Abstract
Study Objectives:
To assess the efficacy of a novel female-specific autotitrating continuous positive airway pressure (CPAP) algorithm (AutoSet for her, AfH) in premenopausal women relative to a standard autotitrating algorithm (AutoSet, S9) (ResMed Ltd., Bella Vista, New South Wales, Australia).
Design:
Prospective randomised crossover noninferiority trial.
Setting:
Tertiary hospital sleep clinic and university research sleep laboratory.
Participants:
20 female patients with obstructive sleep apnea (OSA) established on long-term CPAP treatment.
Interventions:
Treatment with 1 night each of AfH and AutoSet while monitored with overnight laboratory-based polysomnography (PSG); order randomly allocated.
Measurements and Results:
The primary outcome variables were the apnea-hypopnea index (AHI) and 3% oxygen desaturation index (ODI 3%) determined from PSG. Treatment efficacy on the AfH night was noninferior to the AutoSet night as assessed by median (IQR) AHI (1.2 [0.60–1.85]/h versus 1.15 [0.40–2.85]/h, respectively, P = 0.51) and 3% ODI (0.85 [0.25–1.5]/h versus 0.5 [0.25–2.55]/h, respectively, P = 0.83). Other PSG measures were similar, except for the percentage of the night spent in flow limitation, which was lower on the AfH (0.14%) than the AutoSet night (0.19%, P = 0.007). The device-downloaded 95th centile pressure on the AfH night was also lower than on the AutoSet night (10.6 ± 1.7 versus 11.6 ± 2.6 cmH2O, respectively; mean difference [95% confidence interval]: −1.1 [−2.13 to −0.01] cm H2O).
Conclusion:
Among premenopausal women a novel female-specific autotitrating algorithm (AfH) is as effective as the standard AutoSet algorithm in controlling obstructive sleep apnea (OSA). The new algorithm may reduce flow limitation more than the standard algorithm and achieve control of OSA at a lower (95th centile) pressure.
Citation:
McArdle N, King S, Shepherd K, Baker V, Ramanan D, Ketheeswaran S, Bateman P, Wimms A, Armitstead J, Richards G, Hillman D, Eastwood P. Study of a novel APAP algorithm for the treatment of obstructive sleep apnea in women. SLEEP 2015;38(11):1775–1781.
Keywords: apnea-hypopnea index, automatic positive airway pressure algorithm, continuous positive airway pressure, obstructive sleep apnea, positive airway pressure titration, premenopausal women
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder characterized by repetitive collapse of the upper airway during sleep and associated nocturnal hypoxia and sleep fragmentation. It is a disorder that has widespread effects on health and is associated with reduced quality of life,1 neurocognitive impairment (including increased risk of motor vehicle accidents2), and increased cardiovascular morbidity and mortality; from ischemic heart disease, congestive heart failure, and stroke.3–5
Early studies of OSA report a high male predominance, with male-to-female ratios ranging between 10:1 and 60:1 in clinic populations.6 Hence, OSA is traditionally thought of as a predominantly male disorder and treatment options have often been developed and tested in male study populations. More recently, several studies have reported a male-to-female ratio closer to 3:1,7,8 and indicate that women may present with different clinical features8 and have different polysomnographic (PSG) patterns of obstructive sleep disordered breathing compared to men. In particular, PSG studies show a relative rapid eye movement (REM) predominance to obstructive events9 and milder disease (i.e., lower apnea-hypopnea index; AHI) in women compared to men.10,11 Women with obstructive sleep apnea are also less likely to manifest complete upper airway collapse (apneas)12 and more likely to have flow limitation, which can manifest as an upper airway resistance syndrome (UARS).11 These sex differences may affect therapeutic decisions and therapeutic effectiveness.
The gold-standard treatment for moderate and severe OSA is continuous positive airway pressure (CPAP),13 which acts as a pneumatic splint to maintain patency of the upper airway. Long-term treatment may be delivered using a standard CPAP device at a set “fixed” pressure, or using automatic positive airway pressure (APAP) devices that vary the pressure throughout the night based on device-monitored physiological signals. The pressure response in these APAP devices is controlled by a computerized algorithm. ResMed Corporation has recently developed a female-specific ‘AutoSet for Her’ (AfH) algorithm; designed to optimize the pressure response to the specific patterns of obstructive sleep disordered breathing seen in women. The AfH algorithm is adapted from the S9 AutoSet algorithm (ResMed Ltd., Bella Vista, Sydney) with a number of modifications, including an increased sensitivity to flow limitation, an optimized internal gain (a slower, and lower, pressure rise and decay in response to flow limitation), a lower cap on the pressure response to obstructive apneas, and an adaptive minimum pressure.
