Abstract
Naïve T-cell activation requires signals from both the T-cell receptor (TCR) and the costimulatory molecule CD28. A central mediator of the TCR and CD28 signals is the scaffold protein CARMA1, which functions by forming a complex with partner proteins, Bcl10 and MALT1. A well-known function of the CARMA1 signaling complex is to mediate activation of IκB kinase (IKK) and its target transcription factor NF-κB, thereby promoting T-cell activation and survival. Recent evidence suggests that CARMA1 also mediates TCR/CD28-stimulated activation of the IKK-related kinase TBK1, which plays a role in regulating the homeostasis and migration of T cells. Moreover, the CARMA1 complex connects the TCR/CD28 signals to the activation of mTORC1, a metabolic kinase regulating various aspects of T-cell functions. This review will discuss the mechanism underlying the activation of the CARMA1-dependent signaling pathways and their roles in regulating T-cell functions.
Keywords: T-cell activation, T-cell tolerance, TCR signaling, NF-κB, mTORC1, CARMA1, Glutamine
1. Introduction
T lymphocytes serve as the central player in adaptive immune responses against infections and cancer and, when deregulated, are also responsible for many autoimmune and inflammatory diseases (Ohashi, 2002; Zhu et al., 2010). Activation of a naïve T cell is initiated upon engagement of the T-cell receptor (TCR) by a specific peptide antigen presented on antigen-presenting cells (APCs). However, full activation of naïve T cells also requires signals elicited from costimulatory receptors, most importantly CD28 that is bound by its ligand, B7, on APCs. It is now clear that the activation and differentiation of T cells also require environmental cues (Maciver et al., 2013; van der Windt and Pearce, 2012; Waickman and Powell, 2012). How the different T-cell activation signals are integrated intracellularly leading to the induction of gene expression and other cellular changes is an active area of research.
A well-defined signaling event stimulated by the TCR and CD28 signals is activation of nuclear factor kB (NF-κB), a family of transcription factors that mediates induction of a large number of target genes by binding to an enhancer element called kB (Hayden and Ghosh, 2012). Mammalian NF-κB includes five members, p50, p52, RelA, RelB, and c-Rel, which function as various homo- and heterodimers. In resting T cells, NF-κB members exist as latent cytoplasmic complexes associated with specific inhibitory proteins, IκBs. Activation of NF-κB involves two major pathways, the canonical and noncanonical pathways, which differ extensively in both the mechanism of regulation and the signaling components. Canonical pathway relies on a trimeric IκB kinase (IKK) complex, composed of IKKα, IKKβ, and a regulatory subunit termed IKKγ or NF-κB essential modulator (NEMO). Upon activation, IKK phosphorylates a prototypical IκB member, IκBα, triggering its ubiquitin-dependent degradation and concomitant nuclear translocation of the liberated NF-κB members, predominantly p50/RelA and p50/c-Rel heterodimers(Hayden and Ghosh, 2012). Noncanonical pathway is based on inducible processing of an NF-κB precursor protein, p100, to generate the mature NF-κB member p52 (Sun, 2012). Due to the presence of an IκB-like structure in its C-terminal portion, p100 inhibits the nuclear translocation of its associated NF-κB members, particularly RelB. The processing of p100 involves proteasomal degradation of its IκB-like portion, leading to generation of p52 and nuclear translocation of p52 and RelB. A central signaling component of the noncanonical NF-κB pathway is NF-κB-inducing kinase (NIK), which functions together with IKKα to induce p100 phosphorylation and ubiquitin-dependent processing (Senftleben et al., 2001; Xiao et al., 2001). A role for canonical NF-κB pathway in mediating T-cell activation and differentiation has been well established (Oh and Ghosh, 2013). In contrast, much less is known regarding the activation and function of noncanonical NF-κB pathway in T cells, although important progress has been made through recent studies.
The activation and subsequent differentiation of T cells are also influenced by environmental signals, such as those induced by growth factors, amino acids, and stress inducers (Maciver et al., 2013; Smith-Garvin et al., 2009; van der Windt and Pearce, 2012). The serine/threonine kinase mTOR plays vital role in integrating the environmental signals (Chi, 2012; Waickman and Powell, 2012). mTOR exists in two different complexes, mTOR complex 1 (mTORC1) and mTORC2, which differ in mechanism of activation and function (Sarbassov et al., 2005). mTORC1 promotes cell growth and proliferation by phosphorylating two major regulators of protein synthesis, S6 kinase 1 (S6K1) and 4 elongation facor-binding protein 1 (4E-BP1) (Fingar et al., 2002; Holz and Blenis, 2005; Sarbassov et al., 2005). In T cells, both mTORC1 and mTORC2 regulate important aspects of T-cell functions, including differentiation and lineage commitment as well as memory responses (Chi, 2012; Waickman and Powell, 2012). Interestingly, mTORC1 is activated not only by the environmental cues but also immune signals, including those elicited from TCR and CD28. Recent studies provide novel insight into the mechanism by which TCR/CD28 signals activate mTORC1, suggesting shared upstream regulators of the mTORC1 and NF-κB pathways (Hamilton et al., 2014; Nakaya et al., 2014). In addition, emerging evidence suggests that the TCR/CD28 signals also activate IKK-related kinases, TBK1 and IKKε, which appear to crosstalk with the mTORC1 pathway in T cells (Gulen et al., 2012; Yu et al., 2015).
2. TCR signaling to NF-κB
2.1. Canonical NF-κB activation: central role of the CBM complex
A hallmark of IKK/NF-κB activation by the TCR and CD28 signals is the requirement of a scaffold protein termed caspase recruitment domain (CARD)-containing MAGUK 1 (CARMA1, also named CARD11) (Blonska and Lin, 2009; Thome et al., 2010). Initial studies using cell line models reveal an essential role for CARMA1 in mediating NF-κB activation and IL-2 gene induction by the TCR and CD28 signals (Gaide et al., 2002; Pomerantz et al., 2002; Wang et al., 2002). CARMA1 contains several domains, including CARD, coiled-coil, SH3, GUK, and PDZ domains (Thome et al., 2010). All of these domains, with the exception of the PDZ domain, appear to be required for the function of CARMA1 in mediating NF-κB activation (Pomerantz et al., 2002). CARMA1 is constitutively associated with plasma membrane and recruited into the lipid rafts following TCR stimulation (Blonska and Lin, 2009). In response to TCR/CD28 signals, CARMA1 forms a complex (called CBM complex) with a CARD-containing adaptor, Bcl10, and the paracaspase MALT1, thereby recruiting these partner proteins to the lipid rafts. In addition to activating IKK, the CBM complex also mediates activation of the MAP kinase JNK, particularly JNK2 (Blonska et al., 2007; Gaide et al., 2002; Hara et al., 2003). The essential role of the CBM complex in NF-κB activation has also been demonstrated using animal models. Mice deficient in any of the three CBM components have a defect in TCR/CD28-stimulated IKK/NF-κB activation, coupled with immunodeficiencies (Blonska and Lin, 2009; Thome et al., 2010).
Phosphorylation serves as a primary mechanism that mediates CARMA1 activation (Blonska and Lin, 2009; Thome et al., 2010). A key phosphorylation event that triggers the signaling function of CARMA1 is mediated by protein kinase C theta (PKCθ), a kinase that integrates the TCR and CD28 signals. Upon activation by the TCR and CD28 signals, PKCθ phosphorylates CARMA1 at serines 552 and 645 in a linker region located between the colied coil and PDZ domains of CARMA1 (Matsumoto et al., 2005; Sommer et al., 2005), which triggers the conformation change of CARMA1 enabling its association with Bcl10 and MALT1. CARMA1 is also phosphorylated by other kinases, such as IKKβ, CaMKII, HPK1, and AKT, which appears to play a modulatory role to fine tune the activation of IKK/NF-κB (Brenner et al., 2009; Cheng et al., 2014; Ishiguro et al., 2006; Shinohara et al., 2005). Based on the studies in B cells, IKKβ-mediated CARMA1 phoshporylation at serine 578 has been implicated as a positive feedback mechanism that enhances the formation of the CBM complex (Shinohara et al., 2005). This idea is further emphasized by a recent study using experimental and mathematical modeling approaches, which suggests a model whereby the IKKβ-mediated CARMA1 phosphorylation functions as a switch mechanism for NF-κB activation (Shinohara et al., 2014). Whether this mechanism also functions in T cells remains to be investigated. A more recent study demonstrates that overexpressed AKT phosphorylates CARMA1 at several serine residues, S551, S637, and S645, which may play a positive tuning role in enhancing the activation of IKK/NF-κB (Cheng et al., 2014). This finding is in line with the previous report that AKT regulates TCR/CD28-stimulated NF-κB activation and NF-κB target gene expression (Cheng et al., 2011). However, it remains to be examined whether the AKT mediates the site-specific phosphorylation of CARMA1 in T cells upon TCR/CD28 stimulation.
How the formation of CBM complex leads to IKK activation is still incompletely understood. Nevertheless, K63 ubiquitination is a crucial event, since genetic deficiency in a K63-specific ubiquitin-conjugating enzyme, Ubc13, abolishes TCR/CD28-stimulated activation of IKK and JNK (Yamamoto et al., 2006). Conversely, loss of the K63-specific deubiquitinase CYLD causes spontaneous activation of IKK and JNK as well as their upstream kinase Tak1 (Reiley et al., 2007). It is generally believed that the CBM complex interacts with and activates a K63-specific E3 ubiquitin ligase that functions together with Ubc13 to conjugate K63-linked polyubiquitin chains to Bcl10, the IKK-regulatory subunit NEMO, and Tak1, which in turn facilitates the recruitment of Tak1, IKK, and JNK to the CBM complex for activation (Liu and Chen, 2011; Sun and Ley, 2008). The E3 ubiquitin ligase mediating this signaling step has not been definitively defined. TRAF6 is a strong candidate, although TRAF6 is not essential for NF-κB activation by the TCR signal (King et al., 2006). It is likely that TRAF6 has functional redundancy with other TRAF members, such as TRAF2 and TRAF5 (Liu and Chen, 2011). Another possible E3 is MIB2, which has been shown to mediate NF-κB activation by Bcl10 in transfection and knockdown experiments (Stempin et al., 2011). However, the role of MIB2 in regulating TCR/CD28-stimulated NF-κB activation and T-cell function has not been investigated in primary T cells.
