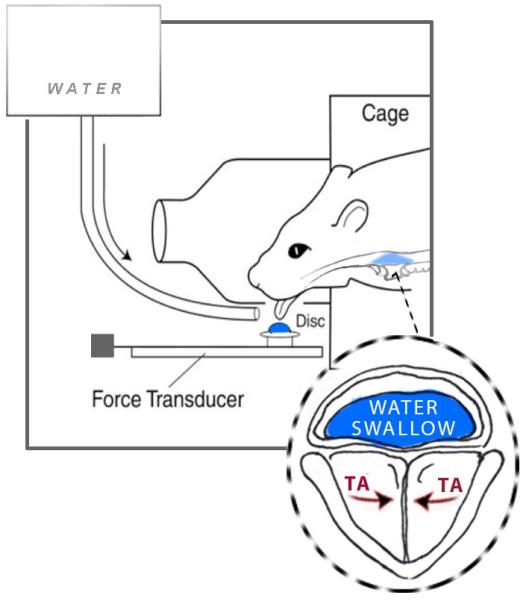
Schematic of tongue force operandum. Image demonstrates tongue exercise followed by a water swallow in the rat. Image highlights activation of the thyroarytenoid muscle (TA) to protect the airway, following licking, during the water swallow. Image adapted by TG.
To examine the lasting benefits of exercise in the cranial sensorimotor system and to provide clinical evidence to support maintenance programs after exercise-based therapy, a detraining protocol was also used in this study. Periods of 2 and 4 weeks of detraining were chosen because they represent both short term (2 weeks) and long term (4 weeks) detraining (Mujika and Padilla, 2000a,Mujika and Padilla, 2000b). It was hypothesized that all outcomes would decrease following detraining, with greater reductions at 4 weeks versus 2 weeks, based on the “use it or lose it” and timing principles of neuroplasticity (Kleim and Jones, 2008). This hypothesis is also supported by previous human clinical studies showing that tongue forces were decreased following 2-4 weeks of detraining following a 9-week lingual strengthening protocol (Clark, et al., 2009).
Therefore, the goal of this study was to obtain a better understanding of the effects of age, exercise specificity, and detraining on neurotrophic factors and behavioral measures within the cranial sensorimotor system, using a rat model. Although this work was performed in an animal model, the level of evidence provided will serve as a foundation for future clinical studies and will provide the evidence needed to direct more focused hypotheses for future human clinical studies related to treatment of dysphagia and dysphonia.
1.2 Methods
All procedures were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory, 2011) and approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee.
A total of 80 Fischer 344/Brown Norway male rats were obtained from a National Institute on Aging (NIA) aging animal colony. This inbred strain of rat is used most frequently in aging research because they are genetically identical and are raised in identical conditions. Two age groups were studied: 40 young adult (9-month old) and 40 old (32-month old). The 32-month old group represented advanced senescence because the median lifespan for this strain of rats is 33 months (Turturro, et al., 1999). Only male rats were used because the female estrus cycle may affect vocalization acoustics (Matochik, et al., 1992). The rats were equally and randomly assigned to either a tongue exercise group (n=20; 10 young adult and 10 old), 2-week detraining group (n=20), 4-week detraining group (n=20) or a no-exercise control group (n=20).
1.2.1 Behavioral Tongue Exercise and Tongue Force Acquisition
Rats had access to water gradually restricted to only 3 hours a day over 14 days (Toth and Gardiner, 2000). Rats also had light and dark cycles reversed to ensure exercise was provided at the time of most activity. All rats were trained during a 2-week acclimation period to lick with 2.0 mN of force against an 18 mm aluminum disk fitted with a force transducer on the shaft of the disk (Sensotec load cell, 0-250 g range) to receive approximately 0.10 mL water reward, using a variable ratio 5 reinforcement schedule (VR5; one reinforcement for approximately every 5 licks). After a 10 minute training session per day, rats were removed from the operandum enclosure (Fig. 1) and given water ad libitum in a water dish for 3 hours. This program of water delivery is in current use in our laboratory and was consistent in this study throughout the acclimation period, exercise program, detraining program, and no-exercise control condition (Connor, et al., 2009,Schaser, et al., 2012).
Following the 2 weeks of preliminary behavioral training, each rat was tested to determine an individual maximum tongue force value by averaging the 10 highest force values for each rat over 3 days. Each rat was placed in the operandum enclosure and force targets were progressively increased within 3 consecutive daily sessions to determine maximum values. Maximum tongue force testing was performed for all exercise, detraining, and control rats at baseline and following the 8 week experimental period.
The progressive resistance exercise program began immediately following the 2 weeks of preliminary behavioral training. The exercise period lasted 8 weeks and was performed 5 days per week. At the end of each 2-week exercise period, the tongue force threshold required for receipt of water reward was progressively increased. For detailed information regarding the exercise program see methods from research from our laboratory (Ciucci, et al., 2013,Connor, et al., 2009,Schaser, et al., 2012).
Within an exercise session, rats were required to produce at least 20 tongue presses greater than or equal to the target threshold for that day to be counted as a successful training session. If an initial training session was not successful, rats were placed in the training cage for subsequent attempts (maximum of 2 repeat trials per day). If the rat did not produce 20 tongue presses greater than or equal to the target threshold for 3 consecutive days of training, the threshold value was reduced and the new threshold was documented. Values for percent of maximum force and number of resistance trials within a session were consistent with recommendations for strength training for humans by the American College of Sports Medicine (Kraemer, et al., 2002). Post-testing identical to that performed at baseline revealed maximum tongue forces associated with training. Testing was also performed in an identical manner for animals in the detraining groups.
No-exercise control rats were placed on a water restriction protocol and light/dark cycle reversal identical to exercise treatment rats. To allow collection of baseline tongue force data, no-exercise control rats also underwent 2 weeks of behavioral training using the operandum without any applied resistance. In this manner, the control rats had a threshold of 2.0 mN for receiving a water reward. These rats did not receive progressive resistance exercise during the 8-week experimental duration, but instead entered the cage and licked from the operandum without any resistance for a set period of time, to ensure skill maintenance. In addition, the no-exercise control rats received water for 3 hours per day throughout the experimental duration. Post-testing identical to that performed at baseline revealed maximum tongue forces associated with skill maintenance alone in the no-exercise control group.
1.2.2 Vocalization Acoustics
Rats produce several types of ultrasonic vocalizations (USVs) in the 50 kHz range. These calls, which have been studied extensively, are semiotic in nature because they have symbolic reference and are capable of changing the behavior of the communication partner (Bialy, et al., 2000,Blanchard, et al., 1992,Brudzynski, 2005,McGinnis and Vakulenko, 2003,Riede, 2014,Wohr, et al., 2008). USVs in rats are produced by a constriction in the larynx that occurs through fine motor control of intrinsic laryngeal muscles (Johnson, et al., 2010,Riede, 2011).