We sought to assess the efficacy of this new algorithm in premenopausal women by comparing it to the standard ResMed S9 AutoSet algorithm. The primary aim of the study was to assess the efficacy of the AfH algorithm, based on a priori PSG outcome measures of the AHI and the 3% oxygen desaturation index (3% ODI). Secondary aims were to compare objective sleep quality measures and patient symptomatic responses between the 2 study nights. We hypothesized that the efficacy (AHI and 3% ODI) of the AfH algorithm would be noninferior to the standard AutoSet (ResMed) algorithm and speculated that its use would be associated with advantages in terms of patient comfort.
METHODS
Overview
A double-blind randomized crossover study design was used (Figure 1), which required participants to undergo 2 overnight laboratory-based PSGs, 1 night using an APAP device set in the AfH mode and the other night set in the standard AutoSet algorithm mode.
Figure 1.
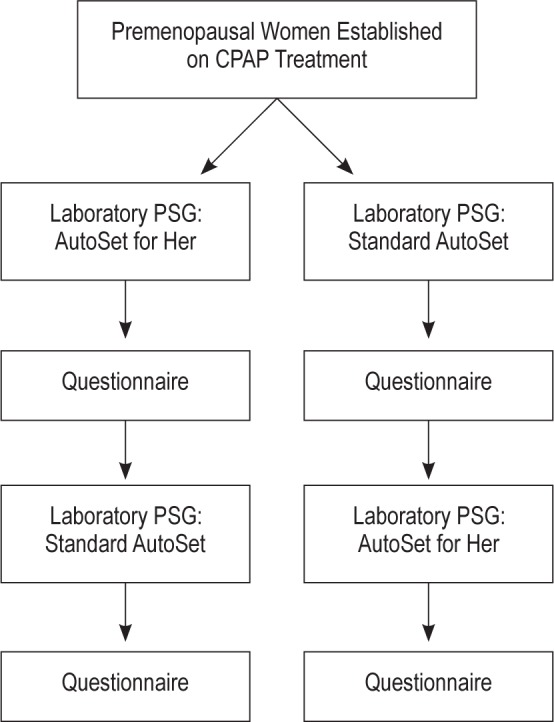
Study flow. Questionnaire asks about sleep quality and comfort using the device. PSG, polysomnography.
Study Participants
Inclusion criteria comprised premenopausal females aged 18 y or older; current positive airway pressure (CPAP or APAP) therapy use, where “current” was defined as on therapy for at least 1 mo prior to study entry; availability of a diagnostic PSG; diagnosis of mild-moderate OSA (5 < AHI ≤ 30); and willingness and ability to give written informed consent
Exclusion criteria comprised current use of bilevel positive airway pressure treatment; current use of supplemental oxygen; pregnancy; a preexisting lung disease or condition that would predispose the participant to pneumothorax (e.g., chronic obstructive pulmonary disease, lung cancer; pulmonary fibrosis; recent (< 2 y) pneumonia or lung infection; other lung injury); and any individual whom the researcher believes is unsuitable for inclusion because that person does not comprehend English or is unable to provide written informed consent or physically unable to comply with the protocol.
Potential participants were identified from the Sleep Clinic database, contacted by phone and asked if they wished to take part in the study. The study was approved by the Institutional Review Boards of the University of Western Australia and Sir Charles Gairdner Hospital. Written informed consent was obtained prior to participation in the study. The trial was registered with ClinicalTrials.gov (Clinical Trials Registry number: NCT01826513).
Study Protocol
A double-blind randomized crossover study design was used (Figure 1). Participants spent 1 night using an APAP device set in the AfH mode and another night set in the standard AutoSet algorithm mode. An in-house questionnaire asking about sleep quality and comfort using the device was completed after each study night. The studies were done on consecutive nights, apart from one patient whose studies were separated by 2 nights. One member of the research team randomly determined the order of the nights, concealed the codes using opaque envelopes, and allocated device modes to each participant. Neither the patient nor the overnight research staff was able to ascertain the device mode because the device appeared identical irrespective of the algorithm used. Furthermore, all outcome analyses were performed by one sleep scientist, blinded to the study arm (i.e., scoring of respiratory events was performed without access to the pressure signal to ensure full blinding, i.e., using other respiratory signals, including mask flow signal). Self-reported menopause status, medical history, and concomitant medications were recorded. Comorbidities were identified based on reported history or treatment for the condition.
