Abstract
Objective
We reviewed our experience of surgery for epileptic spasms (ES) with or without history of infantile spasms.
Methods
Data were reviewed from 65 (33 males) ES patients who underwent surgery between 1993–2014; palliative cases were excluded.
Results
Mean age at surgery was 5.1 (range: 0.2–19) years, with mean post-surgical follow-up of 45.3 (6–120) months. Mean number of anticonvulsants used pre-operatively was 4.2 (2–8) which decreased to 1.2 (0–4) post-operatively (p<0.0001). Total hemispherectomy was the most commonly performed surgery (n=20), followed by subtotal hemispherectomy (n=17), multilobar resection (n=13), lobectomy (n=7), tuberectomy (n=6) and lobectomy+tuberectomy (n=2), with ILAE class-I outcome in 20, 10, 7, 6, 3 and 0 patients, respectively (total=46/65 (71%); 22 off medication). Shorter duration of epilepsy (p=0.022) and presence of MRI lesion (p=0.026) were independently associated with class-I outcome. Of 34 patients operated <3 years after seizure onset, 30 (88%) achieved class-I outcome. 37/47 patients with lesional MRI (79%) had class-I outcome, whereas 9/18 with normal MRI (50%) had class-I outcome. PET scan was abnormal in almost all patients [61/63 (97%) with lateralizing/localizing findings in 56/61 (92%) patients, thus helping in surgical decision-making and guiding subdural grid placements, particularly in patients with non-lesional MRI. Fifteen had post-operative complications, mostly minor.
Significance
Curative epilepsy surgery in ES patients, with or without history of infantile spasms, is best accomplished at an early age and in those with lesional abnormalities on MRI with EEG concordance. Good outcomes can be achieved even when there is no MRI lesion but positive PET localization.
Keywords: Seizure, Epilepsy surgery, Epileptic spasms, FDG PET, West syndrome
Introduction
The triad of epileptic spasms,1 developmental arrest and a pattern of hypsarrhythmia on the electroencephalogram (EEG) is referred to as West syndrome; however, epileptic spasms (hitherto abbreviated as ES) can occur without hypsarrhythmia.2 The recommended initial medical treatment of ES is either vigabatrin or adrenocorticotropic hormone (ACTH).3 If these agents fail to suppress the spasms (and hypsarrhythmia when present), the ketogenic diet or various anticonvulsants may be tried with variable success.3
When ES are refractory to medical management, a surgical option is recommended in selected patients who manifest focal findings on neuroimaging and EEG. Although there had been several case reports of spasms ceasing after surgical removal of a lesion,4–6 the first reported series consisted of 10 patients with ES and porencephalic cysts in whom neurosurgical marsupialization of the cysts and fenestration to the ventricular system resulted in resolution of the spasms.7 Epilepsy surgery for ES in the absence of a visible lesion on magnetic resonance imaging (MRI) was reported in 1990,8 followed by a larger series from the same investigators.9 Both these series focused on subjects without lesions visible on MRI but with metabolic foci detected using glucose metabolism positron emission tomography (PET) scanning. Since then, there have been many studies reporting successful epilepsy surgery for the amelioration of intractable ES.10–13
The best outcomes with surgery for intractable epilepsy in general are achieved when the patients harbor a single lesion detected on MRI and/or PET and there is general concordance with ictal/interictal EEG recordings. Although many centers have gained experience in surgery for intractable ES when there is an MRI visible lesion, there is still a general reluctance to perform surgery guided exclusively by PET and EEG findings, i.e., MRI-negative cases. At our center, we have placed great emphasis on PET imaging in localizing metabolic foci which, if concordant with ictal EEG and when resected, have resulted in excellent outcomes. Sometimes, the MRI scans are interpreted initially as normal but with focal PET findings determined objectively, the MRI is reinterpreted and subtle abnormalities corresponding to the PET localization are discovered in retrospect.14
The present study was conducted in order to review our experience with ES epilepsy surgery with the intent of investigating who might be surgical candidates. Herein, we report our findings in 65 infants and children with intractable ES who were treated with intended curative surgical resections. We have included both MRI-positive and MRI-negative cases, and compared their surgical outcomes.
Methods
Subjects
We reviewed the records of over 500 infants and children with ES evaluated between January 1993 and December 2014 at the Children's Hospital of Michigan, Wayne State University in Detroit, Michigan. Most of them were not considered for surgery due to resolution of spasms with medical treatment or were not good surgical candidates due to bilateral ictal EEG onsets, phenotype of severe developmental delay with no gains in skills over a protracted period, spastic quadriplegia, microcephaly, severe muscle wasting etc. suggesting an underlying neurogenetic condition. Such patients typically had severe bilateral symmetric hypometabolism on PET and some had an underlying inoperable genetic mutation (e.g., ARX, CDKL5, SCN2A, and STXBP1). During this time period, 98 of these infants and children underwent surgical treatment. Of these, one third were excluded from the present series for the following reasons: (i) patients who had less than 6 months follow up, (ii) those who underwent a `palliative resection' (designated as palliative prior to surgery15), and (iii) patients whose seizures had evolved to atonic/tonic semiology and who underwent corpus callosotomy. Complete clinical data from the remaining 65 subjects are described in the present study (Supplementary Table). Ethnic backgrounds were Caucasian (n=44), African-American (n=10), Hispanic (n=4), Middle-Eastern (n=4), and Asian (n=3). Included in the 65 patients are 11 children who were reported in a recent publication on `subtotal hemispherectomy'.16 All 65 had been diagnosed with ES which remained refractory despite aggressive treatment.
