Abstract
Mature skeletal muscle forms from the fusion of skeletal muscle precursor cells, myoblasts. Myoblasts fuse to other myoblasts to generate multinucleate myotubes during myogenesis, and myoblasts also fuse to other myotubes during muscle growth and repair. Proteins within myoblasts and myotubes regulate complex processes such as elongation, migration, cell adherence, cytoskeletal reorganization, membrane coalescence, and ultimately fusion. Recent studies have identified cell surface proteins, intracellular proteins, and extracellular signaling molecules required for the proper fusion of muscle. Many proteins that actively participate in myoblast fusion also coordinate membrane repair. Here we will review mammalian membrane fusion with specific attention to proteins that mediate myoblast fusion and muscle repair.
Keywords: Fusion, Repair, Membrane, Myoblast, Muscle, Development
1. Introduction
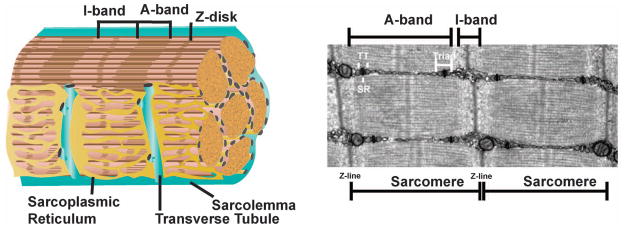
Striated muscle is characterized by a highly ordered intracellular array of sarcomeres optimized for contraction (Figure 1). The sarcolemma protects myofibers and is contiguous with the Transverse (T)-tubules, a network responsible for coordinating excitation and contraction by tightly regulating Ca2+ release into the myoplasm [1]. Ca2+ channels and exchangers reside in the T-tubules, and the T-tubules are flanked by the sarcoplasmic reticulum (SR), a reservoir for the majority of intracellular Ca2+. Protein complexes enriched in the sarcolemma are specialized for attachment to the surrounding matrix, and include the integrins and dystrophin glycoprotein complex (DGC) [2]. The stem cells of muscle, the satellite cells, are located between the sarcolemma and the basal lamina. Upon muscle injury, this regenerative cell population divides and differentiates into myoblasts, which then fuse to regenerate the muscle [3]. Herein, we review membrane fusion events in myoblast fusion, in development and repair.
Fig. 1.
Membranes in muscle. Mature muscle is composed of individual multinucleate myofibers bound by plasma membrane, or sarcolemma. The sarcoplasmic reticulum (SR) is specialized endoplasmic reticulum, and the transverse (T)-tubules are sarcolemmal invaginations evolved for Ca2+ handling and contraction. The SR forms a complex network around the myofiber adjacent to the T-tubules. Muscle striations are generated from the thin-filament (actin) containing I-band and the thick-filament (myosin) containing A-band. The Z-disk defines the edges of the sarcomere. Muscle contraction occurs as myosin slides across the filaments shortening the sarcomere.
Membrane repair and disease
The highly ordered nature of striated muscle is conserved throughout evolution. Skeletal muscle in these organisms mirrors what is found in human muscle, with thick and thin filaments anchored in the Z disc (Figure 1). Improper expression or function of membrane components during development can lead to defects in myoblast fusion and repair, resulting in myopathy. Loss-of function mutations in dystrophin, a membrane stabilizer, result in Duchenne muscular dystrophy (DMD), a progressive muscle wasting disorder characterized by a fragile sarcolemma. Loss-of function mutations in dysferlin, a distinct membrane-associated protein, cause Limb Girdle Muscular Dystrophy Type 2B and other myopathies [4]. In muscular dystrophy, the reparative and regenerative properties of muscle cannot keep pace with muscle degeneration. The genetic mutations responsible for these diseases helped identify the protein machinery responsible for membrane stability and fusion. Much of the research on these proteins and pathways has focused on membrane dynamics occurring during myoblast fusion during muscle development and also during recovery after muscle injury [5, 6].
1.1.2 Overview of myoblast fusion in mammals
During fusion, mononucleated myoblasts fuse generating nascent myotubes. Myoblast to myotube fusion as well as myotube to myotube fusion also occur. Myoblast fusion also occurs during muscle regeneration. Injury is sufficient to activate satellite cells, which follow an asymmetric division process to maintain the satellite cell pool and simultaneously generate myoblasts [3, 7]. This form of cell-mediated repair is distinct from sarcolemmal resealing of membrane disruptions. With smaller sarcolemmal lesions or disruptions, proteins are recruited to the site of damage to repair the torn membrane. The degree to which the machinery governing myoblast fusion is similar to that regulating membrane resealing is not fully known. However, these processes are conserved, and model organisms such as Drosophila melanogaster have provided great insight into the mechanics of the membrane during myoblast fusion [5, 8].
1.1.3 Overview of myoblast fusion in Drosophila
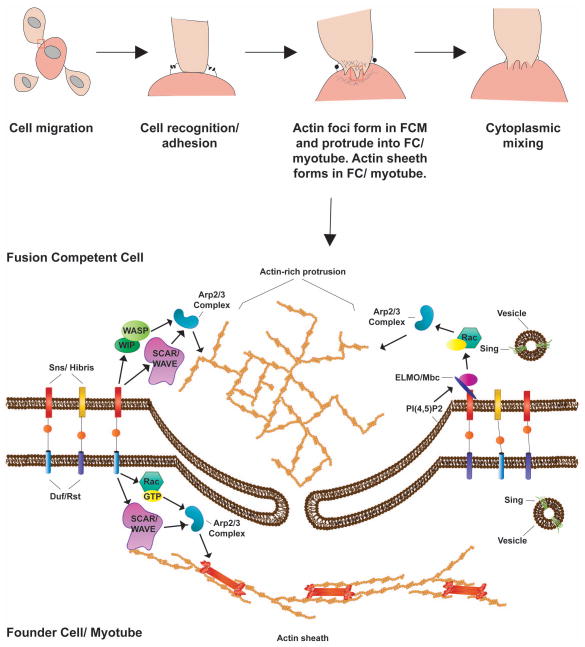
In Drosophila, myoblast fusion occurs around stage 13 of embryogenesis. Two myoblast populations are present, founder cells (FCs) and fusion competent myoblasts (FCMs). FCs establish the destination of the mature muscle. FCMs migrate to FCs requiring secreted signaling molecules and movement through the ECM. Once co-localized, FCs recognize FCMs through signaling and adhesion events. Recognition is accompanied by alignment of the FC and FCM membranes mediated by the immunoglobulin super family of proteins and adhesion molecules. Upon cell alignment, actin polymerization machinery is recruited and the cytoskeleton reorganizes. Actin-rich protrusions form in the FCM, allowing invasion of the FC. Pores form within the membrane, allowing the exchange of cytoplasmic materials and ultimately pore expansion and membrane fusion (Figure 3). Recent work shows the receiving FC can sense mechanical stress at the membrane and is crucial for proper fusion [9, 10].