Signal transduction leading from CBM complex to IKK activation also involves the translocation of the Bcl10-MALT1 signalosome from the plasma membrane to the cytoplasm, where they form aggregates called POLKADOTS (Paul et al., 2012; Rossman et al., 2006; Schaefer et al., 2004). Formation of the POLKADOTS depends on K63 ubiquitination of Bcl10 and the ubiquitin-binding protein p62 (Paul et al., 2012). The ubiquitinated Bcl10 binds to preexisting p62 aggregates, leading to the formation of POLKADOTS. The POLKADOTS seem to be dispensable for IKK/NF-κB activation in naïve T cells, but they do play a role in IKK/NF-κB activation in effector T cells (Paul et al., 2014). The POLKADOTS appear to provide a compartment, in which activated IKK interacts with and phosphorylates its substrate IκBα. In addition, NF-κB members, particularly RelA, are also transiently recruited to the POLKADOTS upon TCR crosslinking. Genetic ablation of p62 reduces the formation of POLKADOTS and activation of IKK in effector T cells (Paul et al., 2014). These findings are consistent with the earlier observations that p62 is important for IKK/NF-κB activation in Th2 effector T cells but not in naïve T cells (Martin et al., 2006). It is thought that the differential roles of p62 in regulating NF-κB signaling in naïve versus effector T cells are due to the low levels of p62 expression in naïve T cells (Paul et al., 2014; Yang et al., 2010). The specific requirement of POLKADOTS signalosome for NF-κB signaling in effector T cells is intriguing, although the precise mechanism of its action requires additional studies.
Another mechanism of CBM function is the activation of protease activity of MALT1. MALT1 was initially identified as a caspase-like protein designated paracaspase (Uren et al., 2000). It cleaves protein substrates after an arginine residue, and the protease activity of MALT1 appears to be activated by its dimerization (Coornaert et al., 2008; Hachmann et al., 2012; Rebeaud et al., 2008). MALT1 has been shown to cleave a number of substrates related to NF-κB regulation, such as the deubiquitinases CYLD and A20, the NF-κB member RelB, and the CBM complex component Bcl10 (Kirchhofer and Vucic, 2012). MALT1 also targets the RNase Regnase-1 and two homologous RNA-binding proteins, Roquin-1 and Roquin-2, which regulate autoimmune and inflammatory processes by controlling the stability of mRNAs for proinflammatory cytokines and T-cell costimulatory molecules (Gewies et al., 2014; Jeltsch et al., 2014; Uehata et al., 2013). Although several of the MALT1 substrates are negative regulators of NF-κB, the precise role of the protease activity of MALT1 in NF-κB regulation is obscure (Thome et al., 2010). Incubation of cells with a cell-permeable peptide inhibitor of MALT1 does not inhibit activation of IKK or JNK, although it impairs the activation of NF-κB transcriptional activity (Duwel et al., 2009; Rebeaud et al., 2008). A more recent work suggests that MALT1 undergoes auto-proteolysis after arginine 149, which removes the N-terminal death domain (Baens et al., 2014). The cleaved MALT1, lacking the N-terminal 149 amino acids, potently stimulates the transcriptional activity of NF-κB in transfected cells, and mutation of arginine-149 creates a MALT1 mutant that is defective in supporting mitogen-stimulated expression of IL-2 and an NF-κB reporter gene (Baens et al., 2014). Consistent with the MALT1 inhibitor studies, R149A mutation of MALT1 has no effect on IκBα phosphorylation or NF-κB nuclear translocation, suggesting an IKK-independent function of MALT1 auto-cleavage.
It is important to note that MALT1 also regulates other signaling functions in T cells in addition to mediating NF-κB activation. For example, MALT1 promotes the expression of transcription factor IRF4 via mediating cleavage of Roquin and Regnase-1, which in turn may contribute to the induction of Th17 differentiation by MALT1 (Jeltsch et al., 2014). Since IRF4 also regulates the metabolic reprogramming of CD8+ T cells (Man et al., 2013; Yao et al., 2013), it raises the question of whether MALT1 also plays a role in modulating the expansion and effector function of CD8+ T cells. The physiological function of MALT1 protease activity has been further studied using knock-in mice expressing a protease-deficient MALT1 mutant (Bornancin et al., 2015; Gewies et al., 2014). Interestingly, these mutant mice develop autoimmune inflammation, characterized by lymphocyte infiltration into multiple organs, body weight loss, and perturbed T-cell homeostasis and cytokine production. These pathological phenotypes may be due to impaired Treg development, although precisely how MALT1 paracaspase activity regulates Treg development is not well understood. T cells expressing the protease-deficient MALT1 mutant have normal activation of IKK and NF-κB as well as MAP kinases (Bornancin et al., 2015; Gewies et al., 2014). However, these mutant T cells have reduced expression of c-Rel and IL-2, both known to mediate Treg cell development. The altered expression of c-Rel and IL-2, and possibly other targets of Roquins and Regnase-1, may contribute to the pathological phenotypes of the MALT1 mutant mice (Bornancin et al., 2015; Gewies et al., 2014).
2.2. Noncanonical NF-κB activation
The noncanonical NF-κB pathway mediates posttranslational production of NF-κB2 p52 and activation of NF-κB members sequestered by the NF-κB2 precursor protein p100 (Sun, 2012). A central signaling factor mediating noncanonical NF-κB activation is NF-κB inducing kinase (NIK), which stimulates site-specific p100 phosphorylation and ubiquitin-dependent p100 processing (Fong and Sun, 2002; Liang et al., 2006; Xiao et al., 2001). NIK functions by activating a downstream kinase, IKKα, which directly mediates the p100 phosphorylation (Senftleben et al., 2001). The signaling pathway mediating noncanonical NF-κB activation differs profoundly from that mediating the canonical NF-κB activation. In contrast to the rapid and transient nature of canonical NF-κB signaling, the activation of noncanonical NF-κB pathway is slow, persistent, and dependent on de novo protein systhesis (Sun, 2012). These characteristics are consistent with the mechanism of NIK activation. The steady level of NIK is extremely low due to its constant degradation by a ubiquitin-dependent mechanism involving its physical association with TRAF3. TRAF3 mediates NIK degradation by recruiting the E3 ubiquitin ligase cIAP (cIAP1 or cIAP2) to NIK, an action that also requires the adaptor TRAF2 that links TRAF3 to cIAP. Signal-stimulated p100 processing involves disruption of the cIAP-TRAF2-TRAF3 E3 ligase complex, mostly via degradation of TRAF3, and accumulation of the stabilized NIK (Sun, 2012) (Liao et al., 2004; Sun, 2012).
Noncanonical NF-κB has been extensively studied in B cells and mouse embryonic fibroblast (MEF) models, in which the p100 processing is induced by a subset of TNF receptor (TNFR) superfamily members, such as BAFF receptor and CD40 on B cells and lymphotoxin beta receptor on MEFs (Sun, 2012). These receptors act by inducing TRAF3 degradation and NIK stabilization. The role of NIK and noncanonical NF-κB in T cells is appreciated only recently. T-cell activation via crosslinking the TCR and CD28 costimulatory molecule induces p100 processing and nuclear translocation of p52 and RelB (Murray et al., 2011; Yu et al., 2014). Although how TCR/CD28 signals induce noncanonical NF-κB activation is incompletely understood, it is clear that this signaling event depends on NIK and C-terminal phosphorylation of p100. NIK deficiency has no effect on the induction of p100 expression, but it completely blocks the induction of p100 processing (Yu et al., 2014). The phosphorylation-dependent p100 processing is also suggested by a study using a mutant mouse model, Lym1 (Tucker et al., 2007), which expresses a p100 mutant lacking the C-terminal phosphorylation region (Yu et al., 2014). In response to the TCR/CD28 signals, the p100 Lym1 mutant is accumulated in the cells without being processed to p52. However, it is unclear whether the TCR/CD28 signals directly induce noncanonical NF-κB signaling or act indirectly through induction of TNFR expression. T-cell activation is associated with induction of several TNFR members, such as CD27, OX40 and 4-1BB, as well as their ligands (Croft, 2009; Watts, 2005). Since these TNFRs are capable of inducing p100 processing (Arima et al., 2010; McPherson et al., 2012; Ramakrishnan et al., 2004), it is possible that the TNFRs may contribute to the induction of p100 processing in activated T cells. This possibility is further suggested by the finding that OX40 crosslinking promotes TCR/CD28-stimulated noncanonical NF-κB activation in T cells (Murray et al., 2011; Xiao et al., 2012). However, careful analysis using NIK-deficient and control naïve T cells reveals that induction of p52 by the T-cell activation signals can be detected as early as 4 h (Yu et al., 2014). This finding suggests that the TCR/CD28 signals may directly trigger noncanonical NF-κB signaling, which is subsequently enhanced through the signaling action of the TNFR family members.
The T-cell activation signals also induce the expression of p100, which is dependent on IKK (Legarda-Addison and Ting, 2007) and its upstream regulator CARMA1 (unpublished data). The steady level of p100 is considerably low in both naïve CD4+ T cells and Jurkat T-cell line but is greatly elevated along with ligation of TCR and CD28 (Legarda-Addison and Ting, 2007; Yu et al., 2014). The TCR/CD28-stimulated p100 expression is coupled with its processing to generate p52. Thus, noncanonical NF-κB activation in T cells requires induction of both NIK-dependent p100 phosphorylation and IKK-dependent p100 expression, suggesting a dynamic cooperation between the canonical and noncanonical NF-κB pathways.