Diagnostic testing
All patients were evaluated with scalp interictal and ictal video-EEG, MRI, and glucose metabolism PET scans. Developmental assessments were performed when feasible. Some children also received PET scans with 11C-flumazenil (FMZ) or 11C-alpha-methyl-L-tryptophan (AMT) under research protocols. In particular, all patients with tuberous sclerosis complex (TSC) underwent AMT PET scans to localize epileptogenic tubers.17 Genetic testing had been performed by referring physicians in some subjects, but none showed a known mutation (at that time) to be associated with ES. Following pre-surgical evaluation, the patients were presented in a weekly held Multidisciplinary Epilepsy Surgery Conference for discussion and consensus regarding surgical candidacy.
Although PET plays an important role in our surgical program, it is used only as a guide for intraoperative or chronic subdural electrocorticography (ECoG) sampling and the actual extent of cortical resection is typically based on the ECoG data. None of the patients in our study was operated based upon PET imaging only. However, subtle ictal EEG changes were often considered more seriously if supported by PET localization/lateralization. On the other hand, patients with structural lesions seen on the MRI scans (e.g., middle cerebral artery infarct in utero) were operated on even if the EEG was discordant due to dipole distortion.
Surgical placements of subdural electrodes
A craniotomy was performed over the location suspected to be epileptogenic based upon the results of pre-surgical investigations. Various combinations of platinum grid and strip subdural electrodes (inter-electrode distance: 1 cm) were placed over the region of brain to be sampled with ECoG. When appropriate, electrode placements included the pre- and post-central gyri as well as medial temporal regions.18 In order to avoid movement of subdural electrodes after placement, all electrode plates were stitched to adjacent plates or the edge of the dura mater. To minimize the risk of infection, electrode leads were then tunneled about an inch from the main wound. Using a digital camera, intraoperative photographs were taken prior to dural closure. Following dural closure in a semi-watertight fashion, the bone flap was replaced but not secured. A subgaleal drain was placed to minimize post-operative scalp swelling. All electrodes were subsequently displayed on the three-dimensional brain surface reconstructed from high-resolution MRI.19
Extraoperative ECoG recording
Patients who had a two-stage surgery underwent extraoperative video-ECoG recordings for 2–5 days (Figure-1). In extraoperative (chronic) ECoG recording, habitual seizures were captured, and visual assessment determined the seizure onset zones, defined as the sites initially exhibiting ictal ECoG changes associated with each seizure event. In all patients, ictal ECoG patterns of epileptic spasms were characterized by widespread fast wave discharges (>30 Hz) with or without a preceding spike.20; 21 Ictal ECoG patterns of focal seizures were characterized by either repetitive spike-wave discharges or focal fast wave discharges.18 In addition to the seizure onset zones, we visually marked the sites showing frequent interictal spike discharges. Following analysis of the ECoG data, the results were discussed with the parents/guardians and the recommendation was made that the extent of resection should include the presumed epileptogenic zones consisting of the seizure onset zones, as well as frequent spiking zones not accounted for by propagation from the seizure onset zone and neuroimaging abnormalities surrounding the seizure onset zones. Thereby, we intended to preserve the eloquent areas defined by electrical stimulation mapping as well as their associated vascular structures.14 In case the presumed epileptogenic zones overlapped with the eloquent areas, resection margin was determined, on a case-by-case basis, after intense discussion with the family of a given patient regarding the pros and cons of complete resection of the presumed epileptogenic zones.
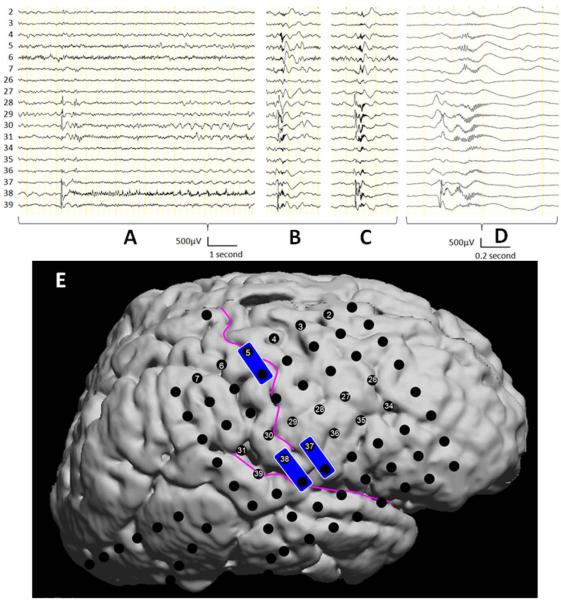
Figure-1.