Baseline and post-intervention acoustic measurements of USVs from all rats were obtained using an existing mating paradigm (Ciucci, et al., 2008). The procedure involved: (1) pairing a male rat with a receptive female in estrus, evidenced by lordosis, darting, and ear wiggling in the female rat; (2) allowing the male to mount the female; and (3) putting both rats into the test cage and removing the female after the male exhibits further interest, such as chasing and vocalization (mounting was not allowed during this stage to control for reduced mounting behavior in aged rats), which induced further vocalization by the male and allowed USVs to be recorded from the male rat in isolation (Ciucci, et al., 2008). Once the female was out of the range of the microphone, two minutes of calling was recorded onto a Windows-based personal computer using an ultrasonic recording system (Avisoft, Germany) with appropriate wide frequency response range (10 to 180 kHz) and the capability of producing spectrograms in real time. All calls within the 50 kHz range, collected during the 2 minute recording period, were analyzed for total number of calls. For subsequent USV characteristic analyses only frequency modulated call (operationally defined as calls with a bandwidth greater than 10 kHZ) were included. Offline acoustic analyses were performed with a customized automated program using SASLab Pro (Avisoft, Germany). Spectrograms were built from each waveform with the frequency resolution set to an FFT of 512 points, frame size of 100%, flat top window, and the temporal resolution set to display 75 % overlap. (For video example and review of previous studies, see Ciucci, et al., 2009,Johnson, et al., 2013,Johnson, et al., 2011).
1.2.3 Detraining
The two detraining rat groups were placed on a water restriction protocol and light/dark cycle reversal identical to exercise rats and no-exercise control rats. They underwent the same exercise treatment protocol as exercise rats. Baseline and post exercise maximum tongue force measures and vocalization acoustic measures were collected as described previously. After collection of the post exercise measures, 2- and 4-week detraining rats remained on water restriction. During the 2-week no-exercise period, 2-week detraining rats entered the cage and licked from the operandum without any resistance for a set period of time to ensure skill maintenance. After the 2-week no-exercise period, post detraining tongue force and vocalization data were collected. Identical procedures were applied to 4-week detraining rats following a 4-week period of no-exercise.
1.2.4 Tissue Collection
All rats were deeply anesthetized with 5% isoflurane until unresponsive to toe pinch or corneal reflex, were humanely euthanized, and their brains and muscle were dissected for analyses. Brain tissue was snap-frozen and embedded in optimum cutting temperature compound (OCT) and stored at -80 degrees. Brains from the level of the spinal cord through the medulla were sliced into 300μm thick slices with a freezing microtome (Leica 2000R, Bannockburn, IL, USA). Using known anatomical markers and methods successfully employed in our lab previously (Behan, et al., 2012,Schaser, et al., 2012,Schwarz, et al., 2009) the hypoglossal nucleus and nucleus ambiguus were grossly microdissected and stored for downstream protein analysis. Neural control tissue from the spinal cord and medulla at levels above and below nuclei of interest was also collected (Brainstem Control (BSC)). The GG and TA muscles were dissected out, as well as extensor digitorum longus (EDL) muscle in the hind limb to serve as muscular control tissue. The specific nuclei and muscles were chosen because of their role in voice and swallowing behaviors. The EDL was chosen because it contains largely rapidly-contracting muscle fiber types analogous to the tongue and larynx (Connor, et al., 2008). The HN is the brainstem nucleus that controls the tongue musculature, including the GG muscle (Nowinski, et al., 2012). The GG is the main protrusive muscle of the tongue, is active during bolus manipulation and transport (Fregosi and Fuller, 1997,Gilliam and Goldberg, 1995,Miller, 2002,Sauerland and Mitchell, 1975), and is active throughout the swallow (Perlman, et al., 1999). The NA is the brainstem nucleus that controls the intrinsic laryngeal musculature, including the TA (Nowinski, et al., 2012). The TA is active during the swallow to protect the airway (Fink, 1974,Gay, et al., 1994,Logemann, et al., 1992,Pressman, 1941,Stuart, 1891), and creates tension in the vocal folds during vocalization (Story and Titze, 1995). Muscle tissue was placed in an Eppendorf tube and frozen in liquid nitrogen. Samples were stored at −80°C until use.
1.2.5 Measurement of Neurotrophins
Neurotrophin levels were measured in the HN, NA, GG, TA, and control samples. Enzyme Linked Immunosorbent Assays (ELISA) were used to detect protein levels. Whole muscles were homogenized in ice cold PBS buffer. Homogenates were centrifuged and supernatants collected. Overall protein concentration levels were normalized using wet tissue weight (w.t.w) mg/volume (μl). BDNF, NT4, and TrkB protein levels were quantified using selected ELISA kits, (BDNF Emax ImmunoAssay System Kit (Promega, Madison, WI); Rat Neurotrophin 4 (NT4) ELISA Kit (Cusabio); Rat TrkB (Tyrosine receptor kinase B) ELISA Kit (MyBioSource), as per the manufacturer’s protocol. Unknown protein concentrations were compared with known protein concentrations using a standardized calibration curve provided by the respective manufacturers.
1.2.6 Statistical Analysis
A two-way analysis of variance (ANOVA) was used to examine main and interaction effects of age (2 levels: young adult and old) and training group (2 levels: tongue exercise and no-exercise controls). For the behavioral data, t-tests were performed on the Δ variables from the post-exercise time point to each respective detraining time point to examine the effect of detraining on tongue force and USV characteristics. In addition, Spearman correlations (ρ) were used to examine the relationship between average participation during tongue training, Δ tongue force, and neurotrophin concentration for the different neurotrophins, age groups, training groups, and regions of interest. All analyses were performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC) or Sigma Plot. An α-level of less than 0.05 was established as the criterion for statistical significance. An α-level correction was not used in this study, due to the limited number of multiple comparisons (6) and the increased chance of type two errors with more conservative α-levels.
For some measures, missing data resulted in smaller sample sizes for comparisons, which is reflected in degrees of freedom for particular tests. Specifically, unexpected animal death accounted for some missing tongue force, USV, and neurotrophin data (n=3). The USV analyses also contained missing data when rats did not vocalize in a testing session (n=10-19, depending on time point). For neurotrophin analyses, missing data also occurred when ELISA assays could not be used because the standard curve needed for a plate was not at an acceptable level (n=4-17, depending on measure). Laryngeal neurotrophin analyses were not performed within 2- and 4-week detraining groups because significant differences were not found as a function of exercise, and this smaller sample size is reflected in the degrees of freedom for those tests (overall n=32-38). All other available data were used in statistical analyses.
1.3 Results
1.3.1 Tongue Force
1.3.1.1 Age-related Deficits in Tongue Force
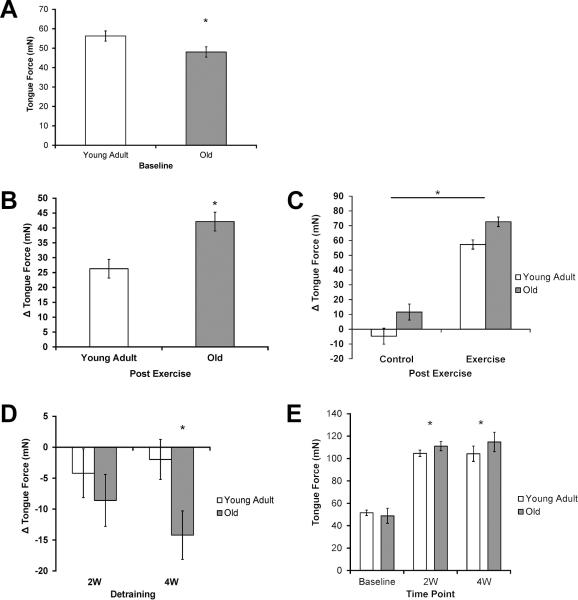
Tongue force (mN) was significantly reduced in the old group compared with the young adult group at baseline (Fig. 2A; F1,73 =4.91, p=0.03), suggesting an age-related tongue weakness.