2.3. Negative regulation of NF-κB signaling by deubiquitinases and E3 ligases
Consistent with the crucial role of NF-κB in mediating the activation, survival, and inflammatory effector functions of T cells, the activation of NF-κB is subjected to tight control in T cells. Many of the NF-κB negative regulators act through modulation of protein ubiquitination. One example is the deubiquitinase (DUB) CYLD, which inhibits NF-κB activation by cleaving K63-linked and linear ubiquitin chains from several signaling components (Sun, 2010; Tokunaga, 2013). Gene targeting studies revealed a crucial role for CYLD in regulating thymocyte development and peripheral T-cell activation (Reiley et al., 2007; Reiley et al., 2006). In particular, CYLD is essential for controlling NF-κB activation under homeostatic conditions (Reiley et al., 2007; Sun, 2010). CYLD-deficient T cells display high levels of constitutive NF-κB activity due to activation of IKK and TAK1. The CYLD-deficient T cells have accumulation of ubiquitinated TAK1, suggesting that CYLD may function by cleaving ubiquitin chains from TAK1, thereby preventing the activation of TAK1 and its downstream target IKK (Reiley et al., 2007). Another DUB, A20, also plays an important role in the regulation of NF-κB signaling in T cells (Coornaert et al., 2008; Duwel et al., 2009; Giordano et al., 2014), although A20 has been most extensively studied in innate immune cells and B cells (Catrysse et al., 2014; Harhaj and Dixit, 2012; Ma and Malynn, 2012). The mechanisms of action for A20 and CYLD appear to be different (Harhaj and Dixit, 2012; Sun, 2008), and they do not seem to share substantially overlapping functions at least in the regulation of B-cell development and activation (Chu et al., 2012). In contrast to CYLD, which acts as a DUB, A20 serves as a component of an ubiquitin-editing complex (Harhaj and Dixit, 2012). Recent evidence suggests that the deubiquitinase activity of A20 may be dispensable for regulating NF-κB signaling (De et al., 2014). Another deubiquitinase, USP34, has been shown to inhibit TCR/CD28-stimulated NF-κB activation in the Jurkat T-cell line (Poalas et al., 2013). How USP34 regulates NF-κB activation is unknown. USP34 knockdown in Jurkat cells promotes mitogen-stimulated IκBα degradation without enhancing IKK activation, suggesting that this deubiquitinase may modulate the proteolytic process involved in IκBα degradation.
Although K63 and linear ubiquitination events are important for the activation of IKK, K48 ubiquitination is involved in controlling the level of nuclear NF-κB during the course of NF-κB activation (Natoli and Chiocca, 2008; Sun et al., 2013). Several E3 ubiquitin ligases have been identified as negative regulators of NF-κB that mediate ubiquitin-dependent degradation of specific NF-κB members (Sun et al., 2013). For example, a multi-subunit E3 ligase, ECSSOCS1, and the E3 ligase PDLIM2 (also called SLIM) have been shown to target nuclear RelA for ubiquitin-dependent degradation (Natoli and Chiocca, 2008). Another E3 ligase, Peli1, is a specific regulator of c-Rel in T cells (Chang et al., 2011). During T-cell activation, c-Rel undergoes ubiquitin-dependent degradation, likely representing a major mechanism that prevents aberrant accumulation of activated c-Rel in the nucleus. Peli1 deficiency attenuates the ubiquitination and degradation of c-Rel and causes a substantial increase in the nuclear level of c-Rel. Consistent with the crucial role of c-Rel in mediating T-cell activation, the Peli1 deficiency renders T cells hyper-responsive to TCR/CD28 stimulation in vitro and perturbs T-cell homeostasis in vivo (Chang et al., 2011).
How the noncanonical NF-κB signaling is negatively regulated in T cells is unclear. Based on the studies in B cells and mouse embryonic fibroblasts (MEFs), a major mechanism of noncanonical NF-κB regulation is to control the fate of the upstream kinase NIK. Under normal conditions, NIK is constantly targeted for ubiquitination and degradation by an E3 ubiquitin ligase complex composed of TRAF2, TRAF3, and cIAP (cIAP1 or cIAP2) (Sun, 2012). Genetic deficiency in either TRAF2 or TRAF3 causes constitutive activation of noncanonical NF-κB. Induction of noncanonical NF-κB signaling involves degradation of TRAF3, a signaling event that is subject to negative control by the TRAF3-specific deubiquitinase Otud7b (also called Cezanne) (Hu et al., 2013). In T cells, loss of TRAF2 or TRAF3 also causes constitutive noncanonical NF-κB activation (Gardam et al., 2008). However, the TCR/CD28 signals do not induce obvious degradation of TRAF3, although they do induce accumulation of NIK (Yu et al., 2014). How the signal-induced noncanonical NF-κB activation is negatively regulated in T cells requires further studies.
3. Regulation of T-cell functions by NF-κB
3.1. Regulation of T-cell activation and homeostasis
The canonical NF-κB pathway is well known for its role in the regulation of T-cell activation, survival, and differentiation (Table 1). Optimal activation of NF-κB requires costimulation of the TCR and CD28, and defect in TCR/CD28-stimulated NF-κB signaling is associated with the induction of T-cell anergy (Clavijo and Frauwirth, 2012; Schmitz and Krappmann, 2006). Conversely, deregulated NF-κB activation in T cells, due to the deficiency in negative regulators, can cause aberrant T-cell activation and autoimmune symptoms (Chang et al., 2011; Coornaert et al., 2008; Peng et al., 2010; Reiley et al., 2007; Sun, 2008). Proper control of NF-κB activation is also important for maintaining T-cell homeostasis (Table 1). Under normal conditions, T cells receive tonic TCR signals via partial recognition of self-ligands, which is important for maintaining T-cell homeostasis (Surh and Sprent, 2008; Theofilopoulos et al., 2001). Aberrant activation of TCR signaling events may reduce the threshold of T-cell activation, which causes activation and expansion of autoimmune T cells and initiation of systemic autoimmunity. Quiescent naïve T cells have basal activity of NF-κB, which is greatly enhanced upon ablation of NF-κB negative regulators, such as the deubiquitinases CYLD and A20 and the E3 ubiquitin ligase Peli1 (Chang et al., 2011; Giordano et al., 2014; Reiley et al., 2007). Consequently, mice deficient in these NF-κB negative regulators have perturbed T-cell homeostasis, associated with autoimmune phenotypes (Chang et al., 2011; Giordano et al., 2014; Reiley et al., 2007). A recent study demonstrates that the basal activity of NF-κB in quiescent T cells promotes expression of the alpha subunit of IL-7 and mediates IL-7-dependent T-cell survival (Miller et al., 2014). These findings suggest that NF-κB not only regulates the activation and survival of antigen-activated T cells but also mediates the homeostatic survival of quiescent T cells.
Table 1.
NF-κB and its regulators in the control of T-cell functions
| NF-κB & regulators |
Function in T cells | References |
|---|---|---|
| RelA | Th1, Th17, Treg differentiation | (Oh and Ghosh, 2013; Ruan and Chen, 2012) |
| RelB | Th1 differentiation | (Corn et al., 2005) |
| c-Rel | Th1, Th17, Treg differentiation | (Oh and Ghosh, 2013; Ruan and Chen, 2012) |
| p50 | Th2 differentiation | (Das et al., 2001) |
| p52 | Th9 differentiation | (Xiao et al., 2012) |
| Bcl-3 | Th1 plasticity and effector function | (Tang et al., 2014) |
| NIK | T-cell memory, Th17 effector function, Treg cell survival | (Murray, 2013; Rowe et al., 2013; Yu et al., 2014) |
| Ubc13 | Peripheral T-cell maintenance, Treg function and stability | (Chang et al., 2012; Yamamoto et al., 2006) |
| IKKβ | Treg function and stability | (Chang et al., 2012) |
| Tak1 | Survival of conventional T and Treg cells | (Chang et al., 2015; Wan et al., 2006) |
| CYLD | Controls NF-κB basal activity, maintains T-cell homeostasis | (Reiley et al., 2007) |
| A20 | Inhibits TCR-stimulated NF-κB activation, suppresses CD8 T-cell activation | (Giordano et al., 2014) |
| Peli1 | Promotes c-Rel degradation, inhibits T-cell activation | (Chang et al., 2011) |
| IκBαΔN | Inhibits NF-κB basal activity, impairs naïve T-cell survival | (Miller et al., 2014) |
| p100 Lym1 | Inhibits Th17 effector function, inhibits EAE induction | (Yu et al., 2014) |
IκBαΔN, a degradation-resistant IκBα mutant lacking its N-terminal region required for phosphorylation and degradation; p100 Lym1, a processing-deficient p100 mutant lacking its C-terminal phosphorylation site.
A role for NF-κB in regulating the differentiation of CD4+ T cells has also been well established (Oh and Ghosh, 2013) (Table 1). Transgenic expression of a degradation-resistant form of IκBα, a potent inhibitor of RelA, or genetic ablation of c-Rel impairs the CD4+ T-cell differentiation to T helper (Th) 1 lineage (Aronica et al., 1999; Hilliard et al., 2002). RelA directly binds to an enhancer region of the IFNγ locus and is required for the induction of IFNγ gene expression in T cells (Balasubramani et al., 2010). Another NF-κB member, p50, has a role in regulating GATA3 expression and Th2 cell differentiation (Das et al., 2001). The p50-deficient mice have a defect in Th2 responses and refractory to the induction of the Th2-dependent allergic airway inflammation (Das et al., 2001). NF-κB also plays an important role in mediating Th17 cell differentiation and the pathogenesis of the Th17-dependent autoimmunity, experimental autoimmune encephalomyelitis (EAE) (Sun et al., 2013). The canonical NF-κB members, c-Rel and RelA, mediate the induction of Th17 cell differentiation and EAE pathogenesis (Chen et al., 2011; Ruan et al., 2011). RelA and c-Rel directly participate in the transcription of the Th17 lineage-specific transcription factor RORgt by binding to the promoter region of the RORrT gene. However, it is important to note that the role of c-Rel in mediating EAE pathogenesis is complex, since c-Rel also functions in differentiated Th17 cells to control the expression of the pathogenic cytokine GM-CSF in cooperation with the noncanonical NF-κB member p52 (Yu et al., 2014). Furthermore, as will be discussed in a following section, c-Rel also mediates the development of Treg cells that negatively regulate the induction of EAE.