Ictal ECoG traces in a patient with intractable seizure and epileptic spasms (patient #5). (A) The focal seizure was associated with a single spike discharge with the maximum amplitude at Channel 38 in the inferior Rolandic region, followed by prolonged fast-wave discharges intermixed with periodic sharp waves in the surrounding regions. (B and C) Forty seconds after the onset of the focal seizure, a cluster of epileptic spasms took place; each of the spasms was associated with a leading focal spike followed by widespread fast wave discharges involving the superior Rolandic region including Channels 4 and 5. (D) Ictal ECoG traces shown in (C) are zoomed; ictal fast wave discharges at Channels 4 and 5 were delayed compared to those in the inferior Rolandic region. (E) The locations of subdural electrodes are presented on the surface rendered image. Electrical stimulation of Channels 5-and-13 elicited left hand twitching, whereas that of Channels 37-and-45 as well as 38-and-46 induced mouth movement. Surgical resection included the inferior Rolandic cortex and frontal lobe containing the cortical tubers, seizure onset zones as well as regions independently showing independent spike discharges. The sensorimotor hand area underlying Channels 5 and 13 was preserved.
Intraoperative ECoG recording
In intraoperative ECoG recordings, we sampled ECoG signals generously from the areas potentially epileptogenic based on the findings of noninvasive presurgical evaluations including scalp video-EEG and neuroimaging. Somatosensory-evoked potentials (SSEPs) were intraoperatively recorded as needed. Surgical resection generally included the regions showing neuroimaging abnormalities as well as frequent spiking on ECoG. If a given patient had profound motor deficits associated with massive structural lesions, we generally recommended hemispherectomy. Otherwise, we generally preserved the normal-appearing cortex as well as the sensorimotor areas determined by intraoperative SSEPs and anatomical landmarks.
Resection procedure
Resective surgery was tailored to the presumed epileptogenic zones. All procedures were performed using a large fronto-tempo-parietal osteoplastic craniotomy based on the temporalis muscle. Trans-sylvian approach was used to perform hemispherectomy (n=20) and subtotal hemispherectomy sparing the primary sensorimotor cortex (n=17). In patients with epileptiform activity involving the primary sensorimotor cortex, extensive multiple subpial transections (MSTs) were performed perpendicular to the sulci. A strip of cortex along the center of the involved gyrus was first coagulated along the entire extent and then MSTs were performed at 5 mm intervals.
Histopathology
All surgical specimens were examined in detail by one of the authors (WJK) after the samples were stained with hematoxylin and eosin, cresyl-violet, Luxol fast-blue and glial fibrillary acidic protein antibody. If indicated for further clarification, the specimens were also stained with periodic-acid Schiff, Bielschowsky, Bodian, Congo red, and various immunostains).
Statistical analysis
Follow-up ranged from 6 months to 120 months (mean: 45.3 months). The surgical outcome was reported based on the International League Against Epilepsy classification.22 Values are reported as mean ± SD for normally distributed continuous variables, median and range for skewed continuous variables, and frequencies and percentages for categorical variables. A multivariate logistic regression analysis was performed to determine if surgical outcome was independently associated with `age at surgery', `duration of epilepsy', `number of antiepileptic drugs', and `presence of MRI lesion'. A p-value of ≤ 0.05 was considered significant. SPSS 21.0 (SPSS Inc, Chicago, IL) was used for the data analyses.
Results
There were 65 patients (33 males), mean age of seizure onset was 1.08 ± 1.4 (median: 0.5; range 0.1 – 7) years, and mean age at surgery was 5.1 ± 4.4 (median: 3.5; range: 0.2 – 19) years. The mean duration of post-surgical follow up was 45.3 ± 30.4 (median: 30; range: 6 – 120) months and mean duration between seizure onset and surgery was 4.21 ± 3.8 (median: 3.0; range: 0.05 – 18.9) years. The mean number of anticonvulsants being taken prior to surgery (including vigabatrin) was 4.22 (range: 2 – 8). Seizure frequency at the time of surgery was multiple daily in 58 patients and at least weekly in the remaining 7 subjects.
Seizure types
Out of the 65 patients, 46 patients had onset of spasms in infancy and 19 patients had late onset spasms ranging from 1.4 to 11 years. At the time of surgery, 37 of the 65 were experiencing active spasms and 28 had a recent history of spasms. Although the onset of spasms ranged from 1 month to 7 years, most had early onset with a median age of 0.7 year (mean: 1.4 ± 1.6). Indeed, out of the 28 patients with recent spasms, 18 had spasm onset in infancy ranging from 1 month to 12 months. The average duration for age of spasm offset in these 28 patients was 4.1 ± 3.3 years (median: 3.0; range 1 year to 14 years).
Fifteen of the 65 had manifested only spasms, whereas 50 had multiple seizure types which include spasms and focal seizures in 38 patients, spasms and generalized seizures (including tonic/myoclonic) in 7 patients, and 5 patients had spasms, focal and generalized seizures (Supplementary Table).
Types of resection
The results from the pre-surgical evaluations are shown in the Supplementary Table. The surgery was performed based upon these data and intra-operative ECoG when applicable (n=23) as well as further data obtained from extraoperative ECoG recordings (n=42). Surgery was quite extensive with total (n=20) or subtotal (n=17) hemispherectomy along with multilobar resection (n=13) performed in the majority of patients (Table-1). Patients who underwent a one-stage hemispherectomy (n=18) had a major structural abnormality and were already significantly hemiparetic. Two patients underwent extraoperative ECoG recording prior to hemispherectomy (patients #16 and 54), since the degree of hemiparesis was subtle/mild and there was a reasonable chance to preserve the sensorimotor cortex. In these two patients, the primary sensorimotor cortex was very much involved in the presumed epileptogenic zone based upon the results of extraoperative ECoG recordings; therefore, hemispherectomy was performed.