Fig. 3.
Myoblast fusion in Drosophila. Two populations of myoblasts exist during Drosophila myoblast fusion: the founder cells (FCs) and the fusion competent myoblasts (FCMs). The FCs are present at sites where mature muscle will eventually form. FCMs migrate to the FCs, adapting a characteristic teardrop form. Adhesion molecules on the cell surface of both FCs and FCMs mediate the initial recognition/adhesion step. Next, the FCM form actin rich podosome-like strictures, which extend into the FCs, while the FCs form a thin actin sheath at the membrane. Tiny pores form and expand allowing cytoplasmic mixing, and ultimately the fusion of myoblasts, resulting in a multinucleated muscle fiber. Importantly, FCMs continue to fuse to this multinucleated fiber, resulting in mature muscle.
1.2.1 The actin cytoskeleton and fusion
The actin cytoskeleton regulates multiple steps in this process. Individual myoblasts must migrate to their sites of fusion, necessitating cytoskeletal shape changes. Second, the cells must recognize each other and adhere. In Drosophila, it has been proposed that an actin-rich protrusion forms in the FCM, which serves to invade the FC membrane. Concomitantly, an actin sheath is formed juxtaposed to the FC membrane, presumably affecting membrane stability for fusion [5, 6]. In mammals, similar actin cytoskeletal rearrangements are required [5, 11]. Formation of these intracellular actin structures involves the conserved Scar/Wave and/or Wip/Wasp pathway, activating the Arp2/3 protein complex and inducing actin polymerization and branching [5, 12]. Furthermore, vesicle transport is required during myoblast fusion and membrane repair. Movement of these vesicles requires reorganization of the actin cytoskeleton [8, 13, 14].
1.2.2 Lipids and fusion
Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidic acid (PA) are the major components of the eukaryotic plasma membrane, with PC making up more than half of the lipid content. Glycolipids and cholesterols are less abundant in the membrane but are important in establishing membrane polarity and fluidity, respectively [15]. Importantly, membrane lipids are fluid, capable of lateral diffusion. Additionally, the sarcolemma has variable regions of lipid concentration. Lipid clusters exist transiently depending on cell function [15]. PS clusters are seen at sites of cell-cell contact during myoblast fusion in primary myoblasts, indicating an important role in membrane ruffling [16]. Furthermore, cholesterol is required to form specialized regions of the membrane termed lipid rafts. In a murine muscle cell line, depletion of cholesterol, and therefore lipid rafts, blocks myoblast fusion. Reintroduction of cholesterol to the media restores fusion [17, 18].
2.1 Known molecules involved in cell recognition and adhesion during myoblast fusion
2.1.1 Cell adhesion molecules and myoblast fusion
In Drosophila, the heterophilic immunoglobulin (Ig) superfamily of proteins is responsible for the initial recognition and adhesion of the FCM to the FC. Specifically, the Ig members Kirre/ Dumbfounded (Duf) and Roughest (Rst/ IrreC) are presented on the FC surface, while Stick and Stones (Sns) and Hibris (Hbs) are presented on the fusion competent cell surface [19–22] (Figure 3). In mice, only one Ig superfamily ortholog has been implicated in the process of myoblast recognition; the Sns ortholog, Nephrin [23]. It should be noted that a vertebrate ortholog of Duf/Kirre in zebrafish is Kirrel, and it has been implicated in myoblast fusion [20, 24]. In mice, specific integrins family members are required for myogenesis. Integrin α3, β1 and α9β1, along with the integrin-associated cytoplasmic adaptor protein, Kindlin 2, are necessary for proper fusion [25–27]. In Drosophila, integrins have not been implicated in the fusion process, but are required for the integrity of muscle attachment sites later in development, as they also are in mammalian muscle [28]. In mice, the cadherin family of Ca2+-dependent adhesion proteins is important for myoblast fusion. Muscle cadherin (M-cad) is expressed in the developing musculature, although this expression pattern is not evident until after the initial fusion steps have occurred. M-cad is also expressed in satellite cells and the underlying sarcolemma. However, M-cad-deficient mice lack obvious skeletal muscle defects, perhaps due to redundancy of other mammalian cadherins [29]. M-cad is observed enriched at cell-cell contact sites and is required for myoblast fusion in the L6 rat myoblast cell line [30]. Cell fusion can be readily imaged in myoblast cultures (Figure 4).
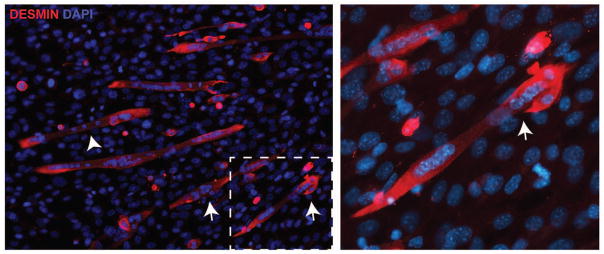
Fig. 4.
Cell culture model of myoblast fusion. Primary myoblasts will differentiate and fuse with serum withdrawal. Desmin (red) is used to mark committed muscle cells. Mono-nucleated myoblasts (arrow) fuse with existing myotubes (arrowhead). The right panel shows a higher magnification view of the dotted box showing the fusion of a myoblast to an existing multinucleate myotube. DAPI (blue) stains nuclei.