CPAP
During the study nights the device was set to a pressure range of 4–20 cm H2O, and the ramp set at the patients' usual value (AutoSet night) or automatic with a maximum of 30 min (AfH night). All other settings (e.g., humidification) were set as per the patients' usual device and the patient used his or her own mask and chin strap (if required) on both study nights. The device was set by research staff in the evening prior to arrival of overnight staff to ensure the latter were blinded to the algorithm used.
PSG
In-laboratory PSG was performed using the Compumedics Grael HD-PSG (Compumedics Ltd., Abbotsford, Australia), which recorded the following signals: F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1 electroencephalogram, bilateral electrooculograms, submental electromyogram, electrocardiogram, device analog outputs (i.e., mask pressure, unintentional leak and flow), oximetry (averaged over three beats, sampling 256 Hz), ribcage and abdominal movement (respiratory inductance plethysmography), body position, sound intensity (dB), and bilateral tibial electromyogram.
Questionnaire
Symptomatic responses to therapy, including questions about participant's perception of their sleep on the device and quality of sleep, were assessed in the morning after each study night using a Likert scale (see supplemental material).
Data Analyses
PSGs were manually scored at the study site by experienced sleep scientists according to standard criteria (AASM 2012).14 Flow limitation was assessed by the site using the sponsor's (ResMed Ltd.) flow limitation tool to perform automatic analyses of high-fidelity flow signals (25 Hz). The flow limitation tool utilizes the shape, tidal volume, and duty cycle (ratio of inspiratory time to total breath time) of each breath and automatically identifies whether each breath is flow limited or not.
Statistical Analyses
Statistical analyses were performed using SigmaStat version 3.5 (Systat, Richmond, CA, USA). Parametric data were described using means and standard deviations (SDs) and paired comparisons were performed using paired t tests and 95th percentile confidence intervals (95% CIs) were reported. Nonparametric variables were described using medians and interquartile ranges and paired comparisons made with the Wilcoxon signed-rank test. Statistical significance was considered to occur when P < 0.05.
Sample Size Calculation
We tested the hypothesis that the AfH algorithm was not worse (but not necessarily better) than the standard AutoSet algorithm. Hence, the null Hypothesis (H0) was: AfH is inferior to standard AutoSet and the alternate Hypothesis (H1) was: AfH is noninferior to standard AutoSet. The expected AHI difference (mu) is 0 events/h and the NonInferiority Margin (delta) is 0.75 events/h (a difference of 1 event/h is seen as clinically significant: 0.75 events/h was chosen to ensure any relevant AHI change was observed). Unpublished data from a trial15 supported by the sponsor showed that the SD of such a dataset is 1.06 events/h. Based on a power of 80% and two-sided alpha of 0.05 (one-sided alpha of 0.025 used in this noninferiority trial), the sample size (for paired Trial)16 = (Z(1 − a.2) + Z(1 − b))2) * (SD / (mu − delta))2. Using our data, the sample size = (1.96 + 0.85)2 * (1.06 / (0 − 0.75))2 = 15.8. On this basis, and allowing for potential dropouts, we chose a sample size of 20 for the study.