Table-1.
Surgical detail and seizure outcome
| Surgery | # | ILAE outcome - # of patients |
|---|---|---|
| Total hemispherectomy | 20 | 1 – 20 |
| Subtotal hemispherectomy | 17 | 1 – 10; 3 - 3; 4 - 4 |
| Multilobar resection | 13 | 1 – 7; 3 – 4; 5 - 2 |
| Lobectomy | 7 | 1 – 6; 3 – 1 |
| Tuberectomy | 6 | 1 – 3; 4 – 3 |
| Lobectomy + Tuberectomy | 2 | 4 – 2 |
In addition to the various resections described above, MSTs were performed on 10 patients (#5, 17, 23, 34, 35, 37, 38, 39, 43 and 60) to preserve motor function. One of the children (patient #26) underwent a partial corpus callosotomy in addition to right multilobar resection. There were seven patients (#9, 1, 23, 26, 42, 43, and 51) who underwent two sets of surgeries (reoperations). Three patients came to us with a history of surgery performed elsewhere and four patients required two sets of surgery at our center.
Surgical outcome
The mean follow-up period after surgery was 45.3 months (range 6 months to 120 months). Forty-six patients were completely seizure-free (Table-1); 22 of them were no longer taking any anticonvulsants, whereas 24 remained on medication. It appeared that patients with larger surgical resection had better surgical outcome (Table-1). The 19 patients with seizure recurrence had Class III, IV or V outcomes and remained on medication. The time of seizure relapse ranged from 1 month to 4.5 years (mean: 1.5 ± 1.3 years; median: 1.0). Two of these 19 patients had been seizure-free for 4 years prior to recurrence of their seizures. The median value number of anticonvulsants being taken by these children decreased to 1 (range 0 to 4) after surgery, compared to 3 (range 2 to 8) before surgery.
Lesional versus non-lesional on MRI
The underlying etiology was somewhat diverse including malformations of cortical development (n=21), neuro-cutaneous syndrome (Klippel-Trenaunay-Weber syndrome=1; linear nevus sebaceous syndrome =1; Tuberous sclerosis=17), infections (n=2), chromosomal abnormalities (n=4), ischemia/hemorrhagic stroke secondary to in utero stroke, MRSA meningitis, aneurysm, MTHFR mutation, Factor V leiden mutation, autoimmune thrombocytopenia (n=12), tumors such as mixed glial tumor and DNET (n=2), perisylvian syndrome (n=1), and Down syndrome (n=1). The imaging findings were diverse with varied pathologies including malformations of cortical development (focal cortical dysplasia, hemimegalencephaly, schizencephaly, polymicrogyria, pachygyria), multiple hamartomas including sub-ependymal giant cell astrocytoma, encephalomalacic changes secondary to ischemic/hemorrhagic stroke, meningitis/encephalitis, and tumors.
Based on the MRI scans, 47 of the 65 patients were considered to have a lesion concordant with ictal EEG localization. Of these 47 lesional cases, 37 had a class I outcome (79%), 4 had class III and 6 had class IV outcomes. The MRI scans did not show lesions in 18 subjects and in this non-lesional group, 9 had class I outcome (50%), 4 had class III outcome, 3 had class IV outcome and 2 had class V outcome. The chance of achievement of class I outcome was 1.6 times greater in lesional than in non-lesional cases. The pathology of the resected tissues in eighteen patients with non-lesional epilepsy (as defined by imaging) revealed FCD in five, of which, four had Class I outcome. These included patient #1 (class I outcome), patient #38 (class I), patient #43 (class V), patient #48 (class I) and patient #60 (class I). In sixteen patients identified as having MCD including FCD by imaging and pathology, fourteen had favorable class I outcomes.
PET Findings
PET scan was abnormal (showed multifocal and widespread abnormalities) in almost all patients [61/63 (97%)], with lateralizing/localizing findings in 56/61 (92%) patients. This greatly helped in surgical decision making and guiding in subdural grid placement, particularly in patients with normal MRI and bilateral EEG abnormalities (Figure-2).
Figure-2.
MRI and FDG PET scan in a 7-year-old right-handed boy (patient #60) with uncontrolled seizures and cluster of epileptic spasms. While MRI was normal (left side), FDG PET (right side) revealed an area of hypometabolism in the right lateral and medial parietal cortex (broken arrows), as well as hypermetabolism (solid arrows) in the right lateral frontal cortex. The patient underwent right fronto-parietal cortical resection, which turned out to be cortical dysplasia on Histopathology. The child is seizure free at two and a half year follow up and is doing well.
Post-operative complications
Of the 65 patients, 15 (patients #3, 7, 18, 19, 20, 23, 24, 26, 28, 31, 32, 38, 51, 53, and 54) had post-operative complications which include isolated hydrocephalus (#3, 18, 19, 20, 26, 28, 32, 38, and 54), anoxic brain injury with hydrocephalus (#7), wound and bone flap infection (#53), hydrocephalus accompanied by post shunt CSF leak with bone flap infection (#51), sub-galeal fluid collection (#23), epidural hematoma (#31), and CSF fluid leak after head trauma (#24). The most severe of these was in one child (patient #7) with left hemiplegia and a history of right middle cerebral artery infarct in the newborn period associated with meningoencephalitis. She had a cardiac arrest intraoperatively after completion of scalp closure, resulting in mild extrapyramidal rigidity, but nevertheless became seizure-free. Out of the 11 patients who required ventricular shunts, the types of surgeries were hemispherectomy (n=7), subtotal hemispherectomy (n=2), multilobar resection (n=1) and tuberectomy (n=1).