Fig. 2.
Tongue Force. A) Reduced tongue force in the old group at baseline. Significantly lower (p=0.03) tongue force was found in the old group than the young adult group. B) Tongue exercise resulted in a greater increase in tongue force in old group. Significantly larger increases (p < 0.001) in tongue force were found in the old group. C) Tongue exercise resulted in greater increases in tongue force than skill maintenance (control). A significantly larger increase (p < 0.001) in tongue force was observed for both ages in the exercise group than the control group. D) Decreased tongue force following 4 weeks of detraining in old group. A significant decrease (p=0.02) in tongue force was observed in the old 4-week (4W) detraining group alone. E) Increased tongue force was found after 2 and 4 weeks of detraining compared to baseline. Tongue force at the 2-week (2W) and 4-week (4W) time points was significantly greater than tongue force at baseline (p < 0.001). * Denotes significant values; error bars represent standard error of the mean.
1.3.1.2 Increased Tongue Force with Exercise
The change in tongue force (Δ mN) from baseline to the post exercise time point was significantly greater in the old group than in young adult group (Fig. 2B, F1, 73 =12.73, p < 0.001) and in the exercise group than in no-exercise control group (Fig. 2C, F1, 73 =192.02, p < 0.001). This finding indicated that progressive-resistance tongue exercise was associated with increased tongue force versus skill maintenance of the task alone, regardless of age.
1.3.1.3 Decreased Tongue Force with 4 weeks of Detraining in the Old
In the old group alone, tongue force (Δ mN) was significantly decreased at the 4-week detraining time point relative to immediate post exercise levels (Fig. 2D, p=0.02). However, as shown in Figure 2E, tongue force at the 2-week and 4-week time points was significantly greater than baseline tongue forces in both age groups (p < .001). In combination, these results suggest that detraining did not eliminate improved tongue forces found following tongue exercise paired with a swallow, but that old rats experienced a relative decrement in maintenance of post-exercise tongue force levels.
1.3.2 Ultrasonic Vocalizations (USVs)
1.3.2.1 Age-related Changes in USVs
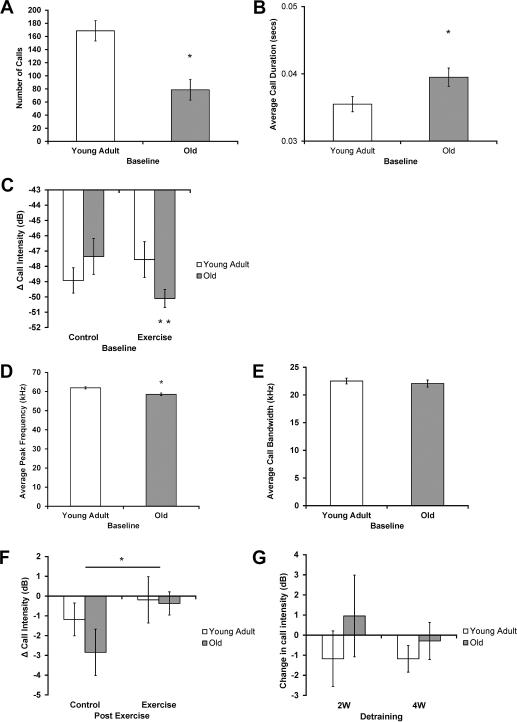
Age-related changes in vocalizations were found in a number of USV variables at baseline. Significant main effects of age were found for number of calls, with a reduced number of calls in the old group (Fig. 3A, F1, 73 =16.68, p < 0.001); average call duration, with an increased call duration in the old group (Fig. 3B, F1, 63 =5.05, p=0.03); and average peak frequency, with a reduced peak frequency in the old group (Fig. 3D, F1, 63 =13.87, p < 0.001). However, there was not a significant main effect of age on average bandwidth (Fig. 3E, F1, 63 =0.31, p=0.58). In addition, a significant interaction effect (age vs condition) was found for average call intensity (F1, 63 =10.32, p=0.002). Post-hoc testing revealed reduced average intensity in the old exercise group (Fig. 3C, p < 0.001), along with decreased average intensity in the old exercise group compared with the old control group (Fig. 3C, p=0.007). These findings indicate that rats in the old exercise group had the quietest calls at baseline.
Fig. 3.
USVs. (A-E). Age-related changes in USV characteristics at baseline. A) Number of calls: Significantly lower (p < 0.001) number of calls in the old group. B) Average call duration: Significantly greater (p=0.03) average duration of calls in the old group. C) Average call intensity: Significantly reduced (p < 0.001) average intensity of calls in the old exercise group, and a decreased average intensity in the old exercise group compared with the old control group (p=0.007). Y axis represents normalized intensity (dB), with smaller numbers representing decreased intensity levels relative to maximum. D) Average Peak Frequency: Significantly lower (p < 0.001) peak frequency of calls in the old group. E) Average bandwidth: No significant age-related differences (p=0.58). F) Maintenance of call intensity found with tongue exercise at the post exercise time point. Significantly smaller (p=0.04) change in intensity of calls in the exercise than the control group. Y axis represents change in normalized intensity (dB). G) No significant difference in intensity following detraining. No difference (Old 2-week [2W]: p=1.0, Young Adult 2W: p=0.28, Old 4-week [4W]: p=0.84, Young Adult 4W: p=0.25) was found in intensity following detraining in either detraining or age group. * Denotes significant values; error bars represent standard error of the mean.
1.3.2.2 Effect of Exercise on USVs
The change in USV characteristics (Δ unit) from baseline to the post exercise time point showed no consistent differences based on condition. There were no significant main effects of condition for number of calls (F1, 73 =1.12, p=0.29), average call duration (F1, 54 =1.05, p=0.31), average peak frequency (F1, 54 =0.27, p=0.60) and average bandwidth (F1, 54 =0.87, p=0.36). There was, however, a significant main effect of condition on average intensity (Fig. 3F, F1, 54 =4.63, p=0.04), with less reductions in average intensity following tongue exercise, indicating that rats in the exercise group maintained intensity of their calls over the 8-week training period, while rats in the control group got quieter.
1.3.2.3 No Change in Intensity with Detraining
There was no significant change in intensity (Δ dB) from the post exercise time point to the 2- and 4-week detraining times points. (Fig. 3G, p=1.0).
As a result of lack of significance found after 8 weeks of tongue exercise with a swallow on all other USV characteristics, changes in all USV characteristic following detraining, except for average intensity (above), were not reported in this manuscript.