2.1.3 Known molecules involved in cell signaling and actin reorganization in myoblast fusion
Dock (Dedicator of Cytokinesis) proteins are conserved throughout evolution. Docks are guanine nucleotide exchange factors (GEFs) that activate small downstream GTPases, namely Rac1 and Cdc42. These GEFs exchange GDP, which when bound to Rac is inactive, for GTP. The vertebrate Dock family has 11 members divided into families; Dock A through D. Dock A subfamily contains the founding members Drosophila Myoblast City (Mbc), C. elegans Ced-5, and mammalian Dock1 (Dock180). Dock1 forms a complex with its obligate binding partner, the adaptor protein ELMO, which is required for Rac1 activation [5, 31, 32]. These proteins are required for myoblast fusion in Drosophila and the initial phase of fusion in mammals [31, 33]. Zebrafish experiments have shown a close Dock1 ortholog is required for fusion of myoblasts [24]. In mammals, this sub-family also consists of Dock5, which is also important for myoblast fusion in mice [34]. The Dock A subfamily has specificity for the small GTPase Rac1. Rac1 activates the WASP pathway, which relieves inhibition of Arp2/3 leading to an increase in actin polymerization and branching. The Dock/ELMO and Rac1 pathway is of particular interest as it has been shown to be involved in myoblast fusion in Drosophila, zebrafish, and mammals [5, 6].
3.1 Proteins involved in both myoblast fusion and membrane repair
3.1.1 Ferlins
The ferlin family has been implicated in disease, specifically muscular dystrophies and non-syndromic deafness. Fer-1 in C. elegans was the first described ferlin protein [35]. Fer-1 mutants are infertile with spermatozoa displaying abnormal intracellular vesicle retention, consistent with defective intracellular membrane fusion. In mammals, there are six ferlin family members Fer1L1 through Fer1L6, and the FER1L1 gene encodes dysferlin [36, 37]. Ferlin family members are characterized has having four to seven C2 domains and a carboxy-terminal transmembrane domain. C2 domains are found in many membrane-associated proteins where they may mediate Ca2+-sensitive phospholipid binding. The C2 domains in ferlin proteins bear resemblance to those in the membrane trafficking proteins, the synaptotagmins [38–40]. Most C2 domain containing proteins generally contain only one or two C2 domains while mammalian ferlin proteins have an excessive number of C2 domains [41].
Dysferlin and myoferlin are 230 kDa proteins that are 57% homologous at the amino acid level [37, 42]. Dysferlin and myoferlin each contain seven conserved C2 domains. The C2A domains of myoferlin and dysferlin bind to PS in a Ca2+-sensitive manner [40, 43]. Structural studies of the C2A domain of dysferlin indicate that these domains alter lipid packing and actively sculpt membranes [44]. The high similarity between myoferlin and dysferlin is balanced by differences in expression within muscle. Myoferlin is highly expressed in the immature myoblast, while dysferlin expression is upregulated as the myoblast differentiates into mature myotubes [43]. Dysferlin was initially described as a plasma membrane-associated protein in mature skeletal muscle [45]. Using an antigen unmasking technique, dysferlin has also been localized at the T-tubule [46]. A pH-sensitive carboxy terminal fusion tag was also used to demonstrate the presence of dysferlin at the T-tubule [46–48]. The expression pattern of dysferlin in mature muscle parallels the presence of T-tubules, as myoblasts lack this membrane structure.
Loss of function, recessive mutations in dysferlin (FER1L1) cause muscular dystrophy, and this form of muscular dystrophy is characterized by defective membrane resealing [39]. To establish dysferlin’s role in membrane repair, Bansal and colleagues used confocal laser microscopy to wound and image isolated myofibers in the presence of a lipophilic dye, FM 1–43. FM 1–43 is a lipophilic styryl dye that fluoresces in the presence of lipids. In the laser wounding assay, FM 1–43 fluorescence persists longer and in a larger region of dysferlin null myofibers compared to wildtype. Laser injury of wildtype fibers in the presence of a Ca2+ chelator appeared similar to dysferlin-null myofibers, consistent with the idea that dysferlin is important for the Ca2+ response and proper timing of membrane repair [39]. Using this same laser wounding assay on myofibers, it was shown that a large patch of dysferlin accumulated at the site of laser wounding and sarcolemmal disruption. It was further shown that caveolin-3 was absent from the site of disruption. Recent work has uncovered a calpain cleavage product, containing the carboxy-terminus of dysferlin termed mini-dysferlin. Mini-dysferlin translocates to the site of damage regulated by L-type calcium channel activity as Diltiazem, a L-type calcium channel inhibitor, prevents mini-dysferlin localization at the site of injury [49].
Dysferlin-null mice develop pathological changes of muscular dystrophy similar to human with dysferlin mutations [39, 50, 51]. In these models, muscle proteins are increased in the serum, as they are in humans with muscular dystrophy and this increase is thought to arise from sarcolemma leak. Myoferlin null mice have defective myoblast fusion and a reduced myofiber diameter [43]. Dysferlin/myoferlin double null mice have enhanced muscular dystrophy accompanied by T-tubule defects and extrusion of lipids from the sarcolemma [52]. Loss of dysferlin is also associated with defective myoblast fusion, and this is reflected by decreased myofiber size in vivo. Myoblast fusion defects were also observed in dysferlin-deficient human myoblasts [53]. Myoferlin and dysferlin also regulate endocytic recycling, a process critical for both fusion and repair [51, 54].
To determine if myoferlin compensates for the loss of dysferlin, transgenic mice over-expressing myoferlin were generated and crossed into a dysferlin mutant background [55]. After laser-induced sarcolemmal ablation, myoferlin overexpression restored efficient resealing in dysferlin null muscle. However despite correction of the membrane repair defect, histological evidence of muscular dystrophy remained. Thus, there was a lack of correlation between the improved resealing and histopathology, suggesting that perhaps dysferlin has additional roles. Protein analysis revealed a 4-fold increase in myoferlin protein levels at 3-weeks of age, while expression at 4-months of age showed a greater than 100-fold increase compared to normal myoferlin levels. Upregulation of membrane-associated proteins can induce ER-stress, and thus it is possible that overexpression of myoferlin itself induced pathology.