Statistical results
A receive operating characteristic (ROC) analysis explored the relationship between the age of surgery and the risk of failing to achieve ILAE Class I outcome. This analysis revealed that the age cut-off of 3 years resulted in the most accurate prediction of seizure outcome; namely, patient age of >3 years was associated with a greater risk of failure to achieve ILAE Class I outcome with sensitivity of 0.95 and specificity of 0.52 (Table-2). In other words, patients who had surgery within 3 years of seizure onset showed a more favorable outcome than those operated more than 3 years after seizure onset. Of the 34 patients operated on prior to 3 years of seizure onset, 30 (88%) had a class I outcome. The remaining 31 patients underwent surgery more than 3 years after seizure onset and these had variable outcomes (Table-2). This observation does not clarify if the surgical outcome was associated with `patient age' or `duration of epilepsy'. To address this issue, we performed multivariate logistic regression analysis. This analysis revealed that both longer duration of epilepsy (p=0.022; Odds ratio: 1.56 [95CI: 1.07–2.28]) and lack of MRI lesion (p=0.026; Odd ratio: 4.54 [95%CI: 1.20–17.2]) were independently associated with a larger risk of failing to achieve ILAE class I outcome in our patient cohort. Patient age (p=0.19) and number of antiepileptic drugs (p=0.98) were not independently associated with surgical outcome in our patient cohort.
Table-2.
Effect of seizure duration on surgical outcome
| Duration between seizure onset and surgery (years) | # of patients | ILAE outcome - # of patients |
|---|---|---|
| <1 | 15 | 1 – 14, 3 – 1 |
| 1–2 | 8 | 1 – 8 |
| 2–3 | 11 | 1 – 8; 3 – 2; 4 – 1 |
| >3 | 31 | 1 – 16; 3 – 5; 4 – 8; 5 – 2 |
Discussion
Several major observations are clear from the present study. First, the presence of an MRI visible lesion in patients with intractable ES is associated with a better surgical outcome than when no lesion can be detected on MRI (79% versus 50% ILAE class I). However, the fact that as many as 50% of MRI-negative cases still had ILAE class I outcome indicates that epilepsy surgery is worth pursuing in this group, if all medical treatments have failed, considering the very poor overall prognosis of intractable ES.12 Second, children who were operated upon within 3 years of presentation with ES had an extremely good result, with 30/34 (88%) achieving ILAE class I outcome. Third, postoperative complications, while not negligible and certainly serious in some cases are, in general, acceptable (considering the gravity of intractable ES) and not too different from those reported in other published series on pediatric epilepsy surgery.
Lesional versus non-lesional based on MRI
Prior to the first cases of resective surgery for ES, these seizures were believed to be of generalized onset rather than localization related. That notion was challenged when focal resections guided by PET studies of glucose metabolism and intraoperative ECoG revealed underlying focal cortical dysplasia not detected on the relatively low resolution MRI scans used at that time.8; 9 Initially, there was much controversy as to whether surgical resections for ES (a `generalized' epilepsy) could be justified based on metabolic imaging,23 but this skepticism has gradually subsided.24 Importantly, Sankar and colleagues25 subsequently demonstrated that MRI scans, if performed in the first year of life at the presentation of West syndrome, may not detect cortical dysplasia in the region of the PET abnormality because poor grey-white matter differentiation may not yet be obvious at this age; however, repeat MRI scans later in the second or third years will often reveal evidence of cortical dysplasia previously missed.25
Indeed, current higher resolution 3-Tesla MRI scanners and advanced scanning sequences are able to detect even very subtle lesions corresponding to the focal metabolic abnormalities shown on PET, and this improvement in MRI technology probably accounts for the relatively larger number of MRI-positive cases (47/65 or 72%) A higher number of patients with MRI visible lesions achieved seizure freedom (79%) compared to those who relied more on PET for neuroimaging localization (50% of these had class I outcome). This is not surprising in view of multiple studies on pediatric epilepsy surgery in general where nonlesional cases typically showed a lower seizure free outcome compared to lesional cases.26 It is likely that the MRI-negative group is heterogeneous, including patients with cortical dysplasia not evident on MRI as well as patients with yet undiagnosed underlying genetic mutations as the etiology for their ES. The notion that mutations causing epilepsy in children may present with focal seizures is exemplified in patients with SCN1A mutations, which may be associated with both focal and generalized epilepsy.27 Still, 50% of MRI-negative cases in the present study attained seizure freedom and, considering that intractable ES is associated with a very poor outcome,12 surgery is well worth pursuing in such cases. A multimodal approach to delineate the epileptogenic zone is often required,28 as evidenced by the contribution of PET in the present cohort. Indeed, PET scan was lateralizing in almost all (92%) patients, and this greatly aided in subdural grid placement, particularly in patients with normal MRI and bilateral EEG abnormalities, and in subsequent surgical decision making, such as the extent of surgical resection, and need for total or sub-total hemispherectomy, which perhaps resulted in better than expected surgical outcomes.