1.3.3 Neurotrophins
1.3.3.1 Age-related Changes in Neurotrophins
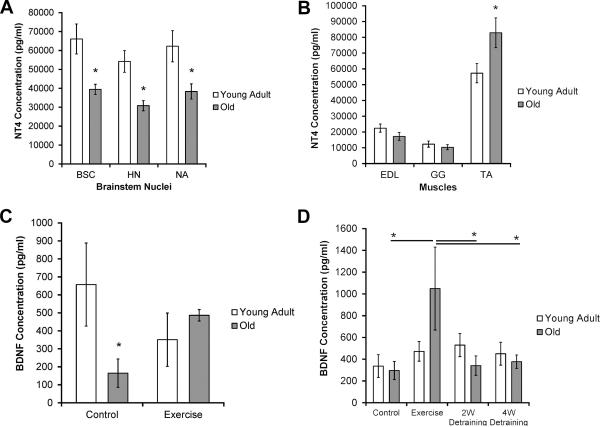
The concentration of NT4 (pg/ml) was significantly lower in the three brainstem nuclei in the old group than in the young adult group. Specifically, the old group had a significantly lower concentration of NT4 in the HN (Fig. 4A, F1, 68 =13.40, p < 0.0001), NA (Fig. 4A, F1, 34 =6.45, p=0.02), and BSC (Fig. 4A, F1, 68 =9.66, p=0.003). There was not a significant difference in NT4 concentration in the old group versus the young adult group in the GG (Fig. 4B, F1, 68 = 0.54, p=0.47) or EDL (Fig. 4B, F1, 68 =2.63, p=0.11). There was a significant increase in the concentration of NT4 in the TA of the old group versus the young adult group (Fig. 4B, F1, 68 =5.29, p=0.03).
Fig. 4.
Neurotrophins. (A-C). Age-related differences in neurotrophins. NT4 differences in (A) brainstem nuclei and (B) muscles of interest, (C) BDNF differences in TA. A) Concentration of NT4 was lower in old groups in all brainstem nuclei: Significantly lower concentration of NT4 in all brainstem nuclei of interest (BSC: p=0.003, HN: p < 0.0001, NA: p=0.02) in the old group than the young adult group. B) Increased concentration of NT4 in the TA muscle with age: Significantly greater (p=0.03) concentration of NT4 in the TA of the old group compared to young adult group. No significant difference in NT4 concentration with age in the EDL (p=0.11) or GG (p=0.47). C) Significantly lower (p=0.03) concentration of BDNF in the TA of old no-exercise control group vs young no-exercise control group. D) Age and exercise interaction effects in the HN. A significant increase (p < 0.001) in BDNF concentration was found in the old exercise group compared with the old control group, and a significant decrease (p < 0.001, p=002) in BDNF concentration was observed between the old exercise group and old 2W and 4W groups, respectively. There was no significant difference between the exercise group and the other training groups (control: p=0.5, 2W: p=0.92, 4W: p=0.44) for the young adult group. * denotes significant values; error bars represent standard error of the mean.
There was a significant interaction effect for age and training group for the concentration of BDNF (pg/ml) in the TA (Fig. 4C, F3,28 =4.70, p=0.04). Post-hoc testing revealed a significantly lower concentration of BDNF in the TA in the old no-exercise control group than in the young adult no-exercise control group (p=0.03). The concentration of BDNF and TrkB did not differ significantly between the old and young adult groups in any of the other brainstem nuclei or muscles of interest in this study. See table in appendix for raw data (means and SEMs).
1.3.3.2 Differences in BDNF with Level of Training in the HN in Old Group Alone
There was a significant interaction effect for age and training group for the concentration of BDNF (pg/ml) in the HN (Fig. 4D, F3, 62 =3.06, p=0.04). Post-hoc testing revealed that the old exercise group had a significantly higher concentration of BDNF in the HN compared to the old no-exercise control group (p < 0.001). In addition, both the old 2-week (p < 0.001) and old 4-week (p=0.002) detraining groups had a significantly lower concentration of BDNF than the old exercise group. However, there was no significant difference between the young adult group and the no-exercise control group (p=0.5) or the young adult 2-week and 4-week detraining groups, respectively (p=0.92, p=0.44). These data suggest that a training effect was observed for BDNF concentration in the HN in only the old group.
The concentration of TrkB and NT4 did not differ significantly with exercise or detraining in any of the brainstem nuclei or muscles in the old or young adult groups. To review all neurotrophin raw data see table in the appendix.
1.3.4 Correlations
1.3.4.1a Participation was Positively Correlated with Tongue Force, but not USV characteristics, regardless of Age
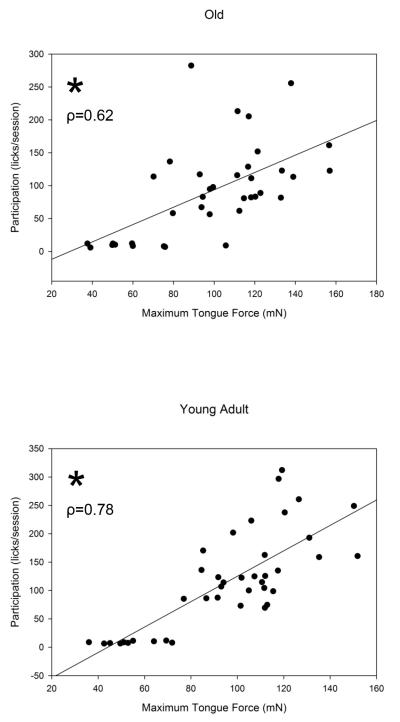
Average participation (i.e., average number of licks above the individual force threshold set during training [licks/session]) was moderately correlated with maximum tongue force in the old group and strongly correlated in the young adult group (Fig. 5, (A); Old: ρ=0.62, p < 0.0001 (B) Young Adult: ρ=0.78, p < 0. 0001). However, there were no significant correlations between average participation and any USV characteristics in this study (p >0.05). These significant correlations suggest increases in behavioral tongue force, but not USV characteristics, were directly related to participation in the tongue exercise protocol and training condition.
Fig. 5.
A&B). Participation was positively correlated with tongue force regardless of age. Tongue force and participation were moderately correlated in the old (A) and strongly correlated in the young adult (B) groups, respectively (Old: ρ=0.62, Young Adult: ρ=0.78, Both: p < 0.0001). * Denotes significant values; ρ value represented by diagonal line.
1.3.4.1b Change in Tongue Force with Tongue Exercise was Positively Correlated with Change in Intensity
For all groups, change in tongue force from baseline to the post-exercise time point (Δ mN) was moderately correlated with change in intensity from baseline to the post-exercise time point (Δ dB) (ρ=0.32, p=0.01). This correlation suggests the significant difference in intensity between the exercise and control groups at the post exercise time point (Fig. 3F) was related to level of improvement in tongue force with tongue exercise.
1.4 Discussion
The hypotheses of this study were two-fold. First, it was hypothesized that tongue exercise paired with a swallow would result in behavioral improvements and an increase in neurotrophin concentration, with greater benefits in lingual vs. laryngeal structures. Second, it was hypothesized that improvements after tongue exercise paired with a swallow would be diminished after detraining. The results of this study support our first hypothesis and partially support our second.
Tongue exercise resulted in greater tongue forces regardless of age than skill maintenance alone (Fig. 2C). In addition, BDNF concentration in the HN was greater in the old exercise group than in the old no-exercise group (Fig. 4D). For ultrasonic vocalizations, there was only one difference shown: call intensity in the exercise group was maintained while a decrease in call intensity was found in the no-exercise group (Fig. 3F). However, there were no significant differences in neurotrophin concentration in the NA with exercise in either age group.. These data support the hypothesis that a greater change would be observed following tongue exercise paired with a swallow in brainstem and muscle regions associated with tongue actions than with laryngeal actions. That is, although laryngeal closure was presumably a key element of airway protection during the tongue exercise/swallow task, this exercise was associated with maintenance, not improvement, in the laryngeal function measure, intensity. In contrast, the exercise employed focused on strengthening the tongue as its main target and resulted in global improvements in maximum tongue force. In addition, the HN, a brainstem nucleus that contains motor neurons specifically innervating the tongue, was the only brainstem nucleus to demonstrate increased BDNF concentration after treatment. As such, our results show tongue exercise paired with a swallow led to specific, neuroplastic benefits in lingual brainstem sensorimotor control structures.