3.1.2 Eps15 homology domain containing proteins (EHDs)
Myoferlin was found to interact with the Eps 15 homology domain containing (EHD) family member, EHD2 [54]. EHD proteins are implicated in endocytic recycling, another membrane fusion process [56]. This interaction was facilitated through binding of the asparagine-proline-phenylalanine (NPF) motif found in the myoferlin’s C2B domain. A similar interaction was documented between EHD2 and Fer1L5, another related ferlin family member. Dysferlin does not contain a classical NPF motif in its C2B domain; however, weak binding to EHD2 was documented likely mediated by the serine-proline-leucine (SPL) amino acid sequence in dysferlin [57]. The EHDs are a family of four highly homologous proteins in mammals. EHD1 through 4 each contain an amino-terminal helical domain responsible for protein-protein interactions, a dynamin-like G-domain that hydrolyzes ATP, a second helical domain, and a carboxy-terminal EH-domain. The EH-domain coordinates Ca2+ through an EF-hand domain and binds to NPF motifs. EHD proteins are expressed in a wide variety of tissues and the expression pattern within muscle is tightly coordinated. EHD1 and EHD4 are expressed at low levels within the myoblast, with expression increasing as muscle matures [57]. EHD2 is highly expressed in the immature myoblast and nascent myotube, and protein expression decreases as differentiation proceeds, similar to the expression pattern of its binding partner myoferlin.
EHD1 and EHD2 are implicated in both membrane fusion and repair [54, 57, 58]. Posey et al showed that depletion of EHD1, EHD2, or the combination of EHD1 and EHD2 in C2C12 cells resulted in decreased fusion efficiency compared to controls [57]. Additionally, myoblasts isolated from Ehd1-null mice had reduced myoblast fusion and this correlated with decreased myofiber size in vivo [59]. An “ATPase-dead” EHD2, G65R, inhibited myoblast fusion and sequestered myoferlin showing the importance of ATP hydrolysis for proper function of the EHD protein in the process of myoblast fusion [54]. EHD1 and EHD2 both are found at the periphery of the myoblast in an actin-free zone localizing with Fer1L5, myoferlin and GRAF1, well positioned for a role in myoblast fusion [54, 57, 60].
EHD1 and EHD2 also are expressed in mature muscle. Specifically, EHD1 is localized to the T-tubule [59]. Live cell microscopy of cultured human myotubes showed that EHD2, but not EHD1, localized to the site of membrane repair after laser-induced membrane damage, and that EHD2 co-localized with actin at the site of repair [58]. The co-localization with actin at the site of sarcolemmal disruption in myotubes differs from what was observed in myoblasts undergoing fusion, where EHDs are enriched in actin-free zones [57]. These observations, made in both myoblasts and myotubes, may differ from what occurs in the mature myofiber. The role of EHD3 and EHD4 in skeletal muscle has not been elucidated. However, EHD3 protein expression is upregulated in mouse and human cardiac muscle after injury suggesting a role for EHD3 in the repair process [61]. The involvement of EHD proteins in both fusion and repair is not surprising as EHD proteins are involved in vesicle trafficking, actin polymerization, and the formation of membrane tubules (referred to as tubulation).
3.1.3 Annexins
In mammals, there are twelve annexin protein family members characterized by the ability to bind phospholipids in a Ca2+- and actin-dependent manner. Annexins are involved in a variety of cellular processes including membrane permeability, mobility, and vesicle fusion. The annexins contain a conserved core domain composed of 4 to 8 annexin repeats and a variable amino-terminal head. Annexins lack a traditional Ca2+-binding EF-hand motif and instead coordinate Ca2+ through the annexin repeats [62]. In the presence of Ca2+, annexin proteins have the ability to oligomerize and bind membrane lipids [63]. Transgenic overexpression of annexin A6 results in abnormal Ca2+ transients in cardiac cells [64].
Annexin A1 and A5 expression is increased during myogenic differentiation in cell culture models [65]. Blocking A1, A5 or both A1 and A5 was shown to inhibit the formation of myotubes in culture [66]. Myoblasts isolated from either the annexin A1-null or A5-null mouse models showed a requirement for annexin A1 and A5 for the formation of proper myotubes [66]. It was also shown that ATP and PIP2 were required at the membrane for proper myoblast fusion [66].
Multiple cellular models have revealed a role for the annexin family in membrane repair, including in skeletal muscle [67–70]. In zebrafish, annexins A1, A2, A5, and A6 accumulate at the sarcolemmal ablation [71]. Annexin A6 was observed to arrive first at the site of sarcolemmal disruption, and differences in Ca2+ and lipid affinity may regulate the timing of translocation. Annexins A1 and A2 have been shown to directly bind dysferlin, where they are hypothesized to facilitate membrane repair in a large membrane repair complex [69, 71]. The role of annexin A6 in mammalian muscle membrane repair was established as it was identified as modifier of muscular dystrophy and shown to translocate to sites of sarcolemmal disruption [72]. Annexin A1 localizes to the site of sarcolemmal damage in cultured myotubes, reiterating the importance of the annexin members in repair [58]. Additionally, annexin A5 binds PS and specifically localizes at the site of sarcolemmal disruption in wounded C2C12 cells [73]. Serum level expression of annexin A1 and A2 are increased in humans with cancer [74, 75]. Annexins A1 and A2 may be useful as biomarkers for muscular dystrophy since these proteins can be detected in the serum of muscular dystrophy patients using immunoblotting [76].
3.1.4 Mitsugumin 53 (MG53)
Mitsugumin 53 (MG53), also known as Trim72, belongs to the family of E3 ubiquitin ligases or Tripartite Motif (TRIM) proteins [77]. MG53 consists of an amino-terminal TRIM domain and a carboxy-terminal SPla and the RYanodine Receptor (SPRY) domain, a structure found in many TRIM family proteins. MG53 is expressed highly in striated muscle [73]. MG53 expression is low in myoblasts and increases in differentiated myotubes, and MG53 is required for proper myotube differentiation [78]. MG53-depleted C2C12 cells had a marked decrease in myotube formation at both 5 and 10 days post differentiation, well into the differentiation and fusion timeline [78]. MG53-null mice develop progressive myopathy. However, MG53-null mice had normal myofiber size until 10 months of age. These data suggest that MG53 may mediate later steps in the fusion and maturation process in muscle.