In contrast to the canonical NF-κB pathway, the noncanonical NF-κB pathway does not seem to play an important role in regulating the initial activation of T cells (Jin et al., 2009; Murray et al., 2011; Yu et al., 2014). Whether noncanonical NF-κB pathway has a role in regulating CD4+ T-cell differentiation is still elusive. Although NIK deficiency reduces the level of Th17 differentiation, this function of NIK seems to be independent of noncanonical NF-κB and may involve regulation of STAT3 activation (Jin et al., 2009). Similarly, IKKα has been shown to regulate Th17 responses, but this is mediated through an NF-κB-independent mechanism involving the association of IKKα to the Il17a locus and histone3 phosphorylation (Li et al., 2011). Nevertheless, it has been shown that induction of noncanonical NF-κB by OX40 signal may contribute to Th9 cell differentiation (Xiao et al., 2012). Furthermore, RelB has been shown to facilitate T-bet expression and Th1 cell differentiation (Corn et al., 2005). It is important to note, though, that RelB activation is not limited to the noncanoical NF-κB pathway and that RelB can function in both the noncanonical and canonical NF-κB pathways by forming heterodimers with p52 and p50, respectively. A useful animal model for studying noncanonical NF-κB function is the nfkb2lym1 mouse, which expresses a p100 mutant defective in phosphorylation-dependent processing (Tucker et al., 2007). It has been shown that impaired p100 processing in nfkb2lym1 mice does not obviously affect the naïve T-cell activation in vitro or T-cell priming in vivo (Yu et al., 2014). Furthermore, CD4+ T cells derived from the nfkb2lym1 mice are also competent in differentiation to Th1 and Th17 cells under in vitro differentiation conditions. However, the nfkb2lym1 mice do show a defect in Th17 cell production in vivo along with EAE induction (Yu et al., 2014). It remains to be examined whether the in vivo role of noncanonical NF-κB in Th17 differentiation is due to indirect function in non-T cells.
Emerging evidence suggests that noncanonical NF-κB regulates the effector function of inflammatory T cells and the generation or maintenance of memory T cells (Rowe et al., 2013; Yu et al., 2014). In particular, Th17 effector T cells from EAE-induced nfkb2lym1 mice are defective in expression of GM-CSF, an effector cytokine that is critically involved in the EAE pathogenesis (Yu et al., 2014). Interestingly, this function of noncanonical NF-κB requires p52, but not RelB. It appears that p52 transactivates the GM-CSF gene in cooperation with the canonical NF-κB member c-Rel, which involves p52-dependent recruitment of c-Rel to the GM-CSF gene promoter (Yu et al., 2014). Consistent with these findings, p100 processing, but not RelB expression, in T cells is required for EAE induction, although RelB plays a role in non-T cells for EAE pahtogenesis (Yu et al., 2014). The role of noncanonical NF-κB in regulating T-cell memory has been demonstrated using NIK bone-marrow chimeric mice infected with lymphocytic choriomeningitis virus (LCMV) (Rowe et al., 2013). Chimeric mice with NIK-deficient T cells display reduced ability to generate LCMV-specific memory CD4+ and CD8+ T cells following prolonged time of LCMV infection.
NF-κB also plays a role in regulating the stability or plasticity of inflammatory effector T cells, as suggested in a recent work that studies the function of an atypical IκB member, Bcl3 (Tang et al., 2014). Unlike the typical IκBs, which inhibit NF-κB nuclear translocation, Bcl-3 functions as a transcriptional modulator by associating with p50 and p52 NF-κB homodimers in the nucleus (Lenardo and Siebenlist, 1994). By T-cell adoptive transfer, a recent study suggests that Bcl-3 has a T cell-intrinsic role in regulating the plasticity and pathogenicity of autoimmune Th1 cells in both EAE and colitis models (Tang et al., 2014). Although Bcl3 is dispensable for Th1 cell differentiation, the Bcl3-deficient Th1 cells convert to less pathogenic Th17-like T cells under inflammatory conditions, characterized by the reduction in GM-CSF expression (Tang et al., 2014). This finding, together with the work described above, raises the question of whether Bcl-3 functions together with the noncanonical NF-κB member p52 in mediating GM-CSF gene transcription.
3.2. NF-κB in T-cell tolerance
Although NF-κB is best known as a mediator of T-cell activation and inflammatory T-cell function, emerging evidence suggests that NF-κB also plays a crucial role in regulating T-cell tolerance (Ruan and Chen, 2012; Sun et al., 2013). This function of NF-κB may serve as another layer of feedback regulation to prevent aberrant T-cell activation and autoimmune/inflammatory responses. T-cell tolerance includes two major mechanisms: central tolerance and peripheral tolerance. The former involves deletion or functional inactivation of autoimmune T cells during their development in the thymus, whereas the latter involves the action of regulatory T (Treg) cells to control the self-reactive T cells that have escaped from central tolerance induction (Hogquist et al., 2005; Josefowicz et al., 2012; Kyewski and Klein, 2006). The noncanonical NF-κB pathway plays a crucial role in regulating central tolerance (Zhu and Fu, 2010). Mice with deficiency in the noncanonical NF-κB members, RelB and NF-κB2, or kinases that mediate noncanonical NF-κB activation (NIK and IKKα) have compromised central tolerance and different levels of autoimmunity. Noncanonical NF-κB regulates central tolerance by mediating the development of medullary epithelial cells (mTEC), which are required for thymic negative selection to delete autoreactive T cells (Zhu and Fu, 2010).
NF-κB also has an important role in regulating peripheral tolerance. Both canonical and noncanonical NF-κB pathways regulate the development or survival of Treg cells, although the underlying mechanisms differ between the two pathways (Table 1). The noncanonical pathway functions mainly via an indirect mechanism through regulation of mTEC development (Zhu and Fu, 2010). The canonical NF-κB, on the other hand, has a cell-intrinsic function in mediating the development of Treg cells in the thymus. Partially impaired Treg cell development has been shown in a number of mice with deficiencies in canonical NF-κB signaling components, such as NF-κB members, IKK, Tak1, and the upstream regulators PKCθ, CARMA1, and Bcl10 (Barnes et al., 2009; Gupta et al., 2008; Isomura et al., 2009; Long et al., 2009; Medoff et al., 2009; Molinero et al., 2009; Ruan et al., 2009; Sato et al., 2006; Schmidt-Supprian et al., 2004). Conversely, loss of the NF-κB negative regulator CYLD promotes the thymic production of Treg cells (Lee et al., 2010a). It is generally believed that c-Rel is the major NF-κB member that mediates Treg cell development. c-Rel promotes Foxp3 expression by facilitating the formation of a enhanceosome, composed of c-Rel, p65, NFAT, Smad, and CREB, on the Foxp3 gene promoter (Ruan et al., 2009). It is important to note that the role of the upstream regulators, particularly the CBM components, in Treg regulation may not solely be mediated through activation of NF-κB. One study revealed that CARMA1 has a role in regulating IL-2 receptor signaling (Lee et al., 2010a). As will be discussed in a later section, CARMA1 is also required for TCR/CD28-stimulated activation of mTORC1 (Hamilton et al., 2014; Nakaya et al., 2014), a transcription factor that couples TCR and IL-2 signals to the immunosuppressive activity of Treg cells (Zeng et al., 2013). It remains to be examined whether the role of CARMA1 in regulating IL-2 signaling in Treg cells involves activation of mTORC1 and whether this signaling axis also regulates the immunosuppressive function of Treg cells.
In addition to regulating Treg cell development, canonical NF-κB signaling pathway plays a role in maintaining the stability and immunosuppressive function of established Treg cells (Table 1). Mice with Treg-specific ablation of Ubc13, a ubiquitin-conjugating enzyme mediating TCR-stimulated IKK/NF-κB activation (Yamamoto et al., 2006), develop autoimmune symptoms due to aberrant activation of conventional T cells (Chang et al., 2012). Although Ubc13 is dispensable for the survival and in vitro immunosuppressive function of Treg cells, the loss of Ubc13 compromises the in vivo functions of Treg cells and renders the Treg cells vulnerable for acquiring Th1- and Th17-like inflammatory effector functions under lymphopenic conditions. In addition to mediating activation of IKK, Ubc13 also targets the activation of the MAP kinases JNK and p38. Genetic evidence suggests that Ubc13 functions in Treg cells by mediating IKK activation, since Treg-specific expression of a constitutively active IKKβ rescues the in vivo function of Ubc13-deficient Treg cells. Furthermore, Treg cells lacking IKKβ resemble the Ubc13-deficient Treg cells in their compromised in vivo function and vulnerability for acquiring Th1- and Th17-like effector functions (Chang et al., 2012). NF-κB pathway appears to mediate the expression of SOCS1, which in turn plays a role in preventing expression of inflammatory cytokines in Treg cells.