The hypothesis that improvement gained following tongue exercise paired with a swallow would diminish after detraining was supported only in the old rat group. We found change in tongue force from the post-exercise time point to the 4-week time point was significantly diminished in old 4-week detraining group alone (Fig. 2D). In addition, BDNF concentration in the HN was significantly lower in the old 2-and 4-week detraining groups than the old exercise group (Fig. 4D). However, tongue force was maintained for up to 4 weeks of detraining in the young adult group, and there were no differences in neurotrophin concentration in the young adult group with detraining. These data demonstrate while young rats maintain performance levels up to 4 weeks following exercise, this is not true for older rats. These data provide initial evidence for the necessity of exercise maintenance programs following tongue exercise protocols in older populations.
Maintenance of USV intensity following tongue exercise paired with a swallow provides evidence for the cross-activation potential of exercise in the cranial sensorimotor system. This argument was previously introduced in the voice and swallowing literature based on clinical findings (Easterling, 2008,El Sharkawi, et al., 2002). We hypothesized some changes within the larynx following tongue exercise paired with a swallow because the TA, a muscle within the larynx important for voice, is activated during swallowing (Perlman, et al., 1999,Van Daele, et al., 2005). Maintenance of USV intensity with tongue exercise paired with a swallow can be explained from a cross-activation perspective considering vocalization intensity is a function of respiratory capacity and glottal closure (Riede, 2011), two elements finely controlled during the exercise task used in this study (Fig. 1). In both the rat and the human, respiratory timing and glottal closure must be precisely coordinated during vocalization to produce voice and during the swallow to allow materials to safely enter the esophagus and avoid aspiration (Perlman, et al., 1999,Riede, 2011). The tongue exercise intervention used in this study was paired with a swallow, which activated and perhaps strengthened glottal closure, and may have contributed to the observed post exercise maintenance of call intensity in the exercise group. However, the goal of a particular exercise protocol may be improvement in a specific function and not solely maintenance. In this work, there were no improvements in USVs found after tongue exercise paired with a swallow. Therefore, cross-activation of the larynx during a swallowing task alone does not appear to be sufficient or specific enough to improve vocal function. As such, based on the results of this initial study, future clinical studies could explore cross-activation for maintenance of function. However, if improvement in a specific function is the goal of therapy, specific exercise appears to be necessary and should be used. Nevertheless, results of this initial study do not rule of the possibility that tongue exercise paired with a swallow could be used in future clinical studies to optimize both swallow and vocal function in individuals at risk for both disorders, such as elderly individuals and people with Parkinson disease, especially in the early stages of the disease where maintenance or slowing of the disease process is the goal.
We observed age-related changes in tongue force (Fig. 2A) and NT4 concentration (Fig. 4A,B). Specifically, we found lower tongue forces in old rats at baseline than in young adult rats. However, reduced tongue force has not been a universal finding (Schaser, et al., 2012). This speaks to the relevance of this animal model to human clinical populations where decreased tongue force, or tongue weakness, is not consistently reported (Steele, 2013). This study did not include a direct measure of functional swallowing. As a result, we are unable to comment on specific age-related changes to swallowing in this cohort of animals. However, work from our laboratory shows that older rats have functional changes to the swallow as a result of normal aging, including reduced bolus transport speed, detected through video fluoroscopic swallowing examination (Russell, et al., 2013). Therefore, age-related changes to the functional swallow may be present in the animals in this study, but it is unknown if the increase in tongue force and BDNF concentration in the HN with tongue exercise has a direct benefit to the functional swallow, such as improved bolus transport speed. Current and future work in our laboratory will explore this hypothesis. We also found a decrease in NT4 concentration in all brainstem nuclei, including our brainstem control nuclei (BSC) and the laryngeal brainstem nucleus of interest (NA). These findings suggest a putative age-related decrease in NT4 concentration in motor and sensory neurons in the brainstem, which has not been shown in previous research.
Differences were sparse in neurotrophin concentration as a function of age or exercise in the TA muscle. Only two significant age-related findings were observed: (1) Increased NT4 concentration in old rats versus young adult rats at baseline (Fig. 4B), and (2) Reduced BDNF concentration in old control rats versus young adult control rats (Fig. 4C). The significantly greater NT4 concentration in old rats versus young adult rats was in opposition to our hypothesis and previous reports of reduced neurotrophin concentration with age (Greising, et al., 2015,Johnson, et al., 1996). However, decreased BDNF concentration was consistent with the hypothesis that neurotrophins are decreased with age in the neuromuscular system (Greising, et al., 2015,Kalinkovich and Livshits, 2015). The unique innervation and sustained activity of the TA (Kuna, et al., 1988) may partially explain our findings. The TA is active during critical life functions of respiration, airway protection, swallowing, and also during vocalization in the rat (Nagai, et al., 2005,Riede, 2011) and the human (Hillel, et al., 1997,Jafari, et al., 2003,Maronian, et al., 2003). Because NT4 is specifically upregulated in an activity-dependent manner in muscle (Funakoshi, et al., 1995) greater NT4 levels might be found in muscles with sustained or frequent activity. The seemingly greater NT4 levels in the TA than in the other muscles analyzed in this study support this view (Fig. 4B). The increased NT4 in old rats may be a compensatory strategy or a sign of growth and remodeling in this muscle. Increased NT4 in old control rats may also be compensatory for reduced BDNF given that both NT4 and BDNF are the ligand for the TrkB receptor (Barbacid, 1995).
In addition to age-related neurotrophin changes found in the NA and TA, we also found age-related changes in USVs. Our findings were similar to age-related changes reported in previous studies examining aging rat vocalizations (Basken, et al., 2012,Johnson, et al., 2013), including reduced number of calls, increased duration, and decreased intensity in old versus young adult groups. In the Basken study, these age-related behavioral changes were correlated with significant motor neuron loss in the NA (Basken, et al., 2012). While motor neuron count in the NA was not examined in our study, it is possible that loss of NA motor neurons contributed to age-related behavioral decrements in the USVs observed to a greater extent than neurotrophin concentration. However, neurotrophin concentration may have a greater role in tongue actions, as we found increased BDNF concentration with exercise. This interpretation is consistent with the results of a prior study showing that motor neuron number in the HN is preserved with age (Schwarz, et al., 2009).
Significant correlations were found between extent of participation in the tongue exercise/swallow task and tongue force in both old and young adult groups (Fig. 5). This finding is proof of principle that participation in the progressive resistance tongue exercise program is associated with increased tongue force, and the increased tongue forces observed are not solely a function of learning or refinements in task performance over time. Participation at or over the individual force threshold set for each individual animal was needed to improve tongue force while decreased participation during the detraining time periods led to decreased tongue force (Fig. 5).