MG53-null muscle fibers display delayed membrane resealing after laser-induced sarcolemmal disruption [73]. Consistent with a role for MG53 in myofiber repair, MG53 preferentially binds PS at the site of membrane damage [73]. MG53 contains multiple cysteine residues that, when reduced, inhibit MG53’s oligomerization and role in membrane repair [73]. MG53 is hypothesized to patch the disrupted sarcolemmal membrane. The addition of recombinant human MG53 to cultured cells or in vivo is sufficient to improve membrane repair capacity as measured by multiple dye permeability assays [79]. Additionally, MG53 localizes with known repair proteins like dysferlin and the annexins, suggesting MG53 acts in a larger repair complex. Upon stretch induced injury, MG53 localized with dysferlin and annexin A1 at the tubule membrane [80]. Using a ballistics approach to disrupt membranes, Lek and colleagues showed that after membrane injury MG53 translocates to the site of damage and colocalizes with dysferlin in a Ca2+-dependent manner. Translocation of annexin or lysosomal-associated membrane protein1 (LAMP1), another protein implicated in membrane resealing, was not evident using this form of muscle injury [49]. Further studies are required to better understand this larger membrane repair complex.
3.1.5 GRAF1
GTPase Regulator Associated with Focal adhesion kinase-1 (GRAF1) is an amino-terminal Bin–Amphiphysin–Rvs (BAR) containing protein that binds lipids including PS through its pleckstrin homology (PH) domain. GRAF1 also contains a catalytic RHO-GAP domain and SH3 domain, through which it interacts with Focal Adhesion Kinase (FAK) [81]. GRAF1 is highly expressed in striated muscle and GRAF1-null mice have reduced myofiber cross-sectional area and force production compared to normal muscle [60]. GRAF1-null myoblasts fuse poorly resulting in smaller myotubes in culture [81]. This is consistent with the increased expression of GRAF1 during muscle development, and its observed downregulation during differentiation of skeletal muscle. GRAF1 colocalizes with the fusogenic proteins myoferlin, Fer1L5, EHD1 and EHD2 at the tip of the elongated myoblasts [60]. GRAF1 is proposed to limit the polymerization of actin in the myoblast periphery. Bin3, another BAR domain-containing protein that regulates myoblast fusion, colocalizes with F-actin [82]. GRAF1 interacts with dynamin, further promoting a role for GRAF1 in modulating membrane scission, a function required during myoblast fusion and repair [81].
3.1.6 Myomaker (Tmem8c)
Myomaker (Tmem8c) is a highly conserved muscle-specific, multipass, transmembrane protein required for myoblast fusion [83]. Myomaker is exclusively expressed in the developing musculature, regulated by the muscle regulatory factor (MRF) myogenin. While myomaker expression is undetectable in mature muscle, transient expression is seen in satellite cells post-injury. Genetic disruption of myomaker in mice inhibits muscle maturation and results in embryonic lethality [83]. Notably, introduction of myomaker into fibroblasts, was sufficient to induce fibroblast fusion to myoblasts, although was insufficient to induce fibroblast to fibroblast fusion [83]. Depletion of myomaker in satellite cells using an inducible Pax7-Cre mouse model demonstrated that expression of myomaker in satellite cells is required for long-term muscle regeneration after injury [84]. Although myomaker has not been studied in membrane repair, its localization at the site of cell-cell contact during muscle fusion suggests a possible role for modulating the muscle membrane at the lesion [83].
3.1.7 Brain-specific Angiogenesis Inhibitors
Brain-specific Angiogenesis Inhibitors are members of the G-protein-coupled receptor family. The transmembrane family member, BAI1, recognizes PS on cells and signals through the Dock1/ELMO/Rac1 pathway [5, 8, 31, 85]. BAI1 KO mice are smaller than their littermates and have impaired muscle regeneration. This pathway is important not only for fusion but also in apoptosis. During muscle development and recovery from injury, apoptotic cells are required for normal myoblast fusion [86]. These apoptotic myoblasts induce a cell-cell signaling cascade to allow the healthy cells to fuse [86]. Transient external localization of PS is required for proper fusion, and the BAI1 PS receptor may serve as an important component for this externalization [16, 86, 87]. Overexpression of BAI1 in C2C12 cells increased myoblast fusion, indicating an important role in this process [86]. A second BAI family member, BAI3, has also been recently implicated in myoblast fusion Expression of mutant BAI3 inhibited myoblast fusion and this defect was unable to be rescued by BAI1 expression. [88]. However, both proteins do function in concert with ELMO [8, 88]. These data identify BAI proteins as important in fusion and regeneration.
3.1.8 Mucolipin-1
Mutations in MCOLN1, which encodes mucolipin-1, a Ca2+-nonselective cation channel, cause autosomal recessive lysosomal storage disease Mucolipidosis type IV (MLIV). While MLIV is generally considered a neurodegenerative disease, the mouse disease model, ML1 KO, displayed features of muscle disease including variable muscle fibers, centralized nuclei, and fibrosis. Muscle uptake of Evan’s Blue Dye and serum CK were increased after exercise, which indicate membrane leakage of the muscle. Laser-induced sarcolemmal disruption revealed a severe delay in membrane repair in a Ca2+-dependent manner, although levels of known repair proteins appeared normal [89]. These data suggest that MCOLN1 function is important for repair and may mediate this effect through alternative pathways.
Conclusions
The machinery that regulates myoblast fusion overlaps with the same protein and lipid components important for resealing a disrupted sarcolemma. After muscle injury, both fusion and resealing occur in order to effectively heal and restore muscle size and strength. The degree to which unique proteins or signaling pathways contribute to only myoblast fusion or sarcolemmal resealing is not fully known. Despite the mechanical aspects that differ between these pathways of membrane fusion, the regulators are at least partially shared. Moreover, these proteins and lipids may generally regulate membrane fusion events in many cell types. Muscle with its unique cellular morphology and physiological demands is highly susceptible to mutations that disrupt the normal processes of cell fusion.