RESULTS
Patient Characteristics
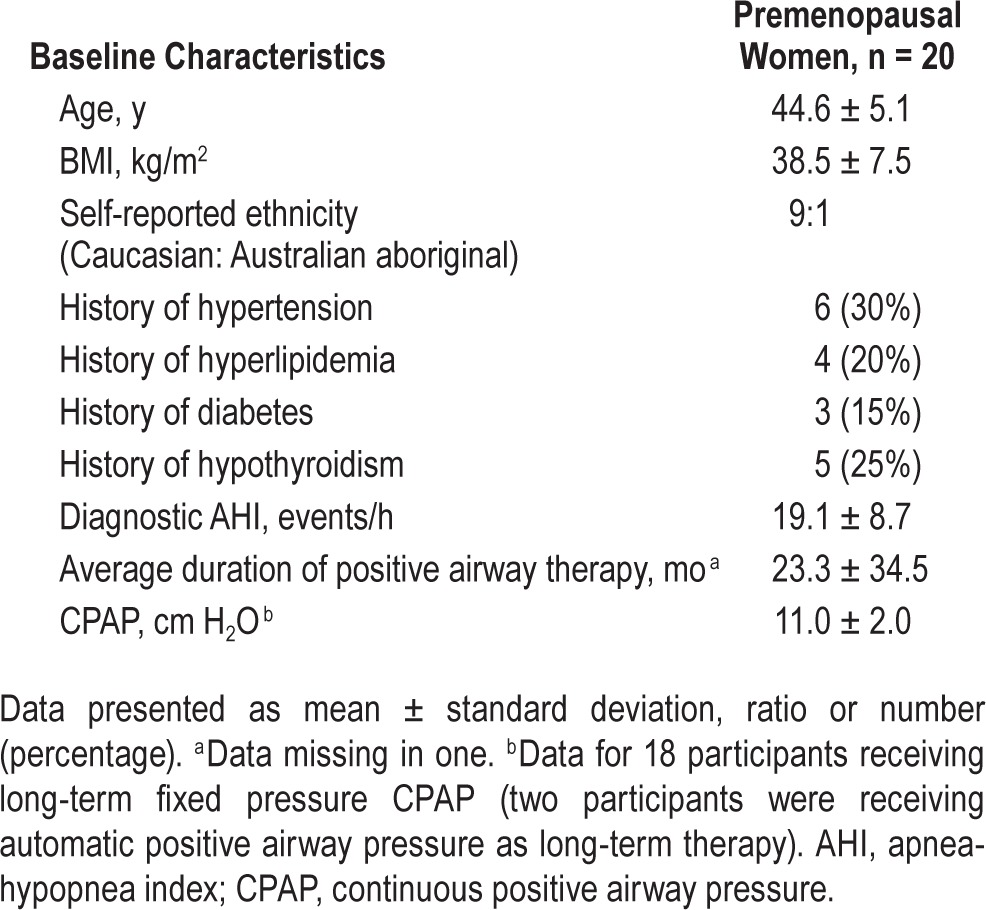
Twenty women participated in the study and all completed the protocol. Participants were premenopausal, obese (body mass index; BMI = 38.5 ± 7.5 kg/m2), predominantly Caucasian females aged 44.6 ± 5.1 y, most of whom received a diagnosis of moderately severe OSA (AHI = 19.1 ± 8.7 events/h) (Table 1). Three participants were recruited with severe OSA after a decision was made by the study investigators to modify the protocol to assist with recruitment. This protocol variation was considered to be safe and was approved by the local ethics review board. Participants had a higher prevalence of cardiovascular risk factors (Table 1) than are typical for similar aged women in the community but similar to that expected in an OSA sleep clinic population. None had severe cardiac or pulmonary comorbidities. Participants had been using CPAP treatment for an average of 23 mo with a mean fixed CPAP pressure of 11.0 ± 2.0 cm H2O, apart from two patients who had been using an APAP device (Table 1). The majority of patients were using a nasal mask (55%), with the remainder using nasal pillows (35%) or a full face mask (15%).
Table 1.
Baseline characteristics of study participants.
Outcomes
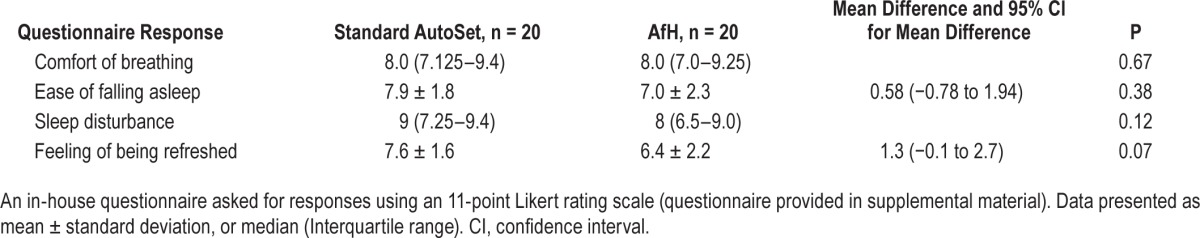
Treatment efficacy on the AfH night was noninferior to the AutoSet night as assessed by AHI (1.2 [0.60–1.85]/h versus 1.15 [0.40–2.85]/h, P = 0.51) and 3% ODI (0.5 [0.25–2.55]/h versus 0.85 [0.25– 1.5]/h, P = 0.83) (Figure 2 and Table 2). In comparison with the patients' diagnostic AHI there was a statistically and clinically significant reduction in AHI with treatment using the AfH (diagnostic versus AfH: 19.07 versus 1.2/h, P < 0.001) and AutoSet algorithms (diagnostic versus AutoSet: 19.07 versus 1.15/h, P < 0.001). Percentage of breaths with flow limitation during sleep was significantly less using the AfH algorithm (0.14%) than the AutoSet (0.20%, P = 0.02) (Table 2). Other PSG measures of sleep quality were similar between study nights (Table 2, all P > 0.05). The downloaded 95th centile pressure from the device on the AfH study night was lower than on the AutoSet night (10.56 ± 1.7 versus 11.63 ± 2.6 cmH2O; mean difference (95% CI): −1.1 (−2.13 to −0.01) cm H2O). The downloaded median pressure delivered by the AfH device was similar to that delivered by the AutoSet (P > 0.05). The downloaded median leak from the device was similar on the AfH and AutoSet nights (P > 0.05), as was the 95th centile leak (AfH: 9.0 ± 9.6 l/min, AutoSet: 12.6 ± 15.6 l/min, P > 0.05). Symptom response to the treatment nights and device tolerance were similar following a night using the AfH compared to AutoSet (all P > 0.05) (Table 3).