A question remaining in this study concerns the underlying changes responsible for increased tongue forces in the young adult group following exercise, given that BDNF concentrations were not higher following exercise. While NT4 levels were not significantly increased in the young adult exercise group, these levels were somewhat higher than those measured in the control group. A possible explanation, therefore, may be that NT4 in combination with other factors not explored in this study contributed to improved neuromuscular transmission in young adult rats. For example, other neurotrophins and their receptors, such as NT3 and TrkC are activity-dependent in nature and upregulated with exercise in the spinal sensorimotor system (Gomez-Pinilla, et al., 2001). Future research should examine this potential contribution and others.
In summary, results from this study support both of the hypotheses established for this work. Tongue exercise paired with a swallow resulted in specific gains in tongue force as well as an increase in BDNF concentration in the HN with exercise in old rats. These findings highlight the neuroplastic potential of tongue exercise paired with a swallow in the cranial sensorimotor system. However, this work did not examine whether an increase in tongue force and BDNF concentration in the HN with exercise resulted in an improvement to the functional swallow in aged rats. It is hypothesized that an increase in tongue strength will result in improved measures of swallowing function, such as bolus transport, but this must be confirmed in future studies. This work also demonstrates the importance of continued examination of current exercise protocols used in clinical populations with voice and swallowing disorders. While cross-activation benefits found in this study were minimal and were tied specifically to behavioral functions directly activated during our exercise task, maintenance of vocalization intensity following tongue exercise may be beneficial in some clinical contexts, as long as tongue exercise is paired with a swallowing activity. Because detraining resulted in loss of tongue force levels, the need for continued maintenance after a therapeutic protocol has been completed is suggested, especially for elderly people.
The results of this study provide evidence for the development of future clinical studies. Human translational and clinical studies should continue to deconstruct our current exercise-based therapy protocols to determine the biological foundations of treatment-based changes in target behaviors. These types of studies will increase the efficacy and efficiency of our current exercise protocols and provide clinicians with the evidence they need to decide which therapy protocol or exercise to use based on the presentation and specific diagnosis of the individual clients they treat.
Acknowledgements
This study was supported by National Institute on Deafness and Other Communication Disorders Grants R01DC005935, R01DC008149, R01 DC014358, T32DC009401, and F31DC013230. We are grateful for the assistance of Dr. Glen Leverson for all statistical analyses, Dr. Tiffany Glass for all illustrations, and Dr. John Russell, Heidi Kletzien, Laura Grant, Brittany Krekeler, and Corey Smith for their guidance and animal behavioral expertise during the completion of this work.
Appendix
Table.
Means and SEM. Values are neurotrophin (NT) concentration (pg/ml), YA= Young Adult. Open cells represent detraining data from the laryngeal brainstem nuclei (NA) and muscle (TA). These data were not analyzed due to the lack of an initial exercise effect on the laryngeal tissue.
| NT | Location | Control | Exercise | 2W | 4W | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |||
| NT4 | BSC | YA | 40447 | 7278 | 84578 | 18838 | 76744 | 19468 | 59983 | 11523 |
| Old | 43113 | 5390 | 41886 | 6800 | 38904 | 5112 | 33402 | 4206 | ||
| HN | YA | 40482 | 8228 | 74547 | 16076 | 52964 | 9872 | 47394 | 7602 | |
| Old | 33745 | 5836 | 34885 | 7833 | 26533 | 3989 | 27782 | 2946 | ||
| NA | YA | 54954 | 11099 | 68808 | 12173 | |||||
| Old | 38618 | 6404 | 37893 | 5072 | ||||||
| EDL | YA | 16542 | 6722 | 29858 | 6707 | 21663 | 2343 | 21168 | 3635 | |
| Old | 22575 | 7825 | 12279 | 2848 | 20235 | 3860 | 12181 | 1770 | ||
| GG | YA | 10783 | 2593 | 7357 | 1830 | 17618 | 4653 | 13375 | 5102 | |
| Old | 8111 | 1943 | 9797 | 1849 | 14536 | 5790 | 9257 | 1610 | ||
| TA | YA | 46989 | 3635 | 66573 | 10547 | |||||
| Old | 82788 | 8724 | 83013 | 18156 | ||||||
| BDNF | BSC | YA Old |
168 | 62 | 133 | 43 | 252 | 80 | 163 | 36 |
| 85 | 28 | 376 | 205 | 137 | 40 | 170 | 34 | |||
| HN | YA Old |
337 | 105 | 471 | 91 | 530 | 107 | 450 | 105 | |
| 296 | 83 | 1049 | 381 | 341 | 89 | 377 | 61 | |||
| NA | YA Old |
107 | 35 | 206 | 77 | |||||
| 144 | 23 | 555 | 308 | |||||||
| EDL | YA Old |
401 | 180 | 584 | 236 | 188 | 62 | 338 | 178 | |
| 228 | 152 | 586 | 279 | 129 | 36 | 351 | 306 | |||
| GG | YA Old |
325 | 133 | 129 | 43 | 311 | 47 | 328 | 90 | |
| 220 | 70 | 375 | 122 | 282 | 98 | 192 | 33 | |||
| TA | YA Old |
657 | 230 | 351 | 148 | |||||
| 165 | 79 | 486 | 32 | |||||||
| TrkB | BSC | YA Old |
645 | 191 | 1277 | 567 | 709 | 262 | 483 | 159 |
| 775 | 324 | 733 | 190 | 771 | 237 | 1379 | 844 | |||
| HN | YA Old |
834 | 323 | 1354 | 612 | 956 | 419 | 792 | 267 | |
| 974 | 414 | 1059 | 352 | 1058 | 280 | 960 | 492 | |||
| NA | YA Old |
920 | 360 | 436 | 203 | |||||
| 506 | 238 | 787 | 279 | |||||||
| EDL | YA Old |
3882 | 613 | 5278 | 834 | 3620 | 413 | 2785 | 427 | |
| 4268 | 773 | 3193 | 889 | 3490 | 788 | 5069 | 1116 | |||
| GG | YA Old |
2584 | 636 | 2815 | 692 | 3210 | 302 | 2184 | 482 | |
| 2041 | 475 | 2575 | 433 | 2496 | 420 | 3145 | 637 | |||
| TA | YA Old |
4635 | 1151 | 8568 | 2204 | |||||
| 6826 | 1851 | 9894 | 4433 | |||||||
References
- Barbacid M. Neurotrophic factors and their receptors. Current opinion in cell biology. 1995;7(2):148–55. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barde YA. The nerve growth factor family. Progress in growth factor research. 1990;2(4):237–48. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- Basken JN, Connor NP, Ciucci MR. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Exp Brain Res. 2012;219(3):351–61. doi: 10.1007/s00221-012-3096-6. doi:10.1007/s00221-012-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Moeser AE, Thomas CF, Russell JA, Wang H, Leverson GE, Connor NP. The effect of tongue exercise on serotonergic input to the hypoglossal nucleus in young and old rats. Journal of speech, language, and hearing research : JSLHR. 2012;55(3):919–29. doi: 10.1044/1092-4388(2011/11-0091). doi:10.1044/1092-4388(2011/11-0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman A, Rutledge J, Hembree A, Sheridan S. Vocal hygiene education, voice production therapy, and the role of patient adherence: a treatment effectiveness study in women with phonotrauma. Journal of speech, language, and hearing research : JSLHR. 2008;51(2):350–66. doi: 10.1044/1092-4388(2008/026). doi:10.1044/1092-4388(2008/026) [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral neuroscience. 2000;114(5):983–90. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC. Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus) Journal of comparative psychology (Washington, DC: 1983) 1992;106(3):270–7. doi: 10.1037/0735-7036.106.3.270. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior genetics. 2005;35(1):85–92. doi: 10.1007/s10519-004-0858-3. doi:10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–65. doi: 10.1007/s00455-006-9074-z. doi:10.1007/s00455-006-9074-z. [DOI] [PubMed] [Google Scholar]