It has been argued, and is now generally accepted, that ES are initiated as cortical epileptic discharges that, during a `critical' developmental period, undergo secondary generalization in an age-dependent mechanism to emerge as spasms.29 Perhaps one of the strongest arguments to support such a concept is the observation that focal seizures may trigger a cluster of ES. This was first reported by Dalla Bernardina et al.30 in the Italian literature, and subsequently replicated by others.31; 32 In this latter study, it was noted that of the 16 patients with clusters of bilateral ES preceded by focal seizures, 3 had complete agenesis of the corpus callosum, suggesting that propagation (or secondary generalization) of the focal seizures to emerge as spasms was not through the corpus callosum in these population. This observation is consistent with the notion that corpus callosotomy is not an effective treatment for ES. In addition, ES can be seen in patients with agenesis of the corpus callosum, such as children with Aicardi syndrome. ECoG on 15 children with subdural electrodes revealed that spasms were associated with either a `leading' spike followed by fast-wave bursts, or fast-wave bursts without a `leading' spike and that the `leading' spike originated in close proximity to the PET abnormality.20 Furthermore, failure to resect cortex showing the `leading' spike was associated with poor surgical outcome, indicating that the `leading' spike may be a biomarker of the trigger zone leading to the spasms.
Occasionally, the epileptogenic zone may be very large and involve much of the hemisphere. If the patient does not exhibit significant hemiparesis, we have elected in such cases to perform a `subtotal' hemispherectomy in order to preserve the primary motor and sensory cortex. We recently reported our experience with this operation in 23 patients with intractable epilepsy, 11 of who had ES as their major seizure type.16 MRI scan showed focal abnormalities in 12 of the 23 children, whereas PET showed focal/lateralized hypometabolism in all but one child. An ILAE class I outcome was achieved in 17 (74%) of the 23 subjects after a mean follow-up period of 65 months in that study. In the present study, 17of the 65 subjects underwent a `subtotal' hemispherectomy. 10 (58.8%) had class I outcome, 3 showed class III outcome and the remaining 4 had class IV outcome.
All too often, however, there are multiple foci seen on the MRI and/or PET, thus precluding a surgical approach. In some instances of multiple cortical epileptic foci, a palliative surgery can be offered if the majority of seizures emanate from one of the foci. We believe that it is important to designate and declare palliative cases prior to surgery in order to analyze surgical results accurately; in the present series, we have excluded such cases. We have recently analyzed our results on palliative epilepsy surgery in children (including 7 cases of ES), and the outcome appears to be quite favorable with 11/18 (61.1%) patients showing seizure reduction and 8/18 (44%) children achieving seizure freedom, including 3 of the 7 with ES.15
Although lesional and non-lesional outcomes in epilepsy surgery are well described,33 there are few such data on patients specifically with ES in this regard. The new information from the current study suggests that ES subjects without an MRI lesion but with a positive FDG PET can still be epilepsy surgery candidates if there is concordance with the EEG, and seizure freedom can still be achieved in half of such subjects. It would be important if the current study were to stimulate a multicenter collaboration of lesional and non-lesional ES cases coming to epilepsy surgery.
Age at surgery
In general, early surgery is advocated in children with medically refractory epilepsy due to greater plasticity and ability to reorganize functions in the developing brain compared to a more mature brain. A review of cognitive outcome following epilepsy surgery in children revealed that the strongest predictors of long-term cognitive outcome were duration of epilepsy, preoperative cognitive status, and postoperative seizure freedom.34 However, many other factors, including age at surgery and underlying etiology, also were related to a change in postoperative cognitive scores.
In the present series, we found that children with intractable ES who had surgery within 3 years of presentation had an excellent outcome. Indeed, of the 34 children in this category, 30 (88%) achieved a class I outcome. This surgical success is higher than average success rates in our own previous series9 as well as recent studies from other centers,10; 12; 13; 35 although the numbers of subjects in most of those reports are small compared to the present series. For example, Lee et al. 35 reported that 9 (60%) of 15 children with ES became seizure free following surgery. In one relatively large series of 30 children with ES and cortical dysplasia who underwent cortical resection, 22 (73.3%) became seizure free.12 Interestingly, that same study found no difference in surgical outcome between MRI-positive and MRI-negative cases before age 1 year.
The reason for a better seizure free outcome (as distinct from cognitive outcome) in younger compared to older children in the present study is not clear, and we can only speculate as to why this might be. A possible explanation is that, since the seizures (and interictal spiking) in patients with ES usually occur with high frequency, the tendency to establish a large epileptic network and secondary epileptic foci from an active primary focus within a short period of time may be greater than with other seizure types. The results of multivariate logistic regression analysis in the present study support this notion. Another possibility could be that the smaller brain is more easily covered by subdural electrodes and hence there may be less sampling error than larger brains. In addition, there is a tendency to perform larger resections in younger infants with the advantage of a more complete removal of epileptogenic brain tissue.
The optimal timing of epilepsy surgery in the pediatric population has been in-debate for some time as the extent and timing of cortical resection is determined “case by case” based on the intractability, developmental concerns and after extensive discussion with the family regarding the pros and cons of surgical resection. The choice of 3 years in our paper was somewhat arbitrary as we explored different time scales. However, it can be safely recommended that earlier surgery can provide early relief from catastrophic epilepsy and may allow resumption of developmental progression during critical stages of brain maturation. From a clinical perspective, our experience is that surgery should be performed no later than 3–4 years, although this has not been rigorously tested. Clearly, large multicenter studies with standardized approaches will be required to address this issue.