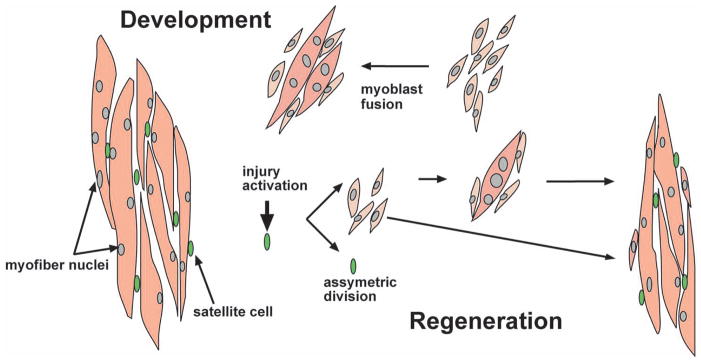
Fig. 2.
Myoblast fusion in mammals. During muscle growth satellite cells (green) give rise to mono-nucleated muscle precursor cells, myoblasts (light pink). Myoblasts fuse with one-another to generate nascent myotubes. During later steps of development, myoblasts fuse with existing myotubes promoting muscle growth. Upon injury, satellite cells are activated and asymmetrically divide generating a new pool of myoblasts. These myoblasts once again fuse with each other and to injured myotubes to promote muscle regeneration.
Table 1.
Proteins that function in myoblast fusion and muscle repair.
| Protein | Myoblast fusion | Regeneration | Membrane repair | Lipid binding | Actin | Fly homologue |
|---|---|---|---|---|---|---|
| EHD1 | Yes | Yes | ? | Yes | No | Past1 |
| EHD2 | Yes | Yes | Yes | Yes | Yes | |
| EHD3 | - | Yes | - | Yes | - | |
| EHD4 | - | - | - | Yes | - | |
| ANXA1 | Yes | Yes | Yes | Yes | Yes | AnnexinB9, B10, B11 |
| ANXA2 | - | - | Yes | Yes | Yes | |
| ANXA5 | Yes | - | Yes | Yes | Yes | |
| ANXA6 | - | Yes | Yes | Yes | Yes | |
| MYOF | Yes | Yes | Yes | Yes | Yes | Misfire |
| DYSF | Yes | Yes | Yes | Yes | Yes | |
| FER1L5 | Yes | - | - | - | - | |
| TMEM8c | Yes | Yes | - | - | - | CG13654 |
| GRAF1 | Yes | Yes | - | - | Yes | GRAF |
| MG53 | Yes | Yes | Yes | Yes | - | Unknown |
| CKB | Yes | - | - | - | - | ArgK |
A minus sign indicates no available data in the literature.
Acknowledgments
This work was supported by National Institutes of Health grants NS047726, NS072027, AR052646.
Abberviation
- CK
Creatine Kinase
- DMD
Duchenne Muscular Dystrophy
- EHD
Eps15 Homology Domain
- FC
Founder Cell
- FCM
Fusion Competent Myoblasts
- LGMD
limb girdle muscular dystrophy
- PH
pleckstrin homology
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PS
phosphatidyl serine
- T-tubule
transverse tubule
Footnotes
Conflict of Interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franzini-Armstrong C, Porter KR. Sarcolemmal Invaginations Constituting the T System in Fish Muscle Fibers. The Journal of cell biology. 1964;22:675–96. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell KP, Stull JT. Skeletal muscle basement membrane-sarcolemma-cytoskeleton interaction minireview series. The Journal of biological chemistry. 2003;278:12599–600. doi: 10.1074/jbc.R300005200. [DOI] [PubMed] [Google Scholar]
- 3.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiological reviews. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper ST, Head SI. Membrane Injury and Repair in the Muscular Dystrophies. Neuroscientist. 2014 doi: 10.1177/1073858414558336. [DOI] [PubMed] [Google Scholar]
- 5.Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012;139:641–56. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: when it takes more to make one. Developmental biology. 2010;341:66–83. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biressi S, Rando TA. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21:845–54. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Jin P, Duan R, Chen EH. Mechanisms of myoblast fusion during muscle development. Curr Opin Genet Dev. 2015;32:162–70. doi: 10.1016/j.gde.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bothe I, Deng S, Baylies M. PI(4,5)P2 regulates myoblast fusion through Arp2/3 regulator localization at the fusion site. Development. 2014;141:2289–301. doi: 10.1242/dev.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Ren Y, Ng WP, Li S, Son S, Kee YS, et al. Mechanical tension drives cell membrane fusion. Dev Cell. 2015;32:561–73. doi: 10.1016/j.devcel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simionescu A, Pavlath GK. Molecular mechanisms of myoblast fusion across species. Advances in experimental medicine and biology. 2011;713:113–35. doi: 10.1007/978-94-007-0763-4_8. [DOI] [PubMed] [Google Scholar]
- 12.San Miguel-Ruiz JE, Letourneau PC. The role of Arp2/3 in growth cone actin dynamics and guidance is substrate dependent. J Neurosci. 2014;34:5895–908. doi: 10.1523/JNEUROSCI.0672-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin NY, Choi H, Neff L, Wu Y, Saito H, Ferguson SM, et al. Dynamin and endocytosis are required for the fusion of osteoclasts and myoblasts. The Journal of cell biology. 2014;207:73–89. doi: 10.1083/jcb.201401137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–86. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Deleu M, Crowet JM, Nasir MN, Lins L. Complementary biophysical tools to investigate lipid specificity in the interaction between bioactive molecules and the plasma membrane: A review. Biochimica et biophysica acta. 2014;1838:3171–90. doi: 10.1016/j.bbamem.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Jeong J, Conboy IM. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochemical and biophysical research communications. 2011;414:9–13. doi: 10.1016/j.bbrc.2011.08.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taulet N, Comunale F, Favard C, Charrasse S, Bodin S, Gauthier-Rouviere C. N-cadherin/p120 catenin association at cell-cell contacts occurs in cholesterol-rich membrane domains and is required for RhoA activation and myogenesis. The Journal of biological chemistry. 2009;284:23137–45. doi: 10.1074/jbc.M109.017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukai A, Kurisaki T, Sato SB, Kobayashi T, Kondoh G, Hashimoto N. Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells. Experimental cell research. 2009;315:3052–63. doi: 10.1016/j.yexcr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Strunkelnberg M, Bonengel B, Moda LM, Hertenstein A, de Couet HG, Ramos RG, et al. rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development. 2001;128:4229–39. doi: 10.1242/dev.128.21.4229. [DOI] [PubMed] [Google Scholar]
- 20.Srinivas BP, Woo J, Leong WY, Roy S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat Genet. 2007;39:781–6. doi: 10.1038/ng2055. [DOI] [PubMed] [Google Scholar]
- 21.Dworak HA, Charles MA, Pellerano LB, Sink H. Characterization of Drosophila hibris, a gene related to human nephrin. Development. 2001;128:4265–76. doi: 10.1242/dev.128.21.4265. [DOI] [PubMed] [Google Scholar]
- 22.Shelton C, Kocherlakota KS, Zhuang S, Abmayr SM. The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development. 2009;136:1159–68. doi: 10.1242/dev.026302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn RL, Huang P, Kawahara G, Mitchell M, Guyon J, Kalluri R, et al. A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9274–9. doi: 10.1073/pnas.0904398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CA, Parkin CA, Bidet Y, Ingham PW. A role for the Myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development. 2007;134:3145–53. doi: 10.1242/dev.001214. [DOI] [PubMed] [Google Scholar]