Figure 2.
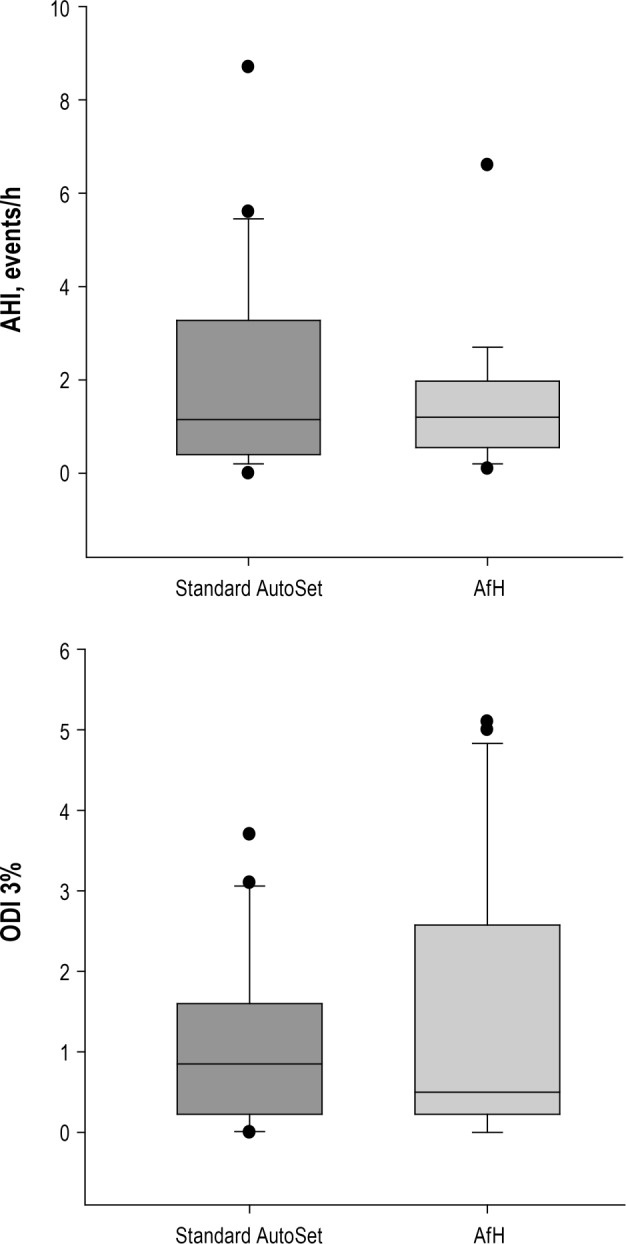
Comparison of efficacy outcomes during standard AutoSet vs AutoSet for Her (AfH) treatment nights.
Table 2.
Polysomnographic sleep characteristics during standard Autoset and AutoSet for her (AfH) treatment nights.