Limitations
Limitations of our study include its retrospective design, and short duration of follow-up in some patients. Larger prospective studies with multicenter collaboration could reveal clinically relevant associations and correlate presurgical variables with outcomes.
Postoperative complications
While early surgery is increasingly being advocated for children with intractable focal epilepsy, and certainly supported by data from the present study, intraoperative and postoperative complications are also the highest in the youngest surgical candidates. In a report of 15 infants who were operated at 6 months of age or younger for intractable severe epilepsy, the most frequently encountered complication was blood loss with a median estimated blood loss of 63% of the total blood volume (range: 3% to 214%). One infant suffered an intraoperative acute ischemic infarct resulting in hemiparesis, yet 46% of the infants became seizure free.36 In a recent analysis of 552 hemispherectomies performed in the United States between 2000 and 2009, in-hospital mortality occurred in 5 subjects (0.9%) and ventricular shunt placement during the surgical admission increased over time from 6.7% to 16.5%.37
The most severe complication encountered in the present study was in a child (patient #7) who suffered a cardiac arrest following right hemispherectomy after scalp closure. Even so, this child became seizure free but manifested mild extrapyramidal rigidity on follow-up examinations. Other complications are not different from what has been reported previously. There were no differences in postoperative complications between the MRI-positive and MRI-negative groups; 11/47 (23.4%) lesional and 4/18 (22.2%) non-lesional patients had post-surgical complications. Further, 12/15 (80%) of them had class I outcome, suggesting no effect of post-surgical complication on surgical outcome. Due to the typical large multilobar resections or even hemispherectomies performed in young children with ES, it is anticipated that some of them will acquire hydrocephalus and require a shunt. In the present series, 11 (16.9%) of the 65 children required cerebrospinal fluid shunting. This number is consistent with the results from a very large multicenter study conducted by the Post-Hemispherectomy Hydrocephalus Workgroup on 690 children who underwent hemispherectomy where 23% required shunt treatment for hydrocephalus.38 Most of the patients had improvement in behavior and developmental parameters including language and social skills with better seizure control.39; 40 Post-operative development was not negatively affected by the surgery. For example, some patients with tuberous sclerosis remained delayed but were no worse. In contrast, most patients made significant developmental gains after surgery.
Curative epilepsy surgery in patients with epileptic spasms (ES), with or without history of infantile spasms, is best accomplished in those with abnormal MRI.
Good outcomes can be achieved even when there is no MRI lesion but positive PET localization.
Surgical outcome is extremely good in children who were operated upon within 3 years of presentation with ES.
Postoperative complications, while not negligible and certainly serious in some cases are, in general, acceptable (considering the gravity of intractable ES).
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health to HTC (R01NS34488, R01NS064989) and EA (K23NS047550, R01NS064033).
Footnotes
Disclosure None of the authors have any conflict of interest to disclose. HTC is a consultant to Lundbeck, LLC.
Ethical Publication Statement The authors confirm that they have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 2.Pavone P, Striano P, Falsaperla R, et al. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36:739–751. doi: 10.1016/j.braindev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;6:CD001770. doi: 10.1002/14651858.CD001770.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branch CE, Dyken PR. Choroid plexus papilloma and infantile spasms. Ann Neurol. 1979;5:302–304. doi: 10.1002/ana.410050315. [DOI] [PubMed] [Google Scholar]
- 5.Mimaki T, Ono J, Yabuuchi H. Temporal lobe astrocytoma with infantile spasms. Ann Neurol. 1983;14:695–696. doi: 10.1002/ana.410140621. [DOI] [PubMed] [Google Scholar]
- 6.Ruggieri V, Caraballo R, Fejerman N. Intracranial tumors and West syndrome. Pediatr Neurol. 1989;5:327–329. doi: 10.1016/0887-8994(89)90029-5. [DOI] [PubMed] [Google Scholar]
- 7.Palm DG, Brandt M, Korinthenberg R. West syndrome and Lennox-Gastaut syndrome in children with porencephalic cysts: long-term follow-up after neurosurgical treatment. In: Niedermeyer E, Degen R, editors. The Lennox-Gastaut syndrome. Alan R. Liss; New York: 1988. pp. 419–426. [Google Scholar]
- 8.Chugani HT, Shields WD, Shewmon DA, et al. Infantile spasms: I. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol. 1990;27:406–413. doi: 10.1002/ana.410270408. [DOI] [PubMed] [Google Scholar]
- 9.Chugani HT, Shewmon DA, Shields WD, et al. Surgery for intractable infantile spasms: neuroimaging perspectives. Epilepsia. 1993;34:764–771. doi: 10.1111/j.1528-1157.1993.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwatani Y, Kagitani-Shimono K, Tominaga K, et al. Long-term developmental outcome in patients with West syndrome after epilepsy surgery. Brain Dev. 2012;34:731–738. doi: 10.1016/j.braindev.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Jonas R, Asarnow RF, LoPresti C, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–750. doi: 10.1212/01.WNL.0000151970.29205.70. [DOI] [PubMed] [Google Scholar]