A recent study examined the role of the IKK-activating kinase Tak1 in Treg function (Chang et al., 2015). Interestingly, unlike Ubc13, which is dispensable for Treg survival, Tak1 is essential for maintaining the peripheral population of Treg cells. Tak1 ablation in committed Treg cells causes their apoptosis. However, the mice with Treg-specific Tak1 deficiency only develop moderate autoimmunity; this is apparently due to the expansion of Tak1-competent Treg cells that have escaped the Cre-mediated Tak1 ablation (Chang et al., 2015). Like Ubc13, Tak1 mediate activation of both IKK/NF-κB and the MAP kinases JNK and p38. It is surprising that Tak1 and Ubc13 have different roles in regulating the survival for Treg cells. One possible reason for this functional difference is that Ubc13 functions more upstream than Tak1 and mainly mediates signaling through the TCR, whereas Tak1 integrates signals from various immune receptors, including both TCR and the gamma chain family of cytokine receptors known to be crucial for T-cell survival (Wan et al., 2006). The survival function of Tak1 in Treg cells may involve both NF-κB and MAP kinases, since expression of a constitutively active IKK only partially rescues the survival of the Tak1-deficient Treg cells. Future studies should determine the role of individual NF-κB members in Treg regulation
The noncanonical NF-κB pathway also has a role in Treg development (Sun et al., 2013; Zhu and Fu, 2010). This function is to a large extent mediated through regulating the development of medullary thymic epithelial cells (mTECs), which are required for generation of natural Treg cells (Aschenbrenner et al., 2007; Zhu and Fu, 2010). Mice deficient in major signaling components of the noncanonical NF-κB pathway, such as NIK and RelB, have impaired mTEC development and reduced number of Treg cells (Kajiura et al., 2004; Zhu and Fu, 2010). The role of noncanonical NF-κB in in mTEC development is further emphasized by the recent finding that TEC-specific ablation of a central inhibitor of noncanonical NF-κB, TRAF3, bypasses the requirement of LTbR and CD40 for mTEC development (Jenkinson et al., 2013). In addition to its role in regulating Treg development, the noncanonical NF-κB pathway is involved in the survival and homeostasis of peripheral Treg cells (Murray, 2013). A study, using mixed bone-marrow adoptive transfer approach, suggests that NIK has a cell-intrinsic role in maintaining the peripheral Treg cells (Murray, 2013). On the other hand, the loss of NIK in T-cell compartment does not seem to impair the development, proliferation, or suppressive function of Treg cells (Murray, 2013). Another study suggests that IKKα not only regulates the homeostasis of Treg cells but also the expansion and effector function of Treg cells (Chen et al., 2015). T cell-specific ablation of IKKα causes more than 60% reduction in the number of Tregs in both the thymus and the peripheral lymphoid organs (Chen et al., 2015). Furthermore, the IKKα-deficient Tregs are impaired in expansion and effector function when adoptively transferred into Rag1 knockout mice. The phenotypic differences between the NIK- and IKKα-deficient mice are likely due to the involvement of IKKα in cellular functions that are independent of noncanonical NF-κB, such as modulation of gene transcription via histone3 phosphorylation (Anest et al., 2003; Huang and Hung, 2013; Yamamoto et al., 2003). As mentioned already, IKKα regulates Th17 responses by an NF-κB-independent mechanism involving direct binding to the Il17a locus and mediating histone3 phosphorylation (Li et al., 2011). Whether some of the functions of IKKα in Treg cells also involve histone modification is unclear.
4. TCR signaling to mTORC1
The serine/threonine kinase mTOR regulates important functions of T cells, including CD4+ T-cell differentiation and the generation of effector versus memory CD8+ T cells (Powell and Delgoffe, 2010). By associating with different adaptor molecules, mTOR forms two complexes, mTORC1 and mTORC2, which differ in both functions and the mechanism of activation (Zoncu et al., 2011). In T cells, mTORC1 can be activated by the TCR/CD28 signals in addition to environmental cues, such as nutrients, growth factors, and stress signals (Chi, 2012; Pollizzi and Powell, 2015). Crosslinking of TCR and CD28 triggers the activation of phosphoinositide 3-kinase (PI3K), a kinase that catalyzes formation of phosphatidylinositol 3,4,5 trisphosphate (PIP3) in the plasma membrane. PIP3 recruits AKT and phosphoinositide depdendent kinase 1 (PDK1) to the plasma membrane, allowing PDK1 to phosphorylate AKT at threonine (T)308 and induce the catalytic activity of AKT. AKT in turn phosphorylates and inactivates a negative regulator of mTORC1, tuberous sclerosis complex 2 (TSC2), thereby causing mTORC1 activation (Inoki et al., 2002; Manning et al., 2002; Potter et al., 2002). A selective inhibitor of PI3K p110δ, IC87114, inhibits TCR/CD28-stimulated AKT phosphorylation and mTORC1 activation in naïve CD4+ T cells (Kurebayashi et al., 2012). Another kinase that activates AKT is mTORC2, which acts through phosphorylating serine (S)473 of AKT (Lee et al., 2010b). Disruption of mTORC2 by genetic ablation of its adaptor protein Rictor inhibits TCR/CD28-stimulated AKT phosphorylation at S473. However, the S473 phosphorylation of AKT is also dependent on PI3K, since pharmacological inhibition of PI3K or genetic ablation of the PI3K regulatory subunit p85α blocks the phosphorylation of AKT at both T308 and S473 (Kurebayashi et al., 2012), likely due to the impaired membrane recruitment of AKT (Andjelkovic et al., 1997).
The mechanism underlying mTORC1 activation in T cells, particularly by the TCR and CD28 signals, is only partially understood. A recent study demonstrates that inhibition of PI3K or AKT does not block the constitutive activity of mTORC1 in CD8+ effector T cells, or cytotoxic T lymphocytes (CTLs), cultured in the presence of the cytokine IL-2 (Finlay et al., 2012). This finding suggests that IL-2-stimulated mTORC1 activation in CTLs may involve an AKT-independent mechanism. The PI3K/AKT inhibitors also do not block glucose uptake in CD8+ T cells stimulated for 18 h with an antigen (Macintyre et al., 2011). It remains unclear whether PI3K and AKT are also dispensable for TCR-stimulated mTORC1 activation in CD8+ T cells, especially during the early phase of stimulation that would not involve secondary contribution from cytokines. The TCR/CD28-stimulated mTORC1 activation is further complicated by the recent finding that this signaling event is dependent on the scaffold molecule CARMA1 (Hamilton et al., 2014; Nakaya et al., 2014). In both naïve CD4+ T cells and Jurkat T-cell line cells, CARMA1 deficiency substantially reduces the activation of mTORC1 by the TCR/CD28 signals. As discussed section 2, CARMA1 functions along with Bcl10 and MALT1 to form an intermediate signaling complex (CBM complex) that mediates TCR/CD28-stimulated activation of IKK/NF-κB signaling. Like CARMA1, MALT1 is crucial for the activation of mTORC1 by TCR/CD28 stimuli, and this function of MALT1 requires its paracaspase activity as revealed by using a small peptide, z-VRPR-fmk, that inhibits the protease activity of MALT1. The role of Bcl10 in mTORC1 signaling is less clear. One study reveals that Bcl10 is dispensable for TCR/CD28-stimulated mTORC1 activation in Jurkat cells and PMA/ionomycin-stimulated mTORC1 activation in primary T cells (Hamilton et al., 2014). However, another study showed a partial, and reproducible, inhibition of mTORC1 activation in Bcl10-deficient naïve CD4+ T cells stimulated by TCR and CD28 stimuli (Nakaya et al., 2014). The discrepancy could be due to the use of different cell conditions and stimuli. In contrast to the CBM components, IKK is dispensable for the activation of mTORC1 (Hamilton et al., 2014; Nakaya et al., 2014). However, the role of IKK in mTORC1 activation may vary between cell types and stimuli, since IKK has been implicated in TNFα-stimulated activation of mTOR in cancer cell lines (Dan and Baldwin, 2008; Lee et al., 2007). In cancer cells, mTORC1 also plays a role in mediating AKT-stimulated activation of NF-κB, suggesting a mechanism of bidirectional regulation (Dan et al., 2008; Dhingra et al., 2013).
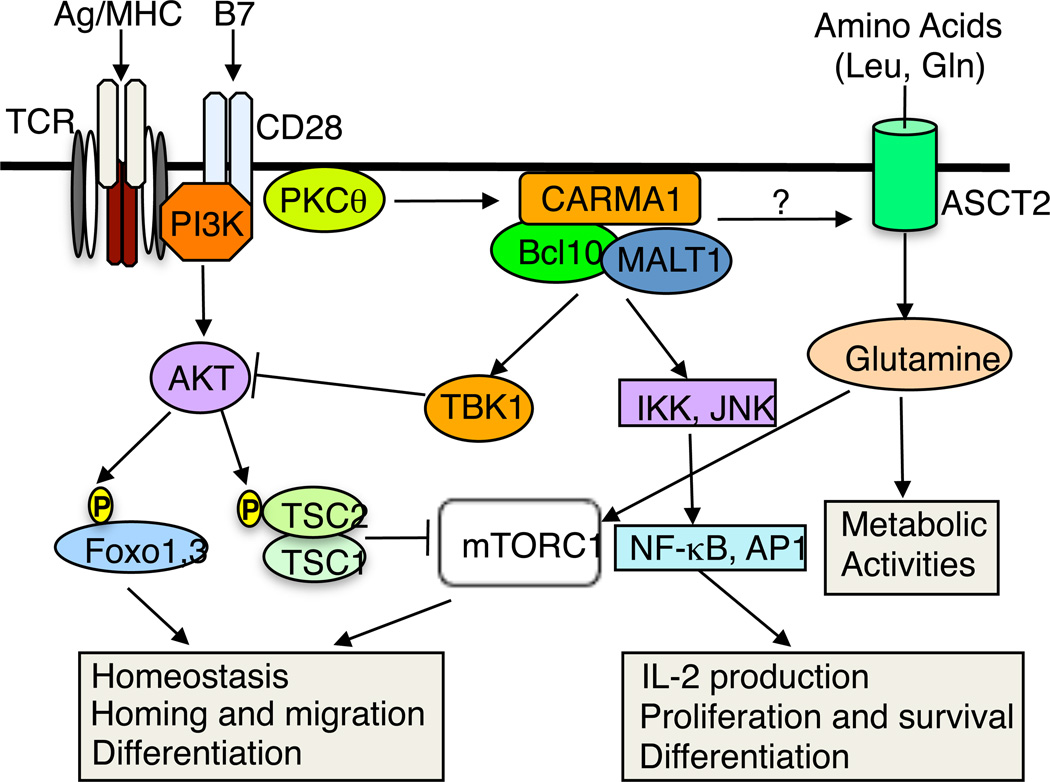
How the CARMA1 complex links the TCR/CD28 signals to mTORC1 activation is still unclear. Since CARMA1 is dispensable for AKT activation (Nakaya et al., 2014; Narayan et al., 2006), it is likely that CARMA1 mediates a novel signaling axis that cooperates with AKT in the activation of mTORC1. This is also consistent with the finding that deficiency in CBM components attenuates, but does not completely block, TCR/CD28-stimulated mTORC1 activation (Nakaya et al., 2014). Activation of mTORC1 involves both TSC2 inactivation and recruitment of mTORC1 to the lysosome, where the lysosomal GTPase Rheb activates mTORC1 (Chi, 2012). The lysosomal recruitment of mTORC1 can be induced by two major amino acids, leucine and glutamine, which function via Rag GTPase-dependent and -independent mechanisms, respectively (Jewell et al., 2015). A recent work demonstrates that crosslinking the TCR and CD28 in naïve CD4+ T cells triggers a rapid increase in glutamine uptake, which is dependent on a glutamine transporter, ASCT2 (Nakaya et al., 2014). ASCT2 deficiency impairs TCR/CD28-stimulated increase in glutamine uptake and activation of mTORC1, although ASCT2 is dispensable for the activation of AKT. Interestingly, the TCR/CD28-stimulated increase in glutamine uptake also requires the CBM components, suggesting the possible involvement of CBM complex in coupling the TCR/CD28 signals to the induction of glutamine uptake (Fig. 1). Although this possibility needs to be examined by additional studies, these studies shed new light on the mechanism by which mTORC1 is activated in T cells and suggests a mechanism that couples the TCR/CD28 signals with environmental cues.