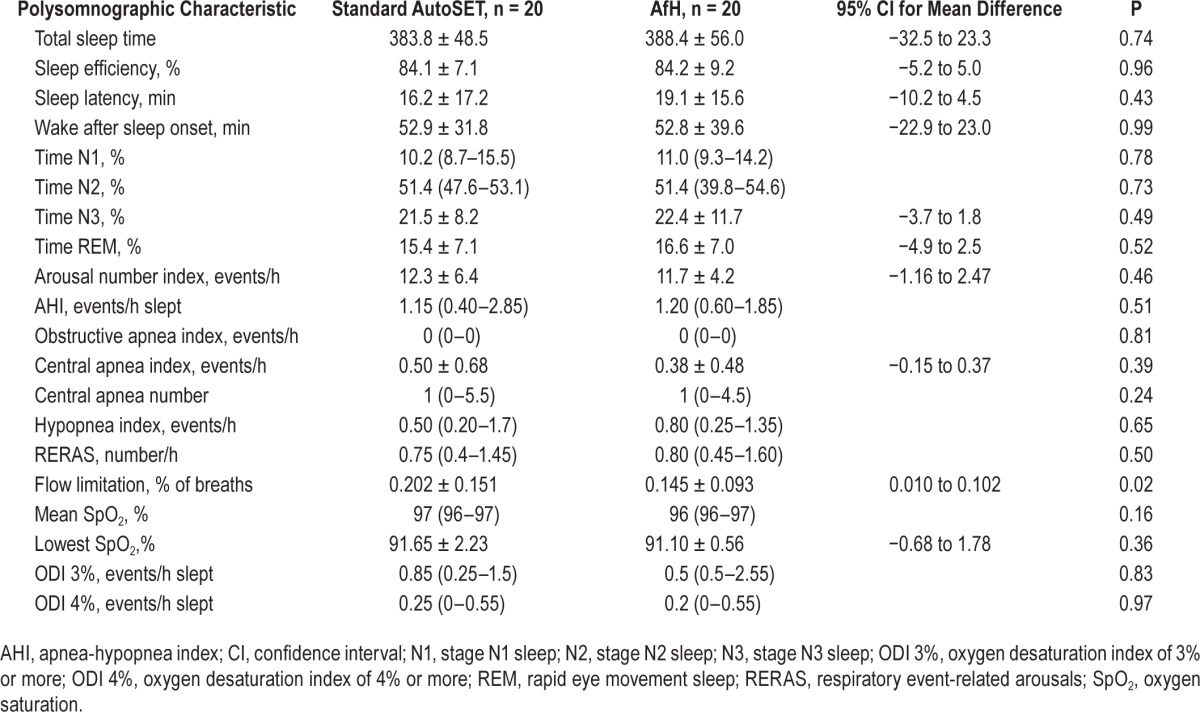
Table 3.
Subjective feedback from participants after standard Autoset and Autoset for her (AfH) treatment nights.
DISCUSSION
This study among premenopausal women shows the AfH algorithm to be as efficacious as the standard AutoSet algorithm, according to overnight full PSG evaluation. Compared to a diagnostic study night (i.e., without treatment) both algorithms reduced the AHI to ‘well controlled’ (P < 0.001), confirming that these are suitable algorithms for CPAP treatment of OSA. In addition, sleep efficiency was high on the AfH and AutoSet nights and other PSG measures of sleep quality were similar on both nights and similar to the quoted normal ranges for middle-aged females.17,18 Notably, there was a statistically significant reduction in flow limitation (% of breaths), achieved at a lower (95th centile) pressure, on the AfH night compared to the AutoSet night.
Many of the early studies showing a high prevalence of OSA among males, compared to females, used clinic-based samples.6,8 By contrast, community studies7,19,20 have consistently shown male-to- female ratios to range from 2:1 to 4:1, suggesting clinical underrecognition of OSA in females, perhaps because females report less classic OSA symptoms such as snoring19,21 and witnessed apneas22 and for other sociocultural reasons.22 The historically high male prevalence in clinical samples has resulted in most treatment options being developed and tested in predominantly male study samples. Moreover, several recent studies have reported sex-specific differences in the patterns of sleep and sleep disordered breathing, particularly among premenopausal women.9,10,23 These differences raise the possibility that tailoring OSA treatment according to sex-specific patterns of obstructive sleep disordered breathing may improve the efficacy of APAP treatment. The current device was, therefore, designed and developed to provide a female-specific APAP algorithm (AfH) with the aim of targeting the breathing abnormalities characteristic of female patients.
The primary aim of the current study was to test the hypothesis that the efficacy of the new AfH algorithm is noninferior to the standard AutoSet algorithm. The AHI and ODI were chosen as the primary outcome measures. AHI is the standard accepted metric used to determine severity of OSA and the ODI may have particular usefulness as a predictor of OSA-related vascular and metabolic consequences.24 On both measures the new AfH algorithm performed similarly to the standard AutoSet algorithm, as assessed by the gold standard of laboratory-based PSG. This finding supports the use of the AfH algorithm as a new efficacious treatment option for mild-moderate OSA among premenopausal patients.