- 25.Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, et al. ADAM12 and alpha9beta1 integrin are instrumental in human myogenic cell differentiation. Mol Biol Cell. 2005;16:861–70. doi: 10.1091/mbc.E04-03-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, et al. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–85. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Dowling JJ, Gibbs EM, Feldman EL. Membrane traffic and muscle: lessons from human disease. Traffic. 2008;9:1035–43. doi: 10.1111/j.1600-0854.2008.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZC, Geisbrecht ER. “Importin” signaling roles for import proteins: the function of Drosophila importin-7 (DIM-7) in muscle-tendon signaling. Cell Adh Migr. 2012;6:4–12. doi: 10.4161/cam.19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollnagel A, Grund C, Franke WW, Arnold HH. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Molecular and cellular biology. 2002;22:4760–70. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. Journal of cell science. 1995;108( Pt 9):2973–81. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]
- 31.Laurin M, Cote JF. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes & development. 2014;28:533–47. doi: 10.1101/gad.236349.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biersmith B, Wang ZH, Geisbrecht ER. Fine-Tuning of the Actin Cytoskeleton and Cell Adhesion During Drosophila Development by the Unconventional Guanine Nucleotide Exchange Factors Myoblast City and Sponge. Genetics. 2015 doi: 10.1534/genetics.115.177063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namekata K, Kimura A, Kawamura K, Harada C, Harada T. Dock GEFs and their therapeutic potential: neuroprotection and axon regeneration. Prog Retin Eye Res. 2014;43:1–16. doi: 10.1016/j.preteyeres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Cote JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15446–51. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. Journal of cell science. 1997;110( Pt 9):1073–81. doi: 10.1242/jcs.110.9.1073. [DOI] [PubMed] [Google Scholar]
- 36.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–6. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 38.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–13. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–72. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 40.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. The Journal of biological chemistry. 2002;277:22883–8. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- 41.Lek A, Evesson FJ, Sutton RB, North KN, Cooper ST. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic. 2012;13:185–94. doi: 10.1111/j.1600-0854.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 42.Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Human molecular genetics. 2000;9:217–26. doi: 10.1093/hmg/9.2.217. [DOI] [PubMed] [Google Scholar]
- 43.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, et al. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565–75. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Therrien C, Di Fulvio S, Pickles S, Sinnreich M. Characterization of lipid binding specificities of dysferlin C2 domains reveals novel interactions with phosphoinositides. Biochemistry. 2009;48:2377–84. doi: 10.1021/bi802242r. [DOI] [PubMed] [Google Scholar]
- 45.Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, et al. Dysferlin is a plasma membrane protein and is expressed early in human development. Human molecular genetics. 1999;8:855–61. doi: 10.1093/hmg/8.5.855. [DOI] [PubMed] [Google Scholar]
- 46.Roche JA, Ru LW, O’Neill AM, Resneck WG, Lovering RM, Bloch RJ. Unmasking potential intracellular roles for dysferlin through improved immunolabeling methods. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59:964–75. doi: 10.1369/0022155411423274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klinge L, Harris J, Sewry C, Charlton R, Anderson L, Laval S, et al. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve. 2010;41:166–73. doi: 10.1002/mus.21166. [DOI] [PubMed] [Google Scholar]
- 48.Kerr JP, Ziman AP, Mueller AL, Muriel JM, Kleinhans-Welte E, Gumerson JD, et al. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20831–6. doi: 10.1073/pnas.1307960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders AK, Turnbull L, et al. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33:5085–94. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, et al. Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Human molecular genetics. 2004;13:1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- 51.Demonbreun AR, Fahrenbach JP, Deveaux K, Earley JU, Pytel P, McNally EM. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Human molecular genetics. 2010 doi: 10.1093/hmg/ddq522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demonbreun AR, Rossi AE, Alvarez MG, Swanson KE, Deveaux HK, Earley JU, et al. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. The American journal of pathology. 2014;184:248–59. doi: 10.1016/j.ajpath.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Luna N, Gallardo E, Soriano M, Dominguez-Perles R, de la Torre C, Rojas-Garcia R, et al. Absence of dysferlin alters myogenin expression and delays human muscle differentiation “in vitro”. The Journal of biological chemistry. 2006;281:17092–8. doi: 10.1074/jbc.M601885200. [DOI] [PubMed] [Google Scholar]
- 54.Doherty KR, Demonbreun AR, Wallace GQ, Cave A, Posey AD, Heretis K, et al. The Endocytic Recycling Protein EHD2 Interacts with Myoferlin to Regulate Myoblast Fusion. The Journal of biological chemistry. 2008;283:20252–60. doi: 10.1074/jbc.M802306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lostal W, Bartoli M, Roudaut C, Bourg N, Krahn M, Pryadkina M, et al. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PloS one. 2012;7:e38036. doi: 10.1371/journal.pone.0038036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–52. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Posey AD, Jr, Pytel P, Gardikiotes K, Demonbreun AR, Rainey M, George M, et al. Endocytic recycling proteins EHD1 and EHD2 interact with fer-1-like-5 (Fer1L5) and mediate myoblast fusion. The Journal of biological chemistry. 2011;286:7379–88. doi: 10.1074/jbc.M110.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marg A, Schoewel V, Timmel T, Schulze A, Shah C, Daumke O, et al. Sarcolemmal repair is a slow process and includes EHD2. Traffic. 2012;13:1286–94. doi: 10.1111/j.1600-0854.2012.01386.x. [DOI] [PubMed] [Google Scholar]