Fig. 1.
CARMA1 in TCR/CD28-stimulated activation of NF-κB and AKT-mTORC1 pathways. Ligation of TCR and CD28 causes activation of protein kinase C theta (PKCθ) and the PI3K/AKT. PKCθ phosphorylates CARMA1 and triggers the assembly of the CBM complex, leading to activation of IKK and JNK and their downstream transcription factors, NF-κB and AP1, important for T-cell activation and survival as well as their subsequent differentiation. A new function of the CBM complex is to mediate TCR/CD28-stimulated activation of mTORC1, despite the dispensable role of CARMA1 in AKT activation. The CARMA1 complex is also important for TCR/CD28-stimulated increase in glutamine uptake, a molecular event that is also dependent on the amino acid transporter ASCT2. It is unclear whether the CBM complex modifies the activity of ASCT2.
5. Regulation of AKT-mTORC1 signaling axis by the IKK-related kinase TBK1
TBK1 and IKKε (also called IKKi) are serine/threonine kinases that are structurally related to IKKα and IKKβ (Peters et al., 2000; Shimada et al., 1999). A well-defined function of TBK1 and IKKε is to mediate type I interferon (IFN) induction by various pattern-recognition receptors (PRRs) in innate immune responses to viral and bacterial infections (Fitzgerald et al., 2003; Hemmi et al., 2004; Hiscott, 2007; McWhirter et al., 2004; Perry et al., 2004; Sharma et al., 2003). However, emerging evidence suggests that TBK1 and IKKε also regulate various other biological or pathological processes, such as autophagy, metabolic activities, oncogenesis, and antibody production (Chiang et al., 2009; Helgason et al., 2013; Jin et al., 2012). It is also increasingly clear that IKKε and TBK1 have non-redundant and even different functions in some biological processes. A role for the IKK-related kinases in regulating T-cell function is suggested by recent findings that TCR/CD28 signals activate both TBK1 and IKKε in T cells (Sgarbanti et al., 2014; Yu et al., 2015). As seen with the canonical IKK activation, the activation of TBK1 and IKKε requires CARMA1 (Yu et al., 2015). Interestingly, IKKε and TBK1 differ in that the former, but not the latter, depends on typical IKK for activation by the T-cell activation signals. IKKε and TBK1 also have different functions in T cells. TBK1, but not IKKε, plays an important role in regulating T-cell activation and homeostasis. TBK1 deficiency promotes the production of cytokines, particularly IFNγ, by naïve CD4+ T cells upon TCR/CD28 ligation in vitro, and T cell-conditional TBK1 knockout (TBK1TKO) mice have an increased frequency of activated or memory-like T cells in peripheral lymphoid organs (Yu et al., 2015). These phenotypes are not detected in mice deficient in IKKε. TBK1 regulates T-cell homeostasis through modulation of the AKT-mTORC1 signaling axis. Under normal conditions, both naïve and memory-like CD4+ T cells have a low basal level of AKT phosphorylation and mTORC1 activation, and such homeostatic AKT-mTORC1 activation is profoundly enhanced in TBK1-deficient T cells (Yu et al., 2015). Injection of TBK1TKO mice with the mTORC1 inhibitor rapamycin partially, although not completely, corrects the CD4+ T-cell homeoastasis phenotype. The primary target of TBK1 appears to be AKT, since TBK1 promotes ubiquitin-dependent degradation of AKT in a manner that depends on a previously defined TBK1 phosphorylation site within AKT, S378. Consistently, the steady level of AKT is elevated in the TBK1-deficient T cells, coupled with enhanced phosphorylation of both the mTORC1-dowstream targets, S6K1 and S6, and the AKT substrate Foxo1 (Yu et al., 2015). These findings establish TBK1 as a key negative regulator of the AKT-mTORC1 signaling axis and explain why the TBK1 deficiency perturbs T-cell homeostasis (Fig. 1), since tight control of the AKT-mTORC1 signaling axis is crucial for maintaining T-cell homeostasis (Chi, 2012). TCR-stimulated mTORC1 activation may also be negatively regulated by adenonsine monophosphate (AMP)-activated protein kinase (AMPK). AMPK is activated by the TCR signaling and thought to negatively regulate mTORC1 activation by phosphorylating and stimulating the activity of the mTORC1 regulator TSC2 (Chi, 2012; Mihaylova and Shaw, 2011; Tamas et al., 2006). However, genetic ablation of AMPKα1, the predominant AMPK isoform in T cells, only has a minor effect on T-cell homeostasis, although ablation of an upstream regulator, liver kinase B1 (LKB1), led to more profound effect on T-cell homeostasis and functions (Chi, 2012; MacIver et al., 2011; Mayer et al., 2008). It is possible that additional kinases targeted by LKB1, as well as TBK1, control the homeostasis of T cells.
T cell-specific TBK1 also has a role in regulating the pathogenesis of EAE, an animal model of the neuroinflammatory disease multiple sclerosis (Yu et al., 2015). Despite the hyper-activation of T cells, the TBK1TKO mice are refractory to EAE induction. The TBK1-deficient T cells have a defect in migration from the draining lymph nodes to the CNS, a crucial step in the induction of EAE. TBK1 appears to regulate the lymph node egress of T cells (Yu et al., 2015). Following activation by an antigen, T cells temporarily down regulate the expression of homing-related factors, including the transcription factor KLF2 and its target gene product S1P receptor 1 (S1PR1), causing their retention in draining lymph nodes (Masopust and Schenkel, 2013). Under normal conditions, the activated T cells gradually regain their expression of KLF2 and S1PR1 and exit the lymphoid organs. The impaired migration of the TBK1-deficient T cells likely results from the aberrant ATK/mTORC1 activation, since this signaling axis is known to negatively regulate the expression of KLF2 and S1PR1 (Finlay and Cantrell, 2010; Groves et al., 2013; Pelletier and Hafler, 2012). Indeed, the TBK1-deficient T cells have reduced expression of KLF2 and S1PR1, which can be restored by incubation with a PI3K inhibitor (Yu et al., 2015).
Although TBK1 and IKKε share the same functions in the regulation of IFN-I induction, these kinases have very different functions in T cells. In contrast to TBK1, IKKε is dispensable for the maintenance of T-cell homeostasis (Yu et al., 2015). IKKε deficiency also does not cause homeostatic activation of AKT and mTORC1. However, IKKε plays an important role in mediating the proliferation and inflammatory function of Th17 cells in response to IL-1 stimulation (Gulen et al., 2012). Interestingly, IKKε is required for IL-1-stimulated activation of AKT-mTORC1 signaling axis, which involves phosphorylation of glycogen synthase kinase 3a (GSK3a). Although GSK3a is generally thought to be a target of AKT, in the IL-1-stimulated Th17 cells, GSK3a negatively regulates AKT activation. The IKKε-mediated GSK3a phosphorylation inactivates its AKT-inhibitory function, resulting in heightened AKT-mTOR activation (Gulen et al., 2012). It is currently unclear whether TBK1 also has a role in regulating the IL-1-stimulated signaling and function of Th17 cells. A more recent study has identified a function of IKKε in the TCR/CD28 signaling pathway (Sgarbanti et al., 2014). Like TBK1, IKKε also responds to the TCR/CD28 signals (Sgarbanti et al., 2014; Yu et al., 2015). In this pathway, an important target of IKKε is IFN regulatory factor 1 (IRF1) (Sgarbanti et al., 2014). IKKε phosphorylates IRF1 at amino acids 215, 219, and 221, a modification that impairs the transcriptional activity of IRF-1 via interfering its binding to the NF-κB member RelA. Consequently, the TCR/CD28-stimulated IKKε activation and IRF-1 phosphorylation suppresses IFNβ induction by poly(I:C) in T cells. This finding raises the question of whether IKKε has a T cell-intrinsic function in regulating T-cell differentiation and responses to viral infection.
5. Concluding remarks
The important role of NF-κB and mTORC1 pathways in mediating antigen-stimulated T-cell functions has been firmly established through extensive studies. However, the signal transduction leading from the TCR ligation to the activation of NF-κB and mTORC1 is still an active area of research. The recent finding of CARMA1 as a common upstream mediator of TCR/CD28-stimulated NF-κB and mTORC1 activation provides novel insight into the mechanism of TCR signaling and also raises important questions. In particular, it is currently unclear how the CARMA1 complex is linked to the activation of mTORC1. Since CARMA1 plays a role in TCR/CD28-stimulated increase in glutamine uptake, it will be important to examine whether the TCR/CD28 signals stimulate mTORC1 lysosomal translocation and whether this molecular event is dependent on CARMA1. Another intriguing question is how the protease activity of MALT1 regulates mTORC1 activation. Does MALT1 cleave a factor in involved in the regulation of mTORC1 signaling?