The AfH algorithm has been designed to be more sensitive to flow limitation by responding to the first identified flow-limited breath rather than requiring three consecutive flow-limited breaths, as occurs with the standard AutoSet algorithm. The basis for this change is the increasing evidence that inspiratory flow limitation is more prevalent in women compared to men. For example, a recent study among consecutive sleep clinic patients referred for evaluation of sleep disordered breathing found women to have more UARS than OSA, whereas among men the prevalence of OSA was greater than UARS.11 Similarly, women attending a sleep clinic appear to have fewer episodes of complete upper airway collapse (lower ratio of apneas to hypopneas) compared to men.12 The precise mechanisms underlying these findings have yet to be resolved, but are most likely related to complex sex-related differences in the structure and function of the upper airway. For example, comparisons between men and women, matched for BMI, found the critical airway closing pressure (Pcrit) was lower in women compared to men without differences in respiratory control stability.25 Overall, these studies indicate that women have a less collapsible upper airway, making obstructive apneas less likely and predisposing to partial airway collapse (hypopnoeas) and flow-limited breathing abnormalities during sleep.
Despite the percentage residual flow limitation being low with both algorithms, the current study showed improved control of flow limitation on the AfH night (Table 2). It is unknown whether a reduction from 0.20 to 0.14% flow-limited breaths using AfH, compared to standard AutoSet, is of clinical significance. However, the participants were compliant users, established on CPAP treatment, who had excellent control of their OSA using APAP, producing ‘floor effects’ that limit the possibility of showing large improvements in disease control with the new algorithm. Studies on consecutive CPAP naïve patients in the standard clinical setting are needed to assess the potential magnitude of improvement in flow limitation obtainable from the AfH algorithm.
Another novel feature of the AfH algorithm is a moving minimum AutoSet pressure (i.e., a minimum pressure is set to which pressure decreases during sleep periods devoid of respiratory events). If apneas occur within a short time period the minimum AfH pressure will automatically increase and the pressure will not decline below this level for the remainder of the night's therapy. The purpose of this is to minimize inappropriate pressure decreases during REM sleep that could occur with the standard AutoSet algorithm. It is possible, for example, that the standard AutoSet algorithm pressure could decay below the critical closing airway pressure during REM sleep, which can result in several apneas at the beginning of REM sleep until the device responds with appropriate pressure increases. This could be particularly important in women, who have been shown to have a predominance of REM-related OSA compared to men.9 During REM sleep CPAP pressures may need to be higher to maintain patency of the upper airway secondary to a REM-related reduction in the tone of upper airway muscles. It is also possible that this algorithm feature could reduce pressure variability, contribute to longer REM sleep, and reduce REM-related respiratory events. However, the current study did not find any statistically significant differences on these measures, although it was not designed or statistically powered to detect these differences and larger studies would be needed in order to demonstrate any such changes.
In order to prevent an excessive pressure rise, the AfH algorithm does not increase pressure above 12 cm H2O in response to detected apneas (but pressure can increase above 12 cm H2O if other respiratory events are present). Furthermore, the AfH algorithm increases pressure in response to flow limitation at a slower rate and to a lesser extent than the standard AutoSet algorithm (similarly the decay in the gain is lower). These features are in response to previous studies that have shown that women tend to have less severe OSA, for a given BMI, compared to men,9–11 and that women appear to require lower CPAP pressures than men as determined by manual attended laboratory PSG titration.26 The current study supports the use of this AfH pressure algorithm strategy among premenopausal female patients with OSA because equivalent control of apneas and hypopneas and improved control of flow limitation was achieved at a lower 95th centile pressure than the standard AutoSet algorithm. The 95th centile pressure is an important index of pressure requirements as it is the value commonly used when setting a fixed pressure from an AutoSet titration.
CPAP devices often incorporate a ramp to increase pressure gradually when the device is first turned on; this aims to keep pressure low and more comfortable when falling asleep. The AfH algorithm incorporates a novel automatic ramp that keeps the pressure at a minimum until there are changes in the breathing pattern indicative of either sleep onset (based on regularity of the breaths); or three obstructive apneas or hypopneas occurring within 2 min; or five consecutive snore breaths. The algorithm will then ramp up to minimum therapy pressure within 1 min of the event occurring at a rate of 1 cm H2O/min. Women with OSA have longer sleep latencies than men with OSA despite no difference in age, respiratory disturbance index, or oxygen saturation.27 Hence, the rationale of the AfH automatic ramp is to allow sufficient time for sleep onset by minimizing disturbance from increasing ramp pressure, while still responding to changes consistent with sleep or obstructive events as necessary. The participants' sleep latency in the current study was not significantly different using the AfH and the standard AutoSet algorithm and similar to values reported in normal middle-aged women.18
In practice, overall treatment effectiveness is determined not only by efficacy but also by compliance with therapy in the home environment. An in-house questionnaire indicated there were no significant differences in symptomatic report and tolerance of the AfH algorithm compared to the AutoSet algorithm. Further studies are needed to assess compliance in the home with the new AfH algorithm.