- Butefisch CM. Neurobiological bases of rehabilitation. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27(Suppl 1):S18–23. doi: 10.1007/s10072-006-0540-z. doi:10.1007/s10072-006-0540-z. [DOI] [PubMed] [Google Scholar]
- Carnaby GD, Harenberg L. What is “usual care” in dysphagia rehabilitation: a survey of USA dysphagia practice patterns. Dysphagia. 2013;28(4):567–74. doi: 10.1007/s00455-013-9467-8. doi:10.1007/s00455-013-9467-8. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behavioral neuroscience. 2009;123(2):328–36. doi: 10.1037/a0014593. doi:10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson's disease: deficit-targeted training. Parkinsonism & related disorders. 2008;14(Suppl 2):S172–5. doi: 10.1016/j.parkreldis.2008.04.027. doi:10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Schaser AJ, Russell JA. Exercise-induced rescue of tongue function without striatal dopamine sparing in a rat neurotoxin model of Parkinson disease. Behav Brain Res. 2013;252:239–45. doi: 10.1016/j.bbr.2013.06.004. doi:10.1016/j.bbr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, O'Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. Journal of speech, language, and hearing research : JSLHR. 2009;52(4):1034–47. doi: 10.1044/1092-4388(2009/08-0062). doi:10.1044/1092-4388(2009/08-0062) [DOI] [PubMed] [Google Scholar]
- Connor NP, Ota F, Nagai H, Russell JA, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. Journal of speech, language, and hearing research : JSLHR. 2008;51(4):818–27. doi: 10.1044/1092-4388(2008/059). doi:10.1044/1092-4388(2008/059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of speech, language, and hearing research : JSLHR. 2009;52(3):732–44. doi: 10.1044/1092-4388(2008/08-0105). doi:10.1044/1092-4388(2008/08-0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, Chen WG, Cohen LG, deCharms C, Duffy CJ, Eden GF, Fetz EE, Filart R, Freund M, Grant SJ, Haber S, Kalivas PW, Kolb B, Kramer AF, Lynch M, Mayberg HS, McQuillen PS, Nitkin R, Pascual-Leone A, Reuter-Lorenz P, Schiff N, Sharma A, Shekim L, Stryker M, Sullivan EV, Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain : a journal of neurology. 2011;134(Pt 6):1591–609. doi: 10.1093/brain/awr039. doi:10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gomez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–80. doi: 10.1016/j.neuroscience.2011.06.032. doi:10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring, Md) 2006;14(3):345–56. doi: 10.1038/oby.2006.46. doi:10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Easterling C. Does an exercise aimed at improving swallow function have an effect on vocal function in the healthy elderly? Dysphagia. 2008;23(3):317–26. doi: 10.1007/s00455-008-9158-z. doi:10.1007/s00455-008-9158-z. [DOI] [PubMed] [Google Scholar]
- El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, Pawlas A, Baum S, Werner C. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT): a pilot study. Journal of neurology, neurosurgery, and psychiatry. 2002;72(1):31–6. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BR. Folding mechanism of the human larynx. Acta Oto-Laryngologica. 1974;78(1-2):124–8. doi: 10.3109/00016487409126336. [DOI] [PubMed] [Google Scholar]
- Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Seminars in speech and language. 2006;27(4):283–99. doi: 10.1055/s-2006-955118. doi:10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respiration physiology. 1997;110(2-3):295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science (New York, NY) 1995;268(5216):1495–9. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gay T, Rendell JK, Spiro J. Oral and laryngeal muscle coordination during swallowing. The Laryngoscope. 1994;104(3 Pt 1):341–9. doi: 10.1288/00005537-199403000-00017. doi:10.1288/00005537-199403000-00017. [DOI] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. Journal of neurophysiology. 1995;74(2):547–55. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. The European journal of neuroscience. 2001;13(6):1078–84. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of neurophysiology. 2002;88(5):2187–95. doi: 10.1152/jn.00152.2002. doi:10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. The European journal of neuroscience. 2011;33(3):383–90. doi: 10.1111/j.1460-9568.2010.07508.x. doi:10.1111/j.1460-9568.2010.07508.x; 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol Aging. 2008;23(4):692–701. doi: 10.1037/a0014345. doi:10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015;593(2):431–40. doi: 10.1113/jphysiol.2014.282244. doi:10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan A, O'Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochemical Society transactions. 2007;35(Pt 2):424–7. doi: 10.1042/BST0350424. doi:10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- Hillel AD, Robinson LR, Waugh P. Laryngeal electromyography for the diagnosis and management of swallowing disorders. Otolaryngol Head Neck Surg. 1997;116(3):344–8. doi: 10.1016/S0194-59989770271-7. [DOI] [PubMed] [Google Scholar]
- Hodges SH, Anderson AL, Connor NP. Remodeling of Neuromuscular Junctions in Aged Rat Genioglossus Muscle. Annals of Otology, Rhinology & Laryngology. 2004;113(3):175–9. doi: 10.1177/000348940411300301. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. doi:10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(Pt 1):287–304. doi: 10.1113/jphysiol.2003.039966. doi:10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Ciucci MR, Connor NP. Vocal training mitigates age-related changes within the vocal mechanism in old rats. J Gerontol A Biol Sci Med Sci. 2013;68(12):1458–68. doi: 10.1093/gerona/glt044. doi:10.1093/gerona/glt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. The Journal of the Acoustical Society of America. 2010;128(2):EL75–9. doi: 10.1121/1.3462234. doi:10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J Vis Exp. 2011;(54) doi: 10.3791/2835. doi:10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T, Ulfhake B. Decreased expression of TrkB and TrkC mRNAs in spinal motoneurons of aged rats. The European journal of neuroscience. 1996;8(3):494–9. doi: 10.1111/j.1460-9568.1996.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Kalinkovich A, Livshits G. Sarcopenia - The search for emerging biomarkers. Ageing Res Rev. 2015;22:58–71. doi: 10.1016/j.arr.2015.05.001. doi:10.1016/j.arr.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of speech, language, and hearing research : JSLHR. 2008;51(1):S225–39. doi: 10.1044/1092-4388(2008/018). doi:10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T, American College of Sports, M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise. 2002;34(2):364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol (1985) 1988;65(3):1332–9. doi: 10.1152/jappl.1988.65.3.1332. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. The American Journal of Physiology. 1992;262(2 Pt 1):G338–44. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Hoit J, Kent R, Ramig LO, Shrivastav R, Strand E, Yorkston K, Sapienza CM. Translating principles of neural plasticity into research on speech motor control recovery and rehabilitation. Journal of speech, language, and hearing research : JSLHR. 2008;51(1):S240–58. doi: 10.1044/1092-4388(2008/019). doi:10.1044/1092-4388(2008/019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronian N, Waugh P, Robinson L, Hillel A. Electromyographic findings in recurrent laryngeal nerve reinnervation. The Annals of Otology, Rhinology, and Laryngology. 2003;112(4):314–23. doi: 10.1177/000348940311200405. [DOI] [PubMed] [Google Scholar]
- Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiology & Behavior. 1992;51(4):783–6. doi: 10.1016/0031-9384(92)90116-j. [DOI] [PubMed] [Google Scholar]
- McCulloch TM, Perlman AL, Palmer PM, Van Daele DJ. Laryngeal activity during swallow, phonation, and the Valsalva maneuver: an electromyographic analysis. The Laryngoscope. 1996;106(11):1351–8. doi: 10.1097/00005537-199611000-00009. [DOI] [PubMed] [Google Scholar]
- McFarland DH, Paydarfar D. Proceedings of the Integrative Neural Systems Underlying Vital Aerodigestive Tract Functions Conference, June 17-19, 2010: work group summary and call to action. Head & neck. 2011;33(Suppl 1):S54–7. doi: 10.1002/hed.21908. doi:10.1002/hed.21908; 10.1002/hed.21908. [DOI] [PubMed] [Google Scholar]
- McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Seminars in speech and language. 2006;27(4):300–9. doi: 10.1055/s-2006-955119. doi:10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;80(1):81–8. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2002;13(5):409–25. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- Mujika I, Padilla S. Detraining: loss of training-induced physiological and performance adaptations. Part I: short term insufficient training stimulus. Sports medicine (Auckland, NZ) 2000a;30(2):79–87. doi: 10.2165/00007256-200030020-00002. [DOI] [PubMed] [Google Scholar]
- Mujika I, Padilla S. Detraining: loss of training-induced physiological and performance adaptations. Part II: Long term insufficient training stimulus. Sports medicine (Auckland, NZ) 2000b;30(3):145–54. doi: 10.2165/00007256-200030030-00001. [DOI] [PubMed] [Google Scholar]
- Nagai H, Ota F, Konopacki R, Connor NP. Discoordination of laryngeal and respiratory movements in aged rats. Am J Otolaryngol. 2005;26(6):377–82. doi: 10.1016/j.amjoto.2005.02.015. doi:10.1016/j.amjoto.2005.02.015. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory, A. Guide for the Care and Use of Laboratory Animals. National Academies Press (US) National Academy of Sciences; Washington (DC): 2011. The National Academies Collection: Reports funded by National Institutes of Health. [Google Scholar]
- Nishio M, Niimi S. Relationship between speech and swallowing disorders in patients with neuromuscular disease. Folia phoniatrica et logopaedica : official organ of the International Association of Logopedics and Phoniatrics (IALP) 2004;56(5):291–304. doi: 10.1159/000080066. doi:10.1159/000080066. [DOI] [PubMed] [Google Scholar]
- Nowinski WL, Johnson A, Chua BC, Nowinska NG. Three-dimensional interactive and stereotactic atlas of the cranial nerves and their nuclei correlated with surface neuroanatomy, vasculature and magnetic resonance imaging. Journal of neuroscience methods. 2012;206(2):205–16. doi: 10.1016/j.jneumeth.2012.02.026. doi:10.1016/j.jneumeth.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Ota F, Connor NP, Konopacki R. Alterations in Contractile Properties of Tongue Muscles in Old Rats. Annals of Otology, Rhinology & Laryngology. 2005;114(10):799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. Journal of applied physiology (Bethesda, Md: 1985) 1999;86(5):1663–9. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- Pressman JJ. SPhincter action of the larynx. 1941:351–77. [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. Journal of neurophysiology. 2011;106(5):2580–92. doi: 10.1152/jn.00478.2011. doi:10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Rat ultrasonic vocalization shows features of a modular behavior. J Neurosci. 2014;34(20):6874–8. doi: 10.1523/JNEUROSCI.0262-14.2014. doi:10.1523/jneurosci.0262-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek J. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. Journal of speech, language, and hearing research : JSLHR. 2008;51(1):S276–300. doi: 10.1044/1092-4388(2008/021). doi:10.1044/1092-4388(2008/021) [DOI] [PubMed] [Google Scholar]
- Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation. 2007;88(2):150–8. doi: 10.1016/j.apmr.2006.11.002. doi:10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Roy N, Weinrich B, Gray SD, Tanner K, Stemple JC, Sapienza CM. Three treatments for teachers with voice disorders: a randomized clinical trial. Journal of speech, language, and hearing research : JSLHR. 2003;46(3):670–88. doi: 10.1044/1092-4388(2003/053). [DOI] [PubMed] [Google Scholar]
- Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28(1):95–104. doi: 10.1007/s00455-012-9417-x. doi:10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Texas reports on biology and medicine. 1975;33(3):444–55. [PubMed] [Google Scholar]
- Schaser AJ, Stang K, Connor NP, Behan M. The effect of age and tongue exercise on BDNF and TrkB in the hypoglossal nucleus of rats. Behav Brain Res. 2012;226(1):235–41. doi: 10.1016/j.bbr.2011.09.027. doi:10.1016/j.bbr.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2011;26(3):256–63. doi: 10.1007/s00455-010-9297-x. doi:10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz EC, Thompson JM, Connor NP, Behan M. The effects of aging on hypoglossal motoneurons in rats. Dysphagia. 2009;24(1):40–8. doi: 10.1007/s00455-008-9169-9. doi:10.1007/s00455-008-9169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM. Optimal approaches for measuring tongue-pressure functional reserve. J Aging Res. 2013;2013:542909. doi: 10.1155/2013/542909. doi:10.1155/2013/542909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple JC, Lee L, D'Amico B, Pickup B. Efficacy of vocal function exercises as a method of improving voice production. J Voice. 1994;8(3):271–8. doi: 10.1016/s0892-1997(05)80299-1. [DOI] [PubMed] [Google Scholar]
- Story BH, Titze IR. Voice simulation with a body-cover model of the vocal folds. The Journal of the Acoustical Society of America. 1995;97(2):1249–60. doi: 10.1121/1.412234. [DOI] [PubMed] [Google Scholar]
- Stuart TPA. On the Mechanism of the Closure of the Larynx. A Preliminary Communication. Proceedings of the Royal Society of London. 1891;50(302-307):323–39. doi:10.1098/rspl.1891.0043. [Google Scholar]
- Suzuki T, Bless DM, Connor NP, Ford CN, Kyungah L, Inagi K. Age-Related Alterations in Myosin Heavy Chain Isoforms in Rat Intrinsic Laryngeal Muscles. Annals of Otology, Rhinology & Laryngology. 2002;111(11):962. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2000;39(6):9–17. [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. The journals of gerontologySeries A, Biological sciences and medical sciences. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of Glottic Closure During Swallowing: A Combined Electromyographic and Endoscopic Analysis. Annals of Otology, Rhinology & Laryngology. 2005;114(6):478–87. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and neural repair. 2005;19(4):283–95. doi: 10.1177/1545968305280753. doi:10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiology & Behavior. 2008;93(4-5):766–76. doi: 10.1016/j.physbeh.2007.11.031. doi:10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Sheng Li Xue Bao. 2003;55(6):617–24. [PubMed] [Google Scholar]