Accumulating evidence suggests the involvement of noncanonical NF-κB pathway in T-cell functions. Unlike the canonical NF-κB pathway, the noncanonical pathway appears to be dispensable for initial activation of naïve T cells, but rather modulates the effector/memory functions of T cells. Better understanding of the T cell-intrinsic functions of noncanonical NF-κB will require future studies using conditional knockout mice with T cell-specific deficiencies in noncanonical NF-κB signaling. Furthermore, the molecular mechanisms underlying the functions of noncanonical NF-κB in T cells are only partially understood. How precisely this pathway is activated by the following TCR/CD28 ligation is another area for further studies.
IKK-related kinases, TBK1 and IKKε, are emerging as important regulators of T-cell homeostasis and functions. These kinases are generally viewed as mediators of antiviral innate immunity. However, it is now clear that they also respond to the TCR/CD28 signals and serve as new targets of the CARMA1 signaling complex. Interestingly, the activation of IKKε is dependent on typical IKK, whereas the activation of TBK1 is largely independent of IKK. Precisely how TBK1 is activated by the CARMA1-dependent signal requires additional studies. The precise role of TBK1 and IKKε in regulating different aspects of T-cell functions also warrants further investigations.
highlights.
T-cell activation and function requires canonical and noncanonical NF-κB pathways
CBM complex mediates TCR activation of both NF-κB and mTORC1 pathways
ASCT2 couples TCR signal to glutamine uptake and mTORC1 activation
IKK-related kinase TBK1 negatively regulates AKT-mTORC1 signaling axis
Acknowledgements
Work in the laboratory of S.-C.S. is supported by grants from the US National Institutes of Health (AI057555, AI064639, GM84459, and AI104519) and the Cancer Prevention and Research Institute of Texas (RP150235).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica MA, Mora AL, Mitchell DB, Finn PW, Johnson JE, Sheller JR, Boothby MR. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116–5124. [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Baens M, Bonsignore L, Somers R, Vanderheydt C, Weeks SD, Gunnarsson J, Nilsson E, Roth RG, Thome M, Marynen P. MALT1 auto-proteolysis is essential for NF-kappaB-dependent gene transcription in activated lymphocytes. PLoS One. 2014;9:e103774. doi: 10.1371/journal.pone.0103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani A, Shibata Y, Crawford GE, Baldwin AS, Hatton RD, Weaver CT. Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli. Immunity. 2010;33:35–47. doi: 10.1016/j.immuni.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, Crozat K, Sovath S, Moresco EM, Theofilopoulos AN, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7:e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol Rev. 2009;228:199–211. doi: 10.1111/j.1600-065X.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Pappu BP, Matsumoto R, Li H, Su B, Wang D, Lin X. The CARMA1-Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity. 2007;26:55–66. doi: 10.1016/j.immuni.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornancin F, Renner F, Touil R, Sic H, Kolb Y, Touil-Allaoui I, Rush JS, Smith PA, Bigaud M, Junker-Walker U, et al. Deficiency of MALT1 Paracaspase Activity Results in Unbalanced Regulatory and Effector T and B Cell Responses Leading to Multiorgan Inflammation. J Immunol. 2015 doi: 10.4049/jimmunol.1402254. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brenner D, Brechmann M, Rohling S, Tapernoux M, Mock T, Winter D, Lehmann WD, Kiefer F, Thome M, Krammer PH, et al. Phosphorylation of CARMA1 by HPK1 is critical for NF-kappaB activation in T cells. Proc Natl Acad Sci U S A. 2009;106:14508–14513. doi: 10.1073/pnas.0900457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35:22–31. doi: 10.1016/j.it.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Chang JH, Hu H, Sun SC. Survival and maintenance of regulatory T cells require the kinase TAK1. Cell Mol Immunol. 2015 doi: 10.1038/cmi.2015.27. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Xiao Y, Hu H, Jin J, Yu J, Zhou X, Wu X, Johnson HM, Akira S, Pasparakis M, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol. 2012;13:481–490. doi: 10.1038/ni.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jin W, Chang JH, Xiao Y, Brittain GC, Yu J, Zhou X, Wang YH, Cheng X, Li P, et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat Immunol. 2011;12:1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S, Daley S, Shannon MF. The NF-kappaB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- Chen X, Willette-Brown J, Wu X, Hu Y, Howard OM, Hu Y, Oppenheim JJ. IKKalpha is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. FASEB J. 2015;29:443–454. doi: 10.1096/fj.14-259564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hamilton KS, Kane LP. Phosphorylation of Carma1, but not Bcl10, by Akt regulates TCR/CD28-mediated NF-kappaB induction and cytokine production. Mol Immunol. 2014;59:110–116. doi: 10.1016/j.molimm.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Phong B, Wilson DC, Hirsch R, Kane LP. Akt fine-tunes NF-kappaB-dependent gene expression during T cell activation. J Biol Chem. 2011;286:36076–36085. doi: 10.1074/jbc.M111.259549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Soberon V, Glockner L, Beyaert R, Massoumi R, van Loo G, Krappmann D, Schmidt-Supprian M. A20 and CYLD do not share significant overlapping functions during B cell development and activation. J Immunol. 2012;189:4437–4443. doi: 10.4049/jimmunol.1200396. [DOI] [PubMed] [Google Scholar]
- Clavijo PE, Frauwirth KA. Anergic CD8+ T lymphocytes have impaired NF-kappaB activation with defects in p65 phosphorylation and acetylation. J Immunol. 2012;188:1213–1221. doi: 10.4049/jimmunol.1100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P, Beyaert R. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J Immunol. 2005;175:2102–2110. doi: 10.4049/jimmunol.175.4.2102. [DOI] [PubMed] [Google Scholar]
- Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Baldwin AS. Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol. 2008;180:7582–7589. doi: 10.4049/jimmunol.180.11.7582. [DOI] [PubMed] [Google Scholar]
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- De A, Dainichi T, Rathinam CV, Ghosh S. The deubiquitinase activity of A20 is dispensable for NF-kappaB signaling. EMBO Rep. 2014;15:775–783. doi: 10.15252/embr.201338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Gang H, Wang Y, Biala AK, Aviv Y, Margulets V, Tee A, Kirshenbaum LA. Bidirectional regulation of nuclear factor-kappaB and mammalian target of rapamycin signaling functionally links Bnip3 gene repression and cell survival of ventricular myocytes. Circ Heart Fail. 2013;6:335–343. doi: 10.1161/CIRCHEARTFAILURE.112.000061. [DOI] [PubMed] [Google Scholar]
- Duwel M, Welteke V, Oeckinghaus A, Baens M, Kloo B, Ferch U, Darnay BG, Ruland J, Marynen P, Krappmann D. A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains. J Immunol. 2009;182:7718–7728. doi: 10.4049/jimmunol.0803313. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Fong A, Sun S-C. Genetic Evidence for the Essential Role of Beta-Transducin Repeat-Containing Protein in the Inducible Processing of NF-κB2/p100. J Biol Chem. 2002;277:22111–22114. doi: 10.1074/jbc.C200151200. [DOI] [PubMed] [Google Scholar]
- Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Gewies A, Gorka O, Bergmann H, Pechloff K, Petermann F, Jeltsch KM, Rudelius M, Kriegsmann M, Weichert W, Horsch M, et al. Uncoupling Malt1 threshold function from paracaspase activity results in destructive autoimmune inflammation. Cell Rep. 2014;9:1292–1305. doi: 10.1016/j.celrep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- Giordano M, Roncagalli R, Bourdely P, Chasson L, Buferne M, Yamasaki S, Beyaert R, van Loo G, Auphan-Anezin N, Schmitt-Verhulst AM, et al. The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells. Proc Natl Acad Sci U S A. 2014;111:11115–11120. doi: 10.1073/pnas.1406259111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulen MF, Bulek K, Xiao H, Yu M, Gao J, Sun L, Beurel E, Kaidanovich-Beilin O, Fox PL, DiCorleto PE, et al. Inactivation of the enzyme GSK3alpha by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37:800–812. doi: 10.1016/j.immuni.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann J, Snipas SJ, van Raam BJ, Cancino EM, Houlihan EJ, Poreba M, Kasperkiewicz P, Drag M, Salvesen GS. Mechanism and specificity of the human paracaspase MALT1. The Biochemical journal. 2012;443:287–295. doi: 10.1042/BJ20120035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KS, Phong B, Corey C, Cheng J, Gorentla B, Zhong X, Shiva S, Kane LP. T cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci Signal. 2014;7:ra55. doi: 10.1126/scisignal.2005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Wada T, Bakal C, Kozieradzki I, Suzuki S, Suzuki N, Nghiem M, Griffiths EK, Krawczyk C, Bauer B, et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–775. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Dixit VM. Regulation of NF-kappaB by deubiquitinases. Immunol Rev. 2012;246:107–124. doi: 10.1111/j.1600-065X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, Kawai T, Hoshino K, Takeda K, Akira S. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clinic Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- Hu H, Brittain GC, Chang JH, Puebla-Osorio N, Jin J, Zal A, Xiao Y, Cheng X, Chang M, Fu YX, et al. OTUD7B controls non-canonical NF-kappaB activation through deubiquitination of TRAF3. Nature. 2013;494:371–374. doi: 10.1038/nature11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Hung MC. Beyond NF-kappaB activation: nuclear functions of IkappaB kinase alpha. J Biomed Sci. 2013;20:3. doi: 10.1186/1423-0127-20-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Green T, Rapley J, Wachtel H, Giallourakis C, Landry A, Cao Z, Lu N, Takafumi A, Goto H, et al. Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-kappaB activation. Mol Cell Biol. 2006;26:5497–5508. doi: 10.1128/MCB.02469-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL, et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol. 2014;15:1079–1089. doi: 10.1038/ni.3008. [DOI] [PubMed] [Google Scholar]
- Jenkinson SR, Williams JA, Jeon H, Zhang J, Nitta T, Ohigashi I, Kruhlak M, Zuklys S, Sharrow S, Adams A, et al. TRAF3 enforces the requirement for T cell cross-talk in thymic medullary epithelial development. Proc Natl Acad Sci U S A. 2013;110:21107–21112. doi: 10.1073/pnas.1314859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Xiao Y, Chang JH, Yu J, Hu H, Starr R, Brittain GC, Chang M, Cheng X, Sun SC. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-kappaB signaling. Nat Immunol. 2012;13:1101–1109. doi: 10.1038/ni.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by the MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, et al. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Immunol. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Vucic D. Protease activity of MALT1: a mystery unravelled. Biochem J. 2012;444:e3–e5. doi: 10.1042/BJ20120414. [DOI] [PubMed] [Google Scholar]
- Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1:360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Wu X, Cheng H, Zhou X, Cheng X, Sun SC. CARMA1 regulation of regulatory T cell development involves modulation of interleukin-2 receptor signaling. J Biol Chem. 2010a;285:15696–15703. doi: 10.1074/jbc.M109.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010b;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarda-Addison D, Ting AT. Negative Regulation of TCR Signaling by NF-{kappa}B2/p100. J Immunol. 2007;178:7767–7778. doi: 10.4049/jimmunol.178.12.7767. [DOI] [PubMed] [Google Scholar]
- Lenardo M, Siebenlist U. Bcl-3-mediated nuclear regulation of the NF-kappa B trans-activating factor. Immunol Today. 1994;15:145–147. doi: 10.1016/0167-5699(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Li L, Ruan Q, Hilliard B, Devirgiliis J, Karin M, Chen YH. Transcriptional regulation of the Th17 immune response by IKK(alpha) J Exp Med. 2011;208:787–796. doi: 10.1084/jem.20091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Zhang M, Sun SC. beta-TrCP binding and processing of NF-kappaB2/p100 involve its phosphorylation at serines 866 and 870. Cell Signal. 2006;18:1309–1317. doi: 10.1016/j.cellsig.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen ZJ. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Martin P, Diaz-Meco MT, Moscat J. The signaling adapter p62 is an important mediator of T helper 2 cell function and allergic airway inflammation. EMBO J. 2006;25:3524–3533. doi: 10.1038/sj.emboj.7601250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Wang D, Lin X. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol. 2008;38:948–956. doi: 10.1002/eji.200738045. [DOI] [PubMed] [Google Scholar]
- McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-kappaB pathway in T cells. J Biol Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci USA. 2004;101:233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, Luster AD, Xavier RJ. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Mashayekhi M, Chen L, Zhou P, Liu X, Michelotti M, Tramontini Gunn N, Powers S, Zhu X, Evaristo C, et al. Basal NF-kappaB controls IL-7 responsiveness of quiescent naive T cells. Proc Natl Acad Sci U S A. 2014;111:7397–7402. doi: 10.1073/pnas.1315398111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol. 2009;182:6736–6743. doi: 10.4049/jimmunol.0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SE. Cell-intrinsic role for NF-kappa B-inducing kinase in peripheral maintenance but not thymic development of Foxp3+ regulatory T cells in mice. PLoS One. 2013;8:e76216. doi: 10.1371/journal.pone.0076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SE, Polesso F, Rowe AM, Basak S, Koguchi Y, Toren KG, Hoffmann A, Parker DC. NF-kappaB-inducing kinase plays an essential T cell-intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121:4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, Sun SC. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G, Chiocca S. Nuclear ubiquitin ligases, NF-kappaB degradation, and the control of inflammation. Sci Sigal. 2008;1(1):1. doi: 10.1126/stke.11pe1. [DOI] [PubMed] [Google Scholar]
- Oh H, Ghosh S. NF-kappaB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev. 2013;252:41–51. doi: 10.1111/imr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi PS. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–438. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- Paul S, Kashyap AK, Jia W, He YW, Schaefer BC. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity. 2012;36:947–958. doi: 10.1016/j.immuni.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Traver MK, Kashyap AK, Washington MA, Latoche JR, Schaefer BC. T cell receptor signals to NF-kappaB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome. Sci Signal. 2014;7:ra45. doi: 10.1126/scisignal.2004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- Peng B, Ling J, Lee AJ, Wang Z, Chang Z, Jin W, Kang Y, Zhang R, Shim D, Wang H, et al. Defective feedback regulation of NF-kappaB underlies Sjogren's syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl Acad Sci U S A. 2010;107:15193–15198. doi: 10.1073/pnas.1005533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J Exp Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- Poalas K, Hatchi EM, Cordeiro N, Dubois SM, Leclair HM, Leveau C, Alexia C, Gavard J, Vazquez A, Bidere N. Negative regulation of NF-kappaB signaling in T lymphocytes by the ubiquitin-specific protease USP34. Cell Commun Signal. 2013;11:25. doi: 10.1186/1478-811X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36:13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. EMBO J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, Rueda D, Gaide O, Guzzardi M, Iancu EM, Rufer N, et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol. 2008;9:272–281. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, Sun SC. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- Rossman JS, Stoicheva NG, Langel FD, Patterson GH, Lippincott-Schwartz J, Schaefer BC. POLKADOTS are foci of functional interactions in T-Cell receptor-mediated signaling to NF-kappaB. Mol Biol Cell. 2006;17:2166–2176. doi: 10.1091/mbc.E05-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AM, Murray SE, Raue HP, Koguchi Y, Slifka MK, Parker DC. A Cell-Intrinsic Requirement for NF-kappaB-Inducing Kinase in CD4 and CD8 T Cell Memory. J Immunol. 2013;191:3663–3672. doi: 10.4049/jimmunol.1301328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Chen YH. Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol. 2012;946:207–221. doi: 10.1007/978-1-4614-0106-3_12. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Tsujimura T, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Takeuchi O, Akira S. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol. 2006;18:1405–1411. doi: 10.1093/intimm/dxl082. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Kappler JW, Kupfer A, Marrack P. Complex and dynamic redistribution of NF-{kappa}B signaling intermediates in response to T cell receptor stimulation. Proc Natl Acad Sci U S A. 2004;101:1004–1009. doi: 10.1073/pnas.0307858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz ML, Krappmann D. Controlling NF-kappaB activation in T cells by costimulatory receptors. Cell Death Differ. 2006;13:834–842. doi: 10.1038/sj.cdd.4401845. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Kraehn G, Greten F, Chen Y, Hu Y, Fong A, Sun S-C, Karin M. Activation of IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Sgarbanti M, Marsili G, Remoli AL, Stellacci E, Mai A, Rotili D, Perrotti E, Acchioni C, Orsatti R, Iraci N, et al. IkappaB kinase epsilon targets interferon regulatory factor 1 in activated T lymphocytes. Mol Cell Biol. 2014;34:1054–1065. doi: 10.1128/MCB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, Kanamaru A, Akira S. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Behar M, Inoue K, Hiroshima M, Yasuda T, Nagashima T, Kimura S, Sanjo H, Maeda S, Yumoto N, et al. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-kappaB activation. Science. 2014;344:760–764. doi: 10.1126/science.1250020. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Yasuda T, Aiba Y, Sanjo H, Hamadate M, Watarai H, Sakurai H, Kurosaki T. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, Rawlings DJ. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–567. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Stempin CC, Chi L, Giraldo-Vela JP, High AA, Hacker H, Redecke V. The E3 ubiquitin ligase mind bomb-2 (MIB2) protein controls B-cell CLL/lymphoma 10 (BCL10)-dependent NF-kappaB activation. J Biol Chem. 2011;286:37147–37157. doi: 10.1074/jbc.M111.263384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8:501–511. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-κB activation. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Chang JH, Jin J. Regulation of nuclear factor-kappaB in autoimmunity. Trends Immunol. 2013;34:282–289. doi: 10.1016/j.it.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wang H, Claudio E, Tassi I, Ha HL, Saret S, Siebenlist U. The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells. Immunity. 2014;41:555–566. doi: 10.1016/j.immuni.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harbor perspectives in biology. 2010;2:a003004. doi: 10.1101/cshperspect.a003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F. Linear ubiquitination-mediated NF-kappaB regulation and its related disorders. J Biochem. 2013;154:313–323. doi: 10.1093/jb/mvt079. [DOI] [PubMed] [Google Scholar]
- Tucker E, O'Donnell K, Fuchsberger M, Hilton AA, Metcalf D, Greig K, Sims NA, Quinn JM, Alexander WS, Hilton DJ, et al. A novel mutation in the Nfkb2 gene generates an NF-kappa B2 "super repressor". J Immunol. 2007;179:7514–7522. doi: 10.4049/jimmunol.179.11.7514. [DOI] [PubMed] [Google Scholar]
- Uehata T, Iwasaki H, Vandenbon A, Matsushita K, Hernandez-Cuellar E, Kuniyoshi K, Satoh T, Mino T, Suzuki Y, Standley DM, et al. Malt1-Induced Cleavage of Regnase-1 in CD4(+) Helper T Cells Regulates Immune Activation. Cell. 2013;153:1036–1049. doi: 10.1016/j.cell.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Uren AG, O'Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J, Lin X. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- Watts TH. Tnf/Tnfr family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky EJ, Fu YX, Choi Y, Walsh MC, Li XC. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Saitoh T, Sakurai H, Uematsu S, Kawai T, Ishii KJ, Takeuchi O, Akira S. Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J Immunol. 2006;177:7520–7524. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- Yang JQ, Liu H, Diaz-Meco MT, Moscat J. NBR1 is a new PB1 signalling adapter in Th2 differentiation and allergic airway inflammation in vivo. EMBO J. 2010;29:3421–3433. doi: 10.1038/emboj.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH, Sun J. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhou X, Chang M, Nakaya M, Chang JH, Xiao Y, William Lindsey J, Dorta-Estremera S, Cao W, Zal A, et al. Regulation of T-cell activation and migration by the kinase TBK1 during neuroinflammation. Nat Commun. 2015;6:6074. doi: 10.1038/ncomms7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhou X, Nakaya M, Jin W, Cheng X, Sun SC. T Cell-Intrinsic Function of the Noncanonical NF-kappaB Pathway in the Regulation of GM-CSF Expression and Experimental Autoimmune Encephalomyelitis. Pathogenesis. J Immunol. 2014;193:422–430. doi: 10.4049/jimmunol.1303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Fu Y. The complicated role of NF-kappaB in T-cell selection. Cell Mol Immunol. 2010;7:89–93. doi: 10.1038/cmi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]