- 12.Kang JW, Rhie SK, Yu R, et al. Seizure outcome of infantile spasms with focal cortical dysplasia. Brain Dev. 2013;35:816–820. doi: 10.1016/j.braindev.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Yum MS, Ko TS, Lee JK, et al. Surgical treatment for localization-related infantile spasms: excellent long-term outcomes. Clin Neurol Neurosurg. 2011;113:213–217. doi: 10.1016/j.clineuro.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Juhasz C, Asano E, et al. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51:1901–1907. doi: 10.2967/jnumed.110.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilyas M, Sivaswamy L, Asano E, et al. Seizure control following palliative resective surgery for intractable epilepsy-a pilot study. Pediatr Neurol. 2014;51:330–335. doi: 10.1016/j.pediatrneurol.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Chugani HT, Asano E, Juhasz C, et al. “Subtotal” hemispherectomy in children with intractable focal epilepsy. Epilepsia. 2014;55:1926–1933. doi: 10.1111/epi.12845. [DOI] [PubMed] [Google Scholar]
- 17.Chugani HT, Luat AF, Kumar A, et al. alpha-[11C]-Methyl-L-tryptophan--PET in 191 patients with tuberous sclerosis complex. Neurology. 2013;81:674–680. doi: 10.1212/WNL.0b013e3182a08f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano E, Juhasz C, Shah A, et al. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–1047. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkonyi B, Juhasz C, Muzik O, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asano E, Juhasz C, Shah A, et al. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–1097. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nariai H, Nagasawa T, Juhasz C, et al. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 23.Hrachovy RA, Frost JD, Jr, Glaze DG, et al. Surgical treatment for infantile spasms? Ann Neurol. 1991;29:110–112. doi: 10.1002/ana.410290123. [DOI] [PubMed] [Google Scholar]
- 24.de la Vaissiere S, Milh M, Scavarda D, et al. Cortical involvement in focal epilepsies with epileptic spasms. Epilepsy Res. 2014;108:1572–1580. doi: 10.1016/j.eplepsyres.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Sankar R, Curran JG, Kevill JW, et al. Microscopic cortical dysplasia in infantile spasms: evolution of white matter abnormalities. AJNR Am J Neuroradiol. 1995;16:1265–1272. [PMC free article] [PubMed] [Google Scholar]
- 26.Englot DJ, Breshears JD, Sun PP, et al. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 27.Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy) Handb Clin Neurol. 2013;111:627–633. doi: 10.1016/B978-0-444-52891-9.00065-8. [DOI] [PubMed] [Google Scholar]
- 28.Hur YJ, Lee JS, Kim DS, et al. Electroencephalography features of primary epileptogenic regions in surgically treated MRI-negative infantile spasms. Pediatr Neurosurg. 2010;46:182–187. doi: 10.1159/000321925. [DOI] [PubMed] [Google Scholar]
- 29.Chugani HT, Shewmon DA, Sankar R, et al. Infantile spasms: II. Lenticular nuclei and brain stem activation on positron emission tomography. Ann Neurol. 1992;31:212–219. doi: 10.1002/ana.410310212. [DOI] [PubMed] [Google Scholar]
- 30.Dalla Bernardina B, Colamaria V, Capoville G. Epileptic syndromes and cerebral malformations in infancy: multicentric study. Boll Lega Ital Epil. 1984;45:65–67. [Google Scholar]
- 31.Carrazana EJ, Lombroso CT, Mikati M, et al. Facilitation of infantile spasms by partial seizures. Epilepsia. 1993;34:97–109. doi: 10.1111/j.1528-1157.1993.tb02381.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto N, Watanabe K, Negoro T, et al. Partial seizures evolving to infantile spasms. Epilepsia. 1988;29:34–40. doi: 10.1111/j.1528-1157.1988.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 33.Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, et al. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Van Schooneveld MM, Braun KP. Cognitive outcome after epilepsy surgery in children. Brain Dev. 2013;35:721–729. doi: 10.1016/j.braindev.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Lee YJ, Lee JS, Kang HC, et al. Outcomes of epilepsy surgery in childhood-onset epileptic encephalopathy. Brain Dev. 2014;36:496–504. doi: 10.1016/j.braindev.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Gowda S, Salazar F, Bingaman WE, et al. Surgery for catastrophic epilepsy in infants 6 months of age and younger. J Neurosurg Pediatr. 2010;5:603–607. doi: 10.3171/2010.1.PEDS08301. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Harris DA, Curry DJ, et al. Trends in outcomes, complications, and hospitalization costs for hemispherectomy in the United States for the years 2000–2009. Epilepsia. 2015;56:139–146. doi: 10.1111/epi.12869. [DOI] [PubMed] [Google Scholar]
- 38.Lew SM, Matthews AE, Hartman AL, et al. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia. 2013;54:383–389. doi: 10.1111/epi.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammed HS, Kaufman CB, Limbrick DD, et al. Impact of epilepsy surgery on seizure control and quality of life: a 26-year follow-up study. Epilepsia. 2012;53:712–720. doi: 10.1111/j.1528-1167.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 40.Zupanc ML, Rubio EJ, Werner RR, et al. Epilepsy surgery outcomes: quality of life and seizure control. Pediatr Neurol. 2010;42:12–20. doi: 10.1016/j.pediatrneurol.2009.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.