The strengths of the current study include the use of a randomized controlled crossover design; with patients acting as their own controls to increase study power. In addition, the patients, therapists, and sleep data scorers were blinded to the study intervention. The gold standard of in-laboratory full PSG assessment was used to ascertain the primary study outcomes, and currently recommended definitions for respiratory events were also used. However, the study was not adequately powered to make conclusive statements about secondary outcomes. Although the study found reduced flow-limited breaths and lower pressure requirements using the AfH algorithm, it is unclear if these changes will translate into measureable clinical benefits to female OSA patients. Further studies, adequately powered for these outcomes, will be needed to answer these questions.
In conclusion, the primary finding of this study is that the efficacy of a novel female-specific (AfH) algorithm among premenopausal women with OSA is noninferior to the standard AutoSet algorithm. The study also suggests the AfH algorithm results in superior control of flow limited breaths in premenopausal women compared to the AutoSet algorithm, and it achieves this at a lower 95th centile pressure.
DISCLOSURE STATEMENT
ResMed Ltd. (Bella Vista, Sydney) sponsored the study but did not influence data collection or interpretation of the outcome data. Dr. McArdle, Stuart King, Dr. Shepherd, Vanessa Baker, David Hillman and Dr. Eastwood received funding support from ResMed Ltd. for this study. Nigel McArdle received an honorarium for participation in a ResMed Ltd. sponsored breakfast symposium. Dinesh Ramanan, Sahisha Ketheeswaran, Peter Bateman, Alison Wimms, Dr. Armitstead, and Dr. Richards receive salary from and/or are shareholders of ResMed Ltd. The study was conducted at the University of Western Australia.
ACKNOWLEDGMENTS
The authors thank the patients who took part in this study.
SUPPLEMENTAL MATERIAL
Questionnaire Used to Assess Symptomatic Responses to Therapy after the Study Nights
REFERENCES
- 1.Engleman HM, Douglas NJ. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008;5:200–6. doi: 10.1513/pats.200708-143MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary BA, Speir WA., Jr Sleep apnea syndromes. South Med J. 1982;75:39–45. doi: 10.1097/00007611-198201000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: clinical features. Sleep. 2002;25:412–9. [PubMed] [Google Scholar]
- 9.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 10.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–70. [PubMed] [Google Scholar]
- 11.Mohsenin V. Gender differences in the expression of sleep-disordered breathing : role of upper airway dimensions. Chest. 2001;120:1442–7. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 12.Leech JA, Onal E, Dulberg C, et al. A comparison of men and women with occlusive sleep apnea syndrome. Chest. 1988;94:983–8. doi: 10.1378/chest.94.5.983. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teschler H, Berthon-Jones M, Thompson AB, et al. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;154:734–40. doi: 10.1164/ajrccm.154.3.8810613. [DOI] [PubMed] [Google Scholar]
- 16.Julious SA. Sample sizes for clinical trials. Boca Raton, FL: Chapman and Hall/CRC Press; 2009. [Google Scholar]
- 17.Krieger J, Maglasiu N, Sforza E, et al. Breathing during sleep in normal middle-aged subjects. Sleep. 1990;13:143–54. [PubMed] [Google Scholar]
- 18.Sahlin C, Franklin KA, Stenlund H, et al. Sleep in women: normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Med. 2009;10:1025–30. doi: 10.1016/j.sleep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Redline S, Kump K, Tishler PV, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 20.Smith R, Ronald J, Delaive K, et al. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121:164–72. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 21.Franklin KA, Sahlin C, Stenlund H, et al. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41:610–5. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]
- 22.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med. 2004;25:257–68. doi: 10.1016/j.ccm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayaraman G, Majid H, Surani S, et al. Influence of gender on continuous positive airway pressure requirements in patients with obstructive sleep apnea syndrome. Sleep Breath. 2011;15:781–4. doi: 10.1007/s11325-010-0436-2. [DOI] [PubMed] [Google Scholar]
- 27.Valencia-Flores M, Bliwise DL, Guilleminault C, et al. Gender differences in sleep architecture in sleep apnoea syndrome. J Sleep Res. 1992;1:51–3. doi: 10.1111/j.1365-2869.1992.tb00009.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