- 59.Posey AD, Jr, Swanson KE, Alvarez MG, Krishnan S, Earley JU, Band H, et al. EHD1 mediates vesicle trafficking required for normal muscle growth and transverse tubule development. Developmental biology. 2014;387:179–90. doi: 10.1016/j.ydbio.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenhart KC, Becherer AL, Li J, Xiao X, McNally EM, Mack CP, et al. GRAF1 promotes ferlin-dependent myoblast fusion. Developmental biology. 2014;393:298–311. doi: 10.1016/j.ydbio.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gudmundsson H, Curran J, Kashef F, Snyder JS, Smith SA, Vargas-Pinto P, et al. Differential regulation of EHD3 in human and mammalian heart failure. Journal of molecular and cellular cardiology. 2012;52:1183–90. doi: 10.1016/j.yjmcc.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blackwood RA, Ernst JD. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. The Biochemical journal. 1990;266:195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaks WJ, Creutz CE. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991;30:9607–15. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]
- 64.Gunteski-Hamblin AM, Song G, Walsh RA, Frenzke M, Boivin GP, Dorn GW, 2nd, et al. Annexin VI overexpression targeted to heart alters cardiomyocyte function in transgenic mice. The American journal of physiology. 1996;270:H1091–100. doi: 10.1152/ajpheart.1996.270.3.H1091. [DOI] [PubMed] [Google Scholar]
- 65.Bizzarro V, Fontanella B, Franceschelli S, Pirozzi M, Christian H, Parente L, et al. Role of Annexin A1 in mouse myoblast cell differentiation. Journal of cellular physiology. 2010;224:757–65. doi: 10.1002/jcp.22178. [DOI] [PubMed] [Google Scholar]
- 66.Leikina E, Melikov K, Sanyal S, Verma SK, Eun B, Gebert C, et al. Extracellular annexins and dynamin are important for sequential steps in myoblast fusion. The Journal of cell biology. 2013;200:109–23. doi: 10.1083/jcb.201207012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Babbin BA, Laukoetter MG, Nava P, Koch S, Lee WY, Capaldo CT, et al. Annexin A1 regulates intestinal mucosal injury, inflammation, and repair. J Immunol. 2008;181:5035–44. doi: 10.4049/jimmunol.181.7.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, Ivanov AI, et al. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. The American journal of pathology. 2007;170:951–66. doi: 10.2353/ajpath.2007.060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. The Journal of biological chemistry. 2003 doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 70.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. The Journal of biological chemistry. 2006;281:35202–7. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 71.Roostalu U, Strahle U. In vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell. 2012;22:515–29. doi: 10.1016/j.devcel.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 72.Swaggart KA, Demonbreun AR, Vo AH, Swanson KE, Kim EY, Fahrenbach JP, et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6004–9. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rong B, Zhao C, Liu H, Ming Z, Cai X, Gao W, et al. Elevated serum annexin A1 as potential diagnostic marker for lung cancer: a retrospective case-control study. American journal of translational research. 2014;6:558–69. [PMC free article] [PubMed] [Google Scholar]
- 75.Gurluler E, Guner OS, Tumay LV, Turkel Kucukmetin N, Hizli B, Zorluoglu A. Serum annexin A2 levels in patients with colon cancer in comparison to healthy controls and in relation to tumor pathology. Medical science monitor : international medical journal of experimental and clinical research. 2014;20:1801–7. doi: 10.12659/MSM.892319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cagliani R, Magri F, Toscano A, Merlini L, Fortunato F, Lamperti C, et al. Mutation finding in patients with dysferlin deficiency and role of the dysferlin interacting proteins annexin A1 and A2 in muscular dystrophies. Hum Mutat. 2005;26:283. doi: 10.1002/humu.9364. [DOI] [PubMed] [Google Scholar]
- 77.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27:1147–57. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- 78.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. The Journal of biological chemistry. 2009;284:15894–902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science translational medicine. 2012;4:139ra85. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, et al. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropathol Exp Neurol. 2011;70:302–13. doi: 10.1097/NEN.0b013e31821350b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doherty JT, Lenhart KC, Cameron MV, Mack CP, Conlon FL, Taylor JM. Skeletal muscle differentiation and fusion are regulated by the BAR-containing Rho-GTPase-activating protein (Rho-GAP), GRAF1. The Journal of biological chemistry. 2011;286:25903–21. doi: 10.1074/jbc.M111.243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simionescu-Bankston A, Leoni G, Wang Y, Pham PP, Ramalingam A, DuHadaway JB, et al. The N-BAR domain protein, Bin3, regulates Rac1- and Cdc42-dependent processes in myogenesis. Developmental biology. 2013;382:160–71. doi: 10.1016/j.ydbio.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Millay DP, O’Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R, et al. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–5. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Millay DP, Sutherland LB, Bassel-Duby R, Olson EN. Myomaker is essential for muscle regeneration. Genes & development. 2014;28:1641–6. doi: 10.1101/gad.247205.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–4. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 86.Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–7. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. Journal of cell science. 2001;114:3631–42. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 88.Hamoud N, Tran V, Croteau LP, Kania A, Cote JF. G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3745–50. doi: 10.1073/pnas.1313886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, et al. The intracellular Ca(2)(+) channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med. 2014;20:1187–92